Zircon halogen geochemistry: Insights into Hadean-Archean fluids
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:3,426Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
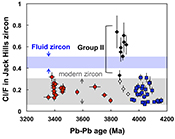
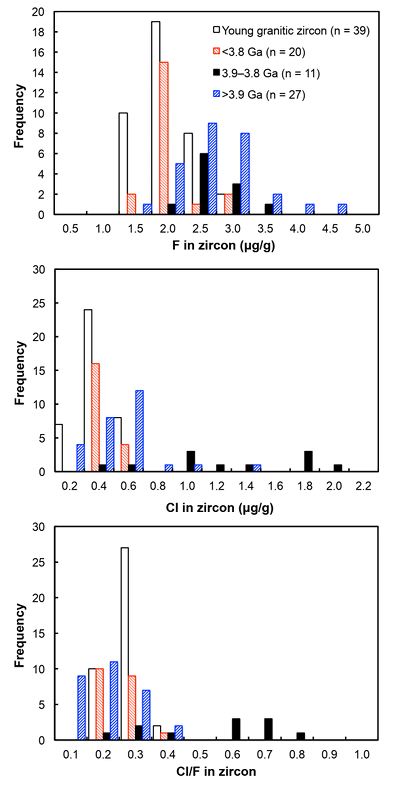 Figure 1 Fluorine concentrations in Jack Hills zircons ([F]average = 2.28 ± 0.19 μg/g) are generally indistinguishable from those in young granitic zircons ([F]average = 1.80 ± 0.11 μg/g). Elevated Cl concentrations observed in eight 3.9–3.8 Ga zircons (black columns) yield distinctly high Cl/F ratios (Cl/F > 0.3) when compared with other Jack Hills zircons. | 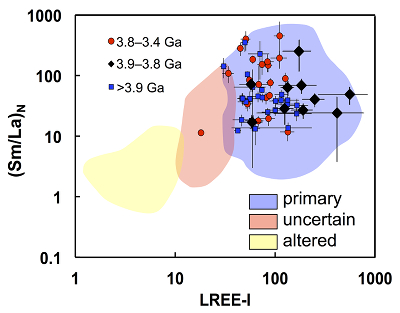 Figure 2 High values of (Sm/La)N and LREE-I [(Dy/Nd) + (Dy/Sm)] in Jack Hills sample grains imply that most of samples, especially Cl-rich zircons, are primary. The elevated Cl concentrations in zircons therefore derived from primary sources. | 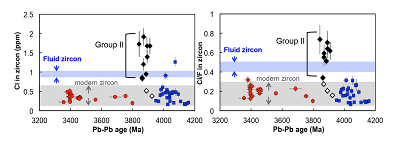 Figure 3 (a) Chlorine distribution and (b) Cl/F fractionation in Jack Hills zircons are mainly identical to the ranges determined from modern magmatic zircons in this study (red circles: <3.8 Ga zircons; white diamonds: 3.9–3.8 Ga Group I zircons; blue rectangles: >3.9 Ga zircons; grey bar: modern zircon ranges) except Group II zircons crystallised at 3.9–3.8 Ga (black diamonds). A synthetic zircon grown in an aqueous-rich fluid exhibits Cl content and Cl/F ratios (blue bars) that fall within the range of Group II. | 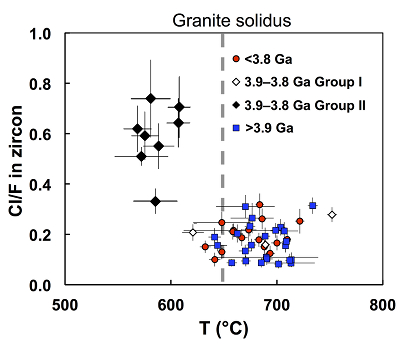 Figure 4 Chlorine-rich Group II zircons are characterised by Ti-in-zircon temperatures below granite solidus. The clearly distinguished cluster of Group II from other magmatic zircons suggests the mechanism of Group II as solid state recrystallisation involving Cl-bearing fluids. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Earliest Earth remains one of the great frontiers for fundamental discovery as little is known about key events such as the mechanisms and timing of life’s origin. Given that >3.8 Ga rocks are exceedingly rare and no rock older than 4.03 Ga is known (cf. O’Neil et al., 2008
O'Neil, J., Carlson, R.W., Francis, D., Stevenson, R.K. (2008) Neodymium-142 evidence for Hadean mafic crust. Science 321, 1828-1831.
), constraining conditions on early Earth has largely relied on the only materials identified from the Hadean eon: ~4.4 to 3.8 Ga detrital zircons (Mojzsis et al., 2001Mojzsis, S.J., Harrison, T.M., Pidgeon, R.T. (2001) Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4,300 Myr ago. Nature 409, 178-181.
; Peck et al., 2001Peck, W.H., Valley, J.W., Wilde, S.A., Graham, C.M. (2001) Oxygen isotope ratios and rare earth elements in 3.3 to 4.4 Ga zircons: Ion microprobe evidence for high δ18O continental crust and oceans in the Early Archean. Geochimica et Cosmochimica Acta 65, 4215-4229.
; Harrison et al., 2008Harrison, T.M., Schmitt, A.K., McCulloch, M.T., Lovera, O.M. (2008) Early (≥ 4.5 Ga) formation of terrestrial crust: Lu–Hf, δ18O, and Ti thermometry results for Hadean zircons. Earth and Planetary Science Letters 268, 476-486.
; Holden et al., 2009Holden, P., Lanc, P., Ireland, T.R., Harrison, T.M., Foster, J.J. Bruce, Z. (2009) Mass-spectrometric mining of Hadean zircons by automated SHRIMP multi-collector and single-collector U/Pb zircon age dating: the first 100,000 grains. International Journal of Mass Spectrometry 286, 53-63.
; Bell et al., 2011Bell, E.A., Harrison, T.M., McCulloch, M.T., Young, E.D. (2011) Early Archean crustal evolution of the Jack Hills Zircon source terrane inferred from Lu–Hf, 207Pb/206Pb, and δ18O systematics of Jack Hills zircons. Geochimica et Cosmochimica Acta 75, 4816-4829.
). Oxygen isotopes, rare earth element (REE) patterns, crystallisation temperatures, and inclusions in detrital zircons from the Jack Hills, Western Australia, are suggestive of the presence of recycled crustal material that had interacted with liquid water at low temperature (e.g., Watson and Harrison, 2005Watson, E.B., Harrison, T.M. (2005) Zircon thermometer reveals minimum melting conditions on earliest Earth. Science 308, 841-844.
; Trail et al., 2011Trail, D., Watson, E.B., Tailby, N.D. (2011) The oxidation state of Hadean magmas and implications for early Earth's atmosphere. Nature 480, 79-82.
; Bell and Harrison 2013Bell, E.A., Harrison, T.M. (2013) Post-Hadean transitions in Jack Hills zircon provenance: A signal of the Late Heavy Bombardment? Earth and Planetary Science Letters 364, 1-11.
; Harrison et al., 2017Harrison, T.M., Bell, E.A., Boehnke, P. (2017) Hadean zircon petrochronology. Reviews in Mineralogy and Geochemistry 83, 329-363.
) and show how experimental calibrations can permit zircon to act as an environmental monitor of lithosphere and surface processes. On the other hand, the lack of a known sedimentary record older than 3.83 Ga (Manning et al., 2006Manning, C.E., Mojzsis, S.J., Harrison, T.M. (2006) Geology, age and origin of supracrustal rocks at Akilia, West Greenland. American Journal of Science 306, 303-366.
