Brucite formation and dissolution in oceanic serpentinite
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:685Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
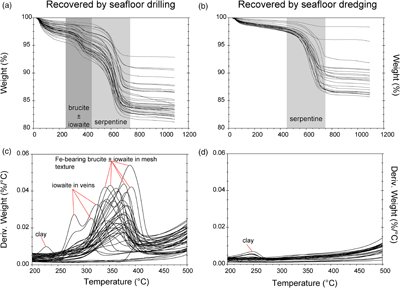 Figure 1 Thermal analysis of drilled (a, c) and dredged (b, d) serpentinites. Upper panels show the decrease in sample mass with temperature. Shading indicates mass loss via dehydroxylation of brucite ± iowaite (dark grey) and serpentine (grey). Lower panels show first derivative (deriv.) of mass loss between 200 and 500 °C. Dehydroxylation of brucite(±iowaite) is apparent in drilled samples but not in dredged samples. | 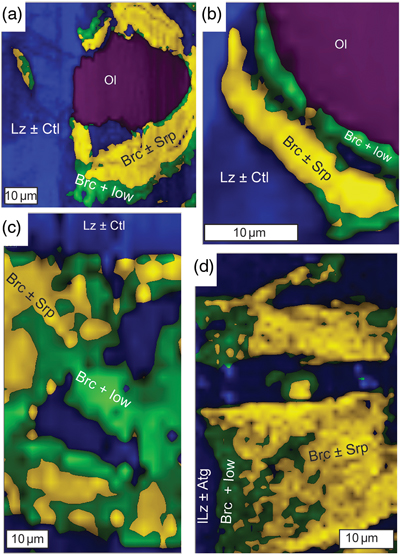 Figure 2 Hyperspectral Raman maps of brucite and serpentine in mesh texture in drilled samples. Some of the brucite is replaced by iowaite. Brucite and iowaite are intergrown with minor amounts of serpentine. (a) Sample 895D-3R-1W 64-66 cm. (b) Sample 1274A-3R1 111-120 cm. (c) Sample 1068A-24R-2W 56-59 cm. (d) 779A-03R-1W, 26–28 cm. | 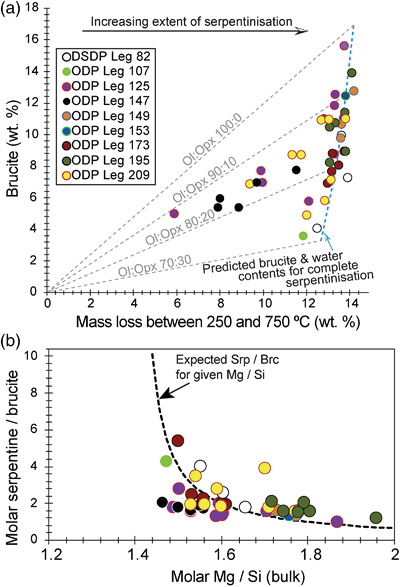 Figure 3 (a) Calculated brucite contents as a function of water content in serpentinite as derived from thermal analysis in comparison to brucite and water contents for serpentinisation of rocks with distinct modal proportions of olivine and orthopyroxene predicted from thermodynamic models (Klein et al., 2013). Note that the trajectories (grey dashed lines) point to averages of predicted brucite contents for complete serpentinisation (blue dashed line) of ultramafic rocks between 250 and 150 °C. They do not imply that the amounts of brucite increase linearly with the extent of serpentinisation. (b) Molar serpentine/brucite ratios in serpentinite as a function of molar Mg/Si ratios of whole rock samples. Molar serpentine/brucite ratios derived from thermal analysis are close to expected ratios for given molar Mg/Si ratios (dashed line) suggesting Mg and Si was conserved during serpentinisation. Only samples that showed evidence for brucite from thermal analysis are included. | 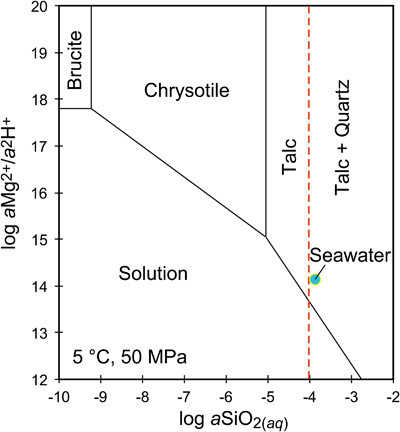 Figure 4 Activity-activity diagram depicting the stability fields of phases in the system MgO-SiO2-H2O at 5 °C and 50 MPa. Brucite is stable only at low activities (a) of H+ (i.e. high pH) and SiO2(aq), and grossly undersaturated in seawater. The red dashed line indicates the solubility of quartz. For simplicity, only Mg end members are illustrated and the water activity is assumed to be unity. Equilibrium constants were calculated using SUPCRT92 (Johnson et al., 1992). |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Tectonic exhumation exposes peridotite to aqueous solutions and, because peridotite is unstable in the presence of H2O over a wide range of temperatures and pressures, it undergoes a series of dissolution-precipitation and redox reactions to form serpentinite. In addition to serpentine, serpentinites commonly contain other minerals in varying proportions, notably brucite, magnetite, talc, chlorite, and tremolite. Brucite is probably one of the most enigmatic minerals formed during serpentinisation. While it is believed to play a central role in modulating SiO2(aq), pH, and the generation of H2 (Klein et al., 2013
Klein, F., Bach, W., McCollom, T.M. (2013) Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69.
), and is inferred to be a major carrier of water into subduction zones (Kawahara et al., 2016Kawahara, H., Endo, S., Wallis, S.R., Nagaya, T., Mori, H., Asahara, Y. (2016) Brucite as an important phase of the shallow mantle wedge: Evidence from the Shiraga unit of the Sanbagawa subduction zone, SW Japan. Lithos 254–255, 53–66.
; Peters et al., 2020Peters, D., Pettke, T., John, T., Scambelluri, M. (2020) The role of brucite in water and element cycling during serpentinite subduction – Insights from Erro Tobbio (Liguria, Italy). Lithos 360–361, 105431.
), few studies have detected brucite in oceanic serpentinite (e.g., Bach et al., 2004Bach, W., Garrido, C.J., Harvey, J., Paulick, H., Rosner, M. (2004) Seawater-peridotite interactions – First insights from ODP Leg 209, MAR 15°N. Geochemistry, Geophysics, Geosystems 5, Q09F26, doi: 10.1029/2004GC000744.
; Klein et al., 2009Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B., Berquó, T. (2009) Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochimica et Cosmochimica Acta 73, 6868–6893.
). In contrast to seemingly brucite-poor seafloor serpentinite, some continental exposures of serpentinite reveal up to 47 mole % brucite (Hostetler et al., 1966Hostetler, P.B., Coleman, R.G., Mumpton, F.A., Evans, B.W. (1966) Brucite in alpine serpentinites. American Mineralogist 51, 75–98.
