Redox state of the convective mantle from CO2-trace element systematics of oceanic basalts
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:5,995Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Frost, D.J., McCammon, C.A. (2008) The Redox State of Earth’s Mantle. Annual Review of Earth and Planetary Sciences 36, 389–420.
). On the other hand, the depth-fO2 profile of the convecting mantle remains poorly known. We compare the CO2-Ba and CO2-Nb systematics of natural oceanic basalts to the CO2-trace element concentrations that can be generated via contributions from depleted peridotite partial melts and graphite-saturated partial melts of subducted lithologies. Results suggest that to produce the CO2 enrichments relative to the depleted end member observed in natural oceanic basalts, subducted lithologies cannot be graphite-saturated at the onset of melting or must undergo oxidative transformation below the respective volatile-free solidi. Therefore, the oxygen fugacity profile of the continental lithospheric mantle may not be applicable to the deep convecting upper mantle, with the convecting upper mantle to at least 150 km depth being more oxidised than the carbonate vs. graphite/diamond buffer.Figures and Tables
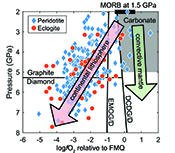
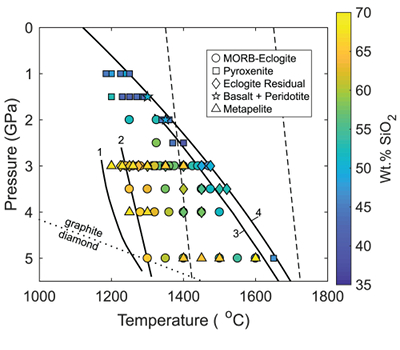 Figure 1 Pressure-temperature conditions for generation of partial melts from crustal/mafic lithologies in the mantle with colour map for wt. % SiO2 in the melt. Also plotted for reference are mantle adiabats for mantle potential temperatures of 1350 and 1650 °C (dashed lines), solidi of various volatile-free lithologies (1-metapelite, 2-MORB-eclogite, 3-silica-deficient garnet pyroxenite, 4-peridotite, references given in footnote of Table S-3 – the table of compiled experiments), and graphite-diamond transition (dotted line). | 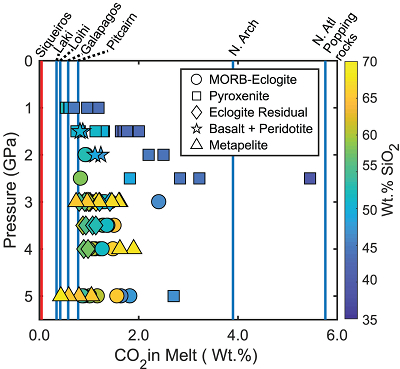 Figure 2 CO2 concentrations calculated at the CCO buffer for each experiment in Figure 1. Vertical blue lines are highest CO2 concentrations measured in natural oceanic basalts from different locations (Table S-2) that are thought to receive contributions from subducted lithologies. Red band is for CO2 contents expected in depleted peridotite-derived partial melts based on undegassed melt inclusions from Siqueiros (Saal et al., 2002) and calculations using an experimental bulk partition coefficient for CO2 and source CO2 estimate of ~75 ppm at F = 10 % (Rosenthal et al., 2015). | 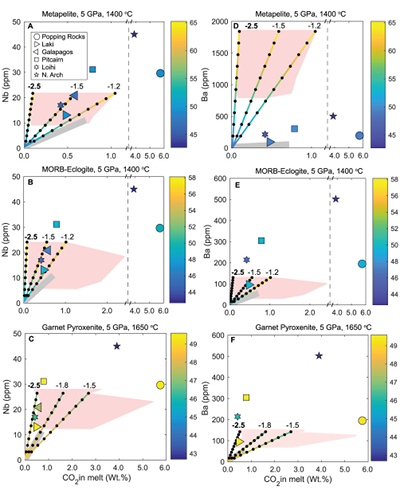 Figure 3 CO2-Nb and CO2-Ba mixing lines between depleted peridotite melts and graphite-saturated melts of subducted lithologies. Mixing lines are calculated at different logfO2s relative to FMQ up to the CCO buffer as denoted by numbers above mixing line (the average fO2 of eclogites based on CLM xenoliths at this pressure is marked with bold text). Each marker along a mixing line represents 10 wt. % incremental contribution from subducted lithology partial melt. Data points are the CO2-Nb/CO2-Ba concentrations recorded in the least degassed natural basalts at each location. Data point and mixings lines coloured for melt SiO2 (wt. %) contents. Pink shaded region shows CO2-Nb-Ba contents at graphite-saturation from CCO to FMQ-2.5 for all relevant experimental partial melts for a particular lithology from Figure 1. Gray band shows CO2-Nb-Ba calculations for a depleted peridotite from 1-10 % melting degree (see also Fig. S-1). Data for generation of Figure 3 given in Supplementary Information. | 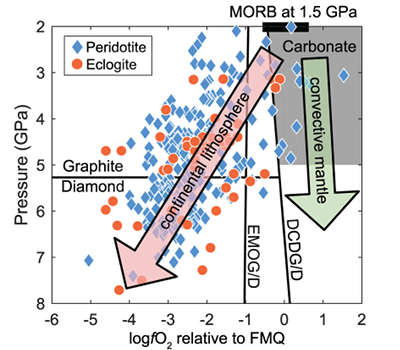 Figure 4 Pressure vs. fO2 computed for continental lithospheric mantle xenoliths versus those that have been estimated here for oceanic mantle sources (gray region). DCDG/D is the graphite/diamond transition in eclogite/pyroxenite (Luth, 1993). Graphite/diamond transition in peridotite is shown for reference (EMOG/D). References for xenolith data are in Table S-4. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
The oxidation state of the mantle plays a critical role in dictating the geochemical and geodynamic evolution of Earth and its atmosphere (e.g., Frost and McCammon, 2008
Frost, D.J., McCammon, C.A. (2008) The Redox State of Earth’s Mantle. Annual Review of Earth and Planetary Sciences 36, 389–420.
). Current understandings of mantle redox are largely based on measurements of Fe3+ and Fe2+ in mantle-derived basalts, which suggest that the oxygen fugacity of Earth’s shallow mantle to about 1.5 GPa is close to the FMQ buffer (e.g., Cottrell and Kelley, 2011Cottrell, E., Kelley, K.A. (2011) The oxidation state of Fe in MORB glasses and the oxygen fugacity of the upper mantle. Earth and Planetary Science Letters 305, 270–282.
; Hartley et al., 2017Hartley, M.E., Shorttle, O., Maclennan, J., Moussallam, Y., Edmonds, M. (2017) Olivine-hosted melt inclusions as an archive of redox heterogeneity in magmatic systems. Earth and Planetary Science Letters 479, 192–205.
). The fO2 of the deeper portions of Earth’s mantle is chiefly known from mantle xenoliths recovered from continental lithospheric mantle (CLM), which suggest fO2 decreases with increasing depth (e.g., Woodland and Koch, 2003Woodland, A.B., Koch, M. (2003) Variation in oxygen fugacity with depth in the upper mantle beneath the Kaapvaal craton, Southern Africa. Earth and Planetary Science Letters 214, 295–310.
