Colloidal origin of microbands in banded iron formations
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:4,540Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
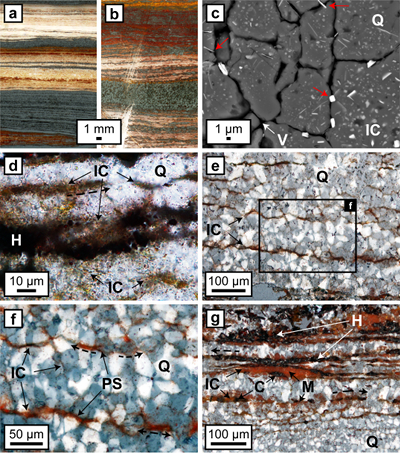 Figure 1 (a, b) Transmitted light images (TL) of microbands of chert/quartz (white), chert with colloidal hematite (red, brown) and hematite bands (blue grey). (c) BSE image with colloidal hematite ‘floating’ in quartz crystals, partly liberated (red arrows) and free hematite particles at grain boundaries. (d) TL and (e, f) reflected light images (XPL) displaying irregular layers of hematite particles at truncated quartz crystals (PS) and along quartz grain boundaries with lateral transitions into equigranular quartz aggregates (dashed arrows). (g) XPL image showing hematite particles that surround layered hematite forming core/mantle textures. C: core, H: hematite, M: mantle, IC: iron oxide colloids/particles, PS: pressure solution, Q: quartz, V: void. | 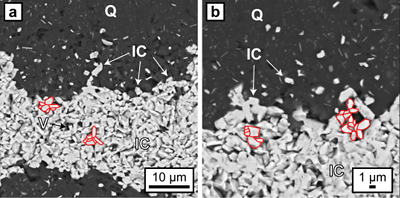 Figure 2 (a, b) BSE images of hematite particles along truncated quartz crystals forming dense aggregates (red outlines) with intergranular pores. Encapsulated colloidal hematite in chert and hematite particles in microbands show similar grain sizes and morphologies. IC: iron oxide colloids/particles, Q: quartz, V: void. | 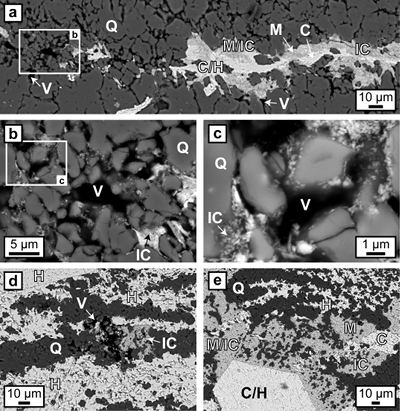 Figure 3 (a–e) BSE images of BIF laminae illustrating the lateral change from porous chert to hematite microbands with irregular boundaries. (a–c) The porosity is (partly) filled with hematite particles encapsulating quartz grains. (d–e) Hematite forms more larger crystals inside these particle agglomerations, as evident by core/mantle structures resulting in new microbands of hematite. C: core, H: hematite, M: mantle, IC: iron oxide colloids/particles, Q: quartz, V: void. | 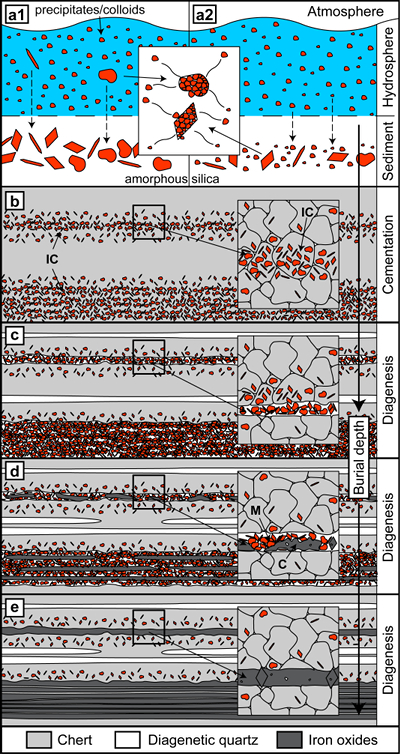 Figure 4 Conceptual evolution from BIF precursor sediments to BIF microbands. (a1, a2). Schematic Precambrian depositional environment of BIF precursor minerals. The inset shows the aggregation of precipitates/colloidal phases to form larger particles by flocculation in the water column (a1) and/or attachment in the sediment prior to lithification (a2). (b–e) Development of BIF textures after lithification. Insets show the microscale to nanoscale formation of minerals and textures. (b) Quartz crystallisation (cementation) leads to the entrapment of randomly dispersed colloidal phases. (c) Progressive compression leads to quartz dissolution by DPC, liberation of encapsulated particles and residual layer-parallel accumulation. Dissolved silica forms new particle-free quartz layers (i.e. diagenetic quartz). (d) Simultaneously, accumulated particles transform into more stable iron oxides resulting in the common BIF texture of pristine chert, diagenetic quartz and iron oxide microbands (e). |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Banded iron formations (BIFs) are chemical sedimentary rocks that originated in Precambrian marine settings. BIFs are characterised by their compositional banding with thicknesses on the scales of metres, centimetres, and millimetres to micrometres, termed macrobands, mesobands, and microbands, respectively. Shale horizons separate individual BIF macrobands, which are subdivided into meso- and microbands dominated by iron oxide minerals (e.g., magnetite, hematite), iron silicates and iron carbonates, alternating with bands dominated by chert (i.e. microcrystalline quartz) (Trendall and Blockley, 1970
Trendall, A.F., Blockley, J.G. (1970) The iron formations of the Precambrian Hamersley Group, Western Australia: Geological Survey of Western Australia Bulletin 119. Geological Survey of Western Australia, Perth.
).The origin of the banded texture of BIFs is still controversial. The majority of models assume that iron-rich micro- and mesobands represent primary layering related to deposition of precursor iron minerals (e.g., ferrihydrite) produced by secular changes in bacterial activity (Posth et al., 2008
Posth, N.R., Hegler, F., Konhauser, K.O., Kappler, A. (2008) Alternating Si and Fe deposition caused by temperature fluctuations in Precambrian oceans. Nature Geoscience 1, 703–708.
), chemical precipitation (Garrels, 1987Garrels, R.M. (1987) A model for the deposition of the microbanded Precambrian iron formations. American Journal of Science 287, 81–106.
), hydrothermal or volcanic influx (Bekker et al., 2010Bekker, A., Slack, J.F., Planavsky, N., Krapež, B., Hofmann, A., Konhauser, K.O., Rouxel, O.J. (2010) Iron Formation: The Sedimentary Product of a Complex Interplay among Mantle, Tectonic, Oceanic, and Biospheric Processes. Economic Geology 105, 467–508.
), and/or re-sedimentation (Krapež et al., 2003Krapež, B., Barley, M.E., Pickard, A.L. (2003) Hydrothermal and resedimented origins of the precursor sediments to banded iron formation: sedimentological evidence from the Early Palaeoproterozoic Brockman Supersequence of Western Australia. Sedimentology 50, 979–1011.
