Co-reduction of Fe(III) and S0 drives Fe-S biomineral formation and phosphate mobilisation
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:492Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Figures
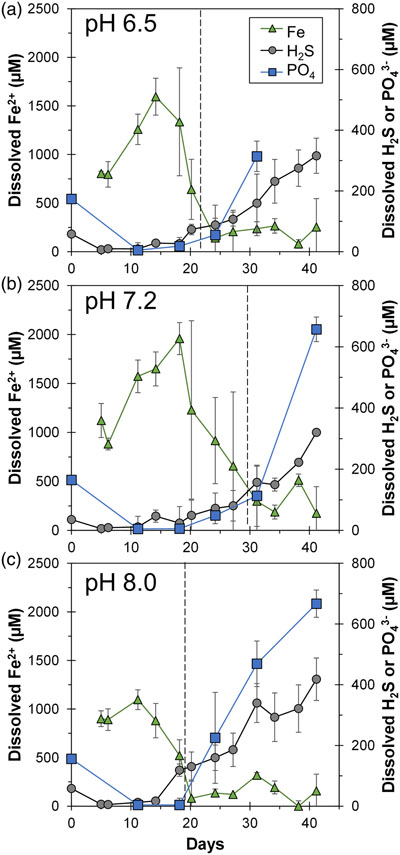 Figure 1 Geochemical evolution of dissolved Fe2+, total sulfide and phosphate at (a) pH 6.5, (b) pH 7.2, and (c) pH 8.0. Dashed vertical lines denote the shift from ferruginous to sulfidic conditions. |  Figure 2 SEM micrographs of microbe-mineral associations. (a) S0 aggregates consisting of ∼20 μm globules and Fe mineral aggregates consisting of nanometre-sized structures. (b) G. sulfurreducens atop a S0 globule. Note the close spatial association with aggregates of Fe-O minerals and FeS-rich minerals with pseudo-honeycomb structure. (c) A naturally fractured sample highlighting the FeS-rich coating on a S0 globule. (d) The FeS-rich coating appeared to peel off, revealing the smoother surface texture of S0 compared to FeS. (e) Typical morphology of Fe-P-O-rich minerals (vivianite). (f) A higher magnification of the radial blade-like morphology of vivianite (from the boxed region in (e)). |  Figure 3 (a) XRD patterns of precipitates at Days (D) 21 and 42. Vertical lines denote 2θ positions specific to certain minerals, with colours corresponding to vivianite (‘V’, light blue), mackinawite (‘M’, black), S0 (‘S’, yellow) and greigite (‘G’, grey). (b) Solid phase Fe distribution based on Mössbauer spectroscopy at 77 K. |  Figure 4 Summary of biogeochemical processes in the cultures. (1) Adsorption of phosphate from growth medium onto ferrihydrite (Fh). (2) Concurrent reduction of Fh and S0 by G. sulfurreducens. (3) Sulfide mediated reduction of Fh. (4) Precipitation of vivianite, mackinawite and greigite. (5) Dissolution of vivianite by H2S, releasing phosphate into solution. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Microbially mediated Fe and S cycling are vital parts of Earth’s history that affected the oceanic transition between ferruginous and sulfidic conditions, as well as playing an integral role in modern biogeochemical cycles of greenhouse gases, nutrients and contaminants (Lepot, 2020
Lepot, K. (2020) Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Science Reviews 209, 103296. https://doi.org/10.1016/j.earscirev.2020.103296
; Kappler et al., 2021Kappler, A., Bryce, C., Mansor, M., Lueder, U., Byrne, J.M., Swanner, E.D. (2021) An evolving view on biogeochemical cycling of iron. Nature Reviews Microbiology 19, 360–374. https://doi.org/10.1038/s41579-020-00502-7
). Many species, including model organisms Geobacter and Shewanella, are capable of linking the Fe and S cycles through the reduction of Fe(III) minerals and elemental sulfur (S0). This metabolic flexibility enables them to survive under alkaline conditions when Fe(III) reduction becomes thermodynamically unfeasible (Flynn et al., 2014Flynn, T.M., O’Loughlin, E.J., Mishra, B., DiChristina, T.J., Kemner, K.M. (2014) Sulfur-mediated electron shuttling during bacterial iron reduction. Science 344, 1039–1042. https://doi.org/10.1126/science.1252066
), resulting in Fe sulfide biomineral formation (Wang et al., 2018Wang, X.-N., Sun, G.-X., Li, X.-M., Clarke, T.A., Zhu, Y.-G. (2018) Electron shuttle-mediated microbial Fe(III) reduction under alkaline conditions. Journal of Soils and Sediments 18, 159–168. https://doi.org/10.1007/s11368-017-1736-y
; Nie et al., 2020Nie, Z., Wang, N., Xia, X., Xia, J., Liu, H., Zhou, Y., Deng, Y., Xue, Z. (2020) Biogenic FeS promotes dechlorination and thus de-cytotoxity of trichloroethylene. Bioprocess and Biosystems Engineering 43, 1791–1800. https://doi.org/10.1007/s00449-020-02369-7
; Ye and Jing, 2022Ye, L., Jing, C. (2022) Iron(III) reducing bacteria immobilise antimonite by respiring elemental sulfur. Geochemical Perspectives Letters 21, 37–41. https://doi.org/10.7185/geochemlet.2215
; Liu et al., 2023Liu, Y., Zhao, Q., Liao, C., Tian, L., Yan, X., Li, N., Wang, X. (2023) Anaerobic bioreduction of elemental sulfur improves bioavailability of Fe(III) oxides for bioremediation. Science of the Total Environment 858, 159794. https://doi.org/10.1016/j.scitotenv.2022.159794
). Investigations of coupled reduction of Fe(III) and S0 are important for mineral biosignatures and their impact on nutrient bioavailability, especially in environments where S0 could be an important electron acceptor such as in microbial mats and sulfate-poor Archean oceans (Troelsen and Jørgensen, 1982Troelsen, H., Jørgensen, B.B. (1982) Seasonal dynamics of elemental sulfur in two coastal sediments. Estuarine, Coastal and Shelf Science 15, 255–266. https://doi.org/10.1016/0272-7714(82)90062-2
; van Gemerden et al., 1989van Gemerden, H., Tughan, C.S., de Wit, R., Herbert, R.A. (1989) Laminated microbial ecosystems on sheltered beaches in Scapa Flow, Orkney Islands. FEMS Microbiology Ecology 5, 87–101. https://doi.org/10.1111/j.1574-6968.1989.tb03661.x
