Not so non-marine? Revisiting the Stoer Group and the Mesoproterozoic biosphere
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:12,413Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract

Figures and Tables
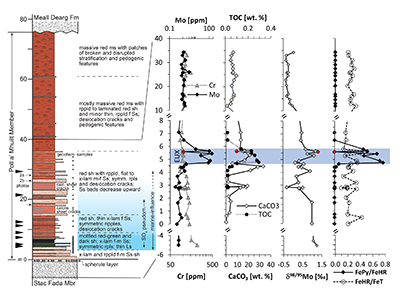 Figure 1 Stratigraphy of the Poll a’Mhuilt Member. EUX = euxinic interval. The horizon at 5.6 m (red symbols) was affected by modern oxidative weathering and is not considered in the discussion. Black arrows = locations of tidal indicators. |  Figure 2 Sedimentary features compatible with a marine setting in the middle Poll a’Mhuilt Member. (a) Flaser- and lenticular-bedding. (b) Superposed sets of herringbone cross-lamination, 3D exposures confirm bi-directional character. Stratigraphic positions are marked in Figure 1. (c) Line drawing of sedimentary features showing superposed sets of bi-polar cross-laminated ripples (herringbone) with multiple reactivation surfaces commonly with thin clay drapes. | 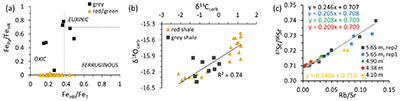 Figure 3 (a) Iron speciation, (b) carbonate C and O isotopes, and (c) carbonate-bound Sr isotopes. Dashed lines in (a) mark redox transitions (Poulton and Canfield, 2011). For comparison to our data in panel (b), values of contemporaneous unaltered marine carbonates fall between -10 ‰ and -7 ‰ for δ18Ocarb and 0 ‰ and +2 ‰ for δ13Ccarb (Shields and Veizer, 2002) (see Fig. S-2 for discussion). In panel (c), data points represent individual leaches increasing acid strength; y-axis intercept = carbonate end-member. |
| Figure 1 | Figure 2 | Figure 3 |
Supplementary Figures and Tables
 Table S-1 Organic carbon isotopes and abundances. TOC = total organic carbon, RE = relative error, SD = standard deviation. |  Table S-2 Carbonate carbon and oxygen isotopes and carbonate abundances (carb.). SD = standard deviation, carb = carbonate content by weight, RE = relative error. | 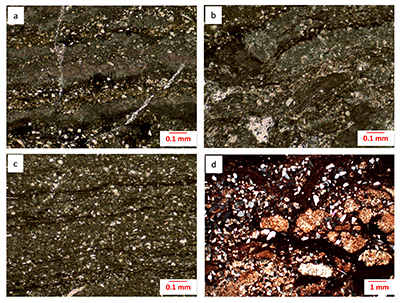 Figure S-1 Photomicrographs of the Poll a’Mhuilt Member. (a) Sample +2.77 m, plane-polarised light; calcareous red shale with 18 % CaCO3 present as microcrystalline laminae, separated by iron-oxide coated siliciclastics. (b) Sample +4.42 m, plane-polarised light; calcareous red shale with 33 % CaCO3 present as disrupted microcrystalline laminae and rare sparry fenestral fillings. (c) Sample +5.30 m, plane-polarised light; calcareous grey shale with 21 % CaCO3 present as microcrystalline laminae, separated by kerogenous siliciclastics. (d) Sample +25.75 m, crossed polars; partially desiccated facies with 6 % CaCO3 present as microcrystalline nodules and cement, stained with iron oxide, separated by iron-oxide coated siliciclastics. All samples contain angular silt grains composed of quartz, plagioclase, and K-feldspar and minor mica. | 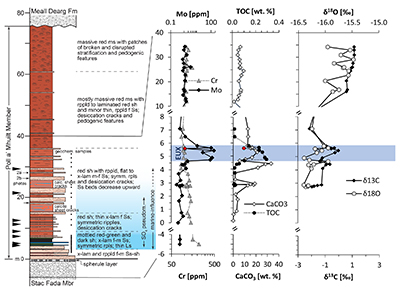 Figure S-2 Stratigraphic section through the Poll a’Mhuilt Member with carbonate C and O isotopes. Lithostratigraphy, Mo, Cr, CaCO3 and TOC abundances are as in Figure 1 in the main text. The last panel shows δ18O and δ13C in carbonate in stratigraphic context. The trend highlights that the heaviest values in both isotopic proxies occur in the upper red shale. This is consistent with the high abundance of desiccation cracks in this part of the section, because evaporation forces these parameters to heavier values. The grey shale, which would have been most continuously flooded, shows relatively light values, consistent with a relatively lesser impact of evaporation. Despite the high degree of evaporation that is implied by the presence of gypsum pseudomorphs in the section (Stewart, 2002; Parnell et al., 2010) the δ18O values are overall light compared to other mid-Proterozoic carbonates of similar age (Shields and Veizer, 2002; Bartley and Kah, 2004), which we attribute to fluid alteration. Oxygen isotopes are much more easily reset than carbon isotopes. However, the overall trend towards heavier values in the most evaporitic part of the section is preserved. |  Table S-3 Bulk elemental abundances. The grey shale unit extends from +4.75 m to +5.8 m; the sample from +5.6 m is likely altered by modern weathering. Samples below 0 m are from the Stac Fada Member and contain volcanic fragments. Abundances are in weight-percent or parts per million (μg/g). |  Table S-4 Iron speciation. FeCarb = carbonate-bound iron, FeOx = ferric oxide-bound iron, FeMag = magnetite-bound iron, FePy = pyrite-bound iron. FeHR = highly reactive iron, which is the sum of all four phases. FeT = total iron from Table S-5. FePy was not determined in most of the red shale facies and assumed to be zero as in the few examples. * = Carbonate content was calculated from the concentration of Ca in the acetic acid extract, assuming a CaCO3 stoichiometry. Note that this calculation is an upper estimate, because it does not account for partial dissolution of silicates, which may become important below a carbonate content of 5 %, as suggested by the low molar Ca/Mg ratios in those samples. |
| Table S-1 | Table S-2 | Figure S-1 | Figure S-2 | Table S-3 | Table S-4 |
 Table S-5 Molybdenum isotopes of bulk rocks, relative to NIST3134 = +0.25 ‰. Samples with reported standard deviations (SD) were prepared and analysed in replicates. |  Table S-6 Dissolution method for carbonate bound Sr analysis. |  Table S-7 Elemental abundances and Sr isotopes of carbonate leaches. N1-N2 = ammonium acetate washing steps, S1-S8 = acetic acid extractions with increasing acid strength. Concentrations are relative to bulk rock. |  Table S-8 Strontium isotope results of basement rocks and impact/volcanic debris layer. 87Sr/86Sr ratios at 1.2 Ga were calculated as described in Section S1.7. |
| Table S-5 | Table S-6 | Table S-7 | Table S-8 |
top
Introduction
Important steps in early biotic evolution may have occurred in lakes that offered distinct environmental conditions compared to the ocean. Support for this hypothesis has been reported from the Mesoproterozoic Poll a’Mhuilt Member (Stoer Group) in Scotland, which is interpreted as a fluvio-lacustrine deposit (Stewart, 2002
Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath, UK.
). Parnell et al. (2010Parnell, J., Boyce, A.J., Mark, D., Bowden, S., Spinks, S. (2010) Early oxygenation of the terrestrial environment during the Mesoproterozoic. Nature 468, 290–293.
; 2015Parnell, J., Spinks, S., Andrews, S., Thayalan, W., Bowden, S. (2015) High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nature Communications 6, doi:10.1038/ncomms7996.
) documented large S isotope fractionations (up to 55 ‰) and Mo concentrations reaching 232 ppm that far exceed those of most contemporaneous marine shales. These features were interpreted as an indication that Mesoproterozoic lacustrine environments were more oxygenated and nutrient-rich than seawater, making them preferable habitats for eukaryotic organisms. However, the supposition that the Poll a’Mhuilt Member was deposited in a lacustrine setting rests on contestable lines of evidence: fluvial sandstones bracketing the proposed lacustrine interval and allegedly high boron concentrations in illite, which were regarded as ambiguous in the original study (Stewart and Parker, 1979Stewart, A.D., Parker, A. (1979) Palaeosalinity and environmental interpretation of red beds from the late Precambrian (‘Torridonian’) of Scotland. Sedimentary Geology 22, 229–241.
; Stewart, 2002Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath, UK.
). Here we present new geochemical data and sedimentological features that indicate a marine influence, particularly during deposition of the Mo- and S-rich interval.top
Geologic Setting
The mostly siliciclastic Stoer Group rests nonconformably on Archaean gneiss in northwest Scotland (Stewart, 1988
Stewart, A.D. (1988) The Stoer Group, Scotland. In: Winchester, J.A. (Ed.) Later Proterozoic stratigraphy of the North Atlantic regions. Blackie, Glasgow, 97–103.
). The depositional age is constrained to 1177 ± 5 Ma based on 40Ar–39Ar dating on diagenetic K-feldspar in the Stac Fada Member, an ancient impact deposit (Parnell et al., 2011Parnell, J., Mark, D., Fallick, A.E., Boyce, A., Thackrey, S. (2011) The age of the Mesoproterozoic Stoer Group sedimentary and impact deposits, NW Scotland. Journal of the Geological Society 168, 349–358.
; Reddy et al., 2015Reddy, S.M., Johnson, T.E., Fischer, S., Rickard, W.D.A., Taylor, R.J.M. (2015) Precambrian reidite discovered in shocked zircon from the Stac Fada impactite, Scotland. Geology 43, 899–902.
) immediately beneath the Poll a’Mhuilt Member (Fig. 1).
Figure 1 Stratigraphy of the Poll a’Mhuilt Member. EUX = euxinic interval. The horizon at 5.6 m (red symbols) was affected by modern oxidative weathering and is not considered in the discussion. Black arrows = locations of tidal indicators.
The basal ~3 m of the Poll a’ Mhuilt Member consist of channeled, trough cross-bedded and planar laminated sandstone overlain by about 1–2 m of mottled grey-red shale with thin limestone beds followed up section by 2–4 m of calcareous dark grey shale. The limestone beds contain small-scale, chicken-wire fabric (calcite and albite pseudomorphs replacing gypsum). The carbonate is mostly micro-crystalline (Fig. S-1); secondary calcite replacements are minor. The next ~25 m consist of red shale and thin sandstone with abundant desiccation cracks and flat-laminated to ripple cross-laminated, 5–50 cm-thick beds of fine to medium sandstone which have abundant symmetrical (wave) ripples and locally developed herringbone cross-lamination (Fig. 2b), as well as flaser and lenticular bedding (Fig. 2a) and evaporite pseudomorphs after gypsum (Parnell et al. 2010
Parnell, J., Boyce, A.J., Mark, D., Bowden, S., Spinks, S. (2010) Early oxygenation of the terrestrial environment during the Mesoproterozoic. Nature 468, 290–293.
). The overlying (and major) part of the Poll a’ Mhuilt Member (>~30 m) comprises massive red mudstone and flat-laminated to ripple cross-laminated fine sandstone and siltstone, all with desiccation cracks and pedogenic structures, such as disrupted and homogenised beds and pseudo-anticlines (Stewart, 2002Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath, UK.
).
Figure 2 Sedimentary features compatible with a marine setting in the middle Poll a’Mhuilt Member. (a) Flaser- and lenticular-bedding. (b) Superposed sets of herringbone cross-lamination, 3D exposures confirm bi-directional character. Stratigraphic positions are marked in Figure 1. (c) Line drawing of sedimentary features showing superposed sets of bi-polar cross-laminated ripples (herringbone) with multiple reactivation surfaces commonly with thin clay drapes.
top
Methods
We collected outcrop samples extending from the upper 5 m of the Stac Fada Member through 35 m of the Poll a’Mhuilt Member, with emphasis on the calcareous grey shale (4.75 to 5.80 m, Fig. 1) (58.202422°N, 5.340425°W). Our analytical methods follow standard protocols as described in the Supplementary Information, with the exception of our carbonate-Sr extraction. As silicate phases can release Sr during acid-dissolution, we extracted carbonate-bound Sr with a ten-step sequential leaching procedure (modified after Liu et al., 2013
Liu, C., Wang, Z., Raub, T.D. (2013) Geochemical constraints on the origin of Marinoan cap dolostones from Nuccaleena Formation, South Australia. Chemical Geology 351, 95–104.
). This approach allowed us to construct a mixing curve between carbonate and silicate phases, where the latter can be monitored with Rb. The pure carbonate end-member was calculated by extrapolation to a Rb/Sr ratio of zero. Bedrock samples were analysed for Sr isotopes after bulk digestions and back-calculated to 1.2 Ga using measured Rb/Sr ratios and the 87Sr←87Rb decay constant to account for 87Rb decay.top
Results
Similar to Parnell et al. (2015)
Parnell, J., Spinks, S., Andrews, S., Thayalan, W., Bowden, S. (2015) High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nature Communications 6, doi:10.1038/ncomms7996.
, we found high Mo concentrations of up to 166 ppm in the grey shale of the Poll a’Mhuilt Member (4.75–5.80 m), which is 180 times higher than in the surrounding red shales (Fig. 1). Other transition metals show weak to no enrichments in the grey shale (Table S-3). Nickel and Cr are elevated in the volcanic/impact breccia of the Stac Fada Member and then decrease slowly into the Poll a’Mhuilt Member.Ratios of highly reactive Fe (FeHR; bound in oxides, sulphides and carbonates) to total Fe (FeT) fall between 0.15 and 0.49 in the grey shale unit, while ratios of pyrite-bound Fe (FePy) to FeHR range from 0.46 to 0.78 (Fig. 3a). Carbon to sulphur ratios range from 0.1 to 1.2 (median 0.2) in the grey shale. Molybdenum isotope data show positive values in the Stac Fada Member (+1.15 ‰), then progressively decrease in the first red shale (~0 m to 4 m), followed up section by an increase to a maximum of +1.19 ‰ in the grey shale (5.65 m, Fig. 1). In the overlying red shale (12 to 33 m), δ98Mo drops to -0.38 ‰. Carbonate δ18O and δ13C values throughout the section covary (Fig. 3b). Carbonate-bound 87Sr/86Sr ratios in the red and grey shale (4.10–5.65 m) trend toward end-members of 0.707–0.710 (Fig. 3c). Two gneiss samples from the Lewisian basement, back-calculated to 1.2 Ga, have 87Sr/86Sr ratios of 0.721 ± 0.008; two amphibolite dyke samples and two Stac Fada samples average around 0.704 ± 0.002 and 0.706 ± 0.0004, respectively.

Figure 3 (a) Iron speciation, (b) carbonate C and O isotopes, and (c) carbonate-bound Sr isotopes. Dashed lines in (a) mark redox transitions (Poulton and Canfield, 2011
Poulton, S.W., Canfield, D.E. (2011) Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7, 107–112.
). For comparison to our data in panel (b), values of contemporaneous unaltered marine carbonates fall between -10 ‰ and -7 ‰ for δ18Ocarb and 0 ‰ and +2 ‰ for δ13Ccarb (Shields and Veizer, 2002Shields, G., Veizer, J. (2002) Precambrian marine carbonate isotope database: Version 1.1. Geochemistry Geophysics Geosystems 3, doi: 10.1029/2001GC000266.
) (see Fig. S-2 for discussion). In panel (c), data points represent individual leaches increasing acid strength; y-axis intercept = carbonate end-member.top
Discussion
Some sedimentary features in the Poll a’Mhuilt Member provide unequivocal evidence for a largely subaerial depositional setting: the basal (<3 m) channelised and trough cross-bedded sandstones and unimodal palaeocurrent indicators imply fluvial deposition (Stewart, 2002
Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath, UK.
) and the abundant pedogenic features in the upper part (30 m) of the member indicate deeply palaeo-weathered alluvium (Stewart, 2002Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath, UK.
). However, within the 3–30 m interval that contains the calcareous and grey shale we observed flaser, pin-stripe and lenticular bedding; multiple reactivation surfaces; mud drapes and herringbone cross-lamination (Fig. 1). These features are strong evidence of tidally influenced sedimentation on marine tidal flats (Davis Jr., 2012Davis Jr., R.A. (2012) Tidal signatures and their preservation potential in stratigraphic sequences. In: Davis Jr., R.A., Dalrymple, R.W.(Eds.) Principles of Tidal Sedimentology. Springer, Netherlands, 35–55.
). Closely interfingered marine and non-marine deposition is not uncommon in the rock record. For example, recent discoveries of tidal indicators in the Ordovician Juniata Formation raised doubts about some of the oldest purported evidence for land colonisation by animal life (Davies et al., 2010Davies, N.S., Rygel, M.C., Gibling, M.R. (2010) Marine influence in the Upper Ordovician Juniata Formation (Potters Mills, Pennsylvania): implications for the history of life on land. Palaios 25, 527–539.
). Our sedimentological observations raise similar concerns for the eukaryotic biota of the Stoer Group (Cloud and Germs, 1971Cloud, P., Germs, A. (1971) New pre-paleozoic nannofossils from the Stoer formation (Torridonian), Northwest Scotland. Geological Society of America Bulletin 82, 3469–3474.
). This view is supported by our geochemical data, which are most parsimoniously explained by a marine influence during the deposition of the middle Poll a’Mhuilt Member (~3–30 m).Carbonate-bound 87Sr/86Sr ratios capture the isotope composition of the water column in which the carbonate precipitated. As typical continental runoff is more radiogenic (87Sr-enriched) than seawater, 87Sr/86Sr values can distinguish between marine and non-marine environments (Veizer et al., 1990
Veizer, J., Clayton, R.N., Hinton, R.W., Von Brunn, V., Mason, T.R., Buck, S.G., Hoefs, J. (1990) Geochemistry of Precambrian carbonates: 3-shelf seas and non-marine environments of the Archean. Geochimica et Cosmochimica Acta 54, 2717–2729.
; Spencer and Patchett, 1997Spencer, J.E., Patchett, P.J. (1997) Sr isotope evidence for a lacustrine origin for the upper Miocene to Pliocene Bouse Formation, lower Colorado River trough, and implications for timing of Colorado Plateau uplift. Geological Society of America Bulletin 109, 767–778.
). However, infiltration of secondary fluids during early or late diagenesis typically increases carbonate 87Sr/86Sr ratios (Banner and Hanson, 1990Banner, J.L., Hanson, G.N. (1990) Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochimica et Cosmochimica Acta 54, 3123–3137.
). Covariation and low values of δ18Ocarb and δ13Ccarb, as seen in our samples, may indicate some degree of alteration by continental fluids (Fig. 3c) (Shields and Veizer, 2002Shields, G., Veizer, J. (2002) Precambrian marine carbonate isotope database: Version 1.1. Geochemistry Geophysics Geosystems 3, doi: 10.1029/2001GC000266.
; Bartley and Kah, 2004Bartley, J.K., Kah, L.C. (2004) Marine carbon reservoir, Corg-Ccarb coupling, and the evolution of the Proterozoic carbon cycle. Geology 32, 129–132.
). However, alteration almost always leads to more radiogenic carbonate 87Sr/86Sr ratios (Banner and Hanson, 1990Banner, J.L., Hanson, G.N. (1990) Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochimica et Cosmochimica Acta 54, 3123–3137.
). Diagenetic fluids were likely sourced from the surrounding land surface and should have reflected the composition of the Lewisian tonalite-trondjemite-granodiorite gneiss (0.740 ± 0.033; Lyon et al., 1975Lyon, T.D.B., Gillen, C., Bowes, D.R. (1975) Rb-Sr isotopic studies near the major Precambrian junction, between Scourie and Loch Laxford, northwest Scotland. Scottish Journal of Geology 11, 333–337.
; this study; and see Supplementary Information for discussion). Therefore, the 87Sr/86Sr ratio of our least radiogenic carbonate end-member (0.707, Fig. 3b), directly from within the sulphide- and Mo-rich interval (Parnell et al., 2015Parnell, J., Spinks, S., Andrews, S., Thayalan, W., Bowden, S. (2015) High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nature Communications 6, doi:10.1038/ncomms7996.
), provides a maximum constraint for the primary 87Sr/86Sr ratio of the water body from which the carbonate precipitated. This value is too low to reflect exclusively continental runoff from the Lewisian basement (>0.715), which should dominate the signal in a lacustrine setting. Instead, this value is better explained by mixing between fluvial and marine waters. The latter have an estimated composition of 0.705–0.706 at 1.2 Ga (Kuznetsov et al., 2014Kuznetsov, A.B., Semikhatov, M.A., Gorokhov, I.M. (2014) The Sr isotope chemostratigraphy as a tool for solving stratigraphic problems of the Upper Proterozoic (Riphean and Vendian). Stratigraphy and Geological Correlation 22, 553–575.
).Repetitive influxes of seawater, followed by evaporation, would favour the precipitation of gypsum as recorded by pseudomorphs in the middle Poll a’Mhuilt Member (3–30 m, Fig. 1). As previously proposed (Parnell et al., 2010
Parnell, J., Boyce, A.J., Mark, D., Bowden, S., Spinks, S. (2010) Early oxygenation of the terrestrial environment during the Mesoproterozoic. Nature 468, 290–293.
, 2015Parnell, J., Spinks, S., Andrews, S., Thayalan, W., Bowden, S. (2015) High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nature Communications 6, doi:10.1038/ncomms7996.
), a combination of proxies—including large S isotope fractionations consistent with pyrite formation in the water column, high Mo/Re ratios and large amounts of pyrite despite low TOC contents (low C/S ratios)—suggest that the water column turned euxinic (sulphidic) during the evaporitic phase, perhaps as a result of salinity stratification and cut-off from seawater inflow. This pattern is supported by the Fe chemistry (see Supplementary Information for detailed discussion). Briefly, in the grey shale, FeHR/FeT ratios at the upper end of the detrital threshold (Raiswell and Canfield, 1998Raiswell, R., Canfield, D.E. (1998) Sources of iron for pyrite formation in marine sediments. American Journal of Science 298, 219–245.