). This trend has been attributed to the increasing stability of the Fe3+-bearing skiagite component of garnet with increasing depth (Gudmundsson and Wood, 1995Gudmundsson, G., Wood, B.J. (1995) Experimental tests of garnet peridotite oxygen barometry. Contributions to Mineralogy and Petrology 119, 56–67.
). The fO2 decrease with increasing depth observed in CLM has been proposed to apply to the convecting mantle (e.g., Frost and McCammon, 2008Frost, D.J., McCammon, C.A. (2008) The Redox State of Earth’s Mantle. Annual Review of Earth and Planetary Sciences 36, 389–420.
). However, several observations offer up the possibility that the fO2 trend estimated for CLM may not be applicable to the convecting mantle. (1) Kimberlites (oxidised, CO2-rich magmas) are generated deep in the mantle, then migrate through overlying cratons, often picking up diamonds, a reduced form of carbon, implying there are regions in the mantle deeper than CLM depths where diamond resides that are more oxidised (Yaxley et al., 2017Yaxley, G.M., Berry, A.J., Rosenthal, A., Woodland, A.B., Paterson, D. (2017) Redox preconditioning deep cratonic lithosphere for kimberlite genesis - Evidence from the central Slave Craton. Scientific Reports 7, doi: 10.1038/s41598-017-00049-3.
; Dasgupta, 2018Dasgupta, R. (2018) Volatile-bearing partial melts beneath oceans and continents–Where, how much, and of what compositions? American Journal of Science 318, 141–165.
). (2) Carbonate inclusions have been found in diamonds from depths in the mantle that should be in the graphite/diamond stability field if the CLM fO2 profile is applied to the convecting upper mantle (Brenker et al., 2007Brenker, F.E., Vollmer, C., Vincze, L., Vekemans, B., Szymanski, A., Janssens, K., Szaloki, I., Nasdala, L., Joswig, W., Kaminsky, F. (2007) Carbonates from the lower part of transition zone or even the lower mantle. Earth and Planetary Science Letters 260, 1–9.
). (3) Kiseeva et al. (2018)Kiseeva, E.S., Vasiukov, D.M., Wood, B.J., McCammon, C., Stachel, T., Bykov, M., Bykova, E., Chumakov, A., Cerantola, V., Harris, J.W., Dubrovinsky, L. (2018) Oxidized iron in garnets from the mantle transition zone. Nature Geoscience 11, 144–147.
argue that fO2 measurements of garnet inclusions in diamonds from the mantle transition zone become more oxidised with depth, and are more oxidised than predicted by the Fe-controlled fO2 profile inferred from CLMs. (4) Komatiites, which are generated deeper in the mantle than basalts have similar redox states to MORB (Gaillard et al., 2015Gaillard, F., Scaillet, B., Pichavant, M., Iacono-Marziano, G. (2015) The redox geodynamics linking basalts and their mantle sources through space and time. Chemical Geology 418, 217–233.
). While these natural observations may be due to special circumstances, they raise the possibility that the CLM fO2 profile may not be applicable to the convecting mantle.top
CO2 Enrichment of Oceanic Basalt – Graphite/Diamond or Carbonate-present Melting?
Many oceanic basalts are argued to contain partial melt contributions of subducted lithologies such as sediments, MORB-eclogite, and pyroxenite (Hofmann and White, 1982
Hofmann, A.W., White, W.M. (1982) Mantle plumes from ancient oceanic crust. Earth and Planetary Science Letters 57, 421–436.
), which begin to melt deeper than peridotite due to their lower solidi (e.g., Spandler et al., 2008Spandler, C., Yaxley, G., Green, D.H., Rosenthal, A. (2008) Phase Relations and Melting of Anhydrous K-bearing Eclogite from 1200 to 1600 C and 3 to 5 GPa. Journal of Petrology 49, 771–795.
). If there are redox sensitive geochemical proxies that track deep melting processes, they may provide constraints on the redox state of deeper portions of the convecting upper mantle, where the recycled lithologies undergo melting. Many of these basalts have elevated CO2 concentrations relative to normal mid-ocean ridge basalts (Aubaud et al., 2006Aubaud, C., Pineau, F., Hékinian, R., Javoy, M. (2006) Carbon and hydrogen isotope constraints on degassing of CO2 and H2O in submarine lavas from the Pitcairn hotspot (South Pacific). Geophysical Research Letters 33, L02308.
), which may be due to a CO2 contribution from subducted lithologies, which have higher CO2 concentrations relative to primitive mantle (Anderson and Poland, 2017Anderson, K.R., Poland, M.P. (2017) Abundant carbon in the mantle beneath Hawai‘i. Nature Geoscience 10, 704–708.
; Hauri et al., 2017Hauri, E.H., Maclennan, J., McKenzie, D., Gronvold, K., Oskarsson, N., Shimizu, N. (2017) CO2 content beneath northern Iceland and the variability of mantle carbon. Geology 46, 55–58.
). At sub-solidus conditions, the stable form of carbon depends on fO2; at low fO2 C exists as graphite/diamond, at high fO2 C exists as carbonate. Graphite-bearing lithologies melt at the lithology’s nominally volatile-free solidus and generate a silicate melt with fO2-dependent CO2 concentrations (e.g., Eguchi and Dasgupta, 2017Eguchi, J., Dasgupta, R. (2017) CO2 content of andesitic melts at graphite-saturated upper mantle conditions with implications for redox state of oceanic basalt source regions and remobilization of reduced carbon from subducted eclogite. Contributions to Mineralogy and Petrology 172, 12.
, 2018Eguchi, J., Dasgupta, R. (2018) A CO2 solubility model for silicate melts from fluid saturation to graphite or diamond saturation. Chemical Geology 487, 23–38.
). In addition, the presence of graphite during partial melting has negligible effect on the composition of generated melts due to the refractory nature of graphite. On the other hand, carbonate-bearing lithologies melt at the lithology’s carbonated solidus and generate a carbonated melt with up to ~45 wt. % CO2 (e.g., Dasgupta and Hirschmann, 2006Dasgupta, R., Hirschmann, M.M. (2006) Melting in the Earth’s deep upper mantle caused by carbon dioxide. Nature 440, 659–662.
). Therefore, oceanic basalts receiving contributions from crustal/mafic lithologies will have different CO2 concentrations depending on whether partial melting of these easily fusible lithologies occurred under graphite/diamond-saturated versus carbonated conditions.In the following, we test whether CO2 and other trace element enrichments observed in natural basalts can be produced by graphite-saturated melting of recycled lithologies. If CO2 concentrations of natural samples are too high to be explained by recycled lithology contributions via graphite-saturated melting, it would suggest that the fO2 of the subducted lithologies at or below their nominally volatile-free solidi must be high enough for carbon to exist as carbonate.
top
CO2-Nb and CO2-Ba Systematics of Oceanic Basalts
Here we use CO2-Nb and CO2-Ba systematics of oceanic basalts argued to be receiving contributions from recycled lithologies (see Table S-2) to investigate the redox state of the deeper portions of the convective upper mantle. We use a new, compositionally dependent CO2 solubility model for silicate melts (Eguchi and Dasgupta, 2018
Eguchi, J., Dasgupta, R. (2018) A CO2 solubility model for silicate melts from fluid saturation to graphite or diamond saturation. Chemical Geology 487, 23–38.