). Indeed, seafloor serpentinites commonly show lower MgO and higher SiO2 concentrations than their continental counterparts, which Snow and Dick (1995)Snow, J.E., Dick, H.J.B. (1995) Pervasive magnesium loss by marine weathering of peridotite. Geochimica et Cosmochimica Acta 59, 4219–4235.
attributed to dissolution of brucite and incongruent dissolution of olivine during seafloor weathering. Alternatively, Malvoisin (2015)Malvoisin, B. (2015) Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth and Planetary Science Letters 430, 75–85.
suggested that the generally lower MgO and higher SiO2 concentrations in seafloor serpentinites are due to the addition of SiO2 from gabbroic rocks, which would limit or even prevent brucite formation during serpentinisation. These models imply contrasting, large scale mass transfers between the oceanic lithosphere and seawater (Snow and Dick, 1995Snow, J.E., Dick, H.J.B. (1995) Pervasive magnesium loss by marine weathering of peridotite. Geochimica et Cosmochimica Acta 59, 4219–4235.
) and between mafic and ultramafic rocks within the oceanic lithosphere (Malvoisin, 2015Malvoisin, B. (2015) Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth and Planetary Science Letters 430, 75–85.
). In this study, we examined the abundance of brucite in oceanic serpentinites. We show that brucite is present in serpentinites recovered by drilling, but absent in serpentinites recovered by dredging directly from the seafloor. We then compare the measured and predicted abundances of brucite in serpentinite and discuss the significance of brucite formation and dissolution in serpentinised oceanic basement.top
Materials & Methods
We examined 22 partially to completely serpentinised, carbonate-free peridotites recovered by dredging and 48 recovered by scientific seafloor drilling. Samples were collected during cruises to the Mid-Cayman Rise, Southwest Indian Ridge, Gakkel Ridge, South American Antarctic Ridge, Mid-Atlantic Ridge, East Pacific Rise, Iberian Margin, Tyrrhenian Sea, and the Mariana forearc (Supplementary Information Table S-1). A description of these samples is provided elsewhere (Klein et al., 2009
Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B., Berquó, T. (2009) Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochimica et Cosmochimica Acta 73, 6868–6893.
, 2014Klein, F., Bach, W., Humphris, S.E., Kahl, W.-A., Jöns, N., Moskowitz, B., Berquó, T.S. (2014) Magnetite in seafloor serpentinite – Some like it hot. Geology 42, 135–138.
) and relevant features are summarised in the Supplementary Information .We used thermogravimetry and differential scanning calorimetry (TGA-DSC) to quantify the abundances of serpentine and brucite, as well as the extents of serpentinisation. TGA-DSC is ideally suited for this purpose because dehydroxylation of serpentine and brucite involves mass loss and heat flow anomalies over distinct temperatures intervals (Lafay et al., 2012
Lafay, R., Montes-Hernandez, G., Janots, E., Chiriac, R., Findling, N., Toche, F. (2012) Mineral replacement rate of olivine by chrysotile and brucite under high alkaline conditions. Journal of Crystal Growth 347, 62–72.
; Okamoto et al., 2011Okamoto, A., Ogasawara, Y., Ogawa, Y., Tsuchiya, N. (2011) Progress of hydration reactions in olivine-H2O and orthopyroxenite-H2O systems at 250 °C and vapor-saturated pressure. Chemical Geology 289, 245–255.
; Viti, 2010Viti, C. (2010) Serpentine minerals discrimination by thermal analysis. American Mineralogist 95, 631–638.
). Raman spectroscopy was performed for non-destructive, high resolution identification of minerals. Major element compositions of whole rock samples were determined by X-ray fluorescence. A description of the analytical techniques used is provided in the Supplementary Information. To assess the stabilities of minerals in the system MgO-SiO2-H2O, equilibrium constants for their dissolution were calculated using SUPCRT92 (Johnson et al., 1992Johnson, J.W., Oelkers, E.H., Helgeson, H.C. (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1-5000 bars and 0-1000 °C. Computers & Geosciences 18, 899–947.
).top
Results
TGA-DSC of serpentinites recovered by seafloor drilling and dredging yielded contrasting results (Figs. 1, S-1). With five exceptions (samples # 173-1068A-22R-1W 30-33 cm, 153-920B-1W-3W 64-66 cm, 153-920B-2R-1W 80-82 cm, 153-920B-10R-1W, 82-86 cm, 153-920B-10R-2W 68–70 cm), drilled serpentinites exhibited significant mass loss between 250 and 450 °C with a maximum between 315 and 380 °C accompanied by a marked endothermic heat flow anomaly. The mass loss and heat flow anomaly over this temperature interval are characteristic for dehydroxylation of Fe-bearing brucite (Okamoto et al., 2011
Okamoto, A., Ogasawara, Y., Ogawa, Y., Tsuchiya, N. (2011) Progress of hydration reactions in olivine-H2O and orthopyroxenite-H2O systems at 250 °C and vapor-saturated pressure. Chemical Geology 289, 245–255.
), which was also identified by means of Raman spectroscopy on the basis of diagnostic Raman bands at 279 cm−1, 444 cm−1, and 3650 cm−1 in pseudomorphic textures after olivine (Fig. 2). The identification of brucite is corroborated by previously published electron microprobe analysis of the same samples (Klein et al., 2009Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B., Berquó, T. (2009) Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochimica et Cosmochimica Acta 73, 6868–6893.
, 2014Klein, F., Bach, W., Humphris, S.E., Kahl, W.-A., Jöns, N., Moskowitz, B., Berquó, T.S. (2014) Magnetite in seafloor serpentinite – Some like it hot. Geology 42, 135–138.
). The mass loss from brucite due to the removal of water from its crystal structure was 1.24 to 4.60 wt. % (average of 2.61 wt. %). Considering that the approximate composition of brucite was Mg0.8Fe0.2(OH)2 (Klein et al., 2009Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B., Berquó, T. (2009) Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochimica et Cosmochimica Acta 73, 6868–6893.
, 2014Klein, F., Bach, W., Humphris, S.E., Kahl, W.-A., Jöns, N., Moskowitz, B., Berquó, T.S. (2014) Magnetite in seafloor serpentinite – Some like it hot. Geology 42, 135–138.
) the measured mass loss suggests brucite contents of 3.56 to 15.61 wt. % (average of 8.58 wt. %). Twenty two of the drilled samples (n = 48) exhibited additional mass loss around 280 °C, which we attributed to dehydroxylation of the layered double hydroxide iowaite (Mg6Fe2Cl2(OH)16). The presence of iowaite was also indicated by Raman spectroscopy based on a strong Raman band at 527 cm−1. Hyperspectral Raman maps suggested that iowaite occurs in mesh texture where it replaces brucite (Fig. 2). To assess the total amount of brucite formed during serpentinisation, we included any mass loss from dehydroxylation of minor iowaite (i.e. >250 °C) to brucite. This is justified because the molar ratio of (Mg + Fe)/OH in brucite and iowaite is 0.5, and iowaite formed at the expense of brucite likely without significantly changing its Mg/Fe ratio.
Figure 1 Thermal analysis of drilled (a, c) and dredged (b, d) serpentinites. Upper panels show the decrease in sample mass with temperature. Shading indicates mass loss via dehydroxylation of brucite ± iowaite (dark grey) and serpentine (grey). Lower panels show first derivative (deriv.) of mass loss between 200 and 500 °C. Dehydroxylation of brucite(±iowaite) is apparent in drilled samples but not in dredged samples.