, also inferred from red shales in our study) are consistent with some iron enrichment under anoxic conditions, and FePy/FeHR ratios of up to 0.8 are consistent with euxinia (Poulton and Canfield, 2011Poulton, S.W., Canfield, D.E. (2011) Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7, 107–112.
). This interpretation is bolstered by the observed high Mo levels that are almost always associated with at least intermittent euxinia in the modern and ancient ocean (Scott and Lyons, 2012Scott, C., Lyons, T.W. (2012) Contrasting molybdenum cycling and isotopic properties in euxinic versus non-euxinic sediments and sedimentary rocks: refining the paleoproxies. Chemical Geology 324, 19–27.
). The red shales lack FeHR/FeT enrichments (Fig. 3a), consistent with oxic deposition at water depths probably shallower than those for the grey shale (Stewart, 2002Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath, UK.
).Although seawater probably had low Mo levels at this time (e.g., 1–10 nM, Reinhard et al., 2013
Reinhard, C.T., Planavsky, N.J., Robbins, L.J., Partin, C.A., Gill, B.C., Lalonde, S.V., Bekker, A., Konhauser, K.O., Lyons, T.W. (2013) Proterozoic ocean redox and biogeochemical stasis. Proceedings of the National Academy of Sciences, 110, 5357–5362.
), the presence of gypsum pseudomorphs implies that the water in this setting evaporated by a factor of up to 11 (assuming 100 % Mesoproterozoic seawater with modern levels of dissolved Ca2+ and ≤ 2–10 mM SO42-; Kah et al., 2004Kah, L.C., Lyons, T.W., Frank, T.D. (2004) Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature 431, 834–838.
; Luo et al., 2014Luo, G., Ono, S., Huang, J., Algeo, T.J., Li, C., Zhou, L., Robinson, A., Lyons, T.W., Xie, S. (2014) Decline in oceanic sulfate levels during the early Mesoproterozoic. Precambrian Research, 258, 36-47.
), which could have locally raised dissolved Mo concentrations (perhaps to near-modern levels of 105 nM). Ensuing euxinia would have pulled this concentrated Mo reservoir into sediments. Repeated seawater incursions, evapo-concentration and euxinia could have acted like a Mo pump, sustaining these sedimentary Mo enrichments.The Mo isotope data are consistent with a marine influence. The δ98Mo of seawater can be effectively captured in sediments when dissolved sulphide levels in the water column are high (Neubert et al., 2008
Neubert, N., Nägler, T.F., Böttcher, M.E. (2008) Sulfidity controls molybdenum isotope fractionation into euxinic sediments: Evidence from the modern Black Sea. Geology 36, 775–778.
). Processes that cause sedimentary archives to deviate from capturing dissolved δ98Mo consistently favour the light isotopes (Siebert et al., 2006Siebert, C., McManus, J., Bice, A., Poulson, R., Berelson, W.M. (2006) Molybdenum isotope signatures in continental margin marine sediments. Earth and Planetary Science Letters 241, 723–733.
). Our maximum value of +1.19 ‰ therefore provides a minimum constraint for the composition of dissolved Mo. This result agrees with previous estimates for seawater from mid-Proterozoic basins (+1.0 ‰ to +1.3 ‰, Kendall et al., 2015Kendall, B., Komiya, T., Lyons, T.W., Bates, S.M., Gordon, G.W., Romaniello, S.J., Jiang, G., Creaser, R.A., Xiao, S., McFadden, K., Sawaki, Y., Tahata, M., Shu, D., Han, J., Li, Y., Chu, X., Anbar, A.D. (2015) Uranium and molybdenum isotope evidence for an episode of widespread ocean oxygenation during the late Ediacaran Period. Geochimica et Cosmochimica Acta 156, 173–193.
). We discount a non-marine interpretation because such heavy δ98Mo values are only known from catchments marked by weathering of pyrite- or sulphate-rich rock (Neubert et al., 2011Neubert, N., Heri, A.R., Voegelin, A.R., Nägler, T.F., Schlunegger, F., Villa, I.M. (2011) The molybdenum isotopic composition in river water: constraints from small catchments. Earth and Planetary Science Letters 304, 180–190.
), which was not the case here. Further, although the Stac Fada Member is isotopically heavy (+1.15 ‰), it cannot be a major Mo source to the Poll a’Mhuilt Member because the up-section decline in Cr concentrations (Fig. 1) indicates a steady decrease in the proportion of material reworked from the Stac Fada into the Poll a’Mhuilt. Lighter δ98Mo values in the remainder of the succession likely resulted from either partial Mo remobilisation under oxic conditions (Kowalski et al., 2013Kowalski, N., Dellwig, O., Beck, M., Gräwe, U., Neubert, N., Nägler, T.F., Badewien, T.H., Brumsack, H.J., van Beusekom, J.E., Böttcher, M.E. (2013) Pelagic molybdenum concentration anomalies and the impact of sediment resuspension on the molybdenum budget in two tidal systems of the North Sea. Geochimica et Cosmochimica Acta 119, 198–211.
) or adsorption of isotopically light MoO42- onto Fe-oxides (Goldberg et al., 2009Goldberg, T., Archer, C., Vance, D., Poulton, S.W. (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr) oxides. Geochimica et Cosmochimica Acta 73, 6502–6516.
).top
Conclusion
The combined geochemical data and sedimentary features characterising the middle Poll a’Mhuilt Member are most parsimoniously interpreted as recording a marine influence on deposition, which calls into question previous inferences that purely non-marine lakes offered particularly favourable conditions for eukaryotic organisms in the Mesoproterozoic (Parnell et al., 2010
Parnell, J., Boyce, A.J., Mark, D., Bowden, S., Spinks, S. (2010) Early oxygenation of the terrestrial environment during the Mesoproterozoic. Nature 468, 290–293.
, 2015Parnell, J., Spinks, S., Andrews, S., Thayalan, W., Bowden, S. (2015) High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nature Communications 6, doi:10.1038/ncomms7996.
). A high bar should be set for arguments favouring non-marine settings in palaeobiological studies because such an assertion carries profound implications for physiological and biochemical characteristics of early life, as well as for its evolutionary history in marine settings. In the light of our data, the importance of non-marine environments in the expansion of eukaryotic life remains unknown.top
Acknowledgements
Funding for this project was provided by the NASA postdoctoral program (EES), the Lewis and Clark Fund (EES), an NSERC PGS-D grant (EJB), the NSF ELT (TWL, NJP) and FESD (TWL) programs, and the NASA Astrobiology Institute (TWL, NJP). We thank Bleuenn Gueguen, Steve Bates and Andy Robinson for technical assistance.
Editor: Liane G. Benning
top
References
Banner, J.L., Hanson, G.N. (1990) Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochimica et Cosmochimica Acta 54, 3123–3137.
 Show in context
Show in context However, infiltration of secondary fluids during early or late diagenesis typically increases carbonate 87Sr/86Sr ratios (Banner and Hanson, 1990).
View in article
However, alteration almost always leads to more radiogenic carbonate 87Sr/86Sr ratios (Banner and Hanson, 1990).
View in article
Bartley, J.K., Kah, L.C. (2004) Marine carbon reservoir, Corg-Ccarb coupling, and the evolution of the Proterozoic carbon cycle. Geology 32, 129–132.
 Show in context
Show in context Covariation and low values of δ18Ocarb and δ13Ccarb, as seen in our samples, may indicate some degree of alteration by continental fluids (Fig. 3c) (Shields and Veizer, 2002; Bartley and Kah, 2004).
View in article
Cloud, P., Germs, A. (1971) New pre-paleozoic nannofossils from the Stoer formation (Torridonian), Northwest Scotland. Geological Society of America Bulletin 82, 3469–3474.
 Show in context
Show in context Our sedimentological observations raise similar concerns for the eukaryotic biota of the Stoer Group (Cloud and Germs, 1971).
View in article
Davies, N.S., Rygel, M.C., Gibling, M.R. (2010) Marine influence in the Upper Ordovician Juniata Formation (Potters Mills, Pennsylvania): implications for the history of life on land. Palaios 25, 527–539.
 Show in context
Show in context For example, recent discoveries of tidal indicators in the Ordovician Juniata Formation raised doubts about some of the oldest purported evidence for land colonisation by animal life (Davies et al., 2010).
View in article
Davis Jr., R.A. (2012) Tidal signatures and their preservation potential in stratigraphic sequences. In: Davis Jr., R.A., Dalrymple, R.W.(Eds.) Principles of Tidal Sedimentology. Springer, Netherlands, 35–55.
 Show in context
Show in context These features are strong evidence of tidally influenced sedimentation on marine tidal flats (Davis Jr., 2012).
View in article
Goldberg, T., Archer, C., Vance, D., Poulton, S.W. (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr) oxides. Geochimica et Cosmochimica Acta 73, 6502–6516.
 Show in context
Show in context Lighter δ98Mo values in the remainder of the succession likely resulted from either partial Mo remobilisation under oxic conditions (Kowalski et al., 2013) or adsorption of isotopically light MoO42- onto Fe-oxides (Goldberg et al., 2009).
View in article
Kah, L.C., Lyons, T.W., Frank, T.D. (2004) Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature 431, 834–838.
 Show in context
Show in context Although seawater probably had low Mo levels at this time (e.g., 1–10 nM, Reinhard et al., 2013), the presence of gypsum pseudomorphs implies that the water in this setting evaporated by a factor of up to 11 (assuming 100 % Mesoproterozoic seawater with modern levels of dissolved Ca2+ and ≤ 2–10 mM SO42-; Kah et al., 2004; Luo et al., 2014), which could have locally raised dissolved Mo concentrations (perhaps to near-modern levels of 105 nM).
View in article
Kendall, B., Komiya, T., Lyons, T.W., Bates, S.M., Gordon, G.W., Romaniello, S.J., Jiang, G., Creaser, R.A., Xiao, S., McFadden, K., Sawaki, Y., Tahata, M., Shu, D., Han, J., Li, Y., Chu, X., Anbar, A.D. (2015) Uranium and molybdenum isotope evidence for an episode of widespread ocean oxygenation during the late Ediacaran Period. Geochimica et Cosmochimica Acta 156, 173–193.
 Show in context
Show in context This result agrees with previous estimates for seawater from mid-Proterozoic basins (+1.0 ‰ to +1.3 ‰, Kendall et al., 2015).
View in article
Kowalski, N., Dellwig, O., Beck, M., Gräwe, U., Neubert, N., Nägler, T.F., Badewien, T.H., Brumsack, H.J., van Beusekom, J.E., Böttcher, M.E. (2013) Pelagic molybdenum concentration anomalies and the impact of sediment resuspension on the molybdenum budget in two tidal systems of the North Sea. Geochimica et Cosmochimica Acta 119, 198–211.
 Show in context
Show in context Lighter δ98Mo values in the remainder of the succession likely resulted from either partial Mo remobilisation under oxic conditions (Kowalski et al., 2013) or adsorption of isotopically light MoO42- onto Fe-oxides (Goldberg et al., 2009).
View in article
Kuznetsov, A.B., Semikhatov, M.A., Gorokhov, I.M. (2014) The Sr isotope chemostratigraphy as a tool for solving stratigraphic problems of the Upper Proterozoic (Riphean and Vendian). Stratigraphy and Geological Correlation 22, 553–575.
 Show in context
Show in context The latter have an estimated composition of 0.705–0.706 at 1.2 Ga (Kuznetsov et al., 2014).
View in article
Liu, C., Wang, Z., Raub, T.D. (2013) Geochemical constraints on the origin of Marinoan cap dolostones from Nuccaleena Formation, South Australia. Chemical Geology 351, 95–104.
 Show in context
Show in context As silicate phases can release Sr during acid-dissolution, we extracted carbonate-bound Sr with a ten-step sequential leaching procedure (modified after Liu et al., 2013).
View in article
Luo, G., Ono, S., Huang, J., Algeo, T.J., Li, C., Zhou, L., Robinson, A., Lyons, T.W., Xie, S. (2014) Decline in oceanic sulfate levels during the early Mesoproterozoic. Precambrian Research, 258, 36-47.
 Show in context
Show in context Although seawater probably had low Mo levels at this time (e.g., 1–10 nM, Reinhard et al., 2013), the presence of gypsum pseudomorphs implies that the water in this setting evaporated by a factor of up to 11 (assuming 100 % Mesoproterozoic seawater with modern levels of dissolved Ca2+ and ≤ 2–10 mM SO42-; Kah et al., 2004; Luo et al., 2014), which could have locally raised dissolved Mo concentrations (perhaps to near-modern levels of 105 nM).
View in article
Lyon, T.D.B., Gillen, C., Bowes, D.R. (1975) Rb-Sr isotopic studies near the major Precambrian junction, between Scourie and Loch Laxford, northwest Scotland. Scottish Journal of Geology 11, 333–337.
 Show in context
Show in context Diagenetic fluids were likely sourced from the surrounding land surface and should have reflected the composition of the Lewisian tonalite-trondjemite-granodiorite gneiss (0.740 ± 0.033; Lyon et al., 1975; this study; and see Supplementary Information for discussion).
View in article
Neubert, N., Heri, A.R., Voegelin, A.R., Nägler, T.F., Schlunegger, F., Villa, I.M. (2011) The molybdenum isotopic composition in river water: constraints from small catchments. Earth and Planetary Science Letters 304, 180–190.
 Show in context
Show in context We discount a non-marine interpretation because such heavy δ98Mo values are only known from catchments marked by weathering of pyrite- or sulphate-rich rock (Neubert et al., 2011), which was not the case here.
View in article
Neubert, N., Nägler, T.F., Böttcher, M.E. (2008) Sulfidity controls molybdenum isotope fractionation into euxinic sediments: Evidence from the modern Black Sea. Geology 36, 775–778.
 Show in context
Show in context The δ98Mo of seawater can be effectively captured in sediments when dissolved sulphide levels in the water column are high (Neubert et al., 2008).
View in article
Parnell, J., Boyce, A.J., Mark, D., Bowden, S., Spinks, S. (2010) Early oxygenation of the terrestrial environment during the Mesoproterozoic. Nature 468, 290–293.
 Show in context
Show in context Parnell et al. (2010; 2015) documented large S isotope fractionations (up to 55 ‰) and Mo concentrations reaching 232 ppm that far exceed those of most contemporaneous marine shales.
View in article
The next ~25 m consist of red shale and thin sandstone with abundant desiccation cracks and flat-laminated to ripple cross-laminated, 5–50 cm-thick beds of fine to medium sandstone which have abundant symmetrical (wave) ripples and locally developed herringbone cross-lamination (Fig. 2b), as well as flaser and lenticular bedding (Fig. 2a) and evaporite pseudomorphs after gypsum (Parnell et al., 2010).
View in article
As previously proposed (Parnell et al., 2010, 2015), a combination of proxies—including large S isotope fractionations consistent with pyrite formation in the water column, high Mo/Re ratios and large amounts of pyrite despite low TOC contents (low C/S ratios)—suggest that the water column turned euxinic (sulphidic) during the evaporitic phase, perhaps as a result of salinity stratification and cut-off from seawater inflow.
View in article
The combined geochemical data and sedimentary features characterising the middle Poll a’Mhuilt Member are most parsimoniously interpreted as recording a marine influence on deposition, which calls into question previous inferences that purely non-marine lakes offered particularly favourable conditions for eukaryotic organisms in the Mesoproterozoic (Parnell et al., 2010, 2015).
View in article
Parnell, J., Mark, D., Fallick, A.E., Boyce, A., Thackrey, S. (2011) The age of the Mesoproterozoic Stoer Group sedimentary and impact deposits, NW Scotland. Journal of the Geological Society 168, 349–358.
 Show in context
Show in context The depositional age is constrained to 1177 ± 5 Ma based on 40Ar–39Ar dating on diagenetic K-feldspar in the Stac Fada Member, an ancient impact deposit (Parnell et al., 2011; Reddy et al., 2015) immediately beneath the Poll a’Mhuilt Member (Fig. 1).
View in article
Parnell, J., Spinks, S., Andrews, S., Thayalan, W., Bowden, S. (2015) High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nature Communications 6, doi:10.1038/ncomms7996.
 Show in context
Show in context Parnell et al. (2010; 2015) documented large S isotope fractionations (up to 55 ‰) and Mo concentrations reaching 232 ppm that far exceed those of most contemporaneous marine shales.
View in article
Similar to Parnell et al. (2015), we found high Mo concentrations of up to 166 ppm in the grey shale of the Poll a’Mhuilt Member (4.75–5.80 m), which is 180 times higher than in the surrounding red shales (Fig. 1).
View in article
Therefore, the 87Sr/86Sr ratio of our least radiogenic carbonate end-member (0.707, Fig. 3b), directly from within the sulphide- and Mo-rich interval (Parnell et al., 2015), provides a maximum constraint for the primary 87Sr/86Sr ratio of the water body from which the carbonate precipitated.
View in article
As previously proposed (Parnell et al., 2010, 2015), a combination of proxies—including large S isotope fractionations consistent with pyrite formation in the water column, high Mo/Re ratios and large amounts of pyrite despite low TOC contents (low C/S ratios)—suggest that the water column turned euxinic (sulphidic) during the evaporitic phase, perhaps as a result of salinity stratification and cut-off from seawater inflow.
View in article
The combined geochemical data and sedimentary features characterising the middle Poll a’Mhuilt Member are most parsimoniously interpreted as recording a marine influence on deposition, which calls into question previous inferences that purely non-marine lakes offered particularly favourable conditions for eukaryotic organisms in the Mesoproterozoic (Parnell et al., 2010, 2015).
View in article
Poulton, S.W., Canfield, D.E. (2011) Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7, 107–112.
 Show in context
Show in context Figure 3 [...] Dashed lines in (a) mark redox transitions (Poulton and Canfield, 2011).
View in article
Briefly, in the grey shale, FeHR/FeT ratios at the upper end of the detrital threshold (Raiswell and Canfield, 1998, also inferred from red shales in our study) are consistent with some iron enrichment under anoxic conditions, and FePy/FeHR ratios of up to 0.8 are consistent with euxinia (Poulton and Canfield, 2011).
View in article
Raiswell, R., Canfield, D.E. (1998) Sources of iron for pyrite formation in marine sediments. American Journal of Science 298, 219–245.
 Show in context
Show in context Briefly, in the grey shale, FeHR/FeT ratios at the upper end of the detrital threshold (Raiswell and Canfield, 1998, also inferred from red shales in our study) are consistent with some iron enrichment under anoxic conditions, and FePy/FeHR ratios of up to 0.8 are consistent with euxinia (Poulton and Canfield, 2011).
View in article
Reddy, S.M., Johnson, T.E., Fischer, S., Rickard, W.D.A., Taylor, R.J.M. (2015) Precambrian reidite discovered in shocked zircon from the Stac Fada impactite, Scotland. Geology 43, 899–902.
 Show in context
Show in context The depositional age is constrained to 1177 ± 5 Ma based on 40Ar–39Ar dating on diagenetic K-feldspar in the Stac Fada Member, an ancient impact deposit (Parnell et al., 2011; Reddy et al., 2015) immediately beneath the Poll a’Mhuilt Member (Fig. 1).
View in article
Reinhard, C.T., Planavsky, N.J., Robbins, L.J., Partin, C.A., Gill, B.C., Lalonde, S.V., Bekker, A., Konhauser, K.O., Lyons, T.W. (2013) Proterozoic ocean redox and biogeochemical stasis. Proceedings of the National Academy of Sciences, 110, 5357–5362.
 Show in context
Show in context Although seawater probably had low Mo levels at this time (e.g., 1–10 nM, Reinhard et al., 2013), the presence of gypsum pseudomorphs implies that the water in this setting evaporated by a factor of up to 11 (assuming 100 % Mesoproterozoic seawater with modern levels of dissolved Ca2+ and ≤ 2–10 mM SO42-; Kah et al., 2004; Luo et al., 2014), which could have locally raised dissolved Mo concentrations (perhaps to near-modern levels of 105 nM).
View in article
Scott, C., Lyons, T.W. (2012) Contrasting molybdenum cycling and isotopic properties in euxinic versus non-euxinic sediments and sedimentary rocks: refining the paleoproxies. Chemical Geology 324, 19–27.
 Show in context
Show in context This interpretation is bolstered by the observed high Mo levels that are almost always associated with at least intermittent euxinia in the modern and ancient ocean (Scott and Lyons, 2012).
View in article
Shields, G., Veizer, J. (2002) Precambrian marine carbonate isotope database: Version 1.1. Geochemistry Geophysics Geosystems 3, doi: 10.1029/2001GC000266.
 Show in context
Show in context For comparison to our data in panel (b), values of contemporaneous unaltered marine carbonates fall between -10 ‰ and -7 ‰ for δ18Ocarb and 0 ‰ and +2 ‰ for δ13Ccarb (Shields and Veizer, 2002) (see Fig. S-2 for discussion)
View in article
Covariation and low values of δ18Ocarb and δ13Ccarb, as seen in our samples, may indicate some degree of alteration by continental fluids (Fig. 3c) (Shields and Veizer, 2002; Bartley and Kah, 2004).
View in article
Siebert, C., McManus, J., Bice, A., Poulson, R., Berelson, W.M. (2006) Molybdenum isotope signatures in continental margin marine sediments. Earth and Planetary Science Letters 241, 723–733.
 Show in context
Show in context Processes that cause sedimentary archives to deviate from capturing dissolved δ98Mo consistently favour the light isotopes (Siebert et al., 2006).
View in article
Spencer, J.E., Patchett, P.J. (1997) Sr isotope evidence for a lacustrine origin for the upper Miocene to Pliocene Bouse Formation, lower Colorado River trough, and implications for timing of Colorado Plateau uplift. Geological Society of America Bulletin 109, 767–778.
 Show in context
Show in context As typical continental runoff is more radiogenic (87Sr-enriched) than seawater, 87Sr/86Sr values can distinguish between marine and non-marine environments (Veizer et al., 1990; Spencer and Patchett, 1997).
View in article
Stewart, A.D. (1988) The Stoer Group, Scotland. In: Winchester, J.A. (Ed.) Later Proterozoic stratigraphy of the North Atlantic regions. Blackie, Glasgow, 97–103.
