Warm Archean oceans reconstructed from oxygen isotope composition of early-life remnants
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: cherts, early-life, kerogen, precambrian surface temperature, seawater oxygen isotope composition
- Share this article





Article views:10,549Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract
Figures and Tables
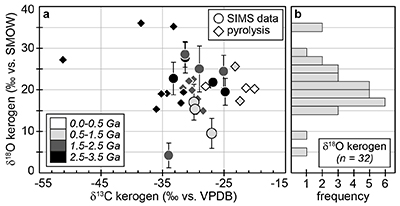 Figure 1 (a) Oxygen isotope compositions of the kerogens analysed by SIMS and by pyrolysis plotted against their C isotope compositions. (b) Frequency distribution of the measured kerogen δ18O values. |  Table 1 Main characteristics of the studied samples. δ13C and the δ18O values are given relative to VPDB and SMOW, respectively. See Supplementary Information for details and references. | 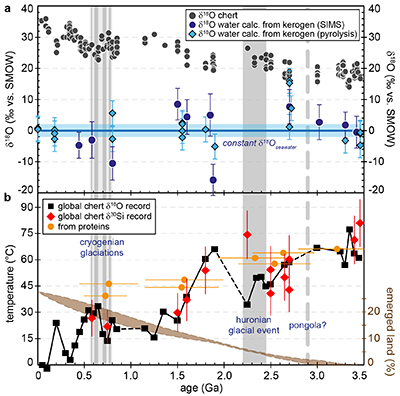 Figure 2 (a) Comparison of the chert and calculated seawater O isotope composition evolution through time, calculated using a Δ18Okerogen-water of 20 ± 4 ‰. Errors bars correspond to the propagated uncertainties on calculated δ18Owater (including the standard deviation – 1 SD – on average kerogen δ18O values and the uncertainty of ±4 ‰ on Δ18Okerogen-water). The horizontal blue band represents a constant δ18Oseawater of 0 ± 2 ‰ through time. (b) Estimates of water T calculated using (i) the available maximum δ18Ochert value (to which 3 ‰ has been added to take into account possible effects induced by diagenesis – see Supplementary Information) per 50 Ma age intervals and using δ18Oseawater = 0 ± 2 ‰, (ii) the chert δ30Si record (Prave et al., 2016) and (iii) resurrected proteins of ancient bacteria (Gaucher et al., 2008). Estimates of the surface of emerged land through time are also shown (Flament et al., 2013). Vertical grey bars running across both panels correspond to the major glaciation events identified in the geological record (after Hambrey and Harland, 1985, Evans et al., 1997 and Young, 2014). | 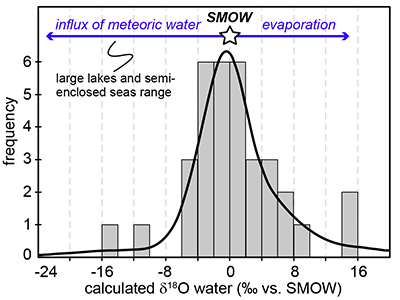 Figure 3 O isotope composition of waters in which precursor biomass of the studied kerogens lived. SMOW corresponds to the present-day mean seawater composition. The range of δ18O values for large lakes and semi-enclosed seas is after Jasechko et al., 2013 |
| Figure 1 | Table 1 | Figure 2 | Figure 3 |
Supplementary Figures and Tables
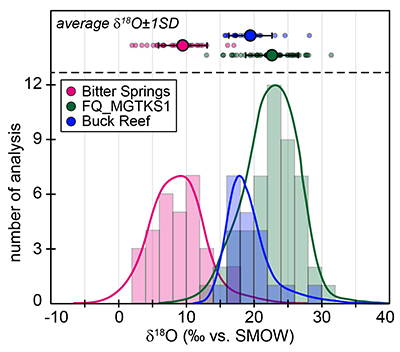 Figure S-1 Distribution of the SIMS δ18O values measured on three selected kerogen samples. To construct histograms the data have been binned into 2 ‰ intervals. All individual analyses are displayed by small circles in the upper part of the panel. Large circles and associated error bars correspond to average δ18O values together with their standard deviation (1 SD). | 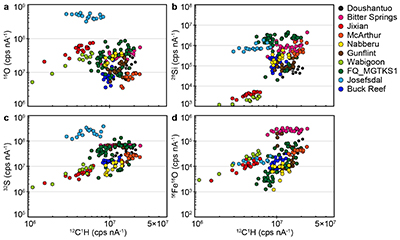 Figure S-2 Measured 16O (a), 28Si (b), 32S (c) and 56Fe16O (d) secondary intensities (normalised to the primary beam intensity) versus 12C1H secondary intensity. For each sample, the relationships between measured 16O, 28Si, 32S and 56Fe16O vs. 12C1H intensities generally trend towards the diagram origin, resulting from variable secondary ion emission for each analysis due to topography and surface flatness effects. | 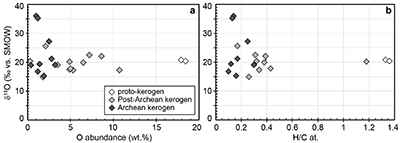 Figure S-3 Oxygen isotope compositions measured in kerogens plotted against their O abundances (a) and their H/C atomic ratios (b). The data are from Wedeking (1983). | 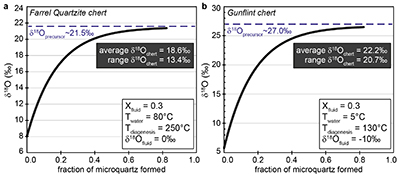 Figure S-4 Evolution of the O isotope composition of microquartz formed from the progressive dissolution of amorphous silica precursors. In (a) the parameters used for the calculation, aimed at modelling the SIMS results obtained on the Farrel Quartzite sample CRT, are; 30 % fluid in the initial porosity of the amorphous silica precursors (Xfluid), temperatures of 80 °C and 250 °C for the water from which it initially precipitated and for metamorphism, respectively, and a δ18Owater value of 0 ‰. In (b) the parameters used for the calculation, aimed at modelling the SIMS results obtained on Gunflint chert samples, are; Xfluid = 30 %, temperatures of 5 °C and 130 °C for the water from which it initially precipitated and for diagenesis/metamorphism, respectively, and a δ18Owater value of -10 ‰ (see text for details). |
| Figure S-1 | Figure S-2 | Figure S-3 | Figure S-4 |
 Table S-1 Bulk H/C, O contents and O and C isotope compositions obtained by pyrolysis on chert-extracted kerogen samples by Wedeking (1983). δ13C and the δ18O values are given relative to VPDB and SMOW, respectively. All the samples, except recent sediments and Lower Toarcian shales, are PPRG samples, whose descriptions are given in Walter et al. (1983). |  Table S-2 O isotope characteristics of the kerogens analysed by SIMS. |  Table S-3 Oxygen isotope results obtained by SIMS on the Farrel Quartzite chert samples. The 2 µm range has been calculated from the range of δ18O values measured at the 20 µm scale and the relationship between analysis scale and δ18O range of Marin et al. (2010). |  Table S-4 All oxygen isotope and chemistry data collected by SIMS. Analyses in purple are those that have been filtered, and grey boxes indicate based on which element between O, Si, S and Fe (see text for details). |  Table S-5 Final SIMS O isotope dataset. |
| Table S-1 | Table S-2 | Table S-3 | Table S-4 | Table S-5 |
top
Introduction
The oxygen isotopic composition (hereafter reported in ‰ using the delta notation, δ18Osample = [(18O/16O)sample/(18O/16O)SMOW – 1] × 1000 where SMOW stands for the present day Standard Mean Ocean Water composition) of cherts has increased systematically during the last 3.5 Ga from ~20 ‰ to ~35 ‰ (Knauth and Epstein, 1976
Knauth, L.P., Epstein, S. (1976) Hydrogen and oxygen isotope ratios in nodular and bedded cherts. Geochimica et Cosmochimica Acta 40, 1095-1108.
; Knauth and Lowe, 2003Knauth, L.P., Lowe, D.R. (2003) High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. Geological Society of America Bulletin 115, 566-580.
; Robert and Chaussidon, 2006Robert, F., Chaussidon, M. (2006) A palaeo-temperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
). Based on the temperature-dependence of the oxygen isotopic fractionation between silica and water (Knauth and Epstein, 1976Knauth, L.P., Epstein, S. (1976) Hydrogen and oxygen isotope ratios in nodular and bedded cherts. Geochimica et Cosmochimica Acta 40, 1095-1108.
), this isotopic record implies that the formation temperature (T) of cherts decreased by 50-80 °C since Archean times, assuming that silica formed from water with a δ18O of ~0 ‰ (Knauth and Epstein, 1976Knauth, L.P., Epstein, S. (1976) Hydrogen and oxygen isotope ratios in nodular and bedded cherts. Geochimica et Cosmochimica Acta 40, 1095-1108.
; Knauth and Lowe, 2003Knauth, L.P., Lowe, D.R. (2003) High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. Geological Society of America Bulletin 115, 566-580.
; Robert and Chaussidon, 2006Robert, F., Chaussidon, M. (2006) A palaeo-temperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
). While this assumption is consistent with δ18O values measured in ca. 3.8 Ga ophiolites (Pope et al., 2012Pope, E.C., Bird, D.K., Rosing, M.T. (2012) Isotope composition and volume of Earth’s early oceans. Proceedings of the National Academy of Science of the United States of America 109, 4371-4376.
) and with geochemical models indicating that δ18Oseawater is buffered to around 0 ± 2 ‰ within a few tens of million years (e.g., Lécuyer and Allemand, 1999Lécuyer, C., Allemand, P. (1999) Modelling of the oxygen isotope evolution of seawater: Implications for the climate interpretation of the δ18O of marine sediments. Geochimica et Cosmochimica Acta 63, 351-361.
), this hypothesis of a near constant δ18Oseawater through time has never been tested by direct measurements on Precambrian samples.Several arguments have been put forward challenging the interpretation of elevated surface T during Precambrian times. Isotopic exchange during diagenesis and chert alteration could have decreased the original δ18Ochert, for example. However, observations of large ranges of δ18Ochert values at the micrometre scale in individual quartz grains (e.g., Marin-Carbonne et al., 2012
Marin-Carbonne, J., Chaussidon, M., Robert, F. (2012) Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts: implications for paleo-temperature reconstructions. Geochimica et Cosmochimica Acta 92, 129-147.
) showed that Precambrian cherts can preserve a record of their pristine O-isotope signature, with diagenetic effects resulting in a limited excess of ~15-20 °C on the crystallisation T calculated previously. Also, the chert O-isotope record of warm T during the Precambrian apparently conflicts with evidence for large scale glaciations, such as the 2.2-2.45 Ga Huronian glaciations (e.g., Evans et al., 1997Evans, D.A., Beukes, N.J., Kirschvink, J.L. (1997) Low-latitude glaciation in the Palaeoproterozoic era. Nature 386, 262-266.
; Young, 2014Young, G.M. (2014) Contradictory correlations of Paleoproterozoic glacial deposits: Local, regional or global controls? Precambrian Research 247, 33-44.
). Therefore, it has been argued that constraints for a constant δ18Oseawater over time were indirect and weak, and that the increase of δ18Ochert values since 3.5 Ga rather reflects a 10-15 ‰ increase of δ18Oseawater, with average surface T remaining around 15-30 °C (e.g., Kasting et al., 2006Kasting, J.F., Tazewell Howard, M., Wallmann, K., Veizer, J., Shields, G., Jaffrés, J. (2006) Paleoclimates, ocean depth, and the oxygen isotopic composition of seawater. Earth and Planetary Science Letters 252, 82-93.
; Jaffrés et al., 2007Jaffrés, J.B.D., Shields, G.A., Wallmann, K. (2007) The oxygen isotope evolution of seawater: A critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years. Earth-Science Reviews 83, 83-122.
). To provide further constraints on this key issue, we have investigated chert-hosted biogenic carbonaceous remnants, whose O isotope composition is directly related to that of the water in which their precursor microorganisms thrived.The isotopic composition of biomolecules is largely determined by metabolic exchange between living organisms and their environment. In aquatic heterotrophic organisms, for example, ca. 70-80 % of organic matter-derived oxygen originates from water (see Supplementary Information). In living organisms, fractionation of O isotopes between water and organic compounds results from both equilibrium and kinetic processes (e.g., Schmidt et al., 2015
Schmidt, H.L., Robins, R.J., Werner, R.A. (2015) Multi-factorial in vivo stable isotope fractionation: causes, correlations, consequences and applications. Isotopes in Environmental and Health Studies 51, 155-199.
). As a result, different organic compounds are characterised by different O isotope compositions: oxygen is enriched in 18O by ~27 ± 5 ‰ compared to ambient water in carbohydrates such as cellulose, while it is enriched by ~19 ± 3 ‰ in carboxyl groups, for example (e.g., Schmidt et al., 2015Schmidt, H.L., Robins, R.J., Werner, R.A. (2015) Multi-factorial in vivo stable isotope fractionation: causes, correlations, consequences and applications. Isotopes in Environmental and Health Studies 51, 155-199.
). Chemical changes occurring during thermal maturation of organic matter (OM) may alter O isotope compositions of various organic compounds. O-rich thermolabile components (e.g., carbohydrates and amino acids) are quickly degraded during maturation, resulting in residual OM (kerogen) dominated by resistant aromatic moieties in which O is mostly bound in hydroxyl (‒OH), ketone (‒C=O) and carboxyl (‒COOH) functional groups (e.g., De Gregorio et al., 2011De Gregorio, B.T., Sharp, T.G., Rushdi, A.I., Simoneit, B.R.T. (2011) Bugs or Gunk? Nanoscale Methods for Assessing the Biogenicity of Ancient Microfossils and Organic Matter. In: Golding, S.D., Glikson, M. (Eds.) Earliest Life on Earth: Habitats, Environments and Methods of Detection. Springer, Netherlands, 239-289.
). Proto-kerogens derived from marine algae collected in recent surface sediments and isolated from Lower Jurassic Toarcian shales are characterised by δ18O values that are on average 20.5 ± 1.1 ‰ (2 SD) higher than the O isotope composition of waters in which the precursor biomass thrived (Supplementary Information). Such a Δ18Okerogen-water is similar to our previous estimates (Tartèse et al., 2016Tartèse, R., Chaussidon, M., Gurenko, A., Delarue, F., Robert, F. (2016) In situ oxygen isotope analysis of fossil organic matter. Geochimica et Cosmochimica Acta 182, 24-39.
) and indicates that the O isotope composition of bulk immature kerogens is consistent with that of O bound to carboxyl functional groups, which appear to be one of the more resistant O-bearing functional group in thermally altered OM (e.g., De Gregorio et al., 2011De Gregorio, B.T., Sharp, T.G., Rushdi, A.I., Simoneit, B.R.T. (2011) Bugs or Gunk? Nanoscale Methods for Assessing the Biogenicity of Ancient Microfossils and Organic Matter. In: Golding, S.D., Glikson, M. (Eds.) Earliest Life on Earth: Habitats, Environments and Methods of Detection. Springer, Netherlands, 239-289.
). This suggests that oxygen in sedimentary OM preserves the δ18O signature of the carboxyl functional groups of its precursor biomass, which does not seem to be fractionated during maturation. Biogenic carbonaceous remnants have, therefore, a great potential to provide direct constraints on the O isotope composition of waters in which their precursor biomass lived.top
Oxygen Isotope Composition of Precambrian Kerogens
Bulk pyrolysis results obtained on 18 kerogens isolated from Precambrian cherts up to ca. 3.5 Ga have consistent δ18O values clustering around 20 ± 5 ‰ (Wedeking, 1983
Wedeking, K.W. (1983) The Biogeochemistry of Precambrian Kerogen. Ph.D. thesis, Indiana University, 167 pp.
) (Fig. 1 and Table S-1). To provide further constraints on the significance of bulk kerogen δ18O values, we used Secondary Ion Mass Spectrometry (SIMS) to analyse additional kerogens isolated by acid-maceration from cherts ranging in age from 0.58 to 3.42 Ga and affected by metamorphic conditions no higher than those of lower greenschist facies (Delarue et al., 2016Delarue, F., Rouzaud, J.-N., Derenne, S., Bourbin, M., Westall, F., Kremer, B., Sugitani, K., Deldicque, D., Robert, F. (2016) The Raman-derived carbonization continuum: A tool to select the best preserved molecular structures in Archean kerogens. Astrobiology 16, 407-417.
) (Supplementary Information). Bulk C isotope compositions of these kerogens are compatible with typical biological signatures (δ13C between -35 and -25 ‰; Fig. 1). Most kerogens have average SIMS δ18O values between ca. 15 and 25 ‰, which is consistent with values obtained by pyrolysis on bulk kerogens (Fig. 1). The kerogens older than 3.0 Ga analysed by SIMS have strikingly consistent average δ18O values between 19.5 ± 3.2 and 22.7 ± 3.9 ‰ (Table 1). In contrast, a few Proterozoic kerogens display large deviations from these older samples, with δ18O values ranging from 4.2 ± 3.0 ‰ for the 1.88 Ga Gunflint samples to 28.5 ± 3.1 ‰ for the 1.5 Ga Jixian sample (Table 1). Other samples, such as Bitter Springs (0.8 Ga), also display a low average δ18O value (8.6 ± 3.7 ‰), while average δ18O values of ca. 25 ± 5 ‰ for the Naberru (1.85 Ga) and McArthur (1.6 Ga) kerogens are consistent within errors with those of Archean kerogens (Table 1). Finally, the average δ18O of 17.0 ± 4.3 ‰ for the Ediacaran (0.58 Ga) Doushantuo kerogen is similar to the O isotope composition previously obtained for the Silurian Zdanow kerogen of 15.3 ± 1.2 ‰ (Tartèse et al., 2016Tartèse, R., Chaussidon, M., Gurenko, A., Delarue, F., Robert, F. (2016) In situ oxygen isotope analysis of fossil organic matter. Geochimica et Cosmochimica Acta 182, 24-39.
). For Bitter Springs kerogens, the δ18O values obtained by SIMS (8.6 ± 3.7 ‰) and by pyrolysis (17.3 ± 0.6 ‰ and 17.5 ± 0.6 ‰) on kerogen residues isolated from two different samples are not consistent with each other. This may indicate that these two Bitter Springs samples correspond to slightly different time periods and/or deposition environments, for example, which can only be thoroughly assessed with further petro-geochemical investigation.
Figure 1 (a) Oxygen isotope compositions of the kerogens analysed by SIMS and by pyrolysis plotted against their C isotope compositions. (b) Frequency distribution of the measured kerogen δ18O values.
Table 1 Main characteristics of the studied samples. δ13C and the δ18O values are given relative to VPDB and SMOW, respectively. See Supplementary Information for details and references.
| Sample | Reference | Age (Ga) | Location | δ18OSIMS (‰) | δ18OTC/EA-IRMS (‰) | d13C (‰) |
| Doushantuo | 1a of 8/25/83 | 0.58 | Doushantuo Fm.. Yangtze Gorges. South China | 17.0 ± 4.3 | 15.0 ± 0.1 | -29.9 |
| Bitter Springs | 3 of 11/2/90 | 0.80 | Bitter Springs Fm.. Amadeus Basin. Australia | 8.6 ± 3.7 | - | -27.0 |
| Jixian | 1 of 8/14/83 | 1.50 | Gaoyuzhuang Group. Jixian section. North China Block. China | 28.5 ± 3.1 | - | -31.4 |
| McArthur | 7 of 6/21/90 | 1.60 | McArthur Basin. Northern Territory. Australia | 24.4 ± 3.9 | - | -25.1 |
| Nabberu | PPRG 089 | 1.85 | Top of Frere Fm.. Earaheedy Group. Nabberu Basin. Western Australia | 25.0 ± 5.5 | - | -29.0 |
| Gunflint | 3 of 6/30/84 | 1.88 | Schreiber Beach. Gunflint Iron Fm.. Ontario. Canada | 4.4 ± 2.2 | 7.3 ± 0.5 | -34.5 |
| PPRG 134 | 4.1 ± 3.4 | - | -33.5 | |||
| Wabigoon | PPRG 325 | 2.70 | Steep Rock Group. Wabigoon Belt. Western Superior Province. Canada | 27.7 ± 3.7 | - | -31.3 |
| Farrel Quartzite | MGTKS1 | 3.02 | Mount Grant. Gorge Creek Group. Pilbara Craton. Australia | 22.7 ± 3.9 | 19.8 ± 0.1 | -33.2 |
| Josefsdal | 99SA07 | 3.30 | Top of Kromberg Fm.. Onverwacht Group. Barberton Greenstone Belt. South Africa | 21.8 ± 1.1 | - | -26.8 |
| Buck Reef | 99SA03 | 3.42 | Base of Kromberg Fm.. Onverwacht Group. Barberton Greenstone Belt. South Africa | 19.5 ± 3.2 | 15.0 ± 0.4 | -24.8 |
The SIMS δ18O values for individual kerogens vary by ~10-15 ‰ at the 20-30 µm spot scale (Fig. S-1 and Table S-2). This variability, and the variations of the measured C, O, S and Fe intensities (Fig. S-2), are similar to the variability observed for Phanerozoic kerogens (Tartèse et al., 2016
Tartèse, R., Chaussidon, M., Gurenko, A., Delarue, F., Robert, F. (2016) In situ oxygen isotope analysis of fossil organic matter. Geochimica et Cosmochimica Acta 182, 24-39.
). It is also comparable with the variations of 5-10 ‰ observed at the micrometre scale for C isotope ratios in Precambrian microfossils (e.g., Williford et al., 2013Williford, K.H., Ushikubo, T., Schopf, J.W., Lepot, K., Kitajima, K., Valley, J.W. (2013) Preservation and detection of microstructural and taxonomic correlations in the carbon isotopic compositions of individual Precambrian microfossils. Geochimica et Cosmochimica Acta 104, 165-182.
). For each sample, individual δ18O values display unimodal Gaussian distributions around their mean value (Fig. S-1), showing that there is no analytical evidence for multiple organic O-bearing components with variable O isotope compositions in the kerogens. The good consistency between combustion and SIMS average δ18O values obtained on selected samples (Table 1) indicates that most of the SIMS δ18O variability at the sample scale can be assigned to analytical effects (e.g., sample topography, sputtering of mineral micro-inclusions; see Supplementary Information). This allows us to use both bulk kerogen pyrolysis and average SIMS δ18Okerogen values (and their standard deviation) to estimate the O isotope composition of water coeval with the kerogen precursor biomass.The last point to consider is the possible effects of diagenesis and metamorphism on preservation of the δ18Okerogen values through time. During heating, kerogen loses O (and other heteroatoms such as N) as H2O, CO and CO2, which could in theory lead to enrichment in 18O in the residual kerogen due to preferential loss of molecules containing light 16O. The bulk kerogens analysed by Wedeking (1983)
Wedeking, K.W. (1983) The Biogeochemistry of Precambrian Kerogen. Ph.D. thesis, Indiana University, 167 pp.
are up to ca. 3.5 billion years old and have large ranges of H/C ratios (from 0.1 to 1.4) and O contents (0.3-18.4 wt. %) (Table S-1). In these samples, δ18Okerogen values are neither correlated with O contents nor with H/C ratios (Fig. S-3). Therefore, there is no evidence that δ18O values measured in the majority of the kerogens have been significantly altered during diagenesis and low grade metamorphism. As indicated previously, analysis of three recent kerogens yielded a Δ18Okerogen-water of 20.5 ± 1.1 ‰ (Wedeking, 1983Wedeking, K.W. (1983) The Biogeochemistry of Precambrian Kerogen. Ph.D. thesis, Indiana University, 167 pp.
). The possible effect of temperature on biochemical O isotope fractionation is uncertain and debated (e.g., Roden and Ehleringer, 2000Roden, J.S., Ehleringer, J.R. (2000) There is no temperature dependence of net biochemical fractionation of Hydrogen and Oxygen isotopes in tree-ring cellulose. Isotopes in Environmental and Health Studies 36, 303-317.
; Sternberg and Ellsworth, 2011Sternberg, L., Ellsworth, P.F.V. (2011) Divergent biochemical fractionation, not convergent temperature, explains cellulose Oxygen isotope enrichment across latitudes. PLoS ONE 6, e28040.
), and this could introduce an additional uncertainty of ca. ±2-3 ‰ on the O isotope composition of OM precursors. Therefore, we used a Δ18Okerogen-water of 20 ± 4 ‰ to calculate δ18Owater from kerogen O isotope compositions.top
Oxygen Isotope Composition of Water Derived from Kerogens
For most samples the calculated δ18Owater are consistent within errors with a value around 0 ± 2 ‰ (Fig. 2a). Overall, the O isotope composition of water reconstructed from the O isotope composition of kerogens up to ca. 3.5 Ga is, therefore, indistinguishable from that of present-day seawater. This is consistent with inferences made from the study of seawater-altered Precambrian oceanic crust remnants (e.g., Lécuyer and Allemand, 1999
Lécuyer, C., Allemand, P. (1999) Modelling of the oxygen isotope evolution of seawater: Implications for the climate interpretation of the δ18O of marine sediments. Geochimica et Cosmochimica Acta 63, 351-361.
; Pope et al., 2012Pope, E.C., Bird, D.K., Rosing, M.T. (2012) Isotope composition and volume of Earth’s early oceans. Proceedings of the National Academy of Science of the United States of America 109, 4371-4376.
), but does not support a ca. 10-15 ‰ progressive increase of δ18Oseawater since 3.5 Ga (Kasting et al., 2006Kasting, J.F., Tazewell Howard, M., Wallmann, K., Veizer, J., Shields, G., Jaffrés, J. (2006) Paleoclimates, ocean depth, and the oxygen isotopic composition of seawater. Earth and Planetary Science Letters 252, 82-93.
; Jaffrés et al., 2007Jaffrés, J.B.D., Shields, G.A., Wallmann, K. (2007) The oxygen isotope evolution of seawater: A critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years. Earth-Science Reviews 83, 83-122.
).
Figure 2 (a) Comparison of the chert and calculated seawater O isotope composition evolution through time, calculated using a Δ18Okerogen-water of 20 ± 4 ‰. Errors bars correspond to the propagated uncertainties on calculated δ18Owater (including the standard deviation – 1 SD – on average kerogen δ18O values and the uncertainty of ±4 ‰ on Δ18Okerogen-water). The horizontal blue band represents a constant δ18Oseawater of 0 ± 2 ‰ through time. (b) Estimates of water T calculated using (i) the available maximum δ18Ochert value (to which 3 ‰ has been added to take into account possible effects induced by diagenesis – see Supplementary Information) per 50 Ma age intervals and using δ18Oseawater = 0 ± 2 ‰, (ii) the chert δ30Si record (Robert and Chaussidon, 2006
Robert, F., Chaussidon, M. (2006) A palaeo-temperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
) and (iii) resurrected proteins of ancient bacteria (Gaucher et al., 2008Gaucher, E.A., Govindarajan, S., Ganesh, O.K. (2008) Palaeotemperature trend for Precambrian life inferred from resurrected proteins. Nature 451, 704-707.
). Estimates of the surface of emerged land through time are also shown (Flament et al., 2013Flament, N., Coltice, N., Rey, P. (2013) The evolution of the 87Sr/86Sr of marine carbonates does not constrain continental growth. Precambrian Research 229, 177-188.
). Vertical grey bars running across both panels correspond to the major glaciation events identified in the geological record (after Hambrey and Harland, 1985Hambrey, M.J., Harland, W.B. (1985) The Late Proterozoic glacial era. Palaeogeography, Palaeoclimatology, Palaeoecology 51, 255-272.
, Evans et al., 1997Evans, D.A., Beukes, N.J., Kirschvink, J.L. (1997) Low-latitude glaciation in the Palaeoproterozoic era. Nature 386, 262-266.
and Young, 2014Young, G.M. (2014) Contradictory correlations of Paleoproterozoic glacial deposits: Local, regional or global controls? Precambrian Research 247, 33-44.
).Kerogens from the 1.88 Ga Gunflint Formation collected at Schreiber Beach yielded low δ18O values of ca. 4 ‰, corresponding to a δ18Owater of around -15 ‰ (Fig. 3). A possible interpretation is that this low δ18Owater corresponds to cold shallow waters with a restricted connection with the open ocean and affected by a large influx of low δ18O continental waters, similar to the present-day Baltic Sea, for example (δ18Owater of -4 to -8 ‰; Jasechko et al., 2013
Jasechko S., Sharp, Z.D., Gibson, J.J. Birks, S.J., Yi, Y., Fawcett, P.J. (2013) Terrestrial water fluxes dominated by transpiration. Nature 496, 347-351.
). This hypothesis would imply that the Gunflint cherts formed at low T < 10 °C, which is not incompatible with the palaeolatitude of ca. 45 °N estimated for that region at 1.88 Ga (see Supplementary Information). On the other hand, kerogens from the 2.7 Ga Belingwe (Manjeri Fm.) and Ventersdorp samples yielded elevated δ18O values of ca. 35 ‰, corresponding to δ18Owater of ~15 ‰ (Fig. 3). A possible interpretation is that precursor OM of these kerogens thrived in warm waters undergoing intense evaporation, which is consistent with the continental depositional environments proposed for these formations (Buck, 1980Buck, S.G. (1980) Stromatolite and ooid deposits within the fluvial and lacustrine sediments of the Precambrian Ventersdorp Supergroup of South Africa. Precambrian Research 12, 311-330.
; Hunter et al., 1998Hunter, M.A., Bickle, M.J., Nisbet, E.G., Martin, A., Chapman, H.J. (1998) Continental extensional setting for the Archean Belingwe Greenstone Belt, Zimbabwe. Geology 26, 883-886.
).
Figure 3 O isotope composition of waters in which precursor biomass of the studied kerogens lived. SMOW corresponds to the present-day mean seawater composition. The range of δ18O values for large lakes and semi-enclosed seas is after Jasechko et al., 2013
Jasechko S., Sharp, Z.D., Gibson, J.J. Birks, S.J., Yi, Y., Fawcett, P.J. (2013) Terrestrial water fluxes dominated by transpiration. Nature 496, 347-351.
.top
Implications for Surface Temperatures on the Earth during the Precambrian
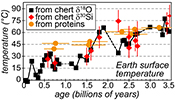
A globally constant δ18Oseawater around 0 ± 5 ‰ through time, as demonstrated by the present results, implies that Precambrian cherts record formation T decreasing from 50-60 °C during the Archean to 0-15 °C for the recent Phanerozoic (Fig. 2b). For some Precambrian formations these elevated precipitation T may reflect mixing of seawater with hot hydrothermal fluids (e.g., de Wit and Furnes, 2016
de Wit, M.J., Furnes, H. (2016) 3.5-Ga hydrothermal fields and diamictites in the Barberton Greenstone Belt – Paleoarchean crust in cold environments. Science Advances 2, e1500368.
). Also, some chert units may have been deposited in environments disconnected from the global oceans. However, taken as a whole the secular decrease of the δ18Ochert record, constructed from 569 individual δ18Ochert analyses representing a worldwide sampling, indicates a global cooling of the conditions on the Earth surface over geological time (Fig. 2b). This is consistent with other estimates such as the temperature of stability measured for resurrected proteins presumably akin those of Precambrian bacteria (Gaucher et al., 2008Gaucher, E.A., Govindarajan, S., Ganesh, O.K. (2008) Palaeotemperature trend for Precambrian life inferred from resurrected proteins. Nature 451, 704-707.
; Fig. 2b). Elevated T around 35-50 °C at 2.2-2.5 Ga appear in conflict with the existence of widespread cold surface conditions during the ‘Huronian Glacial Event’. However, there is still no definitive proof supporting a worldwide extent for Huronian glaciations (Young, 2014Young, G.M. (2014) Contradictory correlations of Paleoproterozoic glacial deposits: Local, regional or global controls? Precambrian Research 247, 33-44.
). Also, global glacial episodes are relatively short (few hundred thousand to a few million years; Prave et al., 2016Prave, A.R., Condon, D.J., Hoffmann, K.H., Tapster, S., Fallick, A.E. (2016) Duration and nature of the end-Cryogenian (Marinoan) glaciation. Geology 44, 631-634.
) so a set of Precambrian chert samples, which are often poorly dated, defining the δ18Ochert for a given 50 Ma time interval, may not have formed contemporaneously with a known glacial period. Finally, it is important to note that reconstructed T have remained below ca. 30 °C for the past 1.5 Ga, well within the range allowing development of complex eukaryotic life (e.g., Clarke, 2014Clarke, A. (2014) The thermal limits to life on Earth. International Journal of Astrobiology 13, 141-154.
).Elevated surface T of ~40-60 °C during Archean times, when the Sun was 20-25 % fainter than today (Gough, 1981
Gough, D.O. (1981) Solar interior structure and luminosity variations. Solar Physics 74, 21-34.
), required an effective greenhouse atmosphere that may have been controlled by high pressures of CO2 (PCO2) and of CH4 (PCH4) (e.g., Kasting and Ono, 2006Kasting, J.F., Ono, S. (2006) Palaeoclimates: the first two billion years. Philosophical Transactions of the Royal Society B 361, 917-929.
). Recent 3D Global Climate Model simulations yielded average surface T of ~20 °C around 3.4 Ga for PCO2 and PCH4 of 0.1 bar and 2 mbar, respectively (Charnay et al., 2013Charnay, B., Forget, F., Wordsworth, R., Leconte, J., Millour, E., Codron, F., Spiga, A. (2013) Exploring the faint young Sun problem and the possible climates of the Archean Earth with a 3-D GCM. Journal of Geophysical Research: Atmospheres 118, 10,414-10,431.
). Further simulations also show that higher mean surface T (up to ca. 50 °C) can be obtained by combining PN2 and PCO2 of 0.5 bar (Supplementary Information). Such atmospheric pressures are not in contradiction with measurements of N2/36Ar in Archean hydrothermal fluids (Marty et al., 2013Marty, B., Zimmermann, L., Pujol, M., Burgess, R., Philippot, P. (2013) Nitrogen isotopic composition and density of the Archean atmosphere. Science 342, 101-104.
) and studies of fossil imprints of 2.7 Ga rain droplets (Som et al., 2012Som, S.M., Catling, D.C., Harnmeijer, J.P., Polivka, P.M., Buick, R. (2012) Air density 2.7 billion years ago limited to less than twice modern levels by fossil raindrop imprints. Nature 484, 359-362.
), which suggest maximum PN2 = 1.1 bar and PCO2 = 0.7 bar around 3.0-3.5 Ga (Marty et al., 2013Marty, B., Zimmermann, L., Pujol, M., Burgess, R., Philippot, P. (2013) Nitrogen isotopic composition and density of the Archean atmosphere. Science 342, 101-104.
). Finally, the progressive decrease of surface T reconstructed from the chert record is inversely correlated with progressive emerging of the continents since ca. 3.0 Ga (Fig. 2b). This relationship suggests that the first order control on Earth surface T at a geological time scale is the consumption and sequestration of atmospheric CO2 by weathering of silicates on continental surfaces followed by carbonate deposition (Berner et al., 1983Berner, R.A., Lasaga, A.C., Garrels, R.M. (1983) The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. American Journal of Science 283, 641-683.
).top
Acknowledgements
This research is supported by the ERC Grant No. 290861 – PaleoNanoLife (PI F. Robert). We thank J.M. Hayes for invaluable discussions, S.M. Awramik, J.W. Schopf, K. Sugitani and F. Westall for providing us with the studied chert samples, and two reviewers for their constructive comments. This is IPGP contribution #3790 and CRPG contribution #2464.
Editor: Bruce Watson
top
References
Berner, R.A., Lasaga, A.C., Garrels, R.M. (1983) The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. American Journal of Science 283, 641-683.
 Show in context
Show in context This relationship suggests that the first order control on Earth surface T at geological time scales is the consumption and sequestration of atmospheric CO2 by weathering of silicates on continental surfaces followed by carbonate deposition (e.g., Berner et al., 1983).
View in article
Buck, S.G. (1980) Stromatolite and ooid deposits within the fluvial and lacustrine sediments of the Precambrian Ventersdorp Supergroup of South Africa. Precambrian Research 12, 311-330.
 Show in context
Show in context A possible interpretation is that precursor OM of these kerogens thrived in warm waters undergoing intense evaporation, which is consistent with the continental depositional environments proposed for these formations (Buck, 1980; Hunter et al., 1998).
View in article
Charnay, B., Forget, F., Wordsworth, R., Leconte, J., Millour, E., Codron, F., Spiga, A. (2013) Exploring the faint young Sun problem and the possible climates of the Archean Earth with a 3-D GCM. Journal of Geophysical Research: Atmospheres 118, 10,414-10,431.
 Show in context
Show in context Recent 3D Global Climate Model simulations yielded average surface T of ~20 °C around 3.4 Ga for PCO2 and PCH4 of 0.1 bar and 2 mbar, respectively (Charnay et al., 2013).
View in article
Clarke, A. (2014) The thermal limits to life on Earth. International Journal of Astrobiology 13, 141-154.
 Show in context
Show in context Finally, it is important to note that reconstructed T have remained below ca. 30 °C for the past 1.5 Ga, well within the range allowing development of complex eukaryotic life (e.g., Clarke, 2014).
View in article
De Gregorio, B.T., Sharp, T.G., Rushdi, A.I., Simoneit, B.R.T. (2011) Bugs or Gunk? Nanoscale Methods for Assessing the Biogenicity of Ancient Microfossils and Organic Matter. In: Golding, S.D., Glikson, M. (Eds.) Earliest Life on Earth: Habitats, Environments and Methods of Detection. Springer, Netherlands, 239-289.
 Show in context
Show in context O-rich thermolabile components (e.g., carbohydrates and amino acids) are quickly degraded during maturation, resulting in residual OM (kerogen) dominated by resistant aromatic moieties in which O is mostly bound in hydroxyl (‒OH), ketone (‒C=O) and carboxyl (‒COOH) functional groups (e.g., De Gregorio et al., 2011)
View in article
Such a Δ18Okerogen-water is consistent with our previous estimates (Tartèse et al., 2016) and indicates that the O isotope composition of bulk immature kerogens is consistent with that of O bound to carboxyl functional groups, which appear to be the most resistant O-bearing functional group in thermally altered OM (e.g., De Gregorio et al., 2011).
View in article
Delarue, F., Rouzaud, J.-N., Derenne, S., Bourbin, M., Westall, F., Kremer, B., Sugitani, K., Deldicque, D., Robert, F. (2016) The Raman-derived carbonization continuum: A tool to select the best preserved molecular structures in Archean kerogens. Astrobiology 16, 407-417.
 Show in context
Show in context To provide further constraints on the significance of bulk kerogen δ18O values, we used Secondary Ion Mass Spectrometry (SIMS) to analyse additional kerogens isolated by acid-maceration from cherts ranging in age from 0.58 to 3.42 Ga and affected by metamorphic conditions no higher than those of lower greenschist facies (Delarue et al., 2016) (Supplementary Information).
View in article
de Wit, M.J., Furnes, H. (2016) 3.5-Ga hydrothermal fields and diamictites in the Barberton Greenstone Belt – Paleoarchean crust in cold environments. Science Advances 2, e1500368.
 Show in context
Show in context For some formations these elevated precipitation T may reflect mixing of seawater with hot hydrothermal fluids (e.g., de Wit and Furnes, 2016).
View in article
Evans, D.A., Beukes, N.J., Kirschvink, J.L. (1997) Low-latitude glaciation in the Palaeoproterozoic era. Nature 386, 262-266.
 Show in context
Show in context Also, the chert O-isotope record of warm T during the Precambrian apparently conflicts with evidence for large scale glaciations, such as the 2.2-2.45 Ga Huronian glaciations (e.g., Evans et al., 1997; Young, 2014).
View in article
Figure 2 [...] Vertical grey bars running across both panels correspond to the major glaciation events identified in the geological record (after Hambrey and Harland, 1985, Evans et al., 1997 and Young, 2014).
View in article
Flament, N., Coltice, N., Rey, P. (2013) The evolution of the 87Sr/86Sr of marine carbonates does not constrain continental growth. Precambrian Research 229, 177-188.
 Show in context
Show in context Figure 2 [...] Estimates of the surface of emerged land through time are also shown (Flament et al., 2013).
View in article
Gaucher, E.A., Govindarajan, S., Ganesh, O.K. (2008) Palaeotemperature trend for Precambrian life inferred from resurrected proteins. Nature 451, 704-707.
 Show in context
Show in context Figure 2 [...] (b) Estimates of water T calculated using (i) the available maximum δ18Ochert value (to which 3 ‰ has been added to take into account possible effects induced by diagenesis – see Supplementary Information) per 50 Ma age intervals and using δ18Oseawater = 0 ± 2 ‰, (ii) the chert δ30Si record (Robert and Chaussidon, 2006) and (iii) resurrected proteins of ancient bacteria (Gaucher et al., 2008).
View in article
This is consistent with other estimates such as the temperature of stability measured for resurrected proteins presumably akin those of Precambrian bacteria (Gaucher et al., 2008; Fig. 2b).
View in article
Gough, D.O. (1981) Solar interior structure and luminosity variations. Solar Physics 74, 21-34.
 Show in context
Show in context Elevated surface T of ~40-60 °C during Archean times, when the Sun was 20-25 % fainter than today (Gough, 1981), required an effective greenhouse atmosphere that may have been controlled by high pressures of CO2 (PCO2) and of CH4 (PCH4) (e.g., Kasting and Ono, 2006).
View in article
Hambrey, M.J., Harland, W.B. (1985) The Late Proterozoic glacial era. Palaeogeography, Palaeoclimatology, Palaeoecology 51, 255-272.
 Show in context
Show in context Figure 2 [...] Vertical grey bars running across both panels correspond to the major glaciation events identified in the geological record (after Hambrey and Harland, 1985, Evans et al., 1997 and Young, 2014).
View in article
Hunter, M.A., Bickle, M.J., Nisbet, E.G., Martin, A., Chapman, H.J. (1998) Continental extensional setting for the Archean Belingwe Greenstone Belt, Zimbabwe. Geology 26, 883-886.