Figure 2 Hyperspectral Raman maps of brucite and serpentine in mesh texture in drilled samples. Some of the brucite is replaced by iowaite. Brucite and iowaite are intergrown with minor amounts of serpentine. (a) Sample 895D-3R-1W 64-66 cm. (b) Sample 1274A-3R1 111-120 cm. (c) Sample 1068A-24R-2W 56-59 cm. (d) 779A-03R-1W, 26–28 cm.
In contrast to drilled serpentinites, dredged serpentinites showed much smaller mass loss between 250 and 450 °C and no discernible heat flow anomaly over this temperature interval (Figs. 1, S-1, Table S-1). Hence, these samples do not contain brucite or iowaite in detectable amounts, i.e. they contain less than 0.4 wt. % brucite. A marked mass loss between 450 and 750 °C in conjunction with pronounced endothermic heat flow anomalies are diagnostic of dehydroxylation of serpentine in drilled and dredged serpentinites (Viti, 2010
Viti, C. (2010) Serpentine minerals discrimination by thermal analysis. American Mineralogist 95, 631–638.
). Raman spectroscopy suggested that most samples are dominated by lizardite with additional chrysotile. However, samples from the Mariana forearc (ODP Leg 125) also contained antigorite, which was reflected by slightly higher dehydroxylation temperatures when compared with lizardite and chrysotile. The mass loss attributed to the removal of water from the crystal structure of serpentine was 4.24 to 11.64 wt. % (average of 9.8 wt. %) in drilled samples, consistent with incomplete serpentinisation and the presence of brucite in these samples. Together, serpentine and brucite in partially to completely serpentinised peridotite examined here contain 5.88 to 14.19 wt. % water (average of 12.22 wt. %). Of the total water content in unweathered serpentinite, on average, 80.28 % is hosted by serpentine and 19.71 % by brucite. In dredged samples, mass loss attributable to water removal from serpentine was 1.0 to 11.2 wt. % (average of 8.1 wt. %). Here, any mass loss lower than the expected ∼12.5 wt. % for pure serpentine is chiefly due to incomplete serpentinisation. Additional mass loss at temperatures lower than 250 °C is related to removal of adsorbed water, destabilisation of clay minerals, or loss of interlayer water molecules in iowaite (Fig. 1).top
Discussion
Brucite formation during serpentinisation. Brucite is present in 90 % of the drilled serpentinites we examined (Figs. 1, S-1, Table S-1). It occurs together with serpentine in mesh texture and veins suggesting it formed at the expense of olivine under static conditions (Fig. 2). While brucite contents of serpentinites are variable, they increase with increasing whole rock water contents and with increasing whole rock Mg/Si ratios (Fig. 3). Since drilled samples examined in this study show no evidence of extensive Si metasomatism (Fig. S-2), our results point to protolith composition and the extent of alteration as the primary controls on brucite contents in serpentinised peridotites. Previous studies using closed system thermodynamic equilibrium models predicted the effect of the protolith olivine/pyroxene ratio on the abundance of brucite in serpentinite (Klein et al., 2009
Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B., Berquó, T. (2009) Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochimica et Cosmochimica Acta 73, 6868–6893.
, 2013Klein, F., Bach, W., McCollom, T.M. (2013) Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69.
; Malvoisin, 2015Malvoisin, B. (2015) Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth and Planetary Science Letters 430, 75–85.
). Models for serpentinisation of pure olivine (Mg1.8Fe0.2SiO4) by heated seawater predicted brucite contents ranging from 16.3 to 17.4 wt. % (or 46 to 49 mole %) between 250 and 150 °C, respectively, i.e. the temperature interval where much of seafloor serpentinisation takes place (Klein et al., 2013Klein, F., Bach, W., McCollom, T.M. (2013) Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69.
, 2014Klein, F., Bach, W., Humphris, S.E., Kahl, W.-A., Jöns, N., Moskowitz, B., Berquó, T.S. (2014) Magnetite in seafloor serpentinite – Some like it hot. Geology 42, 135–138.
). For comparison, complete serpentinisation of dunite produced 15.6 wt. % brucite (Fig. 3, Table S-1). With increasing orthopyroxene content of the protolith, the modelled brucite contents for serpentinisation decreased due to the overall decreased Mg/Si ratios. Harzburgite with 20 vol. % orthopyroxene was predicted to yield 7.6 to 8.2 wt. % brucite (or 26 to 28 mole %) between 250 and 150 °C, while serpentinisation of harzburgite with 30 vol. % orthopyroxene would yield 3.0 to 3.5 wt. % (12 to 14 mole %) brucite over the same temperature range (Klein et al., 2013Klein, F., Bach, W., McCollom, T.M. (2013) Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69.
). Drilled serpentinites contained 3.56 wt. % (or 14 mole %) brucite or more, and 8.58 wt. % (29 mole %) on average. If serpentinisation was quasi-isochemical with respect to Mg, Fe, and Si, their protoliths would have contained approximately 30 vol. % orthopyroxene or less and 20 vol. % orthopyroxene on average. Independent estimates from point counting of serpentinised abyssal peridotite (Michael and Bonatti, 1985Michael, P.J., Bonatti, E. (1985) Peridotite composition from the North Atlantic: regional and tectonic variations and implications for partial melting. Earth and Planetary Science Letters 73, 91–104.
; Dick, 1989Dick, H.J.B. (1989) Abyssal peridotites, very slow spreading ridges and ocean ridge magmatism. In: Saunders, A.D., Norry, M.J. (Eds) Magmatism in the Ocean Basins. Blackwell, Oxford, 71–105.
; Snow and Dick, 1995Snow, J.E., Dick, H.J.B. (1995) Pervasive magnesium loss by marine weathering of peridotite. Geochimica et Cosmochimica Acta 59, 4219–4235.
; Ohara et al., 2002Ohara, Y., Stern, R.J., Ishii, T., Yurimoto, H., Yamazaki, T. (2002) Peridotites from the Mariana Trough: first look at the mantle beneath an active back-arc basin. Contributions to Mineralogy and Petrology 143, 1–18.
) corroborate this estimate with an average orthopyroxene content of 20.8 vol. %. In general, therefore, the range in brucite contents of fully serpentinised peridotites that were recovered by seafloor drilling (Figs. 1, 3) can be explained by variations of the initial olivine/orthopyroxene ratio of the peridotite protolith.
Figure 3 (a) Calculated brucite contents as a function of water content in serpentinite as derived from thermal analysis in comparison to brucite and water contents for serpentinisation of rocks with distinct modal proportions of olivine and orthopyroxene predicted from thermodynamic models (Klein et al., 2013
Klein, F., Bach, W., McCollom, T.M. (2013) Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69.
