Xenon compatibility in magmatic processes: Hadean to current contexts
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:410Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
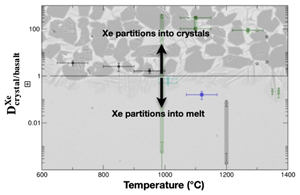
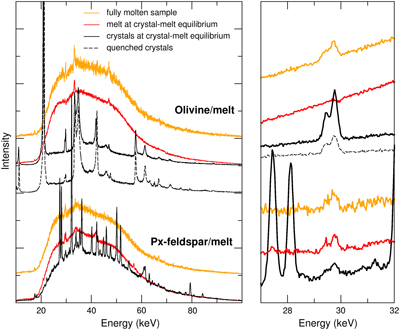 Figure 1 Energy-dispersive X-ray data sets. (Left) Full data sets collected at 10.031°. For each crystal/melt system, data collected at different T are vertically spaced for clarity: fully molten sample (orange), partially molten sample (red for molten zone, black for crystalline or crystal-rich zone), and quenched crystals (black dashed). Peaks at 33 keV and 47 keV are MgO diffraction peaks from the cell-assembly. (Right) Zoom on the Xe Kα1 and Kα2 fluorescence lines (29.4 keV and 29.7 keV). For olivine-melt experiments, data sets in the zoomed panel were collected at 4.0285° to avoid diffraction peaks from crystals overlapping with Xe fluorescence lines. | 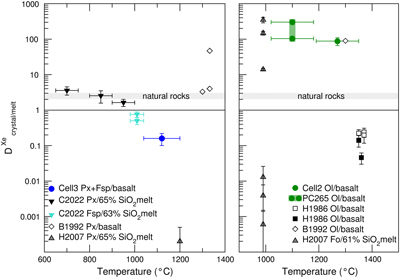 Figure 2 Summary of Xe crystal/melt partition coefficients. Data abbreviations: H1986, Hiyagon and Ozima (1986); B1992, Broadhurst et al. (1992); H2007, Heber et al. (2007); C2022, Chen et al. (2022); PC265, Cell2, and Cell3 are data from this study (Table 2). Empty symbols indicate room P data; filled symbols indicate data collected between 1.0 and 2.0 GPa (cf. Table 1). Note that Heber et al. (2007) discarded all values above unity due to the observation of gas bubbles in crystals, while at least some might form from exsolution upon quenching. Natural rocks dataset: residual rock/basalt partition coefficients from Batiza et al. (1979). | 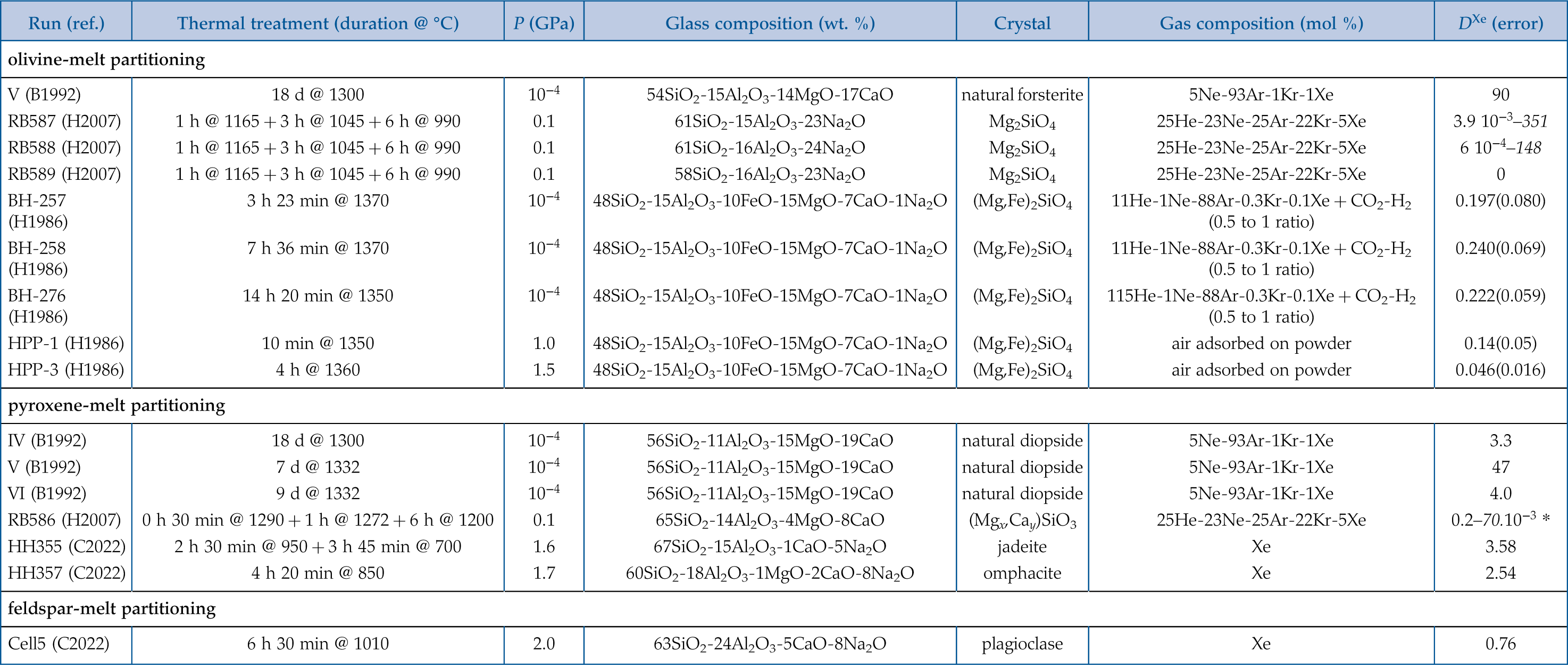 Table 1 Compilation of literature datasets. Chemical compositions have been rounded at unity, given in wt. % for glass and mol % for gas. Analytical techniques: UV laser ablation (Heber et al., 2007), step heating (Hiyagon and Ozima, 1986; Broadhurst et al., 1992). Data discarded by authors due to contamination of crystals with bubbles are given in italics. For Hiyagon and Ozima (1986), glass composition is bulk sample starting composition. Data where lithium borate was added to lower melting T are not included, as borate modifies melt polymerisation which controls noble gases solubility. Error bars on D are given when available; * error equals D values. |  Table 2 Run conditions, coexisting phases, crystalline fraction in crystal-rich areas, Xe content in each phase (wt. %), and partitioning coefficients. Note that crystal and melt fractions do not apply to the whole sample but only to the volume probed by the X-ray beam. *Mass spectrometry measurement. Values in parentheses are errors on the last reported digits. |
| Figure 1 | Figure 2 | Table 1 | Table 2 |
top
Introduction
Noble gases provide unique clues to unravel the geochemical evolution of volatile elements upon Earth’s formation to present day geodynamics (Ozima et al., 2002
Ozima, M., Miura, Y., Podosek, F. (2002) Revisiting I-Xe systematics, an early solar system chronometer. Geochimica et Cosmochimica Acta 66, A576.
and references therein), assuming their inertness and volatile behaviour. Xenon is also unique for its enigmatic atmospheric depletion relative to lighter noble gases (Anders and Owen, 1977Anders, E., Owen, T. (1977) Mars and Earth: Origin and Abundance of Volatiles. Science 198, 453–465. https://doi.org/10.1126/science.198.4316.453
