Goethite, a tailor-made host for the critical metal scandium: The FexSc(1-x)OOH solid solution
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:2,906Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
 Table 1 Relative abundances of Sc measured by ICP-AES and EDX/TEM. | 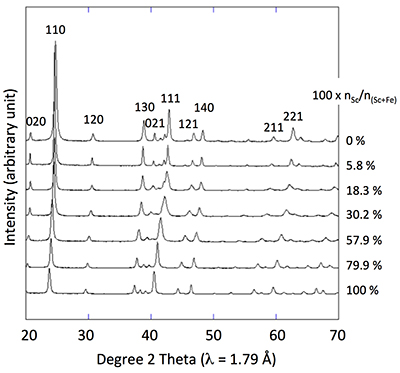 Figure 1 X-ray diffractograms for goethite, ScOOH and Sc-doped goethite samples. | 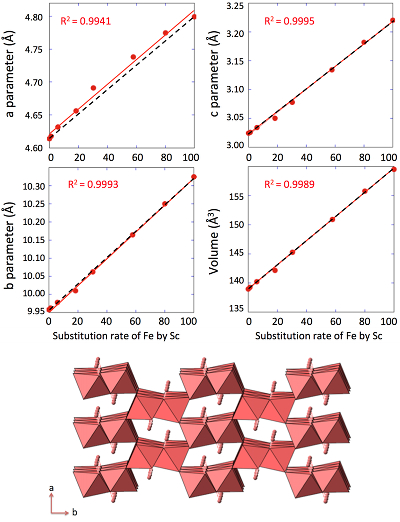 Figure 2 Top: Changes in cell parameters and in cell volume as a function of rate of substitution of Fe by Sc (numerical data for cell parameters and errors can be found in SI). Dashed lines represent ideal Vegard’s law behaviour. Bottom: Crystal structure of goethite. The octahedrons represent the Fe surrounded by 6 oxygen atoms. Spheres represent the hydrogen atoms. | 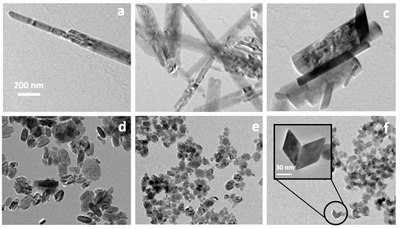 Figure 3 TEM images of samples #1 (a) (0 % Sc), #2 (b) (1.2 % Sc), #3 (c) (5.8 % Sc) #5 (d) (30.2 % Sc), #6 (e) (57.9 % Sc) and #8 (f) (100 % Sc). All images are at the same scale (a) except the inset in image (f). |
| Table 1 | Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Most countries have identified a number of materials they consider as particular challenges. Among them, access to critical metals has been identified as a growing concern worldwide because of their low concentrations in ores and their limited stock in the Earth’s crust, but also because of complex geostrategic conflicts and technological obstacles to their recovery. To overcome these limitations, some countries have started to explore the possibility of mining these metals from primary or secondary sources. This is the case of scandium (Sc), which is coveted by several countries including Australia, India, Russia, and the Philippines (U.S. Geological Survey, 2018
U.S. Geological Survey (2018) Scandium. In: Mineral Commodity Summaries. U.S. Geological Survey, Reston, USA, 144–145
). Scandium was listed by the European Union as a critical metal in 2017 and in February 2018, Sc was listed by the U.S. Department of the Interior as one of the 35 elements considered as “essential to the economic and national security” of the U.S. Despite a few Sc-rich minerals (thortveitite, euxenite, and gadolinite), Sc does not selectively combine with the common ore-forming anions, which explains why Sc only forms small deposits, thereby preventing its widespread use (Wang et al., 2011Wang, W., Pranolo, Y., Cheng, C.Y. (2011) Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 108, 100-108.
). Sc is dispersed in many minerals and tends to form solid solutions (Frondel, 1968Frondel, C. (1968) Crystal chemistry of scandium as a trace element in minerals. Zeitschrift für Kristallographie 127, 121-138.
) but in such small amounts that its structural effect on the host mineral in most cases, remains unexplored.Nevertheless, Sc is of economic value and therefore mining and co-extraction of this element along with other elements is now gaining interest. For example, among all critical elements present in coal ash, Sc was reported to have the highest economic value (Das et al., 2018
Das, S., Gaustad, G., Sekar, A., Williams, E. (2018) Techno-economic analysis of supercritical extraction of rare earth elements from coal ash. Journal of Cleaner Production 189, 539-551.
). Scandium is mainly used in aluminium-scandium alloys destined for aerospace industry components due to the strength and corrosion-resistant properties it gives to the alloy. The automotive industry has also been predicted to be a major consumer of aluminium-scandium alloy, which will further increase the demand for scandium (U.S. Geological Survey, 2018U.S. Geological Survey (2018) Scandium. In: Mineral Commodity Summaries. U.S. Geological Survey, Reston, USA, 144–145
). Scandium is also used in solid oxide fuel cells (SOFCs), where it is added to a zirconia-base electrolyte to improve the power density and lower the reaction temperature of the cell. Other applications include ceramics, electronics, lasers, lighting, and radioactive isotopes (U.S. Geological Survey, 2018U.S. Geological Survey (2018) Scandium. In: Mineral Commodity Summaries. U.S. Geological Survey, Reston, USA, 144–145
).Efficient extraction of scandium requires a good knowledge of its speciation in the deposits. Recently, scandium speciation was investigated in a lateritic deposit in eastern Australia and a strong association with iron oxides was demonstrated (Chassé et al., 2017
Chassé, M., Griffin, W.L., O'Reilly, S.Y., Calas, G. (2017) Scandium speciation in a world-class lateritic deposit. Geochemical Perspectives Letters 3, 105-114.