). Note that the trajectories (grey dashed lines) point to averages of predicted brucite contents for complete serpentinisation (blue dashed line) of ultramafic rocks between 250 and 150 °C. They do not imply that the amounts of brucite increase linearly with the extent of serpentinisation. (b) Molar serpentine/brucite ratios in serpentinite as a function of molar Mg/Si ratios of whole rock samples. Molar serpentine/brucite ratios derived from thermal analysis are close to expected ratios for given molar Mg/Si ratios (dashed line) suggesting Mg and Si was conserved during serpentinisation. Only samples that showed evidence for brucite from thermal analysis are included.Brucite dissolution during weathering. None of the dredged serpentinites examined here contain brucite or iowaite (Figs. 1, S-1). The lack of these minerals may be due to the addition of Si during serpentinisation. However, this would require that dredged serpentinite is affected by Si metasomatism whereas drilled serpentinite is not, an unlikely scenario. It has long been established that seawater is undersaturated with respect to brucite (Fig. 4; Nesbitt and Bricker, 1978
Nesbitt, H.W., Bricker, O.P. (1978) Low temperature alteration processes affecting ultramafic bodies. Geochimica et Cosmochimica Acta 42, 403–409.
). Consequently, serpentinites exposed to seawater on the seafloor for periods of time sufficient for low temperature weathering to occur are free of brucite – as our study demonstrates.
Figure 4 Activity-activity diagram depicting the stability fields of phases in the system MgO-SiO2-H2O at 5 °C and 50 MPa. Brucite is stable only at low activities (a) of H+ (i.e. high pH) and SiO2(aq), and grossly undersaturated in seawater. The red dashed line indicates the solubility of quartz. For simplicity, only Mg end members are illustrated and the water activity is assumed to be unity. Equilibrium constants were calculated using SUPCRT92 (Johnson et al., 1992
Johnson, J.W., Oelkers, E.H., Helgeson, H.C. (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1-5000 bars and 0-1000 °C. Computers & Geosciences 18, 899–947.
).While drilled serpentinites examined in this study do contain brucite, it is conceivable that seafloor weathering penetrates deeper into the subseafloor and dissolves brucite as the plate matures. In serpentinised dunite from ODP Site 1271 south of the 15° 20’ N Fracture Zone on the Mid-Atlantic Ridge, brucite is missing in the topmost core sections, but is abundant (10–15 vol. %) in serpentinite from >10 m sub-seafloor depth (Bach et al., 2004
Bach, W., Garrido, C.J., Harvey, J., Paulick, H., Rosner, M. (2004) Seawater-peridotite interactions – First insights from ODP Leg 209, MAR 15°N. Geochemistry, Geophysics, Geosystems 5, Q09F26, doi: 10.1029/2004GC000744.
). The basement at Site 1271 was exposed about 1.5 Ma (Bach et al., 2011Bach, W., Rosner, M., Jöns, N., Rausch, S., Robinson, L.F., Paulick, H., Erzinger, J. (2011) Carbonate veins trace seawater circulation during exhumation and uplift of mantle rock: Results from ODP Leg 209. Earth and Planetary Science Letters 311, 242–252.
), suggesting that the brucite dissolution front propagated downward at a rate of <10 m per Myr. If we assume that 5 % (Carlson, 2001Carlson, R.L. (2001) The abundance of ultramafic rocks in Atlantic Ocean crust. Geophysical Journal International 144, 37–48.
) of the seafloor in the Arctic, Atlantic, and Indian Oceans consists of serpentinite (∼8,562,550 km2), and that weathering leads to quantitative dissolution of brucite in the uppermost 100 m (i.e. in 856,255 km3) with a 4.0 wt. % MgO loss on average (assuming serpentinite contains 8.58 wt. % brucite), we calculate a MgO mass of ∼9 × 1019 g transferred from the lithosphere to the ocean since the breakup of Pangaea, circa 175 million years ago. This would translate to an average flux of MgO of ∼5 × 1011 g/yr (∼1 × 1010 mol/yr). An upper estimate, where the seafloor of oceanic lithosphere accreted at slow and ultra-slow spreading ridges contains 25 % serpentinite and the depth of weathering is 200 m would result in an average annual MgO flux of ∼5 × 1012 g/yr (∼1 × 1011 mol/yr). These fluxes are significant, but noticeably smaller than the continental runoff flux of 5 × 1012 mol/yr (e.g., Spencer and Hardie, 1990Spencer, R.J., Hardie, L.A. (1990) Control of seawater composition by mixing of river waters and mid-ocean ridge hydrothermal brines. In: Spencer, R.J., Chou, I.-M. (Eds). Fluid-Mineral Interactions: A Tribute to H.P. Eugster. The Geochemical Society, Special Publication No. 2, 409–419.
). With an approximate composition of Mg0.8Fe0.2(OH)2, the potential loss of FeO from dissolution of brucite is 1.8 wt. % on average. Much of this Fe may get oxidised and remain within the rock as ferric (oxyhydr)oxide alongside carbonate (Jöns et al., 2017Jöns, N., Kahl, W.-A., Bach, W. (2017) Reaction-induced porosity and onset of low-temperature carbonation in abyssal peridotites: Insights from 3D high-resolution microtomography. Lithos 268–271, 274–284.
; Templeton and Ellison, 2020Templeton, A.S., Ellison, E.T. (2020) Formation and loss of metastable brucite: does Fe(II)-bearing brucite support microbial activity in serpentinizing ecosystems? Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 378, 20180423.
). Each mole of brucite that dissolves adds two moles of alkalinity to the oceans, which will facilitate the sequestration of CO2 in the seabed. Complete oxidation and precipitation of Fe2+, which is also released when brucite dissolves, will add 0.4 moles H+ per mole Mg0.8Fe0.2(OH)2, so the net alkalinity production per mole brucite dissolving is 1.6 moles. Hence, a net alkalinity flux on the order of 1011 mol/yr can be expected. The related CO2 sink flux should be of similar magnitude, which is small (but perhaps not insignificant) compared to an annual oceanic outgassing flux of 2 × 1012 mole CO2 (Marty and Tolstikhin, 1998Marty, B., Tolstikhin, I.N. (1998) CO2 fluxes from mid-ocean ridges, arcs and plumes. Chemical Geology 145, 233–248.
).top
Conclusions
Brucite in oceanic unweathered serpentinite recovered by drilling is common and widespread. This is at odds with the notion that oceanic serpentinite is brucite-poor, which resulted from a sampling bias toward dredged rocks that had undergone weathering. Brucite dissolution during weathering releases Mg; however, the resulting fluxes are small compared with the riverine input. If brucite dissolution in old ultramafic basement reached as deep as 200 m, then as much as 5 % of the CO2 outgassing at mid-ocean ridges could be compensated by the related enhancement of alkalinity. Iron released via brucite dissolution is rapidly oxidised and largely conserved within the rock. Dissolution of olivine is an additional source of Mg and alkalinity to the oceans, but it is uncertain how much of that released flux is consumed by reverse weathering reactions.