). The origin of chert bands in BIFs is also controversial. Although it is widely acknowledged that chert layers form from amorphous silica of abiotic origin (Posth et al., 2008Posth, N.R., Hegler, F., Konhauser, K.O., Kappler, A. (2008) Alternating Si and Fe deposition caused by temperature fluctuations in Precambrian oceans. Nature Geoscience 1, 703–708.
), it is unknown whether they form from silica deposited alongside ferric oxide precipitates (Fischer and Knoll, 2009Fischer, W.W., Knoll, A.H. (2009) An iron shuttle for deepwater silica in Late Archean and early Paleoproterozoic iron formation. Geological Society of America Bulletin 121, 222–235.
), by seafloor silicification during depositional hiatuses (Rasmussen et al., 2015aRasmussen, B., Krapež, B., Muhling, J.R. (2015a) Seafloor silicification and hardground development during deposition of 2.5 Ga banded iron formations. Geology 43, 235–238.
), or below the seafloor during compaction (Krapež et al., 2003Krapež, B., Barley, M.E., Pickard, A.L. (2003) Hydrothermal and resedimented origins of the precursor sediments to banded iron formation: sedimentological evidence from the Early Palaeoproterozoic Brockman Supersequence of Western Australia. Sedimentology 50, 979–1011.
).Alternative models interpret the banded texture of BIF as a secondary differentiation product generated during burial metamorphism by ion diffusion through a homogeneous mass of silica gel (Pecoits et al., 2009
Pecoits, E., Gingras, M.K., Barley, M.E., Kappler, A., Posth, N.R., Konhauser, K.O. (2009) Petrography and geochemistry of the Dales Gorge banded iron formation: Paragenetic sequence, source and implications for palaeo-ocean chemistry. Precambrian Research 172, 163–187.
), or by differential compaction (Trendall and Blockley, 1970Trendall, A.F., Blockley, J.G. (1970) The iron formations of the Precambrian Hamersley Group, Western Australia: Geological Survey of Western Australia Bulletin 119. Geological Survey of Western Australia, Perth.
). The critical problem for all of these interpretations lies in establishing whether the observed mineral assemblages are of diagenetic origin and thus whether or not they can be directly related to palaeoenvironmental conditions (Bekker et al., 2010Bekker, A., Slack, J.F., Planavsky, N., Krapež, B., Hofmann, A., Konhauser, K.O., Rouxel, O.J. (2010) Iron Formation: The Sedimentary Product of a Complex Interplay among Mantle, Tectonic, Oceanic, and Biospheric Processes. Economic Geology 105, 467–508.
).New nanoscale studies on ’dusty’ chert bands interpreted the presence of abundant colloidal hematite as a product of early sedimentary ferrihydrite (Sun et al., 2015
Sun, S., Konhauser, K.O., Kappler, A., Li, Y. (2015) Primary hematite in Neoarchean to Paleoproterozoic oceans. Geological Society of America Bulletin 127, 850–861.
; Sun and Li, 2017Sun, S., Li, Y.-L. (2017) Geneses and evolutions of iron-bearing minerals in banded iron formations of >3760 to ca. 2200 million-year-old: Constraints from electron microscopic, X-ray diffraction and Mössbauer spectroscopic investigations. Precambrian Research 289, 1–17.
), or post-depositional oxidation of primary iron silicates (Rasmussen et al., 2015bRasmussen, B., Krapež, B., Muhling, J.R., Suvorova, A. (2015b) Precipitation of iron silicate nanoparticles in early Precambrian oceans marks Earth’s first iron age. Geology 43, 303–306.
, 2016Rasmussen, B., Muhling, J.R., Suvorova, A., Krapez, B. (2016) Dust to dust: Evidence for the formation of “primary” hematite dust in banded iron formations via oxidation of iron silicate nanoparticles. Precambrian Research 284, 49–63.
) (The term ‘colloid’ refers to a phase that has a least one dimension in the nanometre range, which is dispersed in another phase of different composition or state; i.e. here solid hematite particles dispersed in solid quartz grains). Although laboratory experiments have demonstrated that diagenetic recrystallisation of ferrihydrite to iron oxides can occur (Posth et al., 2013Posth, N.R., Konhauser, K., Kappler, A. (2013) Simulating Precambrian banded iron formation diagenesis. Chemical Geology 362, 66–73.
), no study has yet comprehensively described the role of deformation in controlling the evolution of (micro-) banding in BIFs.Here, we investigate the fate of colloidal hematite during deformation and low temperature metamorphism to clarify the evolution of BIF banding. Our results show that post-depositional physicochemical processes have obscured primary information and may be responsible for much of the microbanding seen in these rocks.
top
Samples and Methods
Samples were collected from BIF outcrops of the ~2.48 Ga Dales Gorge Member, NW Australia, from the central part of the Hamersley Province (Turner Syncline), which has been affected by maximum lower greenschist facies metamorphic conditions (Smith et al., 1982
Smith, R.E., Perdrix, J.L., Parks, T.C. (1982) Burial metamorphism in the Hamersley basin, Western Australia. Journal of Petrology 23, 75–102.
; Fig. S-1). We selected samples that contain alternating chert bands with brown, orange, pink or red colours and fine- to coarse-grained iron oxide bands for scanning electron microscopy (SEM) and X-ray diffraction (XRD) analyses (Fig. 1; Supplementary Information). Polished samples for SEM observation were thoroughly washed with ultrasonic cleaners to eliminate sample preparation contaminants, although this procedure may have inadvertently removed some original particles from the rocks.
Figure 1 (a, b) Transmitted light images (TL) of microbands of chert/quartz (white), chert with colloidal hematite (red, brown) and hematite bands (blue grey). (c) BSE image with colloidal hematite ‘floating’ in quartz crystals, partly liberated (red arrows) and free hematite particles at grain boundaries. (d) TL and (e, f) reflected light images (XPL) displaying irregular layers of hematite particles at truncated quartz crystals (PS) and along quartz grain boundaries with lateral transitions into equigranular quartz aggregates (dashed arrows). (g) XPL image showing hematite particles that surround layered hematite forming core/mantle textures. C: core, H: hematite, M: mantle, IC: iron oxide colloids/particles, PS: pressure solution, Q: quartz, V: void.
top
Hematite Particles and Microbands
Optical microscopy and SEM analyses show that differences in chert band colouration are caused by inclusion of varying amounts of randomly oriented subhedral to euhedral nm- to µm-sized iron oxide particles within microcrystalline quartz grains (Fig. 1a-c). Particles occasionally protrude from quartz grains or occur along narrow grain boundaries (Fig. 1c). XRD analyses of coloured chert bands primarily identified highly crystalline hematite with crystallite sizes of around 100 nm; goethite was absent or present at very low abundances (≤0.5 wt. %). The extensive colour variation likely corresponds to differences in size and morphology of hematite particles, shifting from the characteristic red colour of macroscopic crystals to orange for sub-µm-sized particles (Cornell and Schwertmann, 2003
Cornell, R.M., Schwertmann, U. (2003) The iron oxides - structure, properties, reactions, occurences and uses. Wiley-VCH, Weinheim.