 Show in context
Show in context The mostly siliciclastic Stoer Group rests nonconformably on Archaean gneiss in northwest Scotland (Stewart, 1988).
View in article
Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath, UK.
 Show in context
Show in context Support for this hypothesis has been reported from the Mesoproterozoic Poll a’Mhuilt Member (Stoer Group) in Scotland, which is interpreted as a fluvio-lacustrine deposit (Stewart, 2002).
View in article
However, the supposition that the Poll a’Mhuilt Member was deposited in a lacustrine setting rests on contestable lines of evidence: fluvial sandstones bracketing the proposed lacustrine interval and allegedly high boron concentrations in illite, which were regarded as ambiguous in the original study (Stewart and Parker, 1979; Stewart, 2002).
View in article
The overlying (and major) part of the Poll a’ Mhuilt Member (>~30 m) comprises massive red mudstone and flat-laminated to ripple cross-laminated fine sandstone and siltstone, all with desiccation cracks and pedogenic structures, such as disrupted and homogenised beds and pseudo-anticlines (Stewart, 2002).
View in article
Some sedimentary features in the Poll a’Mhuilt Member provide unequivocal evidence for a largely subaerial depositional setting: the basal (<3 m) channelised and trough cross-bedded sandstones and unimodal palaeocurrent indicators imply fluvial deposition (Stewart, 2002) and the abundant pedogenic features in the upper part (30 m) of the member indicate deeply palaeo-weathered alluvium (Stewart, 2002).
View in article
The red shales lack FeHR/FeT enrichments (Fig. 3a), consistent with oxic deposition at water depths probably shallower than those for the grey shale (Stewart, 2002).
View in article
Stewart, A.D., Parker, A. (1979) Palaeosalinity and environmental interpretation of red beds from the late Precambrian (‘Torridonian’) of Scotland. Sedimentary Geology 22, 229–241.
 Show in context
Show in context However, the supposition that the Poll a’Mhuilt Member was deposited in a lacustrine setting rests on contestable lines of evidence: fluvial sandstones bracketing the proposed lacustrine interval and allegedly high boron concentrations in illite, which were regarded as ambiguous in the original study (Stewart and Parker, 1979; Stewart, 2002).
View in article
Veizer, J., Clayton, R.N., Hinton, R.W., Von Brunn, V., Mason, T.R., Buck, S.G., Hoefs, J. (1990) Geochemistry of Precambrian carbonates: 3-shelf seas and non-marine environments of the Archean. Geochimica et Cosmochimica Acta 54, 2717–2729.
 Show in context
Show in context As typical continental runoff is more radiogenic (87Sr-enriched) than seawater, 87Sr/86Sr values can distinguish between marine and non-marine environments (Veizer et al., 1990; Spencer and Patchett, 1997).
View in article
top
Supplementary Information
S1. Analytical Methods
1.1 Rock preparation
Samples were cut with a rock saw to remove weathered surfaces and then hammered into cm-sized chips. The chips were transferred into acid-washed glass beakers, cleaned twice with 2 N HCl (trace metal grade) for 10–15 seconds, and washed thoroughly with 18 MΩ DI-H2O. The clean chips were air-dried for two days with light cover, and finally pulverised in a ball mill. Powders were stored in acid-washed scintillation vials.
1.2 Organic carbon analyses
Organic carbon and carbonate carbon and oxygen isotopes were analysed at the University of Washington, following established techniques (e.g., Stüeken, 2013). For organic carbon isotopes and total organic carbon (TOC) content, powders were first decarbonated with 6 N HCl (reagent grade) at 80 °C for three days. The decarbonated powders were washed three times with 18 MΩ DI-H2O and then dried in a closed oven and finally transferred into muffled scintillation vials. For the analyses, powders were weighed into tin capsules and analysed by flash combustion with an elemental analyser (Costech) coupled to a continuous flow IR-MS (Thermo MAT253). Results (Table S-1) are expressed relative to VPDB for δ13Corg. Average reproducibility of replicate samples (1 standard deviation, SD) was 0.18 ‰ (δ13Corg) and average accuracy, as determined with calibrated in-house standards, was -0.04 ‰. The peak area was calibrated for carbon quantities with an average relative error of 2.5 %.
Table S-1 Organic carbon isotopes and abundances. TOC = total organic carbon, RE = relative error, SD = standard deviation.
| position | TOC | RE | δ13Corg | SD |
| [m] | [%] | [%] | [‰] | [‰] |
| 2.73 | 0.01 | 4.73 | -27.22 | 1.14 |
| 2.77 | 0.02 | 4.62 | -26.48 | 0.28 |
| 2.83 | 0.01 | 2.46 | -26.80 | 0.23 |
| 2.87 | 0.01 | 0.21 | -25.98 | 0.62 |
| 3.7 | 0.01 | 15.62 | -28.11 | 0.84 |
| 4.1 | 0.02 | 2.71 | -28.68 | 0.00 |
| 4.38 | 0.04 | 1.44 | -28.85 | 0.12 |
| 4.42 | 0.07 | 2.61 | -29.73 | 0.05 |
| 4.65 | 0.12 | 0.33 | -30.96 | 0.07 |
| 4.75 | 0.36 | 1.02 | -31.23 | 0.08 |
| 4.9 | 0.34 | 0.44 | -30.86 | 0.01 |
| 5.3 | 0.32 | 1.10 | -30.50 | 0.01 |
| 5.45 | 0.28 | 0.51 | -30.38 | 0.01 |
| 5.6 | 0.12 | 0.79 | -29.94 | 0.05 |
| 5.65 | 0.28 | 0.36 | -30.29 | 0.05 |
| 5.8 | 0.17 | 1.70 | -30.33 | 0.04 |
| 6.5 | 0.02 | 0.06 | -26.27 | 0.46 |
| 7.1 | 0.01 | 5.30 | -25.04 | 0.14 |
1.3 Inorganic carbon and oxygen isotopes
For carbonate analyses, untreated powders were weighed into glass vials, reacted with phosphoric acid at 80 °C for 10 minutes in a Kiel III Carbonate Device, and analysed with a dual-inlet IR-MS (Thermo Finnigan Delta Plus) (Stüeken, 2013). For oxygen isotopes, the mineralogy was assumed to be calcite based on staining pink with alizarin red and reacting strongly with 2 M HCl. The calcite mineralogy is confirmed by Ca/Mg ratios mostly >10 measured in acetic acid extracts (see below). Results (Table S-2, Fig. S-2) are expressed relative to VPDP for both δ18Ocarb and δ13Ccarb. Average reproducibility (1 SD of replicate sample analyses) was 0.02 ‰ for δ18Ocarb and 0.01 ‰ for δ13Ccarb, and average accuracy, as determined with calibrated in-house standards was <-0.01 ‰ and -0.01 ‰. The CO2 pressure in the mass spectrometer was calibrated for carbonate quantity with an average relative error of 2.7 %. Stratigraphic patterns in the δ18Ocarb and δ13Ccarb data are further discussed in Figure S-2. Although the δ18Ocarb data have probably been altered to lower values (see Shields and Veizer, 2002; Bartley and Kah, 2004 for comparison), an overall trend is preserved that shows the heaviest values in the most evaporitic facies, as expected from evaporitic enrichment (Fig. S-2).
Table S-2 Carbonate carbon and oxygen isotopes and carbonate abundances (carb.). SD = standard deviation, carb = carbonate content by weight, RE = relative error.
| position | δ13Ccarb | SD | δ18Ocarb | SD | % carb. | RE |
| [m] | [‰] | [‰] | [‰] | [‰] | [%] | |
| 2.73 | -1.94 | 0.01 | -16.36 | 0.03 | 12.8 | 0.9 |
| 2.77 | -1.78 | 0.00 | -16.31 | 0.02 | 18.0 | 2.8 |
| 2.83 | -1.54 | 0.01 | -16.30 | 0.00 | 15.3 | 5.5 |
| 2.87 | -1.22 | 0.04 | -16.39 | 0.02 | 19.0 | 0.3 |
| 4.10 | -1.14 | 0.01 | -16.25 | 0.01 | 21.8 | 0.8 |
| 4.38 | -0.90 | 0.00 | -16.30 | 0.00 | 28.3 | 3.8 |
| 4.42 | -0.61 | 0.01 | -16.20 | 0.02 | 33.3 | 5.1 |
| 4.65 | -1.35 | 0.00 | -15.94 | 0.00 | 5.7 | 2.6 |
| 4.75 | -1.25 | 0.01 | -16.28 | 0.01 | 10.6 | 2.2 |
| 4.90 | -1.16 | 0.00 | -16.38 | 0.03 | 16.6 | 8.4 |
| 5.30 | -0.23 | 0.00 | -16.15 | 0.00 | 21.0 | 1.5 |
| 5.45 | 0.02 | 0.01 | -16.05 | 0.05 | 19.7 | 0.0 |
| 5.60 | -0.52 | 0.00 | -16.18 | 0.03 | 15.4 | 0.5 |
| 5.65 | -0.27 | 0.01 | -15.93 | 0.01 | 16.6 | 0.4 |
| 5.80 | -0.86 | 0.01 | -16.01 | 0.03 | 12.6 | 0.7 |
| 6.50 | -2.02 | 0.01 | -15.06 | 0.02 | 13.2 | 0.5 |
| 7.10 | -2.12 | 0.01 | -16.24 | 0.02 | 13.0 | 6.2 |
| 16.05 | 0.58 | 0.01 | -15.86 | 0.01 | 7.3 | 7.2 |
| 16.85 | 0.65 | -15.75 | 7.5 | |||
| 20.45 | 0.58 | -16.00 | 5.7 | |||
| 22.75 | 0.77 | -15.57 | 7.7 | |||
| 24.55 | 0.88 | 0.02 | -15.57 | 0.02 | 9.7 | 0.2 |
| 25.75 | 0.96 | -15.67 | 6.4 | |||
| 25.95 | 1.03 | -15.65 | 7.0 | |||
| 27.55 | 1.07 | -15.84 | 6.6 | |||
| 29.15 | 1.15 | 0.00 | -15.47 | 0.02 | 6.3 | 7.0 |
| 30.95 | 1.16 | -15.71 | 4.7 | |||
| 32.95 | 1.16 | -15.54 | 5.4 | |||
| 34.25 | 1.16 | 0.02 | -15.96 | 0.03 | 6.9 | 0.1 |

Figure S-1 Photomicrographs of the Poll a’Mhuilt Member. (a) Sample +2.77 m, plane-polarised light; calcareous red shale with 18 % CaCO3 present as microcrystalline laminae, separated by iron-oxide coated siliciclastics. (b) Sample +4.42 m, plane-polarised light; calcareous red shale with 33 % CaCO3 present as disrupted microcrystalline laminae and rare sparry fenestral fillings. (c) Sample +5.30 m, plane-polarised light; calcareous grey shale with 21 % CaCO3 present as microcrystalline laminae, separated by kerogenous siliciclastics. (d) Sample +25.75 m, crossed polars; partially desiccated facies with 6 % CaCO3 present as microcrystalline nodules and cement, stained with iron oxide, separated by iron-oxide coated siliciclastics. All samples contain angular silt grains composed of quartz, plagioclase, and K-feldspar and minor mica.

Figure S-2 Stratigraphic section through the Poll a’Mhuilt Member with carbonate C and O isotopes. Lithostratigraphy, Mo, Cr, CaCO3 and TOC abundances are as in Figure 1 in the main text. The last panel shows δ18O and δ13C in carbonate in stratigraphic context. The trend highlights that the heaviest values in both isotopic proxies occur in the upper red shale. This is consistent with the high abundance of desiccation cracks in this part of the section, because evaporation forces these parameters to heavier values. The grey shale, which would have been most continuously flooded, shows relatively light values, consistent with a relatively lesser impact of evaporation. Despite the high degree of evaporation that is implied by the presence of gypsum pseudomorphs in the section (Stewart, 2002; Parnell et al., 2010) the δ18O values are overall light compared to other mid-Proterozoic carbonates of similar age (Shields and Veizer, 2002; Bartley and Kah, 2004), which we attribute to fluid alteration. Oxygen isotopes are much more easily reset than carbon isotopes. However, the overall trend towards heavier values in the most evaporitic part of the section is preserved.
1.4 Bulk elemental abundances
Bulk digests were prepared at UC Riverside, following a method adapted from Reinhard et al. (2013a). Powders were first ashed at 800 °C in acid-washed ceramic crucibles, weighed before and after to determine the loss on ignition (LOI). Ashed powders were transferred into screw-top Teflon beakers and dissolved with 5ml HNO3 + 1ml HF at 130 °C overnight. The acids were evaporated at 110–130 °C. Fluoride precipitates were removed through 1–2 treatments with aqua regia (3ml HCl + 1ml HNO3) at 120 °C. Samples were stored in 5 % (v/v) HNO3. All acids were trace metal grade and used in concentrated form.
Elemental concentrations (Table S-3) were measured by ICP-MS (Agilent 7500ce). Reproducibility of replicate samples was 5 % on average. For Mo, which showed the largest range of concentrations, reproducibility was 20 % below 2 ppm and 4 % or better above 10 ppm. Accuracy was monitored with the USGS rocks standard SCo-1 and was within 5 % for minor elements and within 10 % for major elements.
Table S-3 Bulk elemental abundances. The grey shale unit extends from +4.75 m to +5.8 m; the sample from +5.6 m is likely altered by modern weathering. Samples below 0 m are from the Stac Fada Member and contain volcanic fragments. Abundances are in weight-percent or parts per million (μg/g).
| position | Na | Mg | Al | P | K | Ca | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Sr | Mo | Cd | Pb | Th | U |
| [m] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [ppm] | [ppm] | [ppm] | [%] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] |
| Poll a'Mhuilt section: | ||||||||||||||||||||||
| -5.00 | 3.36 | 2.23 | 6.78 | 0.07 | 1.80 | 0.46 | 0.37 | 72.3 | 217.3 | 712.9 | 4.43 | 29.8 | 400.6 | 9.8 | 78.6 | 2.4 | 170.0 | 0.4 | 0.0 | 9.6 | 4.1 | 0.7 |
| -4.50 | 3.32 | 2.37 | 7.32 | 0.07 | 1.51 | 0.61 | 0.38 | 70.0 | 156.7 | 774.7 | 3.88 | 25.3 | 270.8 | 10.7 | 70.1 | 1.6 | 202.2 | 0.4 | 0.0 | 7.3 | 3.4 | 0.7 |
| 0.00 | 3.03 | 2.55 | 8.18 | 0.07 | 1.97 | 0.83 | 0.49 | 89.9 | 139.9 | 694.7 | 5.36 | 24.6 | 178.8 | 12.5 | 96.6 | 5.5 | 133.1 | 0.3 | 0.0 | 11.6 | 6.3 | 1.2 |
| 0.50 | 3.98 | 1.86 | 6.40 | 0.08 | 1.40 | 0.94 | 0.48 | 97.9 | 128.0 | 654.5 | 4.01 | 25.4 | 170.8 | 16.1 | 73.7 | 3.9 | 136.6 | 0.8 | 0.0 | 9.1 | 5.4 | 1.4 |
| 0.75 | 3.59 | 1.88 | 7.66 | 0.05 | 1.51 | 1.45 | 0.36 | 66.1 | 117.5 | 625.7 | 3.87 | 18.8 | 136.4 | 16.1 | 69.9 | 3.6 | 109.1 | 0.6 | 0.0 | 9.0 | 4.8 | 1.2 |
| 1.65 | 4.45 | 1.33 | 6.92 | 0.08 | 0.93 | 1.89 | 0.39 | 82.3 | 139.8 | 540.9 | 3.48 | 17.5 | 170.3 | 16.1 | 47.5 | 4.5 | 107.3 | 0.4 | 0.0 | 7.4 | 4.2 | 0.7 |
| 2.05 | 2.98 | 1.60 | 6.34 | 0.10 | 1.98 | 1.04 | 0.46 | 95.2 | 130.6 | 529.0 | 5.45 | 23.7 | 165.7 | 16.1 | 88.8 | 6.5 | 87.7 | 0.7 | 0.0 | 9.1 | 6.2 | 2.2 |
| 2.73 | 4.22 | 1.86 | 8.83 | 0.08 | 1.01 | 5.63 | 0.48 | 114.2 | 114.4 | 1025.6 | 3.70 | 21.1 | 94.3 | 16.1 | 63.6 | 4.2 | 116.1 | 0.5 | 0.0 | 14.4 | 5.5 | 5.2 |
| 2.77 | 3.32 | 1.41 | 7.22 | 0.07 | 1.15 | 7.49 | 0.43 | 87.9 | 98.2 | 1062.4 | 2.93 | 18.2 | 84.8 | 16.1 | 52.7 | 2.8 | 107.3 | 0.4 | 0.1 | 24.2 | 5.0 | 3.8 |
| 2.80 | 1.78 | 3.27 | 8.74 | 0.10 | 2.77 | 0.58 | 0.56 | 115.9 | 134.2 | 663.1 | 5.87 | 39.5 | 197.5 | 16.1 | 103.9 | 5.5 | 76.1 | 0.5 | 0.0 | 24.2 | 8.1 | 3.2 |
| 2.83 | 2.61 | 1.87 | 7.21 | 0.09 | 1.67 | 5.80 | 0.54 | 96.5 | 116.0 | 1125.6 | 3.84 | 27.4 | 129.6 | 16.1 | 78.4 | 4.3 | 101.1 | 0.6 | 0.1 | 19.4 | 5.5 | 4.7 |
| 2.87 | 3.12 | 1.78 | 7.19 | 0.07 | 1.38 | 7.58 | 0.43 | 89.4 | 110.0 | 1142.6 | 3.23 | 22.7 | 128.0 | 16.1 | 64.2 | 2.3 | 111.7 | 0.2 | 0.0 | 11.7 | 5.5 | 3.8 |
| 3.20 | 2.44 | 2.35 | 7.80 | 0.08 | 1.48 | 1.33 | 0.45 | 87.8 | 127.7 | 554.1 | 5.15 | 18.6 | 140.6 | 16.1 | 61.1 | 2.6 | 116.5 | 0.5 | 0.0 | 9.6 | 6.5 | 1.5 |
| 3.70 | 2.69 | 2.08 | 7.39 | 0.07 | 1.92 | 1.09 | 0.46 | 82.2 | 119.6 | 589.7 | 4.57 | 15.8 | 136.8 | 16.1 | 76.4 | 1.7 | 277.3 | 0.4 | 0.0 | 6.4 | 5.8 | 1.6 |
| 4.10 | 2.74 | 0.95 | 5.99 | 0.05 | 1.52 | 6.95 | 0.28 | 80.0 | 83.9 | 960.9 | 2.73 | 10.8 | 86.1 | 16.1 | 45.1 | 4.6 | 107.1 | 1.3 | 0.0 | 15.0 | 4.4 | 4.9 |
| 4.38 | 2.62 | 1.07 | 5.34 | 0.07 | 0.69 | 10.69 | 0.32 | 100.8 | 100.4 | 1171.7 | 2.95 | 16.9 | 99.6 | 16.1 | 48.7 | 3.1 | 142.5 | 0.7 | 0.1 | 13.8 | 4.6 | 4.7 |
| 4.42 | 2.42 | 0.85 | 4.60 | 0.07 | 0.70 | 12.84 | 0.32 | 82.3 | 83.6 | 1176.7 | 2.14 | 12.9 | 78.5 | 16.1 | 35.7 | 3.8 | 161.9 | 1.1 | 0.1 | 12.0 | 4.1 | 5.8 |
| 4.65 | 2.49 | 2.05 | 6.88 | 0.11 | 1.62 | 2.36 | 0.53 | 150.9 | 117.5 | 622.5 | 3.59 | 12.1 | 127.0 | 16.1 | 74.1 | 2.3 | 119.2 | 1.7 | 0.0 | 9.9 | 6.7 | 7.7 |
| 4.75 | 3.06 | 1.81 | 6.74 | 0.09 | 1.26 | 4.15 | 0.45 | 174.3 | 110.3 | 824.3 | 4.27 | 26.0 | 106.1 | 16.1 | 88.0 | 18.5 | 110.2 | 89.2 | 0.3 | 32.7 | 6.4 | 8.5 |
| 4.90 | 2.84 | 1.75 | 6.39 | 0.08 | 1.01 | 5.85 | 0.43 | 153.4 | 95.2 | 925.0 | 4.41 | 36.1 | 100.9 | 16.1 | 97.0 | 15.7 | 168.4 | 122.2 | 0.4 | 24.8 | 5.9 | 7.1 |
| 5.30 | 2.67 | 1.56 | 5.79 | 0.08 | 1.24 | 7.78 | 0.41 | 138.2 | 100.8 | 962.7 | 3.27 | 15.3 | 98.0 | 16.1 | 197.9 | 3.4 | 163.8 | 23.5 | 0.9 | 5.4 | 6.0 | 5.5 |