 Show in context
Show in context A possible interpretation is that precursor OM of these kerogens thrived in warm waters undergoing intense evaporation, which is consistent with the continental depositional environments proposed for these formations (Buck, 1980; Hunter et al., 1998).
View in article
Jaffrés, J.B.D., Shields, G.A., Wallmann, K. (2007) The oxygen isotope evolution of seawater: A critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years. Earth-Science Reviews 83, 83-122.
 Show in context
Show in context Therefore, it has been argued that constraints for a constant δ18Oseawater over time were indirect and weak, and that the increase of δ18Ochert values since 3.5 Ga rather reflects a 10-15 ‰ increase of δ18Oseawater, with average surface T remaining around 15-30 °C (e.g., Kasting et al., 2006; Jaffrés et al., 2007).
View in article
This is consistent with inferences made from the study of seawater-altered Precambrian oceanic crust remnants (e.g., Lécuyer and Allemand, 1999; Pope et al., 2012), but does not support a ca. 10-15 ‰ progressive increase of δ18Oseawater since 3.5 Ga (Kasting et al., 2006; Jaffrés et al., 2007).
View in article
Jasechko S., Sharp, Z.D., Gibson, J.J. Birks, S.J., Yi, Y., Fawcett, P.J. (2013) Terrestrial water fluxes dominated by transpiration. Nature 496, 347-351.
 Show in context
Show in context A possible interpretation is that this low δ18Owater corresponds to cold shallow waters with a restricted connection with the open ocean and affected by a large influx of low δ18O continental waters, similar to the present-day Baltic Sea, for example (δ18Owater of -4 to -8 ‰; Jasechko et al., 2013.
View in article
Figure 3 [...] The range of δ18O values for large lakes and semi-enclosed seas is after Jasechko et al. (2013).
View in article
Kasting, J.F., Ono, S. (2006) Palaeoclimates: the first two billion years. Philosophical Transactions of the Royal Society B 361, 917-929.
 Show in context
Show in context Elevated surface T of ~40-60 °C during Archean times, when the Sun was 20-25 % fainter than today (Gough, 1981), required an effective greenhouse atmosphere that may have been controlled by high pressures of CO2 (PCO2) and of CH4 (PCH4) (e.g., Kasting and Ono, 2006).
View in article
Kasting, J.F., Tazewell Howard, M., Wallmann, K., Veizer, J., Shields, G., Jaffrés, J. (2006) Paleoclimates, ocean depth, and the oxygen isotopic composition of seawater. Earth and Planetary Science Letters 252, 82-93.
 Show in context
Show in context Therefore, it has been argued that constraints for a constant δ18Oseawater over time were indirect and weak, and that the increase of δ18Ochert values since 3.5 Ga rather reflects a 10-15 ‰ increase of δ18Oseawater, with average surface T remaining around 15-30 °C (e.g., Kasting et al., 2006; Jaffrés et al., 2007).
View in article
This is consistent with inferences made from the study of seawater-altered Precambrian oceanic crust remnants (e.g., Lécuyer and Allemand, 1999; Pope et al., 2012), but does not support a ca. 10-15 ‰ progressive increase of δ18Oseawater since 3.5 Ga (Kasting et al., 2006; Jaffrés et al., 2007).
View in article
Knauth, L.P., Epstein, S. (1976) Hydrogen and oxygen isotope ratios in nodular and bedded cherts. Geochimica et Cosmochimica Acta 40, 1095-1108.
 Show in context
Show in context The oxygen isotopic composition (hereafter reported in ‰ using the delta notation, δ18Osample = [(18O/16O)sample/(18O/16O)SMOW – 1] × 1000 where SMOW stands for the present day Standard Mean Ocean Water composition) of cherts has increased systematically during the last 3.5 Ga from ~20 ‰ to ~35 ‰ (Knauth and Epstein, 1976; Knauth and Lowe, 2003; Robert and Chaussidon, 2006).
View in article
Based on the temperature-dependence of the oxygen isotopic fractionation between silica and water (Knauth and Epstein, 1976), this isotopic record implies that the formation temperature (T) of cherts decreased by 50-80 °C since Archean times, assuming that silica formed from water with a δ18O of ~0 ‰ (Knauth and Epstein, 1976; Knauth and Lowe, 2003; Robert and Chaussidon, 2006).
View in article
Knauth, L.P., Lowe, D.R. (2003) High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. Geological Society of America Bulletin 115, 566-580.
 Show in context
Show in context The oxygen isotopic composition (hereafter reported in ‰ using the delta notation, δ18Osample = [(18O/16O)sample/(18O/16O)SMOW – 1] × 1000 where SMOW stands for the present day Standard Mean Ocean Water composition) of cherts has increased systematically during the last 3.5 Ga from ~20 ‰ to ~35 ‰ (Knauth and Epstein, 1976; Knauth and Lowe, 2003; Robert and Chaussidon, 2006).
View in article
Based on the temperature-dependence of the oxygen isotopic fractionation between silica and water (Knauth and Epstein, 1976), this isotopic record implies that the formation temperature (T) of cherts decreased by 50-80 °C since Archean times, assuming that silica formed from water with a δ18O of ~0 ‰ (Knauth and Epstein, 1976; Knauth and Lowe, 2003; Robert and Chaussidon, 2006).
View in article
Lécuyer, C., Allemand, P. (1999) Modelling of the oxygen isotope evolution of seawater: Implications for the climate interpretation of the δ18O of marine sediments. Geochimica et Cosmochimica Acta 63, 351-361.
 Show in context
Show in context While this assumption is consistent with δ18O values measured in ca. 3.8 Ga ophiolites (Pope et al., 2012) and with geochemical models indicating that δ18Oseawater is buffered to around 0 ± 2 ‰ within a few tens of million years (e.g., Lécuyer and Allemand, 1999), this hypothesis of a near constant δ18Oseawater through time has never been tested by direct measurements on Precambrian samples.
View in article
This is consistent with inferences made from the study of seawater-altered Precambrian oceanic crust remnants (e.g., Lécuyer and Allemand, 1999; Pope et al., 2012), but does not support a ca. 10-15 ‰ progressive increase of δ18Oseawater since 3.5 Ga (Kasting et al., 2006; Jaffrés et al., 2007).
View in article
Marin-Carbonne, J., Chaussidon, M., Robert, F. (2012) Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts: implications for paleo-temperature reconstructions. Geochimica et Cosmochimica Acta 92, 129-147.
 Show in context
Show in context However, observations of large ranges of δ18Ochert values at the micrometre scale in individual quartz grains (e.g., Marin-Carbonne et al., 2012) showed that Precambrian cherts can preserve a record of their pristine O-isotope signature, with diagenetic effects resulting in a limited excess of ~15-20 °C on the crystallisation T calculated previously.
View in article
Marty, B., Zimmermann, L., Pujol, M., Burgess, R., Philippot, P. (2013) Nitrogen isotopic composition and density of the Archean atmosphere. Science 342, 101-104.
 Show in context
Show in context Such atmospheric pressures are not in contradiction with measurements of N2/36Ar in Archean hydrothermal fluids (Marty et al., 2013) and studies of fossil imprints of 2.7 Ga rain droplets (Som et al., 2012), which suggest maximum PN2 = 1.1 bar and PCO2 = 0.7 bar around 3.0-3.5 Ga (Marty et al., 2013).
View in article
Pope, E.C., Bird, D.K., Rosing, M.T. (2012) Isotope composition and volume of Earth’s early oceans. Proceedings of the National Academy of Science of the United States of America 109, 4371-4376.
 Show in context
Show in context While this assumption is consistent with δ18O values measured in ca. 3.8 Ga ophiolites (Pope et al., 2012) and with geochemical models indicating that δ18Oseawater is buffered to around 0 ± 2 ‰ within a few tens of million years (e.g., Lécuyer and Allemand, 1999), this hypothesis of a near constant δ18Oseawater through time has never been tested by direct measurements on Precambrian samples.
View in article
This is consistent with inferences made from the study of seawater-altered Precambrian oceanic crust remnants (e.g., Lécuyer and Allemand, 1999; Pope et al., 2012), but does not support a ca. 10-15 ‰ progressive increase of δ18Oseawater since 3.5 Ga (Kasting et al., 2006; Jaffrés et al., 2007).
View in article
Prave, A.R., Condon, D.J., Hoffmann, K.H., Tapster, S., Fallick, A.E. (2016) Duration and nature of the end-Cryogenian (Marinoan) glaciation. Geology 44, 631-634.
 Show in context
Show in context Also, global glacial episodes are relatively short (few hundred thousand to a few million years; Prave et al., 2016) so a set of Precambrian chert samples, which are often poorly dated, defining the δ18Ochert for a given 50 Ma time interval, may not have formed contemporaneously with a known glacial period.
View in article
Robert, F., Chaussidon, M. (2006) A palaeo-temperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
 Show in context
Show in context The oxygen isotopic composition (hereafter reported in ‰ using the delta notation, δ18Osample = [(18O/16O)sample/(18O/16O)SMOW – 1] × 1000 where SMOW stands for the present day Standard Mean Ocean Water composition) of cherts has increased systematically during the last 3.5 Ga from ~20 ‰ to ~35 ‰ (Knauth and Epstein, 1976; Knauth and Lowe, 2003; Robert and Chaussidon, 2006).
View in article
Based on the temperature-dependence of the oxygen isotopic fractionation between silica and water (Knauth and Epstein, 1976), this isotopic record implies that the formation temperature (T) of cherts decreased by 50-80 °C since Archean times, assuming that silica formed from water with a δ18O of ~0 ‰ (Knauth and Epstein, 1976; Knauth and Lowe, 2003; Robert and Chaussidon, 2006).
View in article
Figure 2 [...] (b) Estimates of water T calculated using (i) the available maximum δ18Ochert value (to which 3 ‰ has been added to take into account possible effects induced by diagenesis – see Supplementary Information) per 50 Ma age intervals and using δ18Oseawater = 0 ± 2 ‰, (ii) the chert δ30Si record (Robert and Chaussidon, 2006) and (iii) resurrected proteins of ancient bacteria (Gaucher et al., 2008).
View in article
Roden, J.S., Ehleringer, J.R. (2000) There is no temperature dependence of net biochemical fractionation of Hydrogen and Oxygen isotopes in tree-ring cellulose. Isotopes in Environmental and Health Studies 36, 303-317.
 Show in context
Show in context The possible effect of temperature on biochemical O isotope fractionation is uncertain and debated (e.g., Roden and Ehleringer, 2000; Sternberg and Ellsworth, 2011), and this could introduce an additional uncertainty of ca. ±2-3 ‰ on the O isotope composition of OM precursors.
View in article
Schmidt, H.L., Robins, R.J., Werner, R.A. (2015) Multi-factorial in vivo stable isotope fractionation: causes, correlations, consequences and applications. Isotopes in Environmental and Health Studies 51, 155-199.
 Show in context
Show in context In living organisms, fractionation of O isotopes between water and organic compounds results from both equilibrium and kinetic processes (e.g., Schmidt et al., 2015).
View in article
As a result, different organic compounds are characterised by different O isotope compositions: oxygen is enriched in 18O by ~27 ± 5 ‰ compared to ambient water in carbohydrates such as cellulose, while it is enriched by ~19 ± 3 ‰ in carboxyl groups, for example (e.g., Schmidt et al., 2015).
View in article
Som, S.M., Catling, D.C., Harnmeijer, J.P., Polivka, P.M., Buick, R. (2012) Air density 2.7 billion years ago limited to less than twice modern levels by fossil raindrop imprints. Nature 484, 359-362.
 Show in context
Show in context Such atmospheric pressures are not in contradiction with measurements of N2/36Ar in Archean hydrothermal fluids (Marty et al., 2013) and studies of fossil imprints of 2.7 Ga rain droplets (Som et al., 2012), which suggest maximum PN2 = 1.1 bar and PCO2 = 0.7 bar around 3.0-3.5 Ga (Marty et al., 2013).
View in article
Sternberg, L., Ellsworth, P.F.V. (2011) Divergent biochemical fractionation, not convergent temperature, explains cellulose Oxygen isotope enrichment across latitudes. PLoS ONE 6, e28040.
 Show in context
Show in context The possible effect of temperature on biochemical O isotope fractionation is uncertain and debated (e.g., Roden and Ehleringer, 2000; Sternberg and Ellsworth, 2011), and this could introduce an additional uncertainty of ca. ±2-3 ‰ on the O isotope composition of OM precursors.
View in article
Tartèse, R., Chaussidon, M., Gurenko, A., Delarue, F., Robert, F. (2016) In situ oxygen isotope analysis of fossil organic matter. Geochimica et Cosmochimica Acta 182, 24-39.
 Show in context
Show in context Such a Δ18Okerogen-water is consistent with our previous estimates (Tartèse et al., 2016) and indicates that the O isotope composition of bulk immature kerogens is consistent with that of O bound to carboxyl functional groups, which appear to be the most resistant O-bearing functional group in thermally altered OM (e.g., De Gregorio et al., 2011).
View in article
Finally, the average δ18O of 17.0 ± 4.3 ‰ for the Ediacaran (0.58 Ga) Doushantuo kerogen is similar to the O isotope composition previously obtained for the Silurian Zdanow kerogen of 15.3 ± 1.2 ‰ (Tartèse et al., 2016).
View in article
This variability, and the variations of the measured C, O, S and Fe intensities (Fig. S-2), are similar to the variability observed for Phanerozoic kerogens (Tartèse et al., 2016).
View in article
Wedeking, K.W. (1983) The Biogeochemistry of Precambrian Kerogen. Ph.D. thesis, Indiana University, 167 pp.
 Show in context
Show in context Bulk pyrolysis results obtained on 18 kerogens isolated from Precambrian cherts up to ca. 3.5 Ga have consistent δ18O values clustering around 20 ± 5 ‰ (Wedeking, 1983) (Fig. 1 and Table S-1).
View in article
The bulk kerogens analysed by Wedeking (1983) are up to ca. 3.5 Ga and have large ranges of H/C ratios (from 0.1 to 1.4) and O contents (0.3-18.4 wt. %) (Table S-1).
View in article
As indicated previously, analysis of three recent kerogens yielded a Δ18Okerogen-water of 20.5 ± 1.1 ‰ (Wedeking (1983)).
View in article
Williford, K.H., Ushikubo, T., Schopf, J.W., Lepot, K., Kitajima, K., Valley, J.W. (2013) Preservation and detection of microstructural and taxonomic correlations in the carbon isotopic compositions of individual Precambrian microfossils. Geochimica et Cosmochimica Acta 104, 165-182.
 Show in context
Show in context It is also comparable with the variations of 5-10 ‰ observed at the micrometre scale for C isotope ratios in Precambrian microfossils (e.g., Williford et al., 2013).
View in article
Young, G.M. (2014) Contradictory correlations of Paleoproterozoic glacial deposits: Local, regional or global controls? Precambrian Research 247, 33-44.
 Show in context
Show in context Also, the chert O-isotope record of warm T during the Precambrian apparently conflicts with evidence for large scale glaciations, such as the 2.2-2.45 Ga Huronian glaciations (e.g., Evans et al., 1997; Young, 2014).
View in article
Figure 2 [...] Vertical grey bars running across both panels correspond to the major glaciation events identified in the geological record (after Hambrey and Harland, 1985, Evans et al., 1997 and Young, 2014).
View in article
However, there is still no definitive proof supporting a worldwide extent for Huronian glaciations (Young, 2014).
View in article
top
Supplementary Information
Studied Samples
Doushantuo. The Doushantuo chert sample 1a of 8/25/83, sampled by S.M. Awramik, comes from the Yangtze Gorges, South China (Awramik et al., 1985). The Doushantuo Formation comprises dark dolomite with chert interbedded with shale. It is famous for the occurrence of well-preserved, abundant and diverse microfossils forming benthic mat biota (bacteria, cyanobacteria) and planktonic remnants that thrived in the overlying water column. The age of the Doushantuo microfossils is not precisely known. The Doushantuo Formation itself has been dated between ca. 635 Ma and 550-555 Ma (Condon et al., 2005; Zhang et al., 2005) and we have attributed here an age of ~575 Ma for the studied chert sample.
Bitter Springs. The Bitter Springs chert sample 3 of 11/2/90, sampled by S.M. Awramik, comes from the Ross River Area in the Bitter Springs Formation, Amadeus Basin, Northern Territory, Australia, and is known to contain colonial and filamentous cyanobacteria microfossils (Barghoorn and Schopf, 1965; Schopf, 1968). As summarised by Schopf (1968), the Bitter Springs Formation is predominantly composed of dark grey laminated cherty limestone and dolomite, interbedded with thin units of siltstone and dark shale. Evaporitic deposits (gypsum, halite) are locally abundant at the bottom of the Bitter Springs Formation, while algal stromatolites, commonly associated with dark chert lenses and nodules, are particularly developed in its middle and upper portions. The Bitter Springs Formation, whose age is relatively well constrained at ca. 800 Ma, has been subdivided into the lower Gillen Member and upper Loves Creek Member, the latter being further subdivided into three units, the uppermost of which containing the studied microfossil assemblages (Barghoorn and Schopf, 1965; Schopf, 1968). The depositional environment of the microfossil-bearing units of the Bitter Springs Formation is debated. Initially, Schopf (1968) described the Bitter Springs Formation deposition as starting with sand to silt deposition in a gradually subsiding basin in which evaporites precipitated in restricted, disconnected, areas, followed by the widespread development of algae in a shallow sea environment. Based notably on C and S isotope evidence, recent studies have argued that the microfossil-bearing upper unit of the Loves Creek Member was deposited in a non-marine environment such as a hypersaline lake (Hill and Walter, 2000; Hill et al., 2000), an interpretation refuted by Lindsay et al. (2005) who favoured a shallow marine tidal environment.
Jixian. The Jixian chert sample 1 of 8/14/83, sampled by S.M. Awramik, comes from the Mesoproterozoic Gaoyuzhuang Group, which constitutes one of the four groups comprising the ca. 10 km thick Proterozoic sedimentary succession outcropping near the city of Jixian on the northern margin of the North China Block (Lu et al., 2008). At the type section the Gaoyuzhuang Group is ~1600 m thick and subdivided into 4 formations that mostly consist of varied dolostone units, stromatolite assemblages and chert layers frequently hosting carbonaceous microfossils (e.g., Zhang, 1981; Schopf et al., 1984; Xu and Awramick, 2002). A few Pb-Pb dates obtained on galena from Pb-Zn SEDEX mineralised horizons belonging to the Gaoyuzhuang Group yielded dates ranging between ca. 1350 and 1485 Ma (Cheng et al., 1981), suggesting that the depositional age of the group may be close to 1500 Ma. Recently, U-Pb dating on zircon from a tuff bed in the Gaoyuzhuang Group yielded an age of 1560 Ma (Li et al., 2010). Overall, the voluminous carbonate succession of the Gaoyuzhuang Group was deposited between 1.4 and 1.6 Ga.
McArthur. The McArthur chert sample 7 of 6/21/90, sampled by S.M. Awramik, comes from the Palaeoproterozoic to Mesoproterozoic McArthur Basin, Northern Territory, Australia. The McArthur basin is a ~5-15 km thick sequence of mostly unmetamorphosed sedimentary and associated volcanic rocks deposited on the North Australian Craton between around 1.8 and 1.4 Ga (Rawlings, 1999). It comprises a mixture of carbonate and siliciclastic successions, with minor volcanic units near the base (Rawlings, 1999). Several microfossil-rich occurrences have been described in stromatolitic chert horizons throughout the McArthur basin (e.g., Muir, 1976; Oehler, 1978).
Gunflint. Kerogens were isolated from two Gunflint chert samples, sample 3 of 6/30/84, sampled by S.M. Awramik, and sample PPRG 134 (Walter et al., 1983). Both samples were collected from the lower chert horizon of the Gunflint Iron Formation, ca. 5 km west of the Schreiber beach locality, on the northern shore of the Lake Superior (Ontario, Canada). Cherts at this locality are famous for their abundance of well-preserved microfossils (Barghoorn and Tyler, 1965; Cloud, 1965; Awramik and Barghoorn, 1977; Alleon et al., 2016), indicative of the pristine nature of the Gunflint Formation there, and of the fact that it experienced conditions only slightly above burial diagenesis (Winter and Knauth, 1992; Marin et al., 2010). The Gunflint Iron Formation, dated at ca. 1.88 Ga (Fralick et al., 2002), which is part of the Animikie basin, was deposited in shallow waters in a subsiding basin with a restricted connection with the open ocean (Carrigan and Cameron, 1991; Schulz and Cannon, 2007), at a palaeolatitude of around 45 °N (Pesonen et al., 2012).
Nabberu. The Nabberu chert sample PPRG 089 was collected from beds transitional between the Frere and Windidda Formations in the Lake Carnegie area, Earaheedy Basin, Western Australia (Walter et al., 1983). Recent sedimentological and stratigraphic studies carried out in the Earaheedy Basin, synthesised in Pirajno et al. (2009), indicate that the Windidda Member is in fact part of the top of the Frere Formation. The Frere Formation overlies the Yelma Formation, which constitutes the base of the Earaheedy Group, and was deposited ca. 1.89 Ga (Rasmussen et al., 2012) on the Yilgarn continental margin during a marine transgression, in a shallow water environment as indicated by the presence of varied types of stromatolites (Pirajno et al., 2009; Akin et al., 2013). The ca. 500 m thick Frere Formation is unmetamorphosed and consists of alternating beds of chloritic siltstone, haematitic shale and granular iron formation, the latter horizons being typically 0.5-20 cm thick and containing granular iron beds intercalated with shale, siltstone, jasper and chert (Pirajno et al., 2009). These granular iron formations of the Frere Formation are comparable to those of the Lake Superior region in Canada, and host microfossil assemblages similar to those described in the contemporaneous Gunflint Iron Formation (Walter et al., 1976; Tobin, 1990).
Wabigoon. The Wabigoon chert sample PPRG 325 was collected at the South Robert Pit locality (48.798333 °N; 91.639722 °W), in the Steep Rock Group, Wabigoon Belt, Western Superior Province, Canada (Walter et al., 1983). The Steep Rock Group overlies the 3.0 Ga tonalitic gneiss of the Marmion Complex and comprises three units, from base to top, the detrital Wagita Formation (sandstone and conglomerate), the stromatolite-bearing Mosher Carbonate and the iron-rich Jolliffe Ore Zone (e.g., Wilks and Nisbet, 1988; Fralick and Riding, 2015). Units of the Steep Rock Group were regionally metamorphosed in the lower greenschist facies (Wilks and Nisbet, 1985). Detailed sedimentological, petrological and geochemical studies of the ~500 m thick Mosher Carbonate show that it is essentially composed by limestone that accumulated on a shallow marine platform environment on the 3.0 Ga eroded crystalline basement during a marine transgression, precipitating from oxygenated seawater according to REE geochemistry (Fralick and Riding, 2015 and references therein). Therefore, the ca. 2.7-2.8 Ga Steep Rock stromatolites may have hosted oxygenic photosynthetic cyanobacteria and could constitute an early example of a marine oxygen oasis (Fralick and Riding, 2015).
Farrel Quartzite. The chert sample MGTKS1 was collected by K. Sugitani in a black chert horizon from the ca. 3.0 Ga Farrel Quartzite in the Mount Grant area, part of the Mount Goldsworthy greenstone belt in the northeastern Pilbara Craton, Australia (Sugitani et al., 2007). This greenstone belt comprises a lower unit composed essentially of volcanic sequences older than 3.17 Ga and an upper sedimentary succession, the 3.02-2.93 Ga De Grey Supergroup (Van Kranendonk et al., 2007). The lower part of the De Grey Supergroup is known as the 3.02 Ga Gorge Creek Group, which comprises a clastic formation (the Farrel Quartzite), and an upper formation of chert, banded iron formation, black shale, and siltstone (the Cleaverville Formation). The Farrel Quartzite is up to 80 m thick and contains fine- to very coarse-grained sandstone, minor conglomerate units, mafic to ultra-mafic volcanoclastic layers, evaporite beds and black chert layers (Sugitani et al., 2007). These units were metamorphosed to lower greenschist facies and pervasively silicified. The ca. 30 cm thick microfossil-bearing black chert occurs in the uppermost part of the Farrel Quartzite and is closely associated with evaporite beds. This black chert-evaporite association can be traced for ca. 7 km along strike in the central and the western parts of the greenstone belt. Morphologically diverse carbonaceous microstructures have been identified from the black cherts (Sugitani et al., 2007), whose biogenic origin has been determined through multidisciplinary studies (Grey and Sugitani, 2009; Oehler et al., 2009; Sugitani et al., 2009; House et al., 2013).
Josefsdal. The Josefsdal chert sample 99SA07, collected by F. Westall, was sampled from a chert horizon at the top of the Kromberg Formation in the Onverwacht Group in the upper part of the Josefsdal Valley (25.959490 °S; 31.073787 °E), near the village of Msauli in the Barberton Greenstone Belt, South Africa (Westall et al., 2006). This chert horizon consists of two chert layers separated by silicified pillow basalt, and sample 99SA07 was collected from the upper layer. The black and white/green banded chert horizons consist of silicified volcanoclastic sediments displaying sedimentary structures indicative of deposition in a shallow water environment. These units have been subjected to low-grade metamorphism (lowermost greenschist facies). Combined morphological and chemical characteristics of carbonaceous microstructures suggest that they represent the fossilised remains of photosynthesising microbial mats that thrived in a nearshore volcanogenic sedimentary setting, and whose development was strongly influenced by contemporaneous hydrothermal fluids (Westall et al., 2011, 2015).
Buck Reef. The Buck Reef Chert sample 99SA03, collected by F. Westall, was sampled at the base of the Kromberg Formation, Onverwacht Group, in the Barberton Greenstone Belt (South Africa) (Westall et al., 2001). The Buck Reef Chert is ca. 3.42 Ga old, 250-400 m-thick, and has been metamorphosed to lower greenschist facies. Petrological and geochemical studies indicate that the Buck Reef Chert was deposited under gradually increasing water depths in environments that ranged from shallow coastal lagoons to an open marine platform, with limited influence of local hydrothermal systems and detrital input (Tice, 2009; Tice and Love, 2004, 2006). It has been proposed that carbonaceous matter in the Buck Reef Chert originated from photosynthetic mats developed in the euphotic zone, later dispersed as detrital carbonaceous matter by waves and currents (Tice, 2009; Tice and Love, 2004, 2006).
Carbon isotope compositions of the studied samples. The C isotope compositions given in Table 1 are from Guo et al. (2006) for Doushantuo (average of δ13Corg values obtained on 12 chert samples); Beaumont and Robert (1999) for Bitter Springs, Wabigoon and Gunflint 3 of 6/30/84 samples; Hayes et al. (1983) for McArthur (average of δ13C values obtained on samples 103-1, 106-1, 107-1 and 452-1) and Nabberu (PPRG 089) samples; Strauss and Moore (1992) for Jixian (average of δ13C values obtained on PPRG samples 1422, 1432, 2125 and 2126 from the Gaoyuzhuang Fm.) and Gunflint PPRG 134 (labelled PPRG 1289 in their Table 17.1) samples; Sugahara et al. (2010) for the Farell Quartzite sample MGTKS1 (average of δ13C values obtained on samples labelled CE2 in their Table 1); Westall et al. (2006) for Josefsdal and Hren et al. (2009) for Buck Reef (average of δ13C values obtained on chert samples with δ18O > 18 ‰).
Methods
Kerogen analysis. Kerogens were isolated from the host chert samples through successive demineralisations using an HF-HCl acidic treatment (Durand and Nicaise, 1980), and then ground into a fine powder, from which ~10 mg was used for bulk kerogen O isotope analysis and a few mg were pressed into high purity indium and then carbon coated for secondary electron microscopy (SEM) and SIMS investigations.
Bulk kerogen O isotope compositions were determined by thermal conversion elemental analysis – isotope ratio mass spectrometry (TC/EA-IRMS) at Iso-Analytical Ltd. (Cheshire, UK) following the protocol reported in Tartèse et al. (2016).
O isotope compositions of the kerogen samples were also measured using the Cameca IMS 1280 HR and IMS 1270 E7 ion probe instruments at the Centre de Recherches Pétrographiques et Géochimiques (CRPG) in Nancy (France) during several analytical sessions and using identical analytical protocols to those described in detail in Tartèse et al. (2016). Isotopes of 16O and 18O were first analysed using a Cs+ primary beam of ~10 nA with an acceleration voltage of 10 kV rastered over 10 × 10 mm diameter areas. Negative secondary ions were accelerated at 10 kV and measured in multicollection mode using two Faraday cups (FC) on the L'2 trolley for 16O and on the H1 trolley for 18O and a mass resolving power of ~2500 (using slit #1 in multicollection mode). For each analysis, the FC backgrounds were measured during pre-sputtering, and the measured 18O/16O ratios were corrected for FC background using the average of background measurements performed immediately before (during pre-sputtering of analysis n) and immediately after (during pre-sputtering of analysis n+1). The total analytical time for O isotope analysis was ~5-6 min, including pre-sputtering (60 s) and counting the secondary oxygen ions during 50-60 cycles each of 5 s.
The secondary species 12C1H, 16O, 28Si, 32S and 56Fe16O were then collected during separate acquisition using the magnetic peak switching mode on the same analytical spots using a ~1-10 nA Cs+ beam, depending on C and O intensities, in order to identify and filter the data affected by contamination of organic matter (OM) by residual silica minerals, sulphides, Fe-chromite or Fe-oxides (see details in Tartèse et al., 2016). All the measured oxygen isotope compositions and secondary species intensity are reported in Table S-4.
The final uncertainties for individual δ18O values, reported in Table S-5 at the 2σ level, include uncertainties related to counting statistics associated with each individual analysis and the external reproducibility measured on the Clarno kerogen standard (δ18Obulk = 14.3 ± 0.1 ‰; Tartèse et al., 2016), which was also used to correct the measured δ18O values for instrumental mass fractionation.
O isotope analysis in chert samples by SIMS. O isotope compositions of three black bedded chert samples from the 3.0 Ga Farrel Quartzite Formation (samples CRT, GTEV and GW98) were measured using the CAMECA IMS 1280 HR ion probe at the Centre de Recherches Pétrographiques et Géochimiques (CRPG) in Nancy (France) following the analytical protocol described in Marin et al. (2010). The final uncertainties reported for δ18O values, reported in Table S-3 at the 1σ level, include uncertainties related to counting statistics associated with each individual analysis and the external reproducibility measured on the quartz and Miocene chert internal standards, which were also used to correct the measured δ18O values for instrumental mass fractionation.
Compositions of the Studied Kerogens
Bulk O isotope compositions. Thermal combustion EA-IRMS analyses on four selected kerogen samples ranging in age from 0.58 Ga to 3.42 Ga yielded bulk O abundances between 7.0 and 26.1 wt. %, a range similar to the one reported for Phanerozoic coal and kerogen samples (Tartèse et al., 2016). The associated bulk kerogen δ18O values range between 7.3 ± 1.0 ‰ and 19.8 ± 0.1 ‰ (Table 1).
SIMS elemental contents and O isotopes. Large variations of 12C1H, 16O, 28Si, 32S and 56Fe16O intensities are observed at the ~20-30 mm spot scale in each sample (Fig. S-2), which is similar to the results obtained on Phanerozoic coal and kerogen samples (Tartèse et al., 2016). For each sample, the relationships between measured 16O, 28Si, 32S and 56Fe16O vs. 12C1H intensities generally trend towards the diagram origin in Figure S-2, caused by the variable secondary ion emission for each analysis due to topography and surface flatness effects (resulting from the fact that OM powders were pressed into indium). It is thus not possible to convert these measured intensities directly into C (or O, Si, S, etc.) abundances. However, these intensities are typical of those measured on coal and kerogen samples, ranging, for example, between ∼3 × 106 and ∼2-3 × 107 cps nA-1 for 12C1H intensities (Fig. S-2), which roughly correspond to bulk C contents of around 30 to 75 wt. % (Tartèse et al., 2016). For 16O, most analyses range between ~5 × 106 and 108 cps nA-1 (Fig. S-2), corresponding to O contents of ~7-8 to 25 wt. % (Tartèse et al., 2016), which are typical for kerogens (Durand and Monin, 1980). The O abundance measured in Josefsdal is slightly higher than this range. Intensities of 32S mostly fall in the range ~106-108 cps nA-1 (Fig. S-2), indicating bulk organic S contents around 0.5-2.5 wt. % (Tartèse et al., 2016). The kerogens display a large range of 28Si intensities, between ~103 and 3-4 × 106 cps nA-1 (Fig. S-2). The lower end of this range, up to ~105 cps nA-1, is similar to the 28Si intensities obtained on Phanerozoic coals and kerogens (Tartèse et al., 2016). Finally, 56Fe16O intensities on the studied kerogens range between ~103 and 2-3 × 105 cps nA-1 and are similar to those obtained on Phanerozoic coals and kerogens (Tartèse et al., 2016).
The procedure we employed to filter the SIMS analyses contaminated by sputtering of micro-inclusions of mineral phases is described in detail in Tartèse et al. (2016) and only summarised here. In pure, homogeneous, organic matter the effect of sample surface topography should theoretically result in mixing trends pointing to the origin in binary diagrams (where 16O, 28Si, 32S and 56Fe16O signals are plotted against the 12C1H intensity) because of variations in emissivity. We interpret outliers to such trends as reflecting the presence of mineral inclusions in the corresponding sputtered volume. In binary diagrams of 16O, 28Si, 32S and 56Fe16O versus 12C1H, we calculated linear regressions passing through the origin for each sample, together with their 5σ confidence intervals, which ensures that only true outliers are discarded and not analyses resulting from the local chemical variability of OM. We then excluded outliers falling outside these 5σ confidence intervals as they likely correspond to analyses contaminated by the presence of micro-mineral inclusions. We developed this statistical approach to avoid the ambiguity of ‘visually’ filtering out data.
The studied kerogens display several per mille variations in their oxygen isotopic composition at the micrometre scale (Fig. S-1). For each sample, individual δ18O values display unimodal Gaussian distributions around the mean value (Fig. S-1), indicating that there is no evidence for multiple O-bearing organic components with varied O isotope compositions in the kerogens. The good consistency between thermal combustion EA-IRMS and SIMS average δ18O values obtained on selected samples (Table 1) indicates that most of the SIMS δ18O variability at the sample scale can be assigned to the combined contribution of several analytical effects, such as:
- Topography: even though kerogen samples have been pressed into indium so that their surface is flat, this creates porosity between organic matter grains, which may result in a few per mille uncertainty in measured δ18O values.
- Instrumental Mass Fractionation (IMF): measured δ18O values have been corrected for IMF using analyses carried out on the Clarno kerogen standard (Tartèse et al., 2016). Since there is currently only one kerogen O isotope SIMS standard, it is not possible to assess the possible effects of variable H/C and O/C ratios on IMF of O isotopes. It has been shown that variable H/C ratios induce a few per mille variations of IMF for C isotope analysis by SIMS for example (Sangély et al., 2005). Furthermore, it is likely that H/C and O/C ratios are slightly variable from spot-to-spot in each sample. Altogether this may add a few per mille intra-sample δ18O variability.
- Sputtering micro-mineral inclusions: measurements of the 12C1H, 16O, 28Si, 32S and 56Fe16O intensities for each SIMS spot in which O isotopes were measured allow us to filter out the data compromised by sputtering micro-mineral inclusions (Tartèse et al., 2016). However, this process would not filter out compromised data when micro-mineral inclusions have been completely sputtered away during the O isotope analysis that we carried out before analysis of 12C1H, 16O, 28Si, 32S and 56Fe16O.
- Low 18O count rates: due to the low abundance of O in some of the studied kerogen samples, the 18O intensities measured using a Faraday cup were often low. We have shown previously that measurements with these low 18O count rates (typically between 105 to 106 cps) yielded accurate δ18O average values (see Fig. 1 in Tartèse et al., 2016). However, low 18O count rates increase the dispersion of δ18O values around the mean, which may, therefore, increase the δ18O variability measured at the sample scale.
Oxygen Isotope Fractionation between Organic Matter and Water
As stated in the main text, metabolic exchanges with their environment largely determined the isotopic composition of biomolecules synthesised by living organisms. In aquatic organisms ca. 70-80 % of OM oxygen is derived from the water in which they thrive and the remainder from their diet (Kreuzer-Martin and Jarman, 2007; Wang et al., 2009; Soto et al., 2013; Mayr et al., 2015). However, during thermal maturation of OM, chemical and structural changes occur, such as aromatisation due to the loss of hydrogenated and oxygenated groups, which may then alter the original organic O isotope compositions.
O-rich components (e.g., carbohydrates, amino acids) are quickly degraded during early OM maturation, resulting in the formation of insoluble kerogens dominated by thermally-resistant components (e.g., aromatic moieties). Investigating this potential issue, Zech et al. (2012) showed that neither fractionation nor exchange reactions affected the O isotope composition of carbohydrates during cellulose decomposition over a 27 month period. On the other hand, litter decomposition was accompanied by a diminution of the bulk OM δ18O values, reflecting changes in the relative proportions of O-bearing compounds having different O isotope compositions. Similarly, experiments simulating diagenetic alteration of algal biomass at 80 °C and pH 1-2 have shown that ~70 % of O bound to biomolecules during hydrolytic solubilisation of algal biomass does not exchange with water (Wedeking and Hayes, 1983).
Proto-kerogen samples derived from marine algae collected in surface sediments from the Atlantic and Pacific oceans, and isolated from Lower Jurassic Toarcian shales are characterised by consistent δ18O values of 20.4 ± 1.5 ‰, 20.8 ± 1.1 ‰ and 18.9 ± 1.5 ‰ (Wedeking, 1983). Taking δ18Oseawater values of 0 ‰ for the present-day ocean (in which the precursor biomass of the proto-kerogen samples thrived) and -1 ‰ for the Toarcian ocean (an ice-free ocean in which the precursor biomass of the Toarcian proto-kerogens lived), we can calculate a Δ18Oorganics-water (δ18Oorganics – δ18Owater) of 20.4 ± 0.9 ‰ (2 SD). Such a Δ18Okerogen-water is consistent with the value we estimated for the O isotope fractionation between fossilised cyanobacteria and waters from the lakes Magadi-Natron in East Africa in which their precursor biomass lived (Tartèse et al., 2016). Altogether, these data indicate that the O isotope composition of proto-kerogen is similar to that of O bound to carboxyl functional groups, which does not seem to be fractionated during OM maturation.
Oxygen Isotopes in Cherts
Chert-based oxygen isotope thermometry. The O isotope composition of chert (δ18Ochert) is related to the O isotope composition of the fluid (δ18Owater) from which it precipitated, and the temperature T at which precipitation occurred, and follows the empirical equation derived by Knauth and Epstein (1976):
Eq. S-1
1000 lnαchert-water = 3.09 × 106/T2 – 3.29
≈ Δ18Ochert-water = (δ18Ochert – δ18Owater)
Therefore, interpreting the chert O isotope record in terms of precipitation T, or the O isotope composition of surface water (ocean or lake) from which chert precipitated, implies the assumption of one of these two parameters. As a result, two end-member scenarios can explain the ~10-15 ‰ increase of δ18O values measured in cherts from ca. 3.5 Ga to the present-day: (i) cherts precipitated at low T from waters that were characterised by δ18O values ~10-15 ‰ lower compared to today (Perry, 1967; Kasting et al., 2006; Jaffrés et al., 2007; Hren et al., 2009), or (ii) cherts precipitated at T ~50-70 °C higher than today from waters with O isotope compositions comparable to those of present-day surface waters (Knauth and Epstein, 1976; Knauth and Lowe, 1978, 2003; Robert and Chaussidon, 2006). For Precambrian cherts, there are additional uncertainties regarding the type of waters from which they precipitated (e.g., open marine, restricted lakes, evaporative brine, pore water), and the possibility of post-crystallisation exchange of oxygen isotopes with meteoric or formation water during diagenesis.
Microscale heterogeneity of δ18Ochert and implications for temperature reconstructions. The detailed SIMS study carried out by Marin et al. (2010) on five Gunflint chert samples allowed them to identify large and randomly distributed δ18O variations (up to ~14 ‰ at the 2 µm scale) in microquartz, showing that it preserved O isotope heterogeneity acquired during its formation through diagenesis. Considering that microquartz formed by a dissolution/precipitation process from amorphous silica precipitated at equilibrium with seawater, Marin et al. (2010) were able to reproduce the average and the distribution of δ18O values measured in Gunflint cherts. Their results suggest that cherts precipitated at 37 to 52 °C from water with a δ18O of -1 ‰ and continued to exchange with 30 % pore water during diagenesis at temperatures of 130 to 170 °C. One of the major outcomes of this study is that temperatures calculated from bulk and average SIMS δ18O values measured on chert samples may be ca. 15-20 °C too high, since diagenesis results in bulk δ18Ochert values a few per mille lower than those of amorphous silica precursors. The difference is about 3 ‰ for the Gunflint chert samples studied by Marin et al. (2010).
To test whether similar effects also apply to older samples we carried out SIMS analyses at a ~20 µm scale in three chert samples from the Farrel Quartzite Formation (see description of the studied samples above). These three samples yielded very similar results, with average δ18O values of 16.4-17.3 ‰ and ranges of δ18O values of 5.5-6.5 ‰ at the ~20 µm scale (Table S-3). Such ranges are consistent with those measured at the 20 µm scale on Gunflint chert samples, indicating that ‘true’ (i.e. calculated at the 2 µm scale) δ18O ranges for the Farrel Quartzite chert samples are probably around ~12.5 to 13.5 ‰ (Table S-3). The average and the range of δ18O values of Farrel Quartzite chert samples can be reproduced for temperatures of precipitation of around 80 °C (from water with a δ18O of 0 ‰) and diagenetic temperatures around 250 °C (still considering 30 % pore water) (Fig. S-4a), which corresponds to the lower greenschist conditions experienced by the Farrel Quartzite Formation (Sugitani et al., 2007). Similarly to the observations made for the 1.9 Ga Gunflint samples, this simple modelling shows that the amorphous silica precursor of the Farrel Quartzite chert samples was characterised by δ18O values ~3 ‰ higher than the measured bulk/average δ18O values.