) limits our understanding of the volatile evolution on earliest Earth. To address this issue, one approach is to trace those volatile components that partition at measureable levels into zircon. Previous investigations of oxygen isotopes and trace elements in Jack Hills zircons (e.g., Mojzsis et al., 2001Mojzsis, S.J., Harrison, T.M., Pidgeon, R.T. (2001) Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4,300 Myr ago. Nature 409, 178-181.
; Cavosie et al., 2005Cavosie, A.J., Valley, J.W., Wilde, S.A. (2005) Magmatic δ18O in 4400–3900 Ma detrital zircons: A record of the alteration and recycling of crust in the Early Archean. Earth and Planetary Science Letters 235, 663-681.
; Trail et al., 2007Trail, D., Mojzsis, S.J., Harrison, T.M., Schmitt, A.K., Watson, E.B., Young, E.D. (2007) Constraints on Hadean zircon protoliths from oxygen isotopes, Ti‐thermometry, and rare earth elements. Geochemistry, Geophysics, Geosystems 8, 1-22.
; Bell et al., 2011Bell, E.A., Harrison, T.M., McCulloch, M.T., Young, E.D. (2011) Early Archean crustal evolution of the Jack Hills Zircon source terrane inferred from Lu–Hf, 207Pb/206Pb, and δ18O systematics of Jack Hills zircons. Geochimica et Cosmochimica Acta 75, 4816-4829.
) indicate two distinctive protolith sources: Group I and Group II (Bell and Harrison 2013Bell, E.A., Harrison, T.M. (2013) Post-Hadean transitions in Jack Hills zircon provenance: A signal of the Late Heavy Bombardment? Earth and Planetary Science Letters 364, 1-11.
). Group I is similar in most respects to >3.9 Ga and <3.8 Ga Jack Hills zircons, with magmatic Th/U and average Ti-in-zircon temperatures ca. 680 °C. Group II is a distinctive subset of the zircons with ages 3.9–3.8 Ga that show distinctive chemistry relative to Group I (lower Th/U, P, and Ce; higher U and Hf). Their Ti-in-zircon temperatures are mostly subsolidus, and these zircons are either dark and homogeneous in cathodoluminescence or display patchy zoning. The Group II characteristics imply recrystallisation during thermal events (Hoskin and Black, 2000Hoskin, P.W.O., Black, L.P. (2000) Metamorphic zircon formation by solid-state recrystallization of protolith igneous zircon. Journal of Metamorphic Geology 18, 423-439.
). Such episodes may have provided a mechanism for volatile transport in early fluids. Given the significance of halogens in lithosphere-hydrosphere-atmosphere interactions, we focus here on their abundances in Jack Hills zircons and examine fractionations between nominally mobile (Cl) and immobile (F) halogens in a variety of reservoirs (e.g., magmatic and metamorphic systems).top
Samples and Methods
Our Jack Hills zircon suite ranges in U-Pb age from 4.2 to 3.4 Ga (Table S-2). Oxygen isotope ratios and REE patterns in some of these zircons have been investigated previously and shown to lack secondary alteration (Table S-2, S-3, and S-4, Fig. S-7 and S-8; Bell et al., 2011
Bell, E.A., Harrison, T.M., McCulloch, M.T., Young, E.D. (2011) Early Archean crustal evolution of the Jack Hills Zircon source terrane inferred from Lu–Hf, 207Pb/206Pb, and δ18O systematics of Jack Hills zircons. Geochimica et Cosmochimica Acta 75, 4816-4829.
, 2014Bell, E.A., Harrison, T.M., Kohl, I.E., Young, E.D. (2014) Eoarchean crustal evolution of the Jack Hills zircon source and loss of Hadean crust. Geochimica et Cosmochimica Acta 146, 27-42.
, 2016Bell, E.A., Boehnke, P., Harrison, T.M. (2016) Recovering the primary geochemistry of Jack Hills zircons through quantitative estimates of chemical alteration. Geochimica et Cosmochimica Acta 191, 187-202.
; Bell and Harrison 2013Bell, E.A., Harrison, T.M. (2013) Post-Hadean transitions in Jack Hills zircon provenance: A signal of the Late Heavy Bombardment? Earth and Planetary Science Letters 364, 1-11.
). Given the possible felsic parent sources of Jack Hills zircon (Bell et al., 2018Bell, E.A., Boehnke, P., Harrison, T.M., Wielicki, M.M. (2018) Mineral inclusion assemblage and detrital zircon provenance. Chemical Geology 477, 151-160.
), zircons from igneous (I-type) and sedimentary (S-type) granitoids from the Phanerozoic Lachlan Fold Belt (LFB), Australia, and Mesozoic batholiths of southern California, USA, were also investigated as analogues for establishing geologic provenance. Detection limits ([F] = 0.78 ± 0.07 μg/g and [Cl] = 0.12 ± 0.02 μg/g with 2 se.; Fig. S-4) were determined by analysing synthetic zircons grown in halogen-free conditions by the 1 atm flux method (e.g., Trail et al., 2016Trail. D., Cherniak, D.J., Watson, E.B., Harrison, T.M., Weiss, B.P., Szumila, I. (2016) Li zoning in zircon as a potential geospeedometer and peak temperature indicator. Contributions to Mineralogy and Petrology 171, 25-40.
). We also synthesised zircon grains in a halogen-rich fluid to explore Cl (and F) partitioning in zircon and to produce an analogue of recrystallised zircon (see Supplementary Information for experimental details).The in situ analyses of Cl and F in zircon were performed using the CAMECA ims1290 ion microprobe. Analytical details are described in the Supplementary Information. Mud Tank zircons implanted with known F or Cl isotopic dosages were analysed as concentration standards. Cracks/inclusions in samples were identified via secondary and backscattered electron SEM imaging. Analysis zones were limited to regions without cracks or inclusions.
top
Results
Young zircons (1.1–0.1 Ga) display uniform F concentrations (Fig. 1) within uncertainty (1.80 ± 0.11 μg/g), except AS3 zircon (Duluth Complex, Minnesota), which we attribute to enrichment during hydrothermal alteration (Takehara et al., 2018
Takehara, M., Horie, K., Hokada, T., Kiyokawa, S. (2018) New insight into disturbance of U-Pb and trace-element systems in hydrothermally altered zircon via SHRIMP analyses of zircon from the Duluth Gabbro. Chemical Geology 484, 168-178.
). All the post-Archean zircons in this study exhibit systematically low Cl contents, some of which cannot be resolved from the baseline. The average concentration is determined as 0.31 ± 0.04 μg/g.Fluorine concentrations in all 58 Jack Hills samples are slightly higher than those in zircons from the LFB and southern Californian granitoids (2.28 ± 0.19 μg/g). Chlorine concentrations and Cl/F ratios in Jack Hills zircons appear to vary with age (Fig. 1). Significantly high Cl concentration ([Cl]average = 1.19 ± 0.32 μg/g) is observed in a subset of zircons within a narrow age range of 3.9–3.8 Ga. Archean grains with ages from 3.8 to 3.4 Ga display Cl contents identical to the young zircons ([Cl]average = 0.34 ± 0.04 μg/g), pointing to a magmatic environment with halogens levels roughly similar to modern igneous systems. Two Jack Hills zircons older than 3.9 Ga yield exceptionally high Cl contents (RSES 178-7.14 and RSES 178-8.8 with Pb-Pb ages of 4012 Ma and 4073 Ma, respectively), although the halogen measurements on the other 25 grains are within error of the range of 0.1-0.6 μg/g in the young zircons.