; Philippot et al., 2007Philippot, P., Van Zuilen, M., Lepot, K., Thomazo, C., Farquhar, J., Van Kranendonk, M.J. (2007) Early Archaean Microorganisms Preferred Elemental Sulfur, Not Sulfate. Science 317, 1534–1537. https://doi.org/10.1126/science.1145861
; Galić et al., 2017Galić, A., Mason, P.R.D., Mogollón, J.M., Wolthers, M., Vroon, P.Z., Whitehouse, M.J. (2017) Pyrite in a sulfate-poor Paleoarchean basin was derived predominantly from elemental sulfur: Evidence from 3.2 Ga sediments in the Barberton Greenstone Belt, Kaapvaal Craton. Chemical Geology 449, 135–146. https://doi.org/10.1016/j.chemgeo.2016.12.006
). The mobilisation and bioavailability of phosphate in particular is affected by Fe and S biogeochemistry with direct consequences to primary productivity, climate and Earth’s redox evolution (Alcott et al., 2022Alcott, L.J., Mills, B.J.W., Bekker, A., Poulton, S.W. (2022) Earth’s Great Oxidation Event facilitated by the rise of sedimentary phosphorus recycling. Nature Geoscience 15, 210–215. https://doi.org/10.1038/s41561-022-00906-5
).top
Microbial Metabolism Drives Geochemical Shifts
To investigate biomineral formation during co-reduction of Fe(III) and S0, G. sulfurreducens was incubated in the co-presence of ferrihydrite (30 mM) and S0 (60 mM) with acetate as excess electron donor. Different initial pH values (6.5, 7.2 or 8.0) were employed. Over 42 days of incubation, all cultures exhibited a colour change from dark brown to black, indicating transformation of ferrihydrite to reduced Fe minerals (Fig. S-1). From initial pH values of 6.5 and 7.2, pH increased to 7.1 and 7.5, respectively. At initial pH of 8.0, the pH slightly decreased to 7.9. From here onward, the cultures will be referred to by their initial pH values for simplicity.
At the beginning, solid phase extractable Fe (6 M HCl) amounted to 29.1 ± 2.2 mM (n = 12) with no Fe(II) components detected. Within 15 days, the Fe(II)/Fe(III) percentages of the HCl extractable Fe approached 100 %, indicating Fe(III) reduction (Fig. S-2). Note that the total HCl extractable Fe decreased over time, and we attributed this primarily to sampling inhomogeneity due to mineral aggregation. Fe(III) reduction was accompanied by the release of Fe2+ into solution, reaching maxima of 1000–2000 μM depending on initial pH (Fig. 1). Dissolved Fe2+ showed a decline to <500 μM within 12 days after the maximum. Once Fe2+ reached low levels, dissolved sulfide started to increase, reaching maxima of 320–420 μM. Solid phase Fe sulfides were not quantified but were detectable by scanning electron microscopy (SEM), X-ray diffraction (XRD) and Mössbauer spectroscopy (Figs. 2, 3).

Figure 1 Geochemical evolution of dissolved Fe2+, total sulfide and phosphate at (a) pH 6.5, (b) pH 7.2, and (c) pH 8.0. Dashed vertical lines denote the shift from ferruginous to sulfidic conditions.

Figure 2 SEM micrographs of microbe-mineral associations. (a) S0 aggregates consisting of ∼20 μm globules and Fe mineral aggregates consisting of nanometre-sized structures. (b) G. sulfurreducens atop a S0 globule. Note the close spatial association with aggregates of Fe-O minerals and FeS-rich minerals with pseudo-honeycomb structure. (c) A naturally fractured sample highlighting the FeS-rich coating on a S0 globule. (d) The FeS-rich coating appeared to peel off, revealing the smoother surface texture of S0 compared to FeS. (e) Typical morphology of Fe-P-O-rich minerals (vivianite). (f) A higher magnification of the radial blade-like morphology of vivianite (from the boxed region in (e)).

Figure 3 (a) XRD patterns of precipitates at Days (D) 21 and 42. Vertical lines denote 2θ positions specific to certain minerals, with colours corresponding to vivianite (‘V’, light blue), mackinawite (‘M’, black), S0 (‘S’, yellow) and greigite (‘G’, grey). (b) Solid phase Fe distribution based on Mössbauer spectroscopy at 77 K.
Overall, cultures at different pH values exhibited similar geochemical trends with differences in timing and the amount of Fe2+ and sulfide released to the solution. Parallel abiotic controls showed neither Fe(II) nor sulfide production (Fig. S-2). The geochemical evolution can be divided into two stages. In the first stage, ferruginous conditions dominated as ferrihydrite was reduced and Fe2+ accumulated in solution. In the second stage, sulfidic conditions were observed once reactive Fe(III) surfaces were exhausted and sulfide accumulated in solution. The time at which the shift from ferruginous to sulfidic conditions occurred differed depending on the pH values, following the order: pH 7.2 (30 days) > pH 6.5 (22 days) > pH 8.0 (19 days). Mirroring this trend, the highest maximum Fe2+ followed the order: pH 7.2 (2000 μM) > pH 6.5 (1500 μM) > pH 8.0 (1100 μM). Maximum dissolved sulfide followed the opposite trend: pH 7.2 ≈ pH 6.5 (320 μM) < pH 8.0 (420 μM).