| 5.45 | 2.52 | 1.87 | 6.53 | 0.07 | 1.08 | 7.37 | 0.39 | 121.6 | 93.6 | 1205.2 | 4.48 | 20.6 | 110.2 | 16.1 | 137.1 | 27.9 | 94.4 | 166.2 | 1.8 | 50.6 | 5.8 | 3.8 |
| 5.60 | 2.39 | 1.87 | 8.71 | 0.09 | 2.19 | 5.73 | 0.46 | 119.1 | 100.1 | 880.1 | 4.48 | 19.0 | 105.0 | 16.1 | 150.9 | 2.2 | 154.3 | 1.1 | 0.3 | 4.9 | 6.1 | 3.4 |
| 5.65 | 2.78 | 1.72 | 6.07 | 0.08 | 1.28 | 6.19 | 0.46 | 119.1 | 97.6 | 1128.9 | 4.29 | 21.6 | 100.5 | 16.1 | 110.6 | 26.1 | 83.4 | 106.1 | 7.4 | 67.8 | 6.2 | 4.3 |
| 5.80 | 2.34 | 2.02 | 6.96 | 0.08 | 1.58 | 5.04 | 0.40 | 125.4 | 95.5 | 882.5 | 3.47 | 23.3 | 85.6 | 16.1 | 79.5 | 11.0 | 142.8 | 9.3 | 0.2 | 13.2 | 6.3 | 3.7 |
| 6.50 | 1.65 | 2.30 | 6.84 | 0.06 | 2.21 | 5.14 | 0.48 | 76.6 | 85.2 | 1056.2 | 4.12 | 15.1 | 75.7 | 16.1 | 99.5 | 0.2 | 147.0 | 1.1 | 0.1 | 4.0 | 7.0 | 1.9 |
| 7.10 | 2.65 | 1.77 | 6.79 | 0.06 | 1.38 | 5.18 | 0.41 | 69.9 | 92.4 | 919.3 | 4.05 | 13.3 | 62.5 | 16.1 | 67.7 | 2.5 | 87.3 | 0.4 | 0.0 | 5.7 | 5.7 | 1.4 |
| 12.10 | 1.49 | 2.92 | 8.46 | 0.09 | 3.29 | 0.80 | 0.57 | 157.0 | 114.3 | 583.1 | 5.83 | 23.9 | 101.6 | 16.1 | 99.9 | 25.5 | 66.4 | 1.4 | 0.0 | 16.3 | 12.2 | 4.0 |
| 13.05 | 1.14 | 3.17 | 8.77 | 0.06 | 3.83 | 1.90 | 0.41 | 110.6 | 106.9 | 702.2 | 6.34 | 24.6 | 102.1 | 16.1 | 99.7 | 15.6 | 52.4 | 1.3 | 0.1 | 17.3 | 10.5 | 3.3 |
| 14.65 | 1.36 | 2.35 | 7.16 | 0.08 | 3.02 | 2.33 | 0.47 | 121.7 | 101.0 | 778.9 | 5.58 | 23.4 | 98.8 | 16.1 | 91.7 | 10.1 | 69.6 | 0.9 | 0.0 | 15.4 | 10.8 | 3.3 |
| 16.05 | 1.82 | 2.35 | 7.24 | 0.09 | 3.07 | 2.99 | 0.45 | 123.5 | 111.9 | 794.7 | 5.40 | 22.9 | 103.5 | 16.1 | 86.4 | 13.2 | 100.2 | 1.1 | 0.1 | 15.5 | 9.6 | 3.1 |
| 16.85 | 1.46 | 2.37 | 6.88 | 0.09 | 2.97 | 3.12 | 0.51 | 114.4 | 104.0 | 824.5 | 5.34 | 22.2 | 99.5 | 16.1 | 89.5 | 9.2 | 93.3 | 0.9 | 0.1 | 15.0 | 9.3 | 3.0 |
| 20.45 | 1.82 | 2.44 | 6.89 | 0.09 | 2.88 | 2.65 | 0.54 | 111.5 | 108.3 | 685.4 | 5.66 | 23.1 | 107.2 | 16.1 | 81.6 | 8.7 | 98.8 | 1.2 | 0.1 | 14.3 | 8.7 | 2.7 |
| 22.75 | 1.55 | 2.77 | 8.57 | 0.09 | 3.05 | 3.17 | 0.48 | 111.7 | 110.5 | 787.1 | 5.24 | 23.0 | 102.3 | 16.1 | 98.6 | 7.6 | 95.5 | 1.2 | 0.1 | 14.1 | 8.4 | 2.7 |
| 24.55 | 1.75 | 2.13 | 6.52 | 0.09 | 2.73 | 4.22 | 0.46 | 110.4 | 116.4 | 922.8 | 5.08 | 22.3 | 103.8 | 16.1 | 82.3 | 9.7 | 99.8 | 0.8 | 0.1 | 13.9 | 8.6 | 2.7 |
| 25.75 | 1.39 | 2.69 | 8.09 | 0.08 | 3.14 | 2.78 | 0.40 | 121.8 | 152.9 | 773.1 | 5.32 | 24.1 | 111.6 | 16.1 | 88.8 | 12.7 | 92.3 | 1.0 | 0.0 | 15.0 | 8.5 | 2.7 |
| 25.95 | 1.59 | 2.25 | 7.19 | 0.08 | 2.91 | 3.06 | 0.39 | 108.4 | 108.3 | 765.4 | 5.33 | 23.0 | 103.5 | 16.1 | 87.6 | 8.8 | 103.6 | 3.0 | 0.1 | 14.7 | 8.6 | 2.7 |
| 27.55 | 1.47 | 2.43 | 7.65 | 0.09 | 2.57 | 3.02 | 0.47 | 121.5 | 111.0 | 747.7 | 4.69 | 23.2 | 108.0 | 16.1 | 85.6 | 7.8 | 111.8 | 0.9 | 0.1 | 14.4 | 8.1 | 2.5 |
| 29.15 | 1.43 | 2.33 | 7.80 | 0.11 | 3.02 | 2.79 | 0.48 | 106.8 | 113.5 | 702.0 | 5.14 | 21.5 | 102.5 | 16.1 | 87.8 | 10.8 | 101.2 | 0.8 | 0.1 | 15.2 | 9.6 | 2.5 |
| 30.95 | 1.62 | 2.57 | 7.83 | 0.07 | 3.16 | 2.32 | 0.48 | 114.1 | 109.4 | 644.4 | 5.60 | 23.0 | 105.7 | 16.1 | 82.8 | 8.1 | 101.0 | 1.6 | 0.1 | 15.5 | 9.2 | 2.6 |
| 32.95 | 1.57 | 2.59 | 7.75 | 0.09 | 3.12 | 2.60 | 0.46 | 126.5 | 125.0 | 762.8 | 5.46 | 24.8 | 117.8 | 16.1 | 94.5 | 6.4 | 114.0 | 1.0 | 0.0 | 14.7 | 8.6 | 2.4 |
| 34.25 | 1.50 | 2.41 | 7.55 | 0.09 | 3.06 | 3.28 | 0.48 | 119.0 | 112.9 | 773.7 | 5.22 | 23.3 | 114.3 | 16.1 | 86.6 | 10.1 | 108.7 | 1.1 | 0.1 | 14.6 | 7.7 | 2.1 |
| basement gneiss: | ||||||||||||||||||||||
| G1 | 2.96 | 0.16 | 7.25 | 0.00 | 5.03 | 0.37 | 0.07 | 9.3 | 3.7 | 119.9 | 0.28 | 2.1 | 7.5 | 15.1 | 17.7 | -0.3 | 269.8 | 0.5 | 0.0 | 10.9 | 1.5 | 0.7 |
| G2 | 5.85 | 0.11 | 7.36 | 0.02 | 1.32 | 0.85 | 0.04 | 9.8 | 7.4 | 94.7 | 0.21 | 2.1 | 2.4 | 4.3 | 7.3 | -0.9 | 252.1 | 0.2 | 0.0 | 9.7 | 3.8 | 0.6 |
| G3 | 4.45 | 0.12 | 7.18 | 0.03 | 2.64 | 1.35 | 0.07 | 8.2 | 2.2 | 156.4 | 0.40 | 1.8 | 3.6 | 0.7 | 11.0 | -1.0 | 475.3 | 0.2 | 0.0 | 6.1 | 1.5 | 0.2 |
| basement amphibolite: | ||||||||||||||||||||||
| A1 | 0.66 | 5.27 | 7.84 | 0.03 | 0.28 | 7.19 | 0.48 | 286.5 | 231.7 | 1856.3 | 10.23 | 49.3 | 77.3 | 71.6 | 78.6 | -0.4 | 78.8 | 0.6 | 0.1 | 3.9 | 0.6 | 0.1 |
| A2 | 1.41 | 3.28 | 7.56 | 0.08 | 0.36 | 6.46 | 0.78 | 283.3 | 195.5 | 1614.9 | 10.18 | 40.4 | 99.0 | 69.7 | 94.9 | -0.8 | 165.8 | 0.4 | 0.0 | 2.1 | 1.3 | 0.3 |
| A3 | 1.82 | 2.95 | 7.27 | 0.03 | 0.51 | 5.29 | 0.47 | 238.0 | 212.0 | 1266.9 | 7.26 | 31.5 | 82.3 | 46.5 | 93.0 | -0.5 | 305.0 | 0.3 | 0.1 | 4.5 | 0.2 | 0.0 |
| A4 | 3.75 | 1.75 | 8.32 | 0.12 | 0.68 | 3.71 | 0.42 | 106.1 | 78.2 | 623.4 | 4.13 | 19.5 | 65.1 | 59.2 | 61.4 | 0.5 | 505.1 | 0.2 | 0.0 | 5.4 | 0.6 | 0.1 |
| A5 | 1.58 | 6.60 | 6.44 | 0.03 | 0.43 | 5.88 | 0.33 | 138.3 | 1252.2 | 1669.8 | 8.40 | 41.4 | 264.8 | 6.0 | 113.9 | -0.5 | 284.3 | 0.1 | 0.2 | 3.0 | 0.3 | 0.2 |
| A6 | 1.64 | 5.19 | 7.19 | 0.13 | 0.44 | 6.32 | 0.60 | 245.9 | 330.4 | 1596.7 | 9.29 | 45.2 | 95.9 | 47.8 | 111.5 | 0.3 | 471.7 | 0.2 | 0.2 | 7.5 | 2.0 | 0.2 |
| additional Stac Fada sample from different locality: | ||||||||||||||||||||||
| SF | 2.36 | 2.38 | 7.26 | 0.07 | 1.98 | 1.14 | 0.43 | 91.7 | 221.3 | 765.5 | 4.39 | 31.3 | 382.8 | 26.7 | 110.0 | 0.7 | 270.2 | 0.2 | 0.0 | 7.4 | 4.4 | 0.9 |
1.5 Iron speciation
Iron speciation (Table S-4, Figs. 1, 3a) was done at UC Riverside, using established methods that sequentially extract carbonate-bound iron, ferric oxides and magnetite (Poulton and Canfield, 2005; Reinhard et al., 2009; Reinhard et al., 2013a). Approximately 100 mg of powder were weighed into 15 ml Falcon centrifuge tubes. The carbonate-bound fraction was extracted with 10ml of Na acetate buffered to pH 4.5 with acetic acid. The tubes were placed on a horizontal shaker table for 48 hours at room temperature. 5 ml of the solution were extracted with a pipette after centrifugation; the rest was discarded. The residual rock powder was then treated with Na dithionite (pH 4.8 for 2 hours) to dissolve the ferric oxide fraction and finally with NH4-oxalate (pH 3.2 for 6 hours) to dissolve magnetite. The extracted solutions were diluted 1:100 with 2 % (v/v) HNO3 and analysed by ICP-MS (Agilent 7500ce). The average reproducibility for iron concentrations in each fraction was 5 %, as determined by sample replicates.
The concentration of Ca and Ca/Mg ratios measured in the acetic acid fraction were used as an additional constraint on the abundance of carbonate in the samples. Assuming a CaCO3 mineralogy, the obtained values agreed within 5 % (RE) on average with the carbonate abundance determined from the CO2 pressure after phosphoric acid dissolution (see above). Carbonate concentrations plotted in Figure 1 are taken from the phosphoric acid treatment, except in cases where δ13Ccarb and δ18Ocarb were not measured (Table S-2).
Sulphide-bound iron was quantified by chromium reduction with Zn-acetate traps, followed by iodometric titration (Canfield et al., 1986). Reproducibility was 4 % for sulphide-rich samples (>0.5 % chromium-reducible sulphur) and 20 % for sulphide-poor samples. To convert from the measured concentration of sulphide in the Zn-acetate traps to iron, we assumed a pyrite stoichiometry of FeS2. To test for the relative importance of other metal sulphides such as pyrrhotite or sphalerite we processed a subset of samples with just concentrated boiling HCl, i.e. without the addition of CrCl2 to the reaction vessels (Reinhard et al., 2013a). The results showed that only up to 7 % (average 4 %) of the sulphide minerals in these samples are in this acid-volatile sulphide (AVS) phase, which is negligible in the summation of iron species in the bulk rock.
Table S-4 Iron speciation. FeCarb = carbonate-bound iron, FeOx = ferric oxide-bound iron, FeMag = magnetite-bound iron, FePy = pyrite-bound iron. FeHR = highly reactive iron, which is the sum of all four phases. FeT = total iron from Table S-5. FePy was not determined in most of the red shale facies and assumed to be zero as in the few examples. * = Carbonate content was calculated from the concentration of Ca in the acetic acid extract, assuming a CaCO3 stoichiometry. Note that this calculation is an upper estimate, because it does not account for partial dissolution of silicates, which may become important below a carbonate content of 5 %, as suggested by the low molar Ca/Mg ratios in those samples.
| position [m] | FeCarb [%] | FeOx [%] | FeMag [%] | FePy [%] | FeHR [%] | FePy/FeHR [by mass] | FeHR/FeT [by mass] | carb.* [%] | molar Ca/Mg |
| 0 | 0.09 | 0.69 | 0.39 | 1.17 | 0.00 | 0.22 | 0.1 | 0.2 | |
| 0.5 | 0.32 | 1.11 | 0.33 | 1.75 | 0.00 | 0.44 | 0.4 | 0.2 | |
| 0.75 | 0.09 | 0.47 | 0.26 | 0.82 | 0.00 | 0.21 | 3.0 | 6.4 | |
| 1.65 | 0.13 | 0.58 | 0.11 | 0.83 | 0.00 | 0.24 | 4.1 | 5.8 | |
| 2.05 | 0.07 | 0.60 | 0.34 | 1.02 | 0.00 | 0.19 | 2.3 | 7.3 | |
| 2.73 | 0.59 | 0.51 | 0.21 | 1.31 | 0.00 | 0.36 | 11.8 | 4.3 | |
| 2.77 | 0.16 | 0.32 | 0.11 | 0.00 | 0.60 | 0.00 | 0.21 | 18.2 | 28.3 |
| 2.8 | 0.10 | 0.35 | 0.18 | 0.64 | 0.00 | 0.11 | 0.5 | 1.2 | |
| 2.83 | 0.17 | 0.47 | 0.14 | 0.78 | 0.00 | 0.20 | 14.7 | 23.2 | |
| 2.87 | 0.17 | 0.27 | 0.13 | 0.00 | 0.58 | 0.00 | 0.18 | 17.4 | 28.3 |
| 3.2 | 0.76 | 0.68 | 0.38 | 1.81 | 0.00 | 0.35 | 1.9 | 0.7 | |
| 3.7 | 0.29 | 0.16 | 0.19 | 0.00 | 0.64 | 0.00 | 0.14 | 1.9 | 2.4 |
| 4.1 | 0.10 | 0.53 | 0.18 | 0.81 | 0.00 | 0.30 | 20.6 | 49.0 | |
| 4.38 | 0.11 | 0.45 | 0.11 | 0.67 | 0.00 | 0.23 | 26.9 | 64.0 | |
| 4.42 | 0.10 | 0.27 | 0.09 | 0.01 | 0.47 | 0.03 | 0.22 | 31.1 | 76.4 |
| 4.65 | 0.49 | 0.17 | 0.23 | 0.07 | 0.95 | 0.07 | 0.26 | 5.3 | 2.9 |
| 4.75 | 0.16 | 0.07 | 0.10 | 1.23 | 1.57 | 0.78 | 0.37 | 10.0 | 21.3 |
| 4.9 | 0.23 | 0.08 | 0.11 | 1.15 | 1.58 | 0.73 | 0.36 | 15.8 | 28.0 |
| 5.3 | 0.13 | 0.08 | 0.08 | 0.27 | 0.56 | 0.48 | 0.17 | 19.3 | 46.6 |
| 5.45 | 0.19 | 0.20 | 0.22 | 1.36 | 1.98 | 0.69 | 0.44 | 19.3 | 8.8 |
| 5.6 | 0.27 | 0.56 | 0.23 | 0.00 | 1.06 | 0.00 | 0.24 | 14.2 | 23.4 |
| 5.65 | 0.79 | 0.07 | 0.12 | 1.11 | 2.09 | 0.53 | 0.49 | 15.2 | 17.7 |
| 5.8 | 0.12 | 0.07 | 0.08 | 0.24 | 0.51 | 0.46 | 0.15 | 11.7 | 23.5 |
| 6.5 | 0.37 | 0.16 | 0.24 | 0.00 | 0.77 | 0.00 | 0.19 | 9.8 | 5.9 |
| 7.1 | 0.18 | 0.38 | 0.17 | 0.73 | 0.00 | 0.18 | 12.6 | 21.7 | |
| 12.1 | 0.03 | 1.55 | 0.32 | 1.89 | 0.00 | 0.32 | 1.5 | 3.5 | |
| 13.05 | 0.02 | 1.33 | 0.24 | 1.60 | 0.00 | 0.25 | 4.8 | 14.0 | |
| 14.65 | 0.02 | 0.99 | 0.30 | 1.31 | 0.00 | 0.23 | 5.6 | 15.8 | |
| 16.05 | 0.03 | 1.14 | 0.25 | 1.42 | 0.00 | 0.26 | 7.3 | 14.3 | |
| 16.85 | 0.04 | 1.27 | 0.26 | 1.57 | 0.00 | 0.29 | 7.4 | 12.3 | |
| 20.45 | 0.02 | 0.83 | 0.26 | 1.11 | 0.00 | 0.20 | 6.1 | 15.6 | |
| 22.75 | 0.02 | 0.98 | 0.28 | 1.28 | 0.00 | 0.24 | 7.8 | 17.5 | |
| 24.55 | 0.02 | 1.05 | 0.33 | 1.40 | 0.00 | 0.28 | 9.2 | 20.0 | |
| 25.75 | 0.02 | 0.95 | 0.25 | 1.22 | 0.00 | 0.23 | 6.5 | 19.0 | |
| 25.95 | 0.03 | 1.00 | 0.26 | 1.29 | 0.00 | 0.24 | 8.2 | 24.7 | |
| 27.55 | 0.03 | 1.09 | 0.28 | 1.40 | 0.00 | 0.30 | 6.8 | 13.9 | |
| 29.15 | 0.03 | 1.03 | 0.27 | 1.32 | 0.00 | 0.26 | 6.3 | 18.7 | |
| 30.95 | 0.02 | 1.18 | 0.29 | 1.49 | 0.00 | 0.27 | 5.2 | 14.0 | |
| 32.95 | 0.02 | 0.88 | 0.26 | 1.16 | 0.00 | 0.21 | 5.8 | 17.6 | |
| 34.25 | 0.01 | 0.84 | 0.22 | 1.08 | 0.00 | 0.21 | 7.4 | 22.4 | |
| 42.1 | 0.01 | 1.79 | 0.31 | | 2.11 | 0.00 | 0.32 | 0.2 | 0.5 |
1.6 Molybdenum isotope measurements
Molybdenum and strontium isotope measurements were performed at the Metal Geochemistry Center at Yale University, New Haven Connecticut. For molybdenum, an aliquot of the bulk digests prepared at UC Riverside split was doped with a Mo double spike. Using the known Mo concentrations (Table S-3), the spike was adjusted to maintain a constant sample to spike ratio. The mixture was evaporated at 100 °C, re-dissolved in 7 M HCl, and then purified by chromatographic separation. The 97Mo-100Mo double spike solution was prepared gravimetrically from Oak Ridge Laboratory metal powders as previously described (Asael et al., 2013). A two-stage column procedure was applied for Mo purification, following standard protocols (Asael et al., 2013; Planavsky et al., 2014): The sample was run through an anion resin (AG-MP-1M) to separate Mo and Fe from the matrix followed by purification through a cation resin (AG50W-X8) to separate Mo from any remaining Fe. The Mo isotopic ratios were analysed using a Thermo Neptune Multi collector MC-ICP-MS instrument.
Molybdenum isotope compositions are reported using the δ notation, where δ98/95Mo (‰) = 1000 ∙ [(98Mo/95Mo)sample/(98Mo/95Mo)NIST*99975 - 1], calculated relative to NIST 3134 (Lot 130418) with a value of +0.25 ‰ (Nägler et al., 2014). A Calibration of the NIST standard relative to Rochester (Lot 862309E) gave: δ98MoROCH = δ98Mo NIST3137 − 0.32 ± 0.12 ‰. For each sample, the target Mo concentration was 50 ppb during each session. In all reported samples the 1SD was <0.05 ‰. Duplicates (n = 6) of reference standard NOD-1 had an average δ98Mo value of -0.41 ‰ and a standard deviation (SD) of 0.06 ‰. For SCo-1, for which there is to our knowledge no published reference value, we obtained a value of -0.07 ± 0.01 ‰ (1SD, n = 3). Values for Mo isotope measurements and associated errors can be found in Table S-5.
Table S-5 Molybdenum isotopes of bulk rocks, relative to NIST3134 = +0.25 ‰. Samples with reported standard deviations (SD) were prepared and analysed in replicates.
| position [m] | δ98/95Mo [‰] | SD [‰] |
| -5.00 | 1.24 | |
| -4.50 | 1.05 | |
| 0.00 | 1.00 | |
| 1.65 | 1.00 | |
| 2.73 | 0.35 | |
| 2.77 | 0.33 | |
| 2.80 | 0.52 | |
| 2.83 | -0.31 | 0.02 |
| 3.20 | 0.30 | |
| 3.70 | 0.20 | 0.04 |
| 4.10 | 0.91 | 0.04 |
| 4.38 | 0.47 | |
| 4.42 | 0.30 | |
| 4.65 | 0.57 | 0.01 |
| 4.75 | 0.13 | 0.02 |
| 4.90 | 0.33 | 0.09 |
| 5.30 | 1.10 | 0.22 |
| 5.45 | 0.76 | 0.02 |
| 5.60 | 1.32 | |
| 5.65 | 1.19 | 0.01 |
| 5.80 | 0.68 | 0.03 |
| 6.50 | 0.43 | |
| 7.10 | 0.46 | |
| 12.10 | -0.05 | |
| 13.05 | -0.17 | 0.05 |
| 14.65 | -0.16 | |
| 16.05 | -0.34 | 0.02 |
| 16.85 | -0.24 | |
| 20.45 | -0.34 | |
| 22.75 | -0.38 | |
| 24.55 | -0.26 | |
| 25.75 | -0.32 | |
| 25.95 | -0.24 | |
| 29.15 | -0.24 | |
| 30.95 | -0.36 | 0.04 |
| 32.95 | -0.34 | |
| 34.25 | 0.03 | |
1.7 Carbonate-bound strontium isotopes
Strontium was extracted from carbonate rocks following the sequential leaching protocol described by Bellefroid et al. (2015) (Table S-6), similar to that of Liu et al. (2013). Powdered samples were first washed with ammonium acetate to liberate any loosely bound Sr from clay surfaces. Around 400–500 mg of powder were weighed into 15 ml Falcon centrifuge tubes, mixed with 10 ml of 1M NH4-acetate and left to react for 30 minutes at room temperature in an ultra-sonic bath. Solutions were centrifuged for 5 minutes at 5000 rpm, and then extracted with a pipette and transferred into a 15 ml screw-top Teflon beaker. Carbonate was then extracted from the same powder aliquot with acetic acid in eight steps with increasing acid strength. Acid quantities and concentrations are listed in Table S-6. Especially with the weakest acid, the solutions were carbonate-buffered, thus limiting the mobilisation of silicate-bound Sr. The silicate-bound fraction was monitored with Rb/Sr ratios, which go up with increasing silicate dissolution. The clean carbonate end-member then corresponds to the extrapolated Rb/Sr ratio of 0.