Our SIMS results obtained in Gunflint OM suggest that Gunflint does not represent an open marine environment but may rather correspond to a mid- to high-latitude basin with restricted connection to the open ocean, in which silica crystallised from low δ18O water. Therefore, considering that this silica formed from water with a δ18O of -10 ‰ and contained 30 % pore water with the same O isotope composition, calculations using the model of Marin et al. (2010) yield a bulk chert δ18O value of 22.2 ‰ and a larger range for δ18O variations of 20.7 ‰, for Twater and Tdiagenesis of 5 °C and 130 °C, respectively (Fig. S-4b).
Considering the observations detailed above, we propose a new palaeotemperature evolution curve ranging from 3.5 Ga to the present-day, recalculated from the chert O isotope record by considering the maximum δ18Ochert values per 50 Ma bin intervals (using δ18Ochert data from Knauth, 2005; Knauth and Epstein, 1976; Knauth and Lowe, 1978, 2003; Robert and Chaussidon, 2006; and this study), and adding 3 ‰ to these maximum δ18O values, since it is likely that δ18O values in most, if not all, chert samples were affected to a similar extent by diagenetic dissolution/precipitation processes. As a result, our new δ18Ochert-derived palaeotemperature curve (see Fig. 2 in the main text) is shifted by 10-20 °C compared to those calculated in previous studies.
Climate Modelling for the Early Earth
Using recent 3D Global Climate Model simulations, Charnay et al. (2013) have argued that atmospheric CO2 and CH4 partial pressures of 0.1 bar and 2 mbar, respectively, could have sustained an average surface T of ~20 °C around 3.4 Ga. Further simulations have also shown that even higher mean surface T of ca. 50 °C can be obtained combining PN2 = 0.5 bar and PCO2 = 0.5 bar (for PCH4 between 0 and 1 mbar), and up to ca. 70 °C combining PN2 = 1 bar and PCO2 = 1 bar (for PCH4 between 0 and 2 mbar) (Charnay, 2014). If elevated atmospheric pressures of 1 bar for N2 and CO2 may appear too high, those around 0.5 bar fit well with geological observations (see main text).
Supplementary Figures

Figure S-1 Distribution of the SIMS δ18O values measured on three selected kerogen samples. To construct histograms the data have been binned into 2 ‰ intervals. All individual analyses are displayed by small circles in the upper part of the panel. Large circles and associated error bars correspond to average δ18O values together with their standard deviation (1 SD).

Figure S-2 Measured 16O (a), 28Si (b), 32S (c) and 56Fe16O (d) secondary intensities (normalised to the primary beam intensity) versus 12C1H secondary intensity. For each sample, the relationships between measured 16O, 28Si, 32S and 56Fe16O vs. 12C1H intensities generally trend towards the diagram origin, resulting from variable secondary ion emission for each analysis due to topography and surface flatness effects.

Figure S-3 Oxygen isotope compositions measured in kerogens plotted against their O abundances (a) and their H/C atomic ratios (b). The data are from Wedeking (1983).

Figure S-4 Evolution of the O isotope composition of microquartz formed from the progressive dissolution of amorphous silica precursors. In (a) the parameters used for the calculation, aimed at modelling the SIMS results obtained on the Farrel Quartzite sample CRT, are; 30 % fluid in the initial porosity of the amorphous silica precursors (Xfluid), temperatures of 80 °C and 250 °C for the water from which it initially precipitated and for metamorphism, respectively, and a δ18Owater value of 0 ‰. In (b) the parameters used for the calculation, aimed at modelling the SIMS results obtained on Gunflint chert samples, are; Xfluid = 30 %, temperatures of 5 °C and 130 °C for the water from which it initially precipitated and for diagenesis/ metamorphism, respectively, and a δ18Owater value of -10 ‰ (see text for details).
Supplementary Tables
Table S-1 Bulk H/C, O contents and O and C isotope compositions obtained by pyrolysis on chert-extracted kerogen samples by Wedeking (1983). δ13C and the δ18O values are given relative to VPDB and SMOW, respectively. All the samples, except recent sediments and Lower Toarcian shales, are PPRG samples, whose descriptions are given in Walter et al. (1983).
| Geological unit | Age (Ga) | Sample | H/C at. | δ13C (‰) | O (wt.%) | δ18O (‰) |
| Sediment. Tanner Basin. Pacific | - | 1.33 | -21.4 | 17.9 | 20.8 | |
| Sediment. Walvis Bay. Atlantic | - | 1.36 | -20.0 | 18.4 | 20.4 | |
| Lower Toarcian shales. Paris Basin. France | 0.18 | 10968 | 1.32 | -28.0 | 10.7 | 17.3 |
| 0.18 | 11933 | 1.18 | 4.9 | 20.2 | ||
| 0.18 | 15035 | 1.05 | 3.5 | 19.1 | ||
| Skillogallee. Adelaide Geosyncline. Australia | 0.80 | 455-1 | 0.17 | -23.2 | 2.1 | 25.6 |
| Bitter Springs Fm.. Amadeus basin. Australia | 0.80 | 133-2 | 0.34 | -22.4 | 5.3 | 17.3 |
| 0.80 | 133-2 | 4.9 | 17.5 | |||
| Bungle Bungle Fm.. Birrindudu basin. Australia | 1.55 | 159-1 | 0.31 | -30.0 | 7.2 | 22.5 |
| 1.55 | 138-1 | 0.38 | -30.2 | 6.5 | 19.9 | |
| 1.55 | 154-1 | 0.39 | -30.7 | 8.6 | 22.1 | |
| 1.55 | 152-1 | 0.43 | -29.3 | 5.0 | 17.8 | |
| Duck Creek dolomite. Wyloo Gr.. Ashburton Trough. Australia | 1.80 | 060-1 | 0.32 | -31.7 | 0.3 | 20.4 |
| McLeary Fm.. Belcher Gr.. Ontario. Canada | 1.90 | 447-1 | 0.26 | -28.4 | 1.8 | 14.9 |
| Ventersdorp Fm. Ventersdorp. South Africa | 2.70 | 293-1 | 0.14 | -38.5 | 1.0 | 36.0 |
| Steeprock Lake Fm.. Wabigoon Belt. Ontario. Canada | 2.70 | 325-1 | 0.17 | -27.2 | 2.8 | 21.2 |
| Manjeri Fm.. Belingwe Greenstone Belt. Zimbabwe. Africa | 2.70 | 225-1 | 0.13 | -33.2 | 1.2 | 35.2 |
| Tumbiana Fm.. Fortescue Gr.. Hamersley Basin. Australia | 2.72 | 027-1 | 0.25 | -51.2 | 2.5 | 27.2 |
| Szwartkoppie Fm.. Barberton Greenstone Belt. South Africa | 3.30 | 198-1 | 0.10 | -32.0 | 1.2 | 16.8 |
| Towers Fm.. Warrawoona Gr.. Pilbara Block. Australia | 3.46 | 002-1 | 0.30 | -34.3 | 3.2 | 19.1 |
| 3.46 | 004-1 | 0.16 | -36.0 | 1.9 | 15.3 | |
| Warrawoona Gr.. Pilbara Block. Australia | 3.46 | 016-1 | 0.30 | -35.2 | 0.4 | 19.0 |
| Hooggenoeg Fm.. Barberton Greenstone Belt. South Africa | 3.46 | 182-1 | 0.12 | -31.6 | 1.4 | 19.4 |
Table S-2 O isotope characteristics of the kerogens analysed by SIMS.
| Sample | Reference | Analyses | SIMS δ18O values (‰) | |||
| Min. | Max. | Average | 1SD | |||
| Doushantuo | 1a of 8/25/83 | 13 | 9.2 | 24.7 | 17.0 | 4.3 |
| Bitter Springs | 3 of 11/2/90 | 30 | 2.0 | 17.0 | 8.6 | 3.7 |
| Jixian | 1 of 8/14/83 | 17 | 21.7 | 33.1 | 28.5 | 3.1 |
| McArthur | 7 of 6/21/90 | 13 | 16.7 | 29.6 | 24.4 | 3.9 |
| Nabberu | PPRG 089 | 14 | 16.1 | 33.3 | 25.0 | 5.5 |
| Gunflint | 3 of 6/30/84 & PPRG 134 | 24 | -2.5 | 10.6 | 4.2 | 3.0 |
| Wabigoon | PPRG 325 | 11 | 19.4 | 33.3 | 27.7 | 3.7 |
| Farrel Quartzite | MGTKS1 | 49 | 13.2 | 31.6 | 22.7 | 3.9 |
| Josefsdal | 99SA07 | 16 | 19.6 | 23.9 | 21.8 | 1.1 |
| Buck Reef | 99SA03 | 20 | 15.9 | 28.4 | 19.5 | 3.2 |
Table S-3 Oxygen isotope results obtained by SIMS on the Farrel Quartzite chert samples. The 2 µm range has been calculated from the range of δ18O values measured at the 20 µm scale and the relationship between analysis scale and δ18O range of Marin et al. (2010).
| Analysis | δ18O (‰) | 1σ (‰) | Analysis | δ18O (‰) | 1σ (‰) | Analysis | δ18O (‰) | 1σ (‰) | ||
| CRT 1 | 14.4 | 0.5 | GTEV 1 | 17.2 | 0.2 | GW98-1-48 A | 18.9 | 1.0 | ||
| CRT 5 | 17.3 | 0.9 | GTEV 2 | 16.9 | 0.3 | GW98-1-48 B | 20.4 | 1.0 | ||
| CRT 6 | 14.2 | 0.7 | GTEV 3 A | 17.4 | 0.3 | GW98-1-50 | 16.8 | 0.5 | ||
| CRT 8 | 18.1 | 0.7 | GTEV 3 B | 17.3 | 0.4 | GW98-1-51 | 14.2 | 1.0 | ||
| CRT 9 | 17.1 | 0.6 | GTEV 4 | 18.5 | 1.1 | GW98-1-53 | 15.1 | 1.1 | ||
| CRT 10 | 17.6 | 0.6 | GTEV 4-2 | 15.3 | 0.3 | GW98-1-54 | 18.7 | 1.1 | ||
| CRT 11 | 18.8 | 0.5 | GTEV 6-1 A | 15.2 | 0.2 | GW98-1-55 | 15.9 | 0.7 | ||
| CRT 12 | 18.8 | 0.7 | GTEV 6-1 B | 15.7 | 0.7 | GW98-1-56 | 13.9 | 1.2 | ||
| CRT 13 | 20.5 | 0.6 | GTEV 6-2 | 15.8 | 0.3 | GW98-1-57 | 14.6 | 0.7 | ||
| CRT 14 | 19.9 | 0.6 | GTEV 8 | 19.5 | 0.6 | GW98-1-58 | 14.8 | 0.4 | ||
| CRT 15 | 16.2 | 0.6 | GTEV 10 | 17.2 | 1.2 | GW98-1-59 | 18.6 | 0.3 | ||
| CRT 17 | 15.1 | 0.3 | GTEV 13-1 | 14.3 | 0.9 | GW98-1-62 | 14.2 | 0.5 | ||
| CRT 18 A | 18.1 | 1.1 | GTEV 13-2 | 14.5 | 0.4 | GW98-1-63 | 16.6 | 0.3 | ||
| CRT 18 B | 17.8 | 0.8 | GTEV 15 | 14.0 | 0.6 | GW98-1-99 | 18.0 | 1.1 | ||
| CRT 19 | 14.6 | 0.6 | GTEV 18-1 | 15.5 | 0.8 | |||||
| CRT 21 | 17.6 | 1.4 | GTEV18-2 | 16.0 | 0.4 | |||||
| CRT 22 | 14.6 | 0.8 | GTEV 21-5 | 18.0 | 0.4 | |||||
| CRT 23 | 19.9 | 1.2 | GTEV 30 | 15.4 | 0.4 | |||||
| CRT 24 | 17.9 | 0.3 | GTEV 32 | 17.4 | 0.8 | |||||
| Average | 17.3 | Average | 16.4 | Average | 16.5 | |||||
| 1SD | 2.0 | 1SD | 1.5 | 1SD | 2.1 | |||||
| Max. | 20.5 | Max. | 19.5 | Max. | 20.4 | |||||
| Min. | 14.2 | Min. | 14.0 | Min. | 13.9 | |||||
| Range | 6.3 | Range | 5.5 | Range | 6.5 | |||||
| 2mm range | 13.4 | 2mm range | 12.5 | 2mm range | 13.6 |
Table S-4 All oxygen isotope and chemistry data collected by SIMS. Analyses in purple are those that have been filtered, and grey boxes indicate based on which element between O, Si, S and Fe (see text for details).
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Doushantuo | Doushantuo@1 | 8751 | -920 | 2.10E+07 | 3.42E+04 | -13.64 | 0.62 | -9.33E+04 | 1.86E+07 | 1.74E+07 | 9.03E+04 | 3.55E+07 | 9.86E+04 |
| 1a of 8/25/83 | Doushantuo@02 | 8631 | -929 | 1.72E+07 | 2.65E+04 | -5.34 | 0.51 | -9.38E+04 | 1.58E+07 | 1.90E+07 | 5.32E+04 | 3.60E+07 | 2.36E+04 |
| Doushantuo@03 | 8537 | -938 | 2.60E+07 | 4.43E+04 | -8.46 | 0.64 | -9.39E+04 | 1.62E+07 | 1.60E+07 | 9.08E+04 | 3.55E+07 | 3.04E+04 | |
| Doushantuo@04 | 8595 | -991 | 1.14E+07 | 1.43E+04 | -15.51 | 1.05 | -9.59E+04 | 1.12E+07 | 6.40E+06 | 3.53E+04 | 2.54E+07 | 8.66E+03 | |
| Doushantuo@05 | 8658 | -1185 | 1.80E+07 | 2.79E+04 | -10.38 | 0.81 | -9.57E+04 | 1.59E+07 | 1.34E+07 | 7.27E+04 | 3.15E+07 | 2.09E+04 | |
| Doushantuo@06 | 8849 | -1195 | 1.91E+07 | 3.01E+04 | -8.72 | 0.58 | -9.47E+04 | 1.38E+07 | 9.96E+06 | 5.96E+04 | 2.74E+07 | 1.19E+04 | |
| Doushantuo@07 | 9022 | -1203 | 4.19E+07 | 7.58E+04 | -16.10 | 0.33 | -9.67E+04 | 1.58E+07 | 9.53E+06 | 4.22E+04 | 4.01E+07 | 1.44E+04 | |
| Doushantuo@08 | 9134 | -1296 | 2.79E+07 | 4.78E+04 | -9.99 | 0.36 | -8.96E+04 | 1.81E+07 | 3.01E+07 | 6.72E+04 | 5.22E+07 | 3.19E+04 | |
| Doushantuo@09 | 8968 | -1328 | 5.42E+07 | 1.01E+05 | -15.52 | 0.26 | -8.86E+04 | 1.91E+07 | 2.76E+07 | 4.32E+04 | 5.17E+07 | 4.85E+04 | |
| Doushantuo@10 | 8767 | -1346 | 2.23E+07 | 3.66E+04 | -12.75 | 0.53 | -9.32E+04 | 2.07E+07 | 2.02E+07 | 7.65E+04 | 4.50E+07 | 1.15E+05 | |
| Doushantuo@11 | 8568 | -1355 | 4.00E+07 | 7.17E+04 | -20.83 | 0.25 | -9.41E+04 | 1.23E+07 | 1.30E+07 | 5.51E+04 | 3.35E+07 | 3.07E+04 | |
| Doushantuo@12 | 8473 | -1445 | 3.45E+07 | 6.11E+04 | -14.99 | 0.37 | -9.30E+04 | 1.71E+07 | 1.32E+07 | 4.73E+04 | 3.27E+07 | 2.04E+04 | |
| Doushantuo@15 | 8620 | -1637 | 1.60E+07 | 2.35E+04 | -16.99 | 0.35 | -9.42E+04 | 1.18E+07 | 1.42E+07 | 2.76E+04 | 2.65E+07 | 1.54E+04 | |
| Doushantuo@16 | 8414 | -1550 | 4.30E+07 | 7.82E+04 | -14.34 | 0.27 | -9.02E+04 | 1.93E+07 | 2.86E+07 | 1.96E+05 | 4.66E+07 | 3.81E+04 | |
| Doushantuo@17 | 8279 | -1639 | 4.80E+07 | 8.80E+04 | -17.24 | 0.23 | -8.82E+04 | 1.57E+07 | 3.08E+07 | 3.31E+04 | 5.34E+07 | 3.15E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Bitter Springs | Bitter@02 | 794 | -554 | 4.69E+07 | 8.54E+04 | -23.50 | 0.34 | -7.62E+04 | 1.47E+07 | 2.97E+07 | 7.87E+05 | 6.47E+07 | 2.52E+05 |
| 3 of 11/2/90 | Bitter@03 | 754 | -554 | 7.01E+07 | 1.32E+05 | -20.70 | 0.24 | -7.48E+04 | 7.54E+06 | 4.70E+07 | 1.39E+06 | 6.97E+07 | 3.87E+05 |
| Bitter@05 | 674 | -554 | 7.09E+07 | 1.34E+05 | -19.38 | 0.62 | -7.51E+04 | 1.31E+07 | 3.03E+07 | 8.29E+05 | 6.63E+07 | 2.30E+05 | |
| Bitter@07 | 594 | -554 | 8.48E+07 | 1.63E+05 | -13.01 | 0.52 | -7.59E+04 | 1.14E+07 | 3.94E+07 | 7.80E+05 | 6.70E+07 | 2.61E+05 | |
| Bitter@08 | 554 | -554 | 4.87E+07 | 8.93E+04 | -18.36 | 0.23 | -7.22E+04 | 1.72E+07 | 4.98E+07 | 9.09E+05 | 6.64E+07 | 2.77E+05 | |
| Bitter@09 | 514 | -554 | 4.48E+07 | 8.07E+04 | -26.44 | 0.36 | -7.62E+04 | 6.47E+06 | 2.68E+07 | 3.74E+05 | 6.65E+07 | 3.04E+05 | |
| Bitter@10 | 514 | -514 | 4.16E+07 | 7.46E+04 | -24.50 | 0.30 | -8.06E+04 | 7.73E+06 | 1.83E+07 | 2.81E+05 | 6.84E+07 | 1.71E+05 | |
| Bitter@11 | 554 | -514 | 3.92E+07 | 6.98E+04 | -23.44 | 0.35 | -7.25E+04 | 1.05E+07 | 4.32E+07 | 7.82E+05 | 6.78E+07 | 3.87E+05 | |
| Bitter@12 | 594 | -514 | 4.26E+07 | 7.62E+04 | -27.55 | 0.30 | -7.39E+04 | 1.35E+07 | 3.51E+07 | 1.03E+06 | 6.78E+07 | 3.18E+05 | |
| Bitter@13 | 634 | -514 | 4.67E+07 | 8.42E+04 | -28.04 | 0.37 | -7.74E+04 | 1.17E+07 | 2.50E+07 | 7.85E+05 | 6.57E+07 | 2.71E+05 | |
| Bitter@14 | 674 | -514 | 1.06E+08 | 2.05E+05 | -13.50 | 0.19 | -6.52E+04 | 8.53E+06 | 6.67E+07 | 2.29E+06 | 6.90E+07 | 4.05E+05 | |
| Bitter@15 | 714 | -514 | 4.01E+07 | 7.17E+04 | -21.98 | 0.35 | -7.48E+04 | 1.95E+07 | 3.09E+07 | 1.03E+06 | 6.57E+07 | 2.27E+05 | |
| Bitter@16 | 754 | -514 | 3.61E+07 | 6.39E+04 | -19.12 | 0.37 | -7.60E+04 | 1.11E+07 | 2.96E+07 | 5.83E+05 | 6.74E+07 | 2.24E+05 | |
| Bitter@17 | 794 | -514 | 2.87E+07 | 4.85E+04 | -26.61 | 0.30 | -7.80E+04 | 1.47E+07 | 3.21E+07 | 5.34E+05 | 6.60E+07 | 2.94E+05 | |
| Bitter@18 | 834 | -514 | 4.37E+07 | 7.91E+04 | -20.01 | 0.37 | -7.60E+04 | 1.10E+07 | 2.84E+07 | 6.60E+05 | 6.72E+07 | 1.89E+05 | |
| Bitter@19 | 834 | -474 | 4.41E+07 | 8.02E+04 | -17.65 | 0.26 | -7.35E+04 | 2.01E+07 | 4.50E+07 | 1.08E+06 | 7.01E+07 | 2.75E+05 | |
| Bitter@20 | 794 | -474 | 6.93E+07 | 1.30E+05 | -26.63 | 0.31 | -7.08E+04 | 9.17E+06 | 5.40E+07 | 1.39E+06 | 7.02E+07 | 3.52E+05 | |
| Bitter@21 | 754 | -474 | 4.49E+07 | 8.15E+04 | -20.20 | 0.42 | -7.80E+04 | 1.22E+07 | 2.66E+07 | 4.70E+05 | 7.01E+07 | 2.55E+05 | |
| Bitter@22 | 714 | -474 | 3.84E+07 | 6.82E+04 | -22.26 | 0.41 | -7.51E+04 | 8.66E+06 | 2.82E+07 | 8.03E+05 | 6.55E+07 | 2.44E+05 | |
| Bitter@23 | 674 | -474 | 6.65E+07 | 1.26E+05 | -13.96 | 0.18 | -7.43E+04 | 1.54E+07 | 3.35E+07 | 8.31E+05 | 6.84E+07 | 2.38E+05 | |
| Bitter@24 | 634 | -474 | 4.00E+07 | 7.15E+04 | -24.37 | 0.54 | -7.39E+04 | 1.05E+07 | 2.59E+07 | 5.28E+05 | 6.37E+07 | 2.08E+05 | |
| Bitter@25 | 594 | -474 | 3.12E+07 | 5.41E+04 | -18.53 | 0.46 | -7.62E+04 | 1.86E+07 | 3.36E+07 | 5.83E+05 | 6.35E+07 | 1.96E+05 | |
| Bitter@26 | 554 | -474 | 4.85E+07 | 8.86E+04 | -20.80 | 0.21 | -7.51E+04 | 1.89E+07 | 3.15E+07 | 9.59E+05 | 6.72E+07 | 2.10E+05 | |
| Bitter@27 | 514 | -474 | 3.53E+07 | 6.20E+04 | -29.11 | 0.45 | -7.81E+04 | 6.25E+06 | 1.97E+07 | 3.08E+05 | 6.58E+07 | 2.56E+05 | |
| Bitter@29 | 554 | -434 | 4.65E+07 | 8.48E+04 | -18.77 | 0.29 | -7.29E+04 | 2.81E+07 | 4.43E+07 | 1.29E+06 | 6.76E+07 | 2.17E+05 | |
| Bitter@30 | 594 | -434 | 3.06E+07 | 5.24E+04 | -23.50 | 0.37 | -7.69E+04 | 1.65E+07 | 2.42E+07 | 6.23E+05 | 6.80E+07 | 2.37E+05 | |
| Bitter@31 | 634 | -434 | 5.60E+07 | 1.03E+05 | -26.35 | 0.28 | -7.29E+04 | 7.65E+06 | 6.10E+07 | 4.00E+05 | 6.55E+07 | 6.28E+05 | |
| Bitter@32 | 674 | -434 | 7.18E+07 | 1.36E+05 | -17.03 | 0.34 | -7.40E+04 | 9.93E+06 | 3.96E+07 | 1.68E+06 | 6.57E+07 | 2.87E+05 | |
| Bitter@33 | 714 | -434 | 3.49E+07 | 6.11E+04 | -25.82 | 0.50 | -7.33E+04 | 1.63E+07 | 4.19E+07 | 1.99E+06 | 6.96E+07 | 2.36E+05 | |
| Bitter@34 | 754 | -434 | 2.95E+07 | 5.02E+04 | -24.92 | 0.39 | -7.65E+04 | 9.12E+06 | 2.11E+07 | 2.34E+05 | 6.63E+07 | 2.26E+05 | |
| Bitter@35 | 794 | -434 | 6.60E+07 | 1.24E+05 | -19.67 | 0.25 | -6.99E+04 | 2.36E+07 | 5.53E+07 | 4.46E+06 | 6.58E+07 | 2.96E+05 | |
| Bitter@36 | 834 | -434 | 3.82E+07 | 6.79E+04 | -22.50 | 0.60 | -7.60E+04 | 3.26E+06 | 2.94E+07 | 2.49E+05 | 6.58E+07 | 2.74E+05 | |
| Bitter@37 | 834 | -394 | 4.01E+07 | 7.18E+04 | -21.29 | 0.37 | -7.51E+04 | 1.79E+07 | 3.47E+07 | 4.88E+05 | 7.07E+07 | 2.85E+05 | |
| Bitter@38 | 794 | -394 | 4.65E+07 | 8.48E+04 | -18.00 | 0.40 | -7.55E+04 | 1.30E+07 | 3.28E+07 | 7.71E+05 | 6.66E+07 | 2.38E+05 | |
| Bitter@39 | 754 | -394 | 5.90E+07 | 1.09E+05 | -27.74 | 0.30 | -7.53E+04 | 5.80E+06 | 2.67E+07 | 2.47E+05 | 6.91E+07 | 2.95E+05 | |
| Bitter@40 | 714 | -394 | 9.39E+07 | 1.80E+05 | -17.01 | 0.17 | -6.88E+04 | 1.08E+07 | 5.58E+07 | 3.76E+06 | 6.75E+07 | 2.86E+05 | |
| Bitter@41 | 674 | -394 | 4.90E+07 | 8.96E+04 | -21.12 | 0.28 | -6.53E+04 | 1.50E+07 | 7.06E+07 | 2.42E+07 | 7.37E+07 | 8.10E+05 | |
| Bitter@42 | 634 | -394 | 5.20E+07 | 9.53E+04 | -23.96 | 0.45 | -7.07E+04 | 1.74E+07 | 3.84E+07 | 1.70E+06 | 6.78E+07 | 3.05E+05 | |
| Bitter@43 | 594 | -394 | 3.82E+07 | 6.77E+04 | -23.37 | 0.57 | -7.56E+04 | 1.18E+07 | 3.47E+07 | 6.33E+05 | 7.06E+07 | 3.13E+05 | |
| Bitter@44 | 554 | -394 | 4.77E+07 | 8.70E+04 | -21.61 | 0.34 | -7.47E+04 | 1.96E+07 | 3.60E+07 | 9.55E+05 | 6.63E+07 | 2.45E+05 | |
| Bitter@45 | 514 | -394 | 3.79E+07 | 6.72E+04 | -22.50 | 0.38 | -7.77E+04 | 2.26E+06 | 2.79E+07 | 4.79E+05 | 6.74E+07 | 2.58E+05 | |
| Bitter@46 | 474 | -394 | 1.86E+07 | 2.83E+04 | -31.67 | 0.74 | -7.83E+04 | 5.71E+06 | 1.89E+07 | 1.99E+05 | 7.26E+07 | 1.99E+05 | |
| Bitter@47 | 434 | -394 | 8.01E+07 | 1.52E+05 | -23.48 | 0.31 | -7.08E+04 | 1.00E+07 | 3.60E+07 | 1.11E+06 | 6.79E+07 | 2.91E+05 | |
| Bitter@49 | 354 | -394 | 4.50E+07 | 8.14E+04 | -23.53 | 0.25 | -7.23E+04 | 9.19E+06 | 4.29E+07 | 1.08E+06 | 6.69E+07 | 2.82E+05 | |
| Bitter@50 | 314 | -394 | 4.33E+07 | 7.81E+04 | -19.80 | 0.44 | -7.63E+04 | 1.11E+07 | 2.90E+07 | 9.34E+05 | 7.09E+07 | 2.31E+05 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Jixian | Jixian@1 | -4218 | -23 | 8.39E+07 | 1.88E+05 | -1.70 | 0.20 | 3.80E+04 | 7.46E+06 | 6.22E+07 | 1.25E+04 | 5.87E+07 | 6.76E+04 |
| 1 of 8/14/83 | Jixian@02 | -4178 | -23 | 9.62E+07 | 2.12E+05 | -0.52 | 0.38 | 3.74E+04 | 5.08E+06 | 6.31E+07 | 1.19E+04 | 1.10E+07 | 2.93E+04 |
| Jixian@03 | -4138 | -23 | 8.03E+07 | 1.80E+05 | -3.34 | 0.34 | 3.89E+04 | 1.34E+06 | 2.51E+07 | 8.28E+03 | 1.39E+06 | 1.30E+04 | |
| Jixian@04 | -4098 | -23 | 1.32E+08 | 2.83E+05 | -6.51 | 0.22 | 3.74E+04 | 1.83E+06 | 7.99E+07 | 9.80E+03 | 3.94E+06 | 4.05E+04 | |
| Jixian@05 | -4058 | -23 | 8.17E+07 | 1.82E+05 | -11.37 | 0.32 | 3.67E+04 | 3.22E+06 | 2.60E+07 | 9.44E+03 | 3.57E+06 | 1.28E+04 | |
| Jixian@06 | -4018 | -23 | 5.49E+07 | 1.30E+05 | -4.06 | 0.41 | 3.70E+04 | 9.65E+05 | 1.97E+07 | 8.43E+03 | 1.20E+06 | 1.13E+04 | |
| Jixian@07 | -3978 | -23 | 1.24E+08 | 2.67E+05 | -2.19 | 0.21 | 3.75E+04 | 4.68E+06 | 7.20E+07 | 1.18E+04 | 4.48E+06 | 2.32E+04 | |
| Jixian@08 | -3938 | -23 | 7.49E+07 | 1.69E+05 | -10.96 | 0.37 | 3.70E+04 | 1.59E+06 | 2.07E+07 | 8.41E+03 | 2.13E+06 | 1.06E+04 | |
| Jixian@09 | -3898 | -23 | 1.37E+08 | 2.93E+05 | -2.11 | 0.14 | 3.92E+04 | 5.42E+06 | 5.52E+07 | 1.27E+04 | 4.81E+06 | 2.04E+04 | |
| Jixian@10 | -3858 | -23 | 7.52E+07 | 1.70E+05 | -4.30 | 0.30 | 3.82E+04 | 4.22E+06 | 6.02E+07 | 1.22E+04 | 5.37E+06 | 1.86E+04 | |
| Jixian@11 | -3858 | 17 | 5.26E+07 | 1.26E+05 | -1.88 | 0.42 | 3.75E+04 | 3.81E+06 | 2.96E+07 | 1.06E+04 | 8.00E+06 | 1.70E+04 | |
| Jixian@12 | -3898 | 17 | 8.63E+06 | 3.88E+04 | -12.38 | 2.27 | 3.75E+04 | 1.54E+05 | 1.73E+06 | 7.61E+03 | 1.98E+05 | 9.05E+03 | |
| Jixian@13 | -3938 | 17 | 6.30E+07 | 1.46E+05 | -7.09 | 0.30 | 3.69E+04 | 2.87E+06 | 3.28E+07 | 9.94E+03 | 4.14E+06 | 1.43E+04 | |
| Jixian@14 | -3978 | 17 | 4.42E+07 | 1.08E+05 | -15.01 | 0.42 | 3.55E+04 | 5.84E+06 | 2.20E+07 | 1.09E+04 | 3.00E+06 | 1.15E+04 | |
| Jixian@15 | -4018 | 17 | 4.93E+07 | 1.19E+05 | -10.53 | 0.43 | 3.68E+04 | 1.75E+06 | 2.18E+07 | 9.15E+03 | 1.83E+06 | 1.26E+04 | |
| Jixian@16 | -4058 | 17 | 8.06E+07 | 1.81E+05 | -7.02 | 0.31 | 4.08E+04 | 6.19E+06 | 4.00E+07 | 1.19E+04 | 9.28E+06 | 2.81E+04 | |
| Jixian@17 | -4098 | 17 | 2.70E+07 | 7.49E+04 | -6.96 | 0.69 | 3.83E+04 | 3.02E+05 | 6.76E+06 | 7.88E+03 | 6.59E+06 | 1.13E+04 | |
| Jixian@18 | -4138 | 17 | 6.62E+07 | 1.52E+05 | -5.97 | 0.36 | 3.86E+04 | 3.37E+05 | 1.89E+07 | 8.27E+03 | 5.01E+05 | 1.24E+04 | |
| Jixian@19 | -4178 | 17 | 7.51E+07 | 1.70E+05 | -4.19 | 0.25 | 3.84E+04 | 5.24E+06 | 5.68E+07 | 1.18E+04 | 7.53E+06 | 2.44E+04 | |
| Jixian@20 | -4218 | 17 | 1.03E+08 | 2.26E+05 | -0.60 | 0.47 | 3.77E+04 | 3.86E+06 | 5.34E+07 | 1.12E+04 | 4.53E+06 | 1.97E+04 | |
| Jixian@21 | -4218 | 57 | 8.71E+07 | 1.94E+05 | -2.61 | 0.32 | 3.77E+04 | 4.66E+06 | 4.52E+07 | 1.21E+04 | 5.11E+06 | 1.80E+04 | |
| Jixian@22 | -4178 | 57 | 1.40E+08 | 3.00E+05 | 0.11 | 0.23 | 3.72E+04 | 5.77E+06 | 7.34E+07 | 1.34E+04 | 7.03E+06 | 2.14E+04 | |
| Jixian@23 | -4138 | 57 | 7.58E+07 | 1.72E+05 | -4.57 | 0.24 | 3.80E+04 | 4.82E+06 | 4.09E+07 | 1.15E+04 | 5.83E+06 | 1.72E+04 | |
| Jixian@24 | -4098 | 57 | 1.02E+08 | 2.25E+05 | -0.89 | 0.18 | 3.80E+04 | 2.81E+06 | 4.59E+07 | 1.04E+04 | 5.22E+06 | 1.95E+04 | |
| Jixian@25 | -4058 | 57 | 1.06E+08 | 2.31E+05 | -5.19 | 0.24 | 3.82E+04 | 4.00E+06 | 6.63E+07 | 1.25E+04 | 1.24E+07 | 3.65E+04 | |
| Jixian@26 | -4018 | 57 | 7.50E+07 | 1.69E+05 | -4.83 | 0.34 | 3.75E+04 | 4.07E+06 | 4.04E+07 | 1.15E+04 | 4.93E+06 | 1.64E+04 | |
| Jixian@27 | -3978 | 57 | 8.74E+07 | 1.94E+05 | -3.05 | 0.21 | 3.75E+04 | 5.47E+06 | 5.13E+07 | 1.29E+04 | 5.05E+06 | 2.11E+04 | |
| Jixian@28 | -3938 | 57 | 9.31E+07 | 2.06E+05 | -4.33 | 0.20 | 3.90E+04 | 4.51E+06 | 6.28E+07 | 1.28E+04 | 6.30E+06 | 2.18E+04 | |
| Jixian@29 | -3898 | 57 | 7.45E+07 | 1.69E+05 | -6.00 | 0.30 | 3.89E+04 | 1.50E+06 | 3.25E+07 | 9.36E+03 | 1.92E+06 | 1.39E+04 | |
| Jixian@30 | -3858 | 57 | 4.15E+07 | 1.03E+05 | -13.00 | 0.46 | 3.83E+04 | 9.02E+05 | 1.70E+07 | 9.12E+03 | 1.46E+06 | 1.18E+04 | |
| Jixian@31 | -3858 | 97 | 2.93E+07 | 7.92E+04 | -8.63 | 0.71 | 3.84E+04 | 7.19E+05 | 1.07E+07 | 8.88E+03 | 1.09E+06 | 1.03E+04 | |
| Jixian@32 | -3898 | 97 | 1.19E+08 | 2.55E+05 | -6.11 | 0.14 | 3.63E+04 | 5.25E+06 | 5.12E+07 | 1.25E+04 | 5.60E+06 | 1.98E+04 | |
| Jixian@33 | -3938 | 97 | 8.38E+07 | 1.87E+05 | -4.75 | 0.18 | 3.58E+04 | 4.18E+06 | 4.72E+07 | 1.10E+04 | 3.10E+06 | 1.71E+04 | |
| Jixian@34 | -4018 | 97 | 1.00E+08 | 2.20E+05 | -1.65 | 0.19 | 3.72E+04 | 4.28E+06 | 6.27E+07 | 1.17E+04 | 6.91E+06 | 2.70E+04 | |
| Jixian@35 | -4058 | 97 | 1.36E+08 | 2.89E+05 | -5.27 | 0.16 | 3.84E+04 | 6.26E+06 | 7.53E+07 | 1.28E+04 | 1.08E+07 | 2.78E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| McArthur | McArthur@1 | -5594 | 656 | 1.43E+08 | 2.92E+05 | -5.96 | 0.08 | 4.32E+04 | 2.33E+07 | 6.04E+07 | 3.50E+06 | 6.04E+07 | 1.53E+05 |
| 7 of 6/21/90 | McArthur@02 | -5734 | 671 | 4.25E+07 | 9.30E+04 | -10.37 | 0.29 | 5.15E+04 | 2.00E+07 | 4.12E+07 | 3.50E+06 | 5.98E+07 | 9.60E+04 |
| McArthur@03 | -5597 | 665 | 1.16E+08 | 2.39E+05 | -3.91 | 0.09 | 4.51E+04 | 1.90E+07 | 5.50E+07 | 1.74E+06 | 5.98E+07 | 1.32E+05 | |
| McArthur@04 | -5602 | 792 | 7.32E+07 | 1.54E+05 | -8.96 | 0.23 | 5.30E+04 | 1.70E+07 | 4.82E+07 | 8.73E+05 | 5.93E+07 | 1.13E+05 | |
| McArthur@05 | -5612 | 867 | 1.02E+08 | 2.10E+05 | -7.25 | 0.21 | 5.53E+04 | 1.45E+07 | 5.93E+07 | 1.35E+06 | 5.93E+07 | 1.18E+05 | |
| McArthur@08 | -5372 | 793 | 1.58E+08 | 3.22E+05 | -3.60 | 0.29 | 4.73E+04 | 1.62E+07 | 5.93E+07 | 1.37E+06 | 5.93E+07 | 1.51E+05 | |
| McArthur@09 | -5389 | 701 | 5.47E+07 | 1.17E+05 | -8.78 | 0.22 | 6.65E+04 | 2.09E+07 | 4.49E+07 | 1.40E+06 | 5.98E+07 | 8.58E+04 | |
| McArthur@10 | -5427 | 642 | 5.13E+07 | 1.11E+05 | -3.72 | 0.49 | 4.89E+04 | 1.14E+07 | 2.87E+07 | 1.10E+06 | 5.98E+07 | 5.25E+04 | |
| McArthur@11 | -5591 | 654 | 8.59E+07 | 1.79E+05 | -5.02 | 0.12 | 4.99E+04 | 1.20E+07 | 4.36E+07 | 1.67E+06 | 5.98E+07 | 8.15E+04 | |
| McArthur@12 | -6509 | 1757 | 5.76E+07 | 1.24E+05 | -5.79 | 0.20 | 5.30E+04 | 2.10E+07 | 4.72E+07 | 1.04E+06 | 5.93E+07 | 9.76E+04 | |
| McArthur@13 | -5805 | 1742 | 4.76E+07 | 1.03E+05 | -16.35 | 0.26 | 5.02E+04 | 1.57E+07 | 3.27E+07 | 1.02E+06 | 5.93E+07 | 7.31E+04 | |
| McArthur@14 | -5814 | 1815 | 5.99E+07 | 1.27E+05 | -13.60 | 0.33 | 5.87E+04 | 1.94E+07 | 4.19E+07 | 1.05E+06 | 5.93E+07 | 9.63E+04 | |
| McArthur@15 | -5881 | 1861 | 3.48E+07 | 7.87E+04 | -5.90 | 0.26 | 5.00E+04 | 1.67E+07 | 3.05E+07 | 2.74E+06 | 5.93E+07 | 8.75E+04 | |
| McArthur@16 | -5858 | 1920 | 6.16E+07 | 1.31E+05 | -12.55 | 0.17 | 5.71E+04 | 1.63E+07 | 3.60E+07 | 8.85E+05 | 5.93E+07 | 7.68E+04 | |
| McArthur@18 | -5822 | 2028 | 6.71E+07 | 1.43E+05 | -1.07 | 0.19 | 5.19E+04 | 1.11E+07 | 4.06E+07 | 3.06E+06 | 5.38E+07 | 5.05E+04 | |
| McArthur@19 | -5838 | 2086 | 7.03E+07 | 1.48E+05 | -9.66 | 0.15 | 6.49E+04 | 1.54E+07 | 4.13E+07 | 7.58E+06 | 5.98E+07 | 8.15E+04 | |
| McArthur@20 | -5803 | 2173 | 2.82E+07 | 6.60E+04 | 0.09 | 0.38 | 4.43E+04 | 1.32E+06 | 1.48E+07 | 3.35E+05 | 6.69E+06 | 2.12E+04 | |
| McArthur@21 | -5844 | 2232 | 5.41E+07 | 1.16E+05 | -11.56 | 0.26 | 4.60E+04 | 2.95E+06 | 2.45E+07 | 4.40E+05 | 2.34E+07 | 2.85E+04 | |
| McArthur@23 | -5589 | 2600 | 3.94E+07 | 8.73E+04 | -10.08 | 0.22 | 5.22E+04 | 1.40E+07 | 4.94E+07 | 1.28E+06 | 5.98E+07 | 1.13E+05 | |
| McArthur@24 | -5509 | 2634 | 7.01E+07 | 1.49E+05 | -3.44 | 0.25 | 6.37E+04 | 1.79E+07 | 4.33E+07 | 8.75E+05 | 5.98E+07 | 1.02E+05 | |