) (Fig. 1d).On the microscale (Fig. 1e,f), accumulations of hematite particles are predominantly located along laminae of truncated quartz crystals sub-parallel to the banding. Back scattered electron (BSE) images show that hematite particles can form microbands that share highly irregular boundaries with adjacent chert layers (Fig. 2). Hematite particles in microbands are randomly oriented and have similar sizes and morphologies to the intragranular colloidal hematite encapsulated within quartz grains (Fig. 2).

Figure 2 (a, b) BSE images of hematite particles along truncated quartz crystals forming dense aggregates (red outlines) with intergranular pores. Encapsulated colloidal hematite in chert and hematite particles in microbands show similar grain sizes and morphologies. IC: iron oxide colloids/particles, Q: quartz, V: void.
Hematite particles form fine-grained coatings on pore walls and/or completely fill the available pore space. Microcrystalline quartz grains are then encapsulated by agglomerations of hematite particles which inherit the irregular shape of the quartz aggregates (Fig. 3). Colloform or vein-like crystal growths that would indicate nucleation points for hematite on pore walls were absent. Thicker layers of hematite particles commonly host larger µm-sized grains of anhedral to euhedral hematite, which are commonly surrounded by a cloud or mantle of smaller hematite particles (Figs. 1g, 3a,e). Some laterally uniform bands consist almost entirely of coarsely crystalline hematite with few remaining hematite particles (Figs. 1g, 3d,e).

Figure 3 (a–e) BSE images of BIF laminae illustrating the lateral change from porous chert to hematite microbands with irregular boundaries. (a–c) The porosity is (partly) filled with hematite particles encapsulating quartz grains. (d–e) Hematite forms more larger crystals inside these particle agglomerations, as evident by core/mantle structures resulting in new microbands of hematite. C: core, H: hematite, M: mantle, IC: iron oxide colloids/particles, Q: quartz, V: void.
top
Liberation of Colloidal Hematite
Our observations indicate that many previously encapsulated hematite particles have been liberated from quartz, as demonstrated by their partial liberation, and the similarity in grain sizes and morphologies to fully liberated particles (Figs. 1c, 2, 3a–c). The layer-parallel truncation of quartz grains indicates that quartz dissolution occurred along a systematic preferred orientation induced by deformation. The importance of dissolution–precipitation creep (DPC, or pressure solution) during deformation of BIF has recently been revealed through the observation of variably developed crystallographic preferred orientations within alternating chert bands (Egglseder et al., 2016
Egglseder, M.S., Cruden, A.R., Tomkins, A.G., Wilson, C.J.L. (2016) Deformation-induced silica redistribution in banded iron formation, Hamersley Province, Australia. Lithos 266–267, 87–97.
).DPC is induced by stress applied to a fluid-bearing polymineralic aggregate where quartz dissolves in fluid at grain boundaries and aqueous silica is subsequently transported by diffusion to precipitate in areas of lower concentration (Bons and den Brok, 2000
Bons, P.D., den Brok, B. (2000) Crystallographic preferred orientation development by dissolution–precipitation creep. Journal of Structural Geology 22, 1713–1722.
). This process ultimately leads to intercalation of strongly oriented secondary quartz and more pristine chert layers that lack preferred orientation (Egglseder et al., 2016Egglseder, M.S., Cruden, A.R., Tomkins, A.G., Wilson, C.J.L. (2016) Deformation-induced silica redistribution in banded iron formation, Hamersley Province, Australia. Lithos 266–267, 87–97.
) (Fig. S-2). DPC can commence at pressures as low as 2–3 bar and it is influenced by electrochemical potential differences between dissimilar minerals such as mineral inclusions (colloidal phases) within quartz (Greene et al., 2009Greene, G.W., Kristiansen, K., Meyer, E.E., Boles, J.R., Israelachvili, J.N. (2009) Role of electrochemical reactions in pressure solution. Geochimica et Cosmochimica Acta 73, 2862–2874.
).top
Transport of Particles
We interpret that the small size of hematite particles allowed them to be transported through the micro-porosity of the BIF, which promoted the development of layer-parallel hematite particle accumulations (Fig. 3a-c). Once quartz was removed by DPC, the liberated particles could have: 1) accumulated in situ (Figs. 1d–g, 2), 2) been transported into connected pore spaces creating porosity-infilling textures (Figs. 3a–c); or 3) been dissolved in the available fluid phase. The latter process is not evident in our samples, as the size and shape of the particles inside and outside quartz grains are not significantly different. The lack of evidence for hematite precipitation also suggests that extensive dissolution of liberated particles is unlikely to have occurred. We suggest that the liberated hematite particles in our samples behaved similarly to colloidal dispersions, in that their transport was controlled by the fluid flow velocity and physicochemical interactions among particles, the porous medium, and the fluid (Tosco et al., 2012
Tosco, T., Bosch, J., Meckenstock, R.U., Sethi, R. (2012) Transport of Ferrihydrite Nanoparticles in Saturated Porous Media: Role of Ionic Strength and Flow Rate. Environmental Science and Technology 46, 4008–4015.
).top
Transformation of Particles
Transported hematite particles appear to have been transformed into larger hematite crystals (Figs. 2, 3a,d,e). Previous studies suggested that this transformation occurred by dehydration of ferrihydrite (Posth et al., 2013
Posth, N.R., Konhauser, K., Kappler, A. (2013) Simulating Precambrian banded iron formation diagenesis. Chemical Geology 362, 66–73.
) or dissolution–precipitation (Ostwald ripening) (Sun et al., 2015Sun, S., Konhauser, K.O., Kappler, A., Li, Y. (2015) Primary hematite in Neoarchean to Paleoproterozoic oceans. Geological Society of America Bulletin 127, 850–861.
). In these cases, larger crystals grow at the expense of smaller crystals by dissolution of the particles within a suitable fluid followed by precipitation of the dissolved ions on an existing nucleus, which eventually leads to the formation of relatively smooth, defect-free crystals (Cölfen and Antonietti, 2008Cölfen, H., Antonietti, M. (2008) Mesocrystals and Nonclassical Crystallization. John Wiley and Sons, Chichester.
). Alternatively, we propose that the large hematite crystals formed predominantly by attachment of previously encapsulated particles (Figs. 1 to 3).The self-assembly of particles and their consequent transformation to larger crystals is a common mechanism of non-classical crystallisation (Penn and Banfield, 1998
Penn, R.L., Banfield, J.F. (1998) Imperfect Oriented Attachment: Dislocation Generation in Defect-Free Nanocrystals. Science 281, 969–971.