The different timings and concentrations of Fe2+ and sulfide observed depend on a number of interrelated pH dependent processes including microbial preference of Fe(III) over S0, Fe2+ adsorption to ferrihydrite, sulfide mediated ferrihydrite reduction and Fe mineral precipitation. These processes can lead to secondary phenomena such as Fe2+ catalysed recrystallisation, decreased reactivity from the FeS surface coating, and formation of polysulfides (Peiffer et al., 2015
Peiffer, S., Behrends, T., Hellige, K., Larese-Casanova, P., Wan, M., Pollok, K. (2015) Pyrite formation and mineral transformation pathways upon sulfidation of ferric hydroxides depend on mineral type and sulfide concentration. Chemical Geology 400, 44–55. https://doi.org/10.1016/j.chemgeo.2015.01.023
). Notably, the observed geochemical trends do not follow straightforward predictions based on initial pH (Supplementary Information). Further extensive investigation at the nanometre (e.g., transmission electron microscopy) and molecular (e.g., speciation via synchrotron) scales, coupled to a biogeochemical model will be needed to elucidate the specific mechanisms.top
Formation of Biogenic Mackinawite and Greigite, but not Pyrite
Mineral products were identified using a combination of magnetic testing, XRD, SEM and Mössbauer spectroscopy. XRD patterns of samples at day 21 and 42 indicated the presence of S0, which decreased over time at all pH values, signifying continuous microbial S0 reduction (Fig. 3a). Additionally, XRD and Mössbauer identified mackinawite (FeS) as a major product (20–70 % of solid phase Fe). Black colourations typical of Fe sulfides were commonly observed to form coatings on S0 (Fig. 2c, d).
We report the first instance of greigite (Fe3S4) formation by Geobacter. The presence of a magnetic mineral was first suggested via attraction of the minerals to a hand magnet held next to the bottles. Strong magnetism was observed at all pH values starting from day 11 (Fig. S-1) but decreased noticeably after 27 days (Supplementary Information). Greigite was identified by Mössbauer spectroscopy, making up 17–53 % of solid phase Fe with the highest percentage at pH 7.2. XRD data showed clear patterns for greigite only at pH 6.5 at day 21. Positive identification in other cultures was complicated due to the overlap of the main greigite signal with mackinawite at ∼35° 2θ and the generally broad patterns resulting from low crystallinity. Consistent with this interpretation, crystalline greigite particles were not observed by SEM. Other minerals such as magnetite (Fe3O4) and pyrite were not detectable within 21–42 days of incubation (Supplementary Information).
The mechanism and kinetics of biogenic Fe sulfide mineral transformation are important to understand with respect to their implications for biosignatures and elemental cycling (Picard et al., 2016
Picard, A., Gartman, A., Girguis, P.R. (2016) What Do We Really Know about the Role of Microorganisms in Iron Sulfide Mineral Formation? Frontiers in Earth Science 4, 68. https://doi.org/10.3389/feart.2016.00068
; Nie et al., 2020Nie, Z., Wang, N., Xia, X., Xia, J., Liu, H., Zhou, Y., Deng, Y., Xue, Z. (2020) Biogenic FeS promotes dechlorination and thus de-cytotoxity of trichloroethylene. Bioprocess and Biosystems Engineering 43, 1791–1800. https://doi.org/10.1007/s00449-020-02369-7
). Poorly-crystalline mackinawite typically forms first from the reaction between Fe2+ and sulfide at low temperatures, followed by its transformation to greigite and pyrite over time (Son et al., 2022Son, S., Hyun, S.P., Charlet, L., Kwon, K.D. (2022) Thermodynamic stability reversal of iron sulfides at the nanoscale: Insights into the iron sulfide formation in low-temperature aqueous solution. Geochimica et Cosmochimica Acta 338, 220–228. https://doi.org/10.1016/j.gca.2022.10.021
). In our cultures, the XRD patterns for mackinawite were most pronounced at pH 7.2, and inspection of the main signal at ∼18° 2θ showed that it became broader over time at all pH values, suggesting an overall decrease in bulk crystallinity. This can be understood in terms of different populations of mackinawite that formed under ferruginous versus sulfidic conditions. Mackinawite formed in excess Fe (ferruginous conditions) tends to exhibit higher crystallinity and sharper diffraction patterns than that formed in excess S (sulfidic conditions) (Bourdoiseau et al., 2008Bourdoiseau, J.-A., Jeannin, M., Sabot, R., Rémazeilles, C., Refait, P. (2008) Characterisation of mackinawite by Raman spectroscopy: Effects of crystallisation, drying and oxidation. Corrosion Science 50, 3247–3255. https://doi.org/10.1016/j.corsci.2008.08.041
). This is consistent with the timing of the stages in our cultures. The pH 7.2 cultures had the longest ferruginous stage that encompassed the period at which the first XRD samples were collected (21 days); therefore, crystalline mackinawite is expected. With longer incubation, the cultures shifted to sulfidic conditions, promoting additional mackinawite formation. However, this mackinawite exhibited lower crystallinity and contributed to broader XRD patterns reflective of the bulk mixture.Greigite is increasingly recognised as a common and stable phase in nature and as an important intermediate for pyrite formation (Subramani et al., 2020