). About 80 % was found to be adsorbed on goethite with the remaining 20 % incorporated in the hematite structure. However, the speciation of Sc in these samples was difficult to assess because of its low concentrations and the complexity of the natural matrix (poor crystallinity of fine grained particles). The authors hypothesise that the incorporation of Sc in the structure of iron oxides is limited by the size difference between six-fold coordinated Sc3+ and Fe3+. Scandium has also been found to be strongly associated with iron (oxyhydr)oxide minerals (goethite and hematite) in Greek bauxite and bauxite residue after extraction of alumina (Vind et al., 2018Vind, J., Malfliet, A., Bonomi, C., Paiste, P., Sajó, I.E., Blanpain, B., Tkaczyk, A.H., Vassiliadou, V., Panias, D. (2018) Modes of occurrences of scandium in Greek bauxite and bauxite residue. Minerals Engineering 123, 35-48.
).To better understand the potential dynamics of Sc in such iron-rich environments, we investigated the substitution of Fe by Sc in the goethite structure. Here we present a continuous solid solution αFeOOH-αScOOH for the first time. To the best of our knowledge, this is also the first report of a continuous solid solution with goethite. We believe these results are a key to a better understanding of the fate of Sc in the environment and also provide important knowledge for the recovery of Sc from Fe-rich primary or secondary deposits.
top
Materials and Methods
The synthesis of Sc-doped goethite was performed using the protocol of Schwertmann and Cornell (1991)
Schwertmann, U., Cornell, R.M. (1991) Iron Oxides in the Laboratory, Preparation and Characterization. VCH. Weinheim.
with modifications. First, 25 mL of a 1 M solution of iron (III) nitrate and scandium (III) nitrate was prepared with ultra-pure (UP) water and varying Sc molar proportions (0, 1, 5, 15, 25, 50, 75 and 100 mol. %). Then, 45 mL of 5 M KOH were added to each solution, which was diluted with UP water to reach a final concentration of Fe + Sc of 0.05 M. The final pH of the solutions was 13.3 ± 0.2. The solutions were heated at 70 °C in Teflon flasks for 10 days. The solutions were cooled at room temperature, centrifuged at 4000 g for 15 minutes and washed several times with UP water until the pH reached 7-8. Finally, the recovered products were dried in an oven at 40 °C for two days.The chemical composition of the samples was determined by induced coupled plasma atomic emission spectrometry (ICP-AES). X-ray diffraction (XRD) was used for phase identification. Profex software (Doebelin and Kleeberg, 2015
Doebelin, N., Kleeberg, R. (2015) Profex: a graphical user interface for the Rietveld refinement program BGMN. Journal of Applied Crystallography 48, 1573-1580.
) was used for Rietveld refinement to determine the cell parameters of the (Fe,Sc)OOH samples. The obtained particles were characterised using a transmission electron microscope (TEM, JEOL JEM 2011) coupled with an energy dispersive X-ray spectrometer (EDX, X-Flash Silicon Drift Detector 5030, Bruker). Further details on sample characterisation are provided in Supplementary Information.top
Results
The relative molar concentrations of Sc were found to be close to the nominal concentration (Table 1). More importantly, the relative abundance of Sc measured at the bulk scale by ICP-AES was similar to the abundances measured at the particle scale (Table 1). This similarity is an indication that, at the particle scale, no demixing occurred whatever the initial Sc amount.
Table 1 Relative abundances of Sc measured by ICP-AES and EDX/TEM.
| Samples | n Sc / (n Sc+Fe ) x 100 | ||
| Nominal | ICP-AES | EDX/TEM | |
| #1 | 0 | ||
| #2 | 1 | 1.2 ± 0.01 | 1.2 ± 0.3 |
| #3 | 5 | 5.8 ± 0.10 | 6.3 ± 1.3 |
| #4 | 15 | 18.3 ± 0.30 | 17.9 ± 5.3 |
| #5 | 25 | 30.2 ± 0.30 | 29.0 ± 4.6 |
| #6 | 50 | 57.9 ± 0.40 | 49.9 ± 10.0 |
| #7 | 75 | 79.9 ± 1.10 | 80.2 ± 1.3 |
| #8 | 100 | 100.0 ± 1.20 | |
Calculated standard deviation based on the measurements of Fe and Sc concentrations on 5 replicates (ICP-AES), and on 10 particle aggregates (EDX/TEM).
Download in Excel
Figure 1 X-ray diffractograms for goethite, ScOOH and Sc-doped goethite samples.
X-ray diffraction of the 0 % Sc sample (Fig. 1) is characteristic of the orthorhombic phase of goethite, which is consistent with Schwertmann and Cornell (1991)
Schwertmann, U., Cornell, R.M. (1991) Iron Oxides in the Laboratory, Preparation and Characterization. VCH. Weinheim.
. Replacing Fe with Sc shifted the peaks to longer distances (shorter angles), but the orthorhombic phase was preserved all through the solid solution. Cell parameters were obtained by Rietveld refinement (the refined diffractograms are presented in Supplementary Information). The changes in cell parameters and in the associated cell volume as a function of % Sc were almost perfectly linear for all parameters and followed Vegard’s law (Fig. 2). Only parameter a deviated slightly positively from Vegard’s law, which can potentially be attributed to the structural properties of goethite. αFeOOH and αScOOH crystallise under a hexagonal close packing of oxygen atoms with 6-fold coordinated metal atoms (octahedral environment). The metal atoms form double chains of octahedra running along the b-c plane (Fig. 2). Within these chains, each octahedron shares four of its edges with neighbouring octahedra, potentially strengthening the bonding in the b-c plane. Conversely, the bonding of the two chains occurs through apical oxygen and hydrogen bonding (Schulze, 1984Schulze, D.G. (1984) The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays and Clay Minerals 32, 36-44.