) applicable to graphite/diamond-saturated conditions to investigate a wide range of lithologies. We compiled partial melting experiments conducted on lithologies which may contribute to the generation of oceanic basalts. We only consider partial melting studies conducted at pressure-temperature conditions below the peridotite solidus to investigate processes which occur deeper than the onset of peridotite partial melting, as previous studies have already demonstrated that at shallow depths where major peridotite melting occurs, fO2 is around the FMQ buffer (e.g., Cottrell and Kelley, 2011Cottrell, E., Kelley, K.A. (2011) The oxidation state of Fe in MORB glasses and the oxygen fugacity of the upper mantle. Earth and Planetary Science Letters 305, 270–282.
). Figure 1 shows compiled experiments in P-T space with colours corresponding to wt. % SiO2 in the melt, which is a major controller of CO2 solubility, with more silica-depleted melts dissolving more CO2.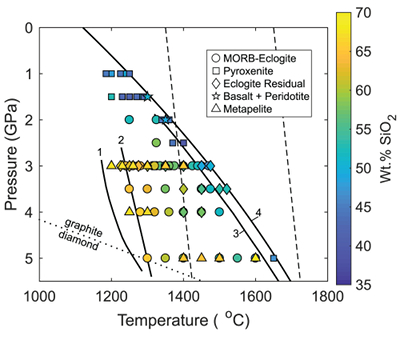
Figure 1 Pressure-temperature conditions for generation of partial melts from crustal/mafic lithologies in the mantle with colour map for wt. % SiO2 in the melt. Also plotted for reference are mantle adiabats for mantle potential temperatures of 1350 and 1650 °C (dashed lines), solidi of various volatile-free lithologies (1-metapelite, 2-MORB-eclogite, 3-silica-deficient garnet pyroxenite, 4-peridotite, references given in footnote of Table S-3 – the table of compiled experiments), and graphite-diamond transition (dotted line).
In Figure 2 we show CO2 concentrations calculated for experimental partial melts at the CCO buffer (maximum amount of CO2 dissolved with graphite present in the source) compared to the highest CO2 concentrations measured in enriched oceanic basalts from several locations. CO2 concentrations from basalts at Siqueiros are measured in undegassed melt inclusions and may be widely representative of CO2 concentrations for basalts from partial melts of normal primitive mantle (Saal et al., 2002
Saal, A.E., Hauri, E.H., Langmuir, C.H., Perfit, M.R. (2002) Vapour undersaturation in primitive mid-ocean-ridge basalt and the volatile content of Earth’s upper mantle. Nature 419, 451–455.
). Therefore, if the elevated CO2 levels observed in enriched, natural oceanic basalts are due to contributions from subducted lithologies at graphite-saturation, then the CO2 concentrations of the natural oceanic basalts must lie between CO2 concentrations of Siqueiros and those calculated for the graphite-saturated experimental partial melts of subducted lithologies. Figure 2 shows that it is unlikely that a graphite/diamond-saturated melt derived from any recycled lithology can dissolve enough CO2 to generate the high CO2 concentrations observed at the North Arch of Hawaii and popping rocks from the North Atlantic (Dixon et al., 1997Dixon, J.E., Clague, D.A., Wallace, P., Poreda, R. (1997) Volatiles in Alkalic Basalts from the North Arch Volcanic Field, Hawaii: Extensive Degassing of Deep Submarine-erupted Alkalic Series Lavas. Journal of Petrology 38, 911–939.
; Cartigny et al., 2008Cartigny, P., Pineau, F., Aubaud, C., Javoy, M. (2008) Towards a consistent mantle carbon flux estimate: Insights from volatile systematics (H2O/Ce, δD, CO2/Nb) in the North Atlantic mantle (14° N and 34° N). Earth and Planetary Science Letters 265, 672–685.
). For locations with lower CO2 concentrations in basalts, such as those from Laki and Loihi, even many of the silica-rich melts may dissolve sufficient CO2 under graphite/diamond-saturated conditions. The question, however, is whether realistic proportions of subducted lithology-derived melt can yield the CO2-trace element systematics of these basalts.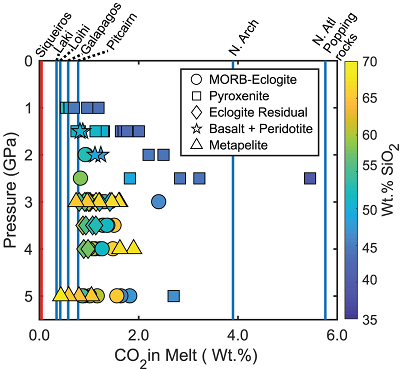
Figure 2 CO2 concentrations calculated at the CCO buffer for each experiment in Figure 1. Vertical blue lines are highest CO2 concentrations measured in natural oceanic basalts from different locations (Table S-2) that are thought to receive contributions from subducted lithologies. Red band is for CO2 contents expected in depleted peridotite-derived partial melts based on undegassed melt inclusions from Siqueiros (Saal et al., 2002
Saal, A.E., Hauri, E.H., Langmuir, C.H., Perfit, M.R. (2002) Vapour undersaturation in primitive mid-ocean-ridge basalt and the volatile content of Earth’s upper mantle. Nature 419, 451–455.
) and calculations using an experimental bulk partition coefficient for CO2 and source CO2 estimate of ~75 ppm at F = 10 % (Rosenthal et al., 2015Rosenthal, A., Hauri, E.H., Hirschmann, M.M. (2015) Experimental determination of C, F, and H partitioning between mantle minerals and carbonated basalt, CO2/Ba and CO2/Nb systematics of partial melting, and the CO2 contents of basaltic source regions. Earth and Planetary Science Letters 412, 77–87.
).Figure 3 shows CO2-Nb and CO2-Ba mixing lines between basalts from Siqueiros (see Fig. S-1 for mixing with variable melting degrees of a depleted peridotite) and partial melts of various graphite-saturated recycled lithologies, compared with the highest CO2-Nb-Ba concentrations of oceanic basalts. The enriched end member melts are generated by calculating graphite-saturated CO2 concentrations at different fO2s (Eguchi and Dasgupta, 2018
Eguchi, J., Dasgupta, R. (2018) A CO2 solubility model for silicate melts from fluid saturation to graphite or diamond saturation. Chemical Geology 487, 23–38.
