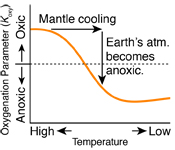
Mantle cooling causes more reducing volcanic gases and gradual reduction of the atmosphere
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:1,836Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
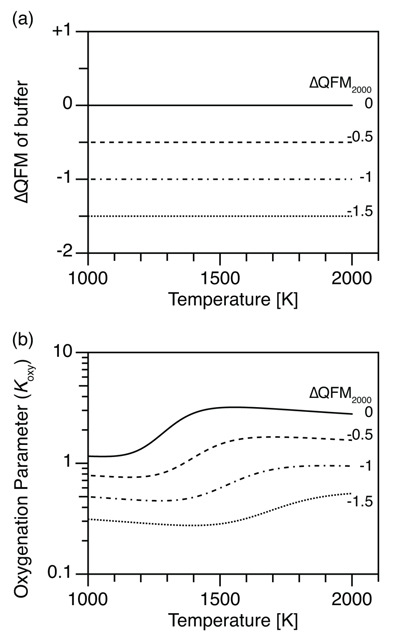 Figure 1 (a) Oxidation state (ΔQFM) buffering volcanic gas composition, and (b) oxygenation parameter (Koxy), as a function of temperature. Here, we assume a system where gases are redox buffered by the surrounding melt and rocks. ΔQFM2000 represents the oxidation state at 2000 K. By definition, ΔQFM is independent of temperature and equal to ΔQFM2000 in (a) whereas cooling tends to decrease Koxy in (b). | 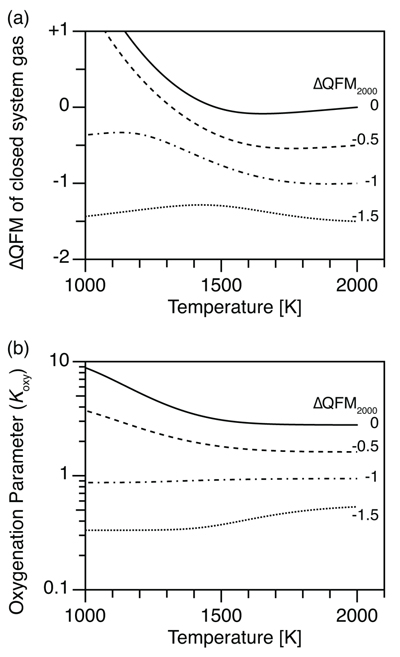 Figure 2 (a) Oxidation state (ΔQFM), and (b) oxygenation parameter (Koxy), as a function of temperature. Here, we assume a closed system of gases, and the ΔQFM of the gases at 2000 K is denoted as ΔQFM2000. Cooling changes ΔQFM unlike the melt buffered case (Fig. 1a) and changes Koxy. However, an initial Koxy that exceeds unity remains >1 with cooling, and an initial Koxy that is less than unity remains <1. |
| Figure 1 | Figure 2 |
top
Introduction
The partial pressure of Archean atmospheric O2 was <0.2 × 10-6 bar and rose during the Great Oxidation Event (GOE), between 2.4 Ga and 2.1 Ga, as indicated by the disappearance of mass independent sulfur isotope fractionation in sedimentary rocks (Farquhar et al., 2000
Farquhar, J., Bao, H.M., Thiemens, M. (2000) Atmospheric influence of Earth's earliest sulfur cycle. Science 289, 756-758.
; Pavlov and Kasting, 2002Pavlov, A.A., Kasting, J.F. (2002) Mass-independent fractionation of sulfur isotopes in Archean sediments: Strong evidence for an anoxic Archean atmosphere. Astrobiology 2, 27-41.
; Zahnle et al., 2006Zahnle, K., Claire, M., Catling, D. (2006) The loss of mass-independent fractionation in sulfur due to a Palaeoproterozoic collapse of atmospheric methane. Geobiology 4, 271-283.
). However, chromium, iron, and molybdenum isotope data suggest the presence of O2 in the marine photic zone (oxygen oases) as early as ~3 Ga (Planavsky et al., 2014Planavsky, N.J., Asael, D., Hofmann, A., Reinhard, C.T., Lalonde, S.V., Knudsen, A., Wang, X.L., Ossa, F.O., Pecoits, E., Smith, A.J.B., Beukes, N.J., Bekker, A., Johnson, T.M., Konhauser, K.O., Lyons, T.W., Rouxel, O.J. (2014) Evidence for oxygenic photosynthesis half a billiion years before the Great Oxidation Event. Nature Geoscience 7, 283-286.
; Satkoski et al., 2015Satkoski, A.M., Beukes, N.J., Li, W.Q., Beard, B.L., Johnson, C.M. (2015) A redox-stratified ocean 3.2 billion years ago. Earth and Planetary Science Letters 430, 43-53.
), and evidence exists for mild oxygenation from these and other proxies at 2.5 Ga (Ostrander et al., 2019Ostrander, C.M., Nielsen, S.G., Owens, J.D., Kendall, B., Gordon, G.W., Romaniello, S.J., Anbar, A.D. (2019) Fully oxygenated water columns over continental shelves before the Great Oxidation Event. Nature Geoscience 12, 186-191.
and references therein). Evidence for free O2 before the GOE is also consistent with phylogenetic inferences that oxygenic photosynthesis evolved by the mid to late Archean (Schirrmeister et al., 2015Schirrmeister, B.E., Gugger, M., Donoghue, P.C.J. (2015) Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology 58, 769-785.
; Magnabosco et al., 2018Magnabosco, C., Moore, K.R., Wolfe, J.M., Fournier, G.P. (2018) Dating phototrophic microbial lineages with reticulate gene histories. Geobiology 16, 179-189.
); earlier, anoxygenic photosynthesis would have been present (Sleep, 2018Sleep, N.H. (2018) Geological and Geochemical Constraints on the Origin and Evolution of Life. Astrobiology 18, 1199-1219.
).The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001
Catling, D.C., Zahnle, K.J., McKay, C.P. (2001) Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science 293, 839-843.
; Holland, 2002Holland, H.D. (2002) Volcanic gases, black smokers, and the Great Oxidation Event. Geochimica et Cosmochimica Acta 66, 3811-3826.
; Kump and Barley, 2007Kump, L.R., Barley, M.E. (2007) Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448, 1033-1036.
; Holland, 2009Holland, H.D. (2009) Why the atmosphere became oxygenated: A proposal. Geochimica et Cosmochimica Acta 73, 5241-5255.
; Gaillard et al., 2011Gaillard, F., Scaillet, B., Arndt, N.T. (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478, 229-U112.
; Kasting, 2013Kasting, J.F. (2013) What caused the rise of atmospheric O-2? Chemical Geology 362, 13-25.
; Ciborowski and Kerr, 2016Ciborowski, T.J.R., Kerr, A.C. (2016) Did mantle plume magmatism help trigger the Great Oxidation Event? Lithos 246, 128-133.