). This bonding along the a axis can be more easily affected by strain or by the presence of defects caused by the substitution and may explain the slight deviation from Vegard’s rule. Additionally, small deviations from Vegard’s law are expected even for ideal solutions (Jacob et al., 2007Jacob, K.T., Raj, S., Rannesh, L. (2007) Vegard's law: a fundamental relation or an approximation? International Journal of Materials Research 98, 776-779.
).
Figure 2 Top: Changes in cell parameters and in cell volume as a function of rate of substitution of Fe by Sc (numerical data for cell parameters and errors can be found in SI). Dashed lines represent ideal Vegard’s law behaviour. Bottom: Crystal structure of goethite. The octahedrons represent the Fe surrounded by 6 oxygen atoms. Spheres represent the hydrogen atoms.
Goethite particles (0 % Sc) have a common needle-like structure (Fig. 3a) widely described in the past. Goethite growth has been described as a two step process (Burleson and Penn, 2006
Burleson, D.J., Penn, R.L. (2006) Two-Step Growth of Goethite from Ferrihydrite. Langmuir 22, 402-409.
). First initially formed six line ferrihydrite nanoparticles convert to goethite nanoparticles and the resulting particles grow by oriented aggregation to produce a goethite nanorod. Any defects and misorientations produced during aggregation are removed by slow recrystallisation and coarsening processes. As Sc is increasingly incorporated into the structure, the anisotropy of the formed particles decreases (Fig. 3). The ScOOH particles (sample #8, Fig. 3f) exhibit faceted structures among which diamond-shaped particles were observed. Zhang et al. (2005)Zhang, Y.-W., Liu, J.-H., Si, R., Yan, Z.-G., Yan, C.-H. (2005) Phase Evolution, Texture Behavior, and Surface Chemistry of Hydrothermally Derived Scandium (Hydrous) Oxide Nanostructures. The Journal of Physical Chemistry B 109, 18324-18331.
showed that the nature and morphologies of Sc (oxyhydr)oxide vary depending on the synthesis conditions (Zhang et al., 2005Zhang, Y.-W., Liu, J.-H., Si, R., Yan, Z.-G., Yan, C.-H. (2005) Phase Evolution, Texture Behavior, and Surface Chemistry of Hydrothermally Derived Scandium (Hydrous) Oxide Nanostructures. The Journal of Physical Chemistry B 109, 18324-18331.
). In particular, these authors investigated the effect of pH on the morphology and crystallinity of the formed particles. In conditions that resembled those in our study (KOH, pH 12 and 14), they observed the formation of αScOOH of similar size and shapes. More interestingly, they obtained αScOOH nanorods at pH 10, similar to those we observed in our study for αFeOOH (Fig. 3a). The loss of anisotropy as Sc is increasingly incorporated in the structure is therefore probably due to the effect of pH. More generally, it is known that oxide nanoparticles shape and size can be tuned by varying the pH which affects surface energies by proton adsorption-desorption, therefore affecting crystal growth (Jolivet et al., 2004Jolivet, J.-P., Froidefond, C., Pottier, A., Chanéac, C., Cassaignon, S., Tronc, E., Euzen, P. (2004) Size tailoring of oxide nanoparticles by precipitation in aqueous medium. A semi-quantitative modelling. Journal of Materials Chemistry 14, 3281-3288.
).
Figure 3 TEM images of samples #1 (a) (0 % Sc), #2 (b) (1.2 % Sc), #3 (c) (5.8 % Sc) #5 (d) (30.2 % Sc), #6 (e) (57.9 % Sc) and #8 (f) (100 % Sc). All images are at the same scale (a) except the inset in image (f).
top
Discussion
Replacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984
Schulze, D.G. (1984) The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays and Clay Minerals 32, 36-44.
; Ebinger and Schulze, 1989Ebinger, M.H., Schulze, D.G. (1989) Mn-substituted goethite and Fe-substituted groutite synthesized at acid pH. Clays and Clay Minerals 37, 151-156.
; Martin et al., 1997Martin, F., Ildefonse, P., Hazemann, J.L., Mathe, P.E., Noack, Y., Grauby, O., Beziat, D., de Parseval, P. (1997) Gallium Crystal Chemistry in Synthetic Goethites. Journal de Physique IV France 7, C2-821-C2-822.
; Scheinost et al., 2001Scheinost, A.C., H., S., Schulze, D.G., Gasser, U., Sparks, D.L. (2001) Structural environment and oxidation state of Mn in goethite-groutite solid-solutions. American Mineralogist 86, 139-146.
; Sileo et al., 2004Sileo, E.E., Ramos, A.Y., Magaz, G.E., Blesa, M.A. (2004) Long-range vs. short-range ordering in synthetic Cr-substituted goethites. Geochimica et Cosmochimica Acta 68, 3053-3063.
; Alvarez et al., 2008Alvarez, M., Sileo, E.E., Rueda, E.H. (2008) Structure and reactivity of synthetic Co-substituted goethites. American Mineralogist 93, 584-590.
; Bazilevskaya et al., 2011Bazilevskaya, E., Archibald, D.D., Aryanpour, M., Kubicki, J.D., Martínez, C.E. (2011) Aluminum coprecipitates with Fe (hydr)oxides: Does isomorphous substitution of Al3+ for Fe3+ in goethite occur? Geochimica et Cosmochimica Acta 75, 4667-4683.
). More generally, iron (oxyhydr)oxides are known to be a major phase controlling the fate of these major and trace elements in the environment (Cornell and Schwertmann, 2003Cornell, R.M., Schwertmann, U. (2003) The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Second edition, WILEY-VCH. Weinheim.