| McArthur@25 | -5414 | 2637 | 4.37E+07 | 9.53E+04 | -14.80 | 0.32 | 6.13E+04 | 1.10E+07 | 5.55E+07 | 6.88E+06 | 5.98E+07 | 1.03E+05 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Gunflint | Gunflint@126 | -134 | 3998 | 1.09E+07 | 2.13E+04 | -26.77 | 0.67 | 8.02E+04 | 1.45E+07 | 7.18E+06 | 2.38E+05 | 5.51E+07 | 5.94E+04 |
| 3 of 6/30/84 | Gunflint@127 | -84 | 3998 | 1.16E+07 | 2.26E+04 | -27.55 | 0.49 | 8.00E+04 | 1.58E+07 | 6.43E+06 | 2.36E+05 | 5.05E+07 | 5.54E+04 |
| Gunflint@128 | -34 | 3998 | 1.96E+07 | 3.82E+04 | -27.15 | 0.37 | 6.20E+04 | 1.01E+07 | 9.88E+06 | 9.11E+04 | 5.66E+07 | 1.91E+05 | |
| Gunflint@129 | 16 | 3998 | 1.83E+07 | 3.59E+04 | -22.13 | 0.38 | 5.73E+04 | 1.02E+07 | 1.04E+07 | 1.57E+05 | 5.15E+07 | 1.33E+05 | |
| Gunflint@130 | 66 | 3998 | 1.01E+07 | 1.99E+04 | -27.52 | 0.86 | 5.26E+04 | 7.08E+06 | 1.13E+07 | 1.21E+06 | 9.64E+06 | 1.43E+04 | |
| Gunflint@131 | 116 | 3998 | 9.68E+06 | 1.91E+04 | -27.42 | 0.66 | 7.47E+04 | 1.62E+07 | 6.59E+06 | 1.48E+05 | 1.50E+07 | 2.20E+04 | |
| Gunflint@133 | 216 | 3998 | 5.75E+06 | 1.16E+04 | -28.69 | 1.21 | 6.11E+04 | 1.02E+07 | 5.45E+06 | 1.42E+05 | 4.63E+07 | 6.26E+04 | |
| Gunflint@134 | 266 | 3998 | 1.07E+07 | 2.10E+04 | -28.61 | 0.49 | 6.80E+04 | 1.16E+07 | 1.02E+07 | 1.15E+05 | 5.68E+07 | 1.89E+05 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Gunflint | PPRG134@01 | 616 | -6533 | 2.50E+07 | 4.09E+04 | -30.35 | 0.74 | -8.81E+04 | 1.15E+07 | 6.86E+06 | 4.91E+04 | 6.95E+07 | 7.41E+03 |
| PPRG 134 | PPRG134@02 | 656 | -6533 | 4.33E+07 | 7.70E+04 | -33.98 | 0.24 | -8.17E+04 | 1.52E+07 | 3.02E+07 | 1.01E+05 | 6.81E+07 | 1.65E+04 |
| PPRG134@04 | 736 | -6533 | 6.70E+06 | 4.71E+03 | -35.65 | 1.37 | -8.75E+04 | 1.14E+07 | 5.78E+06 | 9.33E+04 | 6.88E+07 | 6.12E+03 | |
| PPRG134@06 | 816 | -6533 | 9.85E+06 | 1.08E+04 | -26.60 | 1.02 | -9.28E+04 | 1.39E+07 | 9.72E+06 | 6.30E+04 | 5.93E+07 | 5.32E+03 | |
| PPRG134@09 | 936 | -6533 | 1.68E+07 | 2.46E+04 | -29.51 | 0.87 | -8.19E+04 | 1.57E+07 | 2.00E+07 | 1.12E+05 | 6.95E+07 | 1.02E+04 | |
| PPRG134@10 | 976 | -6533 | 2.13E+07 | 3.39E+04 | -24.18 | 0.52 | -8.45E+04 | 1.34E+07 | 3.08E+07 | 7.25E+04 | 7.00E+07 | 1.74E+04 | |
| PPRG134@11 | 976 | -6613 | 1.87E+07 | 2.86E+04 | -27.04 | 0.71 | -8.50E+04 | 1.36E+07 | 1.26E+07 | 8.77E+04 | 6.94E+07 | 8.20E+03 | |
| PPRG134@12 | 936 | -6613 | 3.01E+07 | 5.13E+04 | -27.44 | 0.46 | -8.43E+04 | 1.46E+07 | 1.56E+07 | 7.27E+04 | 6.99E+07 | 9.73E+03 | |
| PPRG134@13 | 896 | -6613 | 2.89E+07 | 4.89E+04 | -25.27 | 0.39 | -8.38E+04 | 1.48E+07 | 1.77E+07 | 8.85E+04 | 6.96E+07 | 1.13E+04 | |
| PPRG134@14 | 856 | -6613 | 1.48E+07 | 2.07E+04 | -23.89 | 0.76 | -8.86E+04 | 1.40E+07 | 1.03E+07 | 7.34E+04 | 6.95E+07 | 5.46E+03 | |
| PPRG134@15 | 816 | -6613 | 1.56E+07 | 2.24E+04 | -27.04 | 0.47 | -8.43E+04 | 1.52E+07 | 2.68E+07 | 1.02E+05 | 6.90E+07 | 1.22E+04 | |
| PPRG134@16 | 776 | -6613 | 2.57E+07 | 4.25E+04 | -25.53 | 0.53 | -8.90E+04 | 1.39E+07 | 1.23E+07 | 6.49E+04 | 6.94E+07 | 7.54E+03 | |
| PPRG134@17 | 736 | -6613 | 7.58E+06 | 6.36E+03 | -31.62 | 1.41 | -9.28E+04 | 1.20E+07 | 6.14E+06 | 9.34E+04 | 4.97E+07 | 3.11E+03 | |
| PPRG134@18 | 696 | -6613 | 1.29E+07 | 1.70E+04 | -25.10 | 0.68 | -8.86E+04 | 1.32E+07 | 8.67E+06 | 8.21E+04 | 6.92E+07 | 8.31E+03 | |
| PPRG134@19 | 656 | -6613 | 2.46E+07 | 4.04E+04 | -26.80 | 0.49 | -8.31E+04 | 1.43E+07 | 2.28E+07 | 1.21E+05 | 6.82E+07 | 1.28E+04 | |
| PPRG134@22 | 656 | -6653 | 4.41E+07 | 7.90E+04 | -28.05 | 0.26 | -7.87E+04 | 6.70E+06 | 5.49E+07 | 3.98E+04 | 6.94E+07 | 1.93E+04 | |
| PPRG134@23 | 696 | -6653 | 1.87E+07 | 2.87E+04 | -24.92 | 0.67 | -8.31E+04 | 1.17E+07 | 1.84E+07 | 1.05E+05 | 7.06E+07 | 1.43E+04 | |
| PPRG134@24 | 736 | -6653 | 1.01E+07 | 1.13E+04 | -28.26 | 1.01 | -8.37E+04 | 8.40E+06 | 1.15E+07 | 7.95E+04 | 6.81E+07 | 9.30E+03 | |
| PPRG134@25 | 776 | -6653 | 1.26E+07 | 1.65E+04 | -26.90 | 0.85 | -8.59E+04 | 1.15E+07 | 1.14E+07 | 5.65E+04 | 6.98E+07 | 1.06E+04 | |
| PPRG134@26 | 816 | -6653 | 1.89E+07 | 2.92E+04 | -25.86 | 0.73 | -8.67E+04 | 1.39E+07 | 1.77E+07 | 7.42E+04 | 6.92E+07 | 9.84E+03 | |
| PPRG134@27 | 856 | -6653 | 1.40E+07 | 1.92E+04 | -29.29 | 0.68 | -8.36E+04 | 1.39E+07 | 2.09E+07 | 8.99E+04 | 6.84E+07 | 1.01E+04 | |
| PPRG134@28 | 896 | -6653 | 1.20E+07 | 1.52E+04 | -29.17 | 0.85 | -9.13E+04 | 1.37E+07 | 9.72E+06 | 7.50E+04 | 5.78E+07 | 4.40E+03 | |
| PPRG134@29 | 936 | -6653 | 8.44E+06 | 8.26E+03 | -22.25 | 1.24 | -8.67E+04 | 1.19E+07 | 1.01E+07 | 7.93E+04 | 6.91E+07 | 8.15E+03 | |
| PPRG134@30 | 976 | -6653 | 2.93E+07 | 4.97E+04 | -26.49 | 0.45 | -8.12E+04 | 1.49E+07 | 4.01E+07 | 1.57E+05 | 7.12E+07 | 1.89E+04 | |
| PPRG134@31 | 976 | -6733 | 1.88E+07 | 2.86E+04 | -31.30 | 0.50 | -8.31E+04 | 1.44E+07 | 2.55E+07 | 1.19E+05 | 7.10E+07 | 1.69E+04 | |
| PPRG134@32 | 936 | -6733 | 1.70E+07 | 2.53E+04 | -23.80 | 0.61 | -8.41E+04 | 1.33E+07 | 1.86E+07 | 8.71E+04 | 6.99E+07 | 1.08E+04 | |
| PPRG134@33 | 896 | -6733 | 1.21E+07 | 1.53E+04 | -29.84 | 0.80 | -8.67E+04 | 1.30E+07 | 1.13E+07 | 7.84E+04 | 6.98E+07 | 7.95E+03 | |
| PPRG134@34 | 856 | -6733 | 1.99E+07 | 3.10E+04 | -22.51 | 0.57 | -9.15E+04 | 1.33E+07 | 1.08E+07 | 7.83E+04 | 6.87E+07 | 5.17E+03 | |
| PPRG134@35 | 816 | -6733 | 1.29E+07 | 1.68E+04 | -31.08 | 0.75 | -8.59E+04 | 1.34E+07 | 1.32E+07 | 6.63E+04 | 6.96E+07 | 9.63E+03 | |
| PPRG134@36 | 776 | -6733 | 2.82E+07 | 4.74E+04 | -26.94 | 0.49 | -8.27E+04 | 1.50E+07 | 2.03E+07 | 5.39E+04 | 6.91E+07 | 1.36E+04 | |
| PPRG134@37 | 736 | -6733 | 3.12E+07 | 5.31E+04 | -30.91 | 0.33 | -8.45E+04 | 1.60E+07 | 2.13E+07 | 9.01E+04 | 7.01E+07 | 1.40E+04 | |
| PPRG134@38 | 696 | -6733 | 2.45E+07 | 3.96E+04 | -37.42 | 0.52 | -8.35E+04 | 1.10E+07 | 2.39E+07 | 5.51E+04 | 7.00E+07 | 1.55E+04 | |
| PPRG134@39 | 656 | -6733 | 1.59E+07 | 2.29E+04 | -29.76 | 0.70 | -8.51E+04 | 9.48E+06 | 1.25E+07 | 6.14E+04 | 6.94E+07 | 9.01E+03 | |
| PPRG134@42 | 536 | -6733 | 1.20E+07 | 1.53E+04 | -20.89 | 1.21 | -8.74E+04 | 1.15E+07 | 1.06E+07 | 8.18E+04 | 6.93E+07 | 6.26E+03 | |
| PPRG134@43 | 496 | -6733 | 1.44E+07 | 1.99E+04 | -27.18 | 0.90 | -8.61E+04 | 1.16E+07 | 1.11E+07 | 1.17E+05 | 6.92E+07 | 8.19E+03 | |
| PPRG134@45 | 416 | -6733 | 2.36E+07 | 3.77E+04 | -41.90 | 0.66 | -8.36E+04 | 6.03E+06 | 1.55E+07 | 5.45E+04 | 6.67E+07 | 8.98E+03 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Nabberu | Naberu@1 | 1816 | 240 | 9.42E+06 | 2.83E+04 | -9.42 | 1.00 | 4.95E+04 | 6.42E+06 | 4.97E+06 | 3.83E+04 | 2.25E+06 | 8.17E+03 |
| PPRG 089 | Naberu@02 | 1856 | 240 | 7.57E+06 | 2.47E+04 | -11.83 | 1.19 | 5.21E+04 | 1.17E+07 | 4.62E+06 | 9.98E+04 | 4.11E+06 | 8.76E+03 |
| Naberu@03 | 1778 | 230 | 1.11E+07 | 3.16E+04 | -9.52 | 0.77 | 4.89E+04 | 3.84E+06 | 3.67E+06 | 2.41E+04 | 1.61E+06 | 7.57E+03 | |
| Naberu@04 | 1710 | 230 | 9.94E+06 | 2.97E+04 | -3.48 | 1.06 | 5.16E+04 | 4.40E+06 | 3.99E+06 | 4.55E+04 | 1.73E+06 | 6.01E+03 | |
| Naberu@05 | 1739 | 294 | 9.02E+06 | 2.77E+04 | -14.97 | 1.12 | 4.43E+04 | 3.43E+06 | 3.06E+06 | 2.08E+04 | 1.63E+06 | 6.15E+03 | |
| Naberu@06 | 1821 | 285 | 9.07E+06 | 2.79E+04 | -10.67 | 1.17 | 5.42E+04 | 9.09E+06 | 4.99E+06 | 6.33E+04 | 3.07E+06 | 7.19E+03 | |
| Naberu@07 | 1905 | 283 | 8.58E+06 | 2.67E+04 | -14.01 | 0.95 | 6.36E+04 | 1.36E+07 | 4.78E+06 | 4.61E+05 | 3.94E+06 | 7.62E+03 | |
| Naberu@08 | 1988 | 278 | 9.72E+06 | 2.89E+04 | -15.79 | 0.95 | 4.71E+04 | 8.81E+06 | 4.57E+06 | 7.80E+04 | 2.38E+06 | 6.79E+03 | |
| Naberu@09 | 2078 | 272 | 6.35E+06 | 2.24E+04 | -6.97 | 1.50 | 5.11E+04 | 8.91E+06 | 2.89E+06 | 1.18E+05 | 3.44E+06 | 7.10E+03 | |
| Naberu@10 | 2127 | 346 | 7.97E+06 | 2.57E+04 | -4.24 | 1.03 | 5.98E+04 | 1.17E+07 | 3.84E+06 | 1.19E+05 | 3.06E+06 | 6.52E+03 | |
| Naberu@11 | 2042 | 394 | 8.54E+06 | 2.68E+04 | -12.97 | 1.03 | 6.09E+04 | 1.35E+07 | 5.81E+06 | 9.36E+04 | 3.03E+06 | 8.91E+03 | |
| Naberu@12 | 1925 | 402 | 8.51E+06 | 2.69E+04 | -6.01 | 1.01 | 7.59E+04 | 6.67E+06 | 5.03E+06 | 9.75E+04 | 2.15E+06 | 8.78E+03 | |
| Naberu@13 | 1956 | 450 | 7.75E+06 | 2.53E+04 | -10.65 | 0.96 | 4.53E+04 | 4.10E+06 | 3.84E+06 | 2.10E+04 | 1.51E+06 | 6.60E+03 | |
| Naberu@14 | 2039 | 505 | 8.15E+06 | 2.61E+04 | -15.21 | 1.10 | 5.46E+04 | 9.18E+06 | 4.60E+06 | 1.03E+05 | 2.28E+06 | 6.27E+03 | |
| Naberu@15 | 1960 | 530 | 8.77E+06 | 2.74E+04 | -4.75 | 0.85 | 7.53E+04 | 1.43E+07 | 6.32E+06 | 1.20E+05 | 4.95E+06 | 1.07E+04 | |
| Naberu@16 | 1872 | 558 | 7.58E+06 | 2.49E+04 | -0.99 | 1.48 | 5.65E+04 | 1.17E+07 | 4.34E+06 | 1.24E+05 | 3.74E+06 | 7.83E+03 | |
| Naberu@17 | 1975 | 580 | 7.87E+06 | 2.53E+04 | -7.18 | 1.09 | 5.22E+04 | 4.08E+06 | 3.99E+06 | 2.35E+04 | 1.54E+06 | 7.37E+03 | |
| Naberu@18 | 2077 | 589 | 7.34E+06 | 2.46E+04 | 0.21 | 1.15 | 6.01E+04 | 1.08E+07 | 4.75E+06 | 9.04E+04 | 3.28E+06 | 7.85E+03 | |
| Naberu@19 | 2146 | 644 | 1.34E+07 | 3.62E+04 | -16.89 | 0.77 | 1.02E+05 | 1.41E+07 | 6.00E+06 | 1.11E+05 | 4.18E+06 | 8.95E+03 | |
| Naberu@20 | 2182 | 710 | 1.02E+07 | 3.01E+04 | -9.49 | 0.93 | 5.14E+04 | 9.47E+06 | 4.74E+06 | 8.30E+04 | 3.55E+06 | 9.73E+03 | |
| Naberu@21 | 2163 | 833 | 7.36E+06 | 2.47E+04 | -3.96 | 0.99 | 7.41E+04 | 1.27E+07 | 3.39E+06 | 1.26E+05 | 6.58E+06 | 7.94E+03 | |
| Naberu@22 | 2108 | 880 | 7.32E+06 | 2.45E+04 | -4.54 | 1.09 | 8.51E+04 | 1.32E+07 | 5.04E+06 | 3.04E+05 | 4.97E+06 | 9.18E+03 | |
| Naberu@23 | 2100 | 946 | 7.17E+06 | 2.43E+04 | -6.32 | 1.18 | 7.44E+04 | 1.18E+07 | 3.80E+06 | 1.92E+05 | 4.47E+06 | 1.08E+04 | |
| Naberu@24 | 2214 | 991 | 8.15E+06 | 2.63E+04 | -2.06 | 0.82 | 7.14E+04 | 1.40E+07 | 5.27E+06 | 1.36E+05 | 5.49E+06 | 9.19E+03 | |
| Naberu@25 | 2369 | 1226 | 7.15E+06 | 2.42E+04 | -7.78 | 1.45 | 7.09E+04 | 1.10E+07 | 3.84E+06 | 9.53E+04 | 4.61E+06 | 7.12E+03 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Wabigoon | Wabigoon@1 | 4287 | -222 | 3.98E+07 | 9.98E+04 | -6.84 | 0.39 | 4.17E+04 | 4.73E+06 | 2.35E+07 | 9.46E+03 | 1.20E+07 | 2.80E+04 |
| PPRG 325 | Wabigoon@02 | 4327 | -222 | 2.26E+07 | 6.61E+04 | -3.61 | 1.05 | 3.54E+04 | 2.21E+06 | 1.44E+07 | 8.21E+03 | 8.30E+06 | 1.99E+04 |
| Wabigoon@03 | 4367 | -222 | 1.22E+07 | 4.53E+04 | -13.60 | 1.29 | 3.56E+04 | 1.13E+06 | 5.20E+06 | 7.92E+03 | 1.48E+06 | 1.07E+04 | |
| Wabigoon@04 | 4407 | -222 | 5.49E+07 | 1.30E+05 | -4.58 | 0.32 | 3.84E+04 | 5.97E+06 | 3.88E+07 | 1.06E+04 | 1.01E+07 | 4.71E+04 | |
| Wabigoon@05 | 4447 | -222 | 4.28E+07 | 1.07E+05 | 1.49 | 0.49 | 3.76E+04 | 7.78E+05 | 4.16E+06 | 7.93E+03 | 1.46E+06 | 1.08E+04 | |
| Wabigoon@07 | 4527 | -222 | 5.62E+07 | 1.32E+05 | -2.25 | 0.37 | 3.54E+04 | 5.30E+06 | 4.33E+07 | 1.03E+04 | 1.41E+07 | 5.46E+04 | |
| Wabigoon@08 | 4567 | -222 | 5.80E+07 | 1.36E+05 | -1.24 | 0.40 | 3.69E+04 | 4.51E+06 | 4.54E+07 | 9.97E+03 | 6.82E+06 | 4.65E+04 | |
| Wabigoon@09 | 4607 | -222 | 2.87E+07 | 7.78E+04 | -4.68 | 0.61 | 3.82E+04 | 1.72E+06 | 1.52E+07 | 8.79E+03 | 3.69E+06 | 1.76E+04 | |
| Wabigoon@10 | 4647 | -222 | 6.01E+07 | 1.41E+05 | -0.88 | 0.27 | 3.92E+04 | 4.84E+06 | 3.30E+07 | 1.06E+04 | 2.04E+07 | 4.60E+04 | |
| Wabigoon@11 | 4647 | -182 | 5.06E+07 | 1.21E+05 | -8.45 | 0.37 | 3.59E+04 | 3.16E+06 | 2.84E+07 | 9.20E+03 | 8.27E+06 | 2.75E+04 | |
| Wabigoon@12 | 4607 | -182 | 5.60E+07 | 1.32E+05 | -1.83 | 0.39 | 3.54E+04 | 1.59E+06 | 1.57E+07 | 8.39E+03 | 6.40E+06 | 1.98E+04 | |
| Wabigoon@13 | 4567 | -182 | 5.52E+07 | 1.31E+05 | -1.61 | 0.31 | 3.75E+04 | 4.63E+06 | 3.29E+07 | 1.01E+04 | 1.78E+07 | 4.00E+04 | |
| Wabigoon@14 | 4527 | -182 | 6.35E+07 | 1.47E+05 | -2.65 | 0.28 | 3.60E+04 | 4.58E+06 | 3.80E+07 | 1.02E+04 | 7.77E+06 | 4.89E+04 | |
| Wabigoon@17 | 4407 | -182 | 7.12E+07 | 1.63E+05 | -1.40 | 0.30 | 3.71E+04 | 4.63E+06 | 3.35E+07 | 9.80E+03 | 7.88E+06 | 2.76E+04 | |
| Wabigoon@18 | 4367 | -182 | 5.05E+07 | 1.21E+05 | -4.15 | 0.33 | 3.69E+04 | 4.59E+06 | 1.61E+07 | 1.00E+04 | 1.77E+07 | 2.57E+04 | |
| Wabigoon@19 | 4327 | -182 | 4.39E+07 | 1.07E+05 | -8.81 | 0.40 | 3.72E+04 | 5.26E+06 | 2.76E+07 | 1.06E+04 | 5.23E+06 | 3.33E+04 | |
| Wabigoon@20 | 4287 | -182 | 4.60E+07 | 1.12E+05 | -6.55 | 0.40 | 3.81E+04 | 2.87E+06 | 1.57E+07 | 8.89E+03 | 5.16E+06 | 1.85E+04 | |
| Wabigoon@21 | 4247 | -182 | 6.40E+07 | 1.48E+05 | -2.74 | 0.22 | 3.71E+04 | 6.13E+06 | 3.62E+07 | 1.03E+04 | 8.71E+06 | 3.83E+04 | |
| Wabigoon@23 | 4207 | -142 | 5.64E+07 | 1.33E+05 | -5.18 | 0.38 | 3.87E+04 | 4.28E+06 | 2.47E+07 | 9.95E+03 | 7.14E+06 | 3.21E+04 | |
| Wabigoon@24 | 4207 | -102 | 3.89E+07 | 9.82E+04 | -5.45 | 0.62 | 3.89E+04 | 3.55E+06 | 3.03E+07 | 9.92E+03 | 4.71E+06 | 3.58E+04 | |
| Wabigoon@25 | 4207 | -62 | 4.53E+07 | 1.11E+05 | -1.65 | 0.42 | 3.74E+04 | 4.87E+06 | 1.48E+07 | 9.53E+03 | 6.83E+06 | 2.28E+04 | |
| Wabigoon@26 | 4207 | -22 | 6.29E+07 | 1.46E+05 | 0.22 | 0.40 | 3.65E+04 | 5.80E+06 | 2.89E+07 | 1.01E+04 | 9.41E+06 | 3.60E+04 | |
| Wabigoon@27 | 4207 | 18 | 9.08E+07 | 2.02E+05 | 3.37 | 0.24 | 3.67E+04 | 2.45E+06 | 4.08E+07 | 9.03E+03 | 5.86E+06 | 3.32E+04 | |
| Wabigoon@28 | 4207 | 58 | 4.94E+07 | 1.19E+05 | -4.65 | 0.39 | 3.78E+04 | 6.95E+06 | 2.86E+07 | 1.05E+04 | 2.85E+07 | 4.02E+04 | |
| Wabigoon@29 | 4207 | 98 | 3.41E+07 | 8.85E+04 | -4.41 | 0.44 | 3.64E+04 | 1.98E+06 | 9.18E+06 | 8.73E+03 | 1.97E+06 | 1.23E+04 | |
| Wabigoon@30 | 4207 | 138 | 5.17E+07 | 1.23E+05 | -9.53 | 0.31 | 3.68E+04 | 4.58E+06 | 1.77E+07 | 9.93E+03 | 3.47E+06 | 2.09E+04 | |
| Wabigoon@31 | 4207 | 178 | 4.81E+07 | 1.16E+05 | -4.52 | 0.42 | 3.84E+04 | 5.14E+06 | 3.07E+07 | 1.09E+04 | 8.87E+06 | 3.88E+04 | |
| Wabigoon@32 | 4207 | 218 | 3.01E+07 | 8.11E+04 | -5.03 | 0.52 | 3.75E+04 | 5.21E+06 | 1.57E+07 | 9.75E+03 | 1.04E+07 | 2.73E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Farrel Quartzite | MGTKS1@50 | -1917 | -1297 | 2.30E+07 | 4.56E+04 | -13.08 | 0.28 | 3.91E+04 | 1.71E+07 | 3.51E+07 | 2.13E+06 | 1.18E+07 | 2.14E+04 |
| MGTKS1 | MGTKS1@51 | -1917 | -1247 | 3.86E+07 | 7.64E+04 | -9.36 | 0.26 | 4.50E+04 | 1.16E+07 | 4.56E+07 | 2.30E+06 | 5.90E+07 | 4.38E+04 |
| MGTKS1@52 | -1917 | -1197 | 1.05E+08 | 2.07E+05 | -5.49 | 0.07 | 4.02E+04 | 5.86E+06 | 1.27E+08 | 6.00E+06 | 5.41E+07 | 1.01E+05 | |
| MGTKS1@53 | -1917 | -1147 | 2.51E+07 | 5.00E+04 | -10.27 | 0.41 | 3.83E+04 | 1.06E+07 | 1.30E+07 | 3.02E+05 | 1.87E+07 | 2.76E+04 | |
| MGTKS1@54 | -1917 | -1097 | 3.15E+07 | 6.25E+04 | -12.42 | 0.24 | 4.20E+04 | 1.43E+07 | 1.42E+07 | 1.08E+05 | 1.05E+07 | 1.09E+04 | |
| MGTKS1@55 | -1917 | -1047 | 1.57E+07 | 3.14E+04 | -18.17 | 0.39 | 3.98E+04 | 1.26E+07 | 8.33E+06 | 5.46E+04 | 6.96E+06 | 1.07E+04 | |
| MGTKS1@56 | -1917 | -997 | 1.75E+07 | 3.49E+04 | -14.36 | 0.35 | 4.07E+04 | 1.33E+07 | 7.92E+06 | 6.62E+04 | 7.05E+06 | 1.33E+04 | |
| MGTKS1@57 | -1917 | -947 | 2.24E+07 | 4.48E+04 | -14.10 | 0.26 | 4.02E+04 | 1.40E+07 | 9.17E+06 | 1.25E+05 | 7.42E+06 | 1.77E+04 | |
| MGTKS1@58 | -1917 | -897 | 5.33E+07 | 1.06E+05 | -10.60 | 0.17 | 4.14E+04 | 1.68E+07 | 6.53E+07 | 2.99E+06 | 6.02E+07 | 7.96E+04 | |
| MGTKS1@60 | -1917 | -797 | 3.96E+07 | 7.85E+04 | -11.24 | 0.21 | 4.67E+04 | 1.30E+07 | 7.06E+07 | 3.70E+06 | 8.67E+07 | 7.71E+04 | |
| MGTKS1@61 | -1917 | -747 | 5.29E+07 | 1.05E+05 | -9.03 | 0.18 | 4.60E+04 | 1.37E+07 | 4.76E+07 | 3.75E+06 | 1.35E+08 | 5.85E+04 | |
| MGTKS1@62 | -1917 | -697 | 8.83E+07 | 1.75E+05 | -6.84 | 0.10 | 4.60E+04 | 1.53E+07 | 7.98E+07 | 4.48E+06 | 2.47E+08 | 1.14E+05 | |
| MGTKS1@64 | -1917 | -597 | 3.91E+07 | 7.77E+04 | -8.39 | 0.23 | 4.58E+04 | 1.98E+07 | 5.22E+07 | 2.43E+06 | 6.78E+07 | 5.14E+04 | |
| MGTKS1@65 | -1867 | -597 | 8.62E+07 | 1.70E+05 | -10.62 | 0.21 | 4.39E+04 | 1.54E+07 | 8.61E+07 | 2.29E+06 | 7.29E+07 | 5.01E+04 | |
| MGTKS1@66 | -1817 | -597 | 7.53E+07 | 1.49E+05 | -7.98 | 0.24 | 4.52E+04 | 1.72E+07 | 5.57E+07 | 2.64E+06 | 9.24E+07 | 6.77E+04 | |
| MGTKS1@67 | -1767 | -597 | 8.17E+07 | 1.61E+05 | -9.74 | 0.14 | 4.46E+04 | 1.47E+07 | 2.60E+08 | 1.09E+07 | 7.20E+07 | 1.40E+05 | |
| MGTKS1@68 | -1717 | -597 | 9.40E+07 | 1.87E+05 | -3.56 | 0.14 | 4.46E+04 | 8.87E+06 | 8.11E+07 | 1.99E+06 | 2.64E+07 | 2.30E+04 | |
| MGTKS1@69 | -1667 | -597 | 7.09E+07 | 1.40E+05 | -9.57 | 0.13 | 4.40E+04 | 1.41E+07 | 1.41E+08 | 8.83E+06 | 8.30E+07 | 1.12E+05 | |
| MGTKS1@70 | -1667 | -647 | 1.62E+07 | 3.28E+04 | -10.66 | 0.42 | 4.03E+04 | 7.27E+06 | 1.10E+07 | 2.81E+04 | 3.52E+06 | 6.14E+03 | |
| MGTKS1@71 | -1667 | -697 | 4.75E+07 | 9.45E+04 | -7.91 | 0.26 | 4.08E+04 | 7.83E+06 | 3.35E+07 | 1.55E+06 | 3.81E+07 | 2.49E+04 | |
| MGTKS1@72 | -1667 | -747 | 5.74E+07 | 1.14E+05 | -9.97 | 0.15 | 4.49E+04 | 1.54E+07 | 2.13E+08 | 1.14E+07 | 7.60E+07 | 3.10E+05 | |
| MGTKS1@73 | -1667 | -797 | 1.65E+07 | 3.31E+04 | -15.72 | 0.58 | 3.93E+04 | 1.02E+07 | 6.96E+06 | 2.01E+04 | 4.47E+06 | 5.95E+03 | |
| MGTKS1@74 | -1667 | -847 | 2.50E+07 | 5.01E+04 | -10.16 | 0.36 | 4.05E+04 | 8.93E+06 | 8.93E+06 | 5.21E+05 | 9.01E+06 | 8.54E+03 | |
| MGTKS1@75 | -1617 | -847 | 2.70E+08 | 5.34E+05 | -5.65 | 0.07 | 4.47E+04 | 3.95E+06 | 3.50E+08 | 1.84E+07 | 4.35E+08 | 4.89E+05 | |
| MGTKS1@76 | -1567 | -847 | 1.44E+08 | 2.84E+05 | -4.69 | 0.07 | 4.04E+04 | 1.04E+07 | 1.66E+08 | 8.11E+06 | 4.65E+07 | 1.02E+05 | |
| MGTKS1@77 | -1517 | -847 | 6.54E+07 | 1.29E+05 | -8.85 | 0.18 | 4.03E+04 | 9.44E+06 | 5.64E+07 | 3.43E+06 | 5.32E+07 | 5.08E+04 | |
| MGTKS1@78 | -1467 | -847 | 4.47E+07 | 8.89E+04 | -7.21 | 0.22 | 4.53E+04 | 1.30E+07 | 3.31E+07 | 2.99E+06 | 7.16E+07 | 3.87E+04 | |
| MGTKS1@79 | -1417 | -847 | 4.47E+07 | 8.89E+04 | -7.26 | 0.34 | 4.37E+04 | 1.14E+07 | 3.68E+07 | 2.07E+06 | 1.16E+08 | 4.52E+04 | |
| MGTKS1@81 | -1417 | -747 | 3.36E+07 | 6.65E+04 | -14.42 | 0.26 | 4.02E+04 | 1.75E+07 | 1.36E+07 | 2.56E+05 | 1.96E+07 | 1.26E+04 | |
| MGTKS1@82 | -1417 | -697 | 2.32E+07 | 4.61E+04 | -15.65 | 0.23 | 4.05E+04 | 1.33E+07 | 9.28E+06 | 5.58E+04 | 7.68E+06 | 8.48E+03 | |
| MGTKS1@83 | -1417 | -647 | 3.99E+07 | 7.92E+04 | -11.76 | 0.20 | 4.24E+04 | 1.81E+07 | 4.50E+07 | 2.43E+06 | 5.79E+07 | 4.57E+04 | |
| MGTKS1@85 | -1417 | -547 | 1.86E+07 | 3.73E+04 | -13.69 | 0.36 | 4.03E+04 | 1.07E+07 | 8.17E+06 | 3.95E+05 | 2.87E+07 | 9.27E+03 | |
| MGTKS1@86 | -1417 | -497 | 1.09E+08 | 2.15E+05 | -5.42 | 0.09 | 4.35E+04 | 1.59E+07 | 3.51E+07 | 2.47E+06 | 5.56E+07 | 3.57E+04 | |
| MGTKS1@87 | -1417 | -447 | 6.14E+07 | 1.22E+05 | -6.96 | 0.13 | 4.22E+04 | 7.87E+06 | 8.88E+07 | 4.77E+06 | 1.15E+08 | 1.12E+05 | |
| MGTKS1@88 | -1417 | -397 | 5.65E+07 | 1.11E+05 | -11.63 | 0.18 | 4.42E+04 | 1.38E+07 | 8.25E+07 | 4.55E+06 | 6.49E+07 | 7.86E+04 | |
| MGTKS1@89 | -1417 | -347 | 6.38E+07 | 1.27E+05 | -7.43 | 0.14 | 4.30E+04 | 1.63E+07 | 4.43E+07 | 3.06E+06 | 5.54E+07 | 4.76E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Farrel Quartzite | MGTKS1@02 | -70 | 2401 | 2.47E+07 | 4.19E+04 | -6.00 | 0.53 | -8.83E+04 | 7.63E+06 | 2.47E+07 | 1.88E+06 | 3.31E+07 | 2.68E+03 |
| MGTKS1 | MGTKS1@03 | -20 | 2401 | 3.96E+07 | 7.23E+04 | -2.68 | 0.44 | -7.95E+04 | 7.76E+06 | 2.82E+07 | 1.76E+06 | 6.49E+07 | 8.12E+03 |
| MGTKS1@04 | 30 | 2401 | 4.38E+07 | 8.03E+04 | -9.54 | 0.31 | -8.55E+04 | 8.32E+06 | 4.12E+07 | 2.68E+06 | 3.61E+07 | 2.38E+03 | |
| MGTKS1@05 | 80 | 2401 | 4.45E+07 | 8.22E+04 | -3.63 | 0.13 | -8.61E+04 | 7.64E+06 | 3.92E+07 | 3.39E+06 | 4.14E+07 | 3.51E+03 | |
| MGTKS1@06 | 130 | 2401 | 1.16E+07 | 1.52E+04 | -7.27 | 0.67 | -9.88E+04 | 6.43E+06 | 4.17E+06 | 1.54E+05 | 4.17E+06 | -3.38E+02 | |
| MGTKS1@07 | 180 | 2401 | 4.12E+07 | 7.54E+04 | -5.48 | 0.31 | -8.70E+04 | 9.96E+06 | 2.78E+07 | 1.39E+06 | 6.06E+07 | 5.77E+03 | |
| MGTKS1@08 | 230 | 2401 | 3.16E+07 | 5.59E+04 | -5.09 | 0.59 | -9.02E+04 | 8.38E+06 | 1.63E+07 | 1.34E+06 | 3.67E+07 | 1.69E+03 | |
| MGTKS1@09 | 280 | 2401 | 3.26E+07 | 5.78E+04 | -3.61 | 0.52 | -9.35E+04 | 5.73E+06 | 1.60E+07 | 1.53E+06 | 1.17E+07 | 1.05E+03 | |
| MGTKS1@10 | 330 | 2401 | 1.93E+07 | 3.08E+04 | -7.66 | 0.46 | -9.27E+04 | 5.97E+06 | 1.13E+07 | 1.46E+06 | 4.20E+07 | 3.21E+03 | |
| MGTKS1@11 | 330 | 2451 | 2.57E+07 | 4.40E+04 | -6.46 | 0.24 | -8.88E+04 | 7.31E+06 | 3.49E+07 | 5.93E+06 | 3.81E+07 | 3.84E+03 | |
| MGTKS1@14 | 180 | 2451 | 1.83E+07 | 2.90E+04 | -3.19 | 0.69 | -9.32E+04 | 5.67E+06 | 1.95E+07 | 2.74E+06 | 2.15E+07 | 1.97E+03 | |
| MGTKS1@15 | 130 | 2451 | 1.25E+07 | 1.70E+04 | -6.87 | 0.79 | -9.63E+04 | 6.23E+06 | 7.18E+06 | 2.02E+05 | 6.50E+06 | -8.74E+01 | |
| MGTKS1@16 | 80 | 2451 | 3.89E+07 | 7.00E+04 | -15.81 | 0.37 | -8.26E+04 | 5.21E+06 | 1.47E+07 | 5.28E+05 | 6.82E+07 | 1.16E+04 | |
| MGTKS1@17 | 30 | 2451 | 3.26E+07 | 5.81E+04 | 0.07 | 0.46 | -9.21E+04 | 7.33E+06 | 1.81E+07 | 1.37E+06 | 2.70E+07 | 1.23E+03 | |
| MGTKS1@18 | -20 | 2451 | 3.28E+07 | 5.84E+04 | -3.43 | 0.36 | -8.89E+04 | 7.84E+06 | 2.85E+07 | 2.52E+06 | 3.19E+07 | 2.87E+03 | |
| MGTKS1@20 | -120 | 2451 | 1.95E+07 | 3.13E+04 | -2.67 | 0.47 | -8.61E+04 | 7.44E+06 | 4.65E+07 | 4.69E+06 | 2.84E+07 | 2.50E+03 | |
| MGTKS1@21 | -120 | 2501 | 1.43E+07 | 2.04E+04 | -10.89 | 0.78 | -9.12E+04 | 9.01E+06 | 1.89E+07 | 2.06E+06 | 4.40E+07 | 3.15E+03 | |
| MGTKS1@22 | -70 | 2501 | 3.58E+07 | 6.43E+04 | -5.63 | 0.28 | -8.03E+04 | 8.40E+06 | 3.02E+07 | 3.13E+06 | 6.16E+07 | 5.85E+03 | |
| MGTKS1@23 | -20 | 2501 | 3.88E+07 | 7.02E+04 | -9.49 | 0.17 | -8.83E+04 | 8.11E+06 | 1.36E+07 | 7.75E+05 | 3.63E+07 | 1.74E+03 | |
| MGTKS1@24 | 30 | 2501 | 4.30E+07 | 7.92E+04 | -2.83 | 0.25 | -7.78E+04 | 5.84E+06 | 6.15E+07 | 4.47E+06 | 6.18E+07 | 7.06E+03 | |
| MGTKS1@25 | 80 | 2501 | 2.00E+07 | 3.20E+04 | -7.57 | 0.53 | -8.52E+04 | 6.62E+06 | 1.61E+07 | 8.33E+05 | 6.78E+07 | 4.21E+03 | |
| MGTKS1@27 | 180 | 2501 | 2.11E+07 | 3.47E+04 | -4.92 | 0.35 | -9.21E+04 | 8.97E+06 | 1.46E+07 | 1.03E+06 | 3.25E+07 | 2.46E+03 | |
| MGTKS1@28 | 230 | 2501 | 2.58E+07 | 4.42E+04 | -6.31 | 0.44 | -9.09E+04 | 7.09E+06 | 1.86E+07 | 1.39E+06 | 4.26E+07 | 3.47E+03 | |
| MGTKS1@29 | 280 | 2501 | 2.74E+07 | 4.75E+04 | -5.96 | 0.37 | -8.67E+04 | 7.24E+06 | 2.79E+07 | 2.18E+06 | 3.95E+07 | 2.66E+03 | |
| MGTKS1@30 | 330 | 2501 | 2.92E+07 | 5.07E+04 | -9.43 | 0.38 | -8.83E+04 | 7.04E+06 | 2.56E+07 | 2.14E+06 | 3.94E+07 | 2.63E+03 | |
| MGTKS1@31 | 330 | 2551 | 4.22E+07 | 7.73E+04 | -8.13 | 0.29 | -7.82E+04 | 5.79E+06 | 6.57E+07 | 6.12E+06 | 3.08E+07 | 3.60E+03 | |
| MGTKS1@32 | 280 | 2551 | 2.05E+07 | 3.33E+04 | -7.29 | 0.66 | -8.67E+04 | 7.95E+06 | 3.25E+07 | 2.25E+06 | 4.27E+07 | 2.75E+03 | |
| MGTKS1@33 | 230 | 2551 | 2.85E+07 | 4.97E+04 | -4.83 | 0.36 | -8.58E+04 | 7.80E+06 | 2.96E+07 | 3.20E+06 | 4.71E+07 | 3.57E+03 | |
| MGTKS1@34 | 180 | 2551 | 2.48E+07 | 4.20E+04 | -7.87 | 0.60 | -8.79E+04 | 7.31E+06 | 1.56E+07 | 1.63E+06 | 4.71E+07 | 3.00E+03 | |
| MGTKS1@35 | 130 | 2551 | 5.68E+07 | 1.08E+05 | 0.81 | 0.25 | -7.76E+04 | 5.03E+06 | 6.61E+07 | 7.27E+06 | 2.75E+07 | 3.98E+03 | |
| MGTKS1@36 | 80 | 2551 | 2.48E+07 | 4.22E+04 | -6.23 | 0.37 | -7.98E+04 | 6.89E+06 | 2.24E+07 | 2.67E+06 | 6.54E+07 | 5.72E+03 | |
| MGTKS1@39 | -70 | 2551 | 4.82E+07 | 8.99E+04 | -3.52 | 0.25 | -7.97E+04 | 6.83E+06 | 3.19E+07 | 1.62E+06 | 6.46E+07 | 5.69E+03 | |
| MGTKS1@40 | -120 | 2551 | 2.36E+07 | 3.96E+04 | -6.41 | 0.42 | -8.71E+04 | 8.83E+06 | 1.96E+07 | 1.53E+06 | 5.66E+07 | 3.87E+03 | |
| MGTKS1@41 | -170 | 2551 | 6.49E+07 | 1.24E+05 | -1.16 | 0.16 | -7.67E+04 | 4.11E+06 | 6.61E+07 | 1.07E+07 | 2.06E+07 | 3.28E+03 | |
| MGTKS1@42 | -220 | 2551 | 2.69E+07 | 4.64E+04 | -4.66 | 0.42 | -8.43E+04 | 7.78E+06 | 2.13E+07 | 1.70E+06 | 5.90E+07 | 5.04E+03 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Josefsdal | Josefsdal@01 | 361 | 773 | 2.66E+08 | 5.29E+05 | -9.45 | 0.11 | -8.40E+04 | 5.59E+06 | 5.55E+08 | 9.73E+05 | 2.38E+08 | 2.79E+04 |
| 99SA07 | Josefsdal@02 | 411 | 773 | 2.66E+08 | 5.30E+05 | -9.85 | 0.10 | -8.94E+04 | 7.82E+06 | 4.34E+08 | 7.52E+05 | 2.34E+08 | 1.57E+04 |
| Josefsdal@03 | 461 | 773 | 2.98E+08 | 5.95E+05 | -8.74 | 0.12 | -8.64E+04 | 4.36E+06 | 4.65E+08 | 1.62E+06 | 4.44E+08 | 2.85E+04 | |
| Josefsdal@05 | 561 | 773 | 2.39E+08 | 4.76E+05 | -9.15 | 0.11 | -8.77E+04 | 3.94E+06 | 5.62E+08 | 4.94E+05 | 1.42E+08 | 1.64E+04 | |
| Josefsdal@06 | 611 | 773 | 2.60E+08 | 5.18E+05 | -10.29 | 0.13 | -8.98E+04 | 5.16E+06 | 3.36E+08 | 7.14E+05 | 3.39E+08 | 1.98E+04 | |
| Josefsdal@07 | 661 | 773 | 2.85E+08 | 5.68E+05 | -9.06 | 0.06 | -8.62E+04 | 2.95E+06 | 5.74E+08 | 5.08E+05 | 1.51E+08 | 1.26E+04 | |
| Josefsdal@08 | 711 | 773 | 2.92E+08 | 5.84E+05 | -6.51 | 0.15 | -8.55E+04 | 2.00E+06 | 6.02E+08 | 2.62E+06 | 8.76E+07 | 1.30E+04 | |
| Josefsdal@09 | 711 | 823 | 3.04E+08 | 6.09E+05 | -6.76 | 0.23 | -8.73E+04 | 2.71E+06 | 5.99E+08 | 5.07E+05 | 1.72E+08 | 1.38E+04 | |
| Josefsdal@10 | 661 | 823 | 2.20E+08 | 4.36E+05 | -9.94 | 0.08 | -8.66E+04 | 5.40E+06 | 4.23E+08 | 6.28E+05 | 2.79E+08 | 1.79E+04 | |
| Josefsdal@11 | 611 | 823 | 2.23E+08 | 4.42E+05 | -10.49 | 0.10 | -8.60E+04 | 3.46E+06 | 5.47E+08 | 7.48E+05 | 1.21E+08 | 1.23E+04 | |
| Josefsdal@12 | 561 | 823 | 2.80E+08 | 5.57E+05 | -11.07 | 0.11 | -8.60E+04 | 3.47E+06 | 4.61E+08 | 1.10E+06 | 3.47E+08 | 2.34E+04 | |
| Josefsdal@13 | 511 | 823 | 2.92E+08 | 5.83E+05 | -7.79 | 0.14 | -8.50E+04 | 3.91E+06 | 5.62E+08 | 4.37E+05 | 3.17E+08 | 2.02E+04 | |
| Josefsdal@14 | 461 | 823 | 2.53E+08 | 5.02E+05 | -10.75 | 0.07 | -8.65E+04 | 4.20E+06 | 5.18E+08 | 7.39E+05 | 1.91E+08 | 1.53E+04 | |
| Josefsdal@15 | 411 | 823 | 2.56E+08 | 5.07E+05 | -11.59 | 0.10 | -8.74E+04 | 8.28E+06 | 4.07E+08 | 1.01E+06 | 1.53E+08 | 1.23E+04 | |
| Josefsdal@16 | 361 | 823 | 2.42E+08 | 4.80E+05 | -10.56 | 0.16 | -8.83E+04 | 7.65E+06 | 5.16E+08 | 1.37E+06 | 1.52E+08 | 1.70E+04 | |