The solid sample residues after acid treatment were dried at 80 °C for several days and weighed to calculate the cumulative extraction yield, which was on average 103 ± 11 % relative to the known carbonate content. Extracted solutions were evaporated at 80–100 °C overnight. They were then dissolved in weak nitric acid (5 % HNO3 v/v), and 1000-fold dilution splits were measured for major and trace element abundances on a Thermo Element XR ICP-MS at the Yale Metal Geochemistry Center (Table S-7). A second split was taken from each solution and purified for 87Sr/86Sr analysis using an ESI PrepFast-MC-Sr system (Romaniello et al., 2015). Samples were run on a Thermo Neptune MC-ICP-MS with NIST SRM 987 as a bracketing standard (average 87Sr/86Sr ratio of 0.71034 +/- 0.000062, 2SD). A subset of samples of the Lewisian basement and Stac Fada Mbr were analysed after bulk digestion without sequential leaching (Table S-8). The 87Sr/86Sr ratio at 1.2 billion years ago was calculated as (87Sr/86Sr)1.2Ga = (87Sr/86Sr)measured - (87Rb/86Sr)modern ∙ (e(λ∙t) - 1), where λ is the decay constant (1.42∙10-11 yr-1) and t = 1.2∙109 years. The modern 87Rb/86Sr ratio was calculated from elemental concentrations, assuming relative abundances of 27.8 % for 87Rb and 9.86 % for 86Sr. Results are reported in Tables S-7 and S-8. One carbonate sample was processed in duplicate and the calculated carbonate end-member showed a standard deviation of 0.001. Analytical accuracy was assessed with the USGS carbonatite standard COQ-1, for which we obtained values of 0.70336 ± 0.000005, in good agreement with the value of 0.70331 ± 0.00002 (2SD) reported by Grünenfelder et al. (1986) for rocks from the same geologic unit. When COQ-1 was processed through the sequential extraction protocol, we obtained an average value of 0.70340 ± 0.00002 (2SD) for all acetic acid steps.
Table S-6 Dissolution method for carbonate bound Sr analysis.
| Step | Reagent | Volume [ml] | Concentration [M] | pH |
| 1-2 | Ammonium Acetate | 10 | 1 | - |
| 3-6 | Acetic Acid | 7.5 | 0.04 | 3.07 |
| 7-8 | Acetic Acid | 8 | 0.175 | 2.76 |
| 9 | Acetic Acid | 6 | 0.875 | 2.41 |
| 10 | Acetic Acid | 6 | 1.75 | 2.26 |
Table S-7 Elemental abundances and Sr isotopes of carbonate leaches. N1-N2 = ammonium acetate washing steps, S1-S8 = acetic acid extractions with increasing acid strength. Concentrations are relative to bulk rock.
| sample | Mg [ppm] | Al [ppm] | Ca [%] | Mn [ppm] | Fe [ppm] | Rb [ppb] | Sr [ppb] | Rb/Sr | Mn/Sr | 87Sr/86Sr |
| +4.20 m, N1 | 254 | 5 | 1.79 | 72 | 1 | 6044 | 31314 | 0.19 | 2.3 | 0.71553 |
| +4.20 m, N2 | 103 | 4 | 1.59 | 115 | 1 | 523 | 21858 | 0.02 | 5.3 | 0.71272 |
| +4.20 m, S1 | 116 | 2 | 3.80 | 346 | 0 | 185 | 21693 | 0.01 | 15.9 | 0.71072 |
| +4.20 m, S2 | 118 | 2 | 4.39 | 410 | 1 | 201 | 23939 | 0.01 | 17.1 | n.d. |
| +4.20 m, S3 | 93 | 36 | 2.29 | 218 | 4 | 127 | 13855 | 0.01 | 15.7 | 0.71061 |
| +4.20 m, S4 | 108 | 126 | 0.63 | 78 | 8 | 135 | 7814 | 0.02 | 9.9 | 0.71303 |
| +4.20 m, S5 | 145 | 461 | 0.39 | 49 | 23 | 144 | 5007 | 0.03 | 9.9 | 0.71562 |
| +4.20 m, S6 | 169 | 441 | 0.14 | 21 | 34 | 142 | 2514 | 0.06 | 8.5 | n.d. |
| +4.20 m, S7 | 222 | 656 | 0.11 | 19 | 85 | 104 | 1458 | 0.07 | 13.3 | 0.72046 |
| +4.20 m, S8 | 219 | 626 | 0.12 | 17 | 76 | 102 | 1306 | 0.08 | 13.3 | 0.72028 |
| +4.38 m, N1 | 194 | 6 | 1.59 | 55 | 1 | 1645 | 26980 | 0.06 | 2.1 | 0.72807 |
| +4.38 m, N2 | 103 | 14 | 1.58 | 93 | 2 | 207 | 20878 | 0.01 | 4.4 | 0.71223 |
| +4.38 m, S1 | 105 | 2 | 3.79 | 225 | 0 | 74 | 22782 | 0.00 | 9.9 | 0.70989 |
| +4.38 m, S2 | 104 | 4 | 3.99 | 238 | 1 | 85 | 24011 | 0.00 | 9.9 | 0.70977 |
| +4.38 m, S3 | 102 | 5 | 3.97 | 239 | 2 | 66 | 24744 | 0.00 | 9.6 | 0.70964 |
| +4.38 m, S4 | 93 | 33 | 2.53 | 172 | 4 | 61 | 18276 | 0.00 | 9.4 | 0.71005 |
| +4.38 m, S5 | 100 | 183 | 1.10 | 84 | 15 | 63 | 10218 | 0.01 | 8.2 | 0.71061 |
| +4.38 m, S6 | 127 | 200 | 0.36 | 32 | 20 | 59 | 4404 | 0.01 | 7.3 | 0.71258 |
| +4.38 m, S7 | 251 | 507 | 0.51 | 46 | 84 | 68 | 4864 | 0.01 | 9.4 | 0.71242 |
| +4.38 m, S8 | 205 | 333 | 0.36 | 32 | 63 | 54 | 3121 | 0.02 | 10.2 | 0.71223 |
| +4.90 m, N1 | 273 | 17 | 1.48 | 65 | 2 | 3129 | 31046 | 0.10 | 2.1 | 0.71398 |
| +4.90 m, N2 | 124 | 28 | 1.35 | 100 | 5 | 317 | 28622 | 0.01 | 3.5 | 0.71156 |
| +4.90 m, S1 | 115 | 7 | 2.91 | 215 | 1 | 117 | 25023 | 0.00 | 8.6 | 0.70999 |
| +4.90 m, S2 | 157 | 22 | 3.08 | 234 | 3 | 192 | 29003 | 0.01 | 8.1 | 0.71004 |
| +4.90 m, S3 | 161 | 140 | 0.65 | 78 | 10 | 150 | 12266 | 0.01 | 6.3 | n.d. |
| +4.90 m, S4 | 162 | 133 | 0.25 | 30 | 12 | 91 | 6737 | 0.01 | 4.5 | n.d. |
| +4.90 m, S5 | 211 | 348 | 0.26 | 29 | 37 | 91 | 6147 | 0.01 | 4.7 | n.d. |
| +4.90 m, S6 | 248 | 329 | 0.14 | 18 | 48 | 78 | 4910 | 0.02 | 3.7 | n.d. |
| +4.90 m, S7 | 245 | 413 | 0.07 | 11 | 88 | 54 | 1697 | 0.03 | 6.7 | 0.71635 |
| +4.90 m, S8 | 351 | 659 | 0.10 | 15 | 135 | 63 | 1451 | 0.04 | 10.7 | 0.71751 |
| +5.65 m, N1_rep1 | 546 | 15 | 1.66 | 64 | 2 | 5502 | 17301 | 0.32 | 3.7 | 0.72204 |
| +5.65 m, N2_rep1 | 195 | 25 | 1.37 | 99 | 3 | 566 | 13203 | 0.04 | 7.5 | 0.71338 |
| +5.65 m, S1_rep1 | 180 | 12 | 2.96 | 240 | 2 | 176 | 18463 | 0.01 | 13.0 | 0.71000 |
| +5.65 m, S2_rep1 | 215 | 25 | 2.88 | 238 | 4 | 200 | 18912 | 0.01 | 12.6 | 0.71000 |
| +5.65 m, S3_rep1 | 244 | 174 | 0.81 | 109 | 11 | 176 | 6244 | 0.03 | 17.5 | 0.71258 |
| +5.65 m, S4_rep1 | 264 | 181 | 0.36 | 48 | 14 | 105 | 2869 | 0.04 | 16.6 | 0.7162 |
| +5.65 m, S5_rep1 | 337 | 684 | 0.32 | 42 | 59 | 120 | 2419 | 0.05 | 17.2 | 0.72024 |
| +5.65 m, S6_rep1 | 304 | 431 | 0.11 | 19 | 51 | 81 | 824 | 0.10 | 22.7 | 0.72909 |
| +5.65 m, S7_rep1 | 644 | 1275 | 0.09 | 23 | 214 | 88 | 694 | 0.13 | 33.9 | 0.73967 |
| +5.65 m, S8_rep1 | 561 | 1200 | 0.08 | 19 | 179 | 78 | 564 | 0.14 | 33.4 | |
| +5.65 m, N1_rep2 | 533 | 9 | 1.25 | 51 | 1 | 5407 | 16337 | 0.33 | 3.1 | 0.72299 |
| +5.65 m, N2_rep2 | 226 | 12 | 1.28 | 82 | 2 | 709 | 13812 | 0.05 | 5.9 | 0.71374 |
| +5.65 m, S1_rep2 | 189 | 12 | 2.99 | 220 | 1 | 176 | 18944 | 0.01 | 11.6 | 0.70964 |
| +5.65 m, S2_rep2 | 210 | 14 | 3.04 | 230 | 2 | 179 | 18929 | 0.01 | 12.2 | 0.70977 |
| +5.65 m, S3_rep2 | 189 | 79 | 1.24 | 132 | 5 | 124 | 10000 | 0.01 | 13.2 | 0.71075 |
| +5.65 m, S4_rep2 | 257 | 171 | 0.54 | 69 | 11 | 118 | 4531 | 0.03 | 15.3 | 0.71359 |
| +5.65 m, S5_rep2 | 427 | 773 | 0.41 | 58 | 58 | 147 | 3335 | 0.04 | 17.4 | 0.71779 |
| +5.65 m, S6_rep2 | 327 | 540 | 0.15 | 24 | 50 | 98 | 1156 | 0.08 | 21.0 | 0.72447 |
| +5.65 m, S7_rep2 | 704 | 1512 | 0.15 | 31 | 210 | 97 | 1104 | 0.09 | 28.0 | 0.72969 |
| +5.65 m, S8_rep2 | 527 | 1057 | 0.09 | 19 | 153 | 74 | 615 | 0.12 | 31.0 | 0.73106 |
Table S-8 Strontium isotope results of basement rocks and impact/volcanic debris layer. 87Sr/86Sr ratios at 1.2 Ga were calculated as described in Section S1.7.
| ID | Sr [ppm] | Rb [ppm] | (87Sr/86Sr)measured | (87Sr/86Sr)1.2Ga | type |
| A6 | 579.3 | 7.9 | 0.70294 | 0.70227 | mafic |
| A2 | 249.8 | 9.2 | 0.70689 | 0.70508 | mafic |
| G2 | 242.1 | 23.8 | 0.72037 | 0.71554 | felsic |
| G1 | 121.1 | 56.0 | 0.74991 | 0.72717 | felsic |
| -5 m | 198.5 | 46.7 | 0.71822 | 0.70666 | impact/volcanic |
| -4.5 m | 243.5 | 40.5 | 0.71422 | 0.70604 | impact/volcanic |
S2. Preservation of Primary Signatures
2.1 Alteration of Sr isotopes
It has been demonstrated in previous studies that Sr isotopic ratios of carbonates can be altered by later fluid infiltration into the host rock (Banner and Hanson, 1990). This form of alteration can also lead to a covariance between δ18Ocarb and δ13Ccarb and a lowering of absolute δ18Ocarb and δ13Ccarb values (Shields and Veizer, 2002; Bartley and Kah, 2004). Furthermore, anoxic diagenetic fluids can be enriched in Mn, thus leading to elevated Mn/Sr ratios (Gilleaudeau and Kah, 2013a). Our samples do indeed show a covariation between δ18Ocarb and δ13Ccarb, which may reflect some degree of fluid alteration of these rocks. We note, however, that such trends may also have been induced by primary environmental factors such as synchronous evaporation and oxidation of biomass. Mn/Sr ratios in carbonate extracts of our samples (range 3.7–33.9, average 12.4) overlap well with those of unaltered carbonates from the nearly contemporaneous (1.1 Ga) Taoudeni Basin in northwestern Africa (0.7–15.8, average 6.2, in the Aguelt el Mabha and Tourist formations; Gilleaudeau and Kah, 2013a). Even though we cannot fully exclude alteration based on these parameters, the data do not necessarily indicate massive secondary overprint.
Sr isotope fractionation almost exclusively leads to more radiogenic 87Sr/86Sr (Banner and Hanson, 1990; Brand, 2004). There are a few rare exceptions which show non-radiogenic alteration, however these typically show large local abundance of non-radiogenic country rock surrounding the carbonate units (e.g., Miller et al., 2008). In any case, Stoer Group fluid alteration would most likely have elevated the primary 87Sr/86Sr ratios, because meteoric fluids draining the hinterland should have been buffered by the Lewisian basement (0.715–0.851, Lyon et al., 1975, this study). Rare earth element patterns and zircon age distributions of the Stoer sediments match those of the Lewisian gneiss, confirming that there was probably no other significant sediment source (Stewart, 2002 and references therein) and hence probably no other rock type that imparted a significant control on the water chemistry. Mafic rocks (now amphibolites) make up a very small component of the basement. This felsic buffering effect is illustrated by the presence of diagenetic K-feldspar in the Stac Fada Member underneath the Poll a’Mhuilt Member (Parnell et al., 2011). The K-feldspar is thought to have been the first mineral that precipitated in gas escape structures immediately after the proposed impact event that shaped sediment deposition in the Stac Fada Member (Parnell et al., 2011). This event precedes the deposition of the Poll a’Mhuilt Member and could in itself not have contributed to alteration of our samples. Nevertheless, the presence of the K-feldspar is an example of felsic buffering of fluids at regional scale. Although the Stac Fada Member contains mafic volcanic fragments, the diagenetic K-feldspar suggests that the composition of fluids was dominated by that of the largely felsic mineralogy of the Lewisian gneiss and the Stoer Group sandstone. It is therefore unlikely that the mafic components of the Stac Fada Member or other mafic rocks in the Lewisian basement imparted a significant control on the composition of secondary fluids.
2.2 Metamorphic alteration of redox proxies
The metamorphic grade of the Poll a’Mhuilt Member is below greenschist facies (Stewart, 2002) and thus well within the range of other sedimentary rocks that have been studied successfully with the same biogeochemical proxies (e.g., Anbar et al., 2007; Reinhard et al., 2009; Kendall et al., 2011; Gilleaudeau and Kah, 2013b). Metamorphic effects on the Fe redox proxy and on Mo enrichments or isotopes have so far not been investigated systematically, but they are unlikely to have caused the observed patterns. Poulton and Raiswell (2002) showed that sedimentary rocks back to the Cambrian with palaeontological evidence for oxic conditions tend to have slightly lower FeHR/FeT ratios compared to modern oxic marine muds. They interpreted this observation as evidence for post-depositional Fe3+ reduction to mobilise Fe2+ which could subsequently have been lost. Alternatively, or in addition, they speculated that some reactive iron phases may react to form clays and thus get incorporated into the unreactive siliciclastic phase, which would also lower the FeHR/FeT ratio. These processes could potentially also have occurred in the Stoer Group with the implication that our measured FeHR/FeT ratios may originally have been higher, making it more likely that deep waters in the Stoer setting were indeed anoxic as discussed in the main text. Metamorphic addition of Mo is also unlikely, because there is no plausible Mo-rich source rock in the area (see discussion in Section S2.3).
2.3 Metasomatic effects on sulphide phases and Mo isotopes
As mentioned above and noted by Parnell et al. (2010; 2014), the calcareous grey shale in the middle Poll a’Mhuilt Member not only contains pyrite but also minor amounts of other sulphide mineral phases, including chalcopyrite, galena, sphalerite and greenockite. This finding raises the question of whether secondary fluids could have added excess amounts of sulphur and transition metals to these rocks, thus mimicking a euxinic signature with high Mo enrichments. These particular sulphide minerals are part of the acid-volatile sulphide phase (AVS) (Rohwerder and Sand, 2007), but our own measurements of this AVS component revealed that it makes up only between 1.9 and 7.6 % of the total sulphide mineral load. It is thus relatively minor. We further note that Cu, Pb, Zn and Cd show no to weak enrichments in our bulk rock data, which further indicates that they are not anomalously abundant. Furthermore, the sulphide minerals occur as clusters and nodules that appear to be pre-compactional, because depositional laminae are deflected around them (Parnell et al., 2010, 2014; this study). Sulphide laminae or veins are absent. This morphology points to an early diagenetic origin of the sulphide clusters and is inconsistent with precipitation from a late-stage fluid. It is further unlikely that such fluids, if they existed, were the source of the molybdenum, because much of the molybdenum is organic-bound as shown by XRF element maps presented by Parnell et al. (2015). We performed SEM analyses of the sulphide clusters and did not find any Mo above detection limit (<0.1 %). In contrast, if all Mo were sulphide-bound, then a rock with 200 ppm Mo and 2 % pyrite should show around 1 % Mo in the pyrite grains.
Lastly, it is difficult to envision a metasomatic fluid that is so highly enriched in Mo and S but shows only weak enrichments in other elements. On the other hand, seawater could plausibly explain this pattern. We speculate that the seawater that flooded this setting during high-tide or sealevel high-stand would have been from the photic zone, which, as today, was depleted in Cr, Co and Ni due to biological consumption (Yuan-Hui, 1991). Depletions in Zn and Cd may have been less extreme prior to the expansion of eukaryotic consumers (Dupont et al., 2010; Scott et al., 2012), allowing for minor accumulation in the photic zone and small enrichments in our samples. Molybdenum and U behave conservatively today, and As and V are only mildly depleted in the photic zone (Yuan-Hui, 1991). It is probable that Mo, U, As, and V had lower average concentrations in the Mesoproterozoic ocean (Sahoo et al., 2012; Partin et al., 2013; Reinhard et al., 2013b), but that possibility does not preclude moderate abundances in oxic surface waters (e.g., Algeo and Lyons, 2006). Hence, the enrichment patterns in the Poll a’Mhuilt shales may be a product of elemental profiles in seawater at that time.
2.4 Modern oxidative weathering
As noted in Figure 1, we observed a weathered horizon within the calcareous grey shale package, and the sample from that horizon was depleted in pyrite and Mo relative to surrounding samples. We excluded it from the discussion. If outcrop weathering had affected our samples at a larger scale, it would have weakened the primary signature that forms the basis of our interpretation. Modern weathering tends to preferentially mobilise isotopically heavy Mo (Pearce et al., 2010), rendering the residue lighter. The isotopic enrichments of our grey shale samples relative to average crust thus cannot be explained by weathering effects. Overall, the degree of weathering on most of our samples was probably minor, because otherwise this relatively large amount of pyrite would not have been preserved. Pyrite weathers rapidly under the modern atmosphere (Petsch et al., 2000). Oxidative weathering would have lowered the FePy/FeHR ratio as iron sulphide (FePY) would have been converted into iron oxide (part of FeHR). The fact that we still see FePy/FeHR ratios up to 0.8 is thus a strong indication that oxidative weathering has not erased primary signatures.
S3. Potential Caveats for the Iron Redox Proxy
It is important to recognise that the iron redox proxy applied in this study was developed and calibrated with fine-grained marine muds and shales from environments with relatively low sedimentation rates (Berner, 1970; Raiswell et al., 1988; Canfield, 1989; Raiswell et al., 1994; Poulton and Canfield, 2005). Under these conditions, sediments from oxic water columns consistently display FeHR/FeT ratios of <0.38, while anoxic environments are enriched in FeHR/FeT (Canfield et al., 1996; Raiswell and Canfield, 1996, 1998; Raiswell et al., 2001; Wijsman et al., 2001; Lyons et al., 2003). There are two possible sources of reactive iron that can lead to such enrichments: (1) hydrothermal vents on the seafloor, which may have been particularly important sources of iron in the Precambrian when the deep ocean was largely anoxic and dissolved Fe could stay in solution (e.g., Isley and Abbott, 1999; Poulton and Raiswell, 2002; Poulton and Canfield, 2011) and (2) diagenetic mobilisation of iron from pore waters on the continental shelf, followed by redeposition in other parts of the basin (“iron shuttle”, Canfield et al., 1996; Lyons, 1997; Wijsman et al., 2001). Hence, for the FeHR/FeT proxy to be applicable, two conditions have to be fulfilled. First, either one or both of these reactive iron sources have to be present and, second, sedimentation rates need to be sufficiently low such that accumulations of authigenic iron minerals are not diluted by siliciclastic background sedimentation (Lyons and Berner, 1992; Lyons and Severmann, 2006).