; Lee et al., 2016Lee, C.T.A., Yeung, L.Y., McKenzie, N.R., Yokoyama, Y., Ozaki, K., Lenardic, A. (2016) Two-step rise of atmospheric oxygen linked to the growth of continents. Nature Geoscience 9, 417-+.
; Brounce et al., 2017Brounce, M., Stolper, E., Eiler, J. (2017) Redox variations in Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth's oxygenation. Proceedings of the National Academy of Sciences of the United States of America 114, 8997-9002.
; Duncan and Dasgupta, 2017Duncan, M.S., Dasgupta, R. (2017) Rise of Earth's atmospheric oxygen controlled by efficient subduction of organic carbon. Nature Geoscience 10, 387-+.
; Moussallam et al., 2019Moussallam, Y., Oppenheimer, G., Scaillet, B. (2019) On the relationship between oxidation state and temperature of volcanic gas emissions. Earth and Planetary Science Letters 520, 260-267.
). One possibility is that if ancient volcanic gases were sufficiently reducing, they would have overwhelmed O2 production fluxes, limiting O2 to trace levels. If volcanic gases became gradually more oxidised, atmospheric O2 would accumulate rapidly at a tipping point when the reducing volcanic gas flux fell below the O2 production flux (Holland, 2002Holland, H.D. (2002) Volcanic gases, black smokers, and the Great Oxidation Event. Geochimica et Cosmochimica Acta 66, 3811-3826.
; Claire et al., 2006Claire, M.W., Catling, D.C., Zahnle, K.J. (2006) Biogeochemical modelling of the rise in atmospheric oxygen. Geobiology 4, 239-269.
).Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993
Kasting, J.F., Eggler, D.H., Raeburn, S.P. (1993) Mantle Redox Evolution and the Oxidation-State of the Archean Atmosphere. Journal of Geology 101, 245-257.
); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007Kump, L.R., Barley, M.E. (2007) Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448, 1033-1036.
; Gaillard et al., 2011Gaillard, F., Scaillet, B., Arndt, N.T. (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478, 229-U112.
) though this hypothesis is contradicted by Brounce et al. (2017)Brounce, M., Stolper, E., Eiler, J. (2017) Redox variations in Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth's oxygenation. Proceedings of the National Academy of Sciences of the United States of America 114, 8997-9002.
; increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002Holland, H.D. (2002) Volcanic gases, black smokers, and the Great Oxidation Event. Geochimica et Cosmochimica Acta 66, 3811-3826.
, 2009Holland, H.D. (2009) Why the atmosphere became oxygenated: A proposal. Geochimica et Cosmochimica Acta 73, 5241-5255.
) or plume magmatism (Ciborowski and Kerr, 2016Ciborowski, T.J.R., Kerr, A.C. (2016) Did mantle plume magmatism help trigger the Great Oxidation Event? Lithos 246, 128-133.
); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017Duncan, M.S., Dasgupta, R. (2017) Rise of Earth's atmospheric oxygen controlled by efficient subduction of organic carbon. Nature Geoscience 10, 387-+.
).Recently, Moussallam et al. (2019)
Moussallam, Y., Oppenheimer, G., Scaillet, B. (2019) On the relationship between oxidation state and temperature of volcanic gas emissions. Earth and Planetary Science Letters 520, 260-267.
suggested that a decrease in volcanic emission temperature, which they defined as that of the fumarole where gases enter the air, caused volcanic gases to become more oxidised. They argued that the secular cooling of the planetary interior caused a decrease in emission temperatures, oxidation of volcanic gases, and the GOE. Specifically, they considered the cooling of a parcel of gas in a volcanic vent as a closed system separated from a melt.Here, we examine how cooling affected volcanic gas buffered by a surrounding melt and gases in a subsequent closed system. We analyse the effect of inferred changes in the proportions of oxidised and reduced volcanic gases on the redox state of the atmosphere.
top
Model
We begin by describing our model briefly. Supplementary Information Section S-1 contains additional details. We assume that volcanic gas consists of H2O, H2, CO2, CO, CH4, SO2, and H2S in thermodynamic equilibrium at a total pressure of 5 bar, assuming a subaerial volcanic eruption (Holland, 1984
Holland, H.D. (1984) The chemical evolution of the atmosphere and oceans. Princeton University Press, Princeton, N.J.
; p. 47). Section S-8 discusses how the redox state of volcanic gases changes outside of this nominal pressure value. Partial pressures of the gas species are calculated using mass conservation of hydrogen, carbon, and sulfur, and relevant thermodynamic equilibria (see also Section S-1.1).We model two end members of the redox state of the gas mixture. This redox state corresponds to the amount of oxygen within the gas mixture, which is described in our two cases as follows. In one case, the "buffered system", the gas interacts with surrounding melt and rocks. Oxygen exchanges with the melt such that O2 fugacity is fixed at a given temperature and pressure. For the other case, the “closed system”, we assume that the gas and its reactions are isolated from the melt, and since no constituents are supplied or released, we conserve mass for oxygen, hydrogen, carbon, and sulfur (Supplementary Information Eq. S-12; see also Eq. S-6, S-7 and S-8).
We evaluate the oxygenation effect of volcanic gas using an oxygenation parameter, Koxy, introduced in previous studies (Catling and Claire, 2005
Catling, D.C., Claire, M.W. (2005) How Earth's atmosphere evolved to an oxic state: A status report. Earth and Planetary Science Letters 237, 1-20.
; Claire et al., 2006Claire, M.W., Catling, D.C., Zahnle, K.J. (2006) Biogeochemical modelling of the rise in atmospheric oxygen. Geobiology 4, 239-269.
; Kasting, 2013Kasting, J.F. (2013) What caused the rise of atmospheric O-2? Chemical Geology 362, 13-25.
). This parameter is the ratio of the source flux of O2 (Fsource) to the kinetically rapid sink flux of O2 (Fsink):Eq. 1
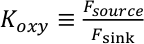
Here, Fsink corresponds to degassing of reductive, i.e. oxidisable, volcanic gases, which can include an excess of reductants beyond that which reacts with O2.
By construction, Fsource and Fsink are not meant to balance each other: they omit fluxes that depend on atmospheric redox state, such as hydrogen escape to space in Fsource and oxidative weathering, e.g., oxidation of Fe2+ to Fe3+, in Fsink (Catling and Claire, 2005
Catling, D.C., Claire, M.W. (2005) How Earth's atmosphere evolved to an oxic state: A status report. Earth and Planetary Science Letters 237, 1-20.
; Kasting, 2013Kasting, J.F. (2013) What caused the rise of atmospheric O-2? Chemical Geology 362, 13-25.
). When Koxy < 1, O2 sinks exceed O2 sources and excess H2 accumulates until balanced by escape to space. When Koxy > 1, O2 sources exceed O2 sinks and O2 accumulates until balanced by oxidative weathering. The evolution of Koxy in a box model coupled to photochemistry shows how atmospheric oxygenation occurs when Koxy reaches unity (Claire et al., 2006Claire, M.W., Catling, D.C., Zahnle, K.J. (2006) Biogeochemical modelling of the rise in atmospheric oxygen. Geobiology 4, 239-269.