; Cornu et al., 2005Cornu, S., Deschatrettes, V., Salvador-Blanes, S., Clozel, B., Hardy, M., Branchut, S., Le Forestier, L. (2005) Trace element accumulation in Mn—Fe—oxide nodules of a planosolic horizon. Geoderma 125, 11-24.
). Many studies have been performed to investigate the effect of replacing different elements on the properties of these iron (oxyhydr)oxides. Replacement of iron by manganese (Mn) in goethite was achieved up to 47 atomic percent (Ebinger and Schulze, 1989Ebinger, M.H., Schulze, D.G. (1989) Mn-substituted goethite and Fe-substituted groutite synthesized at acid pH. Clays and Clay Minerals 37, 151-156.
). However clustering of Mn in the goethite structure was observed from 13 atomic percent (Scheinost et al., 2001Scheinost, A.C., H., S., Schulze, D.G., Gasser, U., Sparks, D.L. (2001) Structural environment and oxidation state of Mn in goethite-groutite solid-solutions. American Mineralogist 86, 139-146.
). The authors attributed clustering to the strong structural distortion as Mn content increases, due to the Jahn Teller effect. Indeed, the electronic configuration of Mn (III) ([Ar] 3d4) causes the distortion of the Mn octahedra thereby preventing the good dispersion of Mn within the goethite structure, despite a similar Mn (III)-Fe (III) atomic radius. Substitution using other transition metals with similar Z and ionic radii to Fe including cobalt (Co) and chromium (Cr) has been attempted (Sileo et al., 2004Sileo, E.E., Ramos, A.Y., Magaz, G.E., Blesa, M.A. (2004) Long-range vs. short-range ordering in synthetic Cr-substituted goethites. Geochimica et Cosmochimica Acta 68, 3053-3063.
; Alvarez et al., 2008Alvarez, M., Sileo, E.E., Rueda, E.H. (2008) Structure and reactivity of synthetic Co-substituted goethites. American Mineralogist 93, 584-590.
). In the case of Cr, substitution was performed up to 12 atomic percent. Failure to incorporate more Cr was again attributed to differences in octahedral distortion but not caused by the Jahn Teller effect (electron configuration for Cr (III) is [Ar] 3d3) (Sileo et al., 2004Sileo, E.E., Ramos, A.Y., Magaz, G.E., Blesa, M.A. (2004) Long-range vs. short-range ordering in synthetic Cr-substituted goethites. Geochimica et Cosmochimica Acta 68, 3053-3063.
). Indeed, in goethite (αFeOOH), the Fe octahedron is distorted because of the nature and charges of the two types of ligands (3OH- and 3O2-), which cause distinct Fe-O distances with three distances of about 1.95 Å for the Fe-O bonds and longer distances (about 2.10 Å) for Fe-OH bonds. Conversely, a single Cr-O distance (1.98 Å) was reported in grimaldiite (αCrOOH). In this case, H atoms were reported to be half way between two O atoms of adjacent corners, leading to a more symmetrical octahedron than in goethite. Substitution was more pronounced with aluminum (Al) and gallium (Ga) with up to 33-40 % substitution (Schulze, 1984Schulze, D.G. (1984) The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays and Clay Minerals 32, 36-44.
; Martin et al., 1997Martin, F., Ildefonse, P., Hazemann, J.L., Mathe, P.E., Noack, Y., Grauby, O., Beziat, D., de Parseval, P. (1997) Gallium Crystal Chemistry in Synthetic Goethites. Journal de Physique IV France 7, C2-821-C2-822.
). In the case of Al, the atomic radius is significantly smaller (53.5 pm) than in iron (64.5 pm) representing a 16 % difference in size (Shannon, 1976Shannon, R. (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32, 751-767.
). Incorporation of Al in the structure resulted in strain and structural defects and a decrease in the cell parameters (Schulze, 1984Schulze, D.G. (1984) The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays and Clay Minerals 32, 36-44.
). This decrease was relatively linear for the b parameter from 0 to 33 atomic percent and followed predicted Vegard’s law. It was also linear for the c parameter but started to deviate from predicted Vegard’s law above 20 atomic percent and strongly deviated for the a parameter even at low substitution rates. A more recent study used both experimental and density functional theory results and showed that above 8 % atomic substitution, Al probably occurs as diaspore-like (αAlOOH) clusters within the goethite structure (Bazilevskaya et al., 2011Bazilevskaya, E., Archibald, D.D., Aryanpour, M., Kubicki, J.D., Martínez, C.E. (2011) Aluminum coprecipitates with Fe (hydr)oxides: Does isomorphous substitution of Al3+ for Fe3+ in goethite occur? Geochimica et Cosmochimica Acta 75, 4667-4683.
). The case of gallium was also investigated (Martin et al., 1997Martin, F., Ildefonse, P., Hazemann, J.L., Mathe, P.E., Noack, Y., Grauby, O., Beziat, D., de Parseval, P. (1997) Gallium Crystal Chemistry in Synthetic Goethites. Journal de Physique IV France 7, C2-821-C2-822.
). Although the chemistry of Ga is similar to that of Al, Ga has an ionic radius closer to that of Fe (62 pm), which, in theory, could facilitate substitution. The authors observed a solid solution up to 40 atomic percent and demixing above this threshold with the formation of two distinct phases, one goethite and one GaOOH similar to that observed with the αAlOOH/αFeOOH system. All these studies confirm that the successful substitution of Fe by trivalent atoms involves a complex interplay between different factors including ionic radii, geometrical distortion of various origins (e.g., the Jahn Teller effect) and the nature of bonding, which can be affected by electronegativity. However, to the best of our knowledge, this is the first time an apparent ideal solid solution has been observed with goethite as an end member despite significant differences in the properties of Fe and Sc.At first glance, the observation of this continuous solid solution between the two end members, FeOOH and ScOOH, may seem surprising, given the marked differences in ionic radii. Indeed, the first Goldschmidt’s rule stipulates that a continuous solid solution is allowed when the ionic radii differ by less than 15 %. The Fe (III) in the goethite structure is in the high spin state and its ionic radius has been reported to be 64.5 pm (Shannon, 1976
Shannon, R. (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32, 751-767.