Subramani, T., Lilova, K., Abramchuk, M., Leinenweber, K.D., Navrotsky, A. (2020) Greigite (Fe3S4) is thermodynamically stable : Implications for its terrestrial and planetary occurrence. Proceedings of the National Academy of Sciences 117, 28645–28648. https://doi.org/10.1073/pnas.2017312117
). We found the highest percentage of greigite at the intermediate pH of 7.2 (53 % of solid phase Fe). This is in contrast with the expectation that lower pH, including around the microenvironments of sulfate reducing bacteria, could promote mackinawite’s transformation to greigite (Bourdoiseau et al., 2011Bourdoiseau, J.-A., Jeannin, M., Rémazeilles, C., Sabot, R., Refait, P. (2011) The transformation of mackinawite into greigite studied by Raman spectroscopy. Journal of Raman Spectroscopy 42, 496–504. https://doi.org/10.1002/jrs.2729
; Mansor et al., 2019Mansor, M., Berti, D., Hochella Jr., M.F., Murayama, M., Xu, J. (2019) Phase, morphology, elemental composition and formation mechanisms of biogenic and abiogenic Fe-Cu-sulfide nanoparticles: A comparative study on their occurrences under anoxic conditions. American Mineralogist 104, 703–717. https://doi.org/10.2138/am-2019-6848
). Instead of pH, we suggest that the crystallinity of the precursor mackinawite is the main controlling factor of transformation kinetics (Csákberényi-Malasics et al., 2012Csákberényi-Malasics, D., Rodriguez-Blanco, J.D., Kis, V.K., Rečnik, A., Benning, L.G., Pósfai, M. (2012) Structural properties and transformations of precipitated FeS. Chemical Geology 294–295, 249–258. https://doi.org/10.1016/j.chemgeo.2011.12.009
; Miller et al., 2020Miller, N., Dougherty, M., Du, R., Sauers, T., Yan, C., et al. (2020) Adsorption of Tetrathiomolybdate to Iron Sulfides and Its Impact on Iron Sulfide Transformations. ACS Earth and Space Chemistry 4, 2246–2260. https://doi.org/10.1021/acsearthspacechem.0c00176
). As discussed, we observed mackinawite with the highest crystallinity at pH 7.2. Our data showed that precipitation under ferruginous conditions enhances mackinawite’s crystallinity and its transformation to greigite, confirming recent predictions from density functional theory (Son et al., 2022Son, S., Hyun, S.P., Charlet, L., Kwon, K.D. (2022) Thermodynamic stability reversal of iron sulfides at the nanoscale: Insights into the iron sulfide formation in low-temperature aqueous solution. Geochimica et Cosmochimica Acta 338, 220–228. https://doi.org/10.1016/j.gca.2022.10.021
).Pyrite, the most common Fe sulfide mineral in the environment, was not formed in our cultures within 42 days, similar to in pure cultures of sulfate reducing bacteria (Picard et al., 2016
Picard, A., Gartman, A., Girguis, P.R. (2016) What Do We Really Know about the Role of Microorganisms in Iron Sulfide Mineral Formation? Frontiers in Earth Science 4, 68. https://doi.org/10.3389/feart.2016.00068
). Nonetheless, studies with sulfur cycling bacteria have demonstrated pyrite formation from sulfidation of Fe(III) phosphates within one month (Berg et al., 2020Berg, J.S., Duverger, A., Cordier, L., Laberty-Robert, C., Guyot, F., Miot, J. (2020) Rapid pyritization in the presence of a sulfur/sulfate-reducing bacterial consortium. Scientific Reports 10, 8264. https://doi.org/10.1038/s41598-020-64990-6
; Duverger et al., 2020Duverger, A., Berg, J.S., Busigny, V., Guyot, F., Bernard, S., Miot, J. (2020) Mechanisms of Pyrite Formation Promoted by Sulfate-Reducing Bacteria in Pure Culture. Frontiers in Earth Science 8, 588310. https://doi.org/10.3389/feart.2020.588310
). Pyrite precipitation was attributed to microbial production of extracellular polymeric substances (EPS) that concentrated key ingredients for pyrite formation locally and the continuous formation of Fe2+, S0 and polysulfides from slow sulfide mediated Fe(III) dissolution. G. sulfurreducens is also known to produce EPS that can bind cations, especially in the presence of Fe(III) minerals (Stöckl et al., 2019Stöckl, M., Teubner, N.C., Holtmann, D., Mangold, K.-M., Sand, W. (2019) Extracellular Polymeric Substances from Geobacter sulfurreducens Biofilms in Microbial Fuel Cells. ACS Applied Materials and Interfaces 11, 8961–8968. https://doi.org/10.1021/acsami.8b14340
; Tomaszewski et al., 2020Tomaszewski, E.J., Olson, L., Obst, M., Byrne, J.M., Kappler, A., Muehe, E.M. (2020) Complexation by cysteine and iron mineral adsorption limit cadmium mobility during metabolic activity of Geobacter sulfurreducens. Environmental Science: Processes and Impacts 22, 1877–1887. https://doi.org/10.1039/D0EM00244E
). Furthermore, the co-presence of Fe(III) and S0 should have led to high polysulfide concentrations that enhance pyrite formation. However, it is possible that rapid reduction of Fe(III) and S0 (and potentially polysulfides) by G. sulfurreducens may have prevented the accumulation of intermediates necessary for fast pyrite formation. Further kinetic based studies and comparison with a pyrite forming culture will be necessary to elucidate factors controlling pyrite formation (Supplementary Information).top
Microbial Fe-S Metabolism leads to Phosphate (Im)Mobilisation
Besides Fe sulfides, XRD and Mössbauer spectroscopy revealed the presence of vivianite [Fe3(PO4)2 · 8 H2O] at all pH values, making up 7–28 % of solid phase Fe (Fig. 3). The formed vivianite had radial blade-like structures up to 50 μm in length (Fig. 2e, f). Vivianite formation is explained by the addition of phosphate to the medium, which resulted in P/Fe ratio of 0.15 in our experiments, comparable to the ∼0.10 ratio typical in nature (Kraal et al., 2022