) and trace element concentrations using the batch melting equation (more details in Supplementary Information). Data points and mixing lines are coloured for wt. % SiO2 measured in natural basalts and wt. % SiO2 resulting from mixing depleted peridotite partial melt and partial melts of recycled lithologies.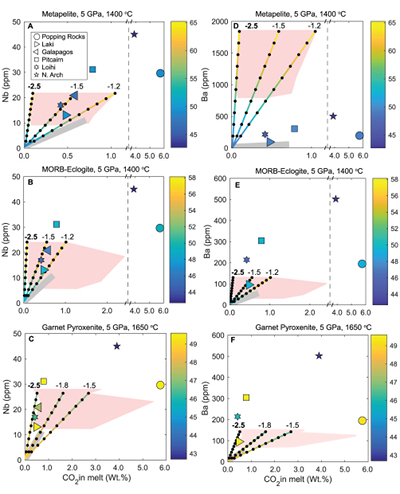
Figure 3 CO2-Nb and CO2-Ba mixing lines between depleted peridotite melts and graphite-saturated melts of subducted lithologies. Mixing lines are calculated at different logfO2s relative to FMQ up to the CCO buffer as denoted by numbers above mixing line (the average fO2 of eclogites based on CLM xenoliths at this pressure is marked with bold text). Each marker along a mixing line represents 10 wt. % incremental contribution from subducted lithology partial melt. Data points are the CO2-Nb/CO2-Ba concentrations recorded in the least degassed natural basalts at each location. Data point and mixings lines coloured for melt SiO2 (wt. %) contents. Pink shaded region shows CO2-Nb-Ba contents at graphite-saturation from CCO to FMQ-2.5 for all relevant experimental partial melts for a particular lithology from Figure 1. Gray band shows CO2-Nb-Ba calculations for a depleted peridotite from 1-10 % melting degree (see also Fig. S-1). Data for generation of Figure 3 given in Supplementary Information.
Figure 3 demonstrates that mixing between (graphite-absent) peridotite melts and partial melts of graphite-saturated subducted lithologies are unlikely to explain CO2-trace element concentrations measured in minimally degassed basalts. While some natural basalt compositions lie along the mixing lines, the required extent of contributions from subducted lithologies would result in reacted (Mallik and Dasgupta, 2012) or mixed melt major element compositions that do not match the natural data, particularly for metapelite and MORB-eclogite (Fig. 3a,b,d,e). In the case of mixing with partial melts of garnet pyroxenite, results demonstrate that CO2-Nb concentrations measured at Laki, Loihi, and Galapagos may be reproduced by mixing of basalts from depleted peridotite and graphite-saturated melts of pyroxenite at ~FMQ -2.5, while also producing major element composition consistent with natural data (Fig. 3c,f). The required mass fractions of melt derived from a pyroxenitic source would be about 50 % at Laki to 80 % at Loihi, which lie at the extreme upper end for estimates made for plume-fed basalts (Shorttle et al., 2014
Shorttle, O., Maclennan, J., Lambart, S. (2014) Quantifying lithological variability in the mantle. Earth and Planetary Science Letters 395, 24–40.
). Therefore, it is possible that for some CO2-enriched plume-fed basalts, melting of pyroxenites under graphite-saturated conditions can deliver enough CO2 to match the observed enrichment.Basalts at Pitcairn, North Arch of Hawaii, and the popping rocks cannot be explained by mixing with a graphite-saturated partial melt of any lithology, and require mixing with carbonated silicate melts, which have been demonstrated to reproduce CO2-trace element systematics of even the popping rocks (e.g., Dasgupta et al., 2009
Dasgupta, R., Hirschmann, M.M., McDonough, W.F., Spiegelman, M., Withers, A.C. (2009) Trace element partitioning between garnet lherzolite and carbonatite at 6.6 and 8.6 GPa with applications to the geochemistry of the mantle and of mantle-derived melts. Chemical Geology 262, 57–77.
). Although Figure 3 only shows results at 5 GPa, the findings in Figure 3 are consistent with all experimental partial melts in Figure 1 which lie between adiabats for mantle potential temperatures of 1350 and 1650 °C and below the peridotite solidus. The CO2-Ba-Nb concentrations for all of these experiments are shown as a pink shaded region in Figure 3 (see also Fig. S-1), which demonstrates that even when partial melts generated over a wide pressure range are considered, graphite-saturated partial melts fail to deliver enough CO2 and trace elements to explain natural, enriched basalts. We acknowledge that the simple binary mixing model presented here is unlikely to represent melt-mixing in nature, however we do not seek to explain the entire array of basalts for a particular location (e.g., Matthews et al., 2017Matthews, S., Shorttle, O., Rudge, J.F., Maclennan, J. (2017) Constraining mantle carbon: CO2-trace element systematics in basalts and the roles of magma mixing and degassing. Earth and Planetary Science Letters 480, 1–14.
). Rather, we only test whether the least degassed basalt CO2-trace element systematics for a given location can be explained by melt contribution from a graphite-saturated crustal lithology, and the simple mixing model used here is appropriate for that test. In addition to melt-melt mixing, we also test another scenario where the graphite-saturated partial melt of the subducted lithology is reactively frozen in upon interaction with ambient peridotite, and this new fertilised lithology melts at shallower oxidised conditions (see Fig. S-2). We find these calculations do not change the main conclusions, especially when the limit of eclogite melt addition through complete reactive crystallisation is considered.top
Oxygen Fugacity of the Mid- to Deep Oceanic Upper Mantle
We show that if CO2-trace element enrichment observed in minimally degassed oceanic basalts is due to contribution from recycled crusts, then the onset of melting must occur at carbonate-saturated conditions, which along mantle adiabats occur at pressures >10 GPa (Dasgupta et al., 2004
Dasgupta, R., Hirschmann, M.M., Withers, A.C. (2004) Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth and Planetary Science Letters 227, 73–85.
); or oxidative transformation of diamond-bearing lithologies must occur deeper than their respective volatile-free solidus. If the lithologic heterogeneity is silica-deficient pyroxenite, then our results do not preclude the possibility that basalts such as those at Laki and Galapagos may derive contributions from graphite-saturated melting. However, more enriched basalts such as those at Pitcairn, North Arch of Hawaii, and the North Atlantic popping rocks require generation of a carbonated silicate melt and therefore relatively oxidised conditions. In addition, previous studies on Iceland have shown a positive correlation between melt enrichment and oxidation state of erupted basalts (Shorttle et al., 2014Shorttle, O., Moussallam, Y., Hartley, M.E., Maclennan, J., Edmonds, M., Murton, B.J. (2015) Fe-XANES analyses of Reykjanes Ridge basalts: Implications for oceanic crust’s role in the solid Earth oxygen cycle. Earth and Planetary Science Letters 427, 272–285.
), lending support for the oxidised nature of source regions for enriched oceanic basalts proposed here.Based on our analyses that graphite-present melting of deeply embedded crustal rocks is mostly inconsistent with geochemistry of oceanic basalts, we show in Figure 4 the plausible fO2 range of oceanic mantle. The DCDD/G buffer, which is the fO2 at which graphite/diamond will coexist with carbonate in eclogite, and may also be applicable to pyroxenite (Luth, 1993
Luth, R.W. (1993) Diamonds, eclogites, and the oxidation state of the Earth’s Mantle. Science 261, 66–68.