Figure 1 Fluorine concentrations in Jack Hills zircons ([F]average = 2.28 ± 0.19 μg/g) are generally indistinguishable from those in young granitic zircons ([F]average = 1.80 ± 0.11 μg/g). Elevated Cl concentrations observed in eight 3.9–3.8 Ga zircons (black columns) yield distinctly high Cl/F ratios (Cl/F > 0.3) when compared with other Jack Hills zircons.
top
Halogens in Jack Hills Zircons: Primary or Secondary?
Given that mobile elements can be substituted into the zircon lattice during secondary alteration (e.g., Bell et al., 2016
Bell, E.A., Boehnke, P., Harrison, T.M. (2016) Recovering the primary geochemistry of Jack Hills zircons through quantitative estimates of chemical alteration. Geochimica et Cosmochimica Acta 191, 187-202.
), care must be taken to ascertain that Cl and F concentrations reported for Jack Hills zircons do not represent post-crystallisation hydrothermal alteration. In our study, U-Pb ages in all Cl-rich zircons are concordant within ±10 % (Bell and Harrison 2013Bell, E.A., Harrison, T.M. (2013) Post-Hadean transitions in Jack Hills zircon provenance: A signal of the Late Heavy Bombardment? Earth and Planetary Science Letters 364, 1-11.
) indicating little Pb loss or U gain after 3.8 Ga. In addition, two indices, including (Sm/La)N (Hoskin, 2005Hoskin P.W.O. (2005) Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia. Geochimica et Cosmochimica Acta 69, 637–648.
) and the LREE-I [= (Dy/Nd) + (Dy/Sm); Bell et al., 2016Bell, E.A., Boehnke, P., Harrison, T.M. (2016) Recovering the primary geochemistry of Jack Hills zircons through quantitative estimates of chemical alteration. Geochimica et Cosmochimica Acta 191, 187-202.
], can test for possible alteration effects leading to crystalline degeneration. The high values of both indices for all grains (except one Archean zircon) are indicative of crystalline zircon, including the Cl-rich grains (see Bell et al., 2016Bell, E.A., Boehnke, P., Harrison, T.M. (2016) Recovering the primary geochemistry of Jack Hills zircons through quantitative estimates of chemical alteration. Geochimica et Cosmochimica Acta 191, 187-202.
) (Fig. 2). The halogen abundances are interpreted as reflecting concentrations present during crystallisation from their primary sources magmas. The distribution of oxygen isotopes in our samples (δ18Oave= 5.87 ± 0.17; Fig. S-7) is consistent with the prevailing populations for their corresponding time periods (Cavosie et al., 2005Cavosie, A.J., Valley, J.W., Wilde, S.A. (2005) Magmatic δ18O in 4400–3900 Ma detrital zircons: A record of the alteration and recycling of crust in the Early Archean. Earth and Planetary Science Letters 235, 663-681.
; Trail et al., 2007Trail, D., Mojzsis, S.J., Harrison, T.M., Schmitt, A.K., Watson, E.B., Young, E.D. (2007) Constraints on Hadean zircon protoliths from oxygen isotopes, Ti‐thermometry, and rare earth elements. Geochemistry, Geophysics, Geosystems 8, 1-22.
; Bell et al., 2016Bell, E.A., Boehnke, P., Harrison, T.M. (2016) Recovering the primary geochemistry of Jack Hills zircons through quantitative estimates of chemical alteration. Geochimica et Cosmochimica Acta 191, 187-202.
) and no obvious correlation between δ18O and Cl concentration is observed. The prominent excursion of Cl content in multiple 3.9–3.8 Ga zircons is higher than that seen thus far in post-Archean grains.
Figure 2 High values of (Sm/La)N and LREE-I [(Dy/Nd) + (Dy/Sm)] in Jack Hills sample grains imply that most of samples, especially Cl-rich zircons, are primary. The elevated Cl concentrations in zircons therefore derived from primary sources.
top
Group II Zircons: Recrystallisation in Cl-rich Fluids?
Bell and Harrison (2013)
Bell, E.A., Harrison, T.M. (2013) Post-Hadean transitions in Jack Hills zircon provenance: A signal of the Late Heavy Bombardment? Earth and Planetary Science Letters 364, 1-11.
classified 3.9–3.8 Ga Jack Hills zircon grains into two categories. Group I exhibits temperatures and compositions similar to other igneous zircons across the age spectrum, and Group II shows high U, lower (Th/U) ratio (<0.25), and anomalously low Ti (1.82 ± 0.47 μg/g). The concordant U-Pb ages in Group II zircons and their igneous protolith similar to Group I suggest that they formed by transgressive recrystallisation, in which recrystallisation occurs across the entire zircon under high temperatures (Hoskin and Black, 2000Hoskin, P.W.O., Black, L.P. (2000) Metamorphic zircon formation by solid-state recrystallization of protolith igneous zircon. Journal of Metamorphic Geology 18, 423-439.
). The distribution of halogens in the Jack Hills zircons is consistent with this classification. The ca. 3.9 Ga zircons in this study contain three Group I zircons ([Cl]average = 0.58 ± 0.28 μg/g) and eight Group II zircons ([Cl]average = 1.43 ± 0.33 μg/g) (Table S-2), in which two Group I zircons (RSES 56-3.17 and RSES 181-2.10) display Cl contents identical to the young zircons, and the other Group I zircon (RSES 178-20.20) has a Cl concentration higher than the young zircons, but consistent with the lowest level of Cl contents in Group II zircons (Fig. 3a).Significant differences in Cl/F ratios are observed between Group I zircons and Group II zircons (Fig. 3b). The Jack Hills zircons with the age of 3.8–3.3 Ga and 4.2–3.9 Ga display Cl/F ratios within the range of young magmatic zircons (0.02-0.30). In addition, in the period of 3.9–3.8 Ga, no distinguishable difference of Cl/F ratios in three Group I zircons are found compared to other Jack Hills zircons. However, Cl/F ratios of Group II zircons are distinctively higher (0.33-0.74) than the ratios of young magmatic zircons.

Figure 3 (a) Chlorine distribution and (b) Cl/F fractionation in Jack Hills zircons are mainly identical to the ranges determined from modern magmatic zircons in this study (red circles: <3.8 Ga zircons; white diamonds: 3.9–3.8 Ga Group I zircons; blue rectangles: >3.9 Ga zircons; grey bar: modern zircon ranges) except Group II zircons crystallised at 3.9–3.8 Ga (black diamonds). A synthetic zircon grown in an aqueous-rich fluid exhibits Cl content and Cl/F ratios (blue bars) that fall within the range of Group II.
The Ti-in-zircon crystallisation temperatures (Txlln) of each Jack Hills zircon are obtained using a protocol developed in previous studies (Watson and Harrison, 2005
Watson, E.B., Harrison, T.M. (2005) Zircon thermometer reveals minimum melting conditions on earliest Earth. Science 308, 841-844.
; Harrison et al., 2008Harrison, T.M., Schmitt, A.K., McCulloch, M.T., Lovera, O.M. (2008) Early (≥ 4.5 Ga) formation of terrestrial crust: Lu–Hf, δ18O, and Ti thermometry results for Hadean zircons. Earth and Planetary Science Letters 268, 476-486.