), known as the ‘Xe paradox’, and its strong depletion in light isotopes (Krummenacher et al., 1962Krummenacher, D., Merrihue, C.M., Pepin, R.O., Reynolds, J.H. (1962) Meteoritic krypton and barium versus the general isotopic anomalies in xenon. Geochimica et Cosmochimica Acta 26, 231–249. https://doi.org/10.1016/0016-7037(62)90014-5
). Atmospheric escape and trapping-at-depth scenarios have been proposed to explain both observations (Ardoin et al., 2022Ardoin, L., Broadley, M.W, Almayrac, M., Avice, G., Byrne, D.J., Tarantola, A., Lepland, A., Saito, T., Komiya, T., Shibuya, T., Marty, B. (2022) The end of the isotopic evolution of atmospheric xenon. Geochemical Perspective Letters 20, 43–47. https://doi.org/10.7185/geochemlet.2207
; Broadley et al., 2022Broadley, M.W., Byrne, D.J., Ardoin, L., Almayrac, M.G., Bekaert, D.V., Marty, B. (2022) High precision noble gas measurements of hydrothermal quartz reveal variable loss rate of Xe from the Archean atmosphere. Earth and Planetary Science Letters 588, 117577. https://doi.org/10.1016/j.epsl.2022.117577
; Rzeplinski et al., 2022Rzeplinski, I., Sanloup, C., Gilabert, E., Horlait, D. (2022) Hadean isotopic fractionation of xenon retained in deep silicates. Nature 606, 713–717. https://doi.org/10.1038/s41586-022-04710-4
and references therein), and are not exclusive. Atmospheric escape models stem from the fact that Xe is the easiest to ionise amongst noble gases and to investigate regarding how this could be triggered by extreme UV radiation of the young Sun if sufficient amount of hydrogen or organic matter were present. However, the main challenge is how to lift the heavy Xe through the atmosphere up to levels where it can be lost (Zahnle et al., 2019Zahnle, K.J., Gacesa, M., Catling, D.C. (2019) Strange messenger: A new history of hydrogen on Earth, as told by Xenon. Geochimica et Cosmochimica Acta 244, 56–85. https://doi.org/10.1016/j.gca.2018.09.017
). Trapping-at-depth scenarios, in turn, suffer from a lack of knowledge on Xe petrological behaviour. Noble gases partitioning between major minerals and melt is indeed a debated issue, with Xe spanning the largest range amongst noble gases crystal/melt partitioning data (noted as DXecrystal/melt), with up to 6 orders of magnitude from 6 × 10−4 to 351 for DXeolivine/basalt (Hiyagon and Ozima, 1986Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
; Broadhurst et al., 1992Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
; Heber et al., 2007Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
).The published experimental DXecrystal/melt values were obtained from samples brought to high T, equilibrated with a noble gas medium either at atmospheric P or ∼110 MPa, and quenched to room conditions for chemical analysis. A few experiments were carried out at higher P up to 1.5 GPa (Hiyagon and Ozima, 1986
Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
) but with only adsorbed air on starting sample as noble gas source. After correction for eventual melt inclusions in crystals, the very large range of DXecrystal/melt values partly results from the interpretation of bubbles in minerals, i.e. whether they should be excluded from measurements or not. Indeed, while noble gas content in melts is homogeneous, such is not the case in minerals, with almost systematic reports of heterogeneous distribution of heavy noble gases, often at the micron or sub-micron scale (Hiyagon and Ozima, 1986Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