The average brucite contents of unweathered serpentinites are close to those predicted from simple thermodynamic equilibrium models assuming primary mineral abundances characteristic of typical oceanic peridotite. Hence, the brucite contents of serpentinite examined in this study can be entirely explained by variations in the olivine/orthopyroxene ratio of the peridotite protolith. This finding also suggests that Mg, Fe, and Si are largely conserved during serpentinisation. The abundance of brucite determined here underscores the previously suggested importance of brucite in affecting aH2(aq), aSiO2(aq), and the pH of oceanic serpentinisation systems. Notably, brucite hosts approximately 20 % of the water in unweathered serpentinite and, therefore, likely represents an important carrier of water into subduction zones.
top
Acknowledgements
We greatly appreciate constructive comments by B. Tutolo and an anonymous reviewer, as well as editorial handling by S. Opfergelt. Support for this project was provided by the Independent Research & Development Program at Woods Hole Oceanographic Institution, the US National Science Foundation (NSF Award # 1059534 and 9986135), and the Special Priority Program 1144 of the German Science Foundation (BA 1605/1-1 and BA 1605/1-2). This research would not have been possible without samples supplied by the Ocean Drilling Program and the Seafloor Samples Laboratory at WHOI.
Editor: Sophie Opfergelt
top
References
Bach, W., Garrido, C.J., Harvey, J., Paulick, H., Rosner, M. (2004) Seawater-peridotite interactions – First insights from ODP Leg 209, MAR 15°N. Geochemistry, Geophysics, Geosystems 5, Q09F26, doi: 10.1029/2004GC000744.
 Show in context
Show in context While it is believed to play a central role in modulating SiO2(aq), pH, and the generation of H2 (Klein et al., 2013), and is inferred to be a major carrier of water into subduction zones (Kawahara et al., 2016; Peters et al., 2020), few studies have detected brucite in oceanic serpentinite (e.g., Bach et al., 2004; Klein et al., 2009).
View in article
In serpentinised dunite from ODP Site 1271 south of the 15° 20’ N Fracture Zone on the Mid-Atlantic Ridge, brucite is missing in the topmost core sections, but is abundant (10–15 vol. %) in serpentinite from >10 m sub-seafloor depth (Bach et al., 2004).
View in article
Bach, W., Rosner, M., Jöns, N., Rausch, S., Robinson, L.F., Paulick, H., Erzinger, J. (2011) Carbonate veins trace seawater circulation during exhumation and uplift of mantle rock: Results from ODP Leg 209. Earth and Planetary Science Letters 311, 242–252.
 Show in context
Show in context The basement at Site 1271 was exposed about 1.5 Ma (Bach et al., 2011), suggesting that the brucite dissolution front propagated downward at a rate of <10 m per Myr.
View in article
Carlson, R.L. (2001) The abundance of ultramafic rocks in Atlantic Ocean crust. Geophysical Journal International 144, 37–48.
 Show in context
Show in context If we assume that 5 % (Carlson, 2001) of the seafloor in the Arctic, Atlantic, and Indian Oceans consists of serpentinite (∼8,562,550 km2), and that weathering leads to quantitative dissolution of brucite in the uppermost 100 m (i.e. in 856,255 km3) with a 4.0 wt. % MgO loss on average (assuming serpentinite contains 8.58 wt. % brucite), we calculate a MgO mass of ∼9 × 1019 g transferred from the lithosphere to the ocean since the breakup of Pangaea, circa 175 million years ago.
View in article
Dick, H.J.B. (1989) Abyssal peridotites, very slow spreading ridges and ocean ridge magmatism. In: Saunders, A.D., Norry, M.J. (Eds) Magmatism in the Ocean Basins. Blackwell, Oxford, 71–105.
 Show in context
Show in context Independent estimates from point counting of serpentinised abyssal peridotite (Michael and Bonatti, 1985; Dick, 1989; Snow and Dick, 1995; Ohara et al., 2002) corroborate this estimate with an average orthopyroxene content of 20.8 vol. %.
View in article
Hostetler, P.B., Coleman, R.G., Mumpton, F.A., Evans, B.W. (1966) Brucite in alpine serpentinites. American Mineralogist 51, 75–98.
 Show in context
Show in context In contrast to seemingly brucite-poor seafloor serpentinite, some continental exposures of serpentinite reveal up to 47 mole % brucite (Hostetler et al., 1966).
View in article
Johnson, J.W., Oelkers, E.H., Helgeson, H.C. (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1-5000 bars and 0-1000 °C. Computers & Geosciences 18, 899–947.
 Show in context
Show in context To assess the stabilities of minerals in the system MgO-SiO2-H2O, equilibrium constants for their dissolution were calculated using SUPCRT92 (Johnson et al., 1992).
View in article
For simplicity, only Mg end members are illustrated and the water activity is assumed to be unity. Equilibrium constants were calculated using SUPCRT92 (Johnson et al., 1992).
View in article
Jöns, N., Kahl, W.-A., Bach, W. (2017) Reaction-induced porosity and onset of low-temperature carbonation in abyssal peridotites: Insights from 3D high-resolution microtomography. Lithos 268–271, 274–284.
 Show in context
Show in context Much of this Fe may get oxidised and remain within the rock as ferric (oxyhydr)oxide alongside carbonate (Jöns et al., 2017; Templeton and Ellison, 2020).
View in article
Kawahara, H., Endo, S., Wallis, S.R., Nagaya, T., Mori, H., Asahara, Y. (2016) Brucite as an important phase of the shallow mantle wedge: Evidence from the Shiraga unit of the Sanbagawa subduction zone, SW Japan. Lithos 254–255, 53–66.
 Show in context
Show in context While it is believed to play a central role in modulating SiO2(aq), pH, and the generation of H2 (Klein et al., 2013), and is inferred to be a major carrier of water into subduction zones (Kawahara et al., 2016; Peters et al., 2020), few studies have detected brucite in oceanic serpentinite (e.g., Bach et al., 2004; Klein et al., 2009).
View in article
Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B., Berquó, T. (2009) Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochimica et Cosmochimica Acta 73, 6868–6893.
 Show in context
Show in context While it is believed to play a central role in modulating SiO2(aq), pH, and the generation of H2 (Klein et al., 2013), and is inferred to be a major carrier of water into subduction zones (Kawahara et al., 2016; Peters et al., 2020), few studies have detected brucite in oceanic serpentinite (e.g., Bach et al., 2004; Klein et al., 2009).
View in article
A description of these samples is provided elsewhere (Klein et al., 2009, 2014) and relevant features are summarised in the Supplementary Information.
View in article
The identification of brucite is corroborated by previously published electron microprobe analysis of the same samples (Klein et al., 2009, 2014).
View in article
Considering that the approximate composition of brucite was Mg0.8Fe0.2(OH)2 (Klein et al., 2009, 2014) the measured mass loss suggests brucite contents of 3.56 to 15.61 wt. % (average of 8.58 wt. %).
View in article
Previous studies using closed system thermodynamic equilibrium models predicted the effect of the protolith olivine/pyroxene ratio on the abundance of brucite in serpentinite (Klein et al., 2009, 2013; Malvoisin, 2015).
View in article
Klein, F., Bach, W., McCollom, T.M. (2013) Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69.
 Show in context
Show in context While it is believed to play a central role in modulating SiO2(aq), pH, and the generation of H2 (Klein et al., 2013), and is inferred to be a major carrier of water into subduction zones (Kawahara et al., 2016; Peters et al., 2020), few studies have detected brucite in oceanic serpentinite (e.g., Bach et al., 2004; Klein et al., 2009).