). As shown in Figure 4, the Jack Hills samples group into two clusters based on their Txlln and Cl/F ratios. All the Cl-rich Group II zircons exhibit distinct Txlln below the nominal granite solidus (~650 °C), with an average Txlln of 596 ± 17 °C. In contrast, the Txlln among all other Jack Hills zircons with low Cl/F ratios overall cluster about an identical average of 680 °C, indistinguishable from the Hadean distribution (Watson and Harrison, 2005Watson, E.B., Harrison, T.M. (2005) Zircon thermometer reveals minimum melting conditions on earliest Earth. Science 308, 841-844.
).
Figure 4 Chlorine-rich Group II zircons are characterised by Ti-in-zircon temperatures below granite solidus. The clearly distinguished cluster of Group II from other magmatic zircons suggests the mechanism of Group II as solid state recrystallisation involving Cl-bearing fluids.
A possible mechanism to interpret the low Ti contents and other characteristics of Group II zircons is metamorphic transgressive recrystallisation of originally igneous zircons (Bell and Harrison, 2013
Bell, E.A., Harrison, T.M. (2013) Post-Hadean transitions in Jack Hills zircon provenance: A signal of the Late Heavy Bombardment? Earth and Planetary Science Letters 364, 1-11.
). High temperature transgressive recrystallisation results in flushing the more incompatible trace elements (e.g., Ce) from the mineral, an increase in compatible elements (e.g., Hf, U), and homogeneous dark textures (Hoskin and Black, 2000Hoskin, P.W.O., Black, L.P. (2000) Metamorphic zircon formation by solid-state recrystallization of protolith igneous zircon. Journal of Metamorphic Geology 18, 423-439.
). These characteristics are all consistent with the chemistry and internal structure of Group II zircons. Moreover, transgressive recrystallisation of zircon occurs during metamorphism at temperatures up to 600 °C. Such conditions can be achieved by the presence of hydrothermal fluids which can quickly elevate and maintain local temperatures for significant durations (Hoskin and Black, 2000Hoskin, P.W.O., Black, L.P. (2000) Metamorphic zircon formation by solid-state recrystallization of protolith igneous zircon. Journal of Metamorphic Geology 18, 423-439.
). Zircon recrystallisation can occur in the contact or absence of fluids, although the elevated Cl concentrations and Cl/F ratios in Group II Jack Hills zircons relative to magmatic zircons strongly suggest the involvement of Cl-rich fluids at ca. 3.9 Ga, which would additionally enhance zircon recrystallisation given high solubility of ZrO2 in saline fluids relative to pure H2O (Bernini et al., 2013Bernini, D., Audétat, A., Dolejš, D., Keppler. H. (2013) Zircon solubility in aqueous fluids at high temperatures and pressures. Geochimica et Cosmochimica Acta 119, 178-187.
).We can roughly estimate Cl content in fluids during Group II recrystallisation based on the analysis of synthetic zircon synthesised in a Cl- and F-bearing aqueous fluid. The zircon synthesised from a fluid with 4000 μg/g Cl and 2000 μg/g F displays a Cl concentration of ~1 μg/g and a Cl/F ratio of 0.46, demonstrating that Cl can substitute into zircon when crystallising in fluid-rock systems (Table S-1). The Cl/F ratio is relatively low compared with ratios in Group II zircons, which could be attributed to the high temperature (1300 °C; 1 GPa) of the experiment relative to Hadean conditions (~680 °C) and/or the different Cl/F ratios compared to the natural fluid ratios. The partition coefficient (Dzircon-fluid) of 2.3 ± 0.2 × 10-4 implies fluids with Cl contents of 4 × 103-1 × 104 μg/g during Group II recrystallisation. This is similar to Cl concentrations in some brine hydrothermal fluids in the present day (e.g., Stefánsson and Barnes, 2016
Stefánsson, A., Barnes, J.D. (2016) Chlorine isotope geochemistry of Icelandic thermal fluids: Implications for geothermal system behavior at divergent plate boundaries. Earth and Planetary Science Letters 449, 69-78.
), though significantly lower than that in modern deep crustal fluids (e.g., Bodnar, 2003Bodnar, R.J. (2003) Introduction to aqueous fluid systems. In: Samson, I., Anderson, A., Marshall, D. (Eds.) Fluid Inclusions: Analysis and Interpretation. Mineralogical Association of Canada Short Course Series Volume 32, 81-99.
). Future systematic experiments are required for precise estimates on Dzircon-fluid under different P-T conditions.top
Implications and Conclusions
Studies of Bulk Silicate Earth (BSE) halogen abundances suggest the anomalous depletions observed in volatile halogens (Cl, Br, and I) relates to their volatility during condensation from the solar nebula (Lodders, 2003
Lodders, K. (2003) Solar system abundances and condensation temperatures of the elements. The Astrophysical Journal 591, 1220-1247.
; Sharp and Draper, 2013Sharp, Z.D., Draper, D.S. (2013) The chlorine abundance of Earth: implications for a habitable planet. Earth and Planetary Science Letters 369, 71-77.
). However, these putative depletions were rendered moot by a recent analysis of the abundances of Cl, Br, and I in meteorites, which indicate much lower average abundances in primitive chondrites (Clay et al., 2017Clay, P.L., Burgess, R., Busemann, H., Ruzié-Hamilton, L., Joachim, B., Day, J.M.D., Ballentine, C.J. (2017) Halogens in chondritic meteorites and terrestrial accretion. Nature 551, 614-618.
) than previously thought. Given the large proportion of heavy halogens in the crustal reservoirs (80–90 % of BSE; Burgess et al., 2002Burgess, R., Layzelle, E., Turner, G., Harris, J.W. (2002) Constraints on the age and halogen composition of mantle fluids in Siberian coated diamonds. Earth and Planetary Science Letters 197, 193-203.
), notwithstanding contributions from a late veneer (ca. 1 % Earth mass) or complete mantle degassing (maximum estimated extraction efficiency is ~50 %; Allègre et al., 1996Allègre, C.J., Hofmann, A., O'Nions, K. (1996) The argon constraints on mantle structure. Geophysical Research Letters 23, 3555-3557.
), additional halogens transported from deep Earth would still be required, likely via early fluids.The enrichment of Cl in all Group II Jack Hills zircons indicates the presence of fluids with Cl concentrations similar to modern metamorphic fluids. The average δ18O identical to prevailing populations of other Jack Hills zircons suggest that rather than recycled crustal material, which would yield distinctly high δ18O values, the Cl-bearing fluids occurred inside of nascent crust, either in the deeper crust or near the surface following impacts. Whether the ~3.9 Ga recrystallisation arises from meteoritic, meteoric or deep sourced fluids, the elevated Cl concentration in Group II zircons provides the first insight into halogen transfer and cycling through metamorphic fluids on early Earth.
top
Acknowledgements
We thank Ming-Chang Liu for assistance with our SIMS analyses and Bruce S. Brunschwig for his assistance with the profilometer at the Molecular Materials Research Center, Caltech. Yanling Wang is also thanked for assistance. The ion microprobe facility at UCLA is partially supported by a grant from the Instrumentation and Facilities Program, Division of Earth Sciences, NSF (1339051). The research received support from EAR-1447404. We thank Veronique Le Roux, an anonymous reviewer, and the editor Horst Marschall for helpful reviews that improved the paper.
Editor: Horst R. Marschall
top
References
Allègre, C.J., Hofmann, A., O'Nions, K. (1996) The argon constraints on mantle structure. Geophysical Research Letters 23, 3555-3557.
