Sulfur loss from subducted altered oceanic crust and implications for mantle oxidation
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:1,923Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
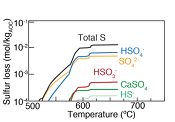
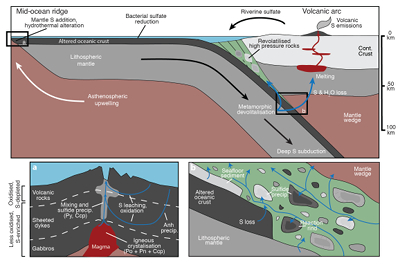 Figure 1 Schematic diagram showing the broader subduction zone system. Sulfur is introduced to the oceanic crust during crystallisation and hydrothermal circulation. (a) Magmatic sulfides react to form pyrite ± chalcopyrite and iron is oxidised (see Supplementary Information S-1). Sulfur is later extracted during metamorphic devolatilisation. (b) Sulfides precipitate in reaction rinds formed upon exhumation of metamorphic rocks along the slab mantle interface, while the remaining sulfur infiltrates the overlying subarc mantle. | 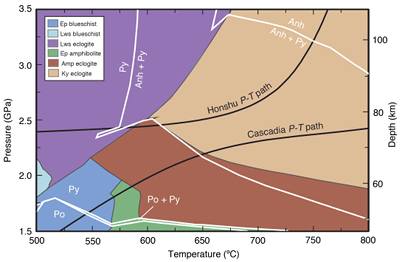 Figure 2 Closed system phase equilibrium modelling results utilising an average AOC composition from ODP hole 504B. The stability fields of common rock types are shaded (see key) and the stable solid sulfur phases are also highlighted. Honshu and Cascadia slab top P-T paths after Syracuse et al. (2010). | 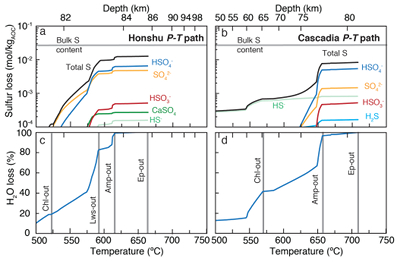 Figure 3 Cumulative sulfur (a,b) and fluid (c,d) loss diagrams for fluids fractionated along the Honshu and Cascadia P-T paths. Both temperature and corresponding slab top depth are plotted on the x-axis. Key dehydration reactions are shown for reference. | 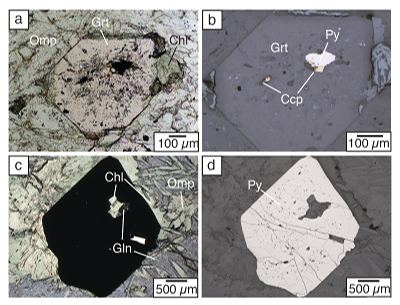 Figure 4 Transmitted (a,c) and reflected (b,d) light images of sulfides in eclogites. In (a,b), pyrite + chalcopyrite are observed as inclusions in garnet from the Junction School eclogite, Franciscan Complex, California. In (c,d), matrix pyrite is associated with retrograde chlorite + glaucophane in a metasomatised metagabbroic eclogite from Syros, Greece. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Subduction may influence the oxygen fugacity (fO2) of the mantle and mantle-derived magmas through the introduction of hydrated and oxidised altered oceanic crust (AOC, Fig. 1; Evans, 2012
Evans, K.A. (2012) The redox budget of subduction zones. Earth Science Reviews 113, 11-32.
). Mantle fO2 can regulate mantle rheology and density through changes in mineralogy; for example, the H2O content of nominally anhydrous minerals is a function of fO2 (McCammon et al., 2004McCammon, C.A., Frost, D.J., Smyth, J.R., Lautsen, H.M.S., Kawamoto, T., Ross, N.L., van Aken, P.A. (2004) Oxidation state of iron in hydrous mantle phases: implications for subduction and mantle oxygen fugacity. Physics of the Earth and Planetary Interiors 143-144, 157-169.
). As a result, secular changes in mantle fO2 induced by plate tectonics may drive variations in mantle circulation (Mackwell, 2008Mackwell, S. (2008) Rheological consequences of redox state. Reviews in Mineralogy and Geochemistry 68, 555-569.
). However, the link between redox sensitive elements in subducting slabs and mantle fO2 at subduction zones remains elusive.Mounting evidence suggests that mantle fO2 evolves in response to a transfer of oxidised slab components. For example, peridotite xenoliths from Mexico record fO2 conditions 1.5–2.4 log units above the quartz-fayalite-magnetite (QFM) buffer (Blatter and Carmichael, 1998
Blatter, D.L., Carmichael, I.S. (1998) Hornblende peridotite xenoliths from central Mexico reveal the highly oxidized nature of subarc mantle. Geology 26, 1035-1038.
), whereas mid-ocean ridge peridotite overlaps with QFM (Birner et al., 2018Birner, S.K., Cottrell, E., Warren, J.M., Kelley, K.A., Davis, F.A. (2018) Peridotites and basalts reveal broad congruence between two independent recorders of mantle fO2 despite local redox heterogeneity. Earth and Planetary Science Letters 494, 172-189.
). Similarly, arc magmas are oxidised relative to mid-ocean ridge basalts (MORB; Kelley and Cottrell, 2009Kelley, K., Cottrell, E. (2009) Water and the oxidation state of subduction zone magmas. Science 325, 605-607.
, 2012Kelley, K., Cottrell, E., (2012) The influence of magmatic differentiation on the oxidation state of Fe in a basaltic arc magma. Earth and Planetary Science Letters 329-330, 109-121.
; Cottrell and Kelley, 2011Cottrell, E., Kelley, K. (2011) The oxidation state of Fe in MORB glasses and the oxygen fugacity of the upper mantle. Earth and Planetary Science Letters 305, 270-282.
; Brounce et al., 2014Brounce, M.N., Kelley, K.A., Cottrell, E. (2014) Variations in Fe3+/ΣFe of Mariana arc basalts and mantle wedge fO2. Journal of Petrology 55, 2514-2536.