), marks the lower fO2 limit (≥FMQ) of mafic/crustal lithologies in the convecting upper mantle up to ~5 GPa. fO2s calculated for CLM peridotite and eclogite xenoliths at similar depths suggest that C will be hosted in graphite/diamond.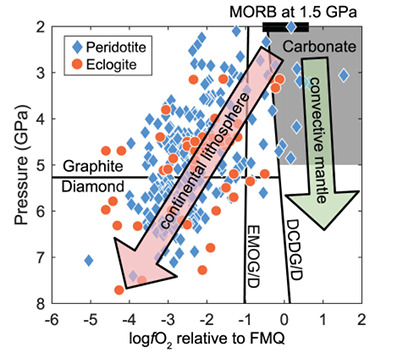
Figure 4 Pressure vs. fO2 computed for continental lithospheric mantle xenoliths versus those that have been estimated here for oceanic mantle sources (gray region). DCDG/D is the graphite/diamond transition in eclogite/pyroxenite (Luth, 1993
Luth, R.W. (1993) Diamonds, eclogites, and the oxidation state of the Earth’s Mantle. Science 261, 66–68.
). Graphite/diamond transition in peridotite is shown for reference (EMOG/D). References for xenolith data are in Table S-4.top
Conclusions
Our results suggest the CLM fO2-depth profile may not be applicable to the convecting upper mantle, with the convecting upper mantle being more oxidised than the continental lithospheric mantle (Fig. 4). We note that our calculations only make predictions on the fO2 of subducted lithologies. However, if the subducted lithologies and ambient mantle are in redox-equilibrium, then the fO2 of the ambient mantle should also be in the carbonate stability field because the carbonate to graphite/diamond transition occurs at higher fO2s in eclogite/pyroxenite (DCDD/G) compared to mantle peridotite (EMOG/D) (Fig. 4) (Luth, 1993
Luth, R.W. (1993) Diamonds, eclogites, and the oxidation state of the Earth’s Mantle. Science 261, 66–68.
).top
Acknowledgements
The authors thank Dr. Oliver Shorttle and an anonymous reviewer for their thoughtful comments which helped improve the manuscript. The authors also thank Dr. Helen Williams for handling the manuscript. The research received support from NSF grants EAR-1255391, OCE-1338842, EAR-1763226, and the Sloan Foundation through Deep Carbon Observatory.
Editor: Helen Williams
top
References
Anderson, K.R., Poland, M.P. (2017) Abundant carbon in the mantle beneath Hawai‘i. Nature Geoscience 10, 704–708.
 Show in context
Show in contextMany of these basalts have elevated CO2 concentrations relative to normal mid-ocean ridge basalts (Aubaud et al., 2006), which may be due to a CO2 contribution from subducted lithologies, which have higher CO2 concentrations relative to primitive mantle (Anderson and Poland, 2017; Hauri et al., 2017).
View in article
Aubaud, C., Pineau, F., Hékinian, R., Javoy, M. (2006) Carbon and hydrogen isotope constraints on degassing of CO2 and H2O in submarine lavas from the Pitcairn hotspot (South Pacific). Geophysical Research Letters 33, L02308.
 Show in context
Show in context Many of these basalts have elevated CO2 concentrations relative to normal mid-ocean ridge basalts (Aubaud et al., 2006), which may be due to a CO2 contribution from subducted lithologies, which have higher CO2 concentrations relative to primitive mantle (Anderson and Poland, 2017; Hauri et al., 2017).
View in article
Brenker, F.E., Vollmer, C., Vincze, L., Vekemans, B., Szymanski, A., Janssens, K., Szaloki, I., Nasdala, L., Joswig, W., Kaminsky, F. (2007) Carbonates from the lower part of transition zone or even the lower mantle. Earth and Planetary Science Letters 260, 1–9.
 Show in context
Show in context(2) Carbonate inclusions have been found in diamonds from depths in the mantle that should be in the graphite/diamond stability field if the CLM fO2 profile is applied to the convecting upper mantle (Brenker et al., 2007).
View in article
Cartigny, P., Pineau, F., Aubaud, C., Javoy, M. (2008) Towards a consistent mantle carbon flux estimate: Insights from volatile systematics (H2O/Ce, δD, CO2/Nb) in the North Atlantic mantle (14° N and 34° N). Earth and Planetary Science Letters 265, 672–685.
 Show in context
Show in context Figure 2 shows that it is unlikely that a graphite/diamond-saturated melt derived from any recycled lithology can dissolve enough CO2 to generate the high CO2 concentrations observed at the North Arch of Hawaii and popping rocks from the North Atlantic (Dixon et al., 1997; Cartigny et al., 2008).
View in article
Cottrell, E., Kelley, K.A. (2011) The oxidation state of Fe in MORB glasses and the oxygen fugacity of the upper mantle. Earth and Planetary Science Letters 305, 270–282.
 Show in context
Show in context Current understandings of mantle redox are largely based on measurements of Fe3+ and Fe2+ in mantle-derived basalts, which suggest that the oxygen fugacity of Earth’s shallow mantle to about 1.5 GPa is close to the FMQ buffer (e.g., Cottrell and Kelley, 2011; Hartley et al., 2017).
View in article
We only consider partial melting studies conducted at pressure-temperature conditions below the peridotite solidus to investigate processes which occur deeper than the onset of peridotite partial melting, as previous studies have already demonstrated that at shallow depths where major peridotite melting occurs, fO2 is around the FMQ buffer (e.g., Cottrell and Kelley, 2011).
View in article
Dasgupta, R. (2018) Volatile-bearing partial melts beneath oceans and continents–Where, how much, and of what compositions? American Journal of Science 318, 141–165.
 Show in context
Show in context(1) Kimberlites (oxidised, CO2-rich magmas) are generated deep in the mantle, then migrate through overlying cratons, often picking up diamonds, a reduced form of carbon, implying there are regions in the mantle deeper than CLM depths where diamond resides that are more oxidised (Yaxley et al., 2017; Dasgupta, 2018).
View in article
Dasgupta, R., Hirschmann, M.M. (2006) Melting in the Earth’s deep upper mantle caused by carbon dioxide. Nature 440, 659–662.
 Show in context
Show in contextOn the other hand, carbonate-bearing lithologies melt at the lithology’s carbonated solidus and generate a carbonated melt with up to ~45 wt. % CO2 (e.g., Dasgupta and Hirschmann, 2006).
View in article
Dasgupta, R., Hirschmann, M.M., Withers, A.C. (2004) Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth and Planetary Science Letters 227, 73–85.
 Show in context
Show in context We show that if CO2-trace element enrichment observed in minimally degassed oceanic basalts is due to contribution from recycled crusts, then the onset of melting must occur at carbonate-saturated conditions, which along mantle adiabats occur at pressures >10 GPa (Dasgupta et al., 2004); or oxidative transformation of diamond-bearing lithologies must occur deeper than their respective volatile-free solidus.
View in article
Dasgupta, R., Hirschmann, M.M., McDonough, W.F., Spiegelman, M., Withers, A.C. (2009) Trace element partitioning between garnet lherzolite and carbonatite at 6.6 and 8.6 GPa with applications to the geochemistry of the mantle and of mantle-derived melts. Chemical Geology 262, 57–77.