). The Sc (III) ionic radius is 74.5 pm (Shannon, 1976Shannon, R. (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32, 751-767.
), i.e. 13.4 % bigger than the Fe (III), which is close to the threshold defined by Goldschmidt for continuous solid solutions. However, when considering the atomic Fe-O or Sc-O distances rather than only the ionic radii of Fe and Sc, successful incorporation of Sc in FeOOH is less surprising. The ionic radius of O2- (135 pm) (Shannon, 1976Shannon, R. (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32, 751-767.
) is almost twice as long as the ionic radius of Fe3+ and Sc3+, Sc-O distance (2.12 Å) is only 4 % longer than the Fe-O distance (2.03 Å). This increase in the Me-O distance matches the observed increase in the cell parameters (3.9 %, 3.6 % and 6.1 % for a, b and c respectively, Figs. 2, S-2). This is in accordance with the study of Jacob and co-workers who assumed that Vegard’s law is valid for ideal solutions when the lattice parameters of the pure components differ by less than 5 % (Jacob et al., 2007Jacob, K.T., Raj, S., Rannesh, L. (2007) Vegard's law: a fundamental relation or an approximation? International Journal of Materials Research 98, 776-779.
).To summarise, synthesis of a solid-solution αFeOOH – αScOOH consistent with an ideal solution was achieved despite significant differences in the ionic radii between Fe (III) and Sc (III). The ideal character of the solid solution should be confirmed by calorimetric measurements in order to ascertain the excess Gibbs free energy relative to the pure phases (Majzlan and Navrotsky, 2003
Majzlan, J., Navrotsky, A. (2003) Thermodynamics of the goethite-diaspore solid solution. European Journal of Mineralogy 15, 495-501.
). Additionally, further investigation is now underway to understand the mechanism behind substitution of Sc in the goethite structure at the atomic scale. In particular, the presence or absence of clusters within the solid solution will be investigated using X-ray spectroscopy. Although synthesised in conditions far from the ones prevailing in many natural environments, these results will potentially bring important information on Sc geochemical behaviour in Fe-rich environments. However, structural analogy between synthetic and natural Sc-doped goethite needs to be further explored. Additionally, these results provide important insights into Sc speciation in primary or secondary sources for the design of extraction processes with a low environmental footprint as an alternative to the currently used energy-intensive technologies.top
Acknowledgements
This work was carried out thanks to financial support from CNRT (French National Centre for Technological Research: “Nickel and its Environment”) for the “Scandium” project.
Editor: Karim Benzerara
top
References
Alvarez, M., Sileo, E.E., Rueda, E.H. (2008) Structure and reactivity of synthetic Co-substituted goethites. American Mineralogist 93, 584-590.
 Show in context
Show in contextReplacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984; Ebinger and Schulze, 1989; Martin et al., 1997; Scheinost et al., 2001; Sileo et al., 2004; Alvarez et al., 2008; Bazilevskaya et al., 2011).
View in article
Substitution using other transition metals with similar Z and ionic radii to Fe including cobalt (Co) and chromium (Cr) has been attempted (Sileo et al., 2004; Alvarez et al., 2008).
View in article
Bazilevskaya, E., Archibald, D.D., Aryanpour, M., Kubicki, J.D., Martínez, C.E. (2011) Aluminum coprecipitates with Fe (hydr)oxides: Does isomorphous substitution of Al3+ for Fe3+ in goethite occur? Geochimica et Cosmochimica Acta 75, 4667-4683.
 Show in context
Show in contextReplacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984; Ebinger and Schulze, 1989; Martin et al., 1997; Scheinost et al., 2001; Sileo et al., 2004; Alvarez et al., 2008; Bazilevskaya et al., 2011).
View in article
A more recent study used both experimental and density functional theory results and showed that above 8 % atomic substitution, Al probably occurs as diaspore-like (αAlOOH) clusters within the goethite structure (Bazilevskaya et al., 2011).
View in article
Burleson, D.J., Penn, R.L. (2006) Two-Step Growth of Goethite from Ferrihydrite. Langmuir 22, 402-409.
 Show in context
Show in context Goethite particles (0 % Sc) have a common needle-like structure (Fig. 3a) widely described in the past. Goethite growth has been described as a two step process (Burleson and Penn, 2006).
View in article
Chassé, M., Griffin, W.L., O'Reilly, S.Y., Calas, G. (2017) Scandium speciation in a world-class lateritic deposit. Geochemical Perspectives Letters 3, 105-114.
 Show in context
Show in contextRecently, scandium speciation was investigated in a lateritic deposit in eastern Australia and a strong association with iron oxides was demonstrated (Chassé et al., 2017).
View in article
Cornell, R.M., Schwertmann, U. (2003) The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Second edition, WILEY-VCH. Weinheim.
 Show in context
Show in context More generally, iron (oxyhydr)oxides are known to be a major phase controlling the fate of these major and trace elements in the environment (Cornell and Schwertmann, 2003; Cornu et al., 2005).
View in article
Cornu, S., Deschatrettes, V., Salvador-Blanes, S., Clozel, B., Hardy, M., Branchut, S., Le Forestier, L. (2005) Trace element accumulation in Mn—Fe—oxide nodules of a planosolic horizon. Geoderma 125, 11-24.
 Show in context
Show in contextMore generally, iron (oxyhydr)oxides are known to be a major phase controlling the fate of these major and trace elements in the environment (Cornell and Schwertmann, 2003; Cornu et al., 2005).