Kraal, P., van Genuchten, C.M., Behrends, T. (2022) Phosphate coprecipitation affects reactivity of iron (oxyhydr)oxides towards dissolved iron and sulfide. Geochimica et Cosmochimica Acta 321, 311–328. https://doi.org/10.1016/j.gca.2021.12.032
).Despite the addition of 4.4 mM phosphate, initial dissolved phosphate was <200 μM (Fig. 1). We attributed this to strong phosphate adsorption to ferrihydrite (Wang et al., 2013
Wang, X., Liu, F., Tan, W., Li, W., Feng, X., Sparks, D.L. (2013) Characteristics of Phosphate Adsorption-Desorption Onto Ferrihydrite: Comparison With Well-Crystalline Fe (Hydr)Oxides. Soil Science 178, 1–11. https://doi.org/10.1097/SS.0b013e31828683f8
; Kraal et al., 2022Kraal, P., van Genuchten, C.M., Behrends, T. (2022) Phosphate coprecipitation affects reactivity of iron (oxyhydr)oxides towards dissolved iron and sulfide. Geochimica et Cosmochimica Acta 321, 311–328. https://doi.org/10.1016/j.gca.2021.12.032
). The concentrations dropped within a few days to near detection limit, coincident with the rise in Fe2+, attributed to vivianite formation. Dissolved phosphate then showed a marked increase coincident with sulfide release to solution.The sequence of biogeochemical processes in the experiments can be summarised as follows (Fig. 4). First, phosphate from the growth medium was rapidly adsorbed to ferrihydrite. G. sulfurreducens reduced ferrihydrite and S0 in the presence of excess electron donor, with more S0 reduction at pH 8.0 compared to lower pH values. In the early ferruginous stage, sulfide concentration was kept low as it reacted rapidly with ferrihydrite, contributing to Fe(III) reduction, Fe2+ release to solution, and the precipitation of mackinawite, greigite and vivianite. The cultures progressed into the late sulfidic stage as reactive Fe(III) became fully exhausted. In addition to further Fe sulfide formation, this biogeochemical switch resulted in vivianite dissolution, releasing phosphate into solution. This supports previous observations in which the switch from ferruginous to sulfidic conditions has been recognised to increase the bioavailability of phosphate (Duverger et al., 2020
Duverger, A., Berg, J.S., Busigny, V., Guyot, F., Bernard, S., Miot, J. (2020) Mechanisms of Pyrite Formation Promoted by Sulfate-Reducing Bacteria in Pure Culture. Frontiers in Earth Science 8, 588310. https://doi.org/10.3389/feart.2020.588310
; Alcott et al., 2022Alcott, L.J., Mills, B.J.W., Bekker, A., Poulton, S.W. (2022) Earth’s Great Oxidation Event facilitated by the rise of sedimentary phosphorus recycling. Nature Geoscience 15, 210–215. https://doi.org/10.1038/s41561-022-00906-5
).
Figure 4 Summary of biogeochemical processes in the cultures. (1) Adsorption of phosphate from growth medium onto ferrihydrite (Fh). (2) Concurrent reduction of Fh and S0 by G. sulfurreducens. (3) Sulfide mediated reduction of Fh. (4) Precipitation of vivianite, mackinawite and greigite. (5) Dissolution of vivianite by H2S, releasing phosphate into solution.
Overall, our study demonstrated that co-reduction of Fe(III) and S0 leads to formation of mackinawite, greigite and vivianite, but not pyrite within 42 days. Initial pH affected the length and timing of the ferruginous-sulfide transition, resulting in differences in the crystallinity and relative abundance of the mineral products. The transition from ferruginous to sulfidic conditions was associated with phosphate release. Similar processes, driven by the activity of whole microbial communities instead of a single species, are likely important to consider for micro-niches in modern sediments and microbial mats. These processes are further applicable to SO4-poor Archean oceans and ultimately the investigation of Fe-S biomineral signatures and the bioavailability of important nutrients that affected primary productivity and Earth’s biogeochemical evolution.
top
Acknowledgements
This study was supported by the DFG (SPP 1833, Emmy Noether Programme, 1450/3-1, DU 1450/3-2, DU 1450/7-1, JPD; INST 37/1027-1 FUGG, AK) as well as the Excellence Strategy of the German Federal and State Governments (EXC2124, 390838134; Tuebingen Structural Microscopy Core Facility; AK, MM, PJ, SF, JS). We thank Prof. D.J. Lunter and Yali Liu for assistance with Raman analysis.
Editor: Juan Liu
top
References
Alcott, L.J., Mills, B.J.W., Bekker, A., Poulton, S.W. (2022) Earth’s Great Oxidation Event facilitated by the rise of sedimentary phosphorus recycling. Nature Geoscience 15, 210–215. https://doi.org/10.1038/s41561-022-00906-5
 Show in context
Show in context The mobilisation and bioavailability of phosphate in particular is affected by Fe and S biogeochemistry with direct consequences to primary productivity, climate and Earth’s redox evolution (Alcott et al., 2022).
View in article
This supports previous observations in which the switch from ferruginous to sulfidic conditions has been recognised to increase the bioavailability of phosphate (Duverger et al., 2020; Alcott et al., 2022).
View in article
Berg, J.S., Duverger, A., Cordier, L., Laberty-Robert, C., Guyot, F., Miot, J. (2020) Rapid pyritization in the presence of a sulfur/sulfate-reducing bacterial consortium. Scientific Reports 10, 8264. https://doi.org/10.1038/s41598-020-64990-6
 Show in context
Show in context Nonetheless, studies with sulfur cycling bacteria have demonstrated pyrite formation from sulfidation of Fe(III) phosphates within one month (Berg et al., 2020; Duverger et al., 2020).