). This behaviour is common to iron oxide particles and has found wide applications in environmental science, material science, and nanotechnology (Guo and Barnard, 2013Guo, H., Barnard, A.S. (2013) Naturally occurring iron oxide nanoparticles: morphology, surface chemistry and environmental stability. Journal of Materials Chemistry A 1, 27–42.
), but has not yet been applied to BIFs. Penn and Banfield (1998)Penn, R.L., Banfield, J.F. (1998) Imperfect Oriented Attachment: Dislocation Generation in Defect-Free Nanocrystals. Science 281, 969–971.
found small misorientations across the interfaces of larger crystals formed by particle attachment, which cannot be explained by classical crystallisation via dissolution–precipitation reactions. Such dislocations have been previously identified using transmission electron microscopy (TEM) within goethite (Morris, 1985Morris, R.C. (1985) Genesis of iron ore in banded iron-formation by supergene and supergene-metamorphic processes - a conceptual model. In: Wolf, K.H. (Ed.) Handbook of Strata-Bound and Stratiform Ore Deposits. Elsevier, Amsterdam, 73–235.
) and hematite crystals (e.g., Sun et al., 2015Sun, S., Konhauser, K.O., Kappler, A., Li, Y. (2015) Primary hematite in Neoarchean to Paleoproterozoic oceans. Geological Society of America Bulletin 127, 850–861.
) in BIFs (images reproduced in Figs. S-3 to S-5). However, these features have not previously been recognised for what they are: textural evidence that non-classical crystallisation plays a role in forming microbands in BIF. Crystal growth by particle aggregation also forms anhedral to euhedral crystals, mineral twinning, defects, and nano- to micro-porosity (Guo and Barnard, 2013Guo, H., Barnard, A.S. (2013) Naturally occurring iron oxide nanoparticles: morphology, surface chemistry and environmental stability. Journal of Materials Chemistry A 1, 27–42.
) of the sort observed in our samples (Fig. 3d).This solid-state process of non-classical crystallisation can be facilitated by internal rearrangements, dehydration, reduction/oxidation, and topotactic transformations until the components (e.g., hematite particles) are exhausted (Cölfen and Antonietti, 2008
Cölfen, H., Antonietti, M. (2008) Mesocrystals and Nonclassical Crystallization. John Wiley and Sons, Chichester.
; Faivre, 2016Faivre, D. (2016) Iron oxides - from nature to applications. Wiley-VCH, Weinheim.
). However, the transformation pathway is sensitive to the environmental conditions (e.g., T, pH, ionic strength) and the precursor material, both of which control the mineralogy and morphology of the final crystals (Guo and Barnard, 2013Guo, H., Barnard, A.S. (2013) Naturally occurring iron oxide nanoparticles: morphology, surface chemistry and environmental stability. Journal of Materials Chemistry A 1, 27–42.
). In principle, non-classical crystallisation can be combined with classical crystallisation mechanisms (Faivre, 2016Faivre, D. (2016) Iron oxides - from nature to applications. Wiley-VCH, Weinheim.
), creating complex pathways for formation of the common hematite and magnetite end products seen in BIF.top
Implications for BIF Formation
The initial precipitation of BIF precursor minerals in the ancient oceans was likely controlled by environmental changes in the depositional environment (Bekker et al., 2010
Bekker, A., Slack, J.F., Planavsky, N., Krapež, B., Hofmann, A., Konhauser, K.O., Rouxel, O.J. (2010) Iron Formation: The Sedimentary Product of a Complex Interplay among Mantle, Tectonic, Oceanic, and Biospheric Processes. Economic Geology 105, 467–508.
). Depending on ambient conditions, iron-bearing precipitates would have remained in suspension until they flocculated and deposited on the seafloor forming thin beds (Fig. 4a1) concomitant or alternating with amorphous silica deposition (Posth et al., 2008Posth, N.R., Hegler, F., Konhauser, K.O., Kappler, A. (2008) Alternating Si and Fe deposition caused by temperature fluctuations in Precambrian oceans. Nature Geoscience 1, 703–708.
). Flocculation could have involved particle aggregation of the sort observed in previous studies (Morris, 1985Morris, R.C. (1985) Genesis of iron ore in banded iron-formation by supergene and supergene-metamorphic processes - a conceptual model. In: Wolf, K.H. (Ed.) Handbook of Strata-Bound and Stratiform Ore Deposits. Elsevier, Amsterdam, 73–235.
; Sun et al., 2015Sun, S., Konhauser, K.O., Kappler, A., Li, Y. (2015) Primary hematite in Neoarchean to Paleoproterozoic oceans. Geological Society of America Bulletin 127, 850–861.
) (Fig. 4a1). Alternatively, iron-bearing precipitates may have initially bonded to silica prior to their deposition (Fig. 4a2) (Fischer and Knoll, 2009Fischer, W.W., Knoll, A.H. (2009) An iron shuttle for deepwater silica in Late Archean and early Paleoproterozoic iron formation. Geological Society of America Bulletin 121, 222–235.
). Both phases could have separated again after deposition to form amorphous silica and iron-bearing colloidal solids, which agglomerated into larger particles (Fig. 4a2). Both routes are not mutually exclusive and the unconsolidated sediments would have been susceptible to reworking by density currents or other sedimentary processes.Subsequent compaction would have led to dehydration of amorphous silica to form microcrystalline quartz, resulting in chert layers containing variable amounts of iron-rich particles (Fig. 4b). Because DPC is enhanced by impurities, diagenesis and deformation likely promoted preferential DPC within chert layers with higher mineral inclusion content (Fig. 4b,c) (Greene et al., 2009
Greene, G.W., Kristiansen, K., Meyer, E.E., Boles, J.R., Israelachvili, J.N. (2009) Role of electrochemical reactions in pressure solution. Geochimica et Cosmochimica Acta 73, 2862–2874.
), creating a banding effect. The release of particles from the chert layers during DPC would have led to residual agglomeration of particles or transport by fluids within pore networks and their concentration into chert and iron oxide microbands (Figs. 1a, 2, 4c). In combination, these processes are expected to obscure primary depositional textures.Aggregation of particles by progressive particle attachment can account for the formation of bands dominated by larger hematite or magnetite crystals (Fig. 4d). Remobilised quartz could have escaped the system by diffusion, or have formed new diagenetic quartz bands such as those observed in our study (Figs. 4c–e, S-2), which are virtually particle-free and possess strong crystallographic preferred orientations, characteristic of quartz formed by DPC (Egglseder et al., 2016
Egglseder, M.S., Cruden, A.R., Tomkins, A.G., Wilson, C.J.L. (2016) Deformation-induced silica redistribution in banded iron formation, Hamersley Province, Australia. Lithos 266–267, 87–97.
).Ongoing segregation led to the enhancement of microbands, possibly including removal of thin chert bands to form iron oxide mesobands (Fig. 1a) (Trendall and Blockley, 1970
Trendall, A.F., Blockley, J.G. (1970) The iron formations of the Precambrian Hamersley Group, Western Australia: Geological Survey of Western Australia Bulletin 119. Geological Survey of Western Australia, Perth.