 Show in context
Show in contextBasalts at Pitcairn, North Arch of Hawaii, and the popping rocks cannot be explained by mixing with a graphite-saturated partial melt of any lithology, and require mixing with carbonated silicate melts, which have been demonstrated to reproduce CO2-trace element systematics of even the popping rocks (e.g., Dasgupta et al., 2009).
View in article
Dixon, J.E., Clague, D.A., Wallace, P., Poreda, R. (1997) Volatiles in Alkalic Basalts from the North Arch Volcanic Field, Hawaii: Extensive Degassing of Deep Submarine-erupted Alkalic Series Lavas. Journal of Petrology 38, 911–939.
 Show in context
Show in contextFigure 2 shows that it is unlikely that a graphite/diamond-saturated melt derived from any recycled lithology can dissolve enough CO2 to generate the high CO2 concentrations observed at the North Arch of Hawaii and popping rocks from the North Atlantic (Dixon et al., 1997; Cartigny et al., 2008).
View in article
Eguchi, J., Dasgupta, R. (2017) CO2 content of andesitic melts at graphite-saturated upper mantle conditions with implications for redox state of oceanic basalt source regions and remobilization of reduced carbon from subducted eclogite. Contributions to Mineralogy and Petrology 172, 12.
 Show in context
Show in contextGraphite-bearing lithologies melt at the lithology’s nominally volatile-free solidus and generate a silicate melt with fO2-dependent CO2 concentrations (e.g., Eguchi and Dasgupta, 2017, 2018).
View in article
Eguchi, J., Dasgupta, R. (2018) A CO2 solubility model for silicate melts from fluid saturation to graphite or diamond saturation. Chemical Geology 487, 23–38.
 Show in context
Show in context Graphite-bearing lithologies melt at the lithology’s nominally volatile-free solidus and generate a silicate melt with fO2-dependent CO2 concentrations (e.g., Eguchi and Dasgupta, 2017, 2018).
View in article
We use a new, compositionally dependent CO2 solubility model for silicate melts (Eguchi and Dasgupta, 2018) applicable to graphite/diamond-saturated conditions to investigate a wide range of lithologies.
View in article
The enriched end member melts are generated by calculating graphite-saturated CO2 concentrations at different fO2s (Eguchi and Dasgupta, 2018) and trace element concentrations using the batch melting equation (more details in Supplementary Information).
View in article
Frost, D.J., McCammon, C.A. (2008) The Redox State of Earth’s Mantle. Annual Review of Earth and Planetary Sciences 36, 389–420.
 Show in context
Show in contextThe redox state of mantle lithologies, based on xenoliths from continental lithospheric mantle, has been shown to decrease with depth and reach oxygen fugacities (fO2) at which graphite/diamond will be the stable form of carbon at pressures greater than about 3-4 GPa (e.g., Frost and McCammon, 2008).
View in article
The oxidation state of the mantle plays a critical role in dictating the geochemical and geodynamic evolution of Earth and its atmosphere (e.g., Frost and McCammon, 2008).
View in article
The fO2 decrease with increasing depth observed in CLM has been proposed to apply to the convecting mantle (e.g., Frost and McCammon, 2008).
View in article
Gaillard, F., Scaillet, B., Pichavant, M., Iacono-Marziano, G. (2015) The redox geodynamics linking basalts and their mantle sources through space and time. Chemical Geology 418, 217–233.
 Show in context
Show in context (4) Komatiites, which are generated deeper in the mantle than basalts have similar redox states to MORB (Gaillard et al., 2015).
View in article
Gudmundsson, G., Wood, B.J. (1995) Experimental tests of garnet peridotite oxygen barometry. Contributions to Mineralogy and Petrology 119, 56–67.
 Show in context
Show in contextThis trend has been attributed to the increasing stability of the Fe3+-bearing skiagite component of garnet with increasing depth (Gudmundsson and Wood, 1995).
View in article
Hartley, M.E., Shorttle, O., Maclennan, J., Moussallam, Y., Edmonds, M. (2017) Olivine-hosted melt inclusions as an archive of redox heterogeneity in magmatic systems. Earth and Planetary Science Letters 479, 192–205.
 Show in context
Show in contextCurrent understandings of mantle redox are largely based on measurements of Fe3+ and Fe2+ in mantle-derived basalts, which suggest that the oxygen fugacity of Earth’s shallow mantle to about 1.5 GPa is close to the FMQ buffer (e.g., Cottrell and Kelley, 2011; Hartley et al., 2017).
View in article
Hauri, E.H., Maclennan, J., McKenzie, D., Gronvold, K., Oskarsson, N., Shimizu, N. (2017) CO2 content beneath northern Iceland and the variability of mantle carbon. Geology 46, 55–58.
 Show in context
Show in contextMany of these basalts have elevated CO2 concentrations relative to normal mid-ocean ridge basalts (Aubaud et al., 2006), which may be due to a CO2 contribution from subducted lithologies, which have higher CO2 concentrations relative to primitive mantle (Anderson and Poland, 2017; Hauri et al., 2017).
View in article
Hofmann, A.W., White, W.M. (1982) Mantle plumes from ancient oceanic crust. Earth and Planetary Science Letters 57, 421–436.
 Show in context
Show in context Many oceanic basalts are argued to contain partial melt contributions of subducted lithologies such as sediments, MORB-eclogite, and pyroxenite (Hofmann and White, 1982), which begin to melt deeper than peridotite due to their lower solidi (e.g., Spandler et al., 2008).
View in article
Kiseeva, E.S., Vasiukov, D.M., Wood, B.J., McCammon, C., Stachel, T., Bykov, M., Bykova, E., Chumakov, A., Cerantola, V., Harris, J.W., Dubrovinsky, L. (2018) Oxidized iron in garnets from the mantle transition zone. Nature Geoscience 11, 144–147.
 Show in context
Show in context (3) Kiseeva et al. (2018) argue that fO2 measurements of garnet inclusions in diamonds from the mantle transition zone become more oxidised with depth, and are more oxidised than predicted by the Fe-controlled fO2 profile inferred from CLMs.
View in article
Luth, R.W. (1993) Diamonds, eclogites, and the oxidation state of the Earth’s Mantle. Science 261, 66–68.
 Show in context
Show in context The DCDD/G buffer, which is the fO2 at which graphite/diamond will coexist with carbonate in eclogite, and may also be applicable to pyroxenite (Luth, 1993), marks the lower fO2 limit (≥FMQ) of mafic/crustal lithologies in the convecting upper mantle up to ~5 GPa.
View in article
Figure 4 [...] DCDG/D is the graphite/diamond transition in eclogite/pyroxenite (Luth, 1993)
View in article
However, if the subducted lithologies and ambient mantle are in redox-equilibrium, then the fO2 of the ambient mantle should also be in the carbonate stability field because the carbonate to graphite/diamond transition occurs at higher fO2s in eclogite/pyroxenite (DCDD/G) compared to mantle peridotite (EMOG/D) (Fig. 4) (Luth, 1993).