 Show in context
Show in contextGiven the large proportion of heavy halogens in the crustal reservoirs (80–90 % of BSE; Burgess et al., 2002), notwithstanding contributions from a late veneer (ca. 1 % Earth mass) or complete mantle degassing (maximum estimated extraction efficiency is ~50 %; Allègre et al., 1996), additional halogens transported from deep Earth would still be required, likely via early fluids.
View in article
Bell, E.A., Harrison, T.M. (2013) Post-Hadean transitions in Jack Hills zircon provenance: A signal of the Late Heavy Bombardment? Earth and Planetary Science Letters 364, 1-11.
 Show in context
Show in contextOxygen isotopes, rare earth element (REE) patterns, crystallisation temperatures, and inclusions in detrital zircons from the Jack Hills, Western Australia, are suggestive of the presence of recycled crustal material that had interacted with liquid water at low temperature (e.g., Watson and Harrison, 2005; Trail et al., 2011; Bell and Harrison 2013; Harrison et al., 2017) and show how experimental calibrations can permit zircon to act as an environmental monitor of lithosphere and surface processes.
View in article
Previous investigations of oxygen isotopes and trace elements in Jack Hills zircons (e.g., Mojzsis et al., 2001; Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2011) indicate two distinctive protolith sources: Group I and Group II (Bell and Harrison, 2013).
View in article
Oxygen isotope ratios and REE patterns in some of these zircons have been investigated previously and shown to lack secondary alteration (Table S-2, S-3, and S-4, Fig. S-7 and S-8; Bell et al., 2011, 2014, 2016; Bell and Harrison 2013).
View in article
In our study, U-Pb ages in all Cl-rich zircons are concordant within ±10 % (Bell and Harrison 2013) indicating little Pb loss or U gain after 3.8 Ga.
View in article
Bell and Harrison (2013) classified 3.9–3.8 Ga Jack Hills zircon grains into two categories.
View in article
A possible mechanism to interpret the low Ti contents and other characteristics of Group II zircons is metamorphic transgressive recrystallisation of originally igneous zircons (Bell and Harrison, 2013).
View in article
Bell, E.A., Harrison, T.M., McCulloch, M.T., Young, E.D. (2011) Early Archean crustal evolution of the Jack Hills Zircon source terrane inferred from Lu–Hf, 207Pb/206Pb, and δ18O systematics of Jack Hills zircons. Geochimica et Cosmochimica Acta 75, 4816-4829.
 Show in context
Show in context Given that >3.8 Ga rocks are exceedingly rare and no rock older than 4.03 Ga is known (cf. O’Neil et al., 2008), constraining conditions on early Earth has largely relied on the only materials identified from the Hadean eon: ~4.4 to 3.8 Ga detrital zircons (Mojzsis et al., 2001; Peck et al., 2001; Harrison et al., 2008; Holden et al., 2009; Bell et al., 2011).
View in article
Previous investigations of oxygen isotopes and trace elements in Jack Hills zircons (e.g., Mojzsis et al., 2001; Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2011) indicate two distinctive protolith sources: Group I and Group II (Bell and Harrison, 2013).
View in article
Oxygen isotope ratios and REE patterns in some of these zircons have been investigated previously and shown to lack secondary alteration (Table S-2, S-3, and S-4, Fig. S-7 and S-8; Bell et al., 2011, 2014, 2016; Bell and Harrison 2013).
View in article
Bell, E.A., Harrison, T.M., Kohl, I.E., Young, E.D. (2014) Eoarchean crustal evolution of the Jack Hills zircon source and loss of Hadean crust. Geochimica et Cosmochimica Acta 146, 27-42.
 Show in context
Show in context Oxygen isotope ratios and REE patterns in some of these zircons have been investigated previously and shown to lack secondary alteration (Table S-2, S-3, and S-4, Fig. S-7 and S-8; Bell et al., 2011, 2014, 2016; Bell and Harrison 2013).
View in article
Bell, E.A., Boehnke, P., Harrison, T.M. (2016) Recovering the primary geochemistry of Jack Hills zircons through quantitative estimates of chemical alteration. Geochimica et Cosmochimica Acta 191, 187-202.
 Show in context
Show in contextOxygen isotope ratios and REE patterns in some of these zircons have been investigated previously and shown to lack secondary alteration (Table S-2, S-3, and S-4, Fig. S-7 and S-8; Bell et al., 2011, 2014, 2016; Bell and Harrison 2013).
View in article
Given that mobile elements can be substituted into the zircon lattice during secondary alteration (e.g., Bell et al., 2016), care must be taken to ascertain that Cl and F concentrations reported for Jack Hills zircons do not represent post-crystallisation hydrothermal alteration.
View in article
In addition, two indices, including (Sm/La)N (Hoskin, 2005) and the LREE-I [= (Dy/Nd) + (Dy/Sm); Bell et al., 2016], can test for possible alteration effects leading to crystalline degeneration.
View in article
The high values of both indices for all grains (except one Archean zircon) are indicative of crystalline zircon, including the Cl-rich grains (see Bell et al., 2016) (Fig. 2).
View in article
The distribution of oxygen isotopes in our samples (δ18Oave= 5.87 ± 0.17; Fig. S-7) is consistent with the prevailing populations for their corresponding time periods (Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2016) and no obvious correlation between δ18O and Cl concentration is observed.
View in article
Bell, E.A., Boehnke, P., Harrison, T.M., Wielicki, M.M. (2018) Mineral inclusion assemblage and detrital zircon provenance. Chemical Geology 477, 151-160.
 Show in context
Show in context Given the possible felsic parent sources of Jack Hills zircon (Bell et al., 2018), zircons from igneous (I-type) and sedimentary (S-type) granitoids from the Phanerozoic Lachlan Fold Belt (LFB), Australia, and Mesozoic batholiths of southern California, USA, were also investigated as analogues for establishing geologic provenance.
View in article
Bernini, D., Audétat, A., Dolejš, D., Keppler. H. (2013) Zircon solubility in aqueous fluids at high temperatures and pressures. Geochimica et Cosmochimica Acta 119, 178-187.
 Show in context
Show in context Zircon recrystallisation can occur in the contact or absence of fluids, although the elevated Cl concentrations and Cl/F ratios in Group II Jack Hills zircons relative to magmatic zircons strongly suggest the involvement of Cl-rich fluids at ca. 3.9 Ga, which would additionally enhance zircon recrystallisation given high solubility of ZrO2 in saline fluids relative to pure H2O (Bernini et al., 2013).
View in article
Bodnar, R.J. (2003) Introduction to aqueous fluid systems. In: Samson, I., Anderson, A., Marshall, D. (Eds.) Fluid Inclusions: Analysis and Interpretation. Mineralogical Association of Canada Short Course Series Volume 32, 81-99.
 Show in context
Show in contextThis is similar to Cl concentrations in some brine hydrothermal fluids in the present day (e.g., Stefánsson and Barnes, 2016), though significantly lower than that in modern deep crustal fluids (e.g., Bodnar, 2003).
View in article
Burgess, R., Layzelle, E., Turner, G., Harris, J.W. (2002) Constraints on the age and halogen composition of mantle fluids in Siberian coated diamonds. Earth and Planetary Science Letters 197, 193-203.
 Show in context
Show in contextGiven the large proportion of heavy halogens in the crustal reservoirs (80–90 % of BSE; Burgess et al., 2002), notwithstanding contributions from a late veneer (ca. 1 % Earth mass) or complete mantle degassing (maximum estimated extraction efficiency is ~50 %; Allègre et al., 1996), additional halogens transported from deep Earth would still be required, likely via early fluids.