View in article
Bourdoiseau, J.-A., Jeannin, M., Sabot, R., Rémazeilles, C., Refait, P. (2008) Characterisation of mackinawite by Raman spectroscopy: Effects of crystallisation, drying and oxidation. Corrosion Science 50, 3247–3255. https://doi.org/10.1016/j.corsci.2008.08.041
 Show in context
Show in context Mackinawite formed in excess Fe (ferruginous conditions) tends to exhibit higher crystallinity and sharper diffraction patterns than that formed in excess S (sulfidic conditions) (Bourdoiseau et al., 2008).
View in article
Bourdoiseau, J.-A., Jeannin, M., Rémazeilles, C., Sabot, R., Refait, P. (2011) The transformation of mackinawite into greigite studied by Raman spectroscopy. Journal of Raman Spectroscopy 42, 496–504. https://doi.org/10.1002/jrs.2729
 Show in context
Show in context This is in contrast with the expectation that lower pH, including around the microenvironments of sulfate reducing bacteria, could promote mackinawite’s transformation to greigite (Bourdoiseau et al., 2011; Mansor et al., 2019).
View in article
Csákberényi-Malasics, D., Rodriguez-Blanco, J.D., Kis, V.K., Rečnik, A., Benning, L.G., Pósfai, M. (2012) Structural properties and transformations of precipitated FeS. Chemical Geology 294–295, 249–258. https://doi.org/10.1016/j.chemgeo.2011.12.009
 Show in context
Show in context Instead of pH, we suggest that the crystallinity of the precursor mackinawite is the main controlling factor of transformation kinetics (Csákberényi-Malasics et al., 2012; Miller et al., 2020).
View in article
Duverger, A., Berg, J.S., Busigny, V., Guyot, F., Bernard, S., Miot, J. (2020) Mechanisms of Pyrite Formation Promoted by Sulfate-Reducing Bacteria in Pure Culture. Frontiers in Earth Science 8, 588310. https://doi.org/10.3389/feart.2020.588310
 Show in context
Show in context Nonetheless, studies with sulfur cycling bacteria have demonstrated pyrite formation from sulfidation of Fe(III) phosphates within one month (Berg et al., 2020; Duverger et al., 2020).
View in article
This supports previous observations in which the switch from ferruginous to sulfidic conditions has been recognised to increase the bioavailability of phosphate (Duverger et al., 2020; Alcott et al., 2022).
View in article
Flynn, T.M., O’Loughlin, E.J., Mishra, B., DiChristina, T.J., Kemner, K.M. (2014) Sulfur-mediated electron shuttling during bacterial iron reduction. Science 344, 1039–1042. https://doi.org/10.1126/science.1252066
 Show in context
Show in context This metabolic flexibility enables them to survive under alkaline conditions when Fe(III) reduction becomes thermodynamically unfeasible (Flynn et al., 2014), resulting in Fe sulfide biomineral formation (Wang et al., 2018; Nie et al., 2020; Ye and Jing, 2022; Liu et al., 2023).
View in article
Galić, A., Mason, P.R.D., Mogollón, J.M., Wolthers, M., Vroon, P.Z., Whitehouse, M.J. (2017) Pyrite in a sulfate-poor Paleoarchean basin was derived predominantly from elemental sulfur: Evidence from 3.2 Ga sediments in the Barberton Greenstone Belt, Kaapvaal Craton. Chemical Geology 449, 135–146. https://doi.org/10.1016/j.chemgeo.2016.12.006
 Show in context
Show in context Investigations of coupled reduction of Fe(III) and S0 are important for mineral biosignatures and their impact on nutrient bioavailability, especially in environments where S0 could be an important electron acceptor such as in microbial mats and sulfate-poor Archean oceans (Troelsen and Jørgensen, 1982; van Gemerden et al., 1989; Philippot et al., 2007; Galić et al., 2017).
View in article
Kappler, A., Bryce, C., Mansor, M., Lueder, U., Byrne, J.M., Swanner, E.D. (2021) An evolving view on biogeochemical cycling of iron. Nature Reviews Microbiology 19, 360–374. https://doi.org/10.1038/s41579-020-00502-7
 Show in context
Show in context Microbially mediated Fe and S cycling are vital parts of Earth’s history that affected the oceanic transition between ferruginous and sulfidic conditions, as well as playing an integral role in modern biogeochemical cycles of greenhouse gases, nutrients and contaminants (Lepot, 2020; Kappler et al., 2021).
View in article
Kraal, P., van Genuchten, C.M., Behrends, T. (2022) Phosphate coprecipitation affects reactivity of iron (oxyhydr)oxides towards dissolved iron and sulfide. Geochimica et Cosmochimica Acta 321, 311–328. https://doi.org/10.1016/j.gca.2021.12.032
 Show in context
Show in context Vivianite formation is explained by the addition of phosphate to the medium, which resulted in P/Fe ratio of 0.15 in our experiments, comparable to the ∼0.10 ratio typical in nature (Kraal et al., 2022).
View in article
We attributed this to strong phosphate adsorption to ferrihydrite (Wang et al., 2013; Kraal et al., 2022).
View in article
Lepot, K. (2020) Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Science Reviews 209, 103296. https://doi.org/10.1016/j.earscirev.2020.103296
 Show in context
Show in context Microbially mediated Fe and S cycling are vital parts of Earth’s history that affected the oceanic transition between ferruginous and sulfidic conditions, as well as playing an integral role in modern biogeochemical cycles of greenhouse gases, nutrients and contaminants (Lepot, 2020; Kappler et al., 2021).