). Although our observations do not exclude the possibility that mesobands formed by primary deposition, we suggest that the processes of particle liberation and non-classical crystallisation enhanced primary layering because initial variations in iron oxide particle concentration within microcrystalline quartz control the post-depositional segregation process. Hence, chemical signals of the Precambrian oceans and Earth’s early biosphere deduced from microbands and possibly mesobands of BIFs have likely been obscured by diagenetic processes; thus, new studies are necessary that integrate physical and chemical post-depositional phenomena to better constrain their effects on geochemical records preserved by BIFs.
Figure 4 Conceptual evolution from BIF precursor sediments to BIF microbands. (a1, a2). Schematic Precambrian depositional environment of BIF precursor minerals. The inset shows the aggregation of precipitates/colloidal phases to form larger particles by flocculation in the water column (a1) and/or attachment in the sediment prior to lithification (a2). (b–e) Development of BIF textures after lithification. Insets show the microscale to nanoscale formation of minerals and textures. (b) Quartz crystallisation (cementation) leads to the entrapment of randomly dispersed colloidal phases. (c) Progressive compression leads to quartz dissolution by DPC, liberation of encapsulated particles and residual layer-parallel accumulation. Dissolved silica forms new particle-free quartz layers (i.e. diagenetic quartz). (d) Simultaneously, accumulated particles transform into more stable iron oxides resulting in the common BIF texture of pristine chert, diagenetic quartz and iron oxide microbands (e).
top
Acknowledgements
We are grateful to Hilke Dalstra (RioTinto Exploration Ltd), Leigh Nicholas and Jim Gordon and their teams (RioTinto Iron Ore Ltd) for their abundant and helpful support. M.S.E. and A.R.C. also acknowledge Monash University for scholarship and research support, respectively. M.S.E. is grateful to Andrea Rielli and Marianne Richter (both Monash University) for assistance with SEM imaging. We thank Nathan Webster, CSIRO Mineral Processing, for his generous help with XRD data collection. We are grateful to Paul Bons, Ariel Anbar and an anonymous reviewer for their helpful comments.
Editor: Ariel Anbar
top
References
Bekker, A., Slack, J.F., Planavsky, N., Krapež, B., Hofmann, A., Konhauser, K.O., Rouxel, O.J. (2010) Iron Formation: The Sedimentary Product of a Complex Interplay among Mantle, Tectonic, Oceanic, and Biospheric Processes. Economic Geology 105, 467–508.
 Show in context
Show in context The majority of models assume that iron-rich micro- and mesobands represent primary layering related to deposition of precursor iron minerals (e.g., ferrihydrite) produced by secular changes in bacterial activity (Posth et al., 2008), chemical precipitation (Garrels, 1987), hydrothermal or volcanic influx (Bekker et al., 2010), and/or re-sedimentation (Krapež et al., 2003).
View in article
The critical problem for all of these interpretations lies in establishing whether the observed mineral assemblages are of diagenetic origin and thus whether or not they can be directly related to palaeoenvironmental conditions (Bekker et al., 2010).
View in article
The initial precipitation of BIF precursor minerals in the ancient oceans was likely controlled by environmental changes in the depositional environment (Bekker et al., 2010).
View in article
Bons, P.D., den Brok, B. (2000) Crystallographic preferred orientation development by dissolution–precipitation creep. Journal of Structural Geology 22, 1713–1722.
 Show in context
Show in context DPC is induced by stress applied to a fluid-bearing polymineralic aggregate where quartz dissolves in fluid at grain boundaries and aqueous silica is subsequently transported by diffusion to precipitate in areas of lower concentration (Bons and den Brok, 2000).
View in article
Cölfen, H., Antonietti, M. (2008) Mesocrystals and Nonclassical Crystallization. John Wiley and Sons, Chichester.
 Show in context
Show in contextIn these cases, larger crystals grow at the expense of smaller crystals by dissolution of the particles within a suitable fluid followed by precipitation of the dissolved ions on an existing nucleus, which eventually leads to the formation of relatively smooth, defect-free crystals (Cölfen and Antonietti, 2008).
View in article
This solid-state process of non-classical crystallisation can be facilitated by internal rearrangements, dehydration, reduction/oxidation, and topotactic transformations until the components (e.g., hematite particles) are exhausted (Cölfen and Antonietti, 2008; Faivre, 2016).
View in article
Cornell, R.M., Schwertmann, U. (2003) The iron oxides - structure, properties, reactions, occurences and uses. Wiley-VCH, Weinheim.
 Show in context
Show in context The extensive colour variation likely corresponds to differences in size and morphology of hematite particles, shifting from the characteristic red colour of macroscopic crystals to orange for sub-µm-sized particles (Cornell and Schwertmann, 2003) (Fig. 1d).
View in article
Egglseder, M.S., Cruden, A.R., Tomkins, A.G., Wilson, C.J.L. (2016) Deformation-induced silica redistribution in banded iron formation, Hamersley Province, Australia. Lithos 266–267, 87–97.
 Show in context
Show in context The importance of dissolution–precipitation creep (DPC, or pressure solution) during deformation of BIF has recently been revealed through the observation of variably developed crystallographic preferred orientations within alternating chert bands (Egglseder et al., 2016).
View in article
This process ultimately leads to intercalation of strongly oriented secondary quartz and more pristine chert layers that lack preferred orientation (Egglseder et al., 2016) (Fig. S-2).
View in article
Remobilised quartz could have escaped the system by diffusion, or have formed new diagenetic quartz bands such as those observed in our study (Figs. 4c–e, S-2), which are virtually particle-free and possess strong crystallographic preferred orientations, characteristic of quartz formed by DPC (Egglseder et al., 2016).
View in article
Faivre, D. (2016) Iron oxides - from nature to applications. Wiley-VCH, Weinheim.
 Show in context
Show in context This solid-state process of non-classical crystallisation can be facilitated by internal rearrangements, dehydration, reduction/oxidation, and topotactic transformations until the components (e.g., hematite particles) are exhausted (Cölfen and Antonietti, 2008; Faivre, 2016).
View in article
In principle, non-classical crystallisation can be combined with classical crystallisation mechanisms (Faivre, 2016), creating complex pathways for formation of the common hematite and magnetite end products seen in BIF.
View in article
Fischer, W.W., Knoll, A.H. (2009) An iron shuttle for deepwater silica in Late Archean and early Paleoproterozoic iron formation. Geological Society of America Bulletin 121, 222–235.
 Show in context
Show in contextAlthough it is widely acknowledged that chert layers form from amorphous silica of abiotic origin (Posth et al., 2008), it is unknown whether they form from silica deposited alongside ferric oxide precipitates (Fischer and Knoll, 2009), by seafloor silicification during depositional hiatuses (Rasmussen et al., 2015a), or below the seafloor during compaction (Krapež et al., 2003).