; Broadhurst et al., 1992Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
; Heber et al., 2007Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
). Some data were discarded on this ground, despite sometimes clear elemental fractionation from the original gas (Hiyagon and Ozima, 1986Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
), which is not expected for passively trapped gas. Indeed, Xe was observed to retro-diffuse out of olivine in high P experiments upon T-quenching (Sanloup et al., 2011Sanloup, C., Schmidt, B.C., Gudfinnsson, G., Dewaele, A., Mezouar, M. (2011) Xenon and Argon: A contrasting behavior in olivine at depth. Geochimica et Cosmochimica Acta 75, 6271–6284. https://doi.org/10.1016/j.gca.2011.08.023
), an exsolution process that could explain at least part of the bubbles observed on quenched samples. It is therefore very challenging to interpret the heterogeneous distribution of Xe in quenched minerals recovered from experiments.Another source of controversy arises from the impact of noble gases adsorption on minerals, i.e. whether or not it is significant in experiments, and therefore if it should be corrected for or not. Step heating experiments show that heavy noble gases are tightly bound in minerals, with largest fractions being released above 1000 °C (Hiyagon and Ozima, 1986
Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
), arguing against physical adsorption.Last but not least, datasets are difficult to compare due to the different compositions used for both melts and crystals (Table 1). For instance, olivine-melt experiment compositions range from synthetic Fe-free forsterite/61 % SiO2-rich melt (Heber et al., 2007
Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
) to natural olivine/basalt (Hiyagon and Ozima, 1986Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
). It is, nonetheless, established that melt composition strongly affects trace element partitioning (Schmidt et al., 2006Schmidt, M.W., Connolly, J.A.D., Günther, D., Bogaerts, M. (2006) Element Partitioning: The Role of Melt Structure and Composition. Science 312, 1646–1650. https://doi.org/10.1126/science.1126690
), with one order of magnitude difference between gabbroic and granitic melts, an effect also expected to be strong for noble gases based on solubility values in melts (Carroll and Stolper, 1993Carroll, M.R., Stolper, E.M. (1993) Noble gas solubilities in silicate melts and glasses: New experimental results for argon and the relationship between solubility and ionic porosity. Geochimica et Cosmochimica Acta 57, 5039–5051. https://doi.org/10.1016/0016-7037(93)90606-W
; Schmidt and Keppler, 2002Schmidt, B.C., Keppler, H. (2002) Experimental evidence for high noble gas solubilities in silicate melts under mantle pressures. Earth and Planetary Science Letters 195, 277–290. https://doi.org/10.1016/S0012-821X(01)00584-2
).Table 1 Compilation of literature datasets. Chemical compositions have been rounded at unity, given in wt. % for glass and mol % for gas. Analytical techniques: UV laser ablation (Heber et al., 2007
Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
), step heating (Hiyagon and Ozima, 1986Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
; Broadhurst et al., 1992Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
). Data discarded by authors due to contamination of crystals with bubbles are given in italics. For Hiyagon and Ozima (1986)Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