). The Fe3+/ΣFe ratios of arc magmas positively correlate with geochemical indicators of material addition from the slab to the magma sources, such as Ba/La ratios (Kelley and Cottrell, 2009Kelley, K., Cottrell, E. (2009) Water and the oxidation state of subduction zone magmas. Science 325, 605-607.
); therefore, elevated mantle fO2 along convergent margins is both spatially and chemically linked to the subducting slab.Such a link may require the transfer of redox sensitive elements from slab lithologies, which are oxidised relative to mantle peridotite. Early studies hypothesised the introduction of slab Fe3+ (e.g., Lecuyer and Ricard, 1999
Lecuyer, C., Ricard, Y. (1999) Long-term fluxes and budget of ferric iron: implications for the redox states of the Earth’s mantle and atmosphere. Earth and Planetary Science Letters 165, 197-211.
); however, the solubility of Fe3+ in hydrous fluids is low (Mungall, 2002Mungall, J.E. (2002) Roasting the mantle: Slab melting and the genesis of major Au and Au-rich Cu deposits. Geology 30, 915-918.
). Instead, volatiles likely play a more important role (Evans, 2012Evans, K.A. (2012) The redox budget of subduction zones. Earth Science Reviews 113, 11-32.
). Of these, only H, C, and S potentially occur in sufficient abundance to influence the redox state of the mantle. Sulfur and carbon are fluid mobile, exhibit an eight electron range in oxidation states, are subducted at global rates on the order of 1012 mol/yr, and may act as important vectors for transferring oxidation state (Evans, 2012Evans, K.A. (2012) The redox budget of subduction zones. Earth Science Reviews 113, 11-32.
). Significant work has focused on decarbonation during subduction, whereas sulfur loss remains less explored. This is despite the fact that the transition from S2- to SO42- occurs at more oxidising conditions relative to the C-CO2 transition (Fig. S-1). Oxidised carbon is stable at normal upper mantle P–T–fO2; therefore, slab-derived CO2 fluxes are unable to initiate Fe oxidising reactions in the mantle wedge (see Supplementary Information S-2). In contrast, a flux of oxidised sulfur may raise log(fO2) of the subarc mantle to ~QFM + 2, consistent with the range commonly observed in subarc mantle xenoliths (e.g., Blatter and Carmichael, 1998Blatter, D.L., Carmichael, I.S. (1998) Hornblende peridotite xenoliths from central Mexico reveal the highly oxidized nature of subarc mantle. Geology 26, 1035-1038.
). Sulfur thus remains the most powerful oxidising agent in subduction zones.Recent studies have favoured either reduced (H2S, HS-) or oxidised (SO42-) sulfur species in slab fluids, with the potential to reduce or oxidise the subarc mantle (e.g., Evans et al., 2014
Evans, K.A., Tomkins, A.G., Cliff, J., Fiorentini, M.L. (2014) Insights into subduction zone sulfur recycling from isotopic analysis of eclogite-hosted sulfides. Chemical Geology 365, 1-19.
; Tomkins and Evans, 2015Tomkins, A.G., Evans, K.A. (2015) Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth and Planetary Science Letters 428, 73-83.
; Walters et al., 2019Walters, J.B., Cruz-Uribe, A.M., Marschall, H.R. (2019) Isotopic compositions of sulfides in exhumed high-pressure terranes: Implications for sulfur cycling in subduction zones. Geochemistry Geophysics Geosystems 20, 1-28.
). We address this discrepancy through thermodynamic modelling using the Gibbs free energy minimisation software Perple_X (Connolly, 2005Connolly, J.A.D. (2005) Computation of phase equilibria by linear programming: a tool for geodynamic modelling and its application to subduction zone decarbonation. Earth and Planetary Science Letters 236, 524-541.
) to predict sulfur speciation in AOC-derived fluids (see Supplementary Information S-3). This enables us to assess the potential of sulfur to influence mantle and arc fO2 as a function of slab composition and subduction zone thermal structure. We predict that oxidised sulfur species are dominant in slab fluids at eclogite facies conditions and may oxidise arc magmas and the subarc mantle.
Figure 1 Schematic diagram showing the broader subduction zone system. Sulfur is introduced to the oceanic crust during crystallisation and hydrothermal circulation. (a) Magmatic sulfides react to form pyrite ± chalcopyrite and iron is oxidised (see Supplementary Information S-1). Sulfur is later extracted during metamorphic devolatilisation. (b) Sulfides precipitate in reaction rinds formed upon exhumation of metamorphic rocks along the slab mantle interface, while the remaining sulfur infiltrates the overlying subarc mantle.
top
Squeezing Sulfur from the Slab
Metamorphic assemblages predicted over a P–T range of 1.5–3.5 GPa and 500–800 °C for average AOC (Fig. 2) show a change from reduced to oxidised sulfur species with increasing depth. Sulfide and sulfate minerals comprise <0.5 vol. % of the rocks, consistent with their low abundance in most high pressure rocks. Pyrrhotite is stable below 1.8 GPa and <680 °C, above which it is replaced by pyrite. Pyrite is converted to anhydrite with increasing P–T (e.g., >2.3 GPa at 650 °C). Anhydrite is the only sulfur phase at pressures above 2.8–3.3 GPa (Fig. 2). Sulfur oxidation is balanced by reduction of ferric iron, resulting in a decrease in Fe3+/ΣFe from 0.28 to 0.13–0.17 (Fig. S-4) in the dehydrated slab residue (i.e. eclogite). Assemblages were also predicted for average MORB (Figs. S-6 and S-7) with an initial Fe3+/ΣFe of 0.14. Although the compositions used here are averages, a comparison of AOC and MORB allows us to assess the degree to which alteration of the oceanic crust may influence sulfur loss. The sulfur oxidising reactions are shifted to higher pressures and Fe3+/ΣFe is constant with depth in the MORB model (Fig. S-10), suggesting that prograde sulfur oxidation varies as a function of pre-subduction seafloor alteration.