View in article
Matthews, S., Shorttle, O., Rudge, J.F., Maclennan, J. (2017) Constraining mantle carbon: CO2-trace element systematics in basalts and the roles of magma mixing and degassing. Earth and Planetary Science Letters 480, 1–14.
 Show in context
Show in context We acknowledge that the simple binary mixing model presented here is unlikely to represent melt-mixing in nature, however we do not seek to explain the entire array of basalts for a particular location (e.g., Matthews et al., 2017).
View in article
Rosenthal, A., Hauri, E.H., Hirschmann, M.M. (2015) Experimental determination of C, F, and H partitioning between mantle minerals and carbonated basalt, CO2/Ba and CO2/Nb systematics of partial melting, and the CO2 contents of basaltic source regions. Earth and Planetary Science Letters 412, 77–87.
 Show in context
Show in contextFigure 2 [...] Red band is for CO2 contents expected in depleted peridotite-derived partial melts based on undegassed melt inclusions from Siqueiros (Saal et al., 2002) and calculations using an experimental bulk partition coefficient for CO2 and source CO2 estimate of ~75 ppm at F = 10 % (Rosenthal et al., 2015).
View in article
Saal, A.E., Hauri, E.H., Langmuir, C.H., Perfit, M.R. (2002) Vapour undersaturation in primitive mid-ocean-ridge basalt and the volatile content of Earth’s upper mantle. Nature 419, 451–455.
 Show in context
Show in contextIn Figure 2 we show CO2 concentrations calculated for experimental partial melts at the CCO buffer (maximum amount of CO2 dissolved with graphite present in the source) compared to the highest CO2 concentrations measured in enriched oceanic basalts from several locations. CO2 concentrations from basalts at Siqueiros are measured in undegassed melt inclusions and may be widely representative of CO2 concentrations for basalts from partial melts of normal primitive mantle (Saal et al., 2002).
View in article
Figure 2 [...] Red band is for CO2 contents expected in depleted peridotite-derived partial melts based on undegassed melt inclusions from Siqueiros (Saal et al., 2002) and calculations using an experimental bulk partition coefficient for CO2 and source CO2 estimate of ~75 ppm at F = 10 % (Rosenthal et al., 2015).
View in article
Shorttle, O., Maclennan, J., Lambart, S. (2014) Quantifying lithological variability in the mantle. Earth and Planetary Science Letters 395, 24–40.
 Show in context
Show in contextThe required mass fractions of melt derived from a pyroxenitic source would be about 50 % at Laki to 80 % at Loihi, which lie at the extreme upper end for estimates made for plume-fed basalts (Shorttle et al., 2014).
View in article
Shorttle, O., Moussallam, Y., Hartley, M.E., Maclennan, J., Edmonds, M., Murton, B.J. (2015) Fe-XANES analyses of Reykjanes Ridge basalts: Implications for oceanic crust’s role in the solid Earth oxygen cycle. Earth and Planetary Science Letters 427, 272–285.
 Show in context
Show in contextIn addition, previous studies on Iceland have shown a positive correlation between melt enrichment and oxidation state of erupted basalts (Shorttle et al., 2015), lending support for the oxidised nature of source regions for enriched oceanic basalts proposed here.
View in article
Spandler, C., Yaxley, G., Green, D.H., Rosenthal, A. (2008) Phase Relations and Melting of Anhydrous K-bearing Eclogite from 1200 to 1600 C and 3 to 5 GPa. Journal of Petrology 49, 771–795.
 Show in context
Show in contextMany oceanic basalts are argued to contain partial melt contributions of subducted lithologies such as sediments, MORB-eclogite, and pyroxenite (Hofmann and White, 1982), which begin to melt deeper than peridotite due to their lower solidi (e.g., Spandler et al., 2008).
View in article
Woodland, A.B., Koch, M. (2003) Variation in oxygen fugacity with depth in the upper mantle beneath the Kaapvaal craton, Southern Africa. Earth and Planetary Science Letters 214, 295–310.
 Show in context
Show in contextThe fO2 of the deeper portions of Earth’s mantle is chiefly known from mantle xenoliths recovered from continental lithospheric mantle (CLM), which suggest fO2 decreases with increasing depth (e.g., Woodland and Koch, 2003).
View in article
Yaxley, G.M., Berry, A.J., Rosenthal, A., Woodland, A.B., Paterson, D. (2017) Redox preconditioning deep cratonic lithosphere for kimberlite genesis - Evidence from the central Slave Craton. Scientific Reports 7, doi: 10.1038/s41598-017-00049-3.
 Show in context
Show in context(1) Kimberlites (oxidised, CO2-rich magmas) are generated deep in the mantle, then migrate through overlying cratons, often picking up diamonds, a reduced form of carbon, implying there are regions in the mantle deeper than CLM depths where diamond resides that are more oxidised (Yaxley et al., 2017; Dasgupta, 2018).
View in article
top
Supplementary Information
The Supplementary Information includes:
- CO2-Nb and CO2-Ba Mixing Calculations
- Mixing with Peridotite Melts at Different Melting Degrees
- Melting of Peridotite Enriched by Partial Melts of Recycled Lithologies
- Tables S-1 to S-4
- Figures S-1 and S-2
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
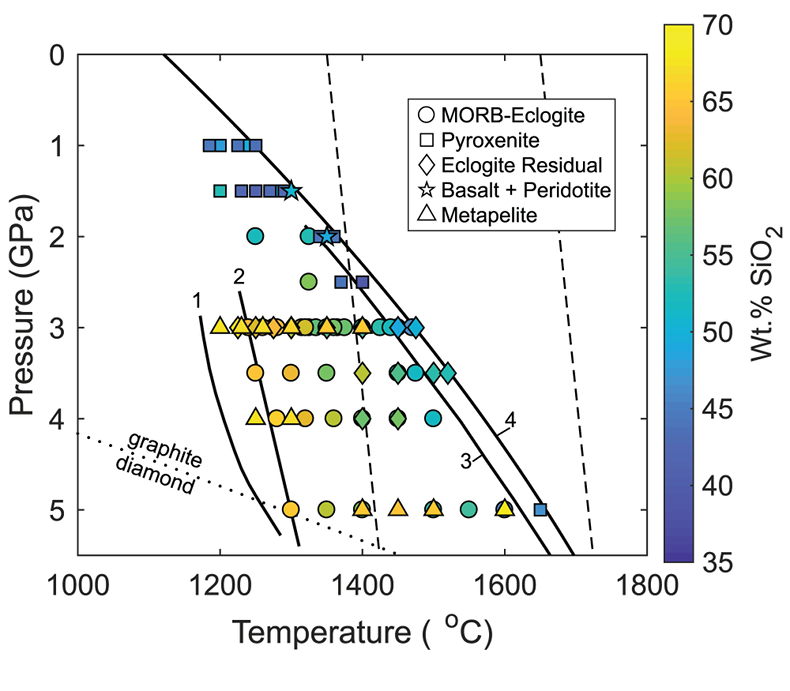
Figure 1 Pressure-temperature conditions for generation of partial melts from crustal/mafic lithologies in the mantle with colour map for wt. % SiO2 in the melt. Also plotted for reference are mantle adiabats for mantle potential temperatures of 1350 and 1650 °C (dashed lines), solidi of various volatile-free lithologies (1-metapelite, 2-MORB-eclogite, 3-silica-deficient garnet pyroxenite, 4-peridotite, references given in footnote of Table S-3 – the table of compiled experiments), and graphite-diamond transition (dotted line).