| Josefsdal@17 | 361 | 873 | 2.33E+08 | 4.62E+05 | -10.28 | 0.09 | -9.16E+04 | 1.14E+06 | 2.75E+08 | 1.24E+05 | 1.30E+08 | 8.93E+03 | |
| Josefsdal@18 | 411 | 873 | 2.63E+08 | 5.23E+05 | -10.02 | 0.11 | -8.85E+04 | 5.76E+06 | 5.47E+08 | 7.34E+05 | 2.87E+08 | 2.02E+04 | |
| Josefsdal@19 | 461 | 873 | 3.21E+08 | 6.40E+05 | -11.94 | 0.13 | -8.61E+04 | 8.36E+06 | 4.38E+08 | 8.30E+05 | 3.83E+08 | 2.51E+04 | |
| Josefsdal@20 | 511 | 873 | 2.92E+08 | 5.84E+05 | -8.67 | 0.25 | -8.14E+04 | 3.17E+06 | 6.06E+08 | 1.33E+06 | 8.73E+07 | 1.62E+04 | |
| Josefsdal@21 | 561 | 873 | 2.36E+08 | 4.68E+05 | -10.54 | 0.12 | -8.60E+04 | 2.99E+06 | 5.50E+08 | 6.89E+06 | 1.66E+08 | 1.23E+04 | |
| Josefsdal@22 | 611 | 873 | 2.90E+08 | 5.79E+05 | -9.49 | 0.07 | -8.34E+04 | 3.14E+06 | 5.73E+08 | 1.34E+06 | 2.92E+08 | 1.94E+04 | |
| Josefsdal@23 | 661 | 873 | 2.39E+08 | 4.76E+05 | -8.91 | 0.16 | -8.81E+04 | 6.29E+06 | 4.11E+08 | 6.90E+05 | 2.49E+08 | 1.83E+04 | |
| Josefsdal@24 | 711 | 873 | 2.11E+08 | 4.21E+05 | -1.42 | 0.10 | -8.59E+04 | 2.48E+06 | 4.94E+08 | 3.02E+06 | 2.39E+08 | 1.34E+04 | |
| Josefsdal@25 | 711 | 923 | 2.54E+08 | 5.07E+05 | -7.63 | 0.10 | -8.93E+04 | 5.57E+06 | 4.52E+08 | 1.30E+06 | 2.59E+08 | 1.67E+04 | |
| Josefsdal@26 | 661 | 923 | 2.44E+08 | 4.84E+05 | -8.80 | 0.08 | -9.02E+04 | 6.89E+06 | 4.06E+08 | 1.87E+06 | 1.77E+08 | 1.32E+04 | |
| Josefsdal@27 | 611 | 923 | 2.67E+08 | 5.31E+05 | -9.26 | 0.26 | -8.71E+04 | 6.45E+06 | 3.95E+08 | 7.12E+05 | 1.96E+08 | 1.54E+04 | |
| Josefsdal@28 | 561 | 923 | 2.25E+08 | 4.47E+05 | -10.17 | 0.19 | -8.68E+04 | 4.63E+06 | 4.87E+08 | 6.54E+05 | 1.72E+08 | 1.26E+04 | |
| Josefsdal@29 | 511 | 923 | 3.55E+08 | 7.11E+05 | -8.42 | 0.11 | -8.45E+04 | 4.40E+06 | 5.70E+08 | 6.86E+05 | 2.55E+08 | 2.03E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Buck Reef | Buck@01 | 5211 | -590 | 2.73E+07 | 4.73E+04 | -8.70 | 0.71 | -7.87E+04 | 8.81E+06 | 6.48E+07 | 1.20E+07 | 1.24E+07 | 5.95E+04 |
| 99SA03 | Buck@03 | 5131 | -590 | 9.92E+06 | 1.18E+04 | -10.31 | 1.18 | -9.86E+04 | 9.30E+06 | 7.77E+06 | 9.77E+04 | 1.49E+07 | 9.54E+03 |
| Buck@05 | 5051 | -590 | 1.67E+07 | 2.57E+04 | -11.36 | 0.66 | -9.55E+04 | 1.11E+07 | 7.66E+06 | 3.83E+04 | 1.59E+07 | 1.96E+04 | |
| Buck@06 | 5011 | -590 | 2.13E+07 | 3.51E+04 | -5.21 | 0.70 | -9.54E+04 | 9.74E+06 | 8.26E+06 | 1.05E+05 | 1.59E+07 | 1.65E+04 | |
| Buck@07 | 4971 | -590 | 1.98E+07 | 3.17E+04 | -10.93 | 0.74 | -9.71E+04 | 8.93E+06 | 3.64E+06 | 5.71E+04 | 1.46E+07 | 6.44E+03 | |
| Buck@08 | 4931 | -590 | 7.06E+07 | 1.35E+05 | -8.82 | 0.17 | -9.15E+04 | 1.10E+07 | 2.30E+07 | 1.51E+05 | 1.61E+07 | 2.21E+04 | |
| Buck@11 | 5011 | -630 | 1.92E+07 | 3.09E+04 | -6.36 | 0.55 | -9.21E+04 | 9.41E+06 | 1.57E+07 | 7.20E+05 | 2.11E+07 | 1.71E+04 | |
| Buck@13 | 5091 | -630 | 1.71E+07 | 2.65E+04 | -9.52 | 0.62 | -9.23E+04 | 1.31E+07 | 1.01E+07 | 1.58E+05 | 2.30E+07 | 3.23E+04 | |
| Buck@14 | 5131 | -630 | 3.69E+07 | 6.62E+04 | -13.64 | 0.48 | -9.57E+04 | 1.03E+07 | 4.10E+06 | 8.83E+04 | 1.76E+07 | 8.32E+03 | |
| Buck@16 | 5211 | -630 | 1.25E+07 | 1.68E+04 | -10.94 | 0.94 | -9.50E+04 | 8.48E+06 | 6.21E+06 | 2.17E+05 | 1.74E+07 | 8.25E+03 | |
| Buck@18 | 5171 | -750 | 1.39E+07 | 1.98E+04 | -12.59 | 0.81 | -9.77E+04 | 8.23E+06 | 9.08E+06 | 2.37E+05 | 1.40E+07 | 8.38E+03 | |
| Buck@19 | 5131 | -750 | 1.19E+07 | 1.58E+04 | -8.33 | 0.70 | -9.16E+04 | 1.10E+07 | 3.14E+07 | 3.79E+05 | 1.54E+07 | 4.67E+04 | |
| Buck@20 | 5091 | -750 | 1.12E+07 | 1.44E+04 | -9.74 | 1.02 | -9.45E+04 | 1.30E+07 | 8.75E+06 | 9.20E+04 | 1.71E+07 | 2.01E+04 | |
| Buck@21 | 5051 | -750 | 1.05E+07 | 1.30E+04 | -14.17 | 0.84 | -9.73E+04 | 8.94E+06 | 7.37E+06 | 4.35E+04 | 1.71E+07 | 1.39E+04 | |
| Buck@22 | 5011 | -750 | 2.11E+07 | 3.46E+04 | -9.01 | 0.60 | -9.55E+04 | 1.03E+07 | 1.12E+07 | 3.08E+05 | 1.64E+07 | 1.63E+04 | |
| Buck@23 | 4971 | -750 | 1.13E+07 | 1.46E+04 | -11.88 | 0.85 | -9.66E+04 | 9.26E+06 | 7.38E+06 | 7.14E+04 | 1.42E+07 | 1.31E+04 | |
| Buck@26 | 4851 | -750 | 1.12E+07 | 1.43E+04 | -12.62 | 1.00 | -9.71E+04 | 9.68E+06 | 5.23E+06 | 2.32E+05 | 1.56E+07 | 8.03E+03 | |
| Buck@27 | 4851 | -710 | 3.21E+07 | 5.74E+04 | -1.62 | 0.44 | -9.64E+04 | 8.77E+06 | 8.31E+06 | 2.64E+05 | 1.40E+07 | 1.08E+04 | |
| Buck@28 | 4851 | -670 | 1.45E+07 | 2.11E+04 | -6.73 | 0.80 | -9.66E+04 | 9.63E+06 | 6.53E+06 | 1.87E+05 | 1.64E+07 | 2.26E+04 | |
| Buck@29 | 4851 | -630 | 1.40E+07 | 1.99E+04 | -19.16 | 0.62 | -9.34E+04 | 9.49E+06 | 1.05E+07 | 7.21E+04 | 2.73E+07 | 1.72E+04 | |
| Buck@31 | 4851 | -550 | 1.54E+07 | 2.29E+04 | -12.57 | 0.47 | -9.68E+04 | 1.19E+07 | 9.77E+06 | 6.72E+04 | 1.38E+07 | 1.72E+04 | |
| Buck@33 | 4851 | -470 | 1.93E+07 | 3.05E+04 | -13.99 | 0.64 | -9.47E+04 | 1.07E+07 | 1.48E+07 | 2.73E+05 | 1.40E+07 | 2.82E+04 | |
| Buck@34 | 4851 | -430 | 1.50E+07 | 2.21E+04 | -12.53 | 1.10 | -9.76E+04 | 8.82E+06 | 7.47E+06 | 5.69E+04 | 1.21E+07 | 1.19E+04 | |
| Buck@35 | 4851 | -390 | 6.26E+07 | 1.20E+05 | -2.24 | 0.27 | -8.51E+04 | 6.72E+06 | 5.35E+07 | 3.37E+06 | 9.83E+06 | 2.45E+04 | |
| Buck@36 | 4851 | -350 | 1.13E+07 | 1.45E+04 | -12.83 | 1.01 | -9.68E+04 | 8.62E+06 | 8.97E+06 | 8.59E+04 | 1.31E+07 | 1.20E+04 | |
Table S-5 Final SIMS O isotope dataset.
| Sample | Analysis Name | Age (Ma) | Bulk δ18O (‰) | δ18O corr. IMF (‰) | 2σ (‰) |
| Doushantuo | Doushantuo@1 | 580 | 15.0 ± 0.2‰ | 16.4 | 1.4 |
| 1a of 8/25/83 | Doushantuo@02 | 24.7 | 1.2 | ||
| Doushantuo@03 | 21.6 | 1.5 | |||
| Doushantuo@04 | 14.6 | 2.2 | |||
| Doushantuo@05 | 19.7 | 1.7 | |||
| Doushantuo@06 | 21.3 | 1.3 | |||
| Doushantuo@07 | 14.0 | 0.9 | |||
| Doushantuo@08 | 20.1 | 1.0 | |||
| Doushantuo@09 | 14.5 | 0.9 | |||
| Doushantuo@10 | 17.3 | 1.3 | |||
| Doushantuo@11 | 9.2 | 0.8 | |||
| Doushantuo@12 | 15.1 | 1.0 | |||
| Doushantuo@15 | 13.1 | 1.0 | |||
| Bitter Springs | Bitter@02 | 800 | 6.6 | 1.0 | |
| 3 of 11/2/90 | Bitter@05 | 10.7 | 1.4 | ||
| Bitter@07 | 17.0 | 1.2 | |||
| Bitter@08 | 11.7 | 0.8 | |||
| Bitter@10 | 5.6 | 0.9 | |||
| Bitter@12 | 2.5 | 0.9 | |||
| Bitter@13 | 2.0 | 1.0 | |||
| Bitter@15 | 8.1 | 1.0 | |||
| Bitter@16 | 10.9 | 1.0 | |||
| Bitter@17 | 3.4 | 0.9 | |||
| Bitter@18 | 10.0 | 1.0 | |||
| Bitter@19 | 12.4 | 0.9 | |||
| Bitter@21 | 9.9 | 1.1 | |||
| Bitter@22 | 7.8 | 1.1 | |||
| Bitter@23 | 16.1 | 0.8 | |||
| Bitter@24 | 5.7 | 1.3 | |||
| Bitter@25 | 11.5 | 1.1 | |||
| Bitter@26 | 9.3 | 0.8 | |||
| Bitter@30 | 6.6 | 1.0 | |||
| Bitter@32 | 13.0 | 1.0 | |||
| Bitter@33 | 4.2 | 1.2 | |||
| Bitter@34 | 5.1 | 1.0 | |||
| Bitter@35 | 10.4 | 0.8 | |||
| Bitter@37 | 8.8 | 1.0 | |||
| Bitter@38 | 12.1 | 1.1 | |||
| Bitter@42 | 6.1 | 1.1 | |||
| Bitter@43 | 6.7 | 1.3 | |||
| Bitter@44 | 8.4 | 1.0 | |||
| Bitter@47 | 6.6 | 0.9 | |||
| Bitter@50 | 10.3 | 1.1 | |||
| Jixian | Jixian@05 | 1500 | 21.7 | 1.0 | |
| 1 of 8/14/83 | Jixian@08 | 22.1 | 1.1 | ||
| Jixian@09 | 30.9 | 0.8 | |||
| Jixian@10 | 28.7 | 1.0 | |||
| Jixian@13 | 25.9 | 1.0 | |||
| Jixian@19 | 28.9 | 0.9 | |||
| Jixian@20 | 32.4 | 1.2 | |||
| Jixian@21 | 30.4 | 1.0 | |||
| Jixian@22 | 33.1 | 0.9 | |||
| Jixian@23 | 28.5 | 0.9 | |||
| Jixian@26 | 28.2 | 1.0 | |||
| Jixian@27 | 30.0 | 0.9 | |||
| Jixian@28 | 28.7 | 0.9 | |||
| Jixian@32 | 26.9 | 0.8 | |||
| Jixian@33 | 28.3 | 0.9 | |||
| Jixian@34 | 31.4 | 0.9 | |||
| Jixian@35 | 27.8 | 0.9 | |||
| McArthur | McArthur@1 | 1600 | 27.1 | 0.8 | |
| 7 of 6/21/90 | McArthur@02 | 22.7 | 1.0 | ||
| McArthur@03 | 29.1 | 0.8 | |||
| McArthur@04 | 24.1 | 0.9 | |||
| McArthur@05 | 25.8 | 0.9 | |||
| McArthur@09 | 24.3 | 0.9 | |||
| McArthur@12 | 27.3 | 0.9 | |||
| McArthur@13 | 16.7 | 1.0 | |||
| McArthur@14 | 19.4 | 1.0 | |||
| McArthur@15 | 27.1 | 1.0 | |||
| McArthur@16 | 20.5 | 0.9 | |||
| McArthur@23 | 23.0 | 0.9 | |||
| McArthur@24 | 29.6 | 0.9 | |||
| Gunflint | Gunflint@126 | 1880 | 7.3 ± 1.0 ‰ | 4.6 | 1.4 |
| 3 of 6/30/84 | Gunflint@127 | 3.8 | 1.1 | ||
| Gunflint@128 | 4.2 | 0.9 | |||
| Gunflint@129 | 9.2 | 0.9 | |||
| Gunflint@131 | 3.9 | 1.4 | |||
| Gunflint@133 | 2.6 | 2.5 | |||
| Gunflint@134 | 2.7 | 1.1 | |||
| Gunflint | PPRG134@02 | -2.5 | 1.9 | ||
| PPRG 134 | PPRG134@09 | 2.0 | 2.5 | ||
| PPRG134@11 | 4.5 | 2.3 | |||
| PPRG134@12 | 4.1 | 2.1 | |||
| PPRG134@13 | 6.3 | 2.0 | |||
| PPRG134@15 | 4.5 | 2.1 | |||
| PPRG134@18 | 6.4 | 2.3 | |||
| PPRG134@25 | 4.6 | 2.5 | |||
| PPRG134@26 | 5.7 | 2.4 | |||
| PPRG134@27 | 2.2 | 2.3 | |||
| PPRG134@29 | 9.3 | 3.1 | |||
| PPRG134@32 | 7.7 | 2.2 | |||
| PPRG134@33 | 1.7 | 2.5 | |||
| PPRG134@35 | 0.4 | 2.4 | |||
| PPRG134@37 | 0.6 | 2.0 | |||
| PPRG134@39 | 1.8 | 2.3 | |||
| PPRG134@42 | 10.6 | 3.1 | |||
| Nabberu | Naberu@02 | 1850 | 21.2 | 2.5 | |
| PPRG 089 | Naberu@06 | 22.4 | 2.5 | ||
| Naberu@08 | 17.2 | 2.1 | |||
| Naberu@09 | 26.1 | 3.1 | |||
| Naberu@10 | 28.8 | 2.2 | |||
| Naberu@14 | 17.8 | 2.3 | |||
| Naberu@15 | 28.3 | 1.9 | |||
| Naberu@16 | 32.0 | 3.1 | |||
| Naberu@18 | 33.3 | 2.4 | |||
| Naberu@19 | 16.1 | 1.7 | |||
| Naberu@20 | 23.6 | 2.0 | |||
| Naberu@23 | 26.7 | 2.5 | |||
| Naberu@24 | 31.0 | 1.8 | |||
| Naberu@25 | 25.3 | 3.0 | |||
| Wabigoon | Wabigoon@1 | 2700 | 26.2 | 0.9 | |
| PPRG 325 | Wabigoon@03 | 19.4 | 1.5 | ||
| Wabigoon@04 | 28.5 | 0.9 | |||
| Wabigoon@17 | 31.6 | 0.9 | |||
| Wabigoon@19 | 24.2 | 0.9 | |||
| Wabigoon@20 | 26.5 | 0.9 | |||
| Wabigoon@21 | 30.3 | 0.8 | |||
| Wabigoon@23 | 27.9 | 0.9 | |||
| Wabigoon@26 | 33.3 | 0.9 | |||
| Wabigoon@29 | 28.6 | 0.9 | |||
| Wabigoon@31 | 28.5 | 0.9 | |||
| Farrel Quartzite | MGTKS1@50 | 3020 | 19.8 ± 0.1‰ | 18.3 | 0.7 |
| MGTKS1 | MGTKS1@51 | 22.0 | 0.7 | ||
| MGTKS1@53 | 21.1 | 0.9 | |||
| MGTKS1@54 | 18.9 | 0.7 | |||
| MGTKS1@55 | 13.2 | 0.9 | |||
| MGTKS1@56 | 17.0 | 0.8 | |||
| MGTKS1@57 | 17.2 | 0.7 | |||
| MGTKS1@58 | 20.7 | 0.6 | |||
| MGTKS1@60 | 20.1 | 0.6 | |||
| MGTKS1@61 | 22.3 | 0.6 | |||
| MGTKS1@64 | 22.9 | 0.6 | |||
| MGTKS1@65 | 20.7 | 0.6 | |||
| MGTKS1@66 | 23.4 | 0.6 | |||
| MGTKS1@68 | 27.8 | 0.5 | |||
| MGTKS1@70 | 20.7 | 0.9 | |||
| MGTKS1@71 | 23.4 | 0.7 | |||
| MGTKS1@73 | 15.6 | 1.2 | |||
| MGTKS1@74 | 21.2 | 0.8 | |||
| MGTKS1@77 | 22.5 | 0.6 | |||
| MGTKS1@78 | 24.1 | 0.6 | |||
| MGTKS1@79 | 24.1 | 0.8 | |||
| MGTKS1@81 | 16.9 | 0.7 | |||
| MGTKS1@82 | 15.7 | 0.6 | |||
| MGTKS1@83 | 19.6 | 0.6 | |||
| MGTKS1@85 | 17.6 | 0.8 | |||
| MGTKS1@86 | 25.9 | 0.5 | |||
| MGTKS1@88 | 19.7 | 0.6 | |||
| MGTKS1@89 | 23.9 | 0.5 | |||
| MGTKS1@02 | 25.5 | 2.1 | |||
| MGTKS1@04 | 22.0 | 2.0 | |||
| MGTKS1@05 | 27.9 | 1.9 | |||
| MGTKS1@07 | 26.0 | 2.0 | |||
| MGTKS1@08 | 26.4 | 2.2 | |||
| MGTKS1@10 | 23.9 | 2.1 | |||
| MGTKS1@14 | 28.3 | 2.3 | |||
| MGTKS1@17 | 31.6 | 2.1 | |||
| MGTKS1@18 | 28.1 | 2.0 | |||
| MGTKS1@21 | 20.6 | 2.4 | |||
| MGTKS1@22 | 25.9 | 1.9 | |||
| MGTKS1@23 | 22.0 | 1.9 | |||
| MGTKS1@27 | 26.6 | 2.0 | |||
| MGTKS1@28 | 25.2 | 2.1 | |||
| MGTKS1@29 | 25.6 | 2.0 | |||
| MGTKS1@30 | 22.1 | 2.0 | |||
| MGTKS1@32 | 24.2 | 2.3 | |||
| MGTKS1@33 | 26.7 | 2.0 | |||
| MGTKS1@34 | 23.7 | 2.2 | |||
| MGTKS1@40 | 25.1 | 2.0 | |||
| MGTKS1@42 | 26.9 | 2.0 | |||
| Josefsdal | Josefsdal@02 | 3300 | 21.7 | 1.9 | |
| 99SA07 | Josefsdal@05 | 22.4 | 1.9 | ||
| Josefsdal@06 | 21.2 | 1.9 | |||
| Josefsdal@07 | 22.5 | 1.9 | |||
| Josefsdal@10 | 21.6 | 1.9 | |||
| Josefsdal@11 | 21.0 | 1.9 | |||
| Josefsdal@14 | 20.8 | 1.9 | |||
| Josefsdal@16 | 21.0 | 1.9 | |||
| Josefsdal@18 | 21.5 | 1.9 | |||
| Josefsdal@19 | 19.6 | 1.9 | |||
| Josefsdal@23 | 22.6 | 1.9 | |||
| Josefsdal@25 | 23.9 | 1.9 | |||
| Josefsdal@26 | 22.7 | 1.9 | |||
| Josefsdal@27 | 22.3 | 1.9 | |||
| Josefsdal@28 | 21.3 | 1.9 | |||
| Josefsdal@29 | 23.1 | 1.9 | |||
| Buck Reef | Buck@03 | 3420 | 15.0 ± 0.7‰ | 19.7 | 2.5 |
| 99SA03 | Buck@05 | 18.7 | 1.5 | ||
| Buck@06 | 24.8 | 1.6 | |||
| Buck@07 | 19.1 | 1.6 | |||
| Buck@08 | 21.2 | 0.8 | |||
| Buck@13 | 20.5 | 1.4 | |||
| Buck@14 | 16.4 | 1.2 | |||
| Buck@16 | 19.1 | 2.0 | |||
| Buck@18 | 17.5 | 1.8 | |||
| Buck@20 | 20.3 | 2.2 | |||
| Buck@21 | 15.9 | 1.8 | |||
| Buck@22 | 21.0 | 1.4 | |||
| Buck@23 | 18.2 | 1.8 | |||
| Buck@26 | 17.4 | 2.1 | |||
| Buck@27 | 28.4 | 1.1 | |||
| Buck@28 | 23.3 | 1.7 | |||
| Buck@31 | 17.5 | 1.2 | |||
| Buck@33 | 16.1 | 1.5 | |||
| Buck@34 | 17.5 | 2.3 | |||
| Buck@36 | 17.2 | 2.1 | |||
Supplementary Information References
Alleon, J., Bernard, S., Le Guillou, C., Marin-Carbonne, J., Pont, S., Beyssac, O., McKeegan, K.D., Robert, F. (2016) Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and mineralogy. Nature Communications 7, 11977.
Awramik, S.M., Barghoorn, E.S. (1977) Gunflint Microbiota. Precambrian Research 5, 121-142.
Awramik, S.M., McMenamin, D.S., Chongyu, Y., Ziqiang, Z., Qixiu, D., Shusen, Z. (1985) Prokaryotic and eukaryotic microfossils from a Proterozoic/Phanerozoic transition in China. Nature 315, 655-658.
Barghoorn, E.S., Schopf, J.W. (1965) Microorganisms from the Late Precambrian of central Australia. Science 150, 337-339.
Barghoorn, E.S., Tyler, S.A. (1965) Microorganisms from the Gunflint chert. Science 147, 563-575.
Beaumont, V., Robert, F. (1999) Nitrogen isotope ratios of kerogens in Precambrian cherts: a record of the evolution of atmosphere chemistry? Precambrian Research 96, 63-82.
Carrigan, W.J., Cameron, E.M. (1991) Petrological and stable isotope studies of carbonate and sulphide minerals from the Gunflint Formation, Ontario: evidence for the origin of early Proterozoic iron-formation. Precambrian Research 52, 347-380.
Charnay, B. (2014) Dynamique troposphérique et évolution climatique de Titan et de la Terre primitive. Ph.D. thesis, Université Pierre et Marie Curie – Paris VI, 240 pp. Available at https://tel.archives-ouvertes.fr/tel-00987546/document.
Charnay, B., Forget, F., Wordsworth, R., Leconte, J., Millour, E., Codron, F., Spiga, A. (2013) Exploring the faint young Sun problem and the possible climates of the Archean Earth with a 3-D GCM. Journal of Geophysical Research: Atmospheres 118, 10,414-10,431.
Cheng, J.B., Zhang, H.M., Xing, Y.S., Ma, G.G. (1981) On the Upper Precambrian (Sinian Suberathem) in China. Precambrian Research 15, 207-228.
Cloud, P.E. (1965) The significance of the Gunflint (Precambrian) microflora. Science 148, 27-35.
Condon, D.J., Zhu, M., Bowring, S.A., Wang, W., Yang, A., Jin, Y. (2005) U-Pb ages from the Neoproterozoic Doushantuo Formation, China. Science 308, 95-98.
Durand, B., Monin, J.C. (1980) Elemental analysis of kerogens (C, H, O, N, S, Fe). In: Durand, B. (Ed.) Kerogen – Insoluble organic matter from sedimentary rocks. Technip, Paris, France, 113-142.
Durand, B., Nicaise, G. (1980) Procedures for kerogen isolation. In: Durand, B. (Ed.) Kerogen – Insoluble organic matter from sedimentary rocks. Technip, Paris, France, 35-54.
Fralick, P.W., Davis, D.W., Kissin, S.A. (2002) The age of the Gunflint Formation, Ontario, Canada: single zircon U-Pb age determinations from reworked volcanic ash. Canadian Journal of Earth Sciences 39, 1085-1091.
Fralick, P.W., Riding, R. (2015) Steep Rock Lake: Sedimentology and geochemistry of an Archean carbonate platform. Earth-Science Reviews 151, 132-175.
Grey, K., Sugitani, K. (2009) Palynology of Archean microfossils (c. 3.0 Ga) from the Mount Grant area, Pilbara Craton, Western Australia: Further evidence of biogenicity. Precambrian Research 173, 60-69.
Guo, Q., Liu, C., Strauss, H., Goldberg, T., Zhu, M., Pi, D., Wang, J. (2006) Organic carbon isotope geochemistry of the Neoproterozoic Doushantuo Formation, South China. Acta Geologica Sinica 80, 670-683.
Hayes, J.M., Kaplan, I.R., Wedeking, K.W. (1983) Precambrian Organic Geochemistry, Preservation of the Record. In: Schopf, J.W. (Ed.) Earth’s Earliest Biosphere. Its Origin and Evolution. Princeton University Press, Princeton, NJ, 93-134.
Hill, A.C., Walter, M.R. (2000) Mid-Neoproterozoic (~830-750 Ma) isotope stratigraphy of Australia and global correlation. Precambrian Research 100, 181-211.
Hill, A.C., Arouri, K., Gorjan, P., Walter, M.R. (2000) Geochemistry of marine and nonmarine environments of a Neoproterozoic cratonic carbonate/evaporate: The Bitter Springs Formation, central Australia. In: Grotzinger, J.P., James, N.P. (Eds.) Carbonate Sedimentation and Diagenesis in the Evolving Precambrian World. Society of Economic Paleontologists and Mineralogists Special Publication 67, Tulsa, OK, 327-344.
House, C.H., Oehler, D.Z., Sugitani, K., Mimura, K. (2013) Carbon isotopic analyses of ca. 3.0 Ga microstructures imply planktonic autotrophs inhabited Earth's early oceans. Geology 41, 651-654.
Hren, M.T., Tice, M.M., Chamberlain, C.P. (2009) Oxygen and hydrogen isotope evidence for a temperate climate 3.42 billion years ago. Nature 462, 205-208.
Jaffrés, J.B.D., Shields, G.A., Wallmann, K. (2007) The oxygen isotope evolution of seawater: A critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years. Earth-Science Reviews 83, 83-122.
Kasting, J.F., Howard, M.T., Wallmann, K., Veizer, J., Shields, G.A., Jaffrés, J.B.D. (2006) Paleoclimates, ocean depth, and the oxygen isotopic composition of seawater. Earth and Planetary Science Letters 252, 82-93.
Knauth, L.P. (2005) Temperature and salinity history of the Precambrian ocean: Implications for the course of microbial evolution. Palaeogeography, Palaeoclimatology, Palaeoecology 219, 53-69.
Knauth, L.P., Epstein, S. (1976) Hydrogen and oxygen isotope ratios in nodular and bedded cherts. Geochimica et Cosmochimica Acta 40, 1095-1108.
Knauth, L.P., Lowe, D.R. (1978) Oxygen isotope geochemistry of cherts from Onverwacht group (3.4 Billion Years), Transvaal, South-Africa, with implications for secular variations in isotopic composition of cherts. Earth and Planetary Science Letters 41, 209-222.
Knauth, L.P., Lowe, D.R. (2003) High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. Geological Society of America Bulletin 115, 566-580.
Kreuzer-Martin, H.W., Jarman, K.H. (2007) Stable isotope ratios and forensic analysis of microorganisms. Applied Environmental Microbiology 73, 3896-3908.
Li, H.K., Zhu, S.X., Xiang, Z.Q., Su, W.B., Lu, S.N., Zhou, H.Y., Geng, J.Z., Li, S., Yang, F.J. (2010) Zircon U-Pb dating on tuff bed from Gaoyuzhuang Formation in Yanqing, Beijing: Further constraints on the new subdivision of the Mesoproterozoic stratigraphy in the North China Craton. Acta Petrologica Sinica 26, 2131-2140.
Lindsay, J.F., Kruse, P.D., Green, O.R., Hawkins, E., Brasier, M.D., Cartlidge, J., Corfield, R.M. (2005) The Neoproterozoic-Cambrian record in Australia: A stable isotope study. Precambrian Research 143, 113-133.
Lu, S., Zhao, G., Wang, H., Hao, G. (2008) Precambrian metamorphic basement and sedimentary cover of the North China Craton: A review. Precambrian Research 160, 77-93.
Marin, J., Chaussidon, M., Robert, F. (2010) Microscale oxygen isotope variations in 1.9 Ga Gunflint cherts: Assessments of diagenesis effects and implications for oceanic paleotemperature reconstructions. Geochimica et Cosmochimica Acta 74, 116-130.
Mayr, C., Laprida, C., Lücke, A., Martín, R.S., Massaferro, J., Ramón-Mercau, J., Wissel, H. (2015) Oxygen isotope ratios of chironomids, aquatic macrophytes and ostracods for lake-water isotopic reconstructions - Results of a calibration study in Patagonia. Journal of Hydrology 529, 600-607.
Muir, M.D. (1976) Proterozoic microfossils from the Amelia Dolomite, McArthur Basin, Northern Territory. Alcheringa 1, 143-158.
Oehler, D.Z. (1978) Microflora of the middle Proterozoic Balbirini Dolomite (McArthur Group) of Australia. Alcheringa 2, 269-309.
Oehler, D.Z., Robert, F., Walter, M.R., Sugitani, K., Allwood, A., Meibom, A., Mostefaoui, S., Selo, M., Thomen, A., Gibson, E.K. (2009) NanoSIMS: Insights to biogenicity and syngeneity of Archaean carbonaceous structures. Precambrian Research 173, 70-78.
Perry Jr., E.C. (1967) The oxygen isotopic chemistry of ancient cherts. Earth and Planetary Science Letters 3, 62-66.
Pesonen, L.J., Mertanen, S., Veikkolainen, T. (2012) Paleo-Mesoproterozoic Supercontinents – A Paleomagnetic View. Geophysica 48, 5-47.
Pirajno, F., Hocking, R.M., Reddy, S.M., Jones, A.J. (2009) A review of the geology and geodynamic evolution of the Paleoproterozoic Earaheedy Basin, Western Australia. Earth-Science Reviews 94, 39-77.
Rasmussen, B., Fletcher, I.R., Bekker, A., Muhling, J.R., Gregory, C.J., Thorne, A.M. (2012) Deposition of 1.88-billion-year-old iron formations as a consequence of rapid crustal growth. Nature 484, 498-501.
Rawlings, D.J. (1999) Stratigraphic resolution of a multiphase intracratonic basin system: the McArthur Basin, northern Australia. Australian Journal of Earth Sciences 46, 703-723.
Robert, F., Chaussidon, M. (2006) A palaeo-temperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
Sangély, L., Chaussidon, M., Michels, R., Huault, V. (2005) Microanalysis of carbon isotope composition in organic matter by secondary ion mass spectrometry. Chemical Geology 223, 179-195.
Schopf, J.W. (1968) Microflora of the Bitter Springs Formation, Late Precambrian, Central Australia. Journal of Paleontology 42, 651-688.
Schopf, J.W., Zhu, W.Q., Xu, Z.L., Hsu, J. (1984) Proterozoic stromatolitic microbiotas of the 1400-1500 Ma-old Gaoyuzhuang Formation near Jixian, Northern China. Precambrian Research 24, 335-349.
Schulz, K.J., Cannon, W.F. (2007) The Penokean orogeny in the Lake Superior region. Precambrian Research 157, 4-25.
Soto, D.X., Wassenaar, L.I., Hobson, K.A. (2013) Stable hydrogen and oxygen isotopes in aquatic food webs are tracers of diet and provenance. Functional Ecology 27, 535-543.
Strauss, H., Moore, T.B. (1992) Abundances and Isotopic Compositions of Carbon and Sulfur Specie Species in Whole Rock and kerogen Samples. In: Schopf, J.W., Klein, C. (Eds.) The Proterozoic Biosphere – A Multidisciplinary Study. Cambridge University Press, Cambridge, NY, 709-798.
Sugahara, H., Sugitani, K., Mimura, K., Yamashita, F., Yamamoto, K. (2010) A systematic rare-earth elements and yttrium study of Archean cherts at the Mount Goldsworthy greenstone belt in the Pilbara Craton: Implications for the origin of microfossil-bearing black cherts. Precambrian Research 177, 73-87.
Sugitani, K., Grey, K., Allwood, A., Nagaoka, T., Mimura, K., Minami, M., Marshall, C.P., Van Kranendonk, M.J., Walter, M.R. (2007) Diverse microstructures from Archaean chert from the Mount Goldsworthy-Mount Grant area, Pilbara Craton, Western Australia: Microfossils, dubiofossils, or pseudofossils? Precambrian Research 158, 228-262.
Sugitani, K., Grey, K., Nagaoka, T., Mimura, K. (2009) Three-dimensional morphological and textural complexity of Archean putative microfossils from the Northeastern Pilbara Craton: Indications of biogenicity of large (>15 µm) spheroidal and spindle-like structures. Astrobiology 9, 603-615.
Tartèse, R., Chaussidon, M., Gurenko, A., Delarue, F., Robert, F. (2016) In situ oxygen isotope analysis of fossil organic matter. Geochimica et Cosmochimica Acta 182, 24-39.
Tice, M.M. (2009) Environmental controls on photosynthetic microbial mat distribution and morphogenesis on a 3.42 Ga clastic-starved platform. Astrobiology 9, 989-1000.
Tice, M.M., Lowe, D.R. (2004) Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature 431, 549-552.
Tice, M.M., Lowe, D.R. (2006) The origin of carbonaceous matter in pre-3.0 Ga greenstone terrains: a review and new evidence from the 3.42 Ga Buck Reef chert. Earth-Science Reviews 76, 259-300.
Tobin, K.J. (1990) The paleoecology and significance of the Gunflint-type microbial assemblages from the Frere Formation (Early Proterozoic), Nabberu Basin, Western Australia. Precambrian Research 47, 71-81.
Van Kranendonk, M.J., Smithies, R.H., Hickman, A.H., Champion, D.C. (2007) Secular tectonic evolution of Archean continental crust: Interplay between horizontal and vertical processes in the formation of the Pilbara Craton, Australia. Terra Nova 19, 1-38.
Walter, M.R., Goode, A.D.T., Hall, W.D.M. (1976) Microfossils from a newly discovered Precambrian stromatolitic iron formation in Western Australia. Nature 261, 221-223.
Walter, M.R., Hofmann, H.J., Schopf, J.W. (1983) Geographic and geological data for processed rock samples. In: Schopf, J.W. (Ed.) Earth's Earliest Biosphere. Its Origin and Evolution. Princeton University Press, Princeton, NJ, 385-413.
Wang, Y.V., O’Brien, D.M., Jenson, J., Francis, D., Wooller, M.J. (2009) The influence of diet and water on the stable oxygen and hydrogen isotope composition of Chironomidae (Diptera) with paleoecological implications. Oecologia 160, 225-233.
Wedeking, K.W. (1983) The Biogeochemistry of Precambrian Kerogen. Ph.D. thesis, Indiana University, 167 pp.
Wedeking, K.W., Hayes, J.M. (1983) Exchange of oxygen isotopes between water and organic material. Chemical Geology 1, 357-370.
Westall, F., de Wit, M.J., Dann, J., van der Gaast, S., de Ronde, C.E.J., Gerneke, D. (2001) Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Research 106, 93-116.
Westall, F., de Ronde, C.E.J., Southam, G., Grassineau, N., Colas, M., Cockell, C., Lammer, H. (2006) Implications of a 3.472-3.333 Ga old subaerial microbial mat from the Barberton Greenstone Belt, South Africa for the UV environmental conditions on the early Earth. Philosophical Transactions of the Royal Society B 361, 1857-1876.