View in article
Das, S., Gaustad, G., Sekar, A., Williams, E. (2018) Techno-economic analysis of supercritical extraction of rare earth elements from coal ash. Journal of Cleaner Production 189, 539-551.
 Show in context
Show in context For example, among all critical elements present in coal ash, Sc was reported to have the highest economic value (Das et al., 2018).
View in article
Doebelin, N., Kleeberg, R. (2015) Profex: a graphical user interface for the Rietveld refinement program BGMN. Journal of Applied Crystallography 48, 1573-1580.
 Show in context
Show in contextProfex software (Doebelin and Kleeberg, 2015) was used for Rietveld refinement to determine the cell parameters of the (Fe,Sc)OOH samples.
View in article
Ebinger, M.H., Schulze, D.G. (1989) Mn-substituted goethite and Fe-substituted groutite synthesized at acid pH. Clays and Clay Minerals 37, 151-156.
 Show in context
Show in context Replacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984; Ebinger and Schulze, 1989; Martin et al., 1997; Scheinost et al., 2001; Sileo et al., 2004; Alvarez et al., 2008; Bazilevskaya et al., 2011).
View in article
Replacement of iron by manganese (Mn) in goethite was achieved up to 47 atomic percent (Ebinger and Schulze, 1989).
View in article
Frondel, C. (1968) Crystal chemistry of scandium as a trace element in minerals. Zeitschrift für Kristallographie 127, 121-138.
 Show in context
Show in context Sc is dispersed in many minerals and tends to form solid solutions (Frondel, 1968) but in such small amounts that its structural effect on the host mineral in most cases, remains unexplored.
View in article
Jacob, K.T., Raj, S., Rannesh, L. (2007) Vegard's law: a fundamental relation or an approximation? International Journal of Materials Research 98, 776-779.
 Show in context
Show in context Additionally, small deviations from Vegard’s law are expected even for ideal solutions (Jacob et al., 2007).
View in article
This is in accordance with the study of Jacob and co-workers who assumed that Vegard’s law is valid for ideal solutions when the lattice parameters of the pure components differ by less than 5 % (Jacob et al., 2007).
View in article
Jolivet, J.-P., Froidefond, C., Pottier, A., Chanéac, C., Cassaignon, S., Tronc, E., Euzen, P. (2004) Size tailoring of oxide nanoparticles by precipitation in aqueous medium. A semi-quantitative modelling. Journal of Materials Chemistry 14, 3281-3288.
 Show in context
Show in contextMore generally, it is known that oxide nanoparticles shape and size can be tuned by varying the pH which affects surface energies by proton adsorption-desorption, therefore affecting crystal growth (Jolivet et al., 2004).
View in article
Majzlan, J., Navrotsky, A. (2003) Thermodynamics of the goethite-diaspore solid solution. European Journal of Mineralogy 15, 495-501.
 Show in context
Show in contextThe ideal character of the solid solution should be confirmed by calorimetric measurements in order to ascertain the excess Gibbs free energy relative to the pure phases (Majzlan and Navrotsky, 2003).
View in article
Martin, F., Ildefonse, P., Hazemann, J.L., Mathe, P.E., Noack, Y., Grauby, O., Beziat, D., de Parseval, P. (1997) Gallium Crystal Chemistry in Synthetic Goethites. Journal de Physique IV France 7, C2-821-C2-822.
 Show in context
Show in contextReplacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984; Ebinger and Schulze, 1989; Martin et al., 1997; Scheinost et al., 2001; Sileo et al., 2004; Alvarez et al., 2008; Bazilevskaya et al., 2011).
View in article
Substitution was more pronounced with aluminum (Al) and gallium (Ga) with up to 33-40 % substitution (Schulze, 1984; Martin et al., 1997).
View in article
The case of gallium was also investigated (Martin et al., 1997).
View in article
Scheinost, A.C., H., S., Schulze, D.G., Gasser, U., Sparks, D.L. (2001) Structural environment and oxidation state of Mn in goethite-groutite solid-solutions. American Mineralogist 86, 139-146.
 Show in context
Show in context Replacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984; Ebinger and Schulze, 1989; Martin et al., 1997; Scheinost et al., 2001; Sileo et al., 2004; Alvarez et al., 2008; Bazilevskaya et al., 2011).
View in article
However clustering of Mn in the goethite structure was observed from 13 atomic percent (Scheinost et al., 2001).
View in article
Schulze, D.G. (1984) The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays and Clay Minerals 32, 36-44.
 Show in context
Show in context Conversely, the bonding of the two chains occurs through apical oxygen and hydrogen bonding (Schulze, 1984).
View in article
Replacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984; Ebinger and Schulze, 1989; Martin et al., 1997; Scheinost et al., 2001; Sileo et al., 2004; Alvarez et al., 2008; Bazilevskaya et al., 2011).
View in article
Substitution was more pronounced with aluminum (Al) and gallium (Ga) with up to 33-40 % substitution (Schulze, 1984; Martin et al., 1997).
View in article
Incorporation of Al in the structure resulted in strain and structural defects and a decrease in the cell parameters (Schulze, 1984).
View in article
Schwertmann, U., Cornell, R.M. (1991) Iron Oxides in the Laboratory, Preparation and Characterization. VCH. Weinheim.
 Show in context
Show in context The synthesis of Sc-doped goethite was performed using the protocol of Schwertmann and Cornell (1991) with modifications.
View in article
X-ray diffraction of the 0 % Sc sample (Fig. 1) is characteristic of the orthorhombic phase of goethite, which is consistent with Schwertmann and Cornell (1991).
View in article
Shannon, R. (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32, 751-767.
 Show in context
Show in contextIn the case of Al, the atomic radius is significantly smaller (53.5 pm) than in iron (64.5 pm) representing a 16 % difference in size (Shannon, 1976).