View in article
Previous studies using closed system thermodynamic equilibrium models predicted the effect of the protolith olivine/pyroxene ratio on the abundance of brucite in serpentinite (Klein et al., 2009, 2013; Malvoisin, 2015).
View in article
Models for serpentinisation of pure olivine (Mg1.8Fe0.2SiO4) by heated seawater predicted brucite contents ranging from 16.3 to 17.4 wt. % (or 46 to 49 mole %) between 250 and 150 °C, respectively, i.e. the temperature interval where much of seafloor serpentinisation takes place (Klein et al., 2013, 2014).
View in article
Harzburgite with 20 vol. % orthopyroxene was predicted to yield 7.6 to 8.2 wt. % brucite (or 26 to 28 mole %) between 250 and 150 °C, while serpentinisation of harzburgite with 30 vol. % orthopyroxene would yield 3.0 to 3.5 wt. % (12 to 14 mole %) brucite over the same temperature range (Klein et al., 2013).
View in article
(a) Calculated brucite contents as a function of water content in serpentinite as derived from thermal analysis in comparison to brucite and water contents for serpentinisation of rocks with distinct modal proportions of olivine and orthopyroxene predicted from thermodynamic models (Klein et al., 2013).
View in article
Klein, F., Bach, W., Humphris, S.E., Kahl, W.-A., Jöns, N., Moskowitz, B., Berquó, T.S. (2014) Magnetite in seafloor serpentinite – Some like it hot. Geology 42, 135–138.
 Show in context
Show in context A description of these samples is provided elsewhere (Klein et al., 2009, 2014) and relevant features are summarised in the Supplementary Information.
View in article
The identification of brucite is corroborated by previously published electron microprobe analysis of the same samples (Klein et al., 2009, 2014).
View in article
Considering that the approximate composition of brucite was Mg0.8Fe0.2(OH)2 (Klein et al., 2009, 2014) the measured mass loss suggests brucite contents of 3.56 to 15.61 wt. % (average of 8.58 wt. %).
View in article
Models for serpentinisation of pure olivine (Mg1.8Fe0.2SiO4) by heated seawater predicted brucite contents ranging from 16.3 to 17.4 wt. % (or 46 to 49 mole %) between 250 and 150 °C, respectively, i.e. the temperature interval where much of seafloor serpentinisation takes place (Klein et al., 2013, 2014).
View in article
Lafay, R., Montes-Hernandez, G., Janots, E., Chiriac, R., Findling, N., Toche, F. (2012) Mineral replacement rate of olivine by chrysotile and brucite under high alkaline conditions. Journal of Crystal Growth 347, 62–72.
 Show in context
Show in context TGA-DSC is ideally suited for this purpose because dehydroxylation of serpentine and brucite involves mass loss and heat flow anomalies over distinct temperatures intervals (Lafay et al., 2012; Okamoto et al., 2011; Viti, 2010).
View in article
Malvoisin, B. (2015) Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth and Planetary Science Letters 430, 75–85.
 Show in context
Show in context Alternatively, Malvoisin (2015) suggested that the generally lower MgO and higher SiO2 concentrations in seafloor serpentinites are due to the addition of SiO2 from gabbroic rocks, which would limit or even prevent brucite formation during serpentinisation.
View in article
These models imply contrasting, large scale mass transfers between the oceanic lithosphere and seawater (Snow and Dick, 1995) and between mafic and ultramafic rocks within the oceanic lithosphere (Malvoisin, 2015).
View in article
Previous studies using closed system thermodynamic equilibrium models predicted the effect of the protolith olivine/pyroxene ratio on the abundance of brucite in serpentinite (Klein et al., 2009, 2013; Malvoisin, 2015).
View in article
Marty, B., Tolstikhin, I.N. (1998) CO2 fluxes from mid-ocean ridges, arcs and plumes. Chemical Geology 145, 233–248.
 Show in context
Show in context The related CO2 sink flux should be of similar magnitude, which is small (but perhaps not insignificant) compared to an annual oceanic outgassing flux of 2 × 1012 mole CO2 (Marty and Tolstikhin, 1998).
View in article
Michael, P.J., Bonatti, E. (1985) Peridotite composition from the North Atlantic: regional and tectonic variations and implications for partial melting. Earth and Planetary Science Letters 73, 91–104.
 Show in context
Show in context Independent estimates from point counting of serpentinised abyssal peridotite (Michael and Bonatti, 1985; Dick, 1989; Snow and Dick, 1995; Ohara et al., 2002) corroborate this estimate with an average orthopyroxene content of 20.8 vol. %.
View in article
Nesbitt, H.W., Bricker, O.P. (1978) Low temperature alteration processes affecting ultramafic bodies. Geochimica et Cosmochimica Acta 42, 403–409.
 Show in context
Show in context It has long been established that seawater is undersaturated with respect to brucite (Fig. 4; Nesbitt and Bricker, 1978).
View in article
Ohara, Y., Stern, R.J., Ishii, T., Yurimoto, H., Yamazaki, T. (2002) Peridotites from the Mariana Trough: first look at the mantle beneath an active back-arc basin. Contributions to Mineralogy and Petrology 143, 1–18.
 Show in context
Show in context Independent estimates from point counting of serpentinised abyssal peridotite (Michael and Bonatti, 1985; Dick, 1989; Snow and Dick, 1995; Ohara et al., 2002) corroborate this estimate with an average orthopyroxene content of 20.8 vol. %.
View in article
Okamoto, A., Ogasawara, Y., Ogawa, Y., Tsuchiya, N. (2011) Progress of hydration reactions in olivine-H2O and orthopyroxenite-H2O systems at 250 °C and vapor-saturated pressure. Chemical Geology 289, 245–255.
 Show in context
Show in context TGA-DSC is ideally suited for this purpose because dehydroxylation of serpentine and brucite involves mass loss and heat flow anomalies over distinct temperatures intervals (Lafay et al., 2012; Okamoto et al., 2011; Viti, 2010).
View in article
The mass loss and heat flow anomaly over this temperature interval are characteristic for dehydroxylation of Fe-bearing brucite (Okamoto et al., 2011), which was also identified by means of Raman spectroscopy on the basis of diagnostic Raman bands at 279 cm−1, 444 cm−1, and 3650 cm−1 in pseudomorphic textures after olivine (Fig. 2).
View in article
Peters, D., Pettke, T., John, T., Scambelluri, M. (2020) The role of brucite in water and element cycling during serpentinite subduction – Insights from Erro Tobbio (Liguria, Italy). Lithos 360–361, 105431.
 Show in context
Show in context While it is believed to play a central role in modulating SiO2(aq), pH, and the generation of H2 (Klein et al., 2013), and is inferred to be a major carrier of water into subduction zones (Kawahara et al., 2016; Peters et al., 2020), few studies have detected brucite in oceanic serpentinite (e.g., Bach et al., 2004; Klein et al., 2009).
View in article
Snow, J.E., Dick, H.J.B. (1995) Pervasive magnesium loss by marine weathering of peridotite. Geochimica et Cosmochimica Acta 59, 4219–4235.
 Show in context
Show in context Indeed, seafloor serpentinites commonly show lower MgO and higher SiO2 concentrations than their continental counterparts, which Snow and Dick (1995) attributed to dissolution of brucite and incongruent dissolution of olivine during seafloor weathering.