View in article
Alternatively, iron-bearing precipitates may have initially bonded to silica prior to their deposition (Fig. 4a2) (Fischer and Knoll, 2009).
View in article
Garrels, R.M. (1987) A model for the deposition of the microbanded Precambrian iron formations. American Journal of Science 287, 81–106.
 Show in context
Show in context The majority of models assume that iron-rich micro- and mesobands represent primary layering related to deposition of precursor iron minerals (e.g., ferrihydrite) produced by secular changes in bacterial activity (Posth et al., 2008), chemical precipitation (Garrels, 1987), hydrothermal or volcanic influx (Bekker et al., 2010), and/or re-sedimentation (Krapež et al., 2003).
View in article
Greene, G.W., Kristiansen, K., Meyer, E.E., Boles, J.R., Israelachvili, J.N. (2009) Role of electrochemical reactions in pressure solution. Geochimica et Cosmochimica Acta 73, 2862–2874.
 Show in context
Show in contextDPC can commence at pressures as low as 2–3 bar and it is influenced by electrochemical potential differences between dissimilar minerals such as mineral inclusions (colloidal phases) within quartz (Greene et al., 2009).
View in article
Because DPC is enhanced by impurities, diagenesis and deformation likely promoted preferential DPC within chert layers with higher mineral inclusion content (Fig. 4b,c) (Greene et al., 2009), creating a banding effect.
View in article
Guo, H., Barnard, A.S. (2013) Naturally occurring iron oxide nanoparticles: morphology, surface chemistry and environmental stability. Journal of Materials Chemistry A 1, 27–42.
 Show in context
Show in contextThis behaviour is common to iron oxide particles and has found wide applications in environmental science, material science, and nanotechnology (Guo and Barnard, 2013), but has not yet been applied to BIFs.
View in article
Crystal growth by particle aggregation also forms anhedral to euhedral crystals, mineral twinning, defects, and nano- to micro-porosity (Guo and Barnard, 2013) of the sort observed in our samples (Fig. 3d).
View in article
However, the transformation pathway is sensitive to the environmental conditions (e.g., T, pH, ionic strength) and the precursor material, both of which control the mineralogy and morphology of the final crystals (Guo and Barnard, 2013).
View in article
Krapež, B., Barley, M.E., Pickard, A.L. (2003) Hydrothermal and resedimented origins of the precursor sediments to banded iron formation: sedimentological evidence from the Early Palaeoproterozoic Brockman Supersequence of Western Australia. Sedimentology 50, 979–1011.
 Show in context
Show in context The majority of models assume that iron-rich micro- and mesobands represent primary layering related to deposition of precursor iron minerals (e.g., ferrihydrite) produced by secular changes in bacterial activity (Posth et al., 2008), chemical precipitation (Garrels, 1987), hydrothermal or volcanic influx (Bekker et al., 2010), and/or re-sedimentation (Krapež et al., 2003).
View in article
Although it is widely acknowledged that chert layers form from amorphous silica of abiotic origin (Posth et al., 2008), it is unknown whether they form from silica deposited alongside ferric oxide precipitates (Fischer and Knoll, 2009), by seafloor silicification during depositional hiatuses (Rasmussen et al., 2015a), or below the seafloor during compaction (Krapež et al., 2003).
View in article
Morris, R.C. (1985) Genesis of iron ore in banded iron-formation by supergene and supergene-metamorphic processes - a conceptual model. In: Wolf, K.H. (Ed.) Handbook of Strata-Bound and Stratiform Ore Deposits. Elsevier, Amsterdam, 73–235.
 Show in context
Show in contextSuch dislocations have been previously identified using transmission electron microscopy (TEM) within goethite (Morris, 1985) and hematite crystals (e.g., Sun et al., 2015) in BIFs (images reproduced in Figs. S-3 to S-6).
View in article
Flocculation could have involved particle aggregation of the sort observed in previous studies (Morris, 1985; Sun et al., 2015) (Fig. 4a1).
View in article
Pecoits, E., Gingras, M.K., Barley, M.E., Kappler, A., Posth, N.R., Konhauser, K.O. (2009) Petrography and geochemistry of the Dales Gorge banded iron formation: Paragenetic sequence, source and implications for palaeo-ocean chemistry. Precambrian Research 172, 163–187.
 Show in context
Show in context Alternative models interpret the banded texture of BIF as a secondary differentiation product generated during burial metamorphism by ion diffusion through a homogeneous mass of silica gel (Pecoits et al., 2009), or by differential compaction (Trendall and Blockley, 1970).
View in article
Penn, R.L., Banfield, J.F. (1998) Imperfect Oriented Attachment: Dislocation Generation in Defect-Free Nanocrystals. Science 281, 969–971.
 Show in context
Show in context The self-assembly of particles and their consequent transformation to larger crystals is a common mechanism of non-classical crystallisation (Penn and Banfield, 1998).
View in article
Penn and Banfield (1998) found small misorientations across the interfaces of larger crystals formed by particle attachment, which cannot be explained by classical crystallisation via dissolution–precipitation reactions.
View in article
Posth, N.R., Hegler, F., Konhauser, K.O., Kappler, A. (2008) Alternating Si and Fe deposition caused by temperature fluctuations in Precambrian oceans. Nature Geoscience 1, 703–708.
 Show in context
Show in context The majority of models assume that iron-rich micro- and mesobands represent primary layering related to deposition of precursor iron minerals (e.g., ferrihydrite) produced by secular changes in bacterial activity (Posth et al., 2008), chemical precipitation (Garrels, 1987), hydrothermal or volcanic influx (Bekker et al., 2010), and/or re-sedimentation (Krapež et al., 2003).
View in article
Although it is widely acknowledged that chert layers form from amorphous silica of abiotic origin (Posth et al., 2008), it is unknown whether they form from silica deposited alongside ferric oxide precipitates (Fischer and Knoll, 2009), by seafloor silicification during depositional hiatuses (Rasmussen et al., 2015a), or below the seafloor during compaction (Krapež et al., 2003).
View in article
Depending on ambient conditions, iron-bearing precipitates would have remained in suspension until they flocculated and deposited on the seafloor forming thin beds (Fig. 4a1) concomitant or alternating with amorphous silica deposition (Posth et al., 2008).
View in article
Posth, N.R., Konhauser, K., Kappler, A. (2013) Simulating Precambrian banded iron formation diagenesis. Chemical Geology 362, 66–73.
 Show in context
Show in context Although laboratory experiments have demonstrated that diagenetic recrystallisation of ferrihydrite to iron oxides can occur (Posth et al., 2013), no study has yet comprehensively described the role of deformation in controlling the evolution of (micro-) banding in BIFs.
View in article
Previous studies suggested that this transformation occurred by dehydration of ferrihydrite (Posth et al., 2013) or dissolution–precipitation (Ostwald ripening) (Sun et al., 2015).
View in article
Rasmussen, B., Krapež, B., Muhling, J.R. (2015a) Seafloor silicification and hardground development during deposition of 2.5 Ga banded iron formations. Geology 43, 235–238.