Figure 2 Closed system phase equilibrium modelling results utilising an average AOC composition from ODP hole 504B. The stability fields of common rock types are shaded (see key) and the stable solid sulfur phases are also highlighted. Honshu and Cascadia slab top P-T paths after Syracuse et al. (2010)
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
.Sulfur concentration and speciation in slab fluids vary as a function of subduction zone thermal structure, which we demonstrate by comparing open system models in which fluids are fractionated at each P–T step along contrasting geothermal gradients (D80; Syracuse et al., 2010
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
). The Honshu (‘cold’) and Cascadia (‘hot’) models for average AOC display some similarity: fluid compositions transition from H2S–HS- to HSO4-–HSO3- dominant as the anhydrite-in reaction is approached with depth. Similarly, sulfur concentrations increase with depth along both paths. In the Honshu model, dissolved sulfur concentrations increase from 2.3 mmol/kg HS-, 0.1 mmol/kg H2S, and 0.5 mmol/kg HSO4- at 2.4 GPa and 500 °C to 481 mmol/kg HSO4-, 51.6 mmol/kg HSO3-, and 93.1 mmol/kg SO42- at 2.5 GPa and 660 °C, the last P-T step before H2O is exhausted (Fig. S-3). Concentration and speciation are similar in the high P–T region of the Cascadia model, yet at low P–T, H2S and HS- concentrations are two orders of magnitude greater than those in the Honshu model. In the Honshu model, sulfur loss is largely in the form of oxidised species during lawsonite breakdown at depths of 82 to 85 km (Fig. 3). In contrast, in the Cascadia model HS- and H2S dominate in the shallow region (<75 km), where nearly 60 % of the H2O is lost (Fig. 3). We conclude from these models that hot subduction zones should exhibit shallow reduced and deep oxidised sulfur fluxes, whereas cold subduction zones will be dominated by the release of oxidised sulfur.
Figure 3 Cumulative sulfur (a,b) and fluid (c,d) loss diagrams for fluids fractionated along the Honshu and Cascadia P-T paths. Both temperature and corresponding slab top depth are plotted on the x-axis. Key dehydration reactions are shown for reference.
Oxidation of the oceanic crust enhances sulfur loss during subduction. Despite higher bulk rock sulfur and similar H2O contents, sulfur fluid concentrations are an order of magnitude lower for MORB compared to AOC, and little oxidised sulfur is released (Figs. S-8 and S-9). These data suggest heterogenous sulfur loss within slabs, where the oxidised volcanic section of the crust is likely to exhibit greater sulfur loss than deeper lithologies (see Supplementary Information S-1). The AOC composition modelled in this study is an average of 6.6 Ma crust formed at a fast spreading centre. More pervasively altered oceanic crust formed at slow spreading centres may exhibit greater sulfur loss. However, these effects will be balanced by the degree to which sulfur is removed during oxidative alteration (see Supplementary Information S-1).
Our results are similar to those of Debret and Sverjensky (2017)
Debret, B., Sverjensky, D.A. (2017) Highly oxidising fluids generated during serpentinite breakdown in subduction zones. Scientific Reports 7,10351.
for subducted serpentinite, where SOx species were calculated to be released at 630–660 °C and 2.0 GPa. Similarly, Tomkins and Evans (2015)Tomkins, A.G., Evans, K.A. (2015) Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth and Planetary Science Letters 428, 73-83.
predicted dissolution of seafloor anhydrite in MORB to also occur over the blueschist-to-eclogite transition modelled here, potentially enhancing the oxidised nature of slab fluids released at these depths. In contrast, Li et al. (2020)Li, J.L., Schwarzenbach, E.M., John, T., Ague, J.J., Huang, F., Gao, J., Klemd, R., Whitehouse, M.J., Wang, X.S. (2020) Uncovering and quantifying the subduction zone sulfur cycle from the slab perspective. Nature Communications 11, 514.
predicted the release of reduced sulfur species during the subduction of mafic crust; however, the reduced nature of these fluids is a result of fixing fO2 at QFM to QFM -- 3 along the dehydration path. We suggest that these models are similar to our MORB models but are inconsistent with the elevated fO2 of AOC.top
The Exhumed Rock Record
Our models are consistent with the petrographic and isotopic record from exhumed high pressure rocks. Under regional metamorphic conditions, pyrite (S-) is commonly replaced by pyrrhotite (S2-) with increasing grade (e.g., Tracy and Robinson, 1988
Tracy, R.J., Robinson, P. (1988) Silicate-sulfide-oxide-fluid reactions in granulite-grade pelitic rocks, central Massachusetts. American Journal of Science 288-A, 45-74.
). This mechanism was adopted in earlier models of pyrite behaviour during subduction of AOC (e.g., Evans et al., 2014Evans, K.A., Tomkins, A.G., Cliff, J., Fiorentini, M.L. (2014) Insights into subduction zone sulfur recycling from isotopic analysis of eclogite-hosted sulfides. Chemical Geology 365, 1-19.
). Such reactions, balanced by the oxidation of Fe2+ to Fe3+ in silicates or oxides, would produce H2S in the presence of water during subduction (see review in Walters et al., 2019Walters, J.B., Cruz-Uribe, A.M., Marschall, H.R. (2019) Isotopic compositions of sulfides in exhumed high-pressure terranes: Implications for sulfur cycling in subduction zones. Geochemistry Geophysics Geosystems 20, 1-28.
). However, our data suggest that sulfur oxidation is balanced by iron reduction. The available Fe3+/ΣFe data for high pressure minerals combined with mass balance constraints show that eclogites are reduced relative to blueschists and AOC (see Supplementary Information S-4). Iron reduction during the blueschist to eclogite facies transition in natural rocks has to be balanced by the oxidation of another element, and we suggest that sulfur is the only possible candidate and its prograde oxidation provides the mechanism.Further support for our model comes from a recent isotopic study of sulfides precipitated from slab-derived fluids during the exhumation of high pressure rocks. The ~36 ‰ (δ34S) variation among metasomatic sulfides suggests that large fractionations (10–20 ‰) were induced by precipitation from oxidised sulfur-bearing slab fluids (Walters et al., 2019
Walters, J.B., Cruz-Uribe, A.M., Marschall, H.R. (2019) Isotopic compositions of sulfides in exhumed high-pressure terranes: Implications for sulfur cycling in subduction zones. Geochemistry Geophysics Geosystems 20, 1-28.