View in article
These models imply contrasting, large scale mass transfers between the oceanic lithosphere and seawater (Snow and Dick, 1995) and between mafic and ultramafic rocks within the oceanic lithosphere (Malvoisin, 2015).
View in article
Independent estimates from point counting of serpentinised abyssal peridotite (Michael and Bonatti, 1985; Dick, 1989; Snow and Dick, 1995; Ohara et al., 2002) corroborate this estimate with an average orthopyroxene content of 20.8 vol. %.
View in article
Spencer, R.J., Hardie, L.A. (1990) Control of seawater composition by mixing of river waters and mid-ocean ridge hydrothermal brines. In: Spencer, R.J., Chou, I.-M. (Eds). Fluid-Mineral Interactions: A Tribute to H.P. Eugster. The Geochemical Society, Special Publication No. 2, 409–419.
 Show in context
Show in context These fluxes are significant, but noticeably smaller than the continental runoff flux of 5 × 1012 mol/yr (e.g., Spencer and Hardie, 1990).
View in article
Templeton, A.S., Ellison, E.T. (2020) Formation and loss of metastable brucite: does Fe(II)-bearing brucite support microbial activity in serpentinizing ecosystems? Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 378, 20180423.
 Show in context
Show in context Much of this Fe may get oxidised and remain within the rock as ferric (oxyhydr)oxide alongside carbonate (Jöns et al., 2017; Templeton and Ellison, 2020).
View in article
Viti, C. (2010) Serpentine minerals discrimination by thermal analysis. American Mineralogist 95, 631–638.
 Show in context
Show in context TGA-DSC is ideally suited for this purpose because dehydroxylation of serpentine and brucite involves mass loss and heat flow anomalies over distinct temperatures intervals (Lafay et al., 2012; Okamoto et al., 2011; Viti, 2010).
View in article
A marked mass loss between 450 and 750 °C in conjunction with pronounced endothermic heat flow anomalies are diagnostic of dehydroxylation of serpentine in drilled and dredged serpentinites (Viti, 2010).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Analytical Methods
- Rock Description
- Figures S-1 and S-2
- Tables S-1 and S-2
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
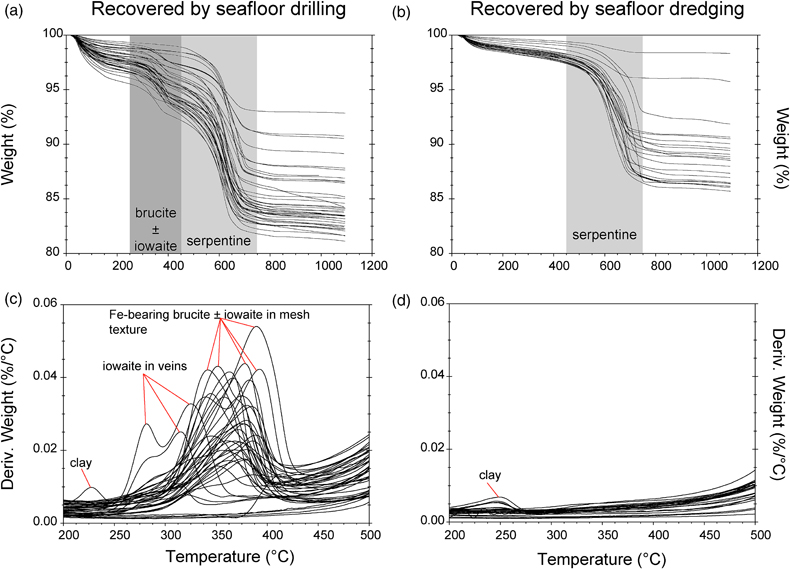
Figure 1 Thermal analysis of drilled (a, c) and dredged (b, d) serpentinites. Upper panels show the decrease in sample mass with temperature. Shading indicates mass loss via dehydroxylation of brucite ± iowaite (dark grey) and serpentine (grey). Lower panels show first derivative (deriv.) of mass loss between 200 and 500 °C. Dehydroxylation of brucite(±iowaite) is apparent in drilled samples but not in dredged samples.
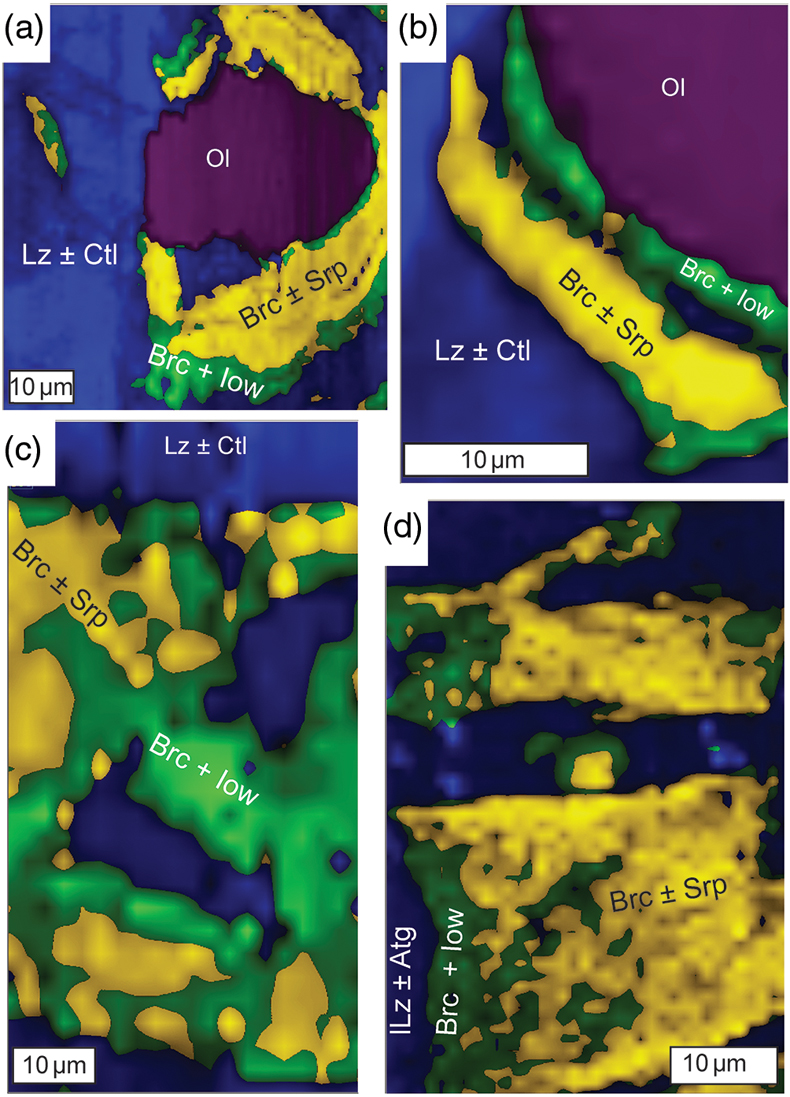
Figure 2 Hyperspectral Raman maps of brucite and serpentine in mesh texture in drilled samples. Some of the brucite is replaced by iowaite. Brucite and iowaite are intergrown with minor amounts of serpentine. (a) Sample 895D-3R-1W 64-66 cm. (b) Sample 1274A-3R1 111-120 cm. (c) Sample 1068A-24R-2W 56-59 cm. (d) 779A-03R-1W, 26–28 cm.