, glass composition is bulk sample starting composition. Data where lithium borate was added to lower melting T are not included, as borate modifies melt polymerisation which controls noble gases solubility. Error bars on D are given when available; * error equals D values.| Run (ref.) | Thermal treatment (duration @ °C) | P (GPa) | Glass composition (wt. %) | Crystal | Gas composition (mol %) | DXe (error) |
| olivine-melt partitioning | ||||||
| V (B1992) | 18 d @ 1300 | 10−4 | 54SiO2-15Al2O3-14MgO-17CaO | natural forsterite | 5Ne-93Ar-1Kr-1Xe | 90 |
| RB587 (H2007) | 1 h @ 1165 + 3 h @ 1045 + 6 h @ 990 | 0.1 | 61SiO2-15Al2O3-23Na2O | Mg2SiO4 | 25He-23Ne-25Ar-22Kr-5Xe | 3.9 × 10−3–351 |
| RB588 (H2007) | 1 h @ 1165 + 3 h @ 1045 + 6 h @ 990 | 0.1 | 61SiO2-16Al2O3-24Na2O | Mg2SiO4 | 25He-23Ne-25Ar-22Kr-5Xe | 6 × 10−4–148 |
| RB589 (H2007) | 1 h @ 1165 + 3 h @ 1045 + 6 h @ 990 | 0.1 | 58SiO2-16Al2O3-23Na2O | Mg2SiO4 | 25He-23Ne-25Ar-22Kr-5Xe | 0 |
| BH-257 (H1986) | 3 h 23 min @ 1370 | 10−4 | 48SiO2-15Al2O3-10FeO-15MgO-7CaO-1Na2O | (Mg,Fe)2SiO4 | 11He-1Ne-88Ar-0.3Kr-0.1Xe + CO2-H2 (0.5 to 1 ratio) | 0.197(0.080) |
| BH-258 (H1986) | 7 h 36 min @ 1370 | 10−4 | 48SiO2-15Al2O3-10FeO-15MgO-7CaO-1Na2O | (Mg,Fe)2SiO4 | 11He-1Ne-88Ar-0.3Kr-0.1Xe + CO2-H2 (0.5 to 1 ratio) | 0.240(0.069) |
| BH-276 (H1986) | 14 h 20 min @ 1350 | 10−4 | 48SiO2-15Al2O3-10FeO-15MgO-7CaO-1Na2O | (Mg,Fe)2SiO4 | 115He-1Ne-88Ar-0.3Kr-0.1Xe + CO2-H2 (0.5 to 1 ratio) | 0.222(0.059) |
| HPP-1 (H1986) | 10 min @ 1350 | 1.0 | 48SiO2-15Al2O3-10FeO-15MgO-7CaO-1Na2O | (Mg,Fe)2SiO4 | air adsorbed on powder | 0.14(0.05) |
| HPP-3 (H1986) | 4 h @ 1360 | 1.5 | 48SiO2-15Al2O3-10FeO-15MgO-7CaO-1Na2O | (Mg,Fe)2SiO4 | air adsorbed on powder | 0.046(0.016) |
| pyroxene-melt partitioning | ||||||
| IV (B1992) | 18 d @ 1300 | 10−4 | 56SiO2-11Al2O3-15MgO-19CaO | natural diopside | 5Ne-93Ar-1Kr-1Xe | 3.3 |
| V (B1992) | 7 d @ 1332 | 10−4 | 56SiO2-11Al2O3-15MgO-19CaO | natural diopside | 5Ne-93Ar-1Kr-1Xe | 47 |
| VI (B1992) | 9 d @ 1332 | 10−4 | 56SiO2-11Al2O3-15MgO-19CaO | natural diopside | 5Ne-93Ar-1Kr-1Xe | 4.0 |
| RB586 (H2007) | 0 h 30 min @ 1290 + 1 h @ 1272 + 6 h @ 1200 | 0.1 | 65SiO2-14Al2O3-4MgO-8CaO | (Mgx,Cay)SiO3 | 25He-23Ne-25Ar-22Kr-5Xe | 0.2–70 × 10−3 * |
| HH355 (C2022) | 2 h 30 min @ 950 + 3 h 45 min @ 700 | 1.6 | 67SiO2-15Al2O3-1CaO-5Na2O | jadeite | Xe | 3.58 |
| HH357 (C2022) | 4 h 20 min @ 850 | 1.7 | 60SiO2-18Al2O3-1MgO-2CaO-8Na2O | omphacite | Xe | 2.54 |
| feldspar-melt partitioning | ||||||
| Cell5 (C2022) | 6 h 30 min @ 1010 | 2.0 | 63SiO2-24Al2O3-5CaO-8Na2O | plagioclase | Xe | 0.76 |
Facing these experimental controversies, the peridotitic database shows an enrichment in Xe over other noble gases in xenoliths (Hennecke and Manuel, 1975
Hennecke, E.W., Manuel, O.K. (1975) Noble gases in an Hawaiian xenolith. Nature 257, 778–780. https://doi.org/10.1038/257778b0
; Poreda and Farley, 1992Poreda, R.J., Farley, K.A. (1992) Rare gases in Samoan xenoliths. Earth and Planetary Science Letters 113, 129–144. https://doi.org/10.1016/0012-821X(92)90215-H
; Czuppon et al., 2009Czuppon, G., Matsumoto, T., Handler, M.R., Matsuda, J.-I. (2009) Noble gases in spinel peridotite xenoliths from Mt Quincan, North Queensland, Australia: Undisturbed MORB-type noble gases in the subcontinental lithospheric mantle. Chemical Geology 266, 19–28. https://doi.org/10.1016/j.chemgeo.2009.03.029