). In addition, a recent study of Fe isotopes in high pressure garnet from Sifnos (Greece) suggests that lawsonite dehydration is accompanied by iron reduction in low temperature eclogites, likely due to a loss of SOx species (Gerrits et al., 2019Gerrits, A.R., Inglis, E., Dragovic, B., Starr, P.G., Baxter, E.F., Burton, K. (2019) Tracing the source of oxidizing fluids in subduction zones using iron isotopes in garnet. Nature Geoscience 12, 1029-1033.
). This is consistent with the predictions of our Honshu model.Sulfates are observed in fluid and solid inclusions in samples of subducted oceanic crust metamorphosed at relatively low pressures of 1.5–2.5 GPa (Frezzotti and Ferrando, 2015
Frezzotti, M.L., Ferrando, S. (2015) The chemical behavior of fluids released during deep subduction based on fluid inclusions. American Mineralogist 100, 352-377.
). This is consistent with our models for AOC, in which the sulfide-sulfate transition occurs at 2.0–2.5 GPa. In contrast, in more reduced (relative to AOC) eclogites embedded in subducted continental crust, sulfate-bearing inclusions are more commonly observed in rocks metamorphosed at pressures >3.0 GPa (Frezzotti and Ferrando, 2015Frezzotti, M.L., Ferrando, S. (2015) The chemical behavior of fluids released during deep subduction based on fluid inclusions. American Mineralogist 100, 352-377.
). In agreement with these observations, we show that the sulfide-sulfate transition is shifted to >3.0 GPa in more reduced lithologies. However, there are limited observations of solid phase inclusions within subducted oceanic crust, and sample preparation with water during cutting, grinding, and polishing is likely to remove anhydrite.Sulfides are a common trace phase found as inclusions in eclogitic minerals (Fig. 4a,b; see review in Walters et al., 2019
Walters, J.B., Cruz-Uribe, A.M., Marschall, H.R. (2019) Isotopic compositions of sulfides in exhumed high-pressure terranes: Implications for sulfur cycling in subduction zones. Geochemistry Geophysics Geosystems 20, 1-28.
). Brown et al. (2014)Brown, J.L., Christy, A.G., Ellis, D.J., Arculus, R.J. (2014) Prograde sulfide metasomatism in blueschist and eclogite, New Caledonia. Journal of Petrology 55, 643-670.
observed prograde replacement of pyrrhotite-bearing inclusion assemblages by pyrite-bearing assemblages between 1.4-1.5 GPa and 450-500 ºC and 1.9 GPa and 600 ºC in New Caledonian HP rocks, consistent with the transformation of pyrrhotite to pyrite predicted by our models (Fig. 2). Matrix sulfides are commonly associated with hydrous metasomatic Fe3+-rich phases, such as epidote, amphibole, and chlorite (Fig. 4c,d), consistent with fluid mediated sulfur reducing reactions.
Figure 4 Transmitted (a,c) and reflected (b,d) light images of sulfides in eclogites. In (a,b), pyrite + chalcopyrite are observed as inclusions in garnet from the Junction School eclogite, Franciscan Complex, California. In (c,d), matrix pyrite is associated with retrograde chlorite + glaucophane in a metasomatised metagabbroic eclogite from Syros, Greece.
The scarcity of peak eclogite facies matrix sulfides combined with remnant prograde sulfide inclusions suggests that sulfur loss is nearly complete following dehydration of AOC; however, our models predict that 55 to 75 % of the sulfur should remain in the slab (Fig. S-4). Dissolved NaCl dramatically increases anhydrite dissolution (Newton and Manning, 2004), but is not so far incorporated into our models. Therefore, SOx concentrations are likely underpredicted in our models. Additional sulfur may also be mobilised by external fluids; however, fluids in these environments are likely channelised (e.g., Zack and John, 2007
Zack, T., John, T. (2007) An evaluation of reactive fluid flow and trace element mobility in subducting slabs. Chemical Geology 239, 199-216.
), such that external fluids will not liberate significant amounts of sulfur from the majority of the subducted crust. Regardless, our current models likely provide a correct estimate of sulfur speciation and a minimum estimate for the total contribution from subducting AOC to the sulfur budget of arcs.top
Oxidising the Arc
The migration of sulfate-bearing fluids from dehydrating AOC will oxidise the upper mantle. Upon contact with more reduced conditions of the mantle (~QFM), sulfides are expected to precipitate from slab fluids to oxidise Fe and hydrate the mantle wedge, a process which accounts for the QFM to QFM + 2 range in fO2 reported for subarc peridotite xenoliths (see Supplementary Information S-2). Metasomatism is likely to strip slab fluids of their oxidising capability before reaching melt generation regions. Fluid loss is predicted in our models at depths (60 to 80 km) less than those of the slab top directly below many volcanic arcs (~100 km; Syracuse et al., 2010
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
). However, deflection of metasomatised mantle or mélange diapirs by corner flow may carry the oxidised mantle to the region of melt generation beneath the arc (Spandler and Pirard, 2013Spandler, C., Pirard, C. (2013) Element recycling from subducting slabs to arc crust: A review. Lithos 170-171, 208-223.
). Bénard et al. (2018)Bénard, A., Klimm, K., Woodland, A.B., Arculus, R.J., Wilke, M., Botcharnikov, R.E., Shimizu, N., Nebel, O., Rivard, C., Ionov, D.A. (2018) Oxidising agents in sub-arc mantle melts link slab devolatilization and arc magmas. Nature Communications 9, 1-10.
identified anhydrite and SO42--bearing inclusions in arc peridotite xenoliths, which may suggest that sulfate-bearing fluids penetrate deep into the subarc mantle. However, the input of sulfur into subduction zones is in excess of the volcanic output (Evans, 2012Evans, K.A. (2012) The redox budget of subduction zones. Earth Science Reviews 113, 11-32.
), suggesting that some sulfur may be stored in the mantle wedge.Our models allow us to predict on a global scale the influence of sulfur loss on the redox state of volcanic arcs. During “hot” subduction, ~30 % of the total sulfur loss occurs as H2S and HS- at shallow depths prior to the sulfide-sulfate transition, followed by a loss of oxidised sulfur at greater depths. Little sulfur is lost prior to the sulfide-sulfate transition in “cold” subduction zones. Therefore, the shallow mantle wedge and forearc magmas may be less oxidised than their arc equivalents. This mechanism is consistent with low Fe3+/ΣFe and sulfur concentrations in Mariana forearc magmas relative to those in the main Mariana arc (Kelley and Cottrell, 2012
Kelley, K., Cottrell, E., (2012) The influence of magmatic differentiation on the oxidation state of Fe in a basaltic arc magma. Earth and Planetary Science Letters 329-330, 109-121.