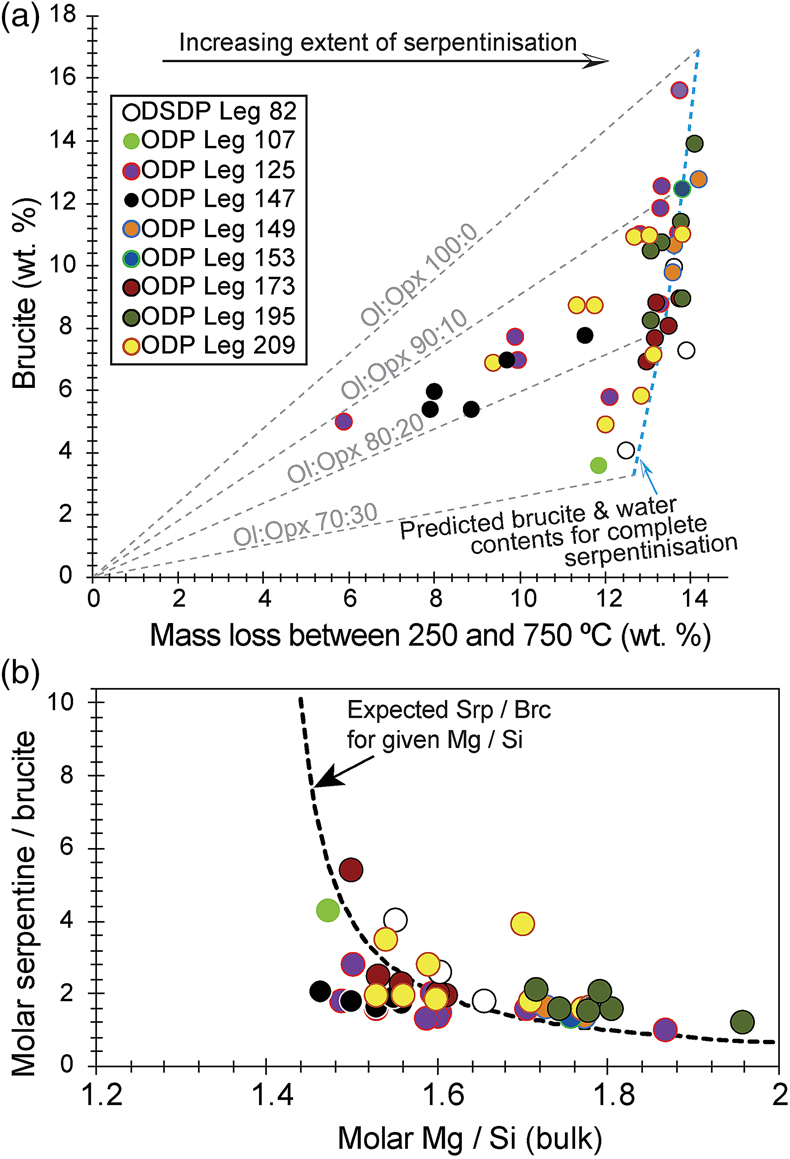
Figure 3 (a) Calculated brucite contents as a function of water content in serpentinite as derived from thermal analysis in comparison to brucite and water contents for serpentinisation of rocks with distinct modal proportions of olivine and orthopyroxene predicted from thermodynamic models (Klein et al., 2013
Klein, F., Bach, W., McCollom, T.M. (2013) Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69.
). Note that the trajectories (grey dashed lines) point to averages of predicted brucite contents for complete serpentinisation (blue dashed line) of ultramafic rocks between 250 and 150 °C. They do not imply that the amounts of brucite increase linearly with the extent of serpentinisation. (b) Molar serpentine/brucite ratios in serpentinite as a function of molar Mg/Si ratios of whole rock samples. Molar serpentine/brucite ratios derived from thermal analysis are close to expected ratios for given molar Mg/Si ratios (dashed line) suggesting Mg and Si was conserved during serpentinisation. Only samples that showed evidence for brucite from thermal analysis are included.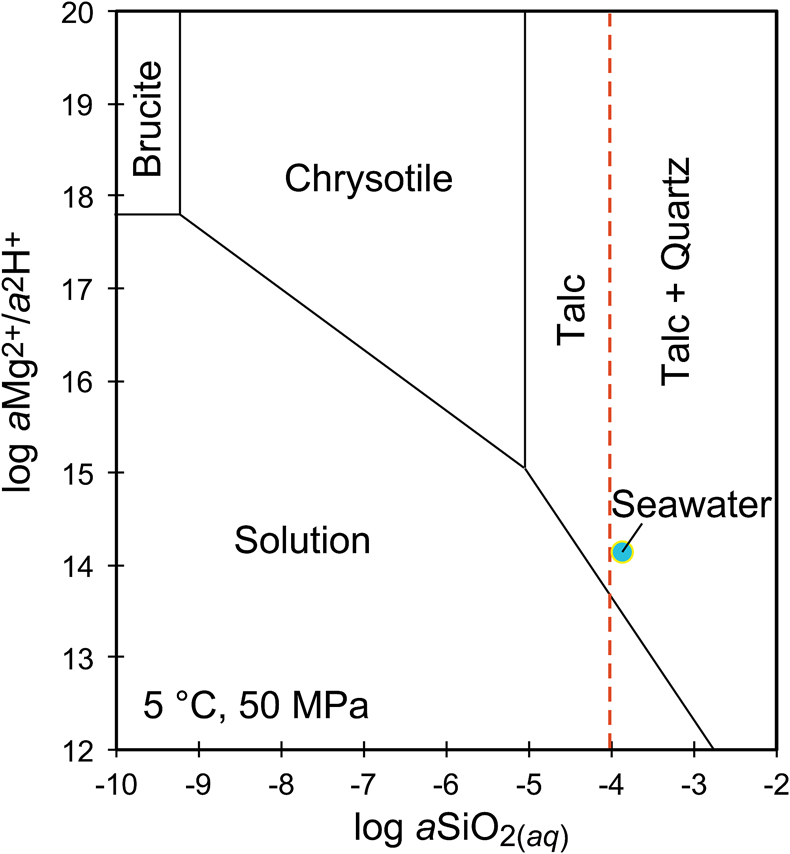
Figure 4 Activity-activity diagram depicting the stability fields of phases in the system MgO-SiO2-H2O at 5 °C and 50 MPa. Brucite is stable only at low activities (a) of H+ (i.e. high pH) and SiO2(aq), and grossly undersaturated in seawater. The red dashed line indicates the solubility of quartz. For simplicity, only Mg end members are illustrated and the water activity is assumed to be unity. Equilibrium constants were calculated using SUPCRT92 (Johnson et al., 1992
Johnson, J.W., Oelkers, E.H., Helgeson, H.C. (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1-5000 bars and 0-1000 °C. Computers & Geosciences 18, 899–947.
).