Figure 2 CO2 concentrations calculated at the CCO buffer for each experiment in Figure 1. Vertical blue lines are highest CO2 concentrations measured in natural oceanic basalts from different locations (Table S-2) that are thought to receive contributions from subducted lithologies. Red band is for CO2 contents expected in depleted peridotite-derived partial melts based on undegassed melt inclusions from Siqueiros (Saal et al., 2002) and calculations using an experimental bulk partition coefficient for CO2 and source CO2 estimate of ~75 ppm at F = 10 % (Rosenthal et al., 2015
Rosenthal, A., Hauri, E.H., Hirschmann, M.M. (2015) Experimental determination of C, F, and H partitioning between mantle minerals and carbonated basalt, CO2/Ba and CO2/Nb systematics of partial melting, and the CO2 contents of basaltic source regions. Earth and Planetary Science Letters 412, 77–87.
).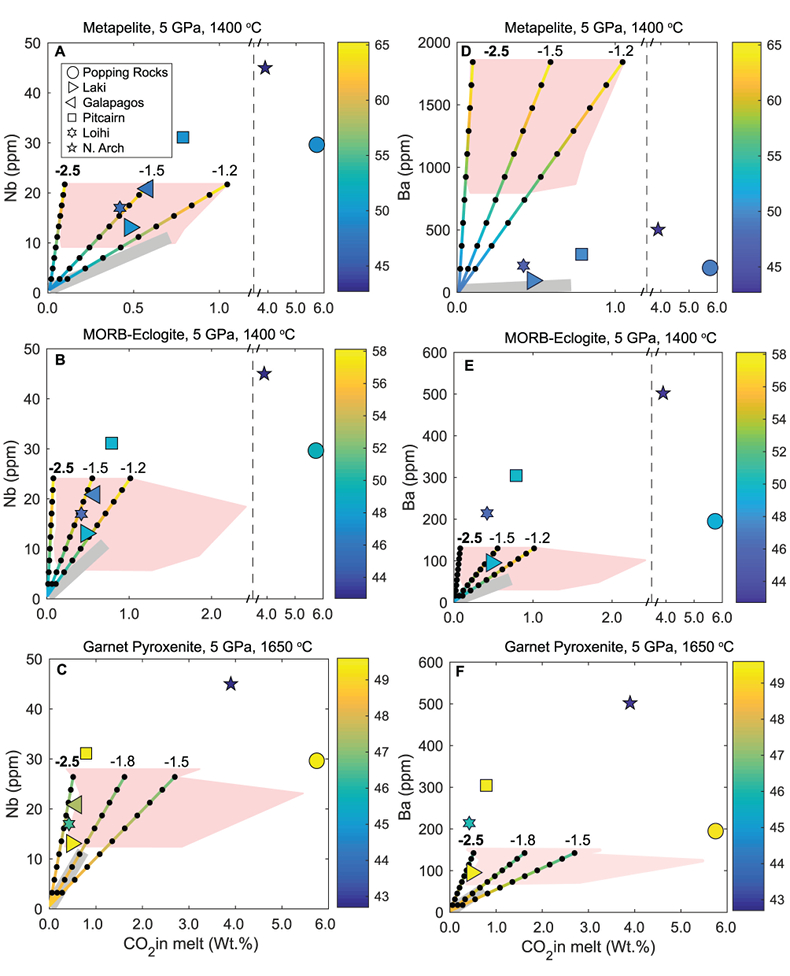
Figure 3 CO2-Nb and CO2-Ba mixing lines between depleted peridotite melts and graphite-saturated melts of subducted lithologies. Mixing lines are calculated at different logfO2s relative to FMQ up to the CCO buffer as denoted by numbers above mixing line (the average fO2 of eclogites based on CLM xenoliths at this pressure is marked with bold text). Each marker along a mixing line represents 10 wt. % incremental contribution from subducted lithology partial melt. Data points are the CO2-Nb/CO2-Ba concentrations recorded in the least degassed natural basalts at each location. Data point and mixings lines coloured for melt SiO2 (wt. %) contents. Pink shaded region shows CO2-Nb-Ba contents at graphite-saturation from CCO to FMQ-2.5 for all relevant experimental partial melts for a particular lithology from Figure 1. Gray band shows CO2-Nb-Ba calculations for a depleted peridotite from 1-10 % melting degree (see also Fig. S-1). Data for generation of Figure 3 given in Supplementary Information.
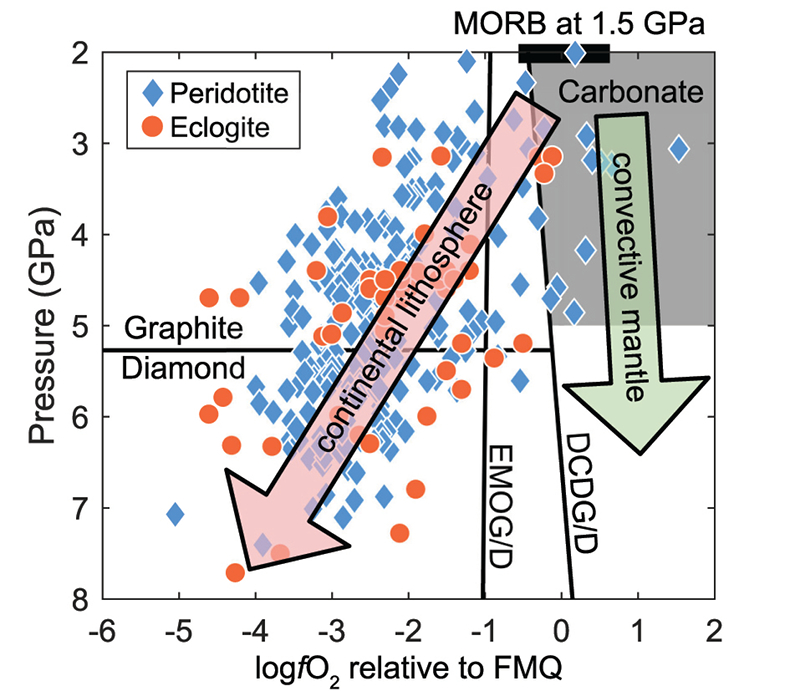
Figure 4 Pressure vs. fO2 computed for continental lithospheric mantle xenoliths versus those that have been estimated here for oceanic mantle sources (gray region). DCDG/D is the graphite/diamond transition in eclogite/pyroxenite (Luth, 1993
Luth, R.W. (1993) Diamonds , eclogites , and the oxidation state of the Earth’s Mantle. Science 261, 66–68.
). Graphite/diamond transition in peridotite is shown for reference (EMOG/D). References for xenolith data are in Table S-4.