; Brounce et al., 2014Brounce, M.N., Kelley, K.A., Cottrell, E. (2014) Variations in Fe3+/ΣFe of Mariana arc basalts and mantle wedge fO2. Journal of Petrology 55, 2514-2536.
, 2016Brounce, M., Kelley, K.A., Stern, R., Martinez, F., Cottrell, E. (2016) The Fina Nagu volcanic complex: Unusual submarine arc volcanism in the rapidly deforming southern Mariana margin. Geochemistry Geophysics Geosystems 17, 4078-4091.
). The predicted SOx fluxes are also consistent with the oxidised nature of trench proximal Cu porphyry deposits in the Andes and elsewhere (e.g., Tomkins and Evans, 2015Tomkins, A.G., Evans, K.A. (2015) Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth and Planetary Science Letters 428, 73-83.
). As a result, arc magmas and subarc mantle in cold subduction zones may be more oxidised relative to hot subduction zones.top
Summary
We have demonstrated that sulfur–iron redox reactions in subducting AOC can produce oxidised fluids. Our model predictions are consistent with the petrographic, isotopic, and geochemical record of exhumed high pressure rocks, mantle xenoliths, and arc magmas. Sulfur release is a function of the redox budget of the slab and subduction zone thermal structure, with cold subduction of oxidised crust most efficiently releasing SOx species. We conclude that sulfur-bearing fluids from AOC may oxidise the arc-mantle system. However, the thermodynamics of sulfur-bearing silicate systems, Fe3+ incorporation in silicate minerals, and aqueous sulfur speciation at high pressures remain poorly constrained. It should be a priority for future studies to constrain further our model through the study of natural samples and high pressure experiments.
top
Acknowledgements
We thank James Connolly for modelling support and Peter van Keken for providing updated P–T paths for the Syracuse et al. (2010)
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
models. The manuscript benefited from the editorial handling by Helen Williams and from constructive reviews of Maryjo Brounce, Katy Evans, and an anonymous reviewer. JBW acknowledges Fulbright and Chase Distinguished Research fellowships. This work was supported by NSF grant EAR1725301 awarded to AMC.Editor: Helen Williams
top
References
Bénard, A., Klimm, K., Woodland, A.B., Arculus, R.J., Wilke, M., Botcharnikov, R.E., Shimizu, N., Nebel, O., Rivard, C., Ionov, D.A. (2018) Oxidising agents in sub-arc mantle melts link slab devolatilization and arc magmas. Nature Communications 9, 1-10.
 Show in context
Show in context Bénard et al. (2018) identified anhydrite and SO42--bearing inclusions in arc peridotite xenoliths, which may suggest that sulfate-bearing fluids penetrate deep into the subarc mantle.
View in article
Birner, S.K., Cottrell, E., Warren, J.M., Kelley, K.A., Davis, F.A. (2018) Peridotites and basalts reveal broad congruence between two independent recorders of mantle fO2 despite local redox heterogeneity. Earth and Planetary Science Letters 494, 172-189.
 Show in context
Show in context For example, peridotite xenoliths from Mexico record fO2 conditions 1.5–2.4 log units above the quartz-fayalite-magnetite (QFM) buffer (Blatter and Carmichael, 1998), whereas mid-ocean ridge peridotite overlaps with QFM (Birner et al., 2018).
View in article
Blatter, D.L., Carmichael, I.S. (1998) Hornblende peridotite xenoliths from central Mexico reveal the highly oxidized nature of subarc mantle. Geology 26, 1035-1038.
 Show in context
Show in context For example, peridotite xenoliths from Mexico record fO2 conditions 1.5–2.4 log units above the quartz-fayalite-magnetite (QFM) buffer (Blatter and Carmichael, 1998), whereas mid-ocean ridge peridotite overlaps with QFM (Birner et al., 2018).
View in article
In contrast, a flux of oxidised sulfur may raise log(fO2) of the subarc mantle to ~QFM + 2, consistent with the range commonly observed in subarc mantle xenoliths (e.g., Blatter and Carmichael, 1998).
View in article
Brounce, M.N., Kelley, K.A., Cottrell, E. (2014) Variations in Fe3+/ΣFe of Mariana arc basalts and mantle wedge fO2. Journal of Petrology 55, 2514-2536.
 Show in context
Show in context Similarly, arc magmas are oxidised relative to mid-ocean ridge basalts (MORB; Kelley and Cottrell, 2009, 2012; Cottrell and Kelley, 2011; Brounce et al., 2014).
View in article
This mechanism is consistent with low Fe3+/ΣFe and sulfur concentrations in Mariana forearc magmas relative to those in the main Mariana arc (Kelley and Cottrell, 2012; Brounce et al., 2014, 2016).
View in article
Brounce, M., Kelley, K.A., Stern, R., Martinez, F., Cottrell, E. (2016) The Fina Nagu volcanic complex: Unusual submarine arc volcanism in the rapidly deforming southern Mariana margin. Geochemistry Geophysics Geosystems 17, 4078-4091.
 Show in context
Show in context This mechanism is consistent with low Fe3+/ΣFe and sulfur concentrations in Mariana forearc magmas relative to those in the main Mariana arc (Kelley and Cottrell, 2012; Brounce et al., 2014, 2016).
View in article
Brown, J.L., Christy, A.G., Ellis, D.J., Arculus, R.J. (2014) Prograde sulfide metasomatism in blueschist and eclogite, New Caledonia. Journal of Petrology 55, 643-670.
 Show in context
Show in context Brown et al. (2014) observed prograde replacement of pyrrhotite-bearing inclusion assemblages by pyrite-bearing assemblages between 1.4-1.5 GPa and 450-500 ºC and 1.9 GPa and 600 ºC in New Caledonian HP rocks, consistent with the transformation of pyrrhotite to pyrite predicted by our models (Fig. 2).
View in article
Cottrell, E., Kelley, K. (2011) The oxidation state of Fe in MORB glasses and the oxygen fugacity of the upper mantle. Earth and Planetary Science Letters 305, 270-282.