In the case of the Poll a’Mhuilt Member, sedimentation rates were probably not exceedingly high because in that case we should not expect to observe such marked enrichments in Mo. However, the first condition, a reactive iron source, may not have been fulfilled in this particular setting. As discussed above and in the main text, the Mo enrichment is most parsimoniously explained by influx of surface seawater, which was likely oxygenated in the mid-Proterozoic (Stüeken, 2013; Reinhard et al., 2016; Hardisty et al., 2017; Koehler et al., 2017). Oxic waters do not carry significant amounts of dissolved iron because of the insolubility of the Fe3+ state. We can therefore rule out an external input of iron into the Stoer setting from the open ocean, including from hydrothermal vents. The burden thus lies on the intrabasinal iron shuttle. In this scenario, iron is mobilised from sediments by diagenetic reduction of Fe3+ to Fe2+, which can then escape into the water column and get transported in the form of colloids or organic complexes (reviewed by Raiswell, 2011). The release requires suppressed sulphate reduction rates in pore waters, such that Fe2+ is not captured in sulphide minerals, and it is aided by bioturbation. Both factors may not have been applicable in the Stoer setting. Sulphate levels high enough to reach gypsum saturation may have fostered diagenetic sulphate reduction and Fe2+ retention. Furthermore, bioturbation would have been absent at 1.2 Ga, making it more difficult for Fe2+ to be released from pore waters into the water column. Lastly, rapid evaporation and desiccation of shallow sedimentary beds where diagenetic Fe3+ reduction may have taken place could further have impeded iron transport and enrichment of FeHR/FeT in deeper sediments. Iron shuttling has so far not been investigated in evaporitic settings, but for these reasons listed above it may not operate as efficiently as it does in open marine systems.
In conclusion, our measured FeHR/FeT ratios likely provide a conservative estimate of the water column redox state. Although only two of the black shales samples fall firmly into the empirically defined anoxic field in Figure 3a (FeHR/FeT > 0.38), it is likely that the lower part of the water column was indeed anoxic, as supported by the high enrichments in Mo, which are highly uncharacteristic of oxic sediments (Scott and Lyons, 2012).
Supplementary Information References
Algeo, T.J., Lyons, T.W. (2006) Mo–total organic carbon covariation in modern anoxic marine environments: Implications for analysis of paleoredox and paleohydrographic conditions. Paleoceanography 21, doi: 10.1029/2004PA001112.
Anbar, A., Duan, Y., Lyons, T.W., Arnold, G.L., Kendall, B., Creaser, R.A., Kaufman, A.J., Gordon, G.W., Scott, C.T., Garvin, J., Buick, R. (2007) A whiff of oxygen before the Great Oxidation Event? Science 317, 1903–1906.
Asael, D., Tissot, F.L., Reinhard, C.T., Rouxel, O., Dauphas, N., Lyons, T.W., Ponzevera, E., Liorzou, C., Chéron, S. (2013) Coupled molybdenum, iron and uranium stable isotopes as oceanic paleoredox proxies during the Paleoproterozoic Shunga Event. Chemical Geology 362, 193–210.
Banner, J.L., Hanson, G.N. (1990) Calculations of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochimica et Cosmochimica Acta 54.
Bartley, J.K., Kah, L.C. (2004) Marine carbon reservoir, Corg-Ccarb coupling, and the evolution of the Proterozoic carbon cycle. Geology 32, 129–132.
Bellefroid, E.J., Planavsky, N., Jiang, G., Hood, A. (2015) A Sequential Leaching Method to Measuring Primary Signatures on Partially Altered Bulk Carbonates. Goldschmidt Conference 2015 (Prague), A248.
Berner, R.A. (1970) Sedimentary pyrite formation. American Journal of Science 268, 1–23.
Brand, U. (2004) Carbon, oxygen and strontium isotopes in Paleozoic carbonate components: an evaluation of original seawater-chemistry proxies. Chemical Geology 204, 23–44.
Canfield, D.E. (1989) Reactive iron in marine sediments. Geochimica et Cosmochimica Acta 53, 619–632.
Canfield, D.E., Lyons, T.W., Raiswell, R. (1996) A model for iron deposition to euxinic Black Sea sediments. American Journal of Science 296, 818–834.
Canfield, D.E., Raiswell, R., Westrich, J.T., Reaves, C.M., Berner, R.A. (1986) The use of chromium reduction in the analysis of reduced inorganic sulphur in sediments and shales. Chemical Geology 54, 149–155.
Dupont, C.L., Butcher, A., Valas, R.E., Bourne, P.E., Caetano-Anollés, G. (2010) History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proceedings of the National Academy of Sciences 107, 10567–10572.
Gilleaudeau, G.J., Kah, L.C. (2013a) Carbon isotope records in a Mesoproterozoic epicratonic sea: carbon cycling in a low-oxygen world. Precambrian Research 228, 85–101.
Gilleaudeau, G.J., Kah, L.C. (2013b) Oceanic molybdenum drawdown by epeiric sea expansion in the Mesoproterozoic. Chemical Geology 356, 21–37.
Grünenfelder, M.H., Tilton, G.R., Bell, K., Blenkinsop, J. (1986) Lead and strontium isotope relationships in the Oka carbonatite complex, Quebec. Geochimica et Cosmochimica Acta 50, 461–468.
Hardisty, D.S., Lu, Z., Bekker, A., Diamond, C.W., Gill, B.C., Jiang, G., Kah, L.C., Knoll, A.H., Loyd, S.J., Osburn, M.R., Planavsky, N.J. (2017) Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth and Planetary Science Letters 463, 159–170.
Isley, A.E., Abbott, D.H. (1999) Plume-related mafic volcanism and the deposition of banded iron formation. Journal of Geophysical Research: Solid Earth 104, 15461–15477.
Kendall, B., Gordon, G.W., Poulton, S.W., Anbar, A.D. (2011) Molybdenum isotope constraints on the extent of late Paleoproterozoic ocean euxinia. Earth and Planetary Science Letters 307, 450–460.
Koehler, M.C., Stüeken, E.E., Kipp, M.A., Buick, R., Knoll, A.H. (2017) Spatial and temporal trends in Precambrian nitrogen cycling: a Mesoproterozoic offshore nitrate minimum. Geochimica et Cosmochimica Acta 198, 315–337.
Liu, C., Wang, Z., Raub, T.D. (2013) Geochemical constraints on the origin of Marinoan cap dolostones from Nuccaleena Formation, South Australia. Chemical Geology 351, 95–104.
Lyon, T.D.B., Gillen, C., Bowes, D.R. (1975) Rb-Sr isotopic studies near the major Precambrian junction, between Scourie and Loch Laxford, northwest Scotland. Scottish Journal of Geology 11, 333–337.
Lyons, T.W. (1997) Sulphur isotopic trends and pathways of iron sulphide formation in upper Holocene sediments of the anoxic Black Sea. Geochimica et Cosmochimica Acta 61, 3367–3382.
Lyons, T.W., Berner, R.A. (1992) Carbon-sulphur-iron systematics of the uppermost deep-water sediments of the Black Sea. Chemical Geology 99, 1–27.
Lyons, T.W., Severmann, S. (2006) A critical look at iron paleoredox proxies: new insights from modern euxinic marine basins. Geochimica et Cosmochimica Acta 70, 5698–5722.
Lyons, T.W., Werne, J.P., Hollander, D.J., Murray, R.W. (2003) Contrasting sulphur geochemistry and Fe/Al and Mo/Al ratios across the last oxic-to-anoxic transition in the Cariaco Basin, Venezuela. Chemical Geology 195, 131–157.
Miller, N., Johnson, P.R., Stern, R.J. (2008) Marine Versus Non-Marine Environments for the Jibalah Group, NW Arabian Shield:--A Sedimentologic and Geochemical Survey and Report of Possible Metazoa in the Dhaiqa Formation. Arabian Journal for Science and Engineering 33, 55–78.
Nägler, T.F., Anbar, A.D., Archer, C., Goldberg, T., Gordon, G.W., Greber, N.D., Siebert, C., Sohrin, Y., Vance, D. (2014) Proposal for an international molybdenum isotope measurement standard and data representation. Geostandards and Geoanalytical Research 38, 149–151.
Parnell, J., Boyce, A.J., Mark, D., Bowden, S., Spinks, S. (2010) Early oxygenation of the terrestrial environment during the Mesoproterozoic. Nature 468, 290–293.
Parnell, J., Mark, D., Fallick, A.E., Boyce, A., Thackrey, S. (2011) The age of the Mesoproterozoic Stoer Group sedimentary and impact deposits, NW Scotland. Journal of the Geological Society 168, 349–358.
Parnell, J., Still, J., Spinks, S., Thayalan, W., Bowden, S. (2014) Cadmium sulphide in a Mesoproterozoic terrestrial environment. Mineralogical Magazine 78, 47–54.
Parnell, J., Spinks, S., Andrews, S., Thayalan, W., Bowden, S. (2015) High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nature Communications 6, doi:10.1038/ncomms7996.
Partin, C.A., Lalonde, S.V., Planavsky, N.J., Bekker, A., Rouxel, O.J., Lyons, T.W., Konhauser, K.O. (2013) Uranium in iron formations and the rise of atmospheric oxygen. Chemical Geology 362, 82–90.
Pearce, C.R., Burton, K.W., von Strandmann, P.A.P., James, R.H., Gíslason, S.R. (2010) Molybdenum isotope behaviour accompanying weathering and riverine transport in a basaltic terrain. Earth and Planetary Science Letters 295, 104–114.
Petsch, S.T., Berner, R.A., Eglinton, T.I. (2000) A field study of the chemical weathering of ancient sedimentary organic matter. Organic Geochemistry 31, 475–487.
Planavsky, N.J., Asael, D., Hofmann, A., Reinhard, C.T., Lalonde, S.V., Knudsen, A., Wang, X., Ossa Ossa, F., Pecoits, E., Smith, A.J.B., Beukes, N.J., Bekker, A., Johnson, T.M., Konhauser, K., Lyons, T.W., Rouxel, O.J. (2014) Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nature Geoscience 7, 283–286.
Poulton, S.W., Canfield, D.E. (2005) Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates. Chemical Geology 214, 209–221.
Poulton, S.W., Canfield, D.E. (2011) Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7, 107–112.
Poulton, S.W., Raiswell, R. (2002) The low-temperature geochemical cycle of iron: from continental fluxes to marine sediment deposition. American Journal of Science 302, 774–805.
Raiswell, R. (2011) Iron transport from the continents to the open ocean: The aging–rejuvenation cycle. Elements 7, 101–106.
Raiswell, R., Canfield, D.E. (1996) Rates of reaction between silicate iron and dissolved sulphide in Peru Margin sediments. Geochimica et Cosmochimica Acta 60, 2777–2787.
Raiswell, R., Canfield, D.E. (1998) Sources of iron for pyrite formation in marine sediments. American Journal of Science 298, 219–245.
Raiswell, R., Buckley, F., Berner, R.A., Anderson, T.F. (1988) Degree of pyritization of iron as a paleoenvironmental indicator of bottom-water oxygenation. Journal of Sedimentary Research 58, 812–819.
Raiswell, R., Canfield, D.E., Berner, R.A. (1994) A comparison of iron extraction methods for the determination of degree of pyritisation and the recognition of iron-limited pyrite formation. Chemical Geology 111, 101–110.
Raiswell, R., Newton, R., Wignall, P.B. (2001) An indicator of water-column anoxia: resolution of biofacies variations in the Kimmeridge Clay (Upper Jurassic, UK). Journal of Sedimentary Research 71, 286–294.
Reinhard, C.T., Raiswell, R., Scott, C.T., Anbar, A., Lyons, T.W. (2009) A late Archaean sulfidic sea stimulated by early oxidative weathering of the continents. Science 326, 713–716.
Reinhard, C.T., Lyons, T.W., Rouxel, O., Asael, D., Dauphas, N., Kump, L.R. (2013a) Iron Speciation and Isotope Perspectives on Palaeoproterozoic Water Column Chemistry. In: Kump, L.R., Kuznetsov, A.B., Gorokhov, I.M., Melezhik, V.A., Farkaš, J., Chakrabarti, R., Jacobsen, S.B., Reinhard, C.T., Lyons, T.W., Rouxel, O., Asael, D., Dauphas, N., van Zuilen, M., Schoenberg, R., Tissot, F.L.H., Hannah, J.L., Stein, H.J. (Eds.) Reading the Archive of Earth’s Oxygenation. Springer, Berlin, 1483–1492.
Reinhard, C.T., Planavsky, N.J., Robbins, L.J., Partin, C.A., Gill, B.C., Lalonde, S.V., Bekker, A., Konhauser, K.O., Lyons, T.W. (2013b) Proterozoic ocean redox and biogeochemical stasis. Proceedings of the National Academy of Sciences, 110, 5357–5362.
Reinhard, C.T., Planavsky, N., Olson, S.L., Lyons, T.W., Erwin, D.H. (2016) Earth's oxygen cycle and the evolution of animal life. Proceedings of the National Academy of Sciences, doi: 10.1073/pnas.1521544113.
Rohwerder, T., Sand, W. (2007) Mechanisms and biochemical fundamentals of bacterial metal sulphide oxidation. In: Donati, E.R., Sand, W. (Eds.) Microbial processing of metal sulphides. Springer Netherlands, Amsterdam, 35–58.
Romaniello, S.J., Field, M.P., Smith, H.B., Gordon, G.W., Kim, M.H., Anbar, A.D. (2015) Fully automated chromatographic purification of Sr and Ca for isotopic analysis. Journal of Analytical Atomic Spectrometry 30, 1906–1912.
Sahoo, S.K., Planavsky, N.J., Kendall, B., Wang, X., Shi, X., Scott, C., Anbar, A.D., Lyons, T.W., Jiang, G. (2012) Ocean oxygenation in the wake of the Marinoan glaciation. Nature 489, 546–549.
Scott, C., Lyons, T.W. (2012) Contrasting molybdenum cycling and isotopic properties in euxinic versus non-euxinic sediments and sedimentary rocks: refining the paleoproxies. Chemical Geology 324, 19–27.
Scott, C., Planavsky, N.J., Dupont, C.L., Kendall, B., Gill, B.C., Robbins, L.J., Husband, K.F., Arnold, G.L., Wing, B., Poulton, S.W., Bekker, A., Anbar, A., Konhauser, K., Lyons, T.W. (2012) Bioavailability of zinc in marine systems through time. Nature Geoscience 6, 125–128.
Shields, G., Veizer, J. (2002) Precambrian marine carbonate isotope database: Version 1.1. Geochemistry Geophysics Geosystems 3, doi: 10.1029/2001GC000266.
Stewart, A.D. (2002) The later Proterozoic Torridonian rocks of Scotland: Their sedimentology, geochemistry and origin. Geological Society, Bath.
Stüeken, E.E. (2013) A test of the nitrogen-limitation hypothesis for retarded eukaryote radiation: nitrogen isotopes across a Mesoproterozoic basinal profile. Geochimica et Cosmochimica Acta 120, 121–139.
Wijsman, J.W., Middelburg, J.J., Herman, P.M., Böttcher, M.E., Heip, C.H. (2001) Sulphur and iron speciation in surface sediments along the northwestern margin of the Black Sea. Marine Chemistry 74, 261–278.
Yuan-Hui, L. (1991) Distribution patterns of the elements in the ocean: A synthesis. Geochimica et Cosmochimica Acta 55, 3223–3240.
Figures and Tables
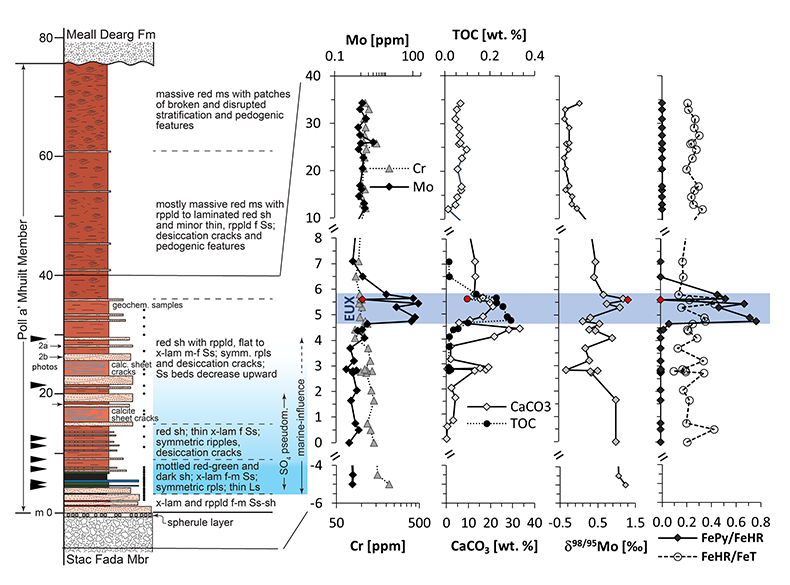
Figure 1 Stratigraphy of the Poll a’Mhuilt Member. EUX = euxinic interval. The horizon at 5.6 m (red symbols) was affected by modern oxidative weathering and is not considered in the discussion. Black arrows = locations of tidal indicators.
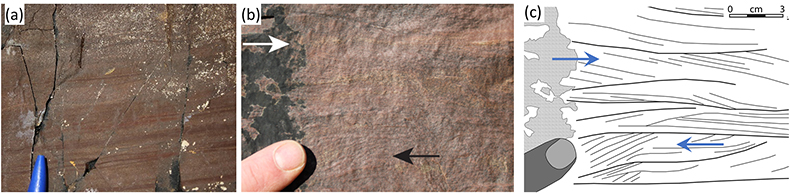
Figure 2 Sedimentary features compatible with a marine setting in the middle Poll a’Mhuilt Member. (a) Flaser- and lenticular-bedding. (b) Superposed sets of herringbone cross-lamination, 3D exposures confirm bi-directional character. Stratigraphic positions are marked in Figure 1. (c) Line drawing of sedimentary features showing superposed sets of bi-polar cross-laminated ripples (herringbone) with multiple reactivation surfaces commonly with thin clay drapes.
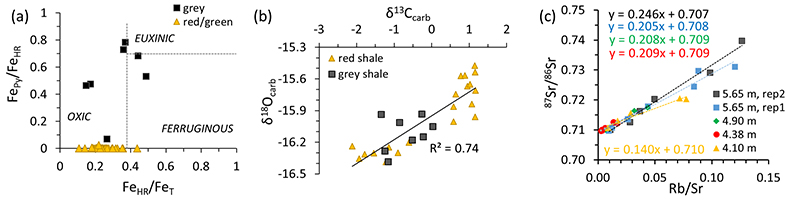
Figure 3 (a) Iron speciation, (b) carbonate C and O isotopes, and (c) carbonate-bound Sr isotopes. Dashed lines in (a) mark redox transitions (Poulton and Canfield, 2011
Poulton, S.W., Canfield, D.E. (2011) Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7, 107–112.
). For comparison to our data in panel (b), values of contemporaneous unaltered marine carbonates fall between -10 ‰ and -7 ‰ for δ18Ocarb and 0 ‰ and +2 ‰ for δ13Ccarb (Shields and Veizer, 2002Shields, G., Veizer, J. (2002) Precambrian marine carbonate isotope database: Version 1.1. Geochemistry Geophysics Geosystems 3, doi: 10.1029/2001GC000266.