Westall, F., Cavalazzi, B., Laurence, L., Marrocchi, Y., Rouzaud, J.-N., Simionovici, A., Salome, G., MacLean, L., Wirick, S., Hofmann, A., Meibom, A., Robert, F., Defarge, C. (2011) Implications of in situ calcification for photosynthesis in a ~3.3 Ga-old microbial biofilm from the Barberton greenstone belt, South Africa. Earth and Planetary Science Letters 310, 468-479.
Westall, F., Campbell, K.A., Bréhéret, J.G., Foucher, F., Gautret, P., Hubert, A., Sorieul, S., Grassineau, N., Guido, D.M. (2015) Archean (3.33 Ga) microbe-sediment systems were diverse and flourished in a hydrothermal context. Geology 43, 615-618.
Wilks, M.E., Nisbet, E.G. (1985) Archaean stromatolites from the Steep Rock Group, northwestern Ontario, Canada. Canadian Journal of Earth Sciences 22, 792-799.
Wilks, M.E., Nisbet, E.G. (1988) Stratigraphy of the Steep Rock Group, northwest Ontario: a major Archaean unconformity and Archaean stromatolites. Canadian Journal of Earth Sciences 25, 370-391.
Winter, B.L., Knauth, L.P. (1992) Stable isotope geochemistry of cherts and carbonates from the 2.0-Ga Gunflint iron formation – implications for the depositional setting, and the effects of diagenesis and metamorphism. Precambrian Research 59, 283-313.
Xu, L.Z., Awramik, S.M. (2002) The Gaoyuzhuang Palaeobiology. Acta Botanica Sinica 44, 617-627.
Zech, M., Werner, R.A., Juchelka, D., Kalbitz, K., Buggle, B., Glaser, B. (2012) Absence of oxygen isotope fractionation/exchange of (hemi-) cellulose derived sugars during litter decomposition. Organic Geochemistry 42, 1470-1475.
Zhang, Y. (1981) Proterozoic stromatolite microfloras of the Gaoyuzhuang Formation (Early Sinian: Riphean), Hebei, China. Journal of Paleontology 55, 485-506.
Zhang, S., Jiang, G., Zhang, J., Song, B., Kennedy, M.J., Christie-Blick, N. (2005) U-Pb sensitive high-resolution ion microprobe ages from the Doushantuo Formation in south China: Constraints on late Neoproterozoic glaciations. Geology 33, 473-476.
Figures and Tables
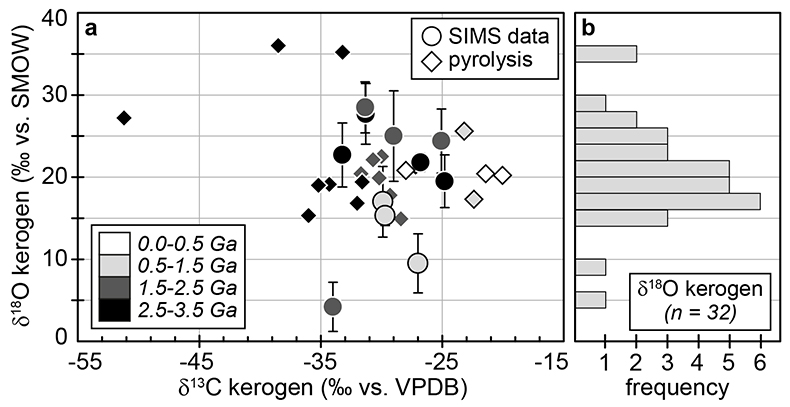
Figure 1 (a) Oxygen isotope compositions of the kerogens analysed by SIMS and by pyrolysis plotted against their C isotope compositions. (b) Frequency distribution of the measured kerogen δ18O values.
Table 1 Main characteristics of the studied samples. δ13C and the δ18O values are given relative to VPDB and SMOW, respectively. See Supplementary Information for details and references.
| Sample | Reference | Age (Ga) | Location | δ18OSIMS (‰) | δ18OTC/EA-IRMS (‰) | d13C (‰) |
| Doushantuo | 1a of 8/25/83 | 0.58 | Doushantuo Fm.. Yangtze Gorges. South China | 17.0 ± 4.3 | 15.0 ± 0.1 | -29.9 |
| Bitter Springs | 3 of 11/2/90 | 0.80 | Bitter Springs Fm.. Amadeus Basin. Australia | 8.6 ± 3.7 | - | -27.0 |
| Jixian | 1 of 8/14/83 | 1.50 | Gaoyuzhuang Group. Jixian section. North China Block. China | 28.5 ± 3.1 | - | -31.4 |
| McArthur | 7 of 6/21/90 | 1.60 | McArthur Basin. Northern Territory. Australia | 24.4 ± 3.9 | - | -25.1 |
| Nabberu | PPRG 089 | 1.85 | Top of Frere Fm.. Earaheedy Group. Nabberu Basin. Western Australia | 25.0 ± 5.5 | - | -29.0 |
| Gunflint | 3 of 6/30/84 | 1.88 | Schreiber Beach. Gunflint Iron Fm.. Ontario. Canada | 4.4 ± 2.2 | 7.3 ± 0.5 | -34.5 |
| PPRG 134 | 4.1 ± 3.4 | - | -33.5 | |||
| Wabigoon | PPRG 325 | 2.70 | Steep Rock Group. Wabigoon Belt. Western Superior Province. Canada | 27.7 ± 3.7 | - | -31.3 |
| Farrel Quartzite | MGTKS1 | 3.02 | Mount Grant. Gorge Creek Group. Pilbara Craton. Australia | 22.7 ± 3.9 | 19.8 ± 0.1 | -33.2 |
| Josefsdal | 99SA07 | 3.30 | Top of Kromberg Fm.. Onverwacht Group. Barberton Greenstone Belt. South Africa | 21.8 ± 1.1 | - | -26.8 |
| Buck Reef | 99SA03 | 3.42 | Base of Kromberg Fm.. Onverwacht Group. Barberton Greenstone Belt. South Africa | 19.5 ± 3.2 | 15.0 ± 0.4 | -24.8 |
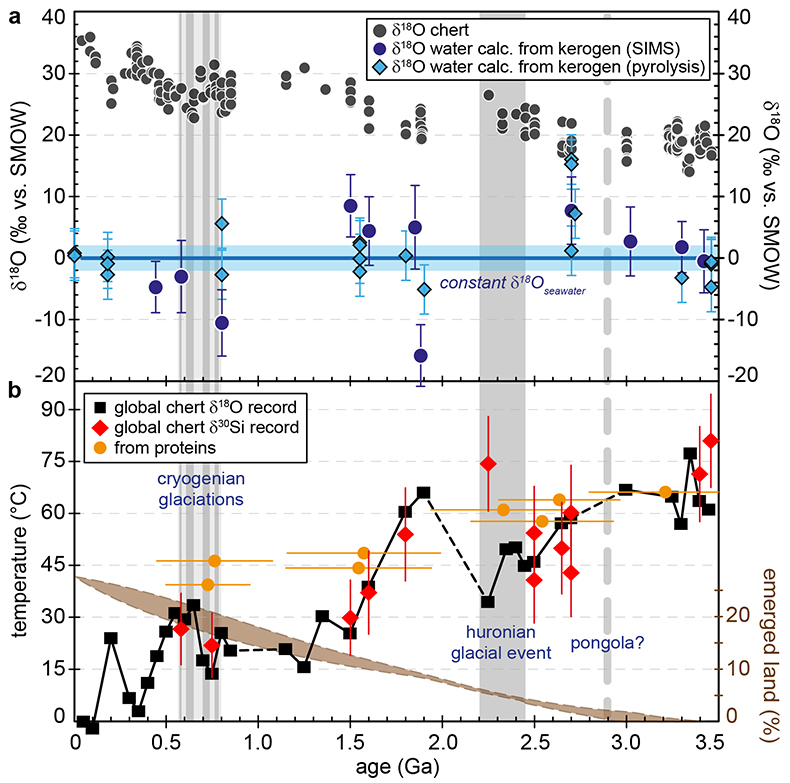
Figure 2 (a) Comparison of the chert and calculated seawater O isotope composition evolution through time, calculated using a Δ18Okerogen-water of 20 ± 4 ‰. Errors bars correspond to the propagated uncertainties on calculated δ18Owater (including the standard deviation – 1 SD – on average kerogen δ18O values and the uncertainty of ±4 ‰ on Δ18Okerogen-water). The horizontal blue band represents a constant δ18Oseawater of 0 ± 2 ‰ through time. (b) Estimates of water T calculated using (i) the available maximum δ18Ochert value (to which 3 ‰ has been added to take into account possible effects induced by diagenesis – see Supplementary Information) per 50 Ma age intervals and using δ18Oseawater = 0 ± 2 ‰, (ii) the chert δ30Si record (Prave et al., 2016
Robert, F., Chaussidon, M. (2006) A palaeo-temperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
) and (iii) resurrected proteins of ancient bacteria (Gaucher et al., 2008Gaucher, E.A., Govindarajan, S., Ganesh, O.K. (2008) Palaeotemperature trend for Precambrian life inferred from resurrected proteins. Nature 451, 704-707.
). Estimates of the surface of emerged land through time are also shown (Flament et al., 2013Flament, N., Coltice, N., Rey, P. (2013) The evolution of the 87Sr/86Sr of marine carbonates does not constrain continental growth. Precambrian Research 229, 177-188.
). Vertical grey bars running across both panels correspond to the major glaciation events identified in the geological record (after Hambrey and Harland, 1985Hambrey, M.J., Harland, W.B. (1985) The Late Proterozoic glacial era. Palaeogeography, Palaeoclimatology, Palaeoecology 51, 255-272.
, Evans et al., 1997Evans, D.A., Beukes, N.J., Kirschvink, J.L. (1997) Low-latitude glaciation in the Palaeoproterozoic era. Nature 386, 262-266.
and Young, 2014Young, G.M. (2014) Contradictory correlations of Paleoproterozoic glacial deposits: Local, regional or global controls? Precambrian Research 247, 33-44.
).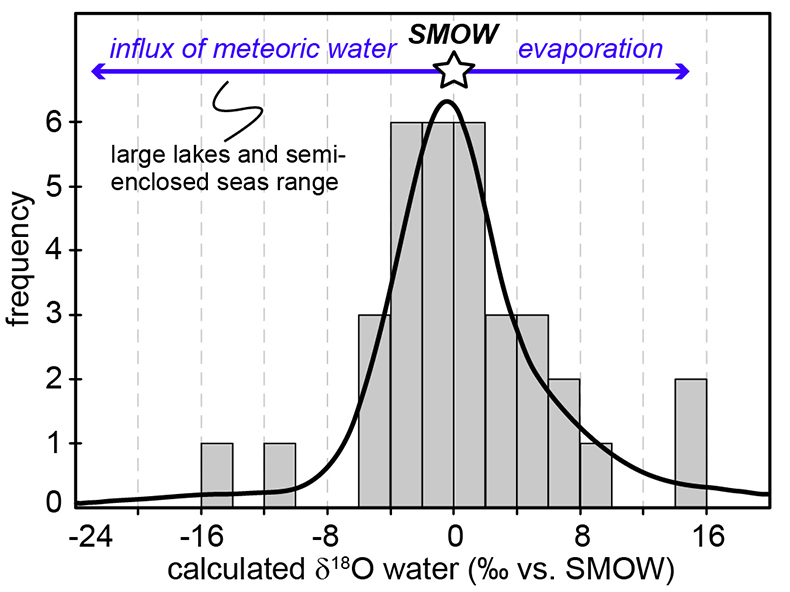
Figure 3 O isotope composition of waters in which precursor biomass of the studied kerogens lived. SMOW corresponds to the present-day mean seawater composition. The range of δ18O values for large lakes and semi-enclosed seas is after Jasechko et al., 2013
Jasechko S., Sharp, Z.D., Gibson, J.J. Birks, S.J., Yi, Y., Fawcett, P.J. (2013) Terrestrial water fluxes dominated by transpiration. Nature 496, 347-351.
.Back to article
Supplementary Figures and Tables
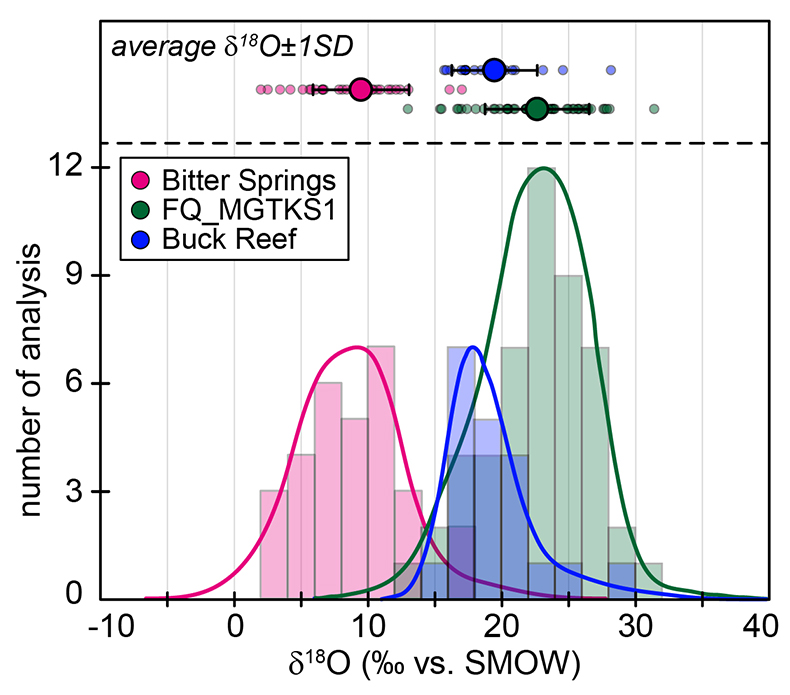
Figure S-1 Distribution of the SIMS δ18O values measured on three selected kerogen samples. To construct histograms the data have been binned into 2 ‰ intervals. All individual analyses are displayed by small circles in the upper part of the panel. Large circles and associated error bars correspond to average δ18O values together with their standard deviation (1 SD).
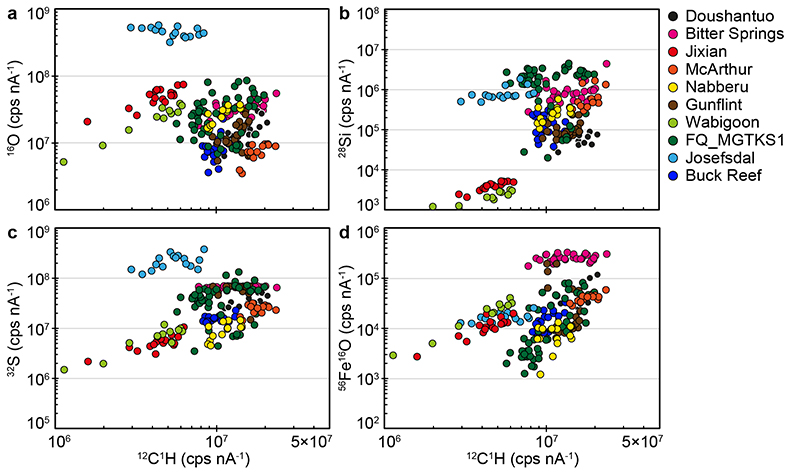
Figure S-2 Measured 16O (a), 28Si (b), 32S (c) and 56Fe16O (d) secondary intensities (normalised to the primary beam intensity) versus 12C1H secondary intensity. For each sample, the relationships between measured 16O, 28Si, 32S and 56Fe16O vs. 12C1H intensities generally trend towards the diagram origin, resulting from variable secondary ion emission for each analysis due to topography and surface flatness effects.
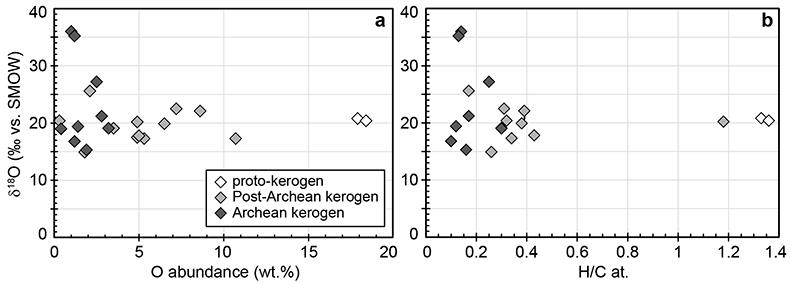
Figure S-3 Oxygen isotope compositions measured in kerogens plotted against their O abundances (a) and their H/C atomic ratios (b). The data are from Wedeking (1983).
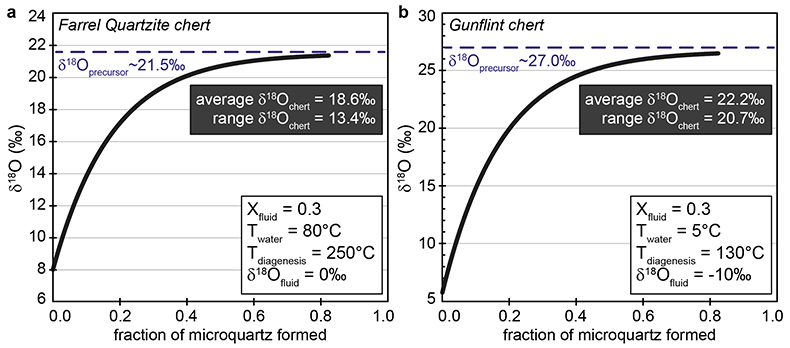
Figure S-4 Evolution of the O isotope composition of microquartz formed from the progressive dissolution of amorphous silica precursors. In (a) the parameters used for the calculation, aimed at modelling the SIMS results obtained on the Farrel Quartzite sample CRT, are; 30 % fluid in the initial porosity of the amorphous silica precursors (Xfluid), temperatures of 80 °C and 250 °C for the water from which it initially precipitated and for metamorphism, respectively, and a δ18Owater value of 0 ‰. In (b) the parameters used for the calculation, aimed at modelling the SIMS results obtained on Gunflint chert samples, are; Xfluid = 30 %, temperatures of 5 °C and 130 °C for the water from which it initially precipitated and for diagenesis/metamorphism, respectively, and a δ18Owater value of -10 ‰ (see text for details).
Table S-1 Bulk H/C, O contents and O and C isotope compositions obtained by pyrolysis on chert-extracted kerogen samples by Wedeking (1983). δ13C and the δ18O values are given relative to VPDB and SMOW, respectively. All the samples, except recent sediments and Lower Toarcian shales, are PPRG samples, whose descriptions are given in Walter et al. (1983).
| Geological unit | Age (Ga) | Sample | H/C at. | δ13C (‰) | O (wt.%) | δ18O (‰) |
| Sediment. Tanner Basin. Pacific | - | 1.33 | -21.4 | 17.9 | 20.8 | |
| Sediment. Walvis Bay. Atlantic | - | 1.36 | -20.0 | 18.4 | 20.4 | |
| Lower Toarcian shales. Paris Basin. France | 0.18 | 10968 | 1.32 | -28.0 | 10.7 | 17.3 |
| 0.18 | 11933 | 1.18 | 4.9 | 20.2 | ||
| 0.18 | 15035 | 1.05 | 3.5 | 19.1 | ||
| Skillogallee. Adelaide Geosyncline. Australia | 0.80 | 455-1 | 0.17 | -23.2 | 2.1 | 25.6 |
| Bitter Springs Fm.. Amadeus basin. Australia | 0.80 | 133-2 | 0.34 | -22.4 | 5.3 | 17.3 |
| 0.80 | 133-2 | 4.9 | 17.5 | |||
| Bungle Bungle Fm.. Birrindudu basin. Australia | 1.55 | 159-1 | 0.31 | -30.0 | 7.2 | 22.5 |
| 1.55 | 138-1 | 0.38 | -30.2 | 6.5 | 19.9 | |
| 1.55 | 154-1 | 0.39 | -30.7 | 8.6 | 22.1 | |
| 1.55 | 152-1 | 0.43 | -29.3 | 5.0 | 17.8 | |
| Duck Creek dolomite. Wyloo Gr.. Ashburton Trough. Australia | 1.80 | 060-1 | 0.32 | -31.7 | 0.3 | 20.4 |
| McLeary Fm.. Belcher Gr.. Ontario. Canada | 1.90 | 447-1 | 0.26 | -28.4 | 1.8 | 14.9 |
| Ventersdorp Fm. Ventersdorp. South Africa | 2.70 | 293-1 | 0.14 | -38.5 | 1.0 | 36.0 |
| Steeprock Lake Fm.. Wabigoon Belt. Ontario. Canada | 2.70 | 325-1 | 0.17 | -27.2 | 2.8 | 21.2 |
| Manjeri Fm.. Belingwe Greenstone Belt. Zimbabwe. Africa | 2.70 | 225-1 | 0.13 | -33.2 | 1.2 | 35.2 |
| Tumbiana Fm.. Fortescue Gr.. Hamersley Basin. Australia | 2.72 | 027-1 | 0.25 | -51.2 | 2.5 | 27.2 |
| Szwartkoppie Fm.. Barberton Greenstone Belt. South Africa | 3.30 | 198-1 | 0.10 | -32.0 | 1.2 | 16.8 |
| Towers Fm.. Warrawoona Gr.. Pilbara Block. Australia | 3.46 | 002-1 | 0.30 | -34.3 | 3.2 | 19.1 |
| 3.46 | 004-1 | 0.16 | -36.0 | 1.9 | 15.3 | |
| Warrawoona Gr.. Pilbara Block. Australia | 3.46 | 016-1 | 0.30 | -35.2 | 0.4 | 19.0 |
| Hooggenoeg Fm.. Barberton Greenstone Belt. South Africa | 3.46 | 182-1 | 0.12 | -31.6 | 1.4 | 19.4 |
Table S-2 O isotope characteristics of the kerogens analysed by SIMS.
| Sample | Reference | Analyses | SIMS δ18O values (‰) | |||
| Min. | Max. | Average | 1SD | |||
| Doushantuo | 1a of 8/25/83 | 13 | 9.2 | 24.7 | 17.0 | 4.3 |
| Bitter Springs | 3 of 11/2/90 | 30 | 2.0 | 17.0 | 8.6 | 3.7 |
| Jixian | 1 of 8/14/83 | 17 | 21.7 | 33.1 | 28.5 | 3.1 |
| McArthur | 7 of 6/21/90 | 13 | 16.7 | 29.6 | 24.4 | 3.9 |
| Nabberu | PPRG 089 | 14 | 16.1 | 33.3 | 25.0 | 5.5 |
| Gunflint | 3 of 6/30/84 & PPRG 134 | 24 | -2.5 | 10.6 | 4.2 | 3.0 |
| Wabigoon | PPRG 325 | 11 | 19.4 | 33.3 | 27.7 | 3.7 |
| Farrel Quartzite | MGTKS1 | 49 | 13.2 | 31.6 | 22.7 | 3.9 |
| Josefsdal | 99SA07 | 16 | 19.6 | 23.9 | 21.8 | 1.1 |
| Buck Reef | 99SA03 | 20 | 15.9 | 28.4 | 19.5 | 3.2 |
Table S-3 Oxygen isotope results obtained by SIMS on the Farrel Quartzite chert samples. The 2 µm range has been calculated from the range of δ18O values measured at the 20 µm scale and the relationship between analysis scale and δ18O range of Marin et al. (2010).
| Analysis | δ18O (‰) | 1σ (‰) | Analysis | δ18O (‰) | 1σ (‰) | Analysis | δ18O (‰) | 1σ (‰) | ||
| CRT 1 | 14.4 | 0.5 | GTEV 1 | 17.2 | 0.2 | GW98-1-48 A | 18.9 | 1.0 | ||
| CRT 5 | 17.3 | 0.9 | GTEV 2 | 16.9 | 0.3 | GW98-1-48 B | 20.4 | 1.0 | ||
| CRT 6 | 14.2 | 0.7 | GTEV 3 A | 17.4 | 0.3 | GW98-1-50 | 16.8 | 0.5 | ||
| CRT 8 | 18.1 | 0.7 | GTEV 3 B | 17.3 | 0.4 | GW98-1-51 | 14.2 | 1.0 | ||
| CRT 9 | 17.1 | 0.6 | GTEV 4 | 18.5 | 1.1 | GW98-1-53 | 15.1 | 1.1 | ||
| CRT 10 | 17.6 | 0.6 | GTEV 4-2 | 15.3 | 0.3 | GW98-1-54 | 18.7 | 1.1 | ||
| CRT 11 | 18.8 | 0.5 | GTEV 6-1 A | 15.2 | 0.2 | GW98-1-55 | 15.9 | 0.7 | ||
| CRT 12 | 18.8 | 0.7 | GTEV 6-1 B | 15.7 | 0.7 | GW98-1-56 | 13.9 | 1.2 | ||
| CRT 13 | 20.5 | 0.6 | GTEV 6-2 | 15.8 | 0.3 | GW98-1-57 | 14.6 | 0.7 | ||
| CRT 14 | 19.9 | 0.6 | GTEV 8 | 19.5 | 0.6 | GW98-1-58 | 14.8 | 0.4 | ||
| CRT 15 | 16.2 | 0.6 | GTEV 10 | 17.2 | 1.2 | GW98-1-59 | 18.6 | 0.3 | ||
| CRT 17 | 15.1 | 0.3 | GTEV 13-1 | 14.3 | 0.9 | GW98-1-62 | 14.2 | 0.5 | ||
| CRT 18 A | 18.1 | 1.1 | GTEV 13-2 | 14.5 | 0.4 | GW98-1-63 | 16.6 | 0.3 | ||
| CRT 18 B | 17.8 | 0.8 | GTEV 15 | 14.0 | 0.6 | GW98-1-99 | 18.0 | 1.1 | ||
| CRT 19 | 14.6 | 0.6 | GTEV 18-1 | 15.5 | 0.8 | |||||
| CRT 21 | 17.6 | 1.4 | GTEV18-2 | 16.0 | 0.4 | |||||
| CRT 22 | 14.6 | 0.8 | GTEV 21-5 | 18.0 | 0.4 | |||||
| CRT 23 | 19.9 | 1.2 | GTEV 30 | 15.4 | 0.4 | |||||
| CRT 24 | 17.9 | 0.3 | GTEV 32 | 17.4 | 0.8 | |||||
| Average | 17.3 | Average | 16.4 | Average | 16.5 | |||||
| 1SD | 2.0 | 1SD | 1.5 | 1SD | 2.1 | |||||
| Max. | 20.5 | Max. | 19.5 | Max. | 20.4 | |||||
| Min. | 14.2 | Min. | 14.0 | Min. | 13.9 | |||||
| Range | 6.3 | Range | 5.5 | Range | 6.5 | |||||
| 2mm range | 13.4 | 2mm range | 12.5 | 2mm range | 13.6 |
Table S-4 All oxygen isotope and chemistry data collected by SIMS. Analyses in purple are those that have been filtered, and grey boxes indicate based on which element between O, Si, S and Fe (see text for details).
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Doushantuo | Doushantuo@1 | 8751 | -920 | 2.10E+07 | 3.42E+04 | -13.64 | 0.62 | -9.33E+04 | 1.86E+07 | 1.74E+07 | 9.03E+04 | 3.55E+07 | 9.86E+04 |
| 1a of 8/25/83 | Doushantuo@02 | 8631 | -929 | 1.72E+07 | 2.65E+04 | -5.34 | 0.51 | -9.38E+04 | 1.58E+07 | 1.90E+07 | 5.32E+04 | 3.60E+07 | 2.36E+04 |
| Doushantuo@03 | 8537 | -938 | 2.60E+07 | 4.43E+04 | -8.46 | 0.64 | -9.39E+04 | 1.62E+07 | 1.60E+07 | 9.08E+04 | 3.55E+07 | 3.04E+04 | |
| Doushantuo@04 | 8595 | -991 | 1.14E+07 | 1.43E+04 | -15.51 | 1.05 | -9.59E+04 | 1.12E+07 | 6.40E+06 | 3.53E+04 | 2.54E+07 | 8.66E+03 | |
| Doushantuo@05 | 8658 | -1185 | 1.80E+07 | 2.79E+04 | -10.38 | 0.81 | -9.57E+04 | 1.59E+07 | 1.34E+07 | 7.27E+04 | 3.15E+07 | 2.09E+04 | |
| Doushantuo@06 | 8849 | -1195 | 1.91E+07 | 3.01E+04 | -8.72 | 0.58 | -9.47E+04 | 1.38E+07 | 9.96E+06 | 5.96E+04 | 2.74E+07 | 1.19E+04 | |
| Doushantuo@07 | 9022 | -1203 | 4.19E+07 | 7.58E+04 | -16.10 | 0.33 | -9.67E+04 | 1.58E+07 | 9.53E+06 | 4.22E+04 | 4.01E+07 | 1.44E+04 | |
| Doushantuo@08 | 9134 | -1296 | 2.79E+07 | 4.78E+04 | -9.99 | 0.36 | -8.96E+04 | 1.81E+07 | 3.01E+07 | 6.72E+04 | 5.22E+07 | 3.19E+04 | |
| Doushantuo@09 | 8968 | -1328 | 5.42E+07 | 1.01E+05 | -15.52 | 0.26 | -8.86E+04 | 1.91E+07 | 2.76E+07 | 4.32E+04 | 5.17E+07 | 4.85E+04 | |
| Doushantuo@10 | 8767 | -1346 | 2.23E+07 | 3.66E+04 | -12.75 | 0.53 | -9.32E+04 | 2.07E+07 | 2.02E+07 | 7.65E+04 | 4.50E+07 | 1.15E+05 | |
| Doushantuo@11 | 8568 | -1355 | 4.00E+07 | 7.17E+04 | -20.83 | 0.25 | -9.41E+04 | 1.23E+07 | 1.30E+07 | 5.51E+04 | 3.35E+07 | 3.07E+04 | |
| Doushantuo@12 | 8473 | -1445 | 3.45E+07 | 6.11E+04 | -14.99 | 0.37 | -9.30E+04 | 1.71E+07 | 1.32E+07 | 4.73E+04 | 3.27E+07 | 2.04E+04 | |
| Doushantuo@15 | 8620 | -1637 | 1.60E+07 | 2.35E+04 | -16.99 | 0.35 | -9.42E+04 | 1.18E+07 | 1.42E+07 | 2.76E+04 | 2.65E+07 | 1.54E+04 | |
| Doushantuo@16 | 8414 | -1550 | 4.30E+07 | 7.82E+04 | -14.34 | 0.27 | -9.02E+04 | 1.93E+07 | 2.86E+07 | 1.96E+05 | 4.66E+07 | 3.81E+04 | |
| Doushantuo@17 | 8279 | -1639 | 4.80E+07 | 8.80E+04 | -17.24 | 0.23 | -8.82E+04 | 1.57E+07 | 3.08E+07 | 3.31E+04 | 5.34E+07 | 3.15E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Bitter Springs | Bitter@02 | 794 | -554 | 4.69E+07 | 8.54E+04 | -23.50 | 0.34 | -7.62E+04 | 1.47E+07 | 2.97E+07 | 7.87E+05 | 6.47E+07 | 2.52E+05 |
| 3 of 11/2/90 | Bitter@03 | 754 | -554 | 7.01E+07 | 1.32E+05 | -20.70 | 0.24 | -7.48E+04 | 7.54E+06 | 4.70E+07 | 1.39E+06 | 6.97E+07 | 3.87E+05 |
| Bitter@05 | 674 | -554 | 7.09E+07 | 1.34E+05 | -19.38 | 0.62 | -7.51E+04 | 1.31E+07 | 3.03E+07 | 8.29E+05 | 6.63E+07 | 2.30E+05 | |
| Bitter@07 | 594 | -554 | 8.48E+07 | 1.63E+05 | -13.01 | 0.52 | -7.59E+04 | 1.14E+07 | 3.94E+07 | 7.80E+05 | 6.70E+07 | 2.61E+05 | |
| Bitter@08 | 554 | -554 | 4.87E+07 | 8.93E+04 | -18.36 | 0.23 | -7.22E+04 | 1.72E+07 | 4.98E+07 | 9.09E+05 | 6.64E+07 | 2.77E+05 | |
| Bitter@09 | 514 | -554 | 4.48E+07 | 8.07E+04 | -26.44 | 0.36 | -7.62E+04 | 6.47E+06 | 2.68E+07 | 3.74E+05 | 6.65E+07 | 3.04E+05 | |
| Bitter@10 | 514 | -514 | 4.16E+07 | 7.46E+04 | -24.50 | 0.30 | -8.06E+04 | 7.73E+06 | 1.83E+07 | 2.81E+05 | 6.84E+07 | 1.71E+05 | |
| Bitter@11 | 554 | -514 | 3.92E+07 | 6.98E+04 | -23.44 | 0.35 | -7.25E+04 | 1.05E+07 | 4.32E+07 | 7.82E+05 | 6.78E+07 | 3.87E+05 | |
| Bitter@12 | 594 | -514 | 4.26E+07 | 7.62E+04 | -27.55 | 0.30 | -7.39E+04 | 1.35E+07 | 3.51E+07 | 1.03E+06 | 6.78E+07 | 3.18E+05 | |
| Bitter@13 | 634 | -514 | 4.67E+07 | 8.42E+04 | -28.04 | 0.37 | -7.74E+04 | 1.17E+07 | 2.50E+07 | 7.85E+05 | 6.57E+07 | 2.71E+05 | |
| Bitter@14 | 674 | -514 | 1.06E+08 | 2.05E+05 | -13.50 | 0.19 | -6.52E+04 | 8.53E+06 | 6.67E+07 | 2.29E+06 | 6.90E+07 | 4.05E+05 | |
| Bitter@15 | 714 | -514 | 4.01E+07 | 7.17E+04 | -21.98 | 0.35 | -7.48E+04 | 1.95E+07 | 3.09E+07 | 1.03E+06 | 6.57E+07 | 2.27E+05 | |
| Bitter@16 | 754 | -514 | 3.61E+07 | 6.39E+04 | -19.12 | 0.37 | -7.60E+04 | 1.11E+07 | 2.96E+07 | 5.83E+05 | 6.74E+07 | 2.24E+05 | |
| Bitter@17 | 794 | -514 | 2.87E+07 | 4.85E+04 | -26.61 | 0.30 | -7.80E+04 | 1.47E+07 | 3.21E+07 | 5.34E+05 | 6.60E+07 | 2.94E+05 | |
| Bitter@18 | 834 | -514 | 4.37E+07 | 7.91E+04 | -20.01 | 0.37 | -7.60E+04 | 1.10E+07 | 2.84E+07 | 6.60E+05 | 6.72E+07 | 1.89E+05 | |
| Bitter@19 | 834 | -474 | 4.41E+07 | 8.02E+04 | -17.65 | 0.26 | -7.35E+04 | 2.01E+07 | 4.50E+07 | 1.08E+06 | 7.01E+07 | 2.75E+05 | |
| Bitter@20 | 794 | -474 | 6.93E+07 | 1.30E+05 | -26.63 | 0.31 | -7.08E+04 | 9.17E+06 | 5.40E+07 | 1.39E+06 | 7.02E+07 | 3.52E+05 | |
| Bitter@21 | 754 | -474 | 4.49E+07 | 8.15E+04 | -20.20 | 0.42 | -7.80E+04 | 1.22E+07 | 2.66E+07 | 4.70E+05 | 7.01E+07 | 2.55E+05 | |
| Bitter@22 | 714 | -474 | 3.84E+07 | 6.82E+04 | -22.26 | 0.41 | -7.51E+04 | 8.66E+06 | 2.82E+07 | 8.03E+05 | 6.55E+07 | 2.44E+05 | |
| Bitter@23 | 674 | -474 | 6.65E+07 | 1.26E+05 | -13.96 | 0.18 | -7.43E+04 | 1.54E+07 | 3.35E+07 | 8.31E+05 | 6.84E+07 | 2.38E+05 | |
| Bitter@24 | 634 | -474 | 4.00E+07 | 7.15E+04 | -24.37 | 0.54 | -7.39E+04 | 1.05E+07 | 2.59E+07 | 5.28E+05 | 6.37E+07 | 2.08E+05 | |
| Bitter@25 | 594 | -474 | 3.12E+07 | 5.41E+04 | -18.53 | 0.46 | -7.62E+04 | 1.86E+07 | 3.36E+07 | 5.83E+05 | 6.35E+07 | 1.96E+05 | |
| Bitter@26 | 554 | -474 | 4.85E+07 | 8.86E+04 | -20.80 | 0.21 | -7.51E+04 | 1.89E+07 | 3.15E+07 | 9.59E+05 | 6.72E+07 | 2.10E+05 | |
| Bitter@27 | 514 | -474 | 3.53E+07 | 6.20E+04 | -29.11 | 0.45 | -7.81E+04 | 6.25E+06 | 1.97E+07 | 3.08E+05 | 6.58E+07 | 2.56E+05 | |
| Bitter@29 | 554 | -434 | 4.65E+07 | 8.48E+04 | -18.77 | 0.29 | -7.29E+04 | 2.81E+07 | 4.43E+07 | 1.29E+06 | 6.76E+07 | 2.17E+05 | |
| Bitter@30 | 594 | -434 | 3.06E+07 | 5.24E+04 | -23.50 | 0.37 | -7.69E+04 | 1.65E+07 | 2.42E+07 | 6.23E+05 | 6.80E+07 | 2.37E+05 | |
| Bitter@31 | 634 | -434 | 5.60E+07 | 1.03E+05 | -26.35 | 0.28 | -7.29E+04 | 7.65E+06 | 6.10E+07 | 4.00E+05 | 6.55E+07 | 6.28E+05 | |
| Bitter@32 | 674 | -434 | 7.18E+07 | 1.36E+05 | -17.03 | 0.34 | -7.40E+04 | 9.93E+06 | 3.96E+07 | 1.68E+06 | 6.57E+07 | 2.87E+05 | |
| Bitter@33 | 714 | -434 | 3.49E+07 | 6.11E+04 | -25.82 | 0.50 | -7.33E+04 | 1.63E+07 | 4.19E+07 | 1.99E+06 | 6.96E+07 | 2.36E+05 | |
| Bitter@34 | 754 | -434 | 2.95E+07 | 5.02E+04 | -24.92 | 0.39 | -7.65E+04 | 9.12E+06 | 2.11E+07 | 2.34E+05 | 6.63E+07 | 2.26E+05 | |
| Bitter@35 | 794 | -434 | 6.60E+07 | 1.24E+05 | -19.67 | 0.25 | -6.99E+04 | 2.36E+07 | 5.53E+07 | 4.46E+06 | 6.58E+07 | 2.96E+05 | |
| Bitter@36 | 834 | -434 | 3.82E+07 | 6.79E+04 | -22.50 | 0.60 | -7.60E+04 | 3.26E+06 | 2.94E+07 | 2.49E+05 | 6.58E+07 | 2.74E+05 | |
| Bitter@37 | 834 | -394 | 4.01E+07 | 7.18E+04 | -21.29 | 0.37 | -7.51E+04 | 1.79E+07 | 3.47E+07 | 4.88E+05 | 7.07E+07 | 2.85E+05 | |
| Bitter@38 | 794 | -394 | 4.65E+07 | 8.48E+04 | -18.00 | 0.40 | -7.55E+04 | 1.30E+07 | 3.28E+07 | 7.71E+05 | 6.66E+07 | 2.38E+05 | |
| Bitter@39 | 754 | -394 | 5.90E+07 | 1.09E+05 | -27.74 | 0.30 | -7.53E+04 | 5.80E+06 | 2.67E+07 | 2.47E+05 | 6.91E+07 | 2.95E+05 | |
| Bitter@40 | 714 | -394 | 9.39E+07 | 1.80E+05 | -17.01 | 0.17 | -6.88E+04 | 1.08E+07 | 5.58E+07 | 3.76E+06 | 6.75E+07 | 2.86E+05 | |
| Bitter@41 | 674 | -394 | 4.90E+07 | 8.96E+04 | -21.12 | 0.28 | -6.53E+04 | 1.50E+07 | 7.06E+07 | 2.42E+07 | 7.37E+07 | 8.10E+05 | |
| Bitter@42 | 634 | -394 | 5.20E+07 | 9.53E+04 | -23.96 | 0.45 | -7.07E+04 | 1.74E+07 | 3.84E+07 | 1.70E+06 | 6.78E+07 | 3.05E+05 | |
| Bitter@43 | 594 | -394 | 3.82E+07 | 6.77E+04 | -23.37 | 0.57 | -7.56E+04 | 1.18E+07 | 3.47E+07 | 6.33E+05 | 7.06E+07 | 3.13E+05 | |
| Bitter@44 | 554 | -394 | 4.77E+07 | 8.70E+04 | -21.61 | 0.34 | -7.47E+04 | 1.96E+07 | 3.60E+07 | 9.55E+05 | 6.63E+07 | 2.45E+05 | |
| Bitter@45 | 514 | -394 | 3.79E+07 | 6.72E+04 | -22.50 | 0.38 | -7.77E+04 | 2.26E+06 | 2.79E+07 | 4.79E+05 | 6.74E+07 | 2.58E+05 | |
| Bitter@46 | 474 | -394 | 1.86E+07 | 2.83E+04 | -31.67 | 0.74 | -7.83E+04 | 5.71E+06 | 1.89E+07 | 1.99E+05 | 7.26E+07 | 1.99E+05 | |
| Bitter@47 | 434 | -394 | 8.01E+07 | 1.52E+05 | -23.48 | 0.31 | -7.08E+04 | 1.00E+07 | 3.60E+07 | 1.11E+06 | 6.79E+07 | 2.91E+05 | |
| Bitter@49 | 354 | -394 | 4.50E+07 | 8.14E+04 | -23.53 | 0.25 | -7.23E+04 | 9.19E+06 | 4.29E+07 | 1.08E+06 | 6.69E+07 | 2.82E+05 | |
| Bitter@50 | 314 | -394 | 4.33E+07 | 7.81E+04 | -19.80 | 0.44 | -7.63E+04 | 1.11E+07 | 2.90E+07 | 9.34E+05 | 7.09E+07 | 2.31E+05 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Jixian | Jixian@1 | -4218 | -23 | 8.39E+07 | 1.88E+05 | -1.70 | 0.20 | 3.80E+04 | 7.46E+06 | 6.22E+07 | 1.25E+04 | 5.87E+07 | 6.76E+04 |
| 1 of 8/14/83 | Jixian@02 | -4178 | -23 | 9.62E+07 | 2.12E+05 | -0.52 | 0.38 | 3.74E+04 | 5.08E+06 | 6.31E+07 | 1.19E+04 | 1.10E+07 | 2.93E+04 |
| Jixian@03 | -4138 | -23 | 8.03E+07 | 1.80E+05 | -3.34 | 0.34 | 3.89E+04 | 1.34E+06 | 2.51E+07 | 8.28E+03 | 1.39E+06 | 1.30E+04 | |
| Jixian@04 | -4098 | -23 | 1.32E+08 | 2.83E+05 | -6.51 | 0.22 | 3.74E+04 | 1.83E+06 | 7.99E+07 | 9.80E+03 | 3.94E+06 | 4.05E+04 | |
| Jixian@05 | -4058 | -23 | 8.17E+07 | 1.82E+05 | -11.37 | 0.32 | 3.67E+04 | 3.22E+06 | 2.60E+07 | 9.44E+03 | 3.57E+06 | 1.28E+04 | |
| Jixian@06 | -4018 | -23 | 5.49E+07 | 1.30E+05 | -4.06 | 0.41 | 3.70E+04 | 9.65E+05 | 1.97E+07 | 8.43E+03 | 1.20E+06 | 1.13E+04 | |
| Jixian@07 | -3978 | -23 | 1.24E+08 | 2.67E+05 | -2.19 | 0.21 | 3.75E+04 | 4.68E+06 | 7.20E+07 | 1.18E+04 | 4.48E+06 | 2.32E+04 | |
| Jixian@08 | -3938 | -23 | 7.49E+07 | 1.69E+05 | -10.96 | 0.37 | 3.70E+04 | 1.59E+06 | 2.07E+07 | 8.41E+03 | 2.13E+06 | 1.06E+04 | |
| Jixian@09 | -3898 | -23 | 1.37E+08 | 2.93E+05 | -2.11 | 0.14 | 3.92E+04 | 5.42E+06 | 5.52E+07 | 1.27E+04 | 4.81E+06 | 2.04E+04 | |
| Jixian@10 | -3858 | -23 | 7.52E+07 | 1.70E+05 | -4.30 | 0.30 | 3.82E+04 | 4.22E+06 | 6.02E+07 | 1.22E+04 | 5.37E+06 | 1.86E+04 | |
| Jixian@11 | -3858 | 17 | 5.26E+07 | 1.26E+05 | -1.88 | 0.42 | 3.75E+04 | 3.81E+06 | 2.96E+07 | 1.06E+04 | 8.00E+06 | 1.70E+04 | |
| Jixian@12 | -3898 | 17 | 8.63E+06 | 3.88E+04 | -12.38 | 2.27 | 3.75E+04 | 1.54E+05 | 1.73E+06 | 7.61E+03 | 1.98E+05 | 9.05E+03 | |
| Jixian@13 | -3938 | 17 | 6.30E+07 | 1.46E+05 | -7.09 | 0.30 | 3.69E+04 | 2.87E+06 | 3.28E+07 | 9.94E+03 | 4.14E+06 | 1.43E+04 | |
| Jixian@14 | -3978 | 17 | 4.42E+07 | 1.08E+05 | -15.01 | 0.42 | 3.55E+04 | 5.84E+06 | 2.20E+07 | 1.09E+04 | 3.00E+06 | 1.15E+04 | |
| Jixian@15 | -4018 | 17 | 4.93E+07 | 1.19E+05 | -10.53 | 0.43 | 3.68E+04 | 1.75E+06 | 2.18E+07 | 9.15E+03 | 1.83E+06 | 1.26E+04 | |
| Jixian@16 | -4058 | 17 | 8.06E+07 | 1.81E+05 | -7.02 | 0.31 | 4.08E+04 | 6.19E+06 | 4.00E+07 | 1.19E+04 | 9.28E+06 | 2.81E+04 | |
| Jixian@17 | -4098 | 17 | 2.70E+07 | 7.49E+04 | -6.96 | 0.69 | 3.83E+04 | 3.02E+05 | 6.76E+06 | 7.88E+03 | 6.59E+06 | 1.13E+04 | |
| Jixian@18 | -4138 | 17 | 6.62E+07 | 1.52E+05 | -5.97 | 0.36 | 3.86E+04 | 3.37E+05 | 1.89E+07 | 8.27E+03 | 5.01E+05 | 1.24E+04 | |
| Jixian@19 | -4178 | 17 | 7.51E+07 | 1.70E+05 | -4.19 | 0.25 | 3.84E+04 | 5.24E+06 | 5.68E+07 | 1.18E+04 | 7.53E+06 | 2.44E+04 | |
| Jixian@20 | -4218 | 17 | 1.03E+08 | 2.26E+05 | -0.60 | 0.47 | 3.77E+04 | 3.86E+06 | 5.34E+07 | 1.12E+04 | 4.53E+06 | 1.97E+04 | |
| Jixian@21 | -4218 | 57 | 8.71E+07 | 1.94E+05 | -2.61 | 0.32 | 3.77E+04 | 4.66E+06 | 4.52E+07 | 1.21E+04 | 5.11E+06 | 1.80E+04 | |
| Jixian@22 | -4178 | 57 | 1.40E+08 | 3.00E+05 | 0.11 | 0.23 | 3.72E+04 | 5.77E+06 | 7.34E+07 | 1.34E+04 | 7.03E+06 | 2.14E+04 | |
| Jixian@23 | -4138 | 57 | 7.58E+07 | 1.72E+05 | -4.57 | 0.24 | 3.80E+04 | 4.82E+06 | 4.09E+07 | 1.15E+04 | 5.83E+06 | 1.72E+04 | |
| Jixian@24 | -4098 | 57 | 1.02E+08 | 2.25E+05 | -0.89 | 0.18 | 3.80E+04 | 2.81E+06 | 4.59E+07 | 1.04E+04 | 5.22E+06 | 1.95E+04 | |
| Jixian@25 | -4058 | 57 | 1.06E+08 | 2.31E+05 | -5.19 | 0.24 | 3.82E+04 | 4.00E+06 | 6.63E+07 | 1.25E+04 | 1.24E+07 | 3.65E+04 | |
| Jixian@26 | -4018 | 57 | 7.50E+07 | 1.69E+05 | -4.83 | 0.34 | 3.75E+04 | 4.07E+06 | 4.04E+07 | 1.15E+04 | 4.93E+06 | 1.64E+04 | |
| Jixian@27 | -3978 | 57 | 8.74E+07 | 1.94E+05 | -3.05 | 0.21 | 3.75E+04 | 5.47E+06 | 5.13E+07 | 1.29E+04 | 5.05E+06 | 2.11E+04 | |
| Jixian@28 | -3938 | 57 | 9.31E+07 | 2.06E+05 | -4.33 | 0.20 | 3.90E+04 | 4.51E+06 | 6.28E+07 | 1.28E+04 | 6.30E+06 | 2.18E+04 | |
| Jixian@29 | -3898 | 57 | 7.45E+07 | 1.69E+05 | -6.00 | 0.30 | 3.89E+04 | 1.50E+06 | 3.25E+07 | 9.36E+03 | 1.92E+06 | 1.39E+04 | |
| Jixian@30 | -3858 | 57 | 4.15E+07 | 1.03E+05 | -13.00 | 0.46 | 3.83E+04 | 9.02E+05 | 1.70E+07 | 9.12E+03 | 1.46E+06 | 1.18E+04 | |
| Jixian@31 | -3858 | 97 | 2.93E+07 | 7.92E+04 | -8.63 | 0.71 | 3.84E+04 | 7.19E+05 | 1.07E+07 | 8.88E+03 | 1.09E+06 | 1.03E+04 | |
| Jixian@32 | -3898 | 97 | 1.19E+08 | 2.55E+05 | -6.11 | 0.14 | 3.63E+04 | 5.25E+06 | 5.12E+07 | 1.25E+04 | 5.60E+06 | 1.98E+04 | |
| Jixian@33 | -3938 | 97 | 8.38E+07 | 1.87E+05 | -4.75 | 0.18 | 3.58E+04 | 4.18E+06 | 4.72E+07 | 1.10E+04 | 3.10E+06 | 1.71E+04 | |
| Jixian@34 | -4018 | 97 | 1.00E+08 | 2.20E+05 | -1.65 | 0.19 | 3.72E+04 | 4.28E+06 | 6.27E+07 | 1.17E+04 | 6.91E+06 | 2.70E+04 | |
| Jixian@35 | -4058 | 97 | 1.36E+08 | 2.89E+05 | -5.27 | 0.16 | 3.84E+04 | 6.26E+06 | 7.53E+07 | 1.28E+04 | 1.08E+07 | 2.78E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| McArthur | McArthur@1 | -5594 | 656 | 1.43E+08 | 2.92E+05 | -5.96 | 0.08 | 4.32E+04 | 2.33E+07 | 6.04E+07 | 3.50E+06 | 6.04E+07 | 1.53E+05 |
| 7 of 6/21/90 | McArthur@02 | -5734 | 671 | 4.25E+07 | 9.30E+04 | -10.37 | 0.29 | 5.15E+04 | 2.00E+07 | 4.12E+07 | 3.50E+06 | 5.98E+07 | 9.60E+04 |
| McArthur@03 | -5597 | 665 | 1.16E+08 | 2.39E+05 | -3.91 | 0.09 | 4.51E+04 | 1.90E+07 | 5.50E+07 | 1.74E+06 | 5.98E+07 | 1.32E+05 | |
| McArthur@04 | -5602 | 792 | 7.32E+07 | 1.54E+05 | -8.96 | 0.23 | 5.30E+04 | 1.70E+07 | 4.82E+07 | 8.73E+05 | 5.93E+07 | 1.13E+05 | |
| McArthur@05 | -5612 | 867 | 1.02E+08 | 2.10E+05 | -7.25 | 0.21 | 5.53E+04 | 1.45E+07 | 5.93E+07 | 1.35E+06 | 5.93E+07 | 1.18E+05 | |
| McArthur@08 | -5372 | 793 | 1.58E+08 | 3.22E+05 | -3.60 | 0.29 | 4.73E+04 | 1.62E+07 | 5.93E+07 | 1.37E+06 | 5.93E+07 | 1.51E+05 | |
| McArthur@09 | -5389 | 701 | 5.47E+07 | 1.17E+05 | -8.78 | 0.22 | 6.65E+04 | 2.09E+07 | 4.49E+07 | 1.40E+06 | 5.98E+07 | 8.58E+04 | |
| McArthur@10 | -5427 | 642 | 5.13E+07 | 1.11E+05 | -3.72 | 0.49 | 4.89E+04 | 1.14E+07 | 2.87E+07 | 1.10E+06 | 5.98E+07 | 5.25E+04 | |
| McArthur@11 | -5591 | 654 | 8.59E+07 | 1.79E+05 | -5.02 | 0.12 | 4.99E+04 | 1.20E+07 | 4.36E+07 | 1.67E+06 | 5.98E+07 | 8.15E+04 | |
| McArthur@12 | -6509 | 1757 | 5.76E+07 | 1.24E+05 | -5.79 | 0.20 | 5.30E+04 | 2.10E+07 | 4.72E+07 | 1.04E+06 | 5.93E+07 | 9.76E+04 | |
| McArthur@13 | -5805 | 1742 | 4.76E+07 | 1.03E+05 | -16.35 | 0.26 | 5.02E+04 | 1.57E+07 | 3.27E+07 | 1.02E+06 | 5.93E+07 | 7.31E+04 | |
| McArthur@14 | -5814 | 1815 | 5.99E+07 | 1.27E+05 | -13.60 | 0.33 | 5.87E+04 | 1.94E+07 | 4.19E+07 | 1.05E+06 | 5.93E+07 | 9.63E+04 | |
| McArthur@15 | -5881 | 1861 | 3.48E+07 | 7.87E+04 | -5.90 | 0.26 | 5.00E+04 | 1.67E+07 | 3.05E+07 | 2.74E+06 | 5.93E+07 | 8.75E+04 | |
| McArthur@16 | -5858 | 1920 | 6.16E+07 | 1.31E+05 | -12.55 | 0.17 | 5.71E+04 | 1.63E+07 | 3.60E+07 | 8.85E+05 | 5.93E+07 | 7.68E+04 | |
| McArthur@18 | -5822 | 2028 | 6.71E+07 | 1.43E+05 | -1.07 | 0.19 | 5.19E+04 | 1.11E+07 | 4.06E+07 | 3.06E+06 | 5.38E+07 | 5.05E+04 | |
| McArthur@19 | -5838 | 2086 | 7.03E+07 | 1.48E+05 | -9.66 | 0.15 | 6.49E+04 | 1.54E+07 | 4.13E+07 | 7.58E+06 | 5.98E+07 | 8.15E+04 | |
| McArthur@20 | -5803 | 2173 | 2.82E+07 | 6.60E+04 | 0.09 | 0.38 | 4.43E+04 | 1.32E+06 | 1.48E+07 | 3.35E+05 | 6.69E+06 | 2.12E+04 | |
| McArthur@21 | -5844 | 2232 | 5.41E+07 | 1.16E+05 | -11.56 | 0.26 | 4.60E+04 | 2.95E+06 | 2.45E+07 | 4.40E+05 | 2.34E+07 | 2.85E+04 | |
| McArthur@23 | -5589 | 2600 | 3.94E+07 | 8.73E+04 | -10.08 | 0.22 | 5.22E+04 | 1.40E+07 | 4.94E+07 | 1.28E+06 | 5.98E+07 | 1.13E+05 | |
| McArthur@24 | -5509 | 2634 | 7.01E+07 | 1.49E+05 | -3.44 | 0.25 | 6.37E+04 | 1.79E+07 | 4.33E+07 | 8.75E+05 | 5.98E+07 | 1.02E+05 | |
| McArthur@25 | -5414 | 2637 | 4.37E+07 | 9.53E+04 | -14.80 | 0.32 | 6.13E+04 | 1.10E+07 | 5.55E+07 | 6.88E+06 | 5.98E+07 | 1.03E+05 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Gunflint | Gunflint@126 | -134 | 3998 | 1.09E+07 | 2.13E+04 | -26.77 | 0.67 | 8.02E+04 | 1.45E+07 | 7.18E+06 | 2.38E+05 | 5.51E+07 | 5.94E+04 |
| 3 of 6/30/84 | Gunflint@127 | -84 | 3998 | 1.16E+07 | 2.26E+04 | -27.55 | 0.49 | 8.00E+04 | 1.58E+07 | 6.43E+06 | 2.36E+05 | 5.05E+07 | 5.54E+04 |
| Gunflint@128 | -34 | 3998 | 1.96E+07 | 3.82E+04 | -27.15 | 0.37 | 6.20E+04 | 1.01E+07 | 9.88E+06 | 9.11E+04 | 5.66E+07 | 1.91E+05 | |
| Gunflint@129 | 16 | 3998 | 1.83E+07 | 3.59E+04 | -22.13 | 0.38 | 5.73E+04 | 1.02E+07 | 1.04E+07 | 1.57E+05 | 5.15E+07 | 1.33E+05 | |
| Gunflint@130 | 66 | 3998 | 1.01E+07 | 1.99E+04 | -27.52 | 0.86 | 5.26E+04 | 7.08E+06 | 1.13E+07 | 1.21E+06 | 9.64E+06 | 1.43E+04 | |
| Gunflint@131 | 116 | 3998 | 9.68E+06 | 1.91E+04 | -27.42 | 0.66 | 7.47E+04 | 1.62E+07 | 6.59E+06 | 1.48E+05 | 1.50E+07 | 2.20E+04 | |
| Gunflint@133 | 216 | 3998 | 5.75E+06 | 1.16E+04 | -28.69 | 1.21 | 6.11E+04 | 1.02E+07 | 5.45E+06 | 1.42E+05 | 4.63E+07 | 6.26E+04 | |
| Gunflint@134 | 266 | 3998 | 1.07E+07 | 2.10E+04 | -28.61 | 0.49 | 6.80E+04 | 1.16E+07 | 1.02E+07 | 1.15E+05 | 5.68E+07 | 1.89E+05 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Gunflint | PPRG134@01 | 616 | -6533 | 2.50E+07 | 4.09E+04 | -30.35 | 0.74 | -8.81E+04 | 1.15E+07 | 6.86E+06 | 4.91E+04 | 6.95E+07 | 7.41E+03 |
| PPRG 134 | PPRG134@02 | 656 | -6533 | 4.33E+07 | 7.70E+04 | -33.98 | 0.24 | -8.17E+04 | 1.52E+07 | 3.02E+07 | 1.01E+05 | 6.81E+07 | 1.65E+04 |
| PPRG134@04 | 736 | -6533 | 6.70E+06 | 4.71E+03 | -35.65 | 1.37 | -8.75E+04 | 1.14E+07 | 5.78E+06 | 9.33E+04 | 6.88E+07 | 6.12E+03 | |
| PPRG134@06 | 816 | -6533 | 9.85E+06 | 1.08E+04 | -26.60 | 1.02 | -9.28E+04 | 1.39E+07 | 9.72E+06 | 6.30E+04 | 5.93E+07 | 5.32E+03 | |
| PPRG134@09 | 936 | -6533 | 1.68E+07 | 2.46E+04 | -29.51 | 0.87 | -8.19E+04 | 1.57E+07 | 2.00E+07 | 1.12E+05 | 6.95E+07 | 1.02E+04 | |
| PPRG134@10 | 976 | -6533 | 2.13E+07 | 3.39E+04 | -24.18 | 0.52 | -8.45E+04 | 1.34E+07 | 3.08E+07 | 7.25E+04 | 7.00E+07 | 1.74E+04 | |
| PPRG134@11 | 976 | -6613 | 1.87E+07 | 2.86E+04 | -27.04 | 0.71 | -8.50E+04 | 1.36E+07 | 1.26E+07 | 8.77E+04 | 6.94E+07 | 8.20E+03 | |
| PPRG134@12 | 936 | -6613 | 3.01E+07 | 5.13E+04 | -27.44 | 0.46 | -8.43E+04 | 1.46E+07 | 1.56E+07 | 7.27E+04 | 6.99E+07 | 9.73E+03 | |
| PPRG134@13 | 896 | -6613 | 2.89E+07 | 4.89E+04 | -25.27 | 0.39 | -8.38E+04 | 1.48E+07 | 1.77E+07 | 8.85E+04 | 6.96E+07 | 1.13E+04 | |
| PPRG134@14 | 856 | -6613 | 1.48E+07 | 2.07E+04 | -23.89 | 0.76 | -8.86E+04 | 1.40E+07 | 1.03E+07 | 7.34E+04 | 6.95E+07 | 5.46E+03 | |
| PPRG134@15 | 816 | -6613 | 1.56E+07 | 2.24E+04 | -27.04 | 0.47 | -8.43E+04 | 1.52E+07 | 2.68E+07 | 1.02E+05 | 6.90E+07 | 1.22E+04 | |
| PPRG134@16 | 776 | -6613 | 2.57E+07 | 4.25E+04 | -25.53 | 0.53 | -8.90E+04 | 1.39E+07 | 1.23E+07 | 6.49E+04 | 6.94E+07 | 7.54E+03 | |
| PPRG134@17 | 736 | -6613 | 7.58E+06 | 6.36E+03 | -31.62 | 1.41 | -9.28E+04 | 1.20E+07 | 6.14E+06 | 9.34E+04 | 4.97E+07 | 3.11E+03 | |
| PPRG134@18 | 696 | -6613 | 1.29E+07 | 1.70E+04 | -25.10 | 0.68 | -8.86E+04 | 1.32E+07 | 8.67E+06 | 8.21E+04 | 6.92E+07 | 8.31E+03 | |
| PPRG134@19 | 656 | -6613 | 2.46E+07 | 4.04E+04 | -26.80 | 0.49 | -8.31E+04 | 1.43E+07 | 2.28E+07 | 1.21E+05 | 6.82E+07 | 1.28E+04 | |
| PPRG134@22 | 656 | -6653 | 4.41E+07 | 7.90E+04 | -28.05 | 0.26 | -7.87E+04 | 6.70E+06 | 5.49E+07 | 3.98E+04 | 6.94E+07 | 1.93E+04 | |
| PPRG134@23 | 696 | -6653 | 1.87E+07 | 2.87E+04 | -24.92 | 0.67 | -8.31E+04 | 1.17E+07 | 1.84E+07 | 1.05E+05 | 7.06E+07 | 1.43E+04 | |
| PPRG134@24 | 736 | -6653 | 1.01E+07 | 1.13E+04 | -28.26 | 1.01 | -8.37E+04 | 8.40E+06 | 1.15E+07 | 7.95E+04 | 6.81E+07 | 9.30E+03 | |
| PPRG134@25 | 776 | -6653 | 1.26E+07 | 1.65E+04 | -26.90 | 0.85 | -8.59E+04 | 1.15E+07 | 1.14E+07 | 5.65E+04 | 6.98E+07 | 1.06E+04 | |
| PPRG134@26 | 816 | -6653 | 1.89E+07 | 2.92E+04 | -25.86 | 0.73 | -8.67E+04 | 1.39E+07 | 1.77E+07 | 7.42E+04 | 6.92E+07 | 9.84E+03 | |
| PPRG134@27 | 856 | -6653 | 1.40E+07 | 1.92E+04 | -29.29 | 0.68 | -8.36E+04 | 1.39E+07 | 2.09E+07 | 8.99E+04 | 6.84E+07 | 1.01E+04 | |
| PPRG134@28 | 896 | -6653 | 1.20E+07 | 1.52E+04 | -29.17 | 0.85 | -9.13E+04 | 1.37E+07 | 9.72E+06 | 7.50E+04 | 5.78E+07 | 4.40E+03 | |
| PPRG134@29 | 936 | -6653 | 8.44E+06 | 8.26E+03 | -22.25 | 1.24 | -8.67E+04 | 1.19E+07 | 1.01E+07 | 7.93E+04 | 6.91E+07 | 8.15E+03 | |
| PPRG134@30 | 976 | -6653 | 2.93E+07 | 4.97E+04 | -26.49 | 0.45 | -8.12E+04 | 1.49E+07 | 4.01E+07 | 1.57E+05 | 7.12E+07 | 1.89E+04 | |
| PPRG134@31 | 976 | -6733 | 1.88E+07 | 2.86E+04 | -31.30 | 0.50 | -8.31E+04 | 1.44E+07 | 2.55E+07 | 1.19E+05 | 7.10E+07 | 1.69E+04 | |
| PPRG134@32 | 936 | -6733 | 1.70E+07 | 2.53E+04 | -23.80 | 0.61 | -8.41E+04 | 1.33E+07 | 1.86E+07 | 8.71E+04 | 6.99E+07 | 1.08E+04 | |
| PPRG134@33 | 896 | -6733 | 1.21E+07 | 1.53E+04 | -29.84 | 0.80 | -8.67E+04 | 1.30E+07 | 1.13E+07 | 7.84E+04 | 6.98E+07 | 7.95E+03 | |
| PPRG134@34 | 856 | -6733 | 1.99E+07 | 3.10E+04 | -22.51 | 0.57 | -9.15E+04 | 1.33E+07 | 1.08E+07 | 7.83E+04 | 6.87E+07 | 5.17E+03 | |
| PPRG134@35 | 816 | -6733 | 1.29E+07 | 1.68E+04 | -31.08 | 0.75 | -8.59E+04 | 1.34E+07 | 1.32E+07 | 6.63E+04 | 6.96E+07 | 9.63E+03 | |
| PPRG134@36 | 776 | -6733 | 2.82E+07 | 4.74E+04 | -26.94 | 0.49 | -8.27E+04 | 1.50E+07 | 2.03E+07 | 5.39E+04 | 6.91E+07 | 1.36E+04 | |
| PPRG134@37 | 736 | -6733 | 3.12E+07 | 5.31E+04 | -30.91 | 0.33 | -8.45E+04 | 1.60E+07 | 2.13E+07 | 9.01E+04 | 7.01E+07 | 1.40E+04 | |
| PPRG134@38 | 696 | -6733 | 2.45E+07 | 3.96E+04 | -37.42 | 0.52 | -8.35E+04 | 1.10E+07 | 2.39E+07 | 5.51E+04 | 7.00E+07 | 1.55E+04 | |
| PPRG134@39 | 656 | -6733 | 1.59E+07 | 2.29E+04 | -29.76 | 0.70 | -8.51E+04 | 9.48E+06 | 1.25E+07 | 6.14E+04 | 6.94E+07 | 9.01E+03 | |
| PPRG134@42 | 536 | -6733 | 1.20E+07 | 1.53E+04 | -20.89 | 1.21 | -8.74E+04 | 1.15E+07 | 1.06E+07 | 8.18E+04 | 6.93E+07 | 6.26E+03 | |
| PPRG134@43 | 496 | -6733 | 1.44E+07 | 1.99E+04 | -27.18 | 0.90 | -8.61E+04 | 1.16E+07 | 1.11E+07 | 1.17E+05 | 6.92E+07 | 8.19E+03 | |
| PPRG134@45 | 416 | -6733 | 2.36E+07 | 3.77E+04 | -41.90 | 0.66 | -8.36E+04 | 6.03E+06 | 1.55E+07 | 5.45E+04 | 6.67E+07 | 8.98E+03 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Nabberu | Naberu@1 | 1816 | 240 | 9.42E+06 | 2.83E+04 | -9.42 | 1.00 | 4.95E+04 | 6.42E+06 | 4.97E+06 | 3.83E+04 | 2.25E+06 | 8.17E+03 |
| PPRG 089 | Naberu@02 | 1856 | 240 | 7.57E+06 | 2.47E+04 | -11.83 | 1.19 | 5.21E+04 | 1.17E+07 | 4.62E+06 | 9.98E+04 | 4.11E+06 | 8.76E+03 |
| Naberu@03 | 1778 | 230 | 1.11E+07 | 3.16E+04 | -9.52 | 0.77 | 4.89E+04 | 3.84E+06 | 3.67E+06 | 2.41E+04 | 1.61E+06 | 7.57E+03 | |
| Naberu@04 | 1710 | 230 | 9.94E+06 | 2.97E+04 | -3.48 | 1.06 | 5.16E+04 | 4.40E+06 | 3.99E+06 | 4.55E+04 | 1.73E+06 | 6.01E+03 | |
| Naberu@05 | 1739 | 294 | 9.02E+06 | 2.77E+04 | -14.97 | 1.12 | 4.43E+04 | 3.43E+06 | 3.06E+06 | 2.08E+04 | 1.63E+06 | 6.15E+03 | |
| Naberu@06 | 1821 | 285 | 9.07E+06 | 2.79E+04 | -10.67 | 1.17 | 5.42E+04 | 9.09E+06 | 4.99E+06 | 6.33E+04 | 3.07E+06 | 7.19E+03 | |
| Naberu@07 | 1905 | 283 | 8.58E+06 | 2.67E+04 | -14.01 | 0.95 | 6.36E+04 | 1.36E+07 | 4.78E+06 | 4.61E+05 | 3.94E+06 | 7.62E+03 | |
| Naberu@08 | 1988 | 278 | 9.72E+06 | 2.89E+04 | -15.79 | 0.95 | 4.71E+04 | 8.81E+06 | 4.57E+06 | 7.80E+04 | 2.38E+06 | 6.79E+03 | |
| Naberu@09 | 2078 | 272 | 6.35E+06 | 2.24E+04 | -6.97 | 1.50 | 5.11E+04 | 8.91E+06 | 2.89E+06 | 1.18E+05 | 3.44E+06 | 7.10E+03 | |
| Naberu@10 | 2127 | 346 | 7.97E+06 | 2.57E+04 | -4.24 | 1.03 | 5.98E+04 | 1.17E+07 | 3.84E+06 | 1.19E+05 | 3.06E+06 | 6.52E+03 | |
| Naberu@11 | 2042 | 394 | 8.54E+06 | 2.68E+04 | -12.97 | 1.03 | 6.09E+04 | 1.35E+07 | 5.81E+06 | 9.36E+04 | 3.03E+06 | 8.91E+03 | |
| Naberu@12 | 1925 | 402 | 8.51E+06 | 2.69E+04 | -6.01 | 1.01 | 7.59E+04 | 6.67E+06 | 5.03E+06 | 9.75E+04 | 2.15E+06 | 8.78E+03 | |
| Naberu@13 | 1956 | 450 | 7.75E+06 | 2.53E+04 | -10.65 | 0.96 | 4.53E+04 | 4.10E+06 | 3.84E+06 | 2.10E+04 | 1.51E+06 | 6.60E+03 | |
| Naberu@14 | 2039 | 505 | 8.15E+06 | 2.61E+04 | -15.21 | 1.10 | 5.46E+04 | 9.18E+06 | 4.60E+06 | 1.03E+05 | 2.28E+06 | 6.27E+03 | |
| Naberu@15 | 1960 | 530 | 8.77E+06 | 2.74E+04 | -4.75 | 0.85 | 7.53E+04 | 1.43E+07 | 6.32E+06 | 1.20E+05 | 4.95E+06 | 1.07E+04 | |
| Naberu@16 | 1872 | 558 | 7.58E+06 | 2.49E+04 | -0.99 | 1.48 | 5.65E+04 | 1.17E+07 | 4.34E+06 | 1.24E+05 | 3.74E+06 | 7.83E+03 | |
| Naberu@17 | 1975 | 580 | 7.87E+06 | 2.53E+04 | -7.18 | 1.09 | 5.22E+04 | 4.08E+06 | 3.99E+06 | 2.35E+04 | 1.54E+06 | 7.37E+03 | |
| Naberu@18 | 2077 | 589 | 7.34E+06 | 2.46E+04 | 0.21 | 1.15 | 6.01E+04 | 1.08E+07 | 4.75E+06 | 9.04E+04 | 3.28E+06 | 7.85E+03 | |
| Naberu@19 | 2146 | 644 | 1.34E+07 | 3.62E+04 | -16.89 | 0.77 | 1.02E+05 | 1.41E+07 | 6.00E+06 | 1.11E+05 | 4.18E+06 | 8.95E+03 | |
| Naberu@20 | 2182 | 710 | 1.02E+07 | 3.01E+04 | -9.49 | 0.93 | 5.14E+04 | 9.47E+06 | 4.74E+06 | 8.30E+04 | 3.55E+06 | 9.73E+03 | |
| Naberu@21 | 2163 | 833 | 7.36E+06 | 2.47E+04 | -3.96 | 0.99 | 7.41E+04 | 1.27E+07 | 3.39E+06 | 1.26E+05 | 6.58E+06 | 7.94E+03 | |
| Naberu@22 | 2108 | 880 | 7.32E+06 | 2.45E+04 | -4.54 | 1.09 | 8.51E+04 | 1.32E+07 | 5.04E+06 | 3.04E+05 | 4.97E+06 | 9.18E+03 | |
| Naberu@23 | 2100 | 946 | 7.17E+06 | 2.43E+04 | -6.32 | 1.18 | 7.44E+04 | 1.18E+07 | 3.80E+06 | 1.92E+05 | 4.47E+06 | 1.08E+04 | |
| Naberu@24 | 2214 | 991 | 8.15E+06 | 2.63E+04 | -2.06 | 0.82 | 7.14E+04 | 1.40E+07 | 5.27E+06 | 1.36E+05 | 5.49E+06 | 9.19E+03 | |
| Naberu@25 | 2369 | 1226 | 7.15E+06 | 2.42E+04 | -7.78 | 1.45 | 7.09E+04 | 1.10E+07 | 3.84E+06 | 9.53E+04 | 4.61E+06 | 7.12E+03 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Wabigoon | Wabigoon@1 | 4287 | -222 | 3.98E+07 | 9.98E+04 | -6.84 | 0.39 | 4.17E+04 | 4.73E+06 | 2.35E+07 | 9.46E+03 | 1.20E+07 | 2.80E+04 |
| PPRG 325 | Wabigoon@02 | 4327 | -222 | 2.26E+07 | 6.61E+04 | -3.61 | 1.05 | 3.54E+04 | 2.21E+06 | 1.44E+07 | 8.21E+03 | 8.30E+06 | 1.99E+04 |
| Wabigoon@03 | 4367 | -222 | 1.22E+07 | 4.53E+04 | -13.60 | 1.29 | 3.56E+04 | 1.13E+06 | 5.20E+06 | 7.92E+03 | 1.48E+06 | 1.07E+04 | |
| Wabigoon@04 | 4407 | -222 | 5.49E+07 | 1.30E+05 | -4.58 | 0.32 | 3.84E+04 | 5.97E+06 | 3.88E+07 | 1.06E+04 | 1.01E+07 | 4.71E+04 | |
| Wabigoon@05 | 4447 | -222 | 4.28E+07 | 1.07E+05 | 1.49 | 0.49 | 3.76E+04 | 7.78E+05 | 4.16E+06 | 7.93E+03 | 1.46E+06 | 1.08E+04 | |
| Wabigoon@07 | 4527 | -222 | 5.62E+07 | 1.32E+05 | -2.25 | 0.37 | 3.54E+04 | 5.30E+06 | 4.33E+07 | 1.03E+04 | 1.41E+07 | 5.46E+04 | |
| Wabigoon@08 | 4567 | -222 | 5.80E+07 | 1.36E+05 | -1.24 | 0.40 | 3.69E+04 | 4.51E+06 | 4.54E+07 | 9.97E+03 | 6.82E+06 | 4.65E+04 | |
| Wabigoon@09 | 4607 | -222 | 2.87E+07 | 7.78E+04 | -4.68 | 0.61 | 3.82E+04 | 1.72E+06 | 1.52E+07 | 8.79E+03 | 3.69E+06 | 1.76E+04 | |
| Wabigoon@10 | 4647 | -222 | 6.01E+07 | 1.41E+05 | -0.88 | 0.27 | 3.92E+04 | 4.84E+06 | 3.30E+07 | 1.06E+04 | 2.04E+07 | 4.60E+04 | |
| Wabigoon@11 | 4647 | -182 | 5.06E+07 | 1.21E+05 | -8.45 | 0.37 | 3.59E+04 | 3.16E+06 | 2.84E+07 | 9.20E+03 | 8.27E+06 | 2.75E+04 | |
| Wabigoon@12 | 4607 | -182 | 5.60E+07 | 1.32E+05 | -1.83 | 0.39 | 3.54E+04 | 1.59E+06 | 1.57E+07 | 8.39E+03 | 6.40E+06 | 1.98E+04 | |
| Wabigoon@13 | 4567 | -182 | 5.52E+07 | 1.31E+05 | -1.61 | 0.31 | 3.75E+04 | 4.63E+06 | 3.29E+07 | 1.01E+04 | 1.78E+07 | 4.00E+04 | |
| Wabigoon@14 | 4527 | -182 | 6.35E+07 | 1.47E+05 | -2.65 | 0.28 | 3.60E+04 | 4.58E+06 | 3.80E+07 | 1.02E+04 | 7.77E+06 | 4.89E+04 | |
| Wabigoon@17 | 4407 | -182 | 7.12E+07 | 1.63E+05 | -1.40 | 0.30 | 3.71E+04 | 4.63E+06 | 3.35E+07 | 9.80E+03 | 7.88E+06 | 2.76E+04 | |
| Wabigoon@18 | 4367 | -182 | 5.05E+07 | 1.21E+05 | -4.15 | 0.33 | 3.69E+04 | 4.59E+06 | 1.61E+07 | 1.00E+04 | 1.77E+07 | 2.57E+04 | |
| Wabigoon@19 | 4327 | -182 | 4.39E+07 | 1.07E+05 | -8.81 | 0.40 | 3.72E+04 | 5.26E+06 | 2.76E+07 | 1.06E+04 | 5.23E+06 | 3.33E+04 | |
| Wabigoon@20 | 4287 | -182 | 4.60E+07 | 1.12E+05 | -6.55 | 0.40 | 3.81E+04 | 2.87E+06 | 1.57E+07 | 8.89E+03 | 5.16E+06 | 1.85E+04 | |
| Wabigoon@21 | 4247 | -182 | 6.40E+07 | 1.48E+05 | -2.74 | 0.22 | 3.71E+04 | 6.13E+06 | 3.62E+07 | 1.03E+04 | 8.71E+06 | 3.83E+04 | |
| Wabigoon@23 | 4207 | -142 | 5.64E+07 | 1.33E+05 | -5.18 | 0.38 | 3.87E+04 | 4.28E+06 | 2.47E+07 | 9.95E+03 | 7.14E+06 | 3.21E+04 | |
| Wabigoon@24 | 4207 | -102 | 3.89E+07 | 9.82E+04 | -5.45 | 0.62 | 3.89E+04 | 3.55E+06 | 3.03E+07 | 9.92E+03 | 4.71E+06 | 3.58E+04 | |
| Wabigoon@25 | 4207 | -62 | 4.53E+07 | 1.11E+05 | -1.65 | 0.42 | 3.74E+04 | 4.87E+06 | 1.48E+07 | 9.53E+03 | 6.83E+06 | 2.28E+04 | |
| Wabigoon@26 | 4207 | -22 | 6.29E+07 | 1.46E+05 | 0.22 | 0.40 | 3.65E+04 | 5.80E+06 | 2.89E+07 | 1.01E+04 | 9.41E+06 | 3.60E+04 | |
| Wabigoon@27 | 4207 | 18 | 9.08E+07 | 2.02E+05 | 3.37 | 0.24 | 3.67E+04 | 2.45E+06 | 4.08E+07 | 9.03E+03 | 5.86E+06 | 3.32E+04 | |
| Wabigoon@28 | 4207 | 58 | 4.94E+07 | 1.19E+05 | -4.65 | 0.39 | 3.78E+04 | 6.95E+06 | 2.86E+07 | 1.05E+04 | 2.85E+07 | 4.02E+04 | |
| Wabigoon@29 | 4207 | 98 | 3.41E+07 | 8.85E+04 | -4.41 | 0.44 | 3.64E+04 | 1.98E+06 | 9.18E+06 | 8.73E+03 | 1.97E+06 | 1.23E+04 | |
| Wabigoon@30 | 4207 | 138 | 5.17E+07 | 1.23E+05 | -9.53 | 0.31 | 3.68E+04 | 4.58E+06 | 1.77E+07 | 9.93E+03 | 3.47E+06 | 2.09E+04 | |
| Wabigoon@31 | 4207 | 178 | 4.81E+07 | 1.16E+05 | -4.52 | 0.42 | 3.84E+04 | 5.14E+06 | 3.07E+07 | 1.09E+04 | 8.87E+06 | 3.88E+04 | |
| Wabigoon@32 | 4207 | 218 | 3.01E+07 | 8.11E+04 | -5.03 | 0.52 | 3.75E+04 | 5.21E+06 | 1.57E+07 | 9.75E+03 | 1.04E+07 | 2.73E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Farrel Quartzite | MGTKS1@50 | -1917 | -1297 | 2.30E+07 | 4.56E+04 | -13.08 | 0.28 | 3.91E+04 | 1.71E+07 | 3.51E+07 | 2.13E+06 | 1.18E+07 | 2.14E+04 |
| MGTKS1 | MGTKS1@51 | -1917 | -1247 | 3.86E+07 | 7.64E+04 | -9.36 | 0.26 | 4.50E+04 | 1.16E+07 | 4.56E+07 | 2.30E+06 | 5.90E+07 | 4.38E+04 |
| MGTKS1@52 | -1917 | -1197 | 1.05E+08 | 2.07E+05 | -5.49 | 0.07 | 4.02E+04 | 5.86E+06 | 1.27E+08 | 6.00E+06 | 5.41E+07 | 1.01E+05 | |
| MGTKS1@53 | -1917 | -1147 | 2.51E+07 | 5.00E+04 | -10.27 | 0.41 | 3.83E+04 | 1.06E+07 | 1.30E+07 | 3.02E+05 | 1.87E+07 | 2.76E+04 | |
| MGTKS1@54 | -1917 | -1097 | 3.15E+07 | 6.25E+04 | -12.42 | 0.24 | 4.20E+04 | 1.43E+07 | 1.42E+07 | 1.08E+05 | 1.05E+07 | 1.09E+04 | |
| MGTKS1@55 | -1917 | -1047 | 1.57E+07 | 3.14E+04 | -18.17 | 0.39 | 3.98E+04 | 1.26E+07 | 8.33E+06 | 5.46E+04 | 6.96E+06 | 1.07E+04 | |
| MGTKS1@56 | -1917 | -997 | 1.75E+07 | 3.49E+04 | -14.36 | 0.35 | 4.07E+04 | 1.33E+07 | 7.92E+06 | 6.62E+04 | 7.05E+06 | 1.33E+04 | |
| MGTKS1@57 | -1917 | -947 | 2.24E+07 | 4.48E+04 | -14.10 | 0.26 | 4.02E+04 | 1.40E+07 | 9.17E+06 | 1.25E+05 | 7.42E+06 | 1.77E+04 | |
| MGTKS1@58 | -1917 | -897 | 5.33E+07 | 1.06E+05 | -10.60 | 0.17 | 4.14E+04 | 1.68E+07 | 6.53E+07 | 2.99E+06 | 6.02E+07 | 7.96E+04 | |
| MGTKS1@60 | -1917 | -797 | 3.96E+07 | 7.85E+04 | -11.24 | 0.21 | 4.67E+04 | 1.30E+07 | 7.06E+07 | 3.70E+06 | 8.67E+07 | 7.71E+04 | |
| MGTKS1@61 | -1917 | -747 | 5.29E+07 | 1.05E+05 | -9.03 | 0.18 | 4.60E+04 | 1.37E+07 | 4.76E+07 | 3.75E+06 | 1.35E+08 | 5.85E+04 | |
| MGTKS1@62 | -1917 | -697 | 8.83E+07 | 1.75E+05 | -6.84 | 0.10 | 4.60E+04 | 1.53E+07 | 7.98E+07 | 4.48E+06 | 2.47E+08 | 1.14E+05 | |
| MGTKS1@64 | -1917 | -597 | 3.91E+07 | 7.77E+04 | -8.39 | 0.23 | 4.58E+04 | 1.98E+07 | 5.22E+07 | 2.43E+06 | 6.78E+07 | 5.14E+04 | |
| MGTKS1@65 | -1867 | -597 | 8.62E+07 | 1.70E+05 | -10.62 | 0.21 | 4.39E+04 | 1.54E+07 | 8.61E+07 | 2.29E+06 | 7.29E+07 | 5.01E+04 | |
| MGTKS1@66 | -1817 | -597 | 7.53E+07 | 1.49E+05 | -7.98 | 0.24 | 4.52E+04 | 1.72E+07 | 5.57E+07 | 2.64E+06 | 9.24E+07 | 6.77E+04 | |
| MGTKS1@67 | -1767 | -597 | 8.17E+07 | 1.61E+05 | -9.74 | 0.14 | 4.46E+04 | 1.47E+07 | 2.60E+08 | 1.09E+07 | 7.20E+07 | 1.40E+05 | |
| MGTKS1@68 | -1717 | -597 | 9.40E+07 | 1.87E+05 | -3.56 | 0.14 | 4.46E+04 | 8.87E+06 | 8.11E+07 | 1.99E+06 | 2.64E+07 | 2.30E+04 | |
| MGTKS1@69 | -1667 | -597 | 7.09E+07 | 1.40E+05 | -9.57 | 0.13 | 4.40E+04 | 1.41E+07 | 1.41E+08 | 8.83E+06 | 8.30E+07 | 1.12E+05 | |
| MGTKS1@70 | -1667 | -647 | 1.62E+07 | 3.28E+04 | -10.66 | 0.42 | 4.03E+04 | 7.27E+06 | 1.10E+07 | 2.81E+04 | 3.52E+06 | 6.14E+03 | |
| MGTKS1@71 | -1667 | -697 | 4.75E+07 | 9.45E+04 | -7.91 | 0.26 | 4.08E+04 | 7.83E+06 | 3.35E+07 | 1.55E+06 | 3.81E+07 | 2.49E+04 | |
| MGTKS1@72 | -1667 | -747 | 5.74E+07 | 1.14E+05 | -9.97 | 0.15 | 4.49E+04 | 1.54E+07 | 2.13E+08 | 1.14E+07 | 7.60E+07 | 3.10E+05 | |
| MGTKS1@73 | -1667 | -797 | 1.65E+07 | 3.31E+04 | -15.72 | 0.58 | 3.93E+04 | 1.02E+07 | 6.96E+06 | 2.01E+04 | 4.47E+06 | 5.95E+03 | |
| MGTKS1@74 | -1667 | -847 | 2.50E+07 | 5.01E+04 | -10.16 | 0.36 | 4.05E+04 | 8.93E+06 | 8.93E+06 | 5.21E+05 | 9.01E+06 | 8.54E+03 | |
| MGTKS1@75 | -1617 | -847 | 2.70E+08 | 5.34E+05 | -5.65 | 0.07 | 4.47E+04 | 3.95E+06 | 3.50E+08 | 1.84E+07 | 4.35E+08 | 4.89E+05 | |
| MGTKS1@76 | -1567 | -847 | 1.44E+08 | 2.84E+05 | -4.69 | 0.07 | 4.04E+04 | 1.04E+07 | 1.66E+08 | 8.11E+06 | 4.65E+07 | 1.02E+05 | |
| MGTKS1@77 | -1517 | -847 | 6.54E+07 | 1.29E+05 | -8.85 | 0.18 | 4.03E+04 | 9.44E+06 | 5.64E+07 | 3.43E+06 | 5.32E+07 | 5.08E+04 | |
| MGTKS1@78 | -1467 | -847 | 4.47E+07 | 8.89E+04 | -7.21 | 0.22 | 4.53E+04 | 1.30E+07 | 3.31E+07 | 2.99E+06 | 7.16E+07 | 3.87E+04 | |
| MGTKS1@79 | -1417 | -847 | 4.47E+07 | 8.89E+04 | -7.26 | 0.34 | 4.37E+04 | 1.14E+07 | 3.68E+07 | 2.07E+06 | 1.16E+08 | 4.52E+04 | |
| MGTKS1@81 | -1417 | -747 | 3.36E+07 | 6.65E+04 | -14.42 | 0.26 | 4.02E+04 | 1.75E+07 | 1.36E+07 | 2.56E+05 | 1.96E+07 | 1.26E+04 | |
| MGTKS1@82 | -1417 | -697 | 2.32E+07 | 4.61E+04 | -15.65 | 0.23 | 4.05E+04 | 1.33E+07 | 9.28E+06 | 5.58E+04 | 7.68E+06 | 8.48E+03 | |
| MGTKS1@83 | -1417 | -647 | 3.99E+07 | 7.92E+04 | -11.76 | 0.20 | 4.24E+04 | 1.81E+07 | 4.50E+07 | 2.43E+06 | 5.79E+07 | 4.57E+04 | |
| MGTKS1@85 | -1417 | -547 | 1.86E+07 | 3.73E+04 | -13.69 | 0.36 | 4.03E+04 | 1.07E+07 | 8.17E+06 | 3.95E+05 | 2.87E+07 | 9.27E+03 | |
| MGTKS1@86 | -1417 | -497 | 1.09E+08 | 2.15E+05 | -5.42 | 0.09 | 4.35E+04 | 1.59E+07 | 3.51E+07 | 2.47E+06 | 5.56E+07 | 3.57E+04 | |
| MGTKS1@87 | -1417 | -447 | 6.14E+07 | 1.22E+05 | -6.96 | 0.13 | 4.22E+04 | 7.87E+06 | 8.88E+07 | 4.77E+06 | 1.15E+08 | 1.12E+05 | |
| MGTKS1@88 | -1417 | -397 | 5.65E+07 | 1.11E+05 | -11.63 | 0.18 | 4.42E+04 | 1.38E+07 | 8.25E+07 | 4.55E+06 | 6.49E+07 | 7.86E+04 | |
| MGTKS1@89 | -1417 | -347 | 6.38E+07 | 1.27E+05 | -7.43 | 0.14 | 4.30E+04 | 1.63E+07 | 4.43E+07 | 3.06E+06 | 5.54E+07 | 4.76E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Farrel Quartzite | MGTKS1@02 | -70 | 2401 | 2.47E+07 | 4.19E+04 | -6.00 | 0.53 | -8.83E+04 | 7.63E+06 | 2.47E+07 | 1.88E+06 | 3.31E+07 | 2.68E+03 |
| MGTKS1 | MGTKS1@03 | -20 | 2401 | 3.96E+07 | 7.23E+04 | -2.68 | 0.44 | -7.95E+04 | 7.76E+06 | 2.82E+07 | 1.76E+06 | 6.49E+07 | 8.12E+03 |
| MGTKS1@04 | 30 | 2401 | 4.38E+07 | 8.03E+04 | -9.54 | 0.31 | -8.55E+04 | 8.32E+06 | 4.12E+07 | 2.68E+06 | 3.61E+07 | 2.38E+03 | |
| MGTKS1@05 | 80 | 2401 | 4.45E+07 | 8.22E+04 | -3.63 | 0.13 | -8.61E+04 | 7.64E+06 | 3.92E+07 | 3.39E+06 | 4.14E+07 | 3.51E+03 | |
| MGTKS1@06 | 130 | 2401 | 1.16E+07 | 1.52E+04 | -7.27 | 0.67 | -9.88E+04 | 6.43E+06 | 4.17E+06 | 1.54E+05 | 4.17E+06 | -3.38E+02 | |
| MGTKS1@07 | 180 | 2401 | 4.12E+07 | 7.54E+04 | -5.48 | 0.31 | -8.70E+04 | 9.96E+06 | 2.78E+07 | 1.39E+06 | 6.06E+07 | 5.77E+03 | |
| MGTKS1@08 | 230 | 2401 | 3.16E+07 | 5.59E+04 | -5.09 | 0.59 | -9.02E+04 | 8.38E+06 | 1.63E+07 | 1.34E+06 | 3.67E+07 | 1.69E+03 | |
| MGTKS1@09 | 280 | 2401 | 3.26E+07 | 5.78E+04 | -3.61 | 0.52 | -9.35E+04 | 5.73E+06 | 1.60E+07 | 1.53E+06 | 1.17E+07 | 1.05E+03 | |
| MGTKS1@10 | 330 | 2401 | 1.93E+07 | 3.08E+04 | -7.66 | 0.46 | -9.27E+04 | 5.97E+06 | 1.13E+07 | 1.46E+06 | 4.20E+07 | 3.21E+03 | |
| MGTKS1@11 | 330 | 2451 | 2.57E+07 | 4.40E+04 | -6.46 | 0.24 | -8.88E+04 | 7.31E+06 | 3.49E+07 | 5.93E+06 | 3.81E+07 | 3.84E+03 | |
| MGTKS1@14 | 180 | 2451 | 1.83E+07 | 2.90E+04 | -3.19 | 0.69 | -9.32E+04 | 5.67E+06 | 1.95E+07 | 2.74E+06 | 2.15E+07 | 1.97E+03 | |
| MGTKS1@15 | 130 | 2451 | 1.25E+07 | 1.70E+04 | -6.87 | 0.79 | -9.63E+04 | 6.23E+06 | 7.18E+06 | 2.02E+05 | 6.50E+06 | -8.74E+01 | |
| MGTKS1@16 | 80 | 2451 | 3.89E+07 | 7.00E+04 | -15.81 | 0.37 | -8.26E+04 | 5.21E+06 | 1.47E+07 | 5.28E+05 | 6.82E+07 | 1.16E+04 | |
| MGTKS1@17 | 30 | 2451 | 3.26E+07 | 5.81E+04 | 0.07 | 0.46 | -9.21E+04 | 7.33E+06 | 1.81E+07 | 1.37E+06 | 2.70E+07 | 1.23E+03 | |
| MGTKS1@18 | -20 | 2451 | 3.28E+07 | 5.84E+04 | -3.43 | 0.36 | -8.89E+04 | 7.84E+06 | 2.85E+07 | 2.52E+06 | 3.19E+07 | 2.87E+03 | |
| MGTKS1@20 | -120 | 2451 | 1.95E+07 | 3.13E+04 | -2.67 | 0.47 | -8.61E+04 | 7.44E+06 | 4.65E+07 | 4.69E+06 | 2.84E+07 | 2.50E+03 | |
| MGTKS1@21 | -120 | 2501 | 1.43E+07 | 2.04E+04 | -10.89 | 0.78 | -9.12E+04 | 9.01E+06 | 1.89E+07 | 2.06E+06 | 4.40E+07 | 3.15E+03 | |
| MGTKS1@22 | -70 | 2501 | 3.58E+07 | 6.43E+04 | -5.63 | 0.28 | -8.03E+04 | 8.40E+06 | 3.02E+07 | 3.13E+06 | 6.16E+07 | 5.85E+03 | |
| MGTKS1@23 | -20 | 2501 | 3.88E+07 | 7.02E+04 | -9.49 | 0.17 | -8.83E+04 | 8.11E+06 | 1.36E+07 | 7.75E+05 | 3.63E+07 | 1.74E+03 | |
| MGTKS1@24 | 30 | 2501 | 4.30E+07 | 7.92E+04 | -2.83 | 0.25 | -7.78E+04 | 5.84E+06 | 6.15E+07 | 4.47E+06 | 6.18E+07 | 7.06E+03 | |
| MGTKS1@25 | 80 | 2501 | 2.00E+07 | 3.20E+04 | -7.57 | 0.53 | -8.52E+04 | 6.62E+06 | 1.61E+07 | 8.33E+05 | 6.78E+07 | 4.21E+03 | |
| MGTKS1@27 | 180 | 2501 | 2.11E+07 | 3.47E+04 | -4.92 | 0.35 | -9.21E+04 | 8.97E+06 | 1.46E+07 | 1.03E+06 | 3.25E+07 | 2.46E+03 | |
| MGTKS1@28 | 230 | 2501 | 2.58E+07 | 4.42E+04 | -6.31 | 0.44 | -9.09E+04 | 7.09E+06 | 1.86E+07 | 1.39E+06 | 4.26E+07 | 3.47E+03 | |
| MGTKS1@29 | 280 | 2501 | 2.74E+07 | 4.75E+04 | -5.96 | 0.37 | -8.67E+04 | 7.24E+06 | 2.79E+07 | 2.18E+06 | 3.95E+07 | 2.66E+03 | |
| MGTKS1@30 | 330 | 2501 | 2.92E+07 | 5.07E+04 | -9.43 | 0.38 | -8.83E+04 | 7.04E+06 | 2.56E+07 | 2.14E+06 | 3.94E+07 | 2.63E+03 | |
| MGTKS1@31 | 330 | 2551 | 4.22E+07 | 7.73E+04 | -8.13 | 0.29 | -7.82E+04 | 5.79E+06 | 6.57E+07 | 6.12E+06 | 3.08E+07 | 3.60E+03 | |
| MGTKS1@32 | 280 | 2551 | 2.05E+07 | 3.33E+04 | -7.29 | 0.66 | -8.67E+04 | 7.95E+06 | 3.25E+07 | 2.25E+06 | 4.27E+07 | 2.75E+03 | |
| MGTKS1@33 | 230 | 2551 | 2.85E+07 | 4.97E+04 | -4.83 | 0.36 | -8.58E+04 | 7.80E+06 | 2.96E+07 | 3.20E+06 | 4.71E+07 | 3.57E+03 | |
| MGTKS1@34 | 180 | 2551 | 2.48E+07 | 4.20E+04 | -7.87 | 0.60 | -8.79E+04 | 7.31E+06 | 1.56E+07 | 1.63E+06 | 4.71E+07 | 3.00E+03 | |
| MGTKS1@35 | 130 | 2551 | 5.68E+07 | 1.08E+05 | 0.81 | 0.25 | -7.76E+04 | 5.03E+06 | 6.61E+07 | 7.27E+06 | 2.75E+07 | 3.98E+03 | |
| MGTKS1@36 | 80 | 2551 | 2.48E+07 | 4.22E+04 | -6.23 | 0.37 | -7.98E+04 | 6.89E+06 | 2.24E+07 | 2.67E+06 | 6.54E+07 | 5.72E+03 | |
| MGTKS1@39 | -70 | 2551 | 4.82E+07 | 8.99E+04 | -3.52 | 0.25 | -7.97E+04 | 6.83E+06 | 3.19E+07 | 1.62E+06 | 6.46E+07 | 5.69E+03 | |
| MGTKS1@40 | -120 | 2551 | 2.36E+07 | 3.96E+04 | -6.41 | 0.42 | -8.71E+04 | 8.83E+06 | 1.96E+07 | 1.53E+06 | 5.66E+07 | 3.87E+03 | |
| MGTKS1@41 | -170 | 2551 | 6.49E+07 | 1.24E+05 | -1.16 | 0.16 | -7.67E+04 | 4.11E+06 | 6.61E+07 | 1.07E+07 | 2.06E+07 | 3.28E+03 | |
| MGTKS1@42 | -220 | 2551 | 2.69E+07 | 4.64E+04 | -4.66 | 0.42 | -8.43E+04 | 7.78E+06 | 2.13E+07 | 1.70E+06 | 5.90E+07 | 5.04E+03 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Josefsdal | Josefsdal@01 | 361 | 773 | 2.66E+08 | 5.29E+05 | -9.45 | 0.11 | -8.40E+04 | 5.59E+06 | 5.55E+08 | 9.73E+05 | 2.38E+08 | 2.79E+04 |
| 99SA07 | Josefsdal@02 | 411 | 773 | 2.66E+08 | 5.30E+05 | -9.85 | 0.10 | -8.94E+04 | 7.82E+06 | 4.34E+08 | 7.52E+05 | 2.34E+08 | 1.57E+04 |
| Josefsdal@03 | 461 | 773 | 2.98E+08 | 5.95E+05 | -8.74 | 0.12 | -8.64E+04 | 4.36E+06 | 4.65E+08 | 1.62E+06 | 4.44E+08 | 2.85E+04 | |
| Josefsdal@05 | 561 | 773 | 2.39E+08 | 4.76E+05 | -9.15 | 0.11 | -8.77E+04 | 3.94E+06 | 5.62E+08 | 4.94E+05 | 1.42E+08 | 1.64E+04 | |
| Josefsdal@06 | 611 | 773 | 2.60E+08 | 5.18E+05 | -10.29 | 0.13 | -8.98E+04 | 5.16E+06 | 3.36E+08 | 7.14E+05 | 3.39E+08 | 1.98E+04 | |
| Josefsdal@07 | 661 | 773 | 2.85E+08 | 5.68E+05 | -9.06 | 0.06 | -8.62E+04 | 2.95E+06 | 5.74E+08 | 5.08E+05 | 1.51E+08 | 1.26E+04 | |
| Josefsdal@08 | 711 | 773 | 2.92E+08 | 5.84E+05 | -6.51 | 0.15 | -8.55E+04 | 2.00E+06 | 6.02E+08 | 2.62E+06 | 8.76E+07 | 1.30E+04 | |
| Josefsdal@09 | 711 | 823 | 3.04E+08 | 6.09E+05 | -6.76 | 0.23 | -8.73E+04 | 2.71E+06 | 5.99E+08 | 5.07E+05 | 1.72E+08 | 1.38E+04 | |
| Josefsdal@10 | 661 | 823 | 2.20E+08 | 4.36E+05 | -9.94 | 0.08 | -8.66E+04 | 5.40E+06 | 4.23E+08 | 6.28E+05 | 2.79E+08 | 1.79E+04 | |
| Josefsdal@11 | 611 | 823 | 2.23E+08 | 4.42E+05 | -10.49 | 0.10 | -8.60E+04 | 3.46E+06 | 5.47E+08 | 7.48E+05 | 1.21E+08 | 1.23E+04 | |
| Josefsdal@12 | 561 | 823 | 2.80E+08 | 5.57E+05 | -11.07 | 0.11 | -8.60E+04 | 3.47E+06 | 4.61E+08 | 1.10E+06 | 3.47E+08 | 2.34E+04 | |
| Josefsdal@13 | 511 | 823 | 2.92E+08 | 5.83E+05 | -7.79 | 0.14 | -8.50E+04 | 3.91E+06 | 5.62E+08 | 4.37E+05 | 3.17E+08 | 2.02E+04 | |
| Josefsdal@14 | 461 | 823 | 2.53E+08 | 5.02E+05 | -10.75 | 0.07 | -8.65E+04 | 4.20E+06 | 5.18E+08 | 7.39E+05 | 1.91E+08 | 1.53E+04 | |
| Josefsdal@15 | 411 | 823 | 2.56E+08 | 5.07E+05 | -11.59 | 0.10 | -8.74E+04 | 8.28E+06 | 4.07E+08 | 1.01E+06 | 1.53E+08 | 1.23E+04 | |
| Josefsdal@16 | 361 | 823 | 2.42E+08 | 4.80E+05 | -10.56 | 0.16 | -8.83E+04 | 7.65E+06 | 5.16E+08 | 1.37E+06 | 1.52E+08 | 1.70E+04 | |
| Josefsdal@17 | 361 | 873 | 2.33E+08 | 4.62E+05 | -10.28 | 0.09 | -9.16E+04 | 1.14E+06 | 2.75E+08 | 1.24E+05 | 1.30E+08 | 8.93E+03 | |
| Josefsdal@18 | 411 | 873 | 2.63E+08 | 5.23E+05 | -10.02 | 0.11 | -8.85E+04 | 5.76E+06 | 5.47E+08 | 7.34E+05 | 2.87E+08 | 2.02E+04 | |
| Josefsdal@19 | 461 | 873 | 3.21E+08 | 6.40E+05 | -11.94 | 0.13 | -8.61E+04 | 8.36E+06 | 4.38E+08 | 8.30E+05 | 3.83E+08 | 2.51E+04 | |
| Josefsdal@20 | 511 | 873 | 2.92E+08 | 5.84E+05 | -8.67 | 0.25 | -8.14E+04 | 3.17E+06 | 6.06E+08 | 1.33E+06 | 8.73E+07 | 1.62E+04 | |
| Josefsdal@21 | 561 | 873 | 2.36E+08 | 4.68E+05 | -10.54 | 0.12 | -8.60E+04 | 2.99E+06 | 5.50E+08 | 6.89E+06 | 1.66E+08 | 1.23E+04 | |
| Josefsdal@22 | 611 | 873 | 2.90E+08 | 5.79E+05 | -9.49 | 0.07 | -8.34E+04 | 3.14E+06 | 5.73E+08 | 1.34E+06 | 2.92E+08 | 1.94E+04 | |
| Josefsdal@23 | 661 | 873 | 2.39E+08 | 4.76E+05 | -8.91 | 0.16 | -8.81E+04 | 6.29E+06 | 4.11E+08 | 6.90E+05 | 2.49E+08 | 1.83E+04 | |
| Josefsdal@24 | 711 | 873 | 2.11E+08 | 4.21E+05 | -1.42 | 0.10 | -8.59E+04 | 2.48E+06 | 4.94E+08 | 3.02E+06 | 2.39E+08 | 1.34E+04 | |
| Josefsdal@25 | 711 | 923 | 2.54E+08 | 5.07E+05 | -7.63 | 0.10 | -8.93E+04 | 5.57E+06 | 4.52E+08 | 1.30E+06 | 2.59E+08 | 1.67E+04 | |
| Josefsdal@26 | 661 | 923 | 2.44E+08 | 4.84E+05 | -8.80 | 0.08 | -9.02E+04 | 6.89E+06 | 4.06E+08 | 1.87E+06 | 1.77E+08 | 1.32E+04 | |
| Josefsdal@27 | 611 | 923 | 2.67E+08 | 5.31E+05 | -9.26 | 0.26 | -8.71E+04 | 6.45E+06 | 3.95E+08 | 7.12E+05 | 1.96E+08 | 1.54E+04 | |
| Josefsdal@28 | 561 | 923 | 2.25E+08 | 4.47E+05 | -10.17 | 0.19 | -8.68E+04 | 4.63E+06 | 4.87E+08 | 6.54E+05 | 1.72E+08 | 1.26E+04 | |
| Josefsdal@29 | 511 | 923 | 3.55E+08 | 7.11E+05 | -8.42 | 0.11 | -8.45E+04 | 4.40E+06 | 5.70E+08 | 6.86E+05 | 2.55E+08 | 2.03E+04 | |
| Sample | Analysis Name | X | Y | 16O (cps/nA) | 18O (cps/nA) | meas. δ18O (‰) | 1σ (‰) | mass 12.8 (cps) | 12CH (cps/nA) | 16O (cps/nA) | 28Si (cps/nA) | 32S (cps/nA) | 56FeO (cps/nA) |
| Buck Reef | Buck@01 | 5211 | -590 | 2.73E+07 | 4.73E+04 | -8.70 | 0.71 | -7.87E+04 | 8.81E+06 | 6.48E+07 | 1.20E+07 | 1.24E+07 | 5.95E+04 |
| 99SA03 | Buck@03 | 5131 | -590 | 9.92E+06 | 1.18E+04 | -10.31 | 1.18 | -9.86E+04 | 9.30E+06 | 7.77E+06 | 9.77E+04 | 1.49E+07 | 9.54E+03 |
| Buck@05 | 5051 | -590 | 1.67E+07 | 2.57E+04 | -11.36 | 0.66 | -9.55E+04 | 1.11E+07 | 7.66E+06 | 3.83E+04 | 1.59E+07 | 1.96E+04 | |
| Buck@06 | 5011 | -590 | 2.13E+07 | 3.51E+04 | -5.21 | 0.70 | -9.54E+04 | 9.74E+06 | 8.26E+06 | 1.05E+05 | 1.59E+07 | 1.65E+04 | |
| Buck@07 | 4971 | -590 | 1.98E+07 | 3.17E+04 | -10.93 | 0.74 | -9.71E+04 | 8.93E+06 | 3.64E+06 | 5.71E+04 | 1.46E+07 | 6.44E+03 | |
| Buck@08 | 4931 | -590 | 7.06E+07 | 1.35E+05 | -8.82 | 0.17 | -9.15E+04 | 1.10E+07 | 2.30E+07 | 1.51E+05 | 1.61E+07 | 2.21E+04 | |
| Buck@11 | 5011 | -630 | 1.92E+07 | 3.09E+04 | -6.36 | 0.55 | -9.21E+04 | 9.41E+06 | 1.57E+07 | 7.20E+05 | 2.11E+07 | 1.71E+04 | |
| Buck@13 | 5091 | -630 | 1.71E+07 | 2.65E+04 | -9.52 | 0.62 | -9.23E+04 | 1.31E+07 | 1.01E+07 | 1.58E+05 | 2.30E+07 | 3.23E+04 | |
| Buck@14 | 5131 | -630 | 3.69E+07 | 6.62E+04 | -13.64 | 0.48 | -9.57E+04 | 1.03E+07 | 4.10E+06 | 8.83E+04 | 1.76E+07 | 8.32E+03 | |
| Buck@16 | 5211 | -630 | 1.25E+07 | 1.68E+04 | -10.94 | 0.94 | -9.50E+04 | 8.48E+06 | 6.21E+06 | 2.17E+05 | 1.74E+07 | 8.25E+03 | |
| Buck@18 | 5171 | -750 | 1.39E+07 | 1.98E+04 | -12.59 | 0.81 | -9.77E+04 | 8.23E+06 | 9.08E+06 | 2.37E+05 | 1.40E+07 | 8.38E+03 | |
| Buck@19 | 5131 | -750 | 1.19E+07 | 1.58E+04 | -8.33 | 0.70 | -9.16E+04 | 1.10E+07 | 3.14E+07 | 3.79E+05 | 1.54E+07 | 4.67E+04 | |
| Buck@20 | 5091 | -750 | 1.12E+07 | 1.44E+04 | -9.74 | 1.02 | -9.45E+04 | 1.30E+07 | 8.75E+06 | 9.20E+04 | 1.71E+07 | 2.01E+04 | |
| Buck@21 | 5051 | -750 | 1.05E+07 | 1.30E+04 | -14.17 | 0.84 | -9.73E+04 | 8.94E+06 | 7.37E+06 | 4.35E+04 | 1.71E+07 | 1.39E+04 | |
| Buck@22 | 5011 | -750 | 2.11E+07 | 3.46E+04 | -9.01 | 0.60 | -9.55E+04 | 1.03E+07 | 1.12E+07 | 3.08E+05 | 1.64E+07 | 1.63E+04 | |
| Buck@23 | 4971 | -750 | 1.13E+07 | 1.46E+04 | -11.88 | 0.85 | -9.66E+04 | 9.26E+06 | 7.38E+06 | 7.14E+04 | 1.42E+07 | 1.31E+04 | |
| Buck@26 | 4851 | -750 | 1.12E+07 | 1.43E+04 | -12.62 | 1.00 | -9.71E+04 | 9.68E+06 | 5.23E+06 | 2.32E+05 | 1.56E+07 | 8.03E+03 | |
| Buck@27 | 4851 | -710 | 3.21E+07 | 5.74E+04 | -1.62 | 0.44 | -9.64E+04 | 8.77E+06 | 8.31E+06 | 2.64E+05 | 1.40E+07 | 1.08E+04 | |
| Buck@28 | 4851 | -670 | 1.45E+07 | 2.11E+04 | -6.73 | 0.80 | -9.66E+04 | 9.63E+06 | 6.53E+06 | 1.87E+05 | 1.64E+07 | 2.26E+04 | |
| Buck@29 | 4851 | -630 | 1.40E+07 | 1.99E+04 | -19.16 | 0.62 | -9.34E+04 | 9.49E+06 | 1.05E+07 | 7.21E+04 | 2.73E+07 | 1.72E+04 | |
| Buck@31 | 4851 | -550 | 1.54E+07 | 2.29E+04 | -12.57 | 0.47 | -9.68E+04 | 1.19E+07 | 9.77E+06 | 6.72E+04 | 1.38E+07 | 1.72E+04 | |
| Buck@33 | 4851 | -470 | 1.93E+07 | 3.05E+04 | -13.99 | 0.64 | -9.47E+04 | 1.07E+07 | 1.48E+07 | 2.73E+05 | 1.40E+07 | 2.82E+04 | |
| Buck@34 | 4851 | -430 | 1.50E+07 | 2.21E+04 | -12.53 | 1.10 | -9.76E+04 | 8.82E+06 | 7.47E+06 | 5.69E+04 | 1.21E+07 | 1.19E+04 | |
| Buck@35 | 4851 | -390 | 6.26E+07 | 1.20E+05 | -2.24 | 0.27 | -8.51E+04 | 6.72E+06 | 5.35E+07 | 3.37E+06 | 9.83E+06 | 2.45E+04 | |
| Buck@36 | 4851 | -350 | 1.13E+07 | 1.45E+04 | -12.83 | 1.01 | -9.68E+04 | 8.62E+06 | 8.97E+06 | 8.59E+04 | 1.31E+07 | 1.20E+04 | |
Table S-5 Final SIMS O isotope dataset.
| Sample | Analysis Name | Age (Ma) | Bulk δ18O (‰) | δ18O corr. IMF (‰) | 2σ (‰) |
| Doushantuo | Doushantuo@1 | 580 | 15.0 ± 0.2‰ | 16.4 | 1.4 |
| 1a of 8/25/83 | Doushantuo@02 | 24.7 | 1.2 | ||
| Doushantuo@03 | 21.6 | 1.5 | |||
| Doushantuo@04 | 14.6 | 2.2 | |||
| Doushantuo@05 | 19.7 | 1.7 | |||
| Doushantuo@06 | 21.3 | 1.3 | |||
| Doushantuo@07 | 14.0 | 0.9 | |||
| Doushantuo@08 | 20.1 | 1.0 | |||
| Doushantuo@09 | 14.5 | 0.9 | |||
| Doushantuo@10 | 17.3 | 1.3 | |||
| Doushantuo@11 | 9.2 | 0.8 | |||
| Doushantuo@12 | 15.1 | 1.0 | |||
| Doushantuo@15 | 13.1 | 1.0 | |||
| Bitter Springs | Bitter@02 | 800 | 6.6 | 1.0 | |
| 3 of 11/2/90 | Bitter@05 | 10.7 | 1.4 | ||
| Bitter@07 | 17.0 | 1.2 | |||
| Bitter@08 | 11.7 | 0.8 | |||
| Bitter@10 | 5.6 | 0.9 | |||
| Bitter@12 | 2.5 | 0.9 | |||
| Bitter@13 | 2.0 | 1.0 | |||
| Bitter@15 | 8.1 | 1.0 | |||
| Bitter@16 | 10.9 | 1.0 | |||
| Bitter@17 | 3.4 | 0.9 | |||
| Bitter@18 | 10.0 | 1.0 | |||
| Bitter@19 | 12.4 | 0.9 | |||
| Bitter@21 | 9.9 | 1.1 | |||
| Bitter@22 | 7.8 | 1.1 | |||
| Bitter@23 | 16.1 | 0.8 | |||
| Bitter@24 | 5.7 | 1.3 | |||
| Bitter@25 | 11.5 | 1.1 | |||
| Bitter@26 | 9.3 | 0.8 | |||
| Bitter@30 | 6.6 | 1.0 | |||
| Bitter@32 | 13.0 | 1.0 | |||
| Bitter@33 | 4.2 | 1.2 | |||
| Bitter@34 | 5.1 | 1.0 | |||
| Bitter@35 | 10.4 | 0.8 | |||
| Bitter@37 | 8.8 | 1.0 | |||
| Bitter@38 | 12.1 | 1.1 | |||
| Bitter@42 | 6.1 | 1.1 | |||
| Bitter@43 | 6.7 | 1.3 | |||
| Bitter@44 | 8.4 | 1.0 | |||
| Bitter@47 | 6.6 | 0.9 | |||
| Bitter@50 | 10.3 | 1.1 | |||
| Jixian | Jixian@05 | 1500 | 21.7 | 1.0 | |
| 1 of 8/14/83 | Jixian@08 | 22.1 | 1.1 | ||
| Jixian@09 | 30.9 | 0.8 | |||
| Jixian@10 | 28.7 | 1.0 | |||
| Jixian@13 | 25.9 | 1.0 | |||
| Jixian@19 | 28.9 | 0.9 | |||
| Jixian@20 | 32.4 | 1.2 | |||
| Jixian@21 | 30.4 | 1.0 | |||
| Jixian@22 | 33.1 | 0.9 | |||
| Jixian@23 | 28.5 | 0.9 | |||
| Jixian@26 | 28.2 | 1.0 | |||
| Jixian@27 | 30.0 | 0.9 | |||
| Jixian@28 | 28.7 | 0.9 | |||
| Jixian@32 | 26.9 | 0.8 | |||
| Jixian@33 | 28.3 | 0.9 | |||
| Jixian@34 | 31.4 | 0.9 | |||
| Jixian@35 | 27.8 | 0.9 | |||
| McArthur | McArthur@1 | 1600 | 27.1 | 0.8 | |
| 7 of 6/21/90 | McArthur@02 | 22.7 | 1.0 | ||
| McArthur@03 | 29.1 | 0.8 | |||
| McArthur@04 | 24.1 | 0.9 | |||
| McArthur@05 | 25.8 | 0.9 | |||
| McArthur@09 | 24.3 | 0.9 | |||
| McArthur@12 | 27.3 | 0.9 | |||
| McArthur@13 | 16.7 | 1.0 | |||
| McArthur@14 | 19.4 | 1.0 | |||
| McArthur@15 | 27.1 | 1.0 | |||
| McArthur@16 | 20.5 | 0.9 | |||
| McArthur@23 | 23.0 | 0.9 | |||
| McArthur@24 | 29.6 | 0.9 | |||
| Gunflint | Gunflint@126 | 1880 | 7.3 ± 1.0 ‰ | 4.6 | 1.4 |
| 3 of 6/30/84 | Gunflint@127 | 3.8 | 1.1 | ||
| Gunflint@128 | 4.2 | 0.9 | |||
| Gunflint@129 | 9.2 | 0.9 | |||
| Gunflint@131 | 3.9 | 1.4 | |||
| Gunflint@133 | 2.6 | 2.5 | |||
| Gunflint@134 | 2.7 | 1.1 | |||
| Gunflint | PPRG134@02 | -2.5 | 1.9 | ||
| PPRG 134 | PPRG134@09 | 2.0 | 2.5 | ||
| PPRG134@11 | 4.5 | 2.3 | |||
| PPRG134@12 | 4.1 | 2.1 | |||
| PPRG134@13 | 6.3 | 2.0 | |||
| PPRG134@15 | 4.5 | 2.1 | |||
| PPRG134@18 | 6.4 | 2.3 | |||
| PPRG134@25 | 4.6 | 2.5 | |||
| PPRG134@26 | 5.7 | 2.4 | |||
| PPRG134@27 | 2.2 | 2.3 | |||
| PPRG134@29 | 9.3 | 3.1 | |||
| PPRG134@32 | 7.7 | 2.2 | |||
| PPRG134@33 | 1.7 | 2.5 | |||
| PPRG134@35 | 0.4 | 2.4 | |||
| PPRG134@37 | 0.6 | 2.0 | |||
| PPRG134@39 | 1.8 | 2.3 | |||
| PPRG134@42 | 10.6 | 3.1 | |||
| Nabberu | Naberu@02 | 1850 | 21.2 | 2.5 | |
| PPRG 089 | Naberu@06 | 22.4 | 2.5 | ||
| Naberu@08 | 17.2 | 2.1 | |||
| Naberu@09 | 26.1 | 3.1 | |||
| Naberu@10 | 28.8 | 2.2 | |||
| Naberu@14 | 17.8 | 2.3 | |||
| Naberu@15 | 28.3 | 1.9 | |||
| Naberu@16 | 32.0 | 3.1 | |||
| Naberu@18 | 33.3 | 2.4 | |||
| Naberu@19 | 16.1 | 1.7 | |||
| Naberu@20 | 23.6 | 2.0 | |||
| Naberu@23 | 26.7 | 2.5 | |||
| Naberu@24 | 31.0 | 1.8 | |||
| Naberu@25 | 25.3 | 3.0 | |||
| Wabigoon | Wabigoon@1 | 2700 | 26.2 | 0.9 | |
| PPRG 325 | Wabigoon@03 | 19.4 | 1.5 | ||
| Wabigoon@04 | 28.5 | 0.9 | |||
| Wabigoon@17 | 31.6 | 0.9 | |||
| Wabigoon@19 | 24.2 | 0.9 | |||
| Wabigoon@20 | 26.5 | 0.9 | |||
| Wabigoon@21 | 30.3 | 0.8 | |||
| Wabigoon@23 | 27.9 | 0.9 | |||
| Wabigoon@26 | 33.3 | 0.9 | |||
| Wabigoon@29 | 28.6 | 0.9 | |||
| Wabigoon@31 | 28.5 | 0.9 | |||
| Farrel Quartzite | MGTKS1@50 | 3020 | 19.8 ± 0.1‰ | 18.3 | 0.7 |
| MGTKS1 | MGTKS1@51 | 22.0 | 0.7 | ||
| MGTKS1@53 | 21.1 | 0.9 | |||
| MGTKS1@54 | 18.9 | 0.7 | |||
| MGTKS1@55 | 13.2 | 0.9 | |||
| MGTKS1@56 | 17.0 | 0.8 | |||
| MGTKS1@57 | 17.2 | 0.7 | |||
| MGTKS1@58 | 20.7 | 0.6 | |||
| MGTKS1@60 | 20.1 | 0.6 | |||
| MGTKS1@61 | 22.3 | 0.6 | |||
| MGTKS1@64 | 22.9 | 0.6 | |||
| MGTKS1@65 | 20.7 | 0.6 | |||
| MGTKS1@66 | 23.4 | 0.6 | |||
| MGTKS1@68 | 27.8 | 0.5 | |||
| MGTKS1@70 | 20.7 | 0.9 | |||
| MGTKS1@71 | 23.4 | 0.7 | |||
| MGTKS1@73 | 15.6 | 1.2 | |||
| MGTKS1@74 | 21.2 | 0.8 | |||
| MGTKS1@77 | 22.5 | 0.6 | |||
| MGTKS1@78 | 24.1 | 0.6 | |||
| MGTKS1@79 | 24.1 | 0.8 | |||
| MGTKS1@81 | 16.9 | 0.7 | |||
| MGTKS1@82 | 15.7 | 0.6 | |||
| MGTKS1@83 | 19.6 | 0.6 | |||
| MGTKS1@85 | 17.6 | 0.8 | |||
| MGTKS1@86 | 25.9 | 0.5 | |||
| MGTKS1@88 | 19.7 | 0.6 | |||
| MGTKS1@89 | 23.9 | 0.5 | |||
| MGTKS1@02 | 25.5 | 2.1 | |||
| MGTKS1@04 | 22.0 | 2.0 | |||
| MGTKS1@05 | 27.9 | 1.9 | |||
| MGTKS1@07 | 26.0 | 2.0 | |||
| MGTKS1@08 | 26.4 | 2.2 | |||
| MGTKS1@10 | 23.9 | 2.1 | |||
| MGTKS1@14 | 28.3 | 2.3 | |||
| MGTKS1@17 | 31.6 | 2.1 | |||
| MGTKS1@18 | 28.1 | 2.0 | |||
| MGTKS1@21 | 20.6 | 2.4 | |||
| MGTKS1@22 | 25.9 | 1.9 | |||
| MGTKS1@23 | 22.0 | 1.9 | |||
| MGTKS1@27 | 26.6 | 2.0 | |||
| MGTKS1@28 | 25.2 | 2.1 | |||
| MGTKS1@29 | 25.6 | 2.0 | |||
| MGTKS1@30 | 22.1 | 2.0 | |||
| MGTKS1@32 | 24.2 | 2.3 | |||
| MGTKS1@33 | 26.7 | 2.0 | |||
| MGTKS1@34 | 23.7 | 2.2 | |||
| MGTKS1@40 | 25.1 | 2.0 | |||
| MGTKS1@42 | 26.9 | 2.0 | |||
| Josefsdal | Josefsdal@02 | 3300 | 21.7 | 1.9 | |
| 99SA07 | Josefsdal@05 | 22.4 | 1.9 | ||
| Josefsdal@06 | 21.2 | 1.9 | |||
| Josefsdal@07 | 22.5 | 1.9 | |||
| Josefsdal@10 | 21.6 | 1.9 | |||
| Josefsdal@11 | 21.0 | 1.9 | |||
| Josefsdal@14 | 20.8 | 1.9 | |||
| Josefsdal@16 | 21.0 | 1.9 | |||
| Josefsdal@18 | 21.5 | 1.9 | |||
| Josefsdal@19 | 19.6 | 1.9 | |||
| Josefsdal@23 | 22.6 | 1.9 | |||
| Josefsdal@25 | 23.9 | 1.9 | |||
| Josefsdal@26 | 22.7 | 1.9 | |||
| Josefsdal@27 | 22.3 | 1.9 | |||
| Josefsdal@28 | 21.3 | 1.9 | |||
| Josefsdal@29 | 23.1 | 1.9 | |||
| Buck Reef | Buck@03 | 3420 | 15.0 ± 0.7‰ | 19.7 | 2.5 |
| 99SA03 | Buck@05 | 18.7 | 1.5 | ||
| Buck@06 | 24.8 | 1.6 | |||
| Buck@07 | 19.1 | 1.6 | |||
| Buck@08 | 21.2 | 0.8 | |||
| Buck@13 | 20.5 | 1.4 | |||
| Buck@14 | 16.4 | 1.2 | |||
| Buck@16 | 19.1 | 2.0 | |||
| Buck@18 | 17.5 | 1.8 | |||
| Buck@20 | 20.3 | 2.2 | |||
| Buck@21 | 15.9 | 1.8 | |||
| Buck@22 | 21.0 | 1.4 | |||
| Buck@23 | 18.2 | 1.8 | |||
| Buck@26 | 17.4 | 2.1 | |||
| Buck@27 | 28.4 | 1.1 | |||
| Buck@28 | 23.3 | 1.7 | |||
| Buck@31 | 17.5 | 1.2 | |||
| Buck@33 | 16.1 | 1.5 | |||
| Buck@34 | 17.5 | 2.3 | |||
| Buck@36 | 17.2 | 2.1 | |||