 Show in context
Show in context Similarly, arc magmas are oxidised relative to mid-ocean ridge basalts (MORB; Kelley and Cottrell, 2009, 2012; Cottrell and Kelley, 2011; Brounce et al., 2014).
View in article
Connolly, J.A.D. (2005) Computation of phase equilibria by linear programming: a tool for geodynamic modelling and its application to subduction zone decarbonation. Earth and Planetary Science Letters 236, 524-541.
 Show in context
Show in context We address this discrepancy through thermodynamic modelling using the Gibbs free energy minimisation software Perple_X (Connolly, 2005) to predict sulfur speciation in AOC-derived fluids (see Supplementary Information S-3).
View in article
Debret, B., Sverjensky, D.A. (2017) Highly oxidising fluids generated during serpentinite breakdown in subduction zones. Scientific Reports 7,10351.
 Show in context
Show in context Our results are similar to those of Debret and Sverjensky (2017) for subducted serpentinite, where SOx species were calculated to be released at 630–660 °C and 2.0 GPa.
View in article
Evans, K.A. (2012) The redox budget of subduction zones. Earth Science Reviews 113, 11-32.
 Show in context
Show in context Subduction may influence the oxygen fugacity (fO2) of the mantle and mantle-derived magmas through the introduction of hydrated and oxidised altered oceanic crust (AOC, Fig. 1; Evans, 2012).
View in article
Instead, volatiles likely play a more important role (Evans, 2012).
View in article
Sulfur and carbon are fluid mobile, exhibit an eight electron range in oxidation states, are subducted at global rates on the order of 1012 mol/yr, and may act as important vectors for transferring oxidation state (Evans, 2012).
View in article
However, the input of sulfur into subduction zones is in excess of the volcanic output (Evans, 2012), suggesting that some sulfur may be stored in the mantle wedge.
View in article
Evans, K.A., Tomkins, A.G., Cliff, J., Fiorentini, M.L. (2014) Insights into subduction zone sulfur recycling from isotopic analysis of eclogite-hosted sulfides. Chemical Geology 365, 1-19.
 Show in context
Show in context Recent studies have favoured either reduced (H2S, HS-) or oxidised (SO42-) sulfur species in slab fluids, with the potential to reduce or oxidise the subarc mantle (e.g., Evans et al., 2014; Tomkins and Evans, 2015; Walters et al., 2019).
View in article
This mechanism was adopted in earlier models of pyrite behaviour during subduction of AOC (e.g., Evans et al., 2014).
View in article
Frezzotti, M.L., Ferrando, S. (2015) The chemical behavior of fluids released during deep subduction based on fluid inclusions. American Mineralogist 100, 352-377.
 Show in context
Show in context Sulfates are observed in fluid and solid inclusions in samples of subducted oceanic crust metamorphosed at relatively low pressures of 1.5–2.5 GPa (Frezzotti and Ferrando, 2015).
View in article
In contrast, in more reduced (relative to AOC) eclogites embedded in subducted continental crust, sulfate-bearing inclusions are more commonly observed in rocks metamorphosed at pressures >3.0 GPa (Frezzotti and Ferrando, 2015).
View in article
Gerrits, A.R., Inglis, E., Dragovic, B., Starr, P.G., Baxter, E.F., Burton, K. (2019) Tracing the source of oxidizing fluids in subduction zones using iron isotopes in garnet. Nature Geoscience 12, 1029-1033.
 Show in context
Show in context In addition, a recent study of Fe isotopes in high pressure garnet from Sifnos (Greece) suggests that lawsonite dehydration is accompanied by iron reduction in low temperature eclogites, likely due to a loss of SOx species (Gerrits et al., 2019).
View in article
Kelley, K., Cottrell, E. (2009) Water and the oxidation state of subduction zone magmas. Science 325, 605-607.
 Show in context
Show in context Similarly, arc magmas are oxidised relative to mid-ocean ridge basalts (MORB; Kelley and Cottrell, 2009, 2012; Cottrell and Kelley, 2011; Brounce et al., 2014).
View in article
The Fe3+/ΣFe ratios of arc magmas positively correlate with geochemical indicators of material addition from the slab to the magma sources, such as Ba/La ratios (Kelley and Cottrell, 2009); therefore, elevated mantle fO2 along convergent margins is both spatially and chemically linked to the subducting slab.
View in article
Kelley, K., Cottrell, E., (2012) The influence of magmatic differentiation on the oxidation state of Fe in a basaltic arc magma. Earth and Planetary Science Letters 329-330, 109-121.
 Show in context
Show in context Similarly, arc magmas are oxidised relative to mid-ocean ridge basalts (MORB; Kelley and Cottrell, 2009, 2012; Cottrell and Kelley, 2011; Brounce et al., 2014).
View in article
This mechanism is consistent with low Fe3+/ΣFe and sulfur concentrations in Mariana forearc magmas relative to those in the main Mariana arc (Kelley and Cottrell, 2012; Brounce et al., 2014, 2016).
View in article
Lecuyer, C., Ricard, Y. (1999) Long-term fluxes and budget of ferric iron: implications for the redox states of the Earth’s mantle and atmosphere. Earth and Planetary Science Letters 165, 197-211.
 Show in context
Show in context Early studies hypothesised the introduction of slab Fe3+ (e.g., Lecuyer and Ricard, 1999); however, the solubility of Fe3+ in hydrous fluids is low (Mungall, 2002).
View in article
Li, J.L., Schwarzenbach, E.M., John, T., Ague, J.J., Huang, F., Gao, J., Klemd, R., Whitehouse, M.J., Wang, X.S. (2020) Uncovering and quantifying the subduction zone sulfur cycle from the slab perspective. Nature Communications 11, 514.
 Show in context
Show in context In contrast, Li et al. (2020) predicted the release of reduced sulfur species during the subduction of mafic crust; however, the reduced nature of these fluids is a result of fixing fO2 at QFM to QFM -- 3 along the dehydration path.
View in article
Mackwell, S. (2008) Rheological consequences of redox state. Reviews in Mineralogy and Geochemistry 68, 555-569.
 Show in context
Show in context As a result, secular changes in mantle fO2 induced by plate tectonics may drive variations in mantle circulation (Mackwell, 2008).