).We assume that oxygenic photosynthesis is present because we are evaluating O2 build up. We consider H2O, CO2, and SO2 to be redox neutral, while H2, CO, CH4, and H2S fluxes consume O2 in atmospheric photochemistry. The burial of organic matter and pyrite (FeS2) are O2 source fluxes. Considering the stoichiometry of O2 consumption and production, we rewrite Eq. 1 as follows (derived in Section S-1):
Eq. 2
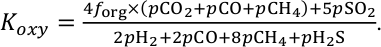
Here, forg represents the fraction of carbon buried as sedimentary organic carbon. Although forg has changed with time, for a nominal case, we set forg to 20 %, which is a rough average over geologic time (Krissansen-Totton et al., 2015
Krissansen-Totton, J., Buick, R., Catling, D.C. (2015) A Statistical Analysis of the Carbon Isotope Record from the Archean to Phanerozoic and Implications for the Rise of Oxygen. American Journal of Science 315, 275-316.
). Section S-7 discusses the dependence of Koxy on variations of forg. The mechanism that sets forg is beyond our scope. However, forg might be controlled by divalent cation fluxes that modulate the carbonate burial flux, which complements the organic burial flux (Sleep, 2005Sleep, N.H. (2005) Dioxygen over geological time. Biogeochemical Cycles of Elements 43, 49-73.
).top
Results and Discussion
The degassing process has two stages. Firstly, a gas bubble emerges from melt. The oxygen fugacity of this gas mixture is buffered by the surrounding melt since gases react with the melt. So, this stage corresponds to the buffered system case. Secondly, the bubble ascends within the melt, and the gas temperature adiabatically decreases with decompression (Oppenheimer et al., 2018
Oppenheimer, C., Scaillet, B., Woods, A., Sutton, A.J., Elias, T., Moussallam, Y. (2018) Influence of eruptive style on volcanic gas emission chemistry and temperature. Nature Geoscience 11, 678-681.
). In this stage, gases react with each other within the closed system bubble. Hereafter, we explain the redox speciation of volcanic gases during each stage.First, we consider the oxidation state of global volcanic gas emissions for the buffered system. We define the redox state as the difference of logarithm of fO2 from that of the Quartz-Fayalite-Magnetite (QFM) buffer: ΔQFM = log10 fO2 – log10 fO2,qfm. Also, we consider 4 different redox states of the surrounding melt (and rocks), and we assume that the redox state of the surroundings in each case is constant and independent of temperature. Since we consider cooling from 2000 K, we denote the oxidation state of the melt as ΔQFM2000. The choice of the initial temperature is arbitrary and does not affect our conclusions.
The ΔQFM of the gas is equal to the ΔQFM of the surroundings and is temperature independent (Fig. 1a) because of buffering by the surrounding melt and rocks. However, since the reference fO2 of the QFM buffer decreases with cooling (Fig. S-1a), the absolute fO2 value of gas and melt decreases with cooling even though their ΔQFM values are constant.
The corresponding Koxy value tells us whether atmospheric oxygenation occurs. Koxy depends on gas composition (Eq. 2), which depends on the equilibrium constant of each reaction in addition to fO2. Equilibrium constants also depend on temperature (Fig. S-1b). Consequently, cooling causes oxidation of CO to CO2 and reduction of SO2 to H2S (Fig. S-1c), even though the redox buffer relative to QFM is constant (see also Section S-2). The net effect of these opposing changes is a step-like decrease in Koxy with cooling, as shown in Figure 1b. In particular, for the case with ΔQFM = -0.5, cooling decreases Koxy from >1 to <1 (dashed line, Fig. 1b), which would cause the atmosphere to flip from oxic to reducing.

Figure 1 (a) Oxidation state (ΔQFM) buffering volcanic gas composition, and (b) oxygenation parameter (Koxy), as a function of temperature. Here, we assume a system where gases are redox buffered by the surrounding melt and rocks. ΔQFM2000 represents the oxidation state at 2000 K. By definition, ΔQFM is independent of temperature and equal to ΔQFM2000 in (a) whereas cooling tends to decrease Koxy in (b).

Figure 2 (a) Oxidation state (ΔQFM), and (b) oxygenation parameter (Koxy), as a function of temperature. Here, we assume a closed system of gases, and the ΔQFM of the gases at 2000 K is denoted as ΔQFM2000. Cooling changes ΔQFM unlike the melt buffered case (Fig. 1a) and changes Koxy. However, an initial Koxy that exceeds unity remains >1 with cooling, and an initial Koxy that is less than unity remains <1.
Now consider a parcel of volcanic gases separated from a melt, e.g., in a volcanic vent. For this closed system gas composition, we calculate an equivalent ΔQFM using the mole ratio of gas species, such as H2 / H2O (Section S-4). Cooling changes the ΔQFM (Fig. 2a), unlike in the buffered system (Fig. 1a). In particular, for relatively oxidised cases (i.e. ΔQFM2000 = 0 and -0.5), ΔQFM increases with cooling (solid and dashed lines in Fig. 2a), consistent with the results of Moussallam et al. (2019)
Moussallam, Y., Oppenheimer, G., Scaillet, B. (2019) On the relationship between oxidation state and temperature of volcanic gas emissions. Earth and Planetary Science Letters 520, 260-267.
. However, for relatively reduced cases (i.e. ΔQFM2000 = -1 and -1.5), the change in ΔQFM is moderate (dash-dot and dashed lines in Fig. 2a). The increase in ΔQFM with cooling in the closed system occurs because reduction of SO2 to H2S is accompanied by oxidation of H2 to H2O by redox conservation (Section S-3). Consequently, the ratio pH2 / pH2O declines, producing a relative increase in fO2 (see Sections S-3 and S-4).Koxy also changes with cooling of the closed system gas (Fig. 2b). However, within the closed system, reduction of one gas is accompanied by oxidation of another gas. Consequently, temperature dependent reactions within a closed system gas mixture do not change the overall sink of O2 in the gas mixture, contrary to the conclusions of Moussallam et al. (2019)
Moussallam, Y., Oppenheimer, G., Scaillet, B. (2019) On the relationship between oxidation state and temperature of volcanic gas emissions. Earth and Planetary Science Letters 520, 260-267.
.For example, consider a mixture initially containing 1 mol of SO2 and 3 mol of H2, where all SO2 is reduced, SO2 + 3H2 → H2S + 2H2O (see also Section S-3). The moles of O2 that can be consumed by the gas mixture do not change. Reduction of 1 mol SO2 accompanied by oxidation of 3 mol H2 decreases the overall sink of O2 by 0.25 mol O2 but the production of 1 mol H2S compensates.
A subtlety is that although the O2 sink cannot change, Koxy shifts because Koxy also accounts for global O2 sources from converted volcanic gases. In our ‘toy’ example, 1 mol SO2 corresponds to a 1.25 mol O2 source (see Section S-1.2), while 3 mol of H2 and 1 mol of H2S correspond to 1.5 mol O2 and 0.25 mol O2 sinks, respectively. Hence, the initial Koxy is 1.25 / 1.5 = 5 / 6, but after reactions, Koxy becomes 0 / 0.25 = 0. Here, the expected reduction of SO2 to pyrite in the global environment (Eq. S-18) is the source of O2 that changes Koxy. The important point is that an initial Koxy of <1 remains less than unity.