). Consistently, natural measurements of Xe mineral/basalt partitioning obtained by analysing parent and partially crystallised magmas (Batiza et al., 1979Batiza, R., Bernatowicz, T.J., Hohenberg, C.M., Podosek, F.A. (1979) Relations of noble gas abundances to petrogenesis and magmatic evolution of some oceanic basalts and related differentiated volcanic rocks. Contributions to Mineralogy and Petrology 69, 301–313. https://doi.org/10.1007/BF00372332
), or coexisting magma and olivine crystals (Kaneoka et al., 1983Kaneoka, I., Takaoka, N., Clague, D.A. (1983) Noble gas systematics for coexisting glass and olivine crystals in basalts and dunite xenoliths from Loihi Seamount. Earth and Planetary Science Letters 66, 427–437. https://doi.org/10.1016/0012-821X(83)90156-5
), show Xe compatibility with DXecrystal/basalt a few-fold above unity. Natural measurements must, nonetheless, be considered with caution (Carroll and Draper, 1994Carroll, M.R., Draper, D.S. (1994) Noble gases as trace elements in magmatic processes. Chemical Geology 117, 37–56. https://doi.org/10.1016/0009-2541(94)90120-1
) due to potential magma degassing processes if minerals and melt did not re-equilibrate, and/or if crystals contain vapour inclusions (Kaneoka et al., 1983Kaneoka, I., Takaoka, N., Clague, D.A. (1983) Noble gas systematics for coexisting glass and olivine crystals in basalts and dunite xenoliths from Loihi Seamount. Earth and Planetary Science Letters 66, 427–437. https://doi.org/10.1016/0012-821X(83)90156-5
), which as for experiments are difficult to interpret.To circumvent these problems, we have recently developed a new method combining synchrotron X-ray fluorescence and diffraction techniques with large volume presses (Chen et al., 2022
Chen, Q., Sanloup, C., Bureau, H., Rzeplinski, I., Glazyrin, K., Farla, R. (2022) Probing the partitioning behaviour of Xe using in situ X-ray synchrotron techniques at high P–T conditions. High Pressure Research 42, 318–335. https://doi.org/10.1080/08957959.2022.2144290
). The method was first tested on crystal/felsic melt Xe partitioning, and Xe was found to be moderately incompatible to compatible, with a T-dependent behaviour (0.50 ± 0.20 for DXeplagioclase/melt at 1010 °C to 3.46 ± 0.25 for DXejadeite/melt at 700 °C). This method is applied here to a Paris-Edinburgh press energy-dispersive set-up, which has the additional advantage of being optimised for the observation of diffuse X-ray scattering signal from melts.top
Mineral/Melt Xenon Partitioning Measurements
We measured Xe partition coefficient between olivine and basalt, and between feldspar-clinopyroxene mix and basalt, in order to target Xe petrological behaviour in early Hadean contexts (e.g., magma oceans in planetary embryos) and present day subduction zone contexts respectively. Xenon crystal/melt partition coefficients were measured by means of in situ synchrotron X-ray diffraction and X-ray fluorescence simultaneously collected on the same spectrum, using an energy-dispersive set-up and a Paris-Edinburgh press to generate high P-T conditions (Supplementary Information). The starting sample (Supplementary Information) is a synthetic glass relevant for lunar-like magma ocean at the stage of anorthite crystallisation, doped by high P-T synthesis with 0.05 wt. % Xe (Table S-1), i.e. well below Xe solubility in tholeiitic melt (Schmidt and Keppler, 2002
Schmidt, B.C., Keppler, H. (2002) Experimental evidence for high noble gas solubilities in silicate melts under mantle pressures. Earth and Planetary Science Letters 195, 277–290. https://doi.org/10.1016/S0012-821X(01)00584-2