View in article
McCammon, C.A., Frost, D.J., Smyth, J.R., Lautsen, H.M.S., Kawamoto, T., Ross, N.L., van Aken, P.A. (2004) Oxidation state of iron in hydrous mantle phases: implications for subduction and mantle oxygen fugacity. Physics of the Earth and Planetary Interiors 143-144, 157-169.
 Show in context
Show in context Mantle fO2 can regulate mantle rheology and density through changes in mineralogy; for example, the H2O content of nominally anhydrous minerals is a function of fO2 (McCammon et al., 2004).
View in article
Mungall, J.E. (2002) Roasting the mantle: Slab melting and the genesis of major Au and Au-rich Cu deposits. Geology 30, 915-918.
 Show in context
Show in context Early studies hypothesised the introduction of slab Fe3+ (e.g., Lecuyer and Ricard, 1999); however, the solubility of Fe3+ in hydrous fluids is low (Mungall, 2002).
View in article
Spandler, C., Pirard, C. (2013) Element recycling from subducting slabs to arc crust: A review. Lithos 170-171, 208-223.
 Show in context
Show in context However, deflection of metasomatised mantle or mélange diapirs by corner flow may carry the oxidised mantle to the region of melt generation beneath the arc (Spandler and Pirard, 2013).
View in article
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
 Show in context
Show in context Figure 2 [...] Honshu and Cascadia slab top P-T paths after Syracuse et al. (2010).
View in article
Sulfur concentration and speciation in slab fluids vary as a function of subduction zone thermal structure, which we demonstrate by comparing open system models in which fluids are fractionated at each P–T step along contrasting geothermal gradients (D80; Syracuse et al., 2010).
View in article
Fluid loss is predicted in our models at depths (60 to 80 km) less than those of the slab top directly below many volcanic arcs (~100 km; Syracuse et al., 2010).
View in article
We thank James Connolly for modelling support and Peter van Keken for providing updated P–T paths for the Syracuse et al. (2010) models.
View in article
Tomkins, A.G., Evans, K.A. (2015) Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth and Planetary Science Letters 428, 73-83.
 Show in context
Show in context Recent studies have favoured either reduced (H2S, HS-) or oxidised (SO42-) sulfur species in slab fluids, with the potential to reduce or oxidise the subarc mantle (e.g., Evans et al., 2014; Tomkins and Evans, 2015; Walters et al., 2019).
View in article
Similarly, Tomkins and Evans (2015) predicted dissolution of seafloor anhydrite in MORB to also occur over the blueschist-to-eclogite transition modelled here, potentially enhancing the oxidised nature of slab fluids released at these depths.
View in article
The predicted SOx fluxes are also consistent with the oxidised nature of trench proximal Cu porphyry deposits in the Andes and elsewhere (e.g., Tomkins and Evans, 2015).
View in article
Tracy, R.J., Robinson, P. (1988) Silicate-sulfide-oxide-fluid reactions in granulite-grade pelitic rocks, central Massachusetts. American Journal of Science 288-A, 45-74.
 Show in context
Show in context Our models are consistent with the petrographic and isotopic record from exhumed high pressure rocks. Under regional metamorphic conditions, pyrite (S-) is commonly replaced by pyrrhotite (S2-) with increasing grade (e.g., Tracy and Robinson, 1988).
View in article
Walters, J.B., Cruz-Uribe, A.M., Marschall, H.R. (2019) Isotopic compositions of sulfides in exhumed high-pressure terranes: Implications for sulfur cycling in subduction zones. Geochemistry Geophysics Geosystems 20, 1-28.
 Show in context
Show in context Recent studies have favoured either reduced (H2S, HS-) or oxidised (SO42-) sulfur species in slab fluids, with the potential to reduce or oxidise the subarc mantle (e.g., Evans et al., 2014; Tomkins and Evans, 2015; Walters et al., 2019).
View in article
Such reactions, balanced by the oxidation of Fe2+ to Fe3+ in silicates or oxides, would produce H2S in the presence of water during subduction (see review in Walters et al., 2019).
View in article
The ~36 ‰ (δ34S) variation among metasomatic sulfides suggests that large fractionations (10–20 ‰) were induced by precipitation from oxidised sulfur-bearing slab fluids (Walters et al., 2019).
View in article
Sulfides are a common trace phase found as inclusions in eclogitic minerals (Fig. 4a,b; see review in Walters et al., 2019).
View in article
Zack, T., John, T. (2007) An evaluation of reactive fluid flow and trace element mobility in subducting slabs. Chemical Geology 239, 199-216.
 Show in context
Show in context Additional sulfur may also be mobilised by external fluids; however, fluids in these environments are likely channelised (e.g., Zack and John, 2007), such that external fluids will not liberate significant amounts of sulfur from the majority of the subducted crust.
View in article
top
Supplementary Information
The Supplementary Information includes:
- S-1 Linking Seafloor Alteration to Pre-subduction Sulfur and Iron Budgets
- S-2 Oxidising the Mantle Wedge – Carbon or Sulfur?
- S-3 Thermodynamic Modelling Methodology
- S-4 Redox Comparison of AOC and High-pressure Equivalents
- Tables S-1 to S-2
- Figures S-1 to S-11
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
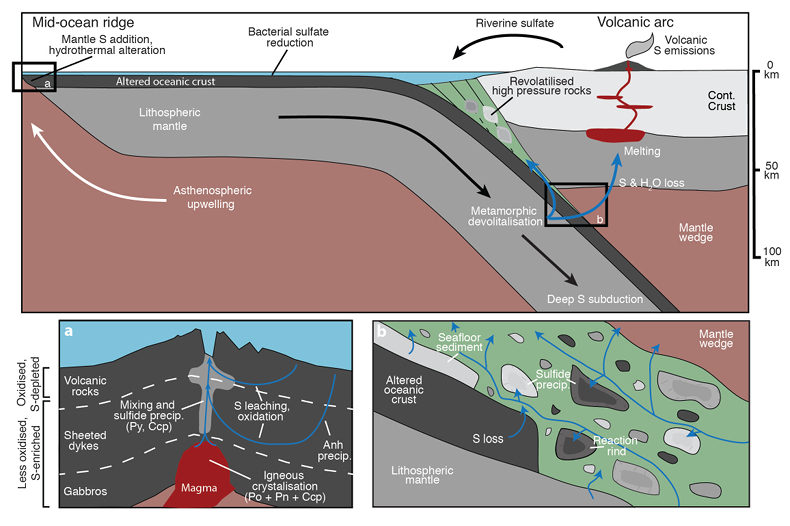
Figure 1 Schematic diagram showing the broader subduction zone system. Sulfur is introduced to the oceanic crust during crystallisation and hydrothermal circulation. (a) Magmatic sulfides react to form pyrite ± chalcopyrite and iron is oxidised (see Supplementary Information S-1). Sulfur is later extracted during metamorphic devolatilisation. (b) Sulfides precipitate in reaction rinds formed upon exhumation of metamorphic rocks along the slab mantle interface, while the remaining sulfur infiltrates the overlying subarc mantle.