Consider again Figure 2. Temperature dependent gas reactions within a closed system do not change the overall sink of O2 in the gas mixture. For relatively oxidised cases (ΔQFM2000 = 0 and -0.5), cooling increases Koxy (solid and dashed lines in Fig. 2b). However, for relatively reduced cases (ΔQFM2000 = -1 and -1.5), cooling decreases Koxy (dash-dot and dotted lines in Fig. 2b). In summary, an initial Koxy of >1 remains larger than unity with cooling, while an initial Koxy of <1 stays less than unity (Fig. 2b).
Therefore, reactions under a melt buffer system change the capacity of the gas to consume O2 and affect atmospheric oxygenation while reactions within the closed system cannot. So, the oxygenation effect of volcanic degassing depends on interactions with the melt.
The Earth’s interior likely cooled with time (Bickle, 1982
Bickle, M.J. (1982) The magnesian contents of komatiitic liquids. In: Arndt, N.T., Nisbet, E.G. (Eds.), Komatiites. Allen and Unqin, London, 477-494.
; Nisbet et al., 1993Nisbet, E.G., Cheadle, M.J., Arndt, N.T., Bickle, M.J. (1993) Constraining the Potential Temperature of the Archean Mantle - a Review of the Evidence from Komatiites. Lithos 30, 291-307.
; Herzberg et al., 2010Herzberg, C., Condie, K., Korenaga, J. (2010) Thermal history of the Earth and its petrological expression. Earth and Planetary Science Letters 292, 79-88.
; Aulbach and Arndt, 2019Aulbach, S., Arndt, N.T. (2019) Eclogites as palaeodynamic archives: Evidence for warm (not hot) and depleted (but heterogeneous) Archaean ambient mantle. Earth and Planetary Science Letters 505, 162-172.
). However, even if the upper mantle’s oxidation state was constant (e.g., ΔQFM = -0.5), its cooling would decrease Koxy and even reduce the atmosphere (Fig. 1b). Thus, processes that dominate over such a Koxy decrease are required to explain the GOE.The trigger for the GOE is debated (Kasting et al., 1993
Kasting, J.F., Eggler, D.H., Raeburn, S.P. (1993) Mantle Redox Evolution and the Oxidation-State of the Archean Atmosphere. Journal of Geology 101, 245-257.
; Catling et al., 2001Catling, D.C., Zahnle, K.J., McKay, C.P. (2001) Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science 293, 839-843.
; Holland, 2002Holland, H.D. (2002) Volcanic gases, black smokers, and the Great Oxidation Event. Geochimica et Cosmochimica Acta 66, 3811-3826.
; Gaillard et al., 2011Gaillard, F., Scaillet, B., Arndt, N.T. (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478, 229-U112.
; Moussallam et al. (2019)Moussallam, Y., Oppenheimer, G., Scaillet, B. (2019) On the relationship between oxidation state and temperature of volcanic gas emissions. Earth and Planetary Science Letters 520, 260-267.
. Proposed secular oxidation of the upper mantle caused by hydrogen escape (Kasting et al., 1993Kasting, J.F., Eggler, D.H., Raeburn, S.P. (1993) Mantle Redox Evolution and the Oxidation-State of the Archean Atmosphere. Journal of Geology 101, 245-257.
) has been dismissed for about two decades because evidence appeared to show a constant oxidation state of the upper mantle (Canil, 1997Canil, D. (1997) Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389, 842-845.
; Delano, 2001Delano, J.W. (2001) Redox history of the Earth's interior since similar to 3900 Ma: Implications for prebiotic molecules. Origins of Life and Evolution of the Biosphere 31, 311-341.
; Canil, 2002Canil, D. (2002) Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth and Planetary Science Letters 195, 75-90.
; Lee et al., 2005Lee, C.T.A., Leeman, W.P., Canil, D., Li, Z.X.A. (2005) Similar V/Sc systematics in MORBs and arc basalts: Implications for oxygen fugacities of their mantle source regions. Geochimica et Cosmochimica Acta 69, A639-A639.
). However, two recent studies suggest that the upper mantle ΔQFM increased by ~1.5 log10 units since the early Archean (Aulbach and Stagno, 2016Aulbach, S., Stagno, V. (2016) Evidence for a reducing Archean ambient mantle and its effects on the carbon cycle. Geology 44, 751-754.
; Nicklas et al., 2019Nicklas, R.W., Puchte, I.S., Ash, R.D., Piccoli, H.M., Hanski, E., Nisbet, E.G., Waterton, P., Pearson, D.G., Anbar, A.D. (2019) Secular mantle oxidation across the Archean-Proterozoic boundary: Evidence from V partitioning in komatiites and picrites. Geochimica et Cosmochimica Acta 250, 49-75.
). Such oxidation would cause Koxy to increase and so possibly triggered the GOE. Regardless, we have shown that if mantle ΔQFM does not increase, mantle cooling actually makes the atmosphere more reducing, contrary to previous claims that mantle cooling would trigger the GOE (Moussallam et al., 2019Moussallam, Y., Oppenheimer, G., Scaillet, B. (2019) On the relationship between oxidation state and temperature of volcanic gas emissions. Earth and Planetary Science Letters 520, 260-267.
).top
Conclusions
We examined the effects of Earth’s secular cooling and volcanic gases on oxygenation of the atmosphere using an oxygenation parameter, Koxy, that is less than unity for an anoxic atmosphere and exceeds unity for an oxic atmosphere (Catling and Claire, 2005
Catling, D.C., Claire, M.W. (2005) How Earth's atmosphere evolved to an oxic state: A status report. Earth and Planetary Science Letters 237, 1-20.
; Kasting, 2013Kasting, J.F. (2013) What caused the rise of atmospheric O-2? Chemical Geology 362, 13-25.
). Low temperature favours H2S more than SO2 because both equilibria constants and the absolute O2 fugacity of the QFM buffer depend on temperature. Hence, for a buffered system, cooling increases the pH2S / pSO2 ratio in volcanic gases and decreases Koxy. For a closed system of gases in a vent that is not melt buffered, cooling also increases the pH2S / pSO2 ratio but this is counteracted by a decrease in pH2 / pH2O. Hence, cooling of a closed system parcel of gas does not change the overall capacity of the volcanic gases to consume O2.We conclude that the long term cooling of the mantle induced changes in volcanic gas composition that reduced the atmosphere. However, other processes dominated because the atmosphere oxygenated with time. Possibilities include secular oxidation of the mantle (Aulbach and Stagno, 2016
Aulbach, S., Stagno, V. (2016) Evidence for a reducing Archean ambient mantle and its effects on the carbon cycle. Geology 44, 751-754.