) of 0.41 wt. % at 2 GPa to avoid supersaturation, while being high enough for the Xe fluorescence signal to be significantly above noise level. The starting sample is used either pure to mimic planetesimal magma ocean stages or mixed with 10 wt. % labradorite feldspar to get an analogue of high alumina basalts, relevant for present day subduction zone settings. At each targeted P, T was first raised until recrystallisation, further raised until full remelting, and lowered until crystals grew in equilibrium with melt to ensure chemical equilibrium. The sample was then scanned perpendicularly to the X-ray beam in order to probe either pure melt or crystallised areas. For the olivine/basalt experiments, crystals were efficiently segregated from melt due to their density difference. This was not the case of the pyroxene-feldspar/basalt experiments for which it was not possible to probe only crystals, and the fraction of melt vs. crystals in crystal-rich areas was determined from the X-ray diffraction signal (Supplementary Information). Xenon content in pure melt or crystals was obtained from the Xe Kα fluorescence intensity signal in the fully molten sample pattern and the relevant pattern, either pure melt or crystals, at crystal-melt equilibrium (Supplementary Information). If a pure crystal pattern could not be obtained, Xe content in crystals from mixed crystals + melt pattern was obtained using mass-balance calculation from Xe Kα fluorescence intensity in crystal-rich area and local melt fraction obtained from X-ray diffraction signal (Eq. S-3).For basaltic melt in equilibrium with feldspar and pyroxene, Xe was enriched in the melt as seen by the stronger Xe fluorescence signal (Fig. 1) and the derived partition coefficient (0.16 ± 0.06; Table 2). Crystal growth partly occurred upon quenching to room T (see microlithic texture of sample in Fig. S-3), hence it is difficult to have a proper estimate of feldspar vs. pyroxene at high P-T conditions, and oriented crystal growth prevents estimate from in situ energy-dispersive X-ray diffraction data. Nonetheless, due to the low global D value, Xe had to be incompatible with both pyroxene and feldspar at our experimental conditions, consistent with the reported T-dependence of D for clinopyroxene/felsic melt (Chen et al., 2022
Chen, Q., Sanloup, C., Bureau, H., Rzeplinski, I., Glazyrin, K., Farla, R. (2022) Probing the partitioning behaviour of Xe using in situ X-ray synchrotron techniques at high P–T conditions. High Pressure Research 42, 318–335. https://doi.org/10.1080/08957959.2022.2144290
). In contrast, Xe fluorescence signal was not detected in basalt coexisting with olivine (Fig. 1), which implies a lower limit of 80 for DXeolivine/basalt, taking the noise oscillations as a maximum for Xe fluorescence signal in melt. Xenon X-ray fluorescence signal in olivine crystals is strongly affected by quenching T at high P, with a 2.9 factor decrease in intensity (Fig. 1a), indicating important—although incomplete—Xe exsolution back to room T, consistent with previous observation from in situ X-ray diffraction (Sanloup et al., 2011Sanloup, C., Schmidt, B.C., Gudfinnsson, G., Dewaele, A., Mezouar, M. (2011) Xenon and Argon: A contrasting behavior in olivine at depth. Geochimica et Cosmochimica Acta 75, 6271–6284. https://doi.org/10.1016/j.gca.2011.08.023
).