 Show in context
Show in context Although it is widely acknowledged that chert layers form from amorphous silica of abiotic origin (Posth et al., 2008), it is unknown whether they form from silica deposited alongside ferric oxide precipitates (Fischer and Knoll, 2009), by seafloor silicification during depositional hiatuses (Rasmussen et al., 2015a), or below the seafloor during compaction (Krapež et al., 2003).
View in article
Rasmussen, B., Krapež, B., Muhling, J.R., Suvorova, A. (2015b) Precipitation of iron silicate nanoparticles in early Precambrian oceans marks Earth’s first iron age. Geology 43, 303–306.
 Show in context
Show in contextNew nanoscale studies on ’dusty’ chert bands interpreted the presence of abundant colloidal hematite as a product of early sedimentary ferrihydrite (Sun et al., 2015; Sun and Li, 2017), or post-depositional oxidation of primary iron silicates (Rasmussen et al., 2015b, 2016) (The term ‘colloid’ refers to a phase that has a least one dimension in the nanometre range, which is dispersed in another phase of different composition or state; i.e. here solid hematite particles dispersed in solid quartz grains).
View in article
Rasmussen, B., Muhling, J.R., Suvorova, A., Krapez, B. (2016) Dust to dust: Evidence for the formation of “primary” hematite dust in banded iron formations via oxidation of iron silicate nanoparticles. Precambrian Research 284, 49–63.
 Show in context
Show in contextNew nanoscale studies on ’dusty’ chert bands interpreted the presence of abundant colloidal hematite as a product of early sedimentary ferrihydrite (Sun et al., 2015; Sun and Li, 2017), or post-depositional oxidation of primary iron silicates (Rasmussen et al., 2015b, 2016) (The term ‘colloid’ refers to a phase that has a least one dimension in the nanometre range, which is dispersed in another phase of different composition or state; i.e. here solid hematite particles dispersed in solid quartz grains).
View in article
Smith, R.E., Perdrix, J.L., Parks, T.C. (1982) Burial metamorphism in the Hamersley basin, Western Australia. Journal of Petrology 23, 75–102.
 Show in context
Show in contextSamples were collected from BIF outcrops of the ~2.48 Ga Dales Gorge Member, NW Australia, from the central part of the Hamersley Province (Turner Syncline), which has been affected by maximum lower greenschist facies metamorphic conditions (Smith et al., 1982; Fig. S-1).
View in article
Sun, S., Li, Y.-L. (2017) Geneses and evolutions of iron-bearing minerals in banded iron formations of >3760 to ca. 2200 million-year-old: Constraints from electron microscopic, X-ray diffraction and Mössbauer spectroscopic investigations. Precambrian Research 289, 1–17.
 Show in context
Show in contextNew nanoscale studies on ’dusty’ chert bands interpreted the presence of abundant colloidal hematite as a product of early sedimentary ferrihydrite (Sun et al., 2015; Sun and Li, 2017), or post-depositional oxidation of primary iron silicates (Rasmussen et al., 2015b, 2016) (The term ‘colloid’ refers to a phase that has a least one dimension in the nanometre range, which is dispersed in another phase of different composition or state; i.e. here solid hematite particles dispersed in solid quartz grains).
View in article
Sun, S., Konhauser, K.O., Kappler, A., Li, Y. (2015) Primary hematite in Neoarchean to Paleoproterozoic oceans. Geological Society of America Bulletin 127, 850–861.
 Show in context
Show in context New nanoscale studies on ’dusty’ chert bands interpreted the presence of abundant colloidal hematite as a product of early sedimentary ferrihydrite (Sun et al., 2015; Sun and Li, 2017), or post-depositional oxidation of primary iron silicates (Rasmussen et al., 2015b, 2016) (The term ‘colloid’ refers to a phase that has a least one dimension in the nanometre range, which is dispersed in another phase of different composition or state; i.e. here solid hematite particles dispersed in solid quartz grains).
View in article
Previous studies suggested that this transformation occurred by dehydration of ferrihydrite (Posth et al., 2013) or dissolution–precipitation (Ostwald ripening) (Sun et al., 2015).
View in article
Such dislocations have been previously identified using transmission electron microscopy (TEM) within goethite (Morris, 1985) and hematite crystals (e.g., Sun et al., 2015) in BIFs (images reproduced in Figs. S-3 to S-6).
View in article
Flocculation could have involved particle aggregation of the sort observed in previous studies (Morris, 1985; Sun et al., 2015) (Fig. 4a1).
View in article
Tosco, T., Bosch, J., Meckenstock, R.U., Sethi, R. (2012) Transport of Ferrihydrite Nanoparticles in Saturated Porous Media: Role of Ionic Strength and Flow Rate. Environmental Science and Technology 46, 4008–4015.
 Show in context
Show in context We suggest that the liberated hematite particles in our samples behaved similarly to colloidal dispersions, in that their transport was controlled by the fluid flow velocity and physicochemical interactions among particles, the porous medium, and the fluid (Tosco et al., 2012).
View in article
Trendall, A.F., Blockley, J.G. (1970) The iron formations of the Precambrian Hamersley Group, Western Australia: Geological Survey of Western Australia Bulletin 119. Geological Survey of Western Australia, Perth.
 Show in context
Show in context Shale horizons separate individual BIF macrobands, which are subdivided into meso- and microbands dominated by iron oxide minerals (e.g., magnetite, hematite), iron silicates and iron carbonates, alternating with bands dominated by chert (i.e. microcrystalline quartz) (Trendall and Blockley, 1970).
View in article
Alternative models interpret the banded texture of BIF as a secondary differentiation product generated during burial metamorphism by ion diffusion through a homogeneous mass of silica gel (Pecoits et al., 2009), or by differential compaction (Trendall and Blockley, 1970).