; Nicklas et al., 2019Nicklas, R.W., Puchte, I.S., Ash, R.D., Piccoli, H.M., Hanski, E., Nisbet, E.G., Waterton, P., Pearson, D.G., Anbar, A.D. (2019) Secular mantle oxidation across the Archean-Proterozoic boundary: Evidence from V partitioning in komatiites and picrites. Geochimica et Cosmochimica Acta 250, 49-75.
) and/or growth in the O2 source flux due to higher rates of organic carbon burial (relative to oxidant burial) (Krissansen-Totton et al., 2015Krissansen-Totton, J., Buick, R., Catling, D.C. (2015) A Statistical Analysis of the Carbon Isotope Record from the Archean to Phanerozoic and Implications for the Rise of Oxygen. American Journal of Science 315, 275-316.
).top
Acknowledgements
Funding support came from NSF Frontiers in Earth System Dynamics award No. 1338810.
Editor: Ambre Luguet
top
References
Aulbach, S., Arndt, N.T. (2019) Eclogites as palaeodynamic archives: Evidence for warm (not hot) and depleted (but heterogeneous) Archaean ambient mantle. Earth and Planetary Science Letters 505, 162-172.
 Show in context
Show in context The Earth’s interior likely cooled with time (Bickle, 1982; Nisbet et al., 1993; Herzberg et al., 2010; Aulbach and Arndt, 2019).
View in article
Aulbach, S., Stagno, V. (2016) Evidence for a reducing Archean ambient mantle and its effects on the carbon cycle. Geology 44, 751-754.
 Show in context
Show in context However, two recent studies suggest that the upper mantle ΔQFM increased by ~1.5 log10 units since the early Archean (Aulbach and Stagno, 2016; Nicklas et al., 2019).
View in article
Possibilities include secular oxidation of the mantle (Aulbach and Stagno, 2016; Nicklas et al., 2019) and/or growth in the O2 source flux due to higher rates of organic carbon burial (relative to oxidant burial) (Krissansen-Totton et al., 2015).
View in article
Bickle, M.J. (1982) The magnesian contents of komatiitic liquids. In: Arndt, N.T., Nisbet, E.G. (Eds.), Komatiites. Allen and Unqin, London, 477-494.
 Show in context
Show in context The Earth’s interior likely cooled with time (Bickle, 1982; Nisbet et al., 1993; Herzberg et al., 2010; Aulbach and Arndt, 2019).
View in article
Brounce, M., Stolper, E., Eiler, J. (2017) Redox variations in Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth's oxygenation. Proceedings of the National Academy of Sciences of the United States of America 114, 8997-9002.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
Canil, D. (1997) Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389, 842-845.
 Show in context
Show in context Proposed secular oxidation of the upper mantle caused by hydrogen escape (Kasting et al., 1993) has been dismissed for about two decades because evidence appeared to show a constant oxidation state of the upper mantle (Canil, 1997; Delano, 2001; Canil, 2002; Lee et al., 2005).
View in article
Canil, D. (2002) Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth and Planetary Science Letters 195, 75-90.
 Show in context
Show in context Proposed secular oxidation of the upper mantle caused by hydrogen escape (Kasting et al., 1993) has been dismissed for about two decades because evidence appeared to show a constant oxidation state of the upper mantle (Canil, 1997; Delano, 2001; Canil, 2002; Lee et al., 2005).
View in article
Catling, D.C., Claire, M.W. (2005) How Earth's atmosphere evolved to an oxic state: A status report. Earth and Planetary Science Letters 237, 1-20.
 Show in context
Show in context We evaluate the oxygenation effect of volcanic gas using an oxygenation parameter, Koxy, introduced in previous studies (Catling and Claire, 2005; Claire et al., 2006; Kasting, 2013).
View in article
By construction, Fsource and Fsink are not meant to balance each other: they omit fluxes that depend on atmospheric redox state, such as hydrogen escape to space in Fsource and oxidative weathering, e.g., oxidation of Fe2+ to Fe3+, in Fsink (Catling and Claire, 2005; Kasting, 2013).
View in article
We examined the effects of Earth’s secular cooling and volcanic gases on oxygenation of the atmosphere using an oxygenation parameter, Koxy, that is less than unity for an anoxic atmosphere and exceeds unity for an oxic atmosphere (Catling and Claire, 2005; Kasting, 2013).
View in article
Catling, D.C., Zahnle, K.J., McKay, C.P. (2001) Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science 293, 839-843.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
The trigger for the GOE is debated (Kasting et al., 1993; Catling et al., 2001; Holland, 2002; Gaillard et al., 2011; Moussallam et al., 2019).
View in article
Ciborowski, T.J.R., Kerr, A.C. (2016) Did mantle plume magmatism help trigger the Great Oxidation Event? Lithos 246, 128-133.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
Claire, M.W., Catling, D.C., Zahnle, K.J. (2006) Biogeochemical modelling of the rise in atmospheric oxygen. Geobiology 4, 239-269.
 Show in context
Show in context If volcanic gases became gradually more oxidised, atmospheric O2 would accumulate rapidly at a tipping point when the reducing volcanic gas flux fell below the O2 production flux (Holland, 2002; Claire et al., 2006).
View in article
We evaluate the oxygenation effect of volcanic gas using an oxygenation parameter, Koxy, introduced in previous studies (Catling and Claire, 2005; Claire et al., 2006; Kasting, 2013).
View in article
The evolution of Koxy in a box model coupled to photochemistry shows how atmospheric oxygenation occurs when Koxy reaches unity (Claire et al., 2006).
View in article
Delano, J.W. (2001) Redox history of the Earth's interior since similar to 3900 Ma: Implications for prebiotic molecules. Origins of Life and Evolution of the Biosphere 31, 311-341.
 Show in context
Show in context Proposed secular oxidation of the upper mantle caused by hydrogen escape (Kasting et al., 1993) has been dismissed for about two decades because evidence appeared to show a constant oxidation state of the upper mantle (Canil, 1997; Delano, 2001; Canil, 2002; Lee et al., 2005).
View in article
Duncan, M.S., Dasgupta, R. (2017) Rise of Earth's atmospheric oxygen controlled by efficient subduction of organic carbon. Nature Geoscience 10, 387-+.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
Farquhar, J., Bao, H.M., Thiemens, M. (2000) Atmospheric influence of Earth's earliest sulfur cycle. Science 289, 756-758.
 Show in context
Show in context The partial pressure of Archean atmospheric O2 was <0.2 × 10-6 bar and rose during the Great Oxidation Event (GOE), between 2.4 Ga and 2.1 Ga, as indicated by the disappearance of mass independent sulfur isotope fractionation in sedimentary rocks (Farquhar et al., 2000; Pavlov and Kasting, 2002; Zahnle et al., 2006).
View in article
Gaillard, F., Scaillet, B., Arndt, N.T. (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478, 229-U112.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
The trigger for the GOE is debated (Kasting et al., 1993; Catling et al., 2001; Holland, 2002; Gaillard et al., 2011; Moussallam et al., 2019).
View in article
Herzberg, C., Condie, K., Korenaga, J. (2010) Thermal history of the Earth and its petrological expression. Earth and Planetary Science Letters 292, 79-88.