) (see Fig. S-2 for discussion). In panel (c), data points represent individual leaches increasing acid strength; y-axis intercept = carbonate end-member.Back to article
Supplementary Figures and Tables
Table S-1 Organic carbon isotopes and abundances. TOC = total organic carbon, RE = relative error, SD = standard deviation.
| position | TOC | RE | δ13Corg | SD |
| [m] | [%] | [%] | [‰] | [‰] |
| 2.73 | 0.01 | 4.73 | -27.22 | 1.14 |
| 2.77 | 0.02 | 4.62 | -26.48 | 0.28 |
| 2.83 | 0.01 | 2.46 | -26.80 | 0.23 |
| 2.87 | 0.01 | 0.21 | -25.98 | 0.62 |
| 3.7 | 0.01 | 15.62 | -28.11 | 0.84 |
| 4.1 | 0.02 | 2.71 | -28.68 | 0.00 |
| 4.38 | 0.04 | 1.44 | -28.85 | 0.12 |
| 4.42 | 0.07 | 2.61 | -29.73 | 0.05 |
| 4.65 | 0.12 | 0.33 | -30.96 | 0.07 |
| 4.75 | 0.36 | 1.02 | -31.23 | 0.08 |
| 4.9 | 0.34 | 0.44 | -30.86 | 0.01 |
| 5.3 | 0.32 | 1.10 | -30.50 | 0.01 |
| 5.45 | 0.28 | 0.51 | -30.38 | 0.01 |
| 5.6 | 0.12 | 0.79 | -29.94 | 0.05 |
| 5.65 | 0.28 | 0.36 | -30.29 | 0.05 |
| 5.8 | 0.17 | 1.70 | -30.33 | 0.04 |
| 6.5 | 0.02 | 0.06 | -26.27 | 0.46 |
| 7.1 | 0.01 | 5.30 | -25.04 | 0.14 |
Table S-2 Carbonate carbon and oxygen isotopes and carbonate abundances (carb.). SD = standard deviation, carb = carbonate content by weight, RE = relative error.
| position | δ13Ccarb | SD | δ18Ocarb | SD | % carb. | RE |
| [m] | [‰] | [‰] | [‰] | [‰] | [%] | |
| 2.73 | -1.94 | 0.01 | -16.36 | 0.03 | 12.8 | 0.9 |
| 2.77 | -1.78 | 0.00 | -16.31 | 0.02 | 18.0 | 2.8 |
| 2.83 | -1.54 | 0.01 | -16.30 | 0.00 | 15.3 | 5.5 |
| 2.87 | -1.22 | 0.04 | -16.39 | 0.02 | 19.0 | 0.3 |
| 4.10 | -1.14 | 0.01 | -16.25 | 0.01 | 21.8 | 0.8 |
| 4.38 | -0.90 | 0.00 | -16.30 | 0.00 | 28.3 | 3.8 |
| 4.42 | -0.61 | 0.01 | -16.20 | 0.02 | 33.3 | 5.1 |
| 4.65 | -1.35 | 0.00 | -15.94 | 0.00 | 5.7 | 2.6 |
| 4.75 | -1.25 | 0.01 | -16.28 | 0.01 | 10.6 | 2.2 |
| 4.90 | -1.16 | 0.00 | -16.38 | 0.03 | 16.6 | 8.4 |
| 5.30 | -0.23 | 0.00 | -16.15 | 0.00 | 21.0 | 1.5 |
| 5.45 | 0.02 | 0.01 | -16.05 | 0.05 | 19.7 | 0.0 |
| 5.60 | -0.52 | 0.00 | -16.18 | 0.03 | 15.4 | 0.5 |
| 5.65 | -0.27 | 0.01 | -15.93 | 0.01 | 16.6 | 0.4 |
| 5.80 | -0.86 | 0.01 | -16.01 | 0.03 | 12.6 | 0.7 |
| 6.50 | -2.02 | 0.01 | -15.06 | 0.02 | 13.2 | 0.5 |
| 7.10 | -2.12 | 0.01 | -16.24 | 0.02 | 13.0 | 6.2 |
| 16.05 | 0.58 | 0.01 | -15.86 | 0.01 | 7.3 | 7.2 |
| 16.85 | 0.65 | -15.75 | 7.5 | |||
| 20.45 | 0.58 | -16.00 | 5.7 | |||
| 22.75 | 0.77 | -15.57 | 7.7 | |||
| 24.55 | 0.88 | 0.02 | -15.57 | 0.02 | 9.7 | 0.2 |
| 25.75 | 0.96 | -15.67 | 6.4 | |||
| 25.95 | 1.03 | -15.65 | 7.0 | |||
| 27.55 | 1.07 | -15.84 | 6.6 | |||
| 29.15 | 1.15 | 0.00 | -15.47 | 0.02 | 6.3 | 7.0 |
| 30.95 | 1.16 | -15.71 | 4.7 | |||
| 32.95 | 1.16 | -15.54 | 5.4 | |||
| 34.25 | 1.16 | 0.02 | -15.96 | 0.03 | 6.9 | 0.1 |
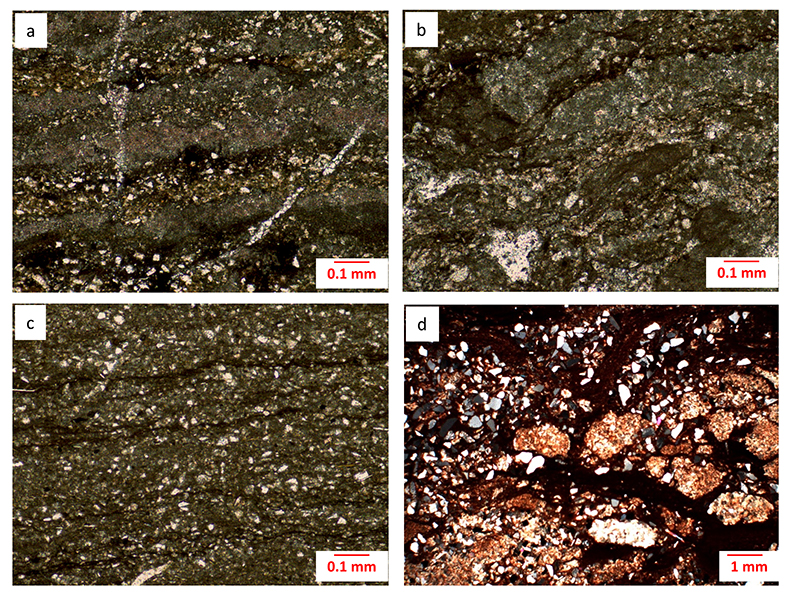
Figure S-1 Photomicrographs of the Poll a’Mhuilt Member. (a) Sample +2.77 m, plane-polarised light; calcareous red shale with 18 % CaCO3 present as microcrystalline laminae, separated by iron-oxide coated siliciclastics. (b) Sample +4.42 m, plane-polarised light; calcareous red shale with 33 % CaCO3 present as disrupted microcrystalline laminae and rare sparry fenestral fillings. (c) Sample +5.30 m, plane-polarised light; calcareous grey shale with 21 % CaCO3 present as microcrystalline laminae, separated by kerogenous siliciclastics. (d) Sample +25.75 m, crossed polars; partially desiccated facies with 6 % CaCO3 present as microcrystalline nodules and cement, stained with iron oxide, separated by iron-oxide coated siliciclastics. All samples contain angular silt grains composed of quartz, plagioclase, and K-feldspar and minor mica.
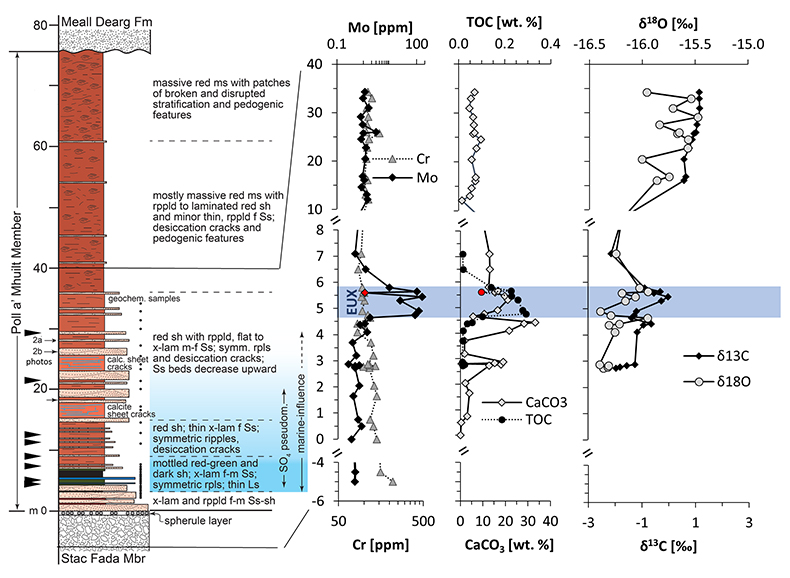
Figure S-2 Stratigraphic section through the Poll a’Mhuilt Member with carbonate C and O isotopes. Lithostratigraphy, Mo, Cr, CaCO3 and TOC abundances are as in Figure 1 in the main text. The last panel shows δ18O and δ13C in carbonate in stratigraphic context. The trend highlights that the heaviest values in both isotopic proxies occur in the upper red shale. This is consistent with the high abundance of desiccation cracks in this part of the section, because evaporation forces these parameters to heavier values. The grey shale, which would have been most continuously flooded, shows relatively light values, consistent with a relatively lesser impact of evaporation. Despite the high degree of evaporation that is implied by the presence of gypsum pseudomorphs in the section (Stewart, 2002; Parnell et al., 2010) the δ18O values are overall light compared to other mid-Proterozoic carbonates of similar age (Shields and Veizer, 2002; Bartley and Kah, 2004), which we attribute to fluid alteration. Oxygen isotopes are much more easily reset than carbon isotopes. However, the overall trend towards heavier values in the most evaporitic part of the section is preserved.
Table S-3 Bulk elemental abundances. The grey shale unit extends from +4.75 m to +5.8 m; the sample from +5.6 m is likely altered by modern weathering. Samples below 0 m are from the Stac Fada Member and contain volcanic fragments. Abundances are in weight-percent or parts per million (μg/g).
| position | Na | Mg | Al | P | K | Ca | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Sr | Mo | Cd | Pb | Th | U |
| [m] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [ppm] | [ppm] | [ppm] | [%] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] |
| Poll a'Mhuilt section: | ||||||||||||||||||||||
| -5.00 | 3.36 | 2.23 | 6.78 | 0.07 | 1.80 | 0.46 | 0.37 | 72.3 | 217.3 | 712.9 | 4.43 | 29.8 | 400.6 | 9.8 | 78.6 | 2.4 | 170.0 | 0.4 | 0.0 | 9.6 | 4.1 | 0.7 |
| -4.50 | 3.32 | 2.37 | 7.32 | 0.07 | 1.51 | 0.61 | 0.38 | 70.0 | 156.7 | 774.7 | 3.88 | 25.3 | 270.8 | 10.7 | 70.1 | 1.6 | 202.2 | 0.4 | 0.0 | 7.3 | 3.4 | 0.7 |
| 0.00 | 3.03 | 2.55 | 8.18 | 0.07 | 1.97 | 0.83 | 0.49 | 89.9 | 139.9 | 694.7 | 5.36 | 24.6 | 178.8 | 12.5 | 96.6 | 5.5 | 133.1 | 0.3 | 0.0 | 11.6 | 6.3 | 1.2 |
| 0.50 | 3.98 | 1.86 | 6.40 | 0.08 | 1.40 | 0.94 | 0.48 | 97.9 | 128.0 | 654.5 | 4.01 | 25.4 | 170.8 | 16.1 | 73.7 | 3.9 | 136.6 | 0.8 | 0.0 | 9.1 | 5.4 | 1.4 |
| 0.75 | 3.59 | 1.88 | 7.66 | 0.05 | 1.51 | 1.45 | 0.36 | 66.1 | 117.5 | 625.7 | 3.87 | 18.8 | 136.4 | 16.1 | 69.9 | 3.6 | 109.1 | 0.6 | 0.0 | 9.0 | 4.8 | 1.2 |
| 1.65 | 4.45 | 1.33 | 6.92 | 0.08 | 0.93 | 1.89 | 0.39 | 82.3 | 139.8 | 540.9 | 3.48 | 17.5 | 170.3 | 16.1 | 47.5 | 4.5 | 107.3 | 0.4 | 0.0 | 7.4 | 4.2 | 0.7 |
| 2.05 | 2.98 | 1.60 | 6.34 | 0.10 | 1.98 | 1.04 | 0.46 | 95.2 | 130.6 | 529.0 | 5.45 | 23.7 | 165.7 | 16.1 | 88.8 | 6.5 | 87.7 | 0.7 | 0.0 | 9.1 | 6.2 | 2.2 |
| 2.73 | 4.22 | 1.86 | 8.83 | 0.08 | 1.01 | 5.63 | 0.48 | 114.2 | 114.4 | 1025.6 | 3.70 | 21.1 | 94.3 | 16.1 | 63.6 | 4.2 | 116.1 | 0.5 | 0.0 | 14.4 | 5.5 | 5.2 |
| 2.77 | 3.32 | 1.41 | 7.22 | 0.07 | 1.15 | 7.49 | 0.43 | 87.9 | 98.2 | 1062.4 | 2.93 | 18.2 | 84.8 | 16.1 | 52.7 | 2.8 | 107.3 | 0.4 | 0.1 | 24.2 | 5.0 | 3.8 |
| 2.80 | 1.78 | 3.27 | 8.74 | 0.10 | 2.77 | 0.58 | 0.56 | 115.9 | 134.2 | 663.1 | 5.87 | 39.5 | 197.5 | 16.1 | 103.9 | 5.5 | 76.1 | 0.5 | 0.0 | 24.2 | 8.1 | 3.2 |
| 2.83 | 2.61 | 1.87 | 7.21 | 0.09 | 1.67 | 5.80 | 0.54 | 96.5 | 116.0 | 1125.6 | 3.84 | 27.4 | 129.6 | 16.1 | 78.4 | 4.3 | 101.1 | 0.6 | 0.1 | 19.4 | 5.5 | 4.7 |
| 2.87 | 3.12 | 1.78 | 7.19 | 0.07 | 1.38 | 7.58 | 0.43 | 89.4 | 110.0 | 1142.6 | 3.23 | 22.7 | 128.0 | 16.1 | 64.2 | 2.3 | 111.7 | 0.2 | 0.0 | 11.7 | 5.5 | 3.8 |
| 3.20 | 2.44 | 2.35 | 7.80 | 0.08 | 1.48 | 1.33 | 0.45 | 87.8 | 127.7 | 554.1 | 5.15 | 18.6 | 140.6 | 16.1 | 61.1 | 2.6 | 116.5 | 0.5 | 0.0 | 9.6 | 6.5 | 1.5 |
| 3.70 | 2.69 | 2.08 | 7.39 | 0.07 | 1.92 | 1.09 | 0.46 | 82.2 | 119.6 | 589.7 | 4.57 | 15.8 | 136.8 | 16.1 | 76.4 | 1.7 | 277.3 | 0.4 | 0.0 | 6.4 | 5.8 | 1.6 |
| 4.10 | 2.74 | 0.95 | 5.99 | 0.05 | 1.52 | 6.95 | 0.28 | 80.0 | 83.9 | 960.9 | 2.73 | 10.8 | 86.1 | 16.1 | 45.1 | 4.6 | 107.1 | 1.3 | 0.0 | 15.0 | 4.4 | 4.9 |
| 4.38 | 2.62 | 1.07 | 5.34 | 0.07 | 0.69 | 10.69 | 0.32 | 100.8 | 100.4 | 1171.7 | 2.95 | 16.9 | 99.6 | 16.1 | 48.7 | 3.1 | 142.5 | 0.7 | 0.1 | 13.8 | 4.6 | 4.7 |
| 4.42 | 2.42 | 0.85 | 4.60 | 0.07 | 0.70 | 12.84 | 0.32 | 82.3 | 83.6 | 1176.7 | 2.14 | 12.9 | 78.5 | 16.1 | 35.7 | 3.8 | 161.9 | 1.1 | 0.1 | 12.0 | 4.1 | 5.8 |
| 4.65 | 2.49 | 2.05 | 6.88 | 0.11 | 1.62 | 2.36 | 0.53 | 150.9 | 117.5 | 622.5 | 3.59 | 12.1 | 127.0 | 16.1 | 74.1 | 2.3 | 119.2 | 1.7 | 0.0 | 9.9 | 6.7 | 7.7 |
| 4.75 | 3.06 | 1.81 | 6.74 | 0.09 | 1.26 | 4.15 | 0.45 | 174.3 | 110.3 | 824.3 | 4.27 | 26.0 | 106.1 | 16.1 | 88.0 | 18.5 | 110.2 | 89.2 | 0.3 | 32.7 | 6.4 | 8.5 |
| 4.90 | 2.84 | 1.75 | 6.39 | 0.08 | 1.01 | 5.85 | 0.43 | 153.4 | 95.2 | 925.0 | 4.41 | 36.1 | 100.9 | 16.1 | 97.0 | 15.7 | 168.4 | 122.2 | 0.4 | 24.8 | 5.9 | 7.1 |
| 5.30 | 2.67 | 1.56 | 5.79 | 0.08 | 1.24 | 7.78 | 0.41 | 138.2 | 100.8 | 962.7 | 3.27 | 15.3 | 98.0 | 16.1 | 197.9 | 3.4 | 163.8 | 23.5 | 0.9 | 5.4 | 6.0 | 5.5 |
| 5.45 | 2.52 | 1.87 | 6.53 | 0.07 | 1.08 | 7.37 | 0.39 | 121.6 | 93.6 | 1205.2 | 4.48 | 20.6 | 110.2 | 16.1 | 137.1 | 27.9 | 94.4 | 166.2 | 1.8 | 50.6 | 5.8 | 3.8 |
| 5.60 | 2.39 | 1.87 | 8.71 | 0.09 | 2.19 | 5.73 | 0.46 | 119.1 | 100.1 | 880.1 | 4.48 | 19.0 | 105.0 | 16.1 | 150.9 | 2.2 | 154.3 | 1.1 | 0.3 | 4.9 | 6.1 | 3.4 |
| 5.65 | 2.78 | 1.72 | 6.07 | 0.08 | 1.28 | 6.19 | 0.46 | 119.1 | 97.6 | 1128.9 | 4.29 | 21.6 | 100.5 | 16.1 | 110.6 | 26.1 | 83.4 | 106.1 | 7.4 | 67.8 | 6.2 | 4.3 |
| 5.80 | 2.34 | 2.02 | 6.96 | 0.08 | 1.58 | 5.04 | 0.40 | 125.4 | 95.5 | 882.5 | 3.47 | 23.3 | 85.6 | 16.1 | 79.5 | 11.0 | 142.8 | 9.3 | 0.2 | 13.2 | 6.3 | 3.7 |
| 6.50 | 1.65 | 2.30 | 6.84 | 0.06 | 2.21 | 5.14 | 0.48 | 76.6 | 85.2 | 1056.2 | 4.12 | 15.1 | 75.7 | 16.1 | 99.5 | 0.2 | 147.0 | 1.1 | 0.1 | 4.0 | 7.0 | 1.9 |
| 7.10 | 2.65 | 1.77 | 6.79 | 0.06 | 1.38 | 5.18 | 0.41 | 69.9 | 92.4 | 919.3 | 4.05 | 13.3 | 62.5 | 16.1 | 67.7 | 2.5 | 87.3 | 0.4 | 0.0 | 5.7 | 5.7 | 1.4 |
| 12.10 | 1.49 | 2.92 | 8.46 | 0.09 | 3.29 | 0.80 | 0.57 | 157.0 | 114.3 | 583.1 | 5.83 | 23.9 | 101.6 | 16.1 | 99.9 | 25.5 | 66.4 | 1.4 | 0.0 | 16.3 | 12.2 | 4.0 |
| 13.05 | 1.14 | 3.17 | 8.77 | 0.06 | 3.83 | 1.90 | 0.41 | 110.6 | 106.9 | 702.2 | 6.34 | 24.6 | 102.1 | 16.1 | 99.7 | 15.6 | 52.4 | 1.3 | 0.1 | 17.3 | 10.5 | 3.3 |
| 14.65 | 1.36 | 2.35 | 7.16 | 0.08 | 3.02 | 2.33 | 0.47 | 121.7 | 101.0 | 778.9 | 5.58 | 23.4 | 98.8 | 16.1 | 91.7 | 10.1 | 69.6 | 0.9 | 0.0 | 15.4 | 10.8 | 3.3 |
| 16.05 | 1.82 | 2.35 | 7.24 | 0.09 | 3.07 | 2.99 | 0.45 | 123.5 | 111.9 | 794.7 | 5.40 | 22.9 | 103.5 | 16.1 | 86.4 | 13.2 | 100.2 | 1.1 | 0.1 | 15.5 | 9.6 | 3.1 |
| 16.85 | 1.46 | 2.37 | 6.88 | 0.09 | 2.97 | 3.12 | 0.51 | 114.4 | 104.0 | 824.5 | 5.34 | 22.2 | 99.5 | 16.1 | 89.5 | 9.2 | 93.3 | 0.9 | 0.1 | 15.0 | 9.3 | 3.0 |
| 20.45 | 1.82 | 2.44 | 6.89 | 0.09 | 2.88 | 2.65 | 0.54 | 111.5 | 108.3 | 685.4 | 5.66 | 23.1 | 107.2 | 16.1 | 81.6 | 8.7 | 98.8 | 1.2 | 0.1 | 14.3 | 8.7 | 2.7 |
| 22.75 | 1.55 | 2.77 | 8.57 | 0.09 | 3.05 | 3.17 | 0.48 | 111.7 | 110.5 | 787.1 | 5.24 | 23.0 | 102.3 | 16.1 | 98.6 | 7.6 | 95.5 | 1.2 | 0.1 | 14.1 | 8.4 | 2.7 |
| 24.55 | 1.75 | 2.13 | 6.52 | 0.09 | 2.73 | 4.22 | 0.46 | 110.4 | 116.4 | 922.8 | 5.08 | 22.3 | 103.8 | 16.1 | 82.3 | 9.7 | 99.8 | 0.8 | 0.1 | 13.9 | 8.6 | 2.7 |
| 25.75 | 1.39 | 2.69 | 8.09 | 0.08 | 3.14 | 2.78 | 0.40 | 121.8 | 152.9 | 773.1 | 5.32 | 24.1 | 111.6 | 16.1 | 88.8 | 12.7 | 92.3 | 1.0 | 0.0 | 15.0 | 8.5 | 2.7 |
| 25.95 | 1.59 | 2.25 | 7.19 | 0.08 | 2.91 | 3.06 | 0.39 | 108.4 | 108.3 | 765.4 | 5.33 | 23.0 | 103.5 | 16.1 | 87.6 | 8.8 | 103.6 | 3.0 | 0.1 | 14.7 | 8.6 | 2.7 |
| 27.55 | 1.47 | 2.43 | 7.65 | 0.09 | 2.57 | 3.02 | 0.47 | 121.5 | 111.0 | 747.7 | 4.69 | 23.2 | 108.0 | 16.1 | 85.6 | 7.8 | 111.8 | 0.9 | 0.1 | 14.4 | 8.1 | 2.5 |
| 29.15 | 1.43 | 2.33 | 7.80 | 0.11 | 3.02 | 2.79 | 0.48 | 106.8 | 113.5 | 702.0 | 5.14 | 21.5 | 102.5 | 16.1 | 87.8 | 10.8 | 101.2 | 0.8 | 0.1 | 15.2 | 9.6 | 2.5 |
| 30.95 | 1.62 | 2.57 | 7.83 | 0.07 | 3.16 | 2.32 | 0.48 | 114.1 | 109.4 | 644.4 | 5.60 | 23.0 | 105.7 | 16.1 | 82.8 | 8.1 | 101.0 | 1.6 | 0.1 | 15.5 | 9.2 | 2.6 |
| 32.95 | 1.57 | 2.59 | 7.75 | 0.09 | 3.12 | 2.60 | 0.46 | 126.5 | 125.0 | 762.8 | 5.46 | 24.8 | 117.8 | 16.1 | 94.5 | 6.4 | 114.0 | 1.0 | 0.0 | 14.7 | 8.6 | 2.4 |
| 34.25 | 1.50 | 2.41 | 7.55 | 0.09 | 3.06 | 3.28 | 0.48 | 119.0 | 112.9 | 773.7 | 5.22 | 23.3 | 114.3 | 16.1 | 86.6 | 10.1 | 108.7 | 1.1 | 0.1 | 14.6 | 7.7 | 2.1 |
| basement gneiss: | ||||||||||||||||||||||
| G1 | 2.96 | 0.16 | 7.25 | 0.00 | 5.03 | 0.37 | 0.07 | 9.3 | 3.7 | 119.9 | 0.28 | 2.1 | 7.5 | 15.1 | 17.7 | -0.3 | 269.8 | 0.5 | 0.0 | 10.9 | 1.5 | 0.7 |
| G2 | 5.85 | 0.11 | 7.36 | 0.02 | 1.32 | 0.85 | 0.04 | 9.8 | 7.4 | 94.7 | 0.21 | 2.1 | 2.4 | 4.3 | 7.3 | -0.9 | 252.1 | 0.2 | 0.0 | 9.7 | 3.8 | 0.6 |
| G3 | 4.45 | 0.12 | 7.18 | 0.03 | 2.64 | 1.35 | 0.07 | 8.2 | 2.2 | 156.4 | 0.40 | 1.8 | 3.6 | 0.7 | 11.0 | -1.0 | 475.3 | 0.2 | 0.0 | 6.1 | 1.5 | 0.2 |
| basement amphibolite: | ||||||||||||||||||||||
| A1 | 0.66 | 5.27 | 7.84 | 0.03 | 0.28 | 7.19 | 0.48 | 286.5 | 231.7 | 1856.3 | 10.23 | 49.3 | 77.3 | 71.6 | 78.6 | -0.4 | 78.8 | 0.6 | 0.1 | 3.9 | 0.6 | 0.1 |
| A2 | 1.41 | 3.28 | 7.56 | 0.08 | 0.36 | 6.46 | 0.78 | 283.3 | 195.5 | 1614.9 | 10.18 | 40.4 | 99.0 | 69.7 | 94.9 | -0.8 | 165.8 | 0.4 | 0.0 | 2.1 | 1.3 | 0.3 |
| A3 | 1.82 | 2.95 | 7.27 | 0.03 | 0.51 | 5.29 | 0.47 | 238.0 | 212.0 | 1266.9 | 7.26 | 31.5 | 82.3 | 46.5 | 93.0 | -0.5 | 305.0 | 0.3 | 0.1 | 4.5 | 0.2 | 0.0 |
| A4 | 3.75 | 1.75 | 8.32 | 0.12 | 0.68 | 3.71 | 0.42 | 106.1 | 78.2 | 623.4 | 4.13 | 19.5 | 65.1 | 59.2 | 61.4 | 0.5 | 505.1 | 0.2 | 0.0 | 5.4 | 0.6 | 0.1 |
| A5 | 1.58 | 6.60 | 6.44 | 0.03 | 0.43 | 5.88 | 0.33 | 138.3 | 1252.2 | 1669.8 | 8.40 | 41.4 | 264.8 | 6.0 | 113.9 | -0.5 | 284.3 | 0.1 | 0.2 | 3.0 | 0.3 | 0.2 |
| A6 | 1.64 | 5.19 | 7.19 | 0.13 | 0.44 | 6.32 | 0.60 | 245.9 | 330.4 | 1596.7 | 9.29 | 45.2 | 95.9 | 47.8 | 111.5 | 0.3 | 471.7 | 0.2 | 0.2 | 7.5 | 2.0 | 0.2 |
| additional Stac Fada sample from different locality: | ||||||||||||||||||||||
| SF | 2.36 | 2.38 | 7.26 | 0.07 | 1.98 | 1.14 | 0.43 | 91.7 | 221.3 | 765.5 | 4.39 | 31.3 | 382.8 | 26.7 | 110.0 | 0.7 | 270.2 | 0.2 | 0.0 | 7.4 | 4.4 | 0.9 |
Table S-4 Iron speciation. FeCarb = carbonate-bound iron, FeOx = ferric oxide-bound iron, FeMag = magnetite-bound iron, FePy = pyrite-bound iron. FeHR = highly reactive iron, which is the sum of all four phases. FeT = total iron from Table S-5. FePy was not determined in most of the red shale facies and assumed to be zero as in the few examples. * = Carbonate content was calculated from the concentration of Ca in the acetic acid extract, assuming a CaCO3 stoichiometry. Note that this calculation is an upper estimate, because it does not account for partial dissolution of silicates, which may become important below a carbonate content of 5 %, as suggested by the low molar Ca/Mg ratios in those samples.