Figure 1 Energy-dispersive X-ray data sets. (Left) Full data sets collected at 10.031°. For each crystal/melt system, data collected at different T are vertically spaced for clarity: fully molten sample (orange), partially molten sample (red for molten zone, black for crystalline or crystal-rich zone), and quenched crystals (black dashed). Peaks at 33 keV and 47 keV are MgO diffraction peaks from the cell-assembly. (Right) Zoom on the Xe Kα1 and Kα2 fluorescence lines (29.4 keV and 29.7 keV). For olivine-melt experiments, data sets in the zoomed panel were collected at 4.0285° to avoid diffraction peaks from crystals overlapping with Xe fluorescence lines.
Table 2 Run conditions, coexisting phases, crystalline fraction in crystal-rich areas, Xe content in each phase (wt. %), and partitioning coefficients. Note that crystal and melt fractions do not apply to the whole sample but only to the volume probed by the X-ray beam. *Mass spectrometry measurement. Values in parentheses are errors on the last reported digits.
| Run | P (GPa) | T (°C) | Phases | Xcrystals | [Xe]crystals (wt. %) | [Xe]melt (wt. %) | DXecrystal/melt |
| Cell2 | 2.0 | 1270 | Ol + melt | 100(5) | 0.145(36) | 1.65(15) × 10−3∗ | 88(22) |
| PC265 | 1.3 | 1100 | Ol + glass | 83(10) | 0.015(6)∗ | 1.5(8) × 10−4∗ | 104(16)–302(46) |
| Cell3 | 1.2 | 1120 | An + Di + melt | 34(4) | 0.007(2) | 0.044(9) | 0.16(6) |
To further constrain Xe concentration in melt coexisting with olivine, pieces of glass recovered from synchrotron experiments and an additional sample synthesised at similar P-T conditions (Supplementary Information) were analysed by noble gas mass spectrometry. From the additional sample, three fragments of glass and two fragments of mostly crystals could be separated, as olivine crystals had sedimented at the bottom of the capsule. Mass spectrometry analyses were done on bulk sample fragments fully melted by laser heating (Supplementary Information), revealing systematic Xe enrichment in olivine-rich fragments compared to glass. Olivine content in olivine-rich fragments is estimated at 83 ± 10 % from analyses of SEM images (Fig. S-3) using ImageJ software. Our reported values of DXeolivine/basalt, 88(22)–302(46) (Table 2), exceed 80 as expected from the lack of Xe X-ray fluorescence signal in the melt. The lower range value was obtained using Xe content in crystals as measured from energy-dispersive X-ray data, and Xe content in glass as measured by mass spectrometry (Table S-2), which is near the 104(16) value obtained on the additional sample using mass spectrometry results only. The upper range corresponds to that value times the intensity ratio between crystals at high P-T and quenched crystals at high P (i.e. 2.9 GPa), to account for Xe exsolution from olivine crystals upon quenching.
top
Olivine Retains Xe in Magmatic Processes
Clinopyroxene-feldspar/melt data are broadly consistent with the T-trend reported for pyroxene/felsic melt under similar P (Chen et al., 2022
Chen, Q., Sanloup, C., Bureau, H., Rzeplinski, I., Glazyrin, K., Farla, R. (2022) Probing the partitioning behaviour of Xe using in situ X-ray synchrotron techniques at high P–T conditions. High Pressure Research 42, 318–335. https://doi.org/10.1080/08957959.2022.2144290
), and intermediate within the range of literature values at ambient or near ambient P (Broadhurst et al., 1992Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
; Heber et al., 2007Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
). Olivine/melt data (Fig. 2) confirm the compatible nature of Xe in olivine predicted from ab initio calculations (Crépisson et al., 2018Crépisson, C., Blanchard, M., Lazzeri, M., Balan, E., Sanloup, C. (2018) New constraints on Xe incorporation mechanisms in olivine from first-principles calculations. Geochimica et Cosmochimica Acta 222, 146–155. https://doi.org/10.1016/j.gca.2017.10.028
), are similar to ambient P data from Broadhurst et al. (1992)Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
, and correspond to the higher range from Heber et al. (2007)Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
. The latter study may underestimate DXeolivine/basalt as 1) the melt was enriched in silica (Table 1) while Xe solubility increases by a factor of five, for instance, between a MORB and a haplogranite (Leroy et al., 2019Leroy, C., Bureau, H., Sanloup, C., Raepsaet, C., Glazyrin, K., Munsch, P., Harmand, M., Prouteau, G., Khodja, H. (2019) Xenon and iodine behaviour in magmas. Earth and Planetary Science Letters 522, 144–154. https://doi.org/10.1016/j.epsl.2019.06.031
), and 2) crystals with bubbles were discarded on the basis of potential contamination while, at least, some of the bubbles are expected to form upon T-quenching, although this effect could be restricted to high P conditions.