 Show in context
Show in context The Earth’s interior likely cooled with time (Bickle, 1982; Nisbet et al., 1993; Herzberg et al., 2010; Aulbach and Arndt, 2019).
View in article
Holland, H.D. (1984) The chemical evolution of the atmosphere and oceans. Princeton University Press, Princeton, N.J.
 Show in context
Show in context We assume that volcanic gas consists of H2O, H2, CO2, CO, CH4, SO2, and H2S in thermodynamic equilibrium at a total pressure of 5 bar, assuming a subaerial volcanic eruption (Holland, 1984; p. 47).
View in article
Holland, H.D. (2002) Volcanic gases, black smokers, and the Great Oxidation Event. Geochimica et Cosmochimica Acta 66, 3811-3826.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
If volcanic gases became gradually more oxidised, atmospheric O2 would accumulate rapidly at a tipping point when the reducing volcanic gas flux fell below the O2 production flux (Holland, 2002; Claire et al., 2006).
View in article
Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
The trigger for the GOE is debated (Kasting et al., 1993; Catling et al., 2001; Holland, 2002; Gaillard et al., 2011; Moussallam et al., 2019).
View in article
Holland, H.D. (2009) Why the atmosphere became oxygenated: A proposal. Geochimica et Cosmochimica Acta 73, 5241-5255.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
Kasting, J.F. (2013) What caused the rise of atmospheric O-2? Chemical Geology 362, 13-25.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
We evaluate the oxygenation effect of volcanic gas using an oxygenation parameter, Koxy, introduced in previous studies (Catling and Claire, 2005; Claire et al., 2006; Kasting, 2013).
View in article
By construction, Fsource and Fsink are not meant to balance each other: they omit fluxes that depend on atmospheric redox state, such as hydrogen escape to space in Fsource and oxidative weathering, e.g., oxidation of Fe2+ to Fe3+, in Fsink (Catling and Claire, 2005; Kasting, 2013).
View in article
We examined the effects of Earth’s secular cooling and volcanic gases on oxygenation of the atmosphere using an oxygenation parameter, Koxy, that is less than unity for an anoxic atmosphere and exceeds unity for an oxic atmosphere (Catling and Claire, 2005; Kasting, 2013).
View in article
Kasting, J.F., Eggler, D.H., Raeburn, S.P. (1993) Mantle Redox Evolution and the Oxidation-State of the Archean Atmosphere. Journal of Geology 101, 245-257.
 Show in context
Show in context Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
The trigger for the GOE is debated (Kasting et al., 1993; Catling et al., 2001; Holland, 2002; Gaillard et al., 2011; Moussallam et al., 2019).
View in article
Proposed secular oxidation of the upper mantle caused by hydrogen escape (Kasting et al., 1993) has been dismissed for about two decades because evidence appeared to show a constant oxidation state of the upper mantle (Canil, 1997; Delano, 2001; Canil, 2002; Lee et al., 2005).
View in article
Krissansen-Totton, J., Buick, R., Catling, D.C. (2015) A Statistical Analysis of the Carbon Isotope Record from the Archean to Phanerozoic and Implications for the Rise of Oxygen. American Journal of Science 315, 275-316.
 Show in context
Show in context Although forg has changed with time, for a nominal case, we set forg to 20 %, which is a rough average over geologic time (Krissansen-Totton et al., 2015).
View in article
Possibilities include secular oxidation of the mantle (Aulbach and Stagno, 2016; Nicklas et al., 2019) and/or growth in the O2 source flux due to higher rates of organic carbon burial (relative to oxidant burial) (Krissansen-Totton et al., 2015).
View in article
Kump, L.R., Barley, M.E. (2007) Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448, 1033-1036.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Several hypotheses account for gradual oxidation of volcanic gases: the oxidation of the mantle as a consequence of hydrogen escape to space (Kasting et al., 1993); a decrease in volcanic degassing pressure associated with an increase in subaerial volcanoes (Kump and Barley, 2007; Gaillard et al., 2011) though this hypothesis is contradicted by Brounce et al. (2017); increasing CO2 and/or SO2 degassing due to increased subduction of carbonate and sulfate sediments (Holland, 2002, 2009) or plume magmatism (Ciborowski and Kerr, 2016); and/or an increase in recycling of organic material (Duncan and Dasgupta, 2017).
View in article
Lee, C.T.A., Leeman, W.P., Canil, D., Li, Z.X.A. (2005) Similar V/Sc systematics in MORBs and arc basalts: Implications for oxygen fugacities of their mantle source regions. Geochimica et Cosmochimica Acta 69, A639-A639.
 Show in context
Show in context Proposed secular oxidation of the upper mantle caused by hydrogen escape (Kasting et al., 1993) has been dismissed for about two decades because evidence appeared to show a constant oxidation state of the upper mantle (Canil, 1997; Delano, 2001; Canil, 2002; Lee et al., 2005).
View in article
Lee, C.T.A., Yeung, L.Y., McKenzie, N.R., Yokoyama, Y., Ozaki, K., Lenardic, A. (2016) Two-step rise of atmospheric oxygen linked to the growth of continents. Nature Geoscience 9, 417-+.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Magnabosco, C., Moore, K.R., Wolfe, J.M., Fournier, G.P. (2018) Dating phototrophic microbial lineages with reticulate gene histories. Geobiology 16, 179-189.
 Show in context
Show in context Evidence for free O2 before the GOE is also consistent with phylogenetic inferences that oxygenic photosynthesis evolved by the mid to late Archean (Schirrmeister et al., 2015; Magnabosco et al., 2018); earlier, anoxygenic photosynthesis would have been present (Sleep, 2018).
View in article
Moussallam, Y., Oppenheimer, G., Scaillet, B. (2019) On the relationship between oxidation state and temperature of volcanic gas emissions. Earth and Planetary Science Letters 520, 260-267.
 Show in context
Show in context The reason for the apparent time lag between the advent of oxygenic photosynthesis and the GOE is debated (Catling et al., 2001; Holland, 2002; Kump and Barley, 2007; Holland, 2009; Gaillard et al., 2011; Kasting, 2013; Ciborowski and Kerr, 2016; Lee et al., 2016; Brounce et al., 2017; Duncan and Dasgupta, 2017; Moussallam et al., 2019).
View in article
Recently, Moussallam et al. (2019) suggested that a decrease in volcanic emission temperature, which they defined as that of the fumarole where gases enter the air, caused volcanic gases to become more oxidised.
View in article
In particular, for relatively oxidised cases (i.e. ΔQFM2000 = 0 and -0.5), ΔQFM increases with cooling (solid and dashed lines in Fig. 2a), consistent with the results of Moussallam et al. (2019).
View in article
Consequently, temperature dependent reactions within a closed system gas mixture do not change the overall sink of O2 in the gas mixture, contrary to the conclusions of Moussallam et al. (2019).
View in article
The trigger for the GOE is debated (Kasting et al., 1993; Catling et al., 2001; Holland, 2002; Gaillard et al., 2011; Moussallam et al., 2019).