View in article
Cavosie, A.J., Valley, J.W., Wilde, S.A. (2005) Magmatic δ18O in 4400–3900 Ma detrital zircons: A record of the alteration and recycling of crust in the Early Archean. Earth and Planetary Science Letters 235, 663-681.
 Show in context
Show in contextPrevious investigations of oxygen isotopes and trace elements in Jack Hills zircons (e.g., Mojzsis et al., 2001; Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2011) indicate two distinctive protolith sources: Group I and Group II (Bell and Harrison, 2013).
View in article
The distribution of oxygen isotopes in our samples (δ18Oave= 5.87 ± 0.17; Fig. S-7) is consistent with the prevailing populations for their corresponding time periods (Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2016) and no obvious correlation between δ18O and Cl concentration is observed.
View in article
Clay, P.L., Burgess, R., Busemann, H., Ruzié-Hamilton, L., Joachim, B., Day, J.M.D., Ballentine, C.J. (2017) Halogens in chondritic meteorites and terrestrial accretion. Nature 551, 614-618.
 Show in context
Show in contextHowever, these putative depletions were rendered moot by a recent analysis of the abundances of Cl, Br, and I in meteorites, which indicate much lower average abundances in primitive chondrites (Clay et al., 2017) than previously thought.
View in article
Harrison, T.M., Schmitt, A.K., McCulloch, M.T., Lovera, O.M. (2008) Early (≥ 4.5 Ga) formation of terrestrial crust: Lu–Hf, δ18O, and Ti thermometry results for Hadean zircons. Earth and Planetary Science Letters 268, 476-486.
 Show in context
Show in contextGiven that >3.8 Ga rocks are exceedingly rare and no rock older than 4.03 Ga is known (cf. O’Neil et al., 2008), constraining conditions on early Earth has largely relied on the only materials identified from the Hadean eon: ~4.4 to 3.8 Ga detrital zircons (Mojzsis et al., 2001; Peck et al., 2001; Harrison et al., 2008; Holden et al., 2009; Bell et al., 2011).
View in article
The Ti-in-zircon crystallisation temperatures (Txlln) of each Jack Hills zircon are obtained using a protocol developed in previous studies (Watson and Harrison, 2005; Harrison et al., 2008).
View in article
Harrison, T.M., Bell, E.A., Boehnke, P. (2017) Hadean zircon petrochronology. Reviews in Mineralogy and Geochemistry 83, 329-363.
 Show in context
Show in contextOxygen isotopes, rare earth element (REE) patterns, crystallisation temperatures, and inclusions in detrital zircons from the Jack Hills, Western Australia, are suggestive of the presence of recycled crustal material that had interacted with liquid water at low temperature (e.g., Watson and Harrison, 2005; Trail et al., 2011; Bell and Harrison 2013; Harrison et al., 2017) and show how experimental calibrations can permit zircon to act as an environmental monitor of lithosphere and surface processes.
View in article
Holden, P., Lanc, P., Ireland, T.R., Harrison, T.M., Foster, J.J. Bruce, Z. (2009) Mass-spectrometric mining of Hadean zircons by automated SHRIMP multi-collector and single-collector U/Pb zircon age dating: the first 100,000 grains. International Journal of Mass Spectrometry 286, 53-63.
 Show in context
Show in context Given that >3.8 Ga rocks are exceedingly rare and no rock older than 4.03 Ga is known (cf. O’Neil et al., 2008), constraining conditions on early Earth has largely relied on the only materials identified from the Hadean eon: ~4.4 to 3.8 Ga detrital zircons (Mojzsis et al., 2001; Peck et al., 2001; Harrison et al., 2008; Holden et al., 2009; Bell et al., 2011).
View in article
Hoskin P.W.O. (2005) Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia. Geochimica et Cosmochimica Acta 69, 637–648.
 Show in context
Show in context In addition, two indices, including (Sm/La)N (Hoskin, 2005) and the LREE-I [= (Dy/Nd) + (Dy/Sm); Bell et al., 2016], can test for possible alteration effects leading to crystalline degeneration.
View in article
Hoskin, P.W.O., Black, L.P. (2000) Metamorphic zircon formation by solid-state recrystallization of protolith igneous zircon. Journal of Metamorphic Geology 18, 423-439.
 Show in context
Show in contextThe Group II characteristics imply recrystallisation during thermal events (Hoskin and Black, 2000).
View in article
The concordant U-Pb ages in Group II zircons and their igneous protolith similar to Group I suggest that they formed by transgressive recrystallisation, in which recrystallisation occurs across the entire zircon under high temperatures (Hoskin and Black, 2000).
View in article
High temperature transgressive recrystallisation results in flushing the more incompatible trace elements (e.g., Ce) from the mineral, an increase in compatible elements (e.g., Hf, U), and homogeneous dark textures (Hoskin and Black, 2000).
View in article
Such conditions can be achieved by the presence of hydrothermal fluids which can quickly elevate and maintain local temperatures for significant durations (Hoskin and Black, 2000).
View in article
Lodders, K. (2003) Solar system abundances and condensation temperatures of the elements. The Astrophysical Journal 591, 1220-1247.
 Show in context
Show in context Studies of Bulk Silicate Earth (BSE) halogen abundances suggest the anomalous depletions observed in volatile halogens (Cl, Br, and I) relates to their volatility during condensation from the solar nebula (Lodders, 2003; Sharp and Draper, 2013).
View in article
Manning, C.E., Mojzsis, S.J., Harrison, T.M. (2006) Geology, age and origin of supracrustal rocks at Akilia, West Greenland. American Journal of Science 306, 303-366.
 Show in context
Show in context On the other hand, the lack of a known sedimentary record older than 3.83 Ga (Manning et al., 2006) limits our understanding of the volatile evolution on earliest Earth.
View in article
Mojzsis, S.J., Harrison, T.M., Pidgeon, R.T. (2001) Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4,300 Myr ago. Nature 409, 178-181.
 Show in context
Show in contextPrevious investigations of oxygen isotopes and trace elements in Jack Hills zircons (e.g., Mojzsis et al., 2001; Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2011) indicate two distinctive protolith sources: Group I and Group II (Bell and Harrison, 2013).
View in article
Given that >3.8 Ga rocks are exceedingly rare and no rock older than 4.03 Ga is known (cf. O’Neil et al., 2008), constraining conditions on early Earth has largely relied on the only materials identified from the Hadean eon: ~4.4 to 3.8 Ga detrital zircons (Mojzsis et al., 2001; Peck et al., 2001; Harrison et al., 2008; Holden et al., 2009; Bell et al., 2011).
View in article
O'Neil, J., Carlson, R.W., Francis, D., Stevenson, R.K. (2008) Neodymium-142 evidence for Hadean mafic crust. Science 321, 1828-1831.
 Show in context
Show in contextGiven that >3.8 Ga rocks are exceedingly rare and no rock older than 4.03 Ga is known (cf. O’Neil et al., 2008), constraining conditions on early Earth has largely relied on the only materials identified from the Hadean eon: ~4.4 to 3.8 Ga detrital zircons (Mojzsis et al., 2001; Peck et al., 2001; Harrison et al., 2008; Holden et al., 2009; Bell et al., 2011).
View in article
Peck, W.H., Valley, J.W., Wilde, S.A., Graham, C.M. (2001) Oxygen isotope ratios and rare earth elements in 3.3 to 4.4 Ga zircons: Ion microprobe evidence for high δ18O continental crust and oceans in the Early Archean. Geochimica et Cosmochimica Acta 65, 4215-4229.