View in article
Liu, Y., Zhao, Q., Liao, C., Tian, L., Yan, X., Li, N., Wang, X. (2023) Anaerobic bioreduction of elemental sulfur improves bioavailability of Fe(III) oxides for bioremediation. Science of the Total Environment 858, 159794. https://doi.org/10.1016/j.scitotenv.2022.159794
 Show in context
Show in context This metabolic flexibility enables them to survive under alkaline conditions when Fe(III) reduction becomes thermodynamically unfeasible (Flynn et al., 2014), resulting in Fe sulfide biomineral formation (Wang et al., 2018; Nie et al., 2020; Ye and Jing, 2022; Liu et al., 2023).
View in article
Mansor, M., Berti, D., Hochella Jr., M.F., Murayama, M., Xu, J. (2019) Phase, morphology, elemental composition and formation mechanisms of biogenic and abiogenic Fe-Cu-sulfide nanoparticles: A comparative study on their occurrences under anoxic conditions. American Mineralogist 104, 703–717. https://doi.org/10.2138/am-2019-6848
 Show in context
Show in context This is in contrast with the expectation that lower pH, including around the microenvironments of sulfate reducing bacteria, could promote mackinawite’s transformation to greigite (Bourdoiseau et al., 2011; Mansor et al., 2019).
View in article
Miller, N., Dougherty, M., Du, R., Sauers, T., Yan, C., et al. (2020) Adsorption of Tetrathiomolybdate to Iron Sulfides and Its Impact on Iron Sulfide Transformations. ACS Earth and Space Chemistry 4, 2246–2260. https://doi.org/10.1021/acsearthspacechem.0c00176
 Show in context
Show in context Instead of pH, we suggest that the crystallinity of the precursor mackinawite is the main controlling factor of transformation kinetics (Csákberényi-Malasics et al., 2012; Miller et al., 2020).
View in article
Nie, Z., Wang, N., Xia, X., Xia, J., Liu, H., Zhou, Y., Deng, Y., Xue, Z. (2020) Biogenic FeS promotes dechlorination and thus de-cytotoxity of trichloroethylene. Bioprocess and Biosystems Engineering 43, 1791–1800. https://doi.org/10.1007/s00449-020-02369-7
 Show in context
Show in context This metabolic flexibility enables them to survive under alkaline conditions when Fe(III) reduction becomes thermodynamically unfeasible (Flynn et al., 2014), resulting in Fe sulfide biomineral formation (Wang et al., 2018; Nie et al., 2020; Ye and Jing, 2022; Liu et al., 2023).
View in article
The mechanism and kinetics of biogenic Fe sulfide mineral transformation are important to understand with respect to their implications for biosignatures and elemental cycling (Picard et al., 2016; Nie et al., 2020).
View in article
Peiffer, S., Behrends, T., Hellige, K., Larese-Casanova, P., Wan, M., Pollok, K. (2015) Pyrite formation and mineral transformation pathways upon sulfidation of ferric hydroxides depend on mineral type and sulfide concentration. Chemical Geology 400, 44–55. https://doi.org/10.1016/j.chemgeo.2015.01.023
 Show in context
Show in context These processes can lead to secondary phenomena such as Fe2+ catalysed recrystallisation, decreased reactivity from the FeS surface coating, and formation of polysulfides (Peiffer et al., 2015).
View in article
Philippot, P., Van Zuilen, M., Lepot, K., Thomazo, C., Farquhar, J., Van Kranendonk, M.J. (2007) Early Archaean Microorganisms Preferred Elemental Sulfur, Not Sulfate. Science 317, 1534–1537. https://doi.org/10.1126/science.1145861
 Show in context
Show in context Investigations of coupled reduction of Fe(III) and S0 are important for mineral biosignatures and their impact on nutrient bioavailability, especially in environments where S0 could be an important electron acceptor such as in microbial mats and sulfate-poor Archean oceans (Troelsen and Jørgensen, 1982; van Gemerden et al., 1989; Philippot et al., 2007; Galić et al., 2017).
View in article
Picard, A., Gartman, A., Girguis, P.R. (2016) What Do We Really Know about the Role of Microorganisms in Iron Sulfide Mineral Formation? Frontiers in Earth Science 4, 68. https://doi.org/10.3389/feart.2016.00068
 Show in context
Show in context The mechanism and kinetics of biogenic Fe sulfide mineral transformation are important to understand with respect to their implications for biosignatures and elemental cycling (Picard et al., 2016; Nie et al., 2020).
View in article
Pyrite, the most common Fe sulfide mineral in the environment, was not formed in our cultures within 42 days, similar to in pure cultures of sulfate reducing bacteria (Picard et al., 2016).
View in article
Son, S., Hyun, S.P., Charlet, L., Kwon, K.D. (2022) Thermodynamic stability reversal of iron sulfides at the nanoscale: Insights into the iron sulfide formation in low-temperature aqueous solution. Geochimica et Cosmochimica Acta 338, 220–228. https://doi.org/10.1016/j.gca.2022.10.021
 Show in context
Show in context Poorly-crystalline mackinawite typically forms first from the reaction between Fe2+ and sulfide at low temperatures, followed by its transformation to greigite and pyrite over time (Son et al., 2022).
View in article
Our data showed that precipitation under ferruginous conditions enhances mackinawite’s crystallinity and its transformation to greigite, confirming recent predictions from density functional theory (Son et al., 2022).
View in article
Stöckl, M., Teubner, N.C., Holtmann, D., Mangold, K.-M., Sand, W. (2019) Extracellular Polymeric Substances from Geobacter sulfurreducens Biofilms in Microbial Fuel Cells. ACS Applied Materials and Interfaces 11, 8961–8968. https://doi.org/10.1021/acsami.8b14340
 Show in context
Show in context G. sulfurreducens is also known to produce EPS that can bind cations, especially in the presence of Fe(III) minerals (Stöckl et al., 2019; Tomaszewski et al., 2020).