View in article
Regardless, we have shown that if mantle ΔQFM does not increase, mantle cooling actually makes the atmosphere more reducing, contrary to previous claims that mantle cooling would trigger the GOE (Moussallam et al., 2019).
View in article
Nicklas, R.W., Puchte, I.S., Ash, R.D., Piccoli, H.M., Hanski, E., Nisbet, E.G., Waterton, P., Pearson, D.G., Anbar, A.D. (2019) Secular mantle oxidation across the Archean-Proterozoic boundary: Evidence from V partitioning in komatiites and picrites. Geochimica et Cosmochimica Acta 250, 49-75.
 Show in context
Show in context However, two recent studies suggest that the upper mantle ΔQFM increased by ~1.5 log10 units since the early Archean (Aulbach and Stagno, 2016; Nicklas et al., 2019).
View in article
Possibilities include secular oxidation of the mantle (Aulbach and Stagno, 2016; Nicklas et al., 2019) and/or growth in the O2 source flux due to higher rates of organic carbon burial (relative to oxidant burial) (Krissansen-Totton et al., 2015).
View in article
Nisbet, E.G., Cheadle, M.J., Arndt, N.T., Bickle, M.J. (1993) Constraining the Potential Temperature of the Archean Mantle - a Review of the Evidence from Komatiites. Lithos 30, 291-307.
 Show in context
Show in context The Earth’s interior likely cooled with time (Bickle, 1982; Nisbet et al., 1993; Herzberg et al., 2010; Aulbach and Arndt, 2019).
View in article
Oppenheimer, C., Scaillet, B., Woods, A., Sutton, A.J., Elias, T., Moussallam, Y. (2018) Influence of eruptive style on volcanic gas emission chemistry and temperature. Nature Geoscience 11, 678-681.
 Show in context
Show in context Secondly, the bubble ascends within the melt, and the gas temperature adiabatically decreases with decompression (Oppenheimer et al., 2018).
View in article
Ostrander, C.M., Nielsen, S.G., Owens, J.D., Kendall, B., Gordon, G.W., Romaniello, S.J., Anbar, A.D. (2019) Fully oxygenated water columns over continental shelves before the Great Oxidation Event. Nature Geoscience 12, 186-191.
 Show in context
Show in context However, chromium, iron, and molybdenum isotope data suggest the presence of O2 in the marine photic zone (oxygen oases) as early as ~3 Ga (Planavsky et al., 2014; Satkoski et al., 2015), and evidence exists for mild oxygenation from these and other proxies at 2.5 Ga (Ostrander et al., 2019 and references therein).
View in article
Pavlov, A.A., Kasting, J.F. (2002) Mass-independent fractionation of sulfur isotopes in Archean sediments: Strong evidence for an anoxic Archean atmosphere. Astrobiology 2, 27-41.
 Show in context
Show in context The partial pressure of Archean atmospheric O2 was <0.2 × 10-6 bar and rose during the Great Oxidation Event (GOE), between 2.4 Ga and 2.1 Ga, as indicated by the disappearance of mass independent sulfur isotope fractionation in sedimentary rocks (Farquhar et al., 2000; Pavlov and Kasting, 2002; Zahnle et al., 2006).
View in article
Planavsky, N.J., Asael, D., Hofmann, A., Reinhard, C.T., Lalonde, S.V., Knudsen, A., Wang, X.L., Ossa, F.O., Pecoits, E., Smith, A.J.B., Beukes, N.J., Bekker, A., Johnson, T.M., Konhauser, K.O., Lyons, T.W., Rouxel, O.J. (2014) Evidence for oxygenic photosynthesis half a billiion years before the Great Oxidation Event. Nature Geoscience 7, 283-286.
 Show in context
Show in context Satkoski, A.M., Beukes, N.J., Li, W.Q., Beard, B.L., Johnson, C.M. (2015) A redox-stratified ocean 3.2 billion years ago. Earth and Planetary Science Letters 430, 43-53.
 Show in context
Show in context However, chromium, iron, and molybdenum isotope data suggest the presence of O2 in the marine photic zone (oxygen oases) as early as ~3 Ga (Planavsky et al., 2014; Satkoski et al., 2015), and evidence exists for mild oxygenation from these and other proxies at 2.5 Ga (Ostrander et al., 2019 and references therein).
View in article
Schirrmeister, B.E., Gugger, M., Donoghue, P.C.J. (2015) Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology 58, 769-785.
 Show in context
Show in context Evidence for free O2 before the GOE is also consistent with phylogenetic inferences that oxygenic photosynthesis evolved by the mid to late Archean (Schirrmeister et al., 2015; Magnabosco et al., 2018); earlier, anoxygenic photosynthesis would have been present (Sleep, 2018).
View in article
Sleep, N.H. (2005) Dioxygen over geological time. Biogeochemical Cycles of Elements 43, 49-73.
 Show in context
Show in context However, forg might be controlled by divalent cation fluxes that modulate the carbonate burial flux, which complements the organic burial flux (Sleep, 2005).
View in article
Sleep, N.H. (2018) Geological and Geochemical Constraints on the Origin and Evolution of Life. Astrobiology 18, 1199-1219.
 Show in context
Show in context Evidence for free O2 before the GOE is also consistent with phylogenetic inferences that oxygenic photosynthesis evolved by the mid to late Archean (Schirrmeister et al., 2015; Magnabosco et al., 2018); earlier, anoxygenic photosynthesis would have been present (Sleep, 2018).
View in article
Zahnle, K., Claire, M., Catling, D. (2006) The loss of mass-independent fractionation in sulfur due to a Palaeoproterozoic collapse of atmospheric methane. Geobiology 4, 271-283.
 Show in context
Show in context The partial pressure of Archean atmospheric O2 was <0.2 × 10-6 bar and rose during the Great Oxidation Event (GOE), between 2.4 Ga and 2.1 Ga, as indicated by the disappearance of mass independent sulfur isotope fractionation in sedimentary rocks (Farquhar et al., 2000; Pavlov and Kasting, 2002; Zahnle et al., 2006).