 Show in context
Show in contextGiven that >3.8 Ga rocks are exceedingly rare and no rock older than 4.03 Ga is known (cf. O’Neil et al., 2008), constraining conditions on early Earth has largely relied on the only materials identified from the Hadean eon: ~4.4 to 3.8 Ga detrital zircons (Mojzsis et al., 2001; Peck et al., 2001; Harrison et al., 2008; Holden et al., 2009; Bell et al., 2011).
View in article
Sharp, Z.D., Draper, D.S. (2013) The chlorine abundance of Earth: implications for a habitable planet. Earth and Planetary Science Letters 369, 71-77.
 Show in context
Show in contextStudies of Bulk Silicate Earth (BSE) halogen abundances suggest the anomalous depletions observed in volatile halogens (Cl, Br, and I) relates to their volatility during condensation from the solar nebula (Lodders, 2003; Sharp and Draper, 2013).
View in article
Stefánsson, A., Barnes, J.D. (2016) Chlorine isotope geochemistry of Icelandic thermal fluids: Implications for geothermal system behavior at divergent plate boundaries. Earth and Planetary Science Letters 449, 69-78.
 Show in context
Show in contextThis is similar to Cl concentrations in some brine hydrothermal fluids in the present day (e.g., Stefánsson and Barnes, 2016), though significantly lower than that in modern deep crustal fluids (e.g., Bodnar, 2003).
View in article
Takehara, M., Horie, K., Hokada, T., Kiyokawa, S. (2018) New insight into disturbance of U-Pb and trace-element systems in hydrothermally altered zircon via SHRIMP analyses of zircon from the Duluth Gabbro. Chemical Geology 484, 168-178.
 Show in context
Show in context Young zircons (1.1–0.1 Ga) display uniform F concentrations (Fig. 1) within uncertainty (1.80 ± 0.11 μg/g), except AS3 zircon (Duluth Complex, Minnesota), which we attribute to enrichment during hydrothermal alteration (Takehara et al., 2018).
View in article
Trail, D., Mojzsis, S.J., Harrison, T.M., Schmitt, A.K., Watson, E.B., Young, E.D. (2007) Constraints on Hadean zircon protoliths from oxygen isotopes, Ti‐thermometry, and rare earth elements. Geochemistry, Geophysics, Geosystems 8, 1-22.
 Show in context
Show in context Previous investigations of oxygen isotopes and trace elements in Jack Hills zircons (e.g., Mojzsis et al., 2001; Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2011) indicate two distinctive protolith sources: Group I and Group II (Bell and Harrison, 2013).
View in article
The distribution of oxygen isotopes in our samples (δ18Oave= 5.87 ± 0.17; Fig. S-7) is consistent with the prevailing populations for their corresponding time periods (Cavosie et al., 2005; Trail et al., 2007; Bell et al., 2016) and no obvious correlation between δ18O and Cl concentration is observed.
View in article
Trail, D., Watson, E.B., Tailby, N.D. (2011) The oxidation state of Hadean magmas and implications for early Earth's atmosphere. Nature 480, 79-82.
 Show in context
Show in contextOxygen isotopes, rare earth element (REE) patterns, crystallisation temperatures, and inclusions in detrital zircons from the Jack Hills, Western Australia, are suggestive of the presence of recycled crustal material that had interacted with liquid water at low temperature (e.g., Watson and Harrison, 2005; Trail et al., 2011; Bell and Harrison 2013; Harrison et al., 2017) and show how experimental calibrations can permit zircon to act as an environmental monitor of lithosphere and surface processes.
View in article
Trail. D., Cherniak, D.J., Watson, E.B., Harrison, T.M., Weiss, B.P., Szumila, I. (2016) Li zoning in zircon as a potential geospeedometer and peak temperature indicator. Contributions to Mineralogy and Petrology 171, 25-40.
 Show in context
Show in context Detection limits ([F] = 0.78 ± 0.07 μg/g and [Cl] = 0.12 ± 0.02 μg/g with 2 se.; Fig. S-4) were determined by analysing synthetic zircons grown in halogen-free conditions by the 1 atm flux method (e.g., Trail et al., 2016).
View in article
Watson, E.B., Harrison, T.M. (2005) Zircon thermometer reveals minimum melting conditions on earliest Earth. Science 308, 841-844.
 Show in context
Show in contextOxygen isotopes, rare earth element (REE) patterns, crystallisation temperatures, and inclusions in detrital zircons from the Jack Hills, Western Australia, are suggestive of the presence of recycled crustal material that had interacted with liquid water at low temperature (e.g., Watson and Harrison, 2005; Trail et al., 2011; Bell and Harrison 2013; Harrison et al., 2017) and show how experimental calibrations can permit zircon to act as an environmental monitor of lithosphere and surface processes.
View in article
The Ti-in-zircon crystallisation temperatures (Txlln) of each Jack Hills zircon are obtained using a protocol developed in previous studies (Watson and Harrison, 2005; Harrison et al., 2008).
View in article
In contrast, the Txlln among all other Jack Hills zircons with low Cl/F ratios overall cluster about an identical average of 680 °C, indistinguishable from the Hadean distribution (Watson and Harrison, 2005).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Sample Description
- Analytical Method
- REE Pattern in Jack Hills Zircons
- Oxygen Isotope Compositions
- Figures S-1 to S-8
- Tables S-1 to S-4
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
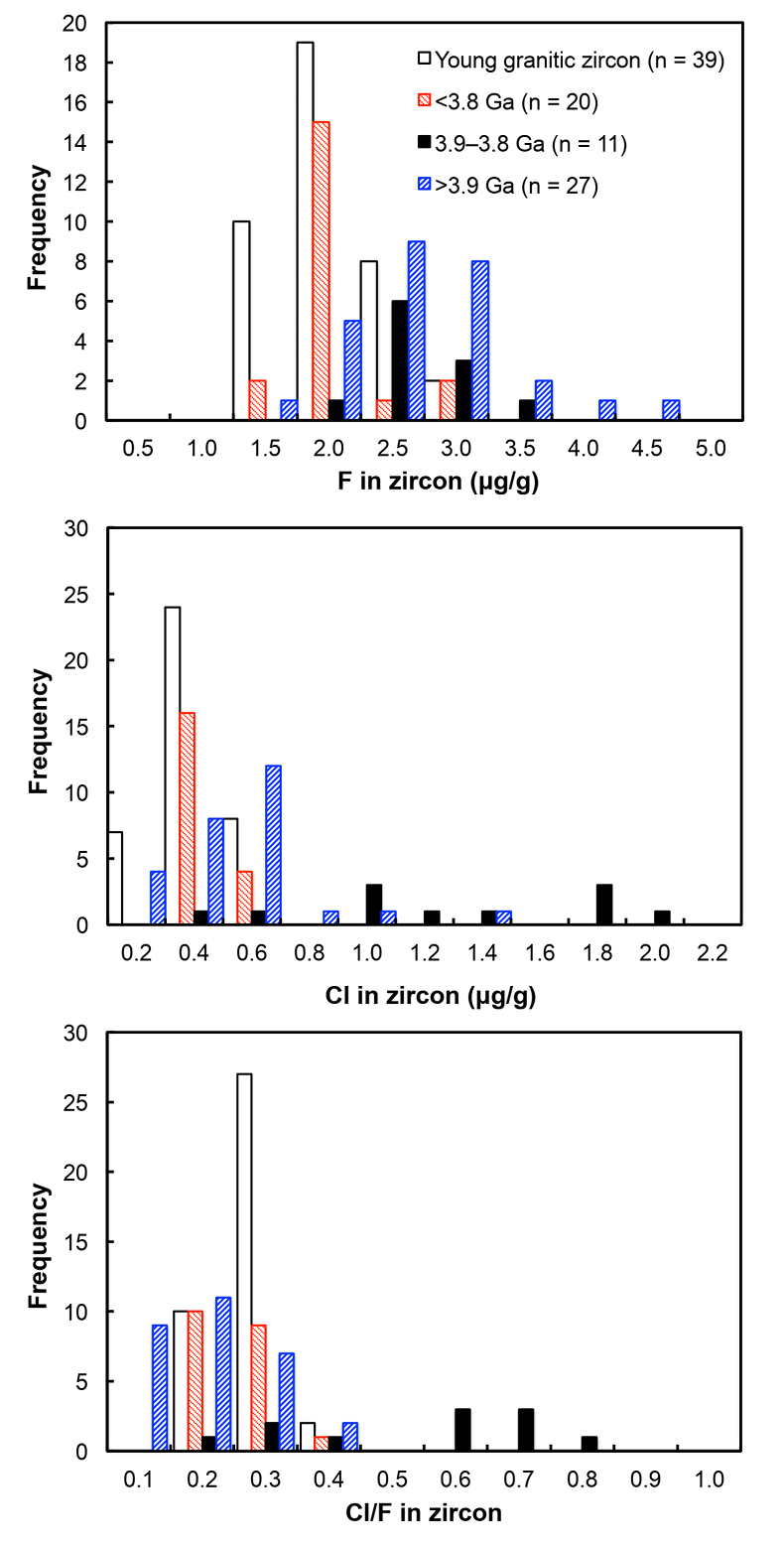
Figure 1 Fluorine concentrations in Jack Hills zircons ([F]average = 2.28 ± 0.19 μg/g) are generally indistinguishable from those in young granitic zircons ([F]average = 1.80 ± 0.11 μg/g). Elevated Cl concentrations observed in eight 3.9–3.8 Ga zircons (black columns) yield distinctly high Cl/F ratios (Cl/F > 0.3) when compared with other Jack Hills zircons.