| position [m] | FeCarb [%] | FeOx [%] | FeMag [%] | FePy [%] | FeHR [%] | FePy/FeHR [by mass] | FeHR/FeT [by mass] | carb.* [%] | molar Ca/Mg |
| 0 | 0.09 | 0.69 | 0.39 | 1.17 | 0.00 | 0.22 | 0.1 | 0.2 | |
| 0.5 | 0.32 | 1.11 | 0.33 | 1.75 | 0.00 | 0.44 | 0.4 | 0.2 | |
| 0.75 | 0.09 | 0.47 | 0.26 | 0.82 | 0.00 | 0.21 | 3.0 | 6.4 | |
| 1.65 | 0.13 | 0.58 | 0.11 | 0.83 | 0.00 | 0.24 | 4.1 | 5.8 | |
| 2.05 | 0.07 | 0.60 | 0.34 | 1.02 | 0.00 | 0.19 | 2.3 | 7.3 | |
| 2.73 | 0.59 | 0.51 | 0.21 | 1.31 | 0.00 | 0.36 | 11.8 | 4.3 | |
| 2.77 | 0.16 | 0.32 | 0.11 | 0.00 | 0.60 | 0.00 | 0.21 | 18.2 | 28.3 |
| 2.8 | 0.10 | 0.35 | 0.18 | 0.64 | 0.00 | 0.11 | 0.5 | 1.2 | |
| 2.83 | 0.17 | 0.47 | 0.14 | 0.78 | 0.00 | 0.20 | 14.7 | 23.2 | |
| 2.87 | 0.17 | 0.27 | 0.13 | 0.00 | 0.58 | 0.00 | 0.18 | 17.4 | 28.3 |
| 3.2 | 0.76 | 0.68 | 0.38 | 1.81 | 0.00 | 0.35 | 1.9 | 0.7 | |
| 3.7 | 0.29 | 0.16 | 0.19 | 0.00 | 0.64 | 0.00 | 0.14 | 1.9 | 2.4 |
| 4.1 | 0.10 | 0.53 | 0.18 | 0.81 | 0.00 | 0.30 | 20.6 | 49.0 | |
| 4.38 | 0.11 | 0.45 | 0.11 | 0.67 | 0.00 | 0.23 | 26.9 | 64.0 | |
| 4.42 | 0.10 | 0.27 | 0.09 | 0.01 | 0.47 | 0.03 | 0.22 | 31.1 | 76.4 |
| 4.65 | 0.49 | 0.17 | 0.23 | 0.07 | 0.95 | 0.07 | 0.26 | 5.3 | 2.9 |
| 4.75 | 0.16 | 0.07 | 0.10 | 1.23 | 1.57 | 0.78 | 0.37 | 10.0 | 21.3 |
| 4.9 | 0.23 | 0.08 | 0.11 | 1.15 | 1.58 | 0.73 | 0.36 | 15.8 | 28.0 |
| 5.3 | 0.13 | 0.08 | 0.08 | 0.27 | 0.56 | 0.48 | 0.17 | 19.3 | 46.6 |
| 5.45 | 0.19 | 0.20 | 0.22 | 1.36 | 1.98 | 0.69 | 0.44 | 19.3 | 8.8 |
| 5.6 | 0.27 | 0.56 | 0.23 | 0.00 | 1.06 | 0.00 | 0.24 | 14.2 | 23.4 |
| 5.65 | 0.79 | 0.07 | 0.12 | 1.11 | 2.09 | 0.53 | 0.49 | 15.2 | 17.7 |
| 5.8 | 0.12 | 0.07 | 0.08 | 0.24 | 0.51 | 0.46 | 0.15 | 11.7 | 23.5 |
| 6.5 | 0.37 | 0.16 | 0.24 | 0.00 | 0.77 | 0.00 | 0.19 | 9.8 | 5.9 |
| 7.1 | 0.18 | 0.38 | 0.17 | 0.73 | 0.00 | 0.18 | 12.6 | 21.7 | |
| 12.1 | 0.03 | 1.55 | 0.32 | 1.89 | 0.00 | 0.32 | 1.5 | 3.5 | |
| 13.05 | 0.02 | 1.33 | 0.24 | 1.60 | 0.00 | 0.25 | 4.8 | 14.0 | |
| 14.65 | 0.02 | 0.99 | 0.30 | 1.31 | 0.00 | 0.23 | 5.6 | 15.8 | |
| 16.05 | 0.03 | 1.14 | 0.25 | 1.42 | 0.00 | 0.26 | 7.3 | 14.3 | |
| 16.85 | 0.04 | 1.27 | 0.26 | 1.57 | 0.00 | 0.29 | 7.4 | 12.3 | |
| 20.45 | 0.02 | 0.83 | 0.26 | 1.11 | 0.00 | 0.20 | 6.1 | 15.6 | |
| 22.75 | 0.02 | 0.98 | 0.28 | 1.28 | 0.00 | 0.24 | 7.8 | 17.5 | |
| 24.55 | 0.02 | 1.05 | 0.33 | 1.40 | 0.00 | 0.28 | 9.2 | 20.0 | |
| 25.75 | 0.02 | 0.95 | 0.25 | 1.22 | 0.00 | 0.23 | 6.5 | 19.0 | |
| 25.95 | 0.03 | 1.00 | 0.26 | 1.29 | 0.00 | 0.24 | 8.2 | 24.7 | |
| 27.55 | 0.03 | 1.09 | 0.28 | 1.40 | 0.00 | 0.30 | 6.8 | 13.9 | |
| 29.15 | 0.03 | 1.03 | 0.27 | 1.32 | 0.00 | 0.26 | 6.3 | 18.7 | |
| 30.95 | 0.02 | 1.18 | 0.29 | 1.49 | 0.00 | 0.27 | 5.2 | 14.0 | |
| 32.95 | 0.02 | 0.88 | 0.26 | 1.16 | 0.00 | 0.21 | 5.8 | 17.6 | |
| 34.25 | 0.01 | 0.84 | 0.22 | 1.08 | 0.00 | 0.21 | 7.4 | 22.4 | |
| 42.1 | 0.01 | 1.79 | 0.31 | | 2.11 | 0.00 | 0.32 | 0.2 | 0.5 |
Table S-5 Molybdenum isotopes of bulk rocks, relative to NIST3134 = +0.25 ‰. Samples with reported standard deviations (SD) were prepared and analysed in replicates.
| position [m] | δ98/95Mo [‰] | SD [‰] |
| -5.00 | 1.24 | |
| -4.50 | 1.05 | |
| 0.00 | 1.00 | |
| 1.65 | 1.00 | |
| 2.73 | 0.35 | |
| 2.77 | 0.33 | |
| 2.80 | 0.52 | |
| 2.83 | -0.31 | 0.02 |
| 3.20 | 0.30 | |
| 3.70 | 0.20 | 0.04 |
| 4.10 | 0.91 | 0.04 |
| 4.38 | 0.47 | |
| 4.42 | 0.30 | |
| 4.65 | 0.57 | 0.01 |
| 4.75 | 0.13 | 0.02 |
| 4.90 | 0.33 | 0.09 |
| 5.30 | 1.10 | 0.22 |
| 5.45 | 0.76 | 0.02 |
| 5.60 | 1.32 | |
| 5.65 | 1.19 | 0.01 |
| 5.80 | 0.68 | 0.03 |
| 6.50 | 0.43 | |
| 7.10 | 0.46 | |
| 12.10 | -0.05 | |
| 13.05 | -0.17 | 0.05 |
| 14.65 | -0.16 | |
| 16.05 | -0.34 | 0.02 |
| 16.85 | -0.24 | |
| 20.45 | -0.34 | |
| 22.75 | -0.38 | |
| 24.55 | -0.26 | |
| 25.75 | -0.32 | |
| 25.95 | -0.24 | |
| 29.15 | -0.24 | |
| 30.95 | -0.36 | 0.04 |
| 32.95 | -0.34 | |
| 34.25 | 0.03 | |
Table S-6 Dissolution method for carbonate bound Sr analysis.
| Step | Reagent | Volume [ml] | Concentration [M] | pH |
| 1-2 | Ammonium Acetate | 10 | 1 | - |
| 3-6 | Acetic Acid | 7.5 | 0.04 | 3.07 |
| 7-8 | Acetic Acid | 8 | 0.175 | 2.76 |
| 9 | Acetic Acid | 6 | 0.875 | 2.41 |
| 10 | Acetic Acid | 6 | 1.75 | 2.26 |
Table S-7 Elemental abundances and Sr isotopes of carbonate leaches. N1-N2 = ammonium acetate washing steps, S1-S8 = acetic acid extractions with increasing acid strength. Concentrations are relative to bulk rock.
| sample | Mg [ppm] | Al [ppm] | Ca [%] | Mn [ppm] | Fe [ppm] | Rb [ppb] | Sr [ppb] | Rb/Sr | Mn/Sr | 87Sr/86Sr |
| +4.20 m, N1 | 254 | 5 | 1.79 | 72 | 1 | 6044 | 31314 | 0.19 | 2.3 | 0.71553 |
| +4.20 m, N2 | 103 | 4 | 1.59 | 115 | 1 | 523 | 21858 | 0.02 | 5.3 | 0.71272 |
| +4.20 m, S1 | 116 | 2 | 3.80 | 346 | 0 | 185 | 21693 | 0.01 | 15.9 | 0.71072 |
| +4.20 m, S2 | 118 | 2 | 4.39 | 410 | 1 | 201 | 23939 | 0.01 | 17.1 | n.d. |
| +4.20 m, S3 | 93 | 36 | 2.29 | 218 | 4 | 127 | 13855 | 0.01 | 15.7 | 0.71061 |
| +4.20 m, S4 | 108 | 126 | 0.63 | 78 | 8 | 135 | 7814 | 0.02 | 9.9 | 0.71303 |
| +4.20 m, S5 | 145 | 461 | 0.39 | 49 | 23 | 144 | 5007 | 0.03 | 9.9 | 0.71562 |
| +4.20 m, S6 | 169 | 441 | 0.14 | 21 | 34 | 142 | 2514 | 0.06 | 8.5 | n.d. |
| +4.20 m, S7 | 222 | 656 | 0.11 | 19 | 85 | 104 | 1458 | 0.07 | 13.3 | 0.72046 |
| +4.20 m, S8 | 219 | 626 | 0.12 | 17 | 76 | 102 | 1306 | 0.08 | 13.3 | 0.72028 |
| +4.38 m, N1 | 194 | 6 | 1.59 | 55 | 1 | 1645 | 26980 | 0.06 | 2.1 | 0.72807 |
| +4.38 m, N2 | 103 | 14 | 1.58 | 93 | 2 | 207 | 20878 | 0.01 | 4.4 | 0.71223 |
| +4.38 m, S1 | 105 | 2 | 3.79 | 225 | 0 | 74 | 22782 | 0.00 | 9.9 | 0.70989 |
| +4.38 m, S2 | 104 | 4 | 3.99 | 238 | 1 | 85 | 24011 | 0.00 | 9.9 | 0.70977 |
| +4.38 m, S3 | 102 | 5 | 3.97 | 239 | 2 | 66 | 24744 | 0.00 | 9.6 | 0.70964 |
| +4.38 m, S4 | 93 | 33 | 2.53 | 172 | 4 | 61 | 18276 | 0.00 | 9.4 | 0.71005 |
| +4.38 m, S5 | 100 | 183 | 1.10 | 84 | 15 | 63 | 10218 | 0.01 | 8.2 | 0.71061 |
| +4.38 m, S6 | 127 | 200 | 0.36 | 32 | 20 | 59 | 4404 | 0.01 | 7.3 | 0.71258 |
| +4.38 m, S7 | 251 | 507 | 0.51 | 46 | 84 | 68 | 4864 | 0.01 | 9.4 | 0.71242 |
| +4.38 m, S8 | 205 | 333 | 0.36 | 32 | 63 | 54 | 3121 | 0.02 | 10.2 | 0.71223 |
| +4.90 m, N1 | 273 | 17 | 1.48 | 65 | 2 | 3129 | 31046 | 0.10 | 2.1 | 0.71398 |
| +4.90 m, N2 | 124 | 28 | 1.35 | 100 | 5 | 317 | 28622 | 0.01 | 3.5 | 0.71156 |
| +4.90 m, S1 | 115 | 7 | 2.91 | 215 | 1 | 117 | 25023 | 0.00 | 8.6 | 0.70999 |
| +4.90 m, S2 | 157 | 22 | 3.08 | 234 | 3 | 192 | 29003 | 0.01 | 8.1 | 0.71004 |
| +4.90 m, S3 | 161 | 140 | 0.65 | 78 | 10 | 150 | 12266 | 0.01 | 6.3 | n.d. |
| +4.90 m, S4 | 162 | 133 | 0.25 | 30 | 12 | 91 | 6737 | 0.01 | 4.5 | n.d. |
| +4.90 m, S5 | 211 | 348 | 0.26 | 29 | 37 | 91 | 6147 | 0.01 | 4.7 | n.d. |
| +4.90 m, S6 | 248 | 329 | 0.14 | 18 | 48 | 78 | 4910 | 0.02 | 3.7 | n.d. |
| +4.90 m, S7 | 245 | 413 | 0.07 | 11 | 88 | 54 | 1697 | 0.03 | 6.7 | 0.71635 |
| +4.90 m, S8 | 351 | 659 | 0.10 | 15 | 135 | 63 | 1451 | 0.04 | 10.7 | 0.71751 |
| +5.65 m, N1_rep1 | 546 | 15 | 1.66 | 64 | 2 | 5502 | 17301 | 0.32 | 3.7 | 0.72204 |
| +5.65 m, N2_rep1 | 195 | 25 | 1.37 | 99 | 3 | 566 | 13203 | 0.04 | 7.5 | 0.71338 |
| +5.65 m, S1_rep1 | 180 | 12 | 2.96 | 240 | 2 | 176 | 18463 | 0.01 | 13.0 | 0.71000 |
| +5.65 m, S2_rep1 | 215 | 25 | 2.88 | 238 | 4 | 200 | 18912 | 0.01 | 12.6 | 0.71000 |
| +5.65 m, S3_rep1 | 244 | 174 | 0.81 | 109 | 11 | 176 | 6244 | 0.03 | 17.5 | 0.71258 |
| +5.65 m, S4_rep1 | 264 | 181 | 0.36 | 48 | 14 | 105 | 2869 | 0.04 | 16.6 | 0.7162 |
| +5.65 m, S5_rep1 | 337 | 684 | 0.32 | 42 | 59 | 120 | 2419 | 0.05 | 17.2 | 0.72024 |
| +5.65 m, S6_rep1 | 304 | 431 | 0.11 | 19 | 51 | 81 | 824 | 0.10 | 22.7 | 0.72909 |
| +5.65 m, S7_rep1 | 644 | 1275 | 0.09 | 23 | 214 | 88 | 694 | 0.13 | 33.9 | 0.73967 |
| +5.65 m, S8_rep1 | 561 | 1200 | 0.08 | 19 | 179 | 78 | 564 | 0.14 | 33.4 | |
| +5.65 m, N1_rep2 | 533 | 9 | 1.25 | 51 | 1 | 5407 | 16337 | 0.33 | 3.1 | 0.72299 |
| +5.65 m, N2_rep2 | 226 | 12 | 1.28 | 82 | 2 | 709 | 13812 | 0.05 | 5.9 | 0.71374 |
| +5.65 m, S1_rep2 | 189 | 12 | 2.99 | 220 | 1 | 176 | 18944 | 0.01 | 11.6 | 0.70964 |
| +5.65 m, S2_rep2 | 210 | 14 | 3.04 | 230 | 2 | 179 | 18929 | 0.01 | 12.2 | 0.70977 |
| +5.65 m, S3_rep2 | 189 | 79 | 1.24 | 132 | 5 | 124 | 10000 | 0.01 | 13.2 | 0.71075 |
| +5.65 m, S4_rep2 | 257 | 171 | 0.54 | 69 | 11 | 118 | 4531 | 0.03 | 15.3 | 0.71359 |
| +5.65 m, S5_rep2 | 427 | 773 | 0.41 | 58 | 58 | 147 | 3335 | 0.04 | 17.4 | 0.71779 |
| +5.65 m, S6_rep2 | 327 | 540 | 0.15 | 24 | 50 | 98 | 1156 | 0.08 | 21.0 | 0.72447 |
| +5.65 m, S7_rep2 | 704 | 1512 | 0.15 | 31 | 210 | 97 | 1104 | 0.09 | 28.0 | 0.72969 |
| +5.65 m, S8_rep2 | 527 | 1057 | 0.09 | 19 | 153 | 74 | 615 | 0.12 | 31.0 | 0.73106 |
Table S-8 Strontium isotope results of basement rocks and impact/volcanic debris layer. 87Sr/86Sr ratios at 1.2 Ga were calculated as described in Section S1.7.
| ID | Sr [ppm] | Rb [ppm] | (87Sr/86Sr)measured | (87Sr/86Sr)1.2Ga | type |
| A6 | 579.3 | 7.9 | 0.70294 | 0.70227 | mafic |
| A2 | 249.8 | 9.2 | 0.70689 | 0.70508 | mafic |
| G2 | 242.1 | 23.8 | 0.72037 | 0.71554 | felsic |
| G1 | 121.1 | 56.0 | 0.74991 | 0.72717 | felsic |
| -5 m | 198.5 | 46.7 | 0.71822 | 0.70666 | impact/volcanic |
| -4.5 m | 243.5 | 40.5 | 0.71422 | 0.70604 | impact/volcanic |