View in article
The Fe (III) in the goethite structure is in the high spin state and its ionic radius has been reported to be 64.5 pm (Shannon, 1976).
View in article
The Sc (III) ionic radius is 74.5 pm (Shannon, 1976), i.e. 13.4 % bigger than the Fe (III), which is close to the threshold defined by Goldschmidt for continuous solid solutions.
View in article
The ionic radius of O2- (135 pm) (Shannon, 1976) is almost twice as long as the ionic radius of Fe3+ and Sc3+, Sc-O distance (2.12 Å) is only 4 % longer than the Fe-O distance (2.03 Å).
View in article
Sileo, E.E., Ramos, A.Y., Magaz, G.E., Blesa, M.A. (2004) Long-range vs. short-range ordering in synthetic Cr-substituted goethites. Geochimica et Cosmochimica Acta 68, 3053-3063.
 Show in context
Show in context Replacing iron with other elements in goethite has been the focus of a number of studies due to iron’s ability to trap a number of cations including transition metals (Co, Cr, Mn) and post-transition metals (Al, Ga) (Schulze, 1984; Ebinger and Schulze, 1989; Martin et al., 1997; Scheinost et al., 2001; Sileo et al., 2004; Alvarez et al., 2008; Bazilevskaya et al., 2011).
View in article
Substitution using other transition metals with similar Z and ionic radii to Fe including cobalt (Co) and chromium (Cr) has been attempted (Sileo et al., 2004; Alvarez et al., 2008).
View in article
Failure to incorporate more Cr was again attributed to differences in octahedral distortion but not caused by the Jahn Teller effect (electron configuration for Cr (III) is [Ar] 3d3) (Sileo et al., 2004).
View in article
U.S. Geological Survey (2018) Scandium. In: Mineral Commodity Summaries. U.S. Geological Survey, Reston, USA, 144–145
 Show in context
Show in contextThis is the case of scandium (Sc), which is coveted by several countries including Australia, India, Russia, and the Philippines (U.S. Geological Survey, 2018).
View in article
The automotive industry has also been predicted to be a major consumer of aluminium-scandium alloy, which will further increase the demand for scandium (U.S. Geological Survey, 2018).
View in article
Other applications include ceramics, electronics, lasers, lighting, and radioactive isotopes (U.S. Geological Survey, 2018).
View in article
Vind, J., Malfliet, A., Bonomi, C., Paiste, P., Sajó, I.E., Blanpain, B., Tkaczyk, A.H., Vassiliadou, V., Panias, D. (2018) Modes of occurrences of scandium in Greek bauxite and bauxite residue. Minerals Engineering 123, 35-48.
 Show in context
Show in context Scandium has also been found to be strongly associated with iron (oxyhydr)oxide minerals (goethite and hematite) in Greek bauxite and bauxite residue after extraction of alumina (Vind et al., 2018).
View in article
Wang, W., Pranolo, Y., Cheng, C.Y. (2011) Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 108, 100-108.
 Show in context
Show in contextDespite a few Sc-rich minerals (thortveitite, euxenite, and gadolinite), Sc does not selectively combine with the common ore-forming anions, which explains why Sc only forms small deposits, thereby preventing its widespread use (Wang et al., 2011).
View in article
Zhang, Y.-W., Liu, J.-H., Si, R., Yan, Z.-G., Yan, C.-H. (2005) Phase Evolution, Texture Behavior, and Surface Chemistry of Hydrothermally Derived Scandium (Hydrous) Oxide Nanostructures. The Journal of Physical Chemistry B 109, 18324-18331.
 Show in context
Show in context Zhang et al. (2005) showed that the nature and morphologies of Sc (oxyhydr)oxide vary depending on the synthesis conditions (Zhang et al., 2005).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Sample Characterisation
- Table S-1
- Figures S-1 and S-2
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
Table 1 Relative abundances of Sc measured by ICP-AES and EDX/TEM.
| Samples | n Sc / (n Sc+Fe ) x 100 | ||
| Nominal | ICP-AES | EDX/TEM | |
| #1 | 0 | ||
| #2 | 1 | 1.2 ± 0.01 | 1.2 ± 0.3 |
| #3 | 5 | 5.8 ± 0.10 | 6.3 ± 1.3 |
| #4 | 15 | 18.3 ± 0.30 | 17.9 ± 5.3 |
| #5 | 25 | 30.2 ± 0.30 | 29.0 ± 4.6 |
| #6 | 50 | 57.9 ± 0.40 | 49.9 ± 10.0 |
| #7 | 75 | 79.9 ± 1.10 | 80.2 ± 1.3 |
| #8 | 100 | 100.0 ± 1.20 | |
Calculated standard deviation based on the measurements of Fe and Sc concentrations on 5 replicates (ICP-AES), and on 10 particle aggregates (EDX/TEM).