View in article
Subramani, T., Lilova, K., Abramchuk, M., Leinenweber, K.D., Navrotsky, A. (2020) Greigite (Fe3S4) is thermodynamically stable : Implications for its terrestrial and planetary occurrence. Proceedings of the National Academy of Sciences 117, 28645–28648. https://doi.org/10.1073/pnas.2017312117
 Show in context
Show in context Greigite is increasingly recognised as a common and stable phase in nature and as an important intermediate for pyrite formation (Subramani et al., 2020).
View in article
Tomaszewski, E.J., Olson, L., Obst, M., Byrne, J.M., Kappler, A., Muehe, E.M. (2020) Complexation by cysteine and iron mineral adsorption limit cadmium mobility during metabolic activity of Geobacter sulfurreducens. Environmental Science: Processes and Impacts 22, 1877–1887. https://doi.org/10.1039/D0EM00244E
 Show in context
Show in context G. sulfurreducens is also known to produce EPS that can bind cations, especially in the presence of Fe(III) minerals (Stöckl et al., 2019; Tomaszewski et al., 2020).
View in article
Troelsen, H., Jørgensen, B.B. (1982) Seasonal dynamics of elemental sulfur in two coastal sediments. Estuarine, Coastal and Shelf Science 15, 255–266. https://doi.org/10.1016/0272-7714(82)90062-2
 Show in context
Show in context Investigations of coupled reduction of Fe(III) and S0 are important for mineral biosignatures and their impact on nutrient bioavailability, especially in environments where S0 could be an important electron acceptor such as in microbial mats and sulfate-poor Archean oceans (Troelsen and Jørgensen, 1982; van Gemerden et al., 1989; Philippot et al., 2007; Galić et al., 2017).
View in article
van Gemerden, H., Tughan, C.S., de Wit, R., Herbert, R.A. (1989) Laminated microbial ecosystems on sheltered beaches in Scapa Flow, Orkney Islands. FEMS Microbiology Ecology 5, 87–101. https://doi.org/10.1111/j.1574-6968.1989.tb03661.x
 Show in context
Show in context Investigations of coupled reduction of Fe(III) and S0 are important for mineral biosignatures and their impact on nutrient bioavailability, especially in environments where S0 could be an important electron acceptor such as in microbial mats and sulfate-poor Archean oceans (Troelsen and Jørgensen, 1982; van Gemerden et al., 1989; Philippot et al., 2007; Galić et al., 2017).
View in article
Wang, X., Liu, F., Tan, W., Li, W., Feng, X., Sparks, D.L. (2013) Characteristics of Phosphate Adsorption-Desorption Onto Ferrihydrite: Comparison With Well-Crystalline Fe (Hydr)Oxides. Soil Science 178, 1–11. https://doi.org/10.1097/SS.0b013e31828683f8
 Show in context
Show in context We attributed this to strong phosphate adsorption to ferrihydrite (Wang et al., 2013; Kraal et al., 2022).
View in article
Wang, X.-N., Sun, G.-X., Li, X.-M., Clarke, T.A., Zhu, Y.-G. (2018) Electron shuttle-mediated microbial Fe(III) reduction under alkaline conditions. Journal of Soils and Sediments 18, 159–168. https://doi.org/10.1007/s11368-017-1736-y
 Show in context
Show in context This metabolic flexibility enables them to survive under alkaline conditions when Fe(III) reduction becomes thermodynamically unfeasible (Flynn et al., 2014), resulting in Fe sulfide biomineral formation (Wang et al., 2018; Nie et al., 2020; Ye and Jing, 2022; Liu et al., 2023).
View in article
Ye, L., Jing, C. (2022) Iron(III) reducing bacteria immobilise antimonite by respiring elemental sulfur. Geochemical Perspectives Letters 21, 37–41. https://doi.org/10.7185/geochemlet.2215
 Show in context
Show in context This metabolic flexibility enables them to survive under alkaline conditions when Fe(III) reduction becomes thermodynamically unfeasible (Flynn et al., 2014), resulting in Fe sulfide biomineral formation (Wang et al., 2018; Nie et al., 2020; Ye and Jing, 2022; Liu et al., 2023).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF)
Figures

Figure 1 Geochemical evolution of dissolved Fe2+, total sulfide and phosphate at (a) pH 6.5, (b) pH 7.2, and (c) pH 8.0. Dashed vertical lines denote the shift from ferruginous to sulfidic conditions.

Figure 2 SEM micrographs of microbe-mineral associations. (a) S0 aggregates consisting of ∼20 μm globules and Fe mineral aggregates consisting of nanometre-sized structures. (b) G. sulfurreducens atop a S0 globule. Note the close spatial association with aggregates of Fe-O minerals and FeS-rich minerals with pseudo-honeycomb structure. (c) A naturally fractured sample highlighting the FeS-rich coating on a S0 globule. (d) The FeS-rich coating appeared to peel off, revealing the smoother surface texture of S0 compared to FeS. (e) Typical morphology of Fe-P-O-rich minerals (vivianite). (f) A higher magnification of the radial blade-like morphology of vivianite (from the boxed region in (e)).

Figure 3 (a) XRD patterns of precipitates at Days (D) 21 and 42. Vertical lines denote 2θ positions specific to certain minerals, with colours corresponding to vivianite (‘V’, light blue), mackinawite (‘M’, black), S0 (‘S’, yellow) and greigite (‘G’, grey). (b) Solid phase Fe distribution based on Mössbauer spectroscopy at 77 K.

Figure 4 Summary of biogeochemical processes in the cultures. (1) Adsorption of phosphate from growth medium onto ferrihydrite (Fh). (2) Concurrent reduction of Fh and S0 by G. sulfurreducens. (3) Sulfide mediated reduction of Fh. (4) Precipitation of vivianite, mackinawite and greigite. (5) Dissolution of vivianite by H2S, releasing phosphate into solution.