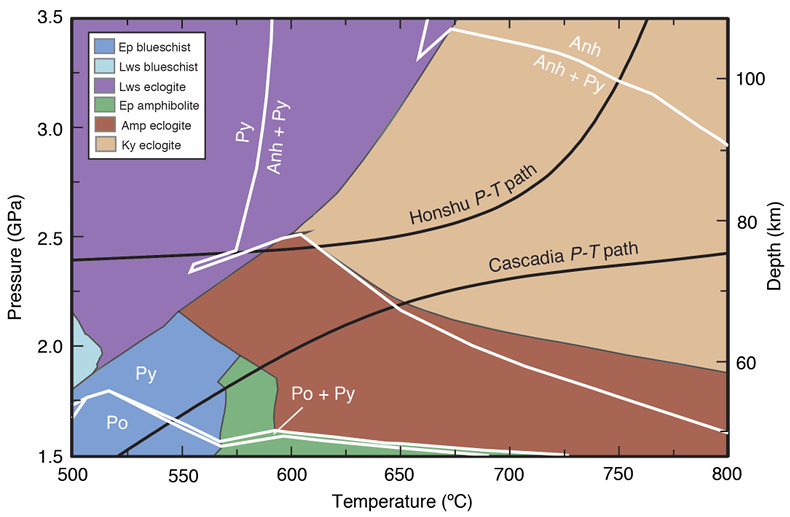
Figure 2 Closed system phase equilibrium modelling results utilising an average AOC composition from ODP hole 504B. The stability fields of common rock types are shaded (see key) and the stable solid sulfur phases are also highlighted. Honshu and Cascadia slab top P-T paths after Syracuse et al. (2010)
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
.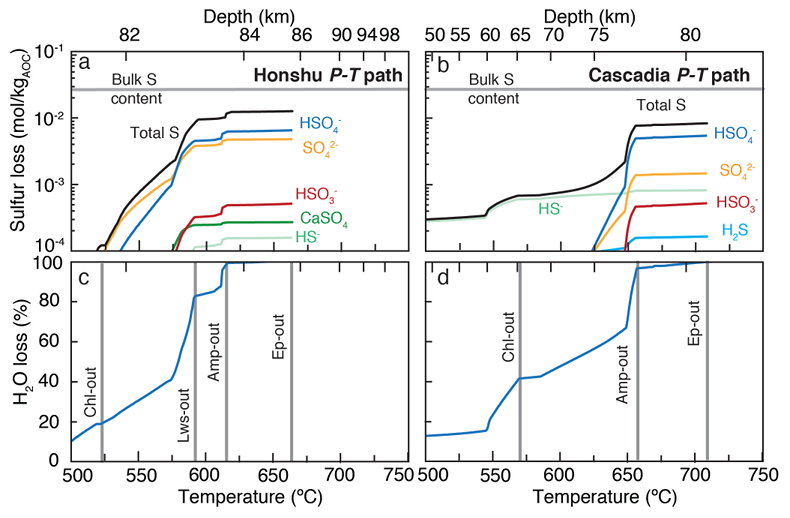
Figure 3 Cumulative sulfur (a,b) and fluid (c,d) loss diagrams for fluids fractionated along the Honshu and Cascadia P-T paths. Both temperature and corresponding slab top depth are plotted on the x-axis. Key dehydration reactions are shown for reference.
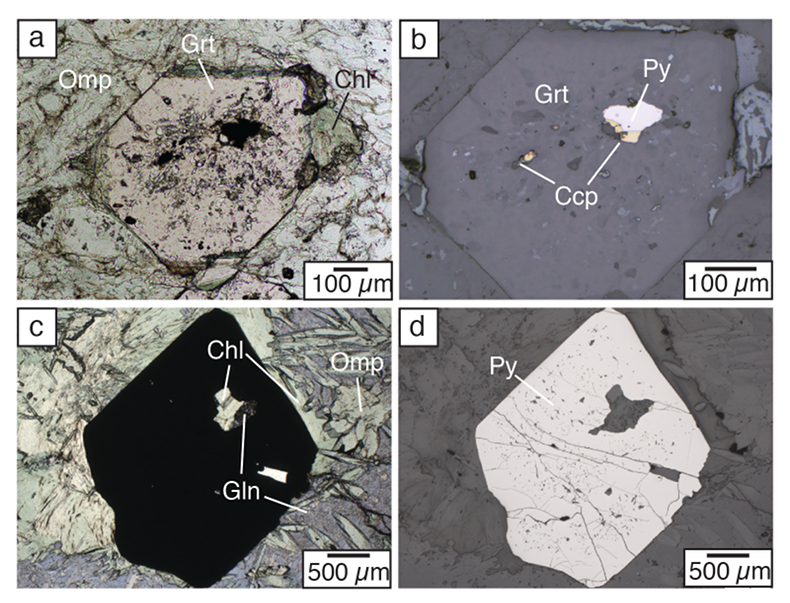
Figure 4 Transmitted (a,c) and reflected (b,d) light images of sulfides in eclogites. In (a,b), pyrite + chalcopyrite are observed as inclusions in garnet from the Junction School eclogite, Franciscan Complex, California. In (c,d), matrix pyrite is associated with retrograde chlorite + glaucophane in a metasomatised metagabbroic eclogite from Syros, Greece.