Figure 2 Summary of Xe crystal/melt partition coefficients. Data abbreviations: H1986, Hiyagon and Ozima (1986)
Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
; B1992, Broadhurst et al. (1992)Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
; H2007, Heber et al. (2007)Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
; C2022, Chen et al. (2022)Chen, Q., Sanloup, C., Bureau, H., Rzeplinski, I., Glazyrin, K., Farla, R. (2022) Probing the partitioning behaviour of Xe using in situ X-ray synchrotron techniques at high P–T conditions. High Pressure Research 42, 318–335. https://doi.org/10.1080/08957959.2022.2144290
; PC265, Cell2, and Cell3 are data from this study (Table 2). Empty symbols indicate room P data; filled symbols indicate data collected between 1.0 and 2.0 GPa (cf. Table 1). Note that Heber et al. (2007)Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
discarded all values above unity due to the observation of gas bubbles in crystals, while at least some might form from exsolution upon quenching. Natural rocks dataset: residual rock/basalt partition coefficients from Batiza et al. (1979)Batiza, R., Bernatowicz, T.J., Hohenberg, C.M., Podosek, F.A. (1979) Relations of noble gas abundances to petrogenesis and magmatic evolution of some oceanic basalts and related differentiated volcanic rocks. Contributions to Mineralogy and Petrology 69, 301–313. https://doi.org/10.1007/BF00372332
.Considered together, the present high P-T clinopyroxene-feldspar/melt and olivine/melt data are consistent with the natural reports of bulk peridotite/melt partitioning coefficient a few-fold above unity from oceanic island basalts (Batiza et al., 1979
Batiza, R., Bernatowicz, T.J., Hohenberg, C.M., Podosek, F.A. (1979) Relations of noble gas abundances to petrogenesis and magmatic evolution of some oceanic basalts and related differentiated volcanic rocks. Contributions to Mineralogy and Petrology 69, 301–313. https://doi.org/10.1007/BF00372332
; Kaneoka et al., 1983Kaneoka, I., Takaoka, N., Clague, D.A. (1983) Noble gas systematics for coexisting glass and olivine crystals in basalts and dunite xenoliths from Loihi Seamount. Earth and Planetary Science Letters 66, 427–437. https://doi.org/10.1016/0012-821X(83)90156-5
). The P effect on DXecrystal/melt could not be investigated here due to the limited P-T stability field of the mineralogical parageneses investigated. It further cannot be inferred from the comparison of the present and previous datasets (Fig. S-5) as data are either at ambient or near ambient P (10−4 and 0.1 GPa) or in a restricted P-range between 1.0 and 2.0 GPa. Additionally, the only high P previous dataset for olivine/melt partitioning was obtained with only air adsorbed on samples as a source of noble gases (Hiyagon and Ozima, 1986Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
). Pressure is, nonetheless, expected to be important as Xe retention in silicates is P-induced. Importantly, Xe retention does not extend to lower mantle silicates (Shcheka and Keppler, 2012Shcheka, S.S., Keppler, H. (2012) The origin of the terrestrial noble-gas signature. Nature 490, 531–534. https://doi.org/10.1038/nature11506
), consistent with the plume source being depleted in Xe compared to MORB source (Parai, 2022Parai, R. (2022) A dry ancient plume mantle from noble gas isotopes. Proceedings of the National Academy of Sciences 119, e2201815119. https://doi.org/10.1073/pnas.2201815119
).As for any element, Xe crystal chemistry controls its partitioning behaviour, and its knowledge is key to understand its repartition in planetary envelopes. In magmas, noble gases enter the ring structure of silicate melts (Carroll and Stolper, 1993
Carroll, M.R., Stolper, E.M. (1993) Noble gas solubilities in silicate melts and glasses: New experimental results for argon and the relationship between solubility and ionic porosity. Geochimica et Cosmochimica Acta 57, 5039–5051. https://doi.org/10.1016/0016-7037(93)90606-W
), eventually leading to oxidation in the case of Xe in compressed silica-rich melt (Leroy et al., 2019Leroy, C., Bureau, H., Sanloup, C., Raepsaet, C., Glazyrin, K., