Experimental evidence for fluid-induced melting in subduction zones
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:3,967Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
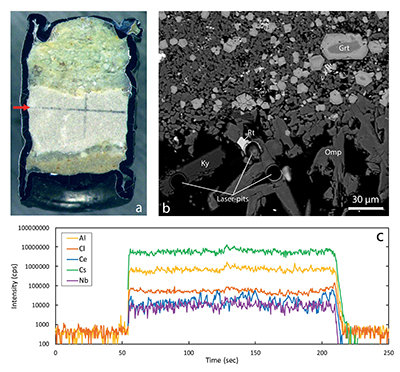 Figure 1 Run products from high pressure experiments. (a) Cross section of a sample capsule after an experiment (image width 5 mm). A white layer of diamond powder is sandwiched between the silicate sample. The red arrow points to a laser ablation trace. (b) Backscatter electron image of the silicate part of a sample, consisting mostly of omphacite (Omp) and garnet (Grt) with minor kyanite (Ky) and rutile (Rt). In the centre of some garnet crystals, remnants of the garnet seeds are visible. (c) Laser ablation analysis of frozen fluid in the diamond trap, demonstrating the homogeneity of the sample. | 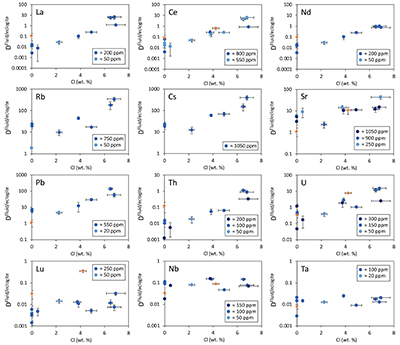 Figure 2 Effect of chloride on fluid/eclogite partition coefficients of trace elements at 4 GPa and 800 ˚C. Blue data points are the results from “forward” experiments, where the trace elements were initially doped into the solid starting material, while orange data points are from “reversed” experiments, which started with all trace elements dissolved in the fluid. For the forward experiments, results for different initial trace element concentrations in the starting material are given. Error bars are one standard deviation. Data for these and additional elements are given in Tables S-1 to S-8. | 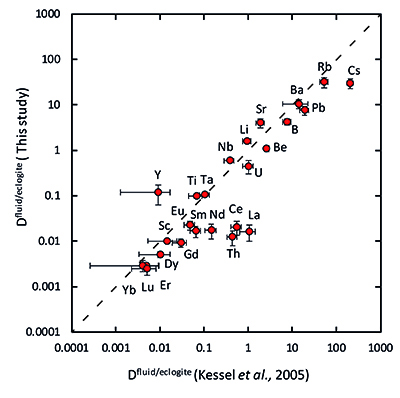 Figure 3 Comparison of the fluid/eclogite partition coefficients for Cl-free fluids measured in this study with those reported by Kessel et al. (2005). Both sets of experiments were carried out at 4 GPa and 800 ˚C, with a bulk composition resembling MORB. | 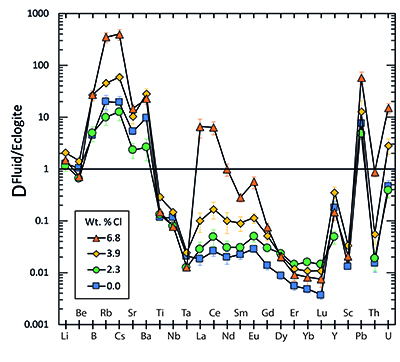 Figure 4 Trace element enrichment patterns in fluids from fluid/eclogite partitioning experiments at 4 GPa and 800 ˚C. Error bars are one standard deviation. Data for these and additional elements are given in Tables S-1 to S-8. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
In subduction zones, oceanic crust is recycled into the mantle. Thermal models show that the temperature of the mantle wedge above the subducting slab is actually considerably lower than in other parts of the shallow upper mantle (Syracuse et al., 2010
Syracuse, E.M., Van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73–90.
). Melting must therefore be caused by other effects, most likely by the addition of water, which may reduce the melting temperatures of mantle peridotite by several 100 ˚C (Kawamoto and Holloway, 1997Kawamoto, T., Holloway, P.R. (1997) Melting temperature and partial melt chemistry of H2O-saturated mantle peridotite to 11 gigapascals. Science 276, 240–243.
; Gaetani and Grove, 1998Gaetani, G.A., Grove, T.L. (1998) The influence of water on melting of mantle peridotite. Contributions to Mineralogy and Petrology 131, 323–346.
). Water may be transferred from the subducted oceanic slab to the mantle wedge in the form of aqueous fluids, released by the dehydration of hydrous minerals, or by sediment melts. Already early studies (Perfit et al., 1980Perfit, M.R., Gust, D.A., Bence, A.E., Arculus, R.J., Taylor, S.R. (1980) Chemical characteristics of island-arc basalts - implications for mantle sources. Chemical Geology 30, 227–256.
; Arculus and Powell, 1986Arculus, R.J., Powell, R. (1986) Source component mixing in the regions of arc magma generation. Journal of Geophysical Research 91, 5913–5926.
) noted that the trace element enrichment pattern in magmas from volcanic arcs above a subduction zone is distinctly different from that observed in magmas at divergent plate boundaries, e.g., mid-ocean ridges. Typical features of arc magmas include high enrichments of large ion lithophile elements (LILE, such as Rb+, Cs+, Sr2+, Ba2+) and light rare earth elements (REE, such as La3+ and Ce3+), but strong depletions of high field strength elements (HFSE, such as Ti4+, Nb5+ and Ta5+). Some experimental studies (Kessel et al., 2005Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727.
; Hermann et al., 2006Hermann, J., Spandler, C., Hack, A., Korsakov, A.V. (2006) Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos 92, 399–417.
) suggested that trace element transport by aqueous fluids is unable to produce the observed trace element enrichment pattern in arc magmas. This led to the suggestion that sediment melts are the main agents of metasomatism in the mantle wedge above subduction zones (Kelemen et al., 2005Kelemen, P.B., Hanghøj, K., Greene, A.R. (2005) One view of the geochemistry of subduction-related magmatic arcs, with an emphasis on primitive andesite and lower crust. In: Rudnick, R.L. (Ed.) Treatise of Geochemistry, Volume 3, The Crust. Elsevier, Amsterdam, 593–659.
; Hermann et al., 2006Hermann, J., Spandler, C., Hack, A., Korsakov, A.V. (2006) Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos 92, 399–417.
; Skora and Blundy, 2010Skora, S., Blundy, J. (2010) High-pressure hydrous phase relations of radiolarian clay and implications for the involvement of subducted sediment in arc magmatism. Journal of Petrology 51, 2211–2243.
; Behn et al., 2011Behn, M.D., Kelemen, P.B., Hirth, G., Hacker, B.R., Massonne, H.J. (2011) Diapirs as the source of the sediment signature in arc lavas. Nature Geoscience 4, 641–646.
; Spandler and Pirard, 2013Spandler, C., Pirard, C. (2013) Element recycling from subducting slabs to arc crust: A review. Lithos 170-171, 208–223.
). Previous studies, however, did not consider the effect of chloride, which may affect the partition behaviour of various trace elements by the formation of chloride complexes in the fluid. As the subducted oceanic crust was in contact with seawater, it is expected to contain chloride and measurements of the Cl/H2O ratio of primitive arc magmas (Métrich and Wallace, 2008Métrich, N., Wallace, P.J. (2008) Volatile abundances in basaltic magmas and their degassing paths tracked by melt inclusions. Reviews in Mineralogy and Geochemistry 69, 363–402.
), as well as other lines of evidence (Kawamoto et al., 2013Kawamoto, T., Yoshikawa, M., Kumagai, Y., Mirabueno, M.H.T., Okuno, M., Kobayashi, T. (2013) Mantle wedge infiltrated with saline fluids from dehydration and decarbonation of subducting slab. Proceedings of the National Academy of Sciences of the USA 110, 9663–9668.
), are consistent with the incorporation of aqueous fluids (Manning, 2004Manning, C.E. (2004) The chemistry of subduction zone fluids. Earth and Planetary Science Letters 223, 1–16.
) containing up to 15 wt. % NaCl. In the present study, we therefore for the first time directly measured the effect of chlorine on the partitioning of trace elements between aqueous fluids and the minerals of the subducted basaltic crust at conditions corresponding to the typical depth of the slab below the volcanic front.Experiments were carried out in an end-loaded piston cylinder apparatus (Boyd and England, 1960
Boyd, F.R., England, J.L. (1960) Apparatus for phase-equilibrium measurements at pressures up to 50 kilobars and temperatures up to 1750˚C. Journal of Geophysical Research 65, 741–748.
) at 4 GPa and 800 ˚C with run durations between 2 and 7 days. Synthetic MORB (mid-ocean ridge basalt) glass doped with a suite of trace elements was loaded together with water or NaCl solutions into platinum capsules. A layer of diamond powder was inserted in the middle of the capsule between the layers of MORB powder to provide some empty pore space between the diamond grains for trapping the fluid (Ryabchikov et al., 1989Ryabchikov, I.D., Orlova, G.P., Kalenchuk, G.Y., Ganeyev, I.I., Udovkina, N.G., Nosik, L.P. (1989) Reactions of spinel lherzolite with H2O-CO2 fluids at 20 kbar and 900 °C. Geochemistry International 26, 56–62.
). After quenching of the experiments, the sample capsules were cooled to liquid nitrogen temperature and cut in half. Both the compositions of the minerals and of the quenched fluid trapped between the diamond grains were then measured in frozen state (Kessel et al., 2005Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727.
) by laser ablation ICP-MS. Additional details about the experimental and analytical methods are given in the Supplementary Information.top
Results and Discussion
During the high pressure experiments, the glasses recrystallised to an eclogitic assemblage of omphacite, garnet, rutile, and kyanite, i.e. the same minerals that are expected to be stable in the subducted basaltic oceanic crust below the volcanic arc (Fig. 1). Other accessory phases likely do not occur in natural MORB at eclogite facies conditions. The solubility of phosphorus in garnet is so high that apatite and other phosphates are unlikely to form (Konzett and Frost, 2009
Konzett, J., Frost, D.J. (2009) The high P-T stability of hydroxyl-apatite in natural and simplified MORB – an experimental study to 15 GPa with implications for transport and storage of phosphorus and halogens in subduction zones. Journal of Petrology 50, 2043–2062.
). Due to the very low K2O content in natural MORB, eclogites of MORB composition either contain no phengite at all or at most traces of this mineral (e.g., Okrusch et al., 1991Okrusch, M., Matthes, S., Klemd, R., O'Brien P.J., Schmidt, K. (1991) Eclogites at the north-western margin of the Bohemian Massif: A review. European Journal of Mineralogy 3, 707–730.
; see also the Supplementary Information for further discussion). Indeed, in sub-solidus experiments with natural MORB at 3 GPa and 800 ˚C, Carter et al. (2015)Carter, L.B., Skora, S., Blundy, J.D., De Hoog, J.C.M., Elliott, T. (2015) An experimental study of trace element fluxes from subducted oceanic crust. Journal of Petrology 56, 1585–1606.
did not observe any phengite or apatite.
Figure 1 Run products from high pressure experiments. (a) Cross section of a sample capsule after an experiment (image width 5 mm). A white layer of diamond powder is sandwiched between the silicate sample. The red arrow points to a laser ablation trace. (b) Backscatter electron image of the silicate part of a sample, consisting mostly of omphacite (Omp) and garnet (Grt) with minor kyanite (Ky) and rutile (Rt). In the centre of some garnet crystals, remnants of the garnet seeds are visible. (c) Laser ablation analysis of frozen fluid in the diamond trap, demonstrating the homogeneity of the sample.
Mineral compositions in our experiments were found to be uniform in the entire sample, consistent with attainment of equilibrium throughout the entire charge. With a few exceptions, as discussed below, laser ablation ICP-MS analyses of trace element concentrations yielded homogeneous compositions of both the quenched fluid phase and the minerals (see Fig. 1 for typical laser ablation signals). Fluid/mineral partition coefficients Dfluid/mineral = cfluid/cmineral were calculated from the measured trace element concentrations in fluid (cfluid) and coexisting minerals (cmineral). Bulk fluid/eclogite partition coefficients were then calculated from the individual fluid/mineral partition coefficients assuming an eclogitic mineralogy with 59 % omphacite, 39 % garnet and 2 % rutile. Experimental details, compositions of all phases and calculated bulk fluid eclogite partition coefficients are compiled in Tables S-1 to S-8 of the Supplementary Information.
A major problem in all studies of element partitioning between minerals and fluid is attainment of equilibrium, since the diffusion coefficients of most of the relevant trace elements in the minerals are very low. In order to circumvent this problem, we introduced periodic temperature fluctuations by ±30 ˚C in our experiments, which enhanced grain growth and equilibration by Ostwald ripening (i.e. the dissolution of smaller grains at higher temperature and the growth of larger grains upon cooling). Indeed, the resulting grain sizes observed after runs with these sinusoidal temperature fluctuations were generally much larger than for experiments at constant temperature, but mineral compositions were not affected. In order to demonstrate conclusively the attainment of equilibrium, we also performed some reversed experiments, starting with a trace element-free MORB glass and trace element-doped solutions. In general, both the normal “forward” experiments starting with trace element-doped MORB glass and the reversed experiments gave very consistent results. We are therefore confident that the trace element partition coefficients reported here represent true chemical equilibrium between aqueous fluid and minerals. Moreover, results from experiments with different concentration levels of trace elements yielded consistent partition coefficients, implying that Henry´s law is fulfilled.
Figure 2 shows the fluid/eclogite partition coefficients for some selected trace elements as a function of the chloride content in the fluid. For the light rare earths, such as La and Ce, there is a striking increase of Dfluid/eclogite by up to three orders of magnitude even for moderate salinities (up to 15 wt. % NaCl). Similar, although smaller effects are seen for the alkalis (e.g., Rb and Cs) and the alkaline earths (Sr). Pb, Th, and U also show striking increases with salinity. On the other hand, both the typical high field strength elements, such as Nb and Ta as well as the heavy rare earth (e.g., Lu) appear to be unaffected by chloride.

Figure 2 Effect of chloride on fluid/eclogite partition coefficients of trace elements at 4 GPa and 800 ˚C. Blue data points are the results from “forward” experiments, where the trace elements were initially doped into the solid starting material, while orange data points are from “reversed” experiments, which started with all trace elements dissolved in the fluid. For the forward experiments, results for different initial trace element concentrations in the starting material are given. Error bars are one standard deviation. Data for these and additional elements are given in Tables S-1 to S-8.
Our data for Cl-free aqueous fluids are generally consistent with those from a previous study (Kessel et al., 2005
Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727.
), as shown in Figure 3. For saline fluids, there are no published data that could be directly compared with our results. However, both studies on mineral solubilities (Bali et al., 2011Bali, E., Audetat, A., Keppler, H. (2011) The mobility of U and Th in subduction zone fluids: an indicator of oxygen fugacity and fluid salinity. Contributions to Mineralogy and Petrology 161, 597–613.
; Tropper et al., 2011Tropper, P., Manning, C.E., Harlov, D.E. (2011) Solubility of CePO4 monazite and YPO4 xenotime in H2O and H2O–NaCl at 800 °C and 1 GPa: Implications for REE and Y transport during high-grade metamorphism. Chemical Geology 282, 58–66.
; Tsay et al., 2014Tsay, A., Zajacz, Z., Sanchez-Valle, C. (2014) Efficient mobilization and fractionation of rare-earth elements by aqueous fluids upon slab dehydration. Earth and Planetary Science Letters 398, 101–112.
) and fluid/melt partitioning (Keppler, 1996Keppler, H. (1996) Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240.
; Kawamoto et al., 2014Kawamoto, T., Mibe, K., Bureau, H., Reguer, S., Mocuta, C., Kubsky, S., Thiaudiere, D., Ono, S., Kogiso, T. (2014) Large-ion lithophile elements delivered by saline fluids to the sub-arc mantle. Earth, Planets and Space 66, doi:10.1186/1880-5981-66-61.
) at lower pressures suggest that those elements that are affected by fluid salinity indeed form stable chloride complexes in aqueous fluids. In particular, Tsay et al. (2014)Tsay, A., Zajacz, Z., Sanchez-Valle, C. (2014) Efficient mobilization and fractionation of rare-earth elements by aqueous fluids upon slab dehydration. Earth and Planetary Science Letters 398, 101–112.
noted an increase of the solubility of La2Si2O7 and Nd2Si2O7 in aqueous fluid by one order of magnitude upon addition of 1.5 M NaCl at 800 ˚C and 2.6 GPa. The formation of chloride complexes will tend to stabilise the trace element in the fluid and therefore increase the fluid/eclogite partition coefficient. Only the sensitivity of Th to chloride is unexpected, as it behaves differently from other HFSE trace elements, such as Nb and Ta. However, the ionic radius of Th4+ is significantly larger than that of Nb5+, Ta5+, or Ti4+, such that its geochemical behaviour may be transitional between a typical high field strength and a large ion lithophile element. We also tried to measure the fluid/eclogite partitioning of Zr and Hf, two important HFSE trace elements, but here we encountered experimental problems. The distribution of these elements in the quenched fluid inside the diamond trap was always highly inhomogeneous, which precluded the reliable determination of fluid concentrations and partition coefficients. A possible reason could be the very low solubility (Bernini et al., 2013Bernini, D., Audetat, A., Dolejs, D., Keppler, H. (2013) Zircon solubility in aqueous fluids at high temperatures and pressures. Geochimica et Cosmochimica Acta 119, 178–187.
) of zircon ZrSiO4 and hafnon HfSiO4, which may have precipitated early during the experiment inside the diamond trap and may have failed to reach equilibrium.
Figure 3 Comparison of the fluid/eclogite partition coefficients for Cl-free fluids measured in this study with those reported by Kessel et al. (2005)
Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727.
. Both sets of experiments were carried out at 4 GPa and 800 ˚C, with a bulk composition resembling MORB.Figure 4 shows the trace element enrichment pattern in the fluid phase from the fluid/eclogite partitioning experiments as a function of salinity. An important observation here is that a pure aqueous fluid would not be able to produce all of the trace element enrichment features observed in arc magmas. While such fluids may effectively transport some large ion lithophile elements, like Rb, Cs, Sr, and Ba (with fluid/eclogite partition coefficients >1), the light rare earths as well as uranium would be retained in the eclogite. This used to be one of the main arguments why aqueous fluids were considered to be “too dilute” to produce the trace element enrichment observed in arc magmas and why alternative mechanisms, such as metasomatism by sediment melt were proposed. However, for elevated salinities the enrichment pattern in aqueous fluid has a striking similarity to that observed in arc magmas, with the light rare earths and U becoming mobile in the fluid together with the large ion lithophile elements, while at the same time, high field strength elements, such as Nb, Ta, and Ti are nearly completely retained in the eclogite. The high Ba/La, Ba/Nb, and U/Th ratios match well with those inferred from primitive arc basalts (see Supplementary Information for further discussion). In particular, the “negative Nb-Ta anomaly” i.e. the strong depletion of Nb and Ta relative to both light rare earths and large ion lithophile elements is a hallmark of subduction zone magmas. Saline fluids can fractionate these elements by three orders of magnitude, mainly through the effect of Cl on rare earth partitioning. In a chloride-free system, the fluid/eclogite partition coefficient of La and Ce could be increased to a similar value by a temperature increase of several 100 ˚C, ultimately leading to melting (Kessel et al. 2005
Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727.
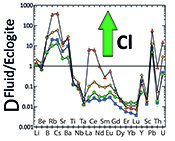
). However, in silicate melts, Nb and Ta would also become mobile and therefore, this effect cannot produce the negative Nb Ta anomaly observed in subduction zone magmas.
Figure 4 Trace element enrichment patterns in fluids from fluid/eclogite partitioning experiments at 4 GPa and 800 ˚C. Error bars are one standard deviation. Data for these and additional elements are given in Tables S-1 to S-8.
top
Conclusions
Our experimental data show that saline fluids released from the basaltic layer of the subducted slab can account for most features in the trace element enrichment pattern observed in subduction zone magmas. In the light of these experiments, the relative importance of aqueous fluids and sediment melts in the formation of arc magmas needs to be reconsidered. Strong evidence for the involvement of sedimentary material comes from isotopic data; already Armstrong (1971)
Armstrong, R.L. (1971) Isotopic and chemical constraints on models of magma genesis in volcanic arcs. Earth and Planetary Science Letters 12, 37–142.
noted a close correlation between the 206Pb/204Pb ratio of arc magmas and the sediments in front of some arcs and similar evidence has been presented for different isotope systems. However, these observations do not necessarily require the involvement of sediment melts. The isotopic signal observed may also have been transported by aqueous fluids; our data suggest that both Pb and Sr may be efficiently transported by saline fluids (Fig. 4) and even Be and Nd may be significantly mobile under some conditions. High Th/La ratios in arc magmas may be inherited from sediments (Plank, 2005Plank, T. (2005) Constraints from thorium/lanthanum on sediment recycling at subduction zones and the evolution of the continents. Journal of Petrology 46, 921–944.
); however, it remains uncertain whether sediment melts could effectively transport these elements, as they are strongly retained in residual monazite and other phases and the fractionation of Th and La between melt and monazite may not always operate in the right direction (Skora and Blundy, 2010Skora, S., Blundy, J. (2010) High-pressure hydrous phase relations of radiolarian clay and implications for the involvement of subducted sediment in arc magmatism. Journal of Petrology 51, 2211–2243.
). On the other hand, experimental data suggest that mantle metasomatism by sediment melts produces distinctly potassic melts (Mallik et al., 2015Mallik, A., Nelson, J., Dasgupta, R. (2015) Partial melting of fertile peridotite fluxed by hydrous rhyolitic melt at 2-3 GPa: implications for mantle wedge hybridization by sediment melt and generation of ultrapotassic magmas in convergent margins. Contributions to Mineralogy and Petrology 169, Article Number 48.
) different from average subduction zone magmas. Thermal models of subduction zones (Syracuse et al., 2010Syracuse, E.M., Van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73–90.
) suggest temperatures below the arc that are lower than those required for dehydration melting (e.g., Mann and Schmidt, 2015Mann, U., Schmidt, M.W. (2015) Melting of pelitic sediments at subarc depths: 1. Flux vs. fluid-absent melting and a parameterization of melt productivity. Chemical Geology 404, 150–167.
). Higher temperatures have been inferred from Ce/H2O ratios. However, the Ce/H2O geothermometer (Plank et al., 2009Plank, T., Cooper, L.B., Manning, C.E. (2009) Emerging geothermometers for estimating slab surface temperatures. Nature Geoscience, 2, 611–615.
) is based on the assumption that the Ce/H2O ratio in fluids and melts is a function of temperature only. Our data (Fig. 2) show that at the same temperature, this ratio may vary by three orders of magnitude as a function of salinity.top
Acknowledgements
We thank Patrick O´Brien for discussing some aspects of eclogite mineralogy with us. Reviews by Matthieu Galvez and by an anonymous referee helped to improve the manuscript. This work was supported by the DFG International Research Training Group “Deep Earth Volatile Cycles” (GRK 2156/1).
Editor: Cin-Ty Lee
top
References
Arculus, R.J., Powell, R. (1986) Source component mixing in the regions of arc magma generation. Journal of Geophysical Research 91, 5913–5926.
 Show in context
Show in contextAlready early studies (Perfit et al., 1980; Arculus and Powell, 1986) noted that the trace element enrichment pattern in magmas from volcanic arcs above a subduction zone is distinctly different from that observed in magmas at divergent plate boundaries, e.g., mid-ocean ridges.
View in article
Armstrong, R.L. (1971) Isotopic and chemical constraints on models of magma genesis in volcanic arcs. Earth and Planetary Science Letters 12, 37–142.
 Show in context
Show in context Strong evidence for the involvement of sedimentary material comes from isotopic data; already Armstrong (1971) noted a close correlation between the 206Pb/204Pb ratio of arc magmas and the sediments in front of some arcs and similar evidence has been presented for different isotope systems.
View in article
Bali, E., Audetat, A., Keppler, H. (2011) The mobility of U and Th in subduction zone fluids: an indicator of oxygen fugacity and fluid salinity. Contributions to Mineralogy and Petrology 161, 597–613.
 Show in context
Show in contextHowever, both studies on mineral solubilities (Bali et al., 2011; Tropper et al., 2011; Tsay et al., 2014) and fluid/melt partitioning (Keppler, 1996; Kawamoto et al., 2014) at lower pressures suggest that those elements that are affected by fluid salinity indeed form stable chloride complexes in aqueous fluids.
View in article
Behn, M.D., Kelemen, P.B., Hirth, G., Hacker, B.R., Massonne, H.J. (2011) Diapirs as the source of the sediment signature in arc lavas. Nature Geoscience 4, 641–646.
 Show in context
Show in contextThis led to the suggestion that sediment melts are the main agents of metasomatism in the mantle wedge above subduction zones (Kelemen et al., 2005; Hermann et al., 2006; Skora and Blundy, 2010; Behn et al., 2011; Spandler and Pirard, 2013).
View in article
Bernini, D., Audetat, A., Dolejs, D., Keppler, H. (2013) Zircon solubility in aqueous fluids at high temperatures and pressures. Geochimica et Cosmochimica Acta 119, 178–187.
 Show in context
Show in contextA possible reason could be the very low solubility (Bernini et al., 2013) of zircon ZrSiO4 and hafnon HfSiO4, which may have precipitated early during the experiment inside the diamond trap and may have failed to reach equilibrium.
View in article
Boyd, F.R., England, J.L. (1960) Apparatus for phase-equilibrium measurements at pressures up to 50 kilobars and temperatures up to 1750˚C. Journal of Geophysical Research 65, 741–748.
 Show in context
Show in context Experiments were carried out in an end-loaded piston cylinder apparatus (Boyd and England, 1960) at 4 GPa and 800 ˚C with run durations between 2 and 7 days. Synthetic MORB (mid-ocean ridge basalt) glass doped with a suite of trace elements was loaded together with water or NaCl solutions into platinum capsules.
View in article
Carter, L.B., Skora, S., Blundy, J.D., De Hoog, J.C.M., Elliott, T. (2015) An experimental study of trace element fluxes from subducted oceanic crust. Journal of Petrology 56, 1585–1606.
 Show in context
Show in context Indeed, in sub-solidus experiments with natural MORB at 3 GPa and 800 ˚C, Carter et al. (2015) did not observe any phengite or apatite.
View in article
Gaetani, G.A., Grove, T.L. (1998) The influence of water on melting of mantle peridotite. Contributions to Mineralogy and Petrology 131, 323–346.
 Show in context
Show in contextMelting must therefore be caused by other effects, most likely by the addition of water, which may reduce the melting temperatures of mantle peridotite by several 100 ˚C (Kawamoto and Holloway, 1997; Gaetani and Grove, 1998).
View in article
Hermann, J., Spandler, C., Hack, A., Korsakov, A.V. (2006) Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos 92, 399–417.
 Show in context
Show in context Some experimental studies (Kessel et al., 2005; Hermann et al., 2006) suggested that trace element transport by aqueous fluids is unable to produce the observed trace element enrichment pattern in arc magmas.
View in article
This led to the suggestion that sediment melts are the main agents of metasomatism in the mantle wedge above subduction zones (Kelemen et al., 2005; Hermann et al., 2006; Skora and Blundy, 2010; Behn et al., 2011; Spandler and Pirard, 2013).
View in article
Kawamoto, T., Holloway, P.R. (1997) Melting temperature and partial melt chemistry of H2O-saturated mantle peridotite to 11 gigapascals. Science 276, 240–243.
 Show in context
Show in context Melting must therefore be caused by other effects, most likely by the addition of water, which may reduce the melting temperatures of mantle peridotite by several 100 ˚C (Kawamoto and Holloway, 1997; Gaetani and Grove, 1998).
View in article
Kawamoto, T., Yoshikawa, M., Kumagai, Y., Mirabueno, M.H.T., Okuno, M., Kobayashi, T. (2013) Mantle wedge infiltrated with saline fluids from dehydration and decarbonation of subducting slab. Proceedings of the National Academy of Sciences of the USA 110, 9663–9668.
 Show in context
Show in context As the subducted oceanic crust was in contact with seawater, it is expected to contain chloride and measurements of the Cl/H2O ratio of primitive arc magmas (Métrich and Wallace, 2008), as well as other lines of evidence (Kawamoto et al., 2013), are consistent with the incorporation of aqueous fluids (Manning, 2004) containing up to 15 wt. % NaCl.
View in article
Kawamoto, T., Mibe, K., Bureau, H., Reguer, S., Mocuta, C., Kubsky, S., Thiaudiere, D., Ono, S., Kogiso, T. (2014) Large-ion lithophile elements delivered by saline fluids to the sub-arc mantle. Earth, Planets and Space 66, doi:10.1186/1880-5981-66-61.
 Show in context
Show in context However, both studies on mineral solubilities (Bali et al., 2011; Tropper et al., 2011; Tsay et al., 2014) and fluid/melt partitioning (Keppler, 1996; Kawamoto et al., 2014) at lower pressures suggest that those elements that are affected by fluid salinity indeed form stable chloride complexes in aqueous fluids.
View in article
Kelemen, P.B., Hanghøj, K., Greene, A.R. (2005) One view of the geochemistry of subduction-related magmatic arcs, with an emphasis on primitive andesite and lower crust. In: Rudnick, R.L. (Ed.) Treatise of Geochemistry, Volume 3, The Crust. Elsevier, Amsterdam, 593–659.
 Show in context
Show in contextThis led to the suggestion that sediment melts are the main agents of metasomatism in the mantle wedge above subduction zones (Kelemen et al., 2005; Hermann et al., 2006; Skora and Blundy, 2010; Behn et al., 2011; Spandler and Pirard, 2013).
View in article
Keppler, H. (1996) Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240.
 Show in context
Show in contextHowever, both studies on mineral solubilities (Bali et al., 2011; Tropper et al., 2011; Tsay et al., 2014) and fluid/melt partitioning (Keppler, 1996; Kawamoto et al., 2014) at lower pressures suggest that those elements that are affected by fluid salinity indeed form stable chloride complexes in aqueous fluids.
View in article
Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727.
 Show in context
Show in contextSome experimental studies (Kessel et al., 2005; Hermann et al., 2006) suggested that trace element transport by aqueous fluids is unable to produce the observed trace element enrichment pattern in arc magmas.
View in article
Both the compositions of the minerals and of the quenched fluid trapped between the diamond grains were then measured in frozen state (Kessel et al., 2005) by laser ablation ICP-MS.
View in article
Our data for Cl-free aqueous fluids are generally consistent with those from a previous study (Kessel et al., 2005), as shown in Figure 3.
View in article
Figure 3 Comparison of the fluid/eclogite partition coefficients for Cl-free fluids measured in this study with those reported by Kessel et al. (2005).
View in article
In a chloride-free system, the fluid/eclogite partition coefficient of La and Ce could be increased to a similar value by a temperature increase of several 100 ˚C, ultimately leading to melting (Kessel et al., 2005).
View in article
Konzett, J., Frost, D.J. (2009) The high P-T stability of hydroxyl-apatite in natural and simplified MORB – an experimental study to 15 GPa with implications for transport and storage of phosphorus and halogens in subduction zones. Journal of Petrology 50, 2043–2062.
 Show in context
Show in contextThe solubility of phosphorus in garnet is so high that apatite and other phosphates are unlikely to form (Konzett and Frost, 2009).
View in article
Mallik, A., Nelson, J., Dasgupta, R. (2015) Partial melting of fertile peridotite fluxed by hydrous rhyolitic melt at 2-3 GPa: implications for mantle wedge hybridization by sediment melt and generation of ultrapotassic magmas in convergent margins. Contributions to Mineralogy and Petrology 169, Article Number 48.
 Show in context
Show in context On the other hand, experimental data suggest that mantle metasomatism by sediment melts produces distinctly potassic melts (Mallik et al., 2015) different from average subduction zone magmas.
View in article
Mann, U., Schmidt, M.W. (2015) Melting of pelitic sediments at subarc depths: 1. Flux vs. fluid-absent melting and a parameterization of melt productivity. Chemical Geology 404, 150–167.
 Show in context
Show in context Thermal models of subduction zones (Syracuse et al., 2010) suggest temperatures below the arc that are lower than those required for dehydration melting (e.g., Mann and Schmidt, 2015).
View in article
Manning, C.E. (2004) The chemistry of subduction zone fluids. Earth and Planetary Science Letters 223, 1–16.
 Show in context
Show in context As the subducted oceanic crust was in contact with seawater, it is expected to contain chloride and measurements of the Cl/H2O ratio of primitive arc magmas (Métrich and Wallace, 2008), as well as other lines of evidence (Kawamoto et al., 2013), are consistent with the incorporation of aqueous fluids (Manning, 2004) containing up to 15 wt. % NaCl.
View in article
Métrich, N., Wallace, P.J. (2008) Volatile abundances in basaltic magmas and their degassing paths tracked by melt inclusions. Reviews in Mineralogy and Geochemistry 69, 363–402.
 Show in context
Show in contextAs the subducted oceanic crust was in contact with seawater, it is expected to contain chloride and measurements of the Cl/H2O ratio of primitive arc magmas (Métrich and Wallace, 2008), as well as other lines of evidence (Kawamoto et al., 2013), are consistent with the incorporation of aqueous fluids (Manning, 2004) containing up to 15 wt. % NaCl.
View in article
Okrusch, M., Matthes, S., Klemd, R., O'Brien P.J., Schmidt, K. (1991) Eclogites at the north-western margin of the Bohemian Massif: A review. European Journal of Mineralogy 3, 707–730.
 Show in context
Show in context Due to the very low K2O content in natural MORB, eclogites of MORB composition either contain no phengite at all or at most traces of this mineral (e.g., Okrusch et al., 1991; see also the Supplementary Information for further discussion).
View in article
Perfit, M.R., Gust, D.A., Bence, A.E., Arculus, R.J., Taylor, S.R. (1980) Chemical characteristics of island-arc basalts - implications for mantle sources. Chemical Geology 30, 227–256.
 Show in context
Show in context Already early studies (Perfit et al., 1980; Arculus and Powell, 1986) noted that the trace element enrichment pattern in magmas from volcanic arcs above a subduction zone is distinctly different from that observed in magmas at divergent plate boundaries, e.g., mid-ocean ridges.
View in article
Plank, T. (2005) Constraints from thorium/lanthanum on sediment recycling at subduction zones and the evolution of the continents. Journal of Petrology 46, 921–944.
 Show in context
Show in contextHigh Th/La ratios in arc magmas may be inherited from sediments (Plank, 2005); however, it remains uncertain whether sediment melts could effectively transport these elements, as they are strongly retained in residual monazite and other phases and the fractionation of Th and La between melt and monazite may not always operate in the right direction (Skora and Blundy, 2010).
View in article
Plank, T., Cooper, L.B., Manning, C.E. (2009) Emerging geothermometers for estimating slab surface temperatures. Nature Geoscience, 2, 611–615.
 Show in context
Show in contextHigher temperatures have been inferred from Ce/H2O ratios. However, the Ce/H2O geothermometer (Plank et al., 2009) is based on the assumption that the Ce/H2O ratio in fluids and melts is a function of temperature only.
View in article
Ryabchikov, I.D., Orlova, G.P., Kalenchuk, G.Y., Ganeyev, I.I., Udovkina, N.G., Nosik, L.P. (1989) Reactions of spinel lherzolite with H2O-CO2 fluids at 20 kbar and 900 °C. Geochemistry International 26, 56–62.
 Show in context
Show in context A layer of diamond powder was inserted in the middle of the capsule between the layers of MORB powder to provide some empty pore space between the diamond grains for trapping the fluid (Ryabchikov et al., 1989).
View in article
Skora, S., Blundy, J. (2010) High-pressure hydrous phase relations of radiolarian clay and implications for the involvement of subducted sediment in arc magmatism. Journal of Petrology 51, 2211–2243.
 Show in context
Show in contextThis led to the suggestion that sediment melts are the main agents of metasomatism in the mantle wedge above subduction zones (Kelemen et al., 2005; Hermann et al., 2006; Skora and Blundy, 2010; Behn et al., 2011; Spandler and Pirard, 2013).
View in article
High Th/La ratios in arc magmas may be inherited from sediments (Plank, 2005); however, it remains uncertain whether sediment melts could effectively transport these elements, as they are strongly retained in residual monazite and other phases and the fractionation of Th and La between melt and monazite may not always operate in the right direction (Skora and Blundy, 2010).
View in article
Spandler, C., Pirard, C. (2013) Element recycling from subducting slabs to arc crust: A review. Lithos 170-171, 208–223.
 Show in context
Show in contextThis led to the suggestion that sediment melts are the main agents of metasomatism in the mantle wedge above subduction zones (Kelemen et al., 2005; Hermann et al., 2006; Skora and Blundy, 2010; Behn et al., 2011; Spandler and Pirard, 2013).
View in article
Syracuse, E.M., Van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73–90.
 Show in context
Show in context Thermal models show that the temperature of the mantle wedge above the subducting slab is actually considerably lower than in other parts of the shallow upper mantle (Syracuse et al., 2010).
View in article
Thermal models of subduction zones (Syracuse et al., 2010) suggest temperatures below the arc that are lower than those required for dehydration melting (e.g., Mann and Schmidt, 2015).
View in article
Tropper, P., Manning, C.E., Harlov, D.E. (2011) Solubility of CePO4 monazite and YPO4 xenotime in H2O and H2O–NaCl at 800 °C and 1 GPa: Implications for REE and Y transport during high-grade metamorphism. Chemical Geology 282, 58–66.
 Show in context
Show in context However, both studies on mineral solubilities (Bali et al., 2011; Tropper et al., 2011; Tsay et al., 2014) and fluid/melt partitioning (Keppler, 1996; Kawamoto et al., 2014) at lower pressures suggest that those elements that are affected by fluid salinity indeed form stable chloride complexes in aqueous fluids.
View in article
Tsay, A., Zajacz, Z., Sanchez-Valle, C. (2014) Efficient mobilization and fractionation of rare-earth elements by aqueous fluids upon slab dehydration. Earth and Planetary Science Letters 398, 101–112.
 Show in context
Show in context However, both studies on mineral solubilities (Bali et al., 2011; Tropper et al., 2011; Tsay et al., 2014) and fluid/melt partitioning (Keppler, 1996; Kawamoto et al., 2014) at lower pressures suggest that those elements that are affected by fluid salinity indeed form stable chloride complexes in aqueous fluids.
View in article
In particular, Tsay et al. (2014) noted an increase of the solubility of La2Si2O7 and Nd2Si2O7 in aqueous fluid by one order of magnitude upon addition of 1.5 M NaCl at 800 ˚C and 2.6 GPa.
View in article
top
Supplementary Information
The Supplementary Information includes:
- Starting Materials and Methods
- Supplementary Discussion
- Tables S-1 to S-8
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
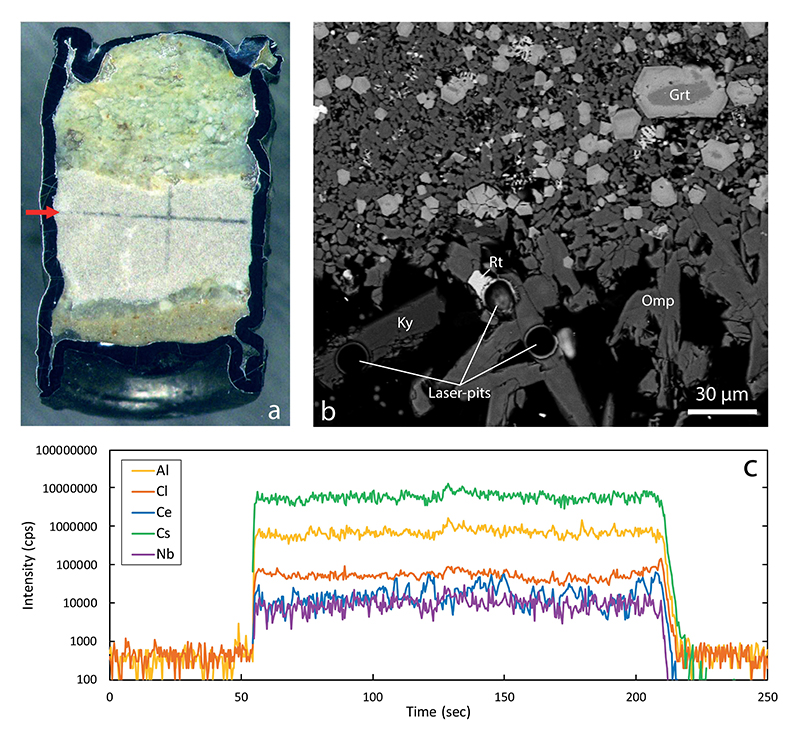
Figure 1 Run products from high pressure experiments. (a) Cross section of a sample capsule after an experiment (image width 5 mm). A white layer of diamond powder is sandwiched between the silicate sample. The red arrow points to a laser ablation trace. (b) Backscatter electron image of the silicate part of a sample, consisting mostly of omphacite (Omp) and garnet (Grt) with minor kyanite (Ky) and rutile (Rt). In the centre of some garnet crystals, remnants of the garnet seeds are visible. (c) Laser ablation analysis of frozen fluid in the diamond trap, demonstrating the homogeneity of the sample.
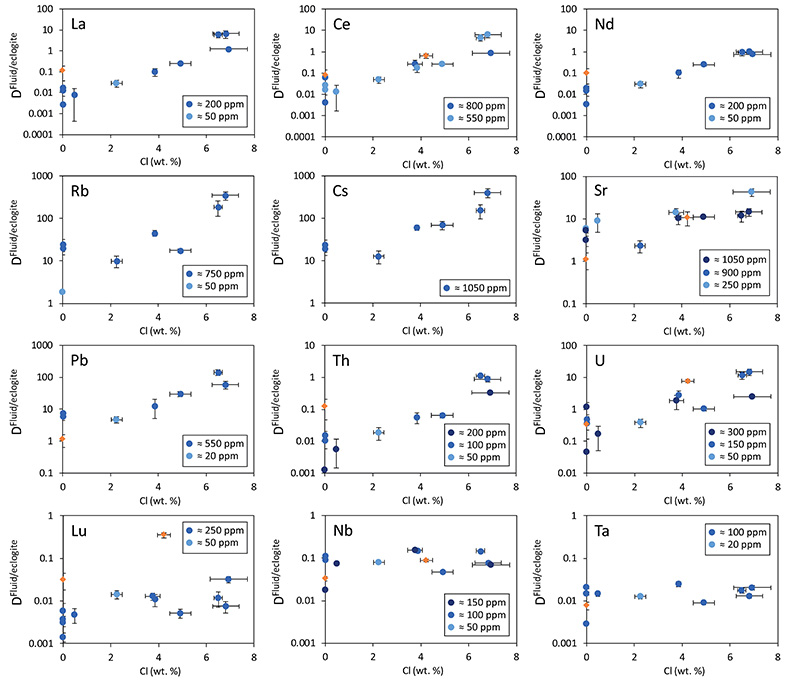
Figure 2 Effect of chloride on fluid/eclogite partition coefficients of trace elements at 4 GPa and 800 ˚C. Blue data points are the results from “forward” experiments, where the trace elements were initially doped into the solid starting material, while orange data points are from “reversed” experiments, which started with all trace elements dissolved in the fluid. For the forward experiments, results for different initial trace element concentrations in the starting material are given. Error bars are one standard deviation. Data for these and additional elements are given in Tables S-1 to S-8.
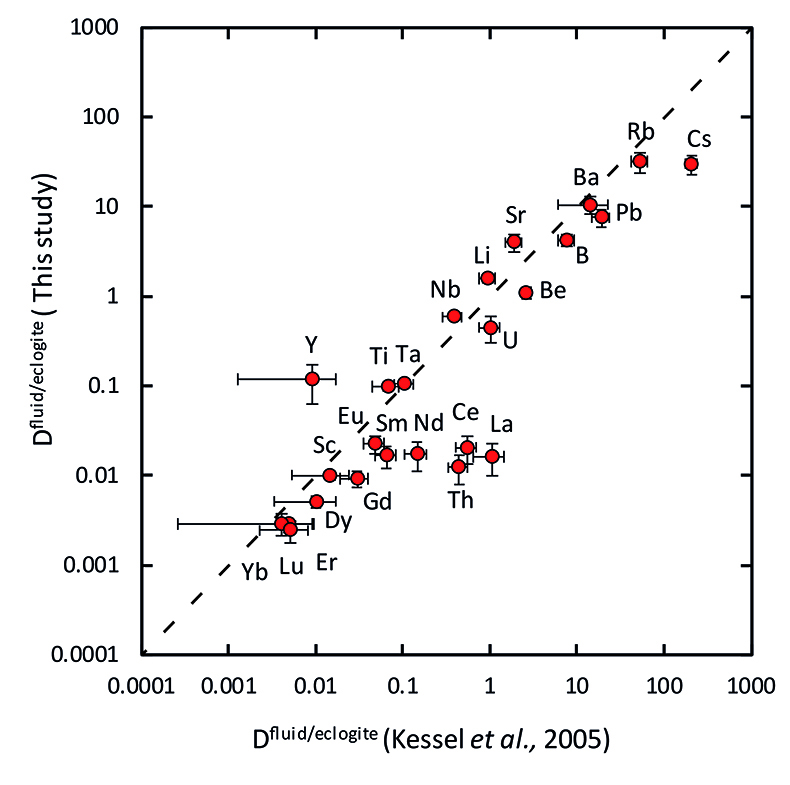
Figure 3 Comparison of the fluid/eclogite partition coefficients for Cl-free fluids measured in this study with those reported by Kessel et al. (2005)
Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727.
. Both sets of experiments were carried out at 4 GPa and 800 ˚C, with a bulk composition resembling MORB.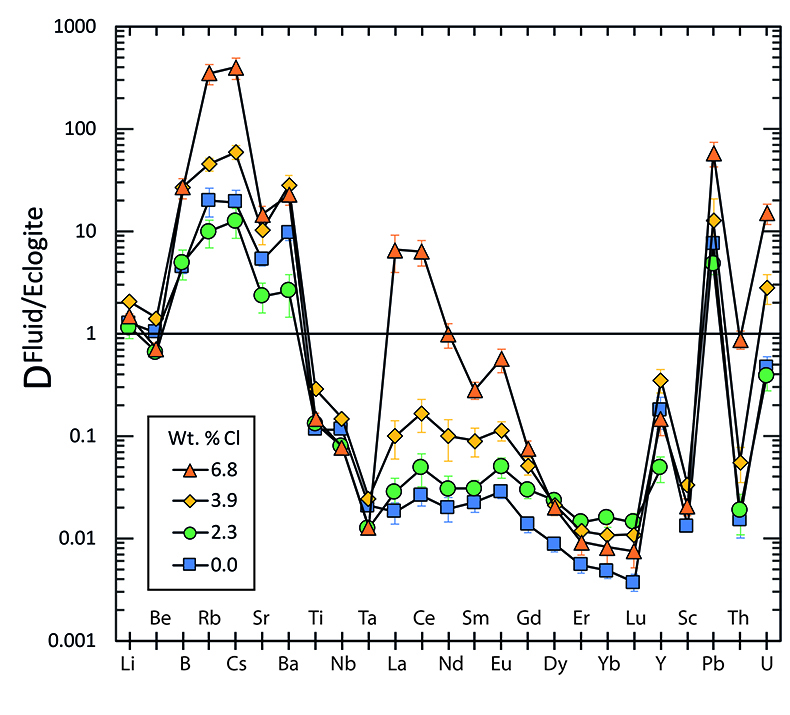
Figure 4 Trace element enrichment patterns in fluids from fluid/eclogite partitioning experiments at 4 GPa and 800 ˚C. Error bars are one standard deviation. Data for these and additional elements are given in Tables S-1 to S-8.