View in article
Ongoing segregation led to the enhancement of microbands, possibly including removal of thin chert bands to form iron oxide mesobands (Fig. 1a) (Trendall and Blockley, 1970).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Geological Overview
- Methods
- Table S-1
- XRD Patterns
- Figures S-1 to S-5
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
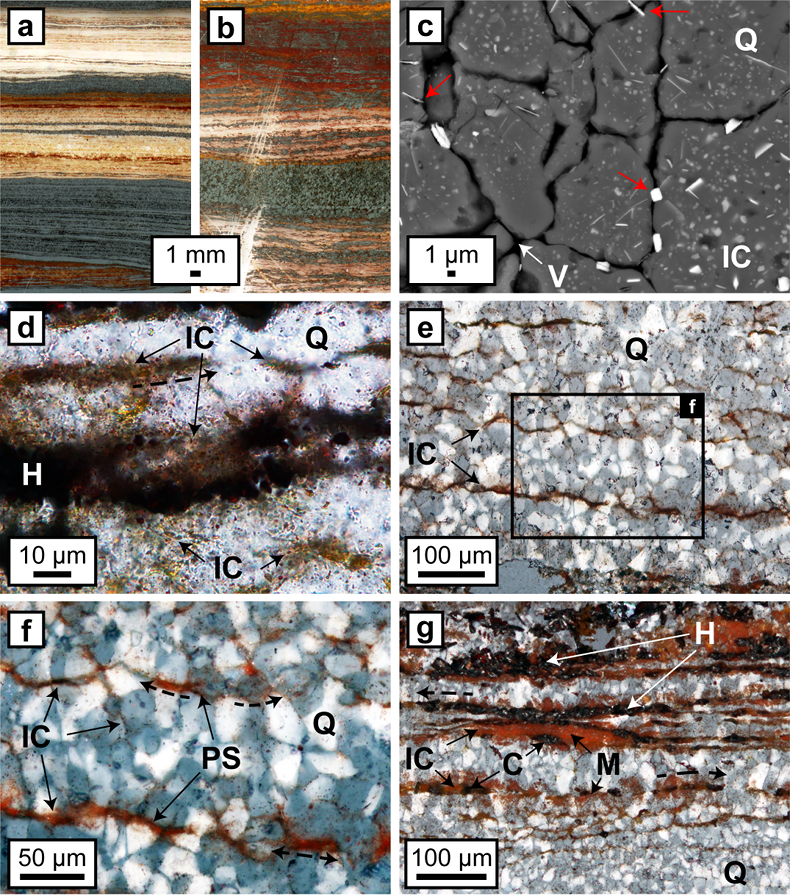
Figure 1 (a, b) Transmitted light images (TL) of microbands of chert/quartz (white), chert with colloidal hematite (red, brown) and hematite bands (blue grey). (c) BSE image with colloidal hematite ‘floating’ in quartz crystals, partly liberated (red arrows) and free hematite particles at grain boundaries. (d) TL and (e, f) reflected light images (XPL) displaying irregular layers of hematite particles at truncated quartz crystals (PS) and along quartz grain boundaries with lateral transitions into equigranular quartz aggregates (dashed arrows). (g) XPL image showing hematite particles that surround layered hematite forming core/mantle textures. C: core, H: hematite, M: mantle, IC: iron oxide colloids/particles, PS: pressure solution, Q: quartz, V: void.
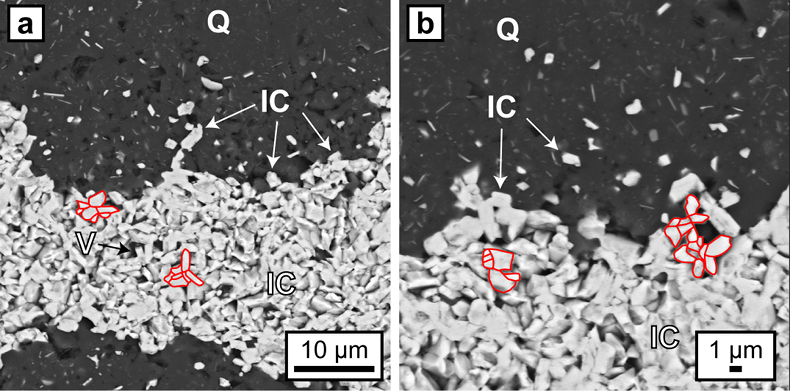
Figure 2 (a, b) BSE images of hematite particles along truncated quartz crystals forming dense aggregates (red outlines) with intergranular pores. Encapsulated colloidal hematite in chert and hematite particles in microbands show similar grain sizes and morphologies. IC: iron oxide colloids/particles, Q: quartz, V: void.
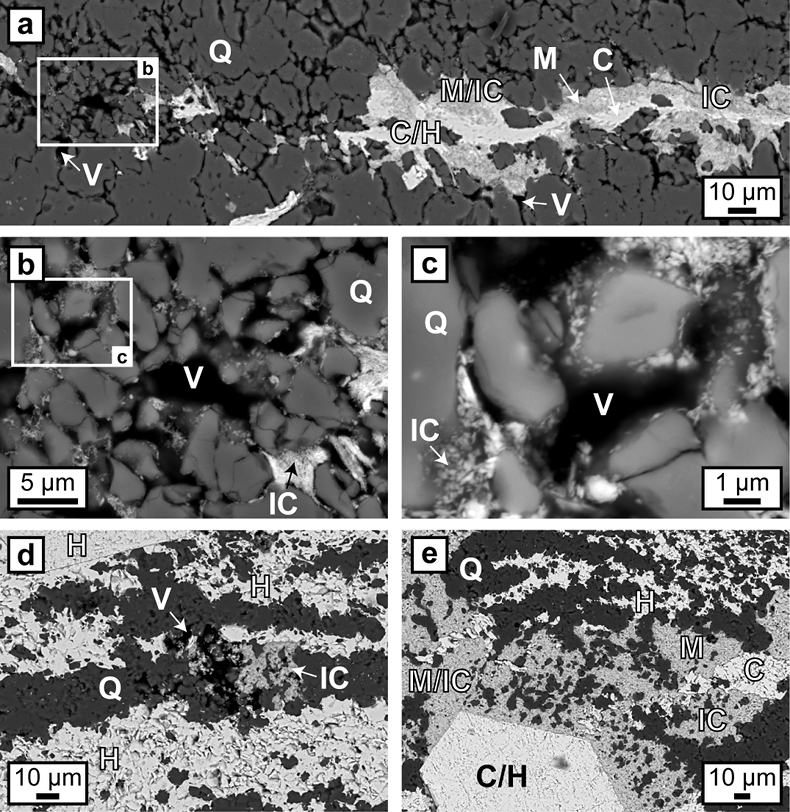
Figure 3 (a–e) BSE images of BIF laminae illustrating the lateral change from porous chert to hematite microbands with irregular boundaries. (a–c) The porosity is (partly) filled with hematite particles encapsulating quartz grains. (d–e) Hematite forms more larger crystals inside these particle agglomerations, as evident by core/mantle structures resulting in new microbands of hematite. C: core, H: hematite, M: mantle, IC: iron oxide colloids/particles, Q: quartz, V: void.

Figure 4 Conceptual evolution from BIF precursor sediments to BIF microbands. (a1, a2). Schematic Precambrian depositional environment of BIF precursor minerals. The inset shows the aggregation of precipitates/colloidal phases to form larger particles by flocculation in the water column (a1) and/or attachment in the sediment prior to lithification (a2). (b–e) Development of BIF textures after lithification. Insets show the microscale to nanoscale formation of minerals and textures. (b) Quartz crystallisation (cementation) leads to the entrapment of randomly dispersed colloidal phases. (c) Progressive compression leads to quartz dissolution by DPC, liberation of encapsulated particles and residual layer-parallel accumulation. Dissolved silica forms new particle-free quartz layers (i.e. diagenetic quartz). (d) Simultaneously, accumulated particles transform into more stable iron oxides resulting in the common BIF texture of pristine chert, diagenetic quartz and iron oxide microbands (e).