View in article
top
Supplementary Information
The Supplementary Information includes:
- S-1 Model Description
- S-2 Temperature Dependence of the QFM Buffer and Equilibrium Constants
- S-3 Temperature Dependence of Gas Composition
- S-4 Temperature Dependence of Oxygen Fugacity
- S-5 Temperature Dependence of Oxygenation Parameter
- S-6 Another Effect of Temperature Decrease
- S-7 Sensitivity Test to the Organic Burial Fraction (forg)
- S-8 Effect of Pressure
- Figures S-1 to S-9
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
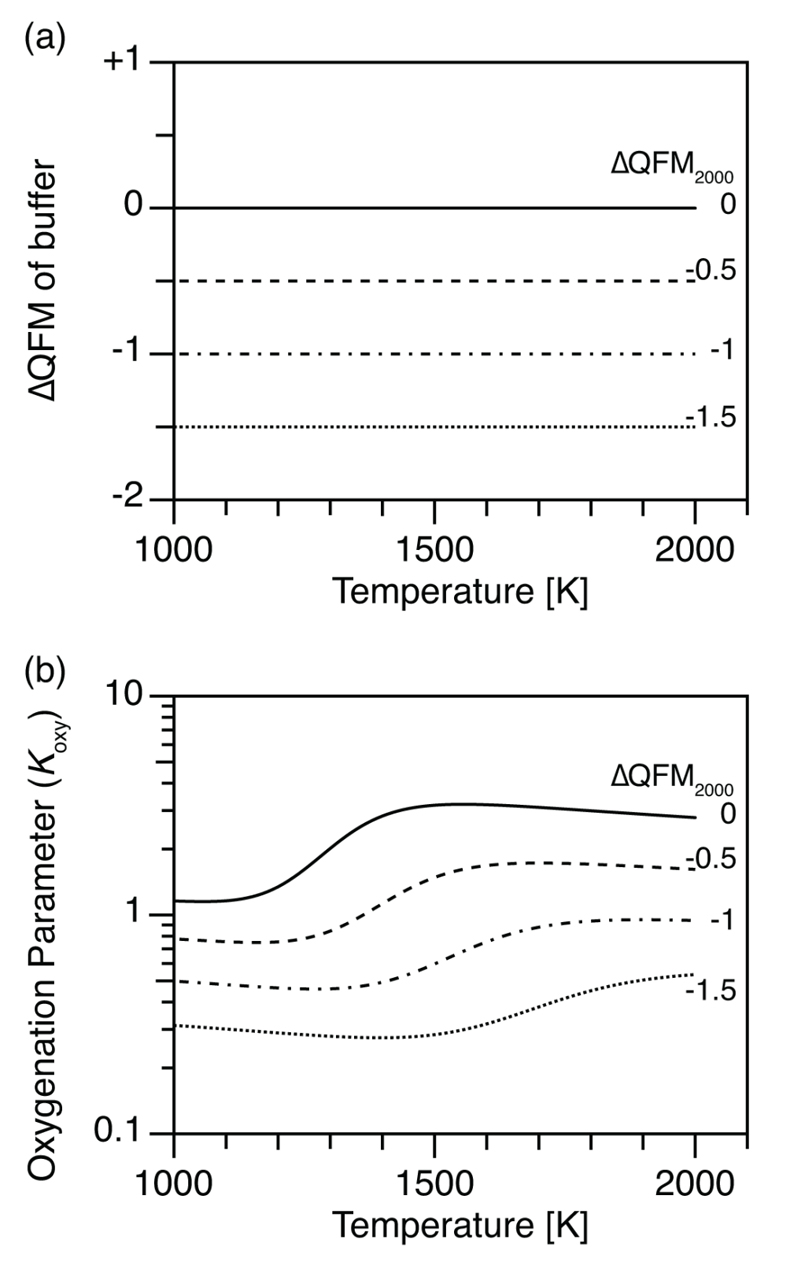
Figure 1 (a) Oxidation state (ΔQFM) buffering volcanic gas composition, and (b) oxygenation parameter (Koxy), as a function of temperature. Here, we assume a system where gases are redox buffered by the surrounding melt and rocks. ΔQFM2000 represents the oxidation state at 2000 K. By definition, ΔQFM is independent of temperature and equal to ΔQFM2000 in (a) whereas cooling tends to decrease Koxy in (b).
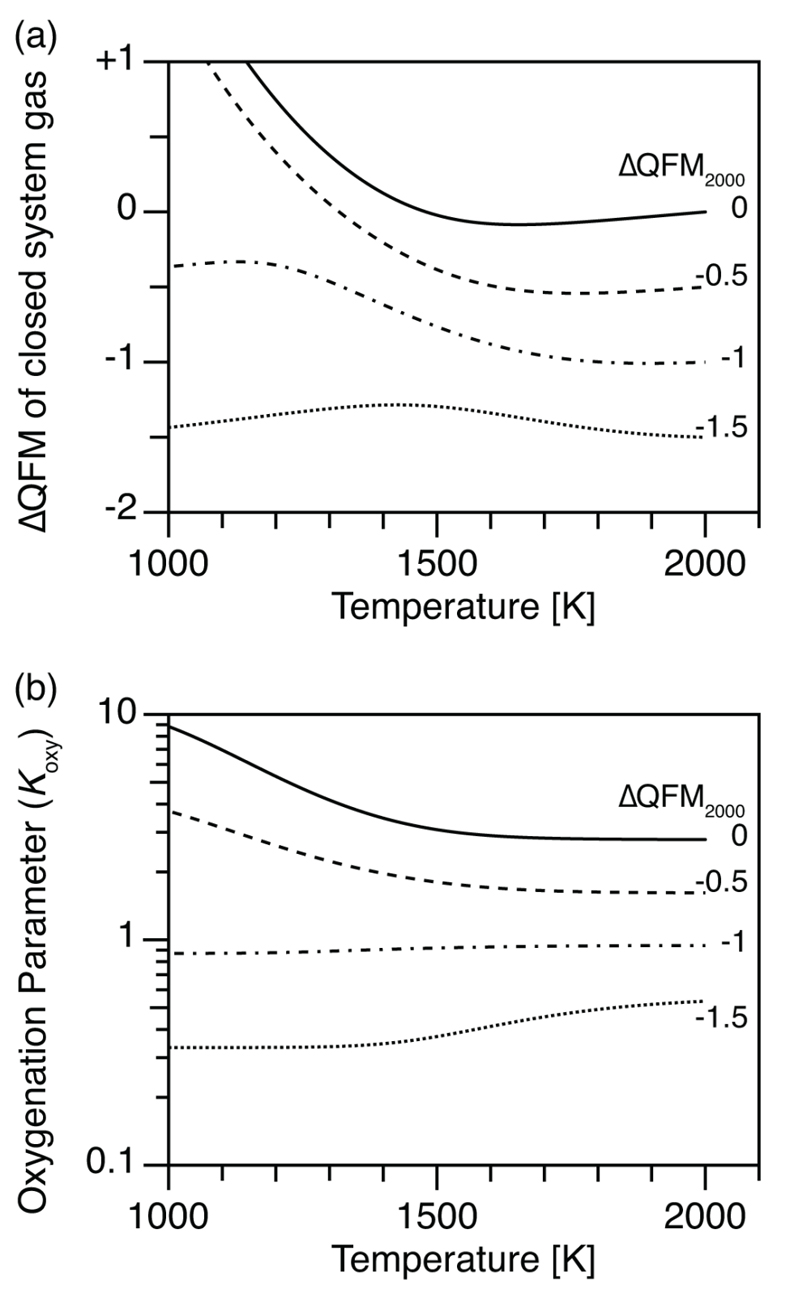
Figure 2 (a) Oxidation state (ΔQFM), and (b) oxygenation parameter (Koxy), as a function of temperature. Here, we assume a closed system of gases, and the ΔQFM of the gases at 2000 K is denoted as ΔQFM2000. Cooling changes ΔQFM unlike the melt buffered case (Fig. 1a) and changes Koxy. However, an initial Koxy that exceeds unity remains >1 with cooling, and an initial Koxy that is less than unity remains <1.