Back to article | Download in Excel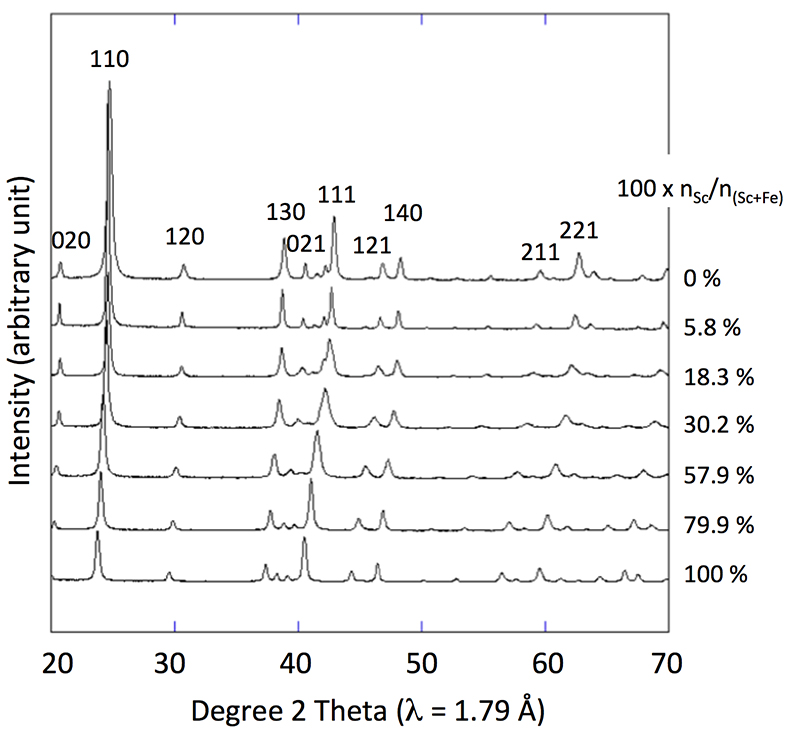
Figure 1 X-ray diffractograms for goethite, ScOOH and Sc-doped goethite samples.
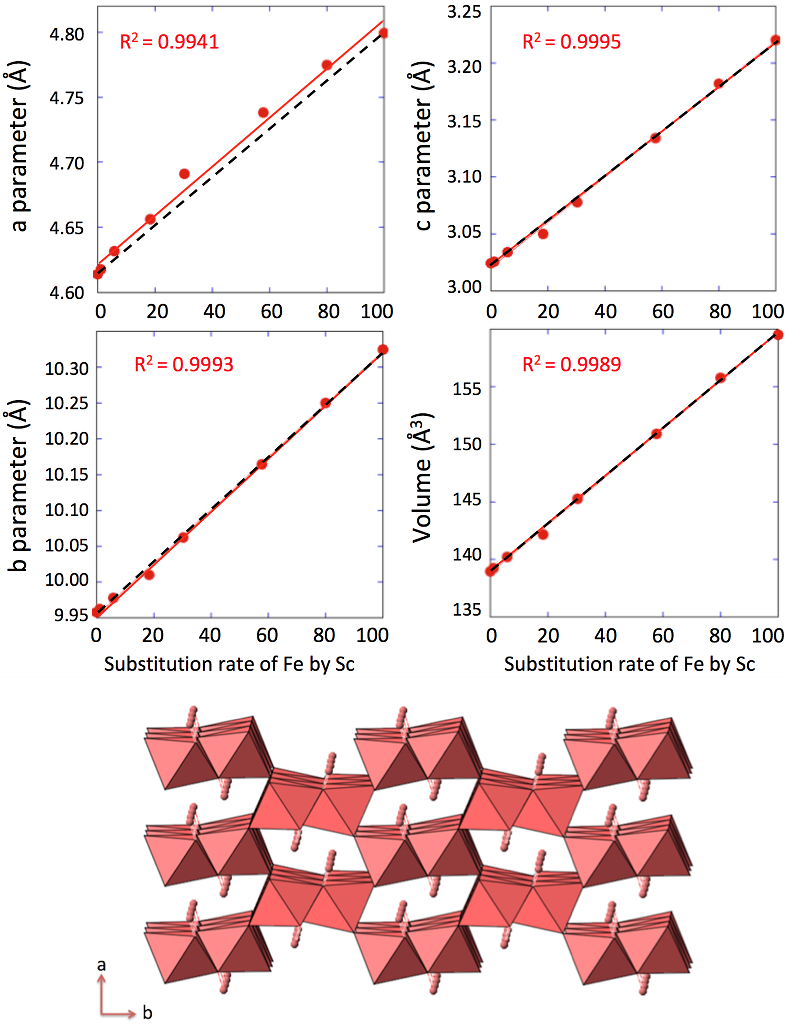
Figure 2 Top: Changes in cell parameters and in cell volume as a function of rate of substitution of Fe by Sc (numerical data for cell parameters and errors can be found in SI). Dashed lines represent ideal Vegard’s law behaviour. Bottom: Crystal structure of goethite. The octahedrons represent the Fe surrounded by 6 oxygen atoms. Spheres represent the hydrogen atoms.
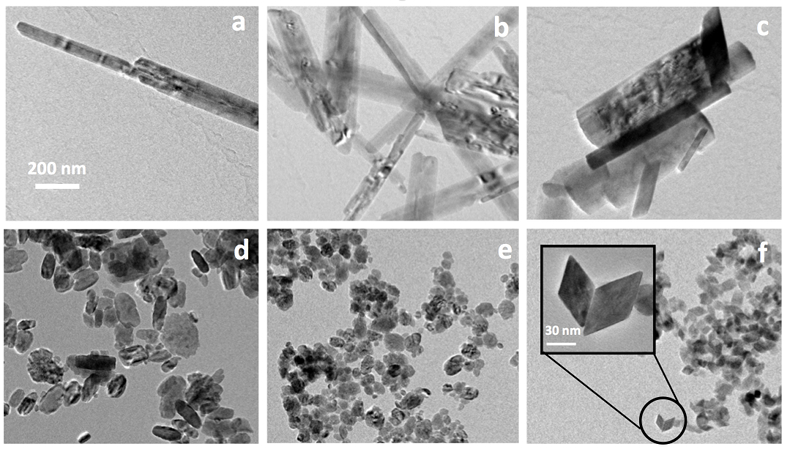
Figure 3 TEM images of samples #1 (a) (0 % Sc), #2 (b) (1.2 % Sc), #3 (c) (5.8 % Sc) #5 (d) (30.2 % Sc), #6 (e) (57.9 % Sc) and #8 (f) (100 % Sc). All images are at the same scale (a) except the inset in image (f).