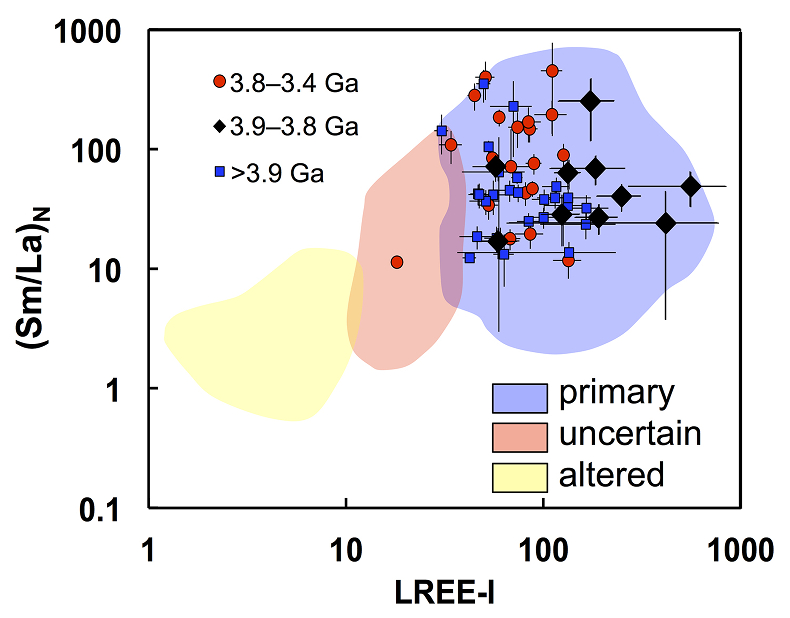
Figure 2 High values of (Sm/La)N and LREE-I [(Dy/Nd) + (Dy/Sm)] in Jack Hills sample grains imply that most of samples, especially Cl-rich zircons, are primary. The elevated Cl concentrations in zircons therefore derived from primary sources.
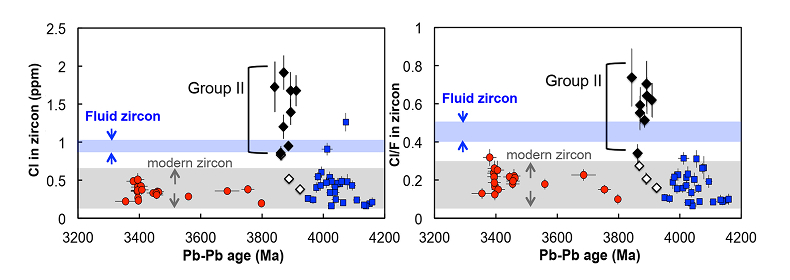
Figure 3 (a) Chlorine distribution and (b) Cl/F fractionation in Jack Hills zircons are mainly identical to the ranges determined from modern magmatic zircons in this study (red circles: <3.8 Ga zircons; white diamonds: 3.9–3.8 Ga Group I zircons; blue rectangles: >3.9 Ga zircons; grey bar: modern zircon ranges) except Group II zircons crystallised at 3.9–3.8 Ga (black diamonds). A synthetic zircon grown in an aqueous-rich fluid exhibits Cl content and Cl/F ratios (blue bars) that fall within the range of Group II.
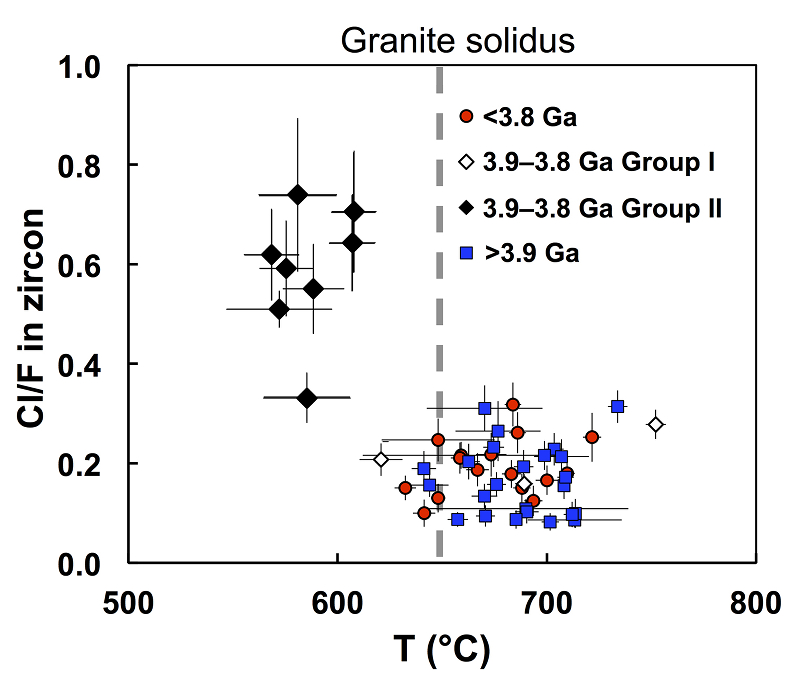
Figure 4 Chlorine-rich Group II zircons are characterised by Ti-in-zircon temperatures below granite solidus. The clearly distinguished cluster of Group II from other magmatic zircons suggests the mechanism of Group II as solid state recrystallisation involving Cl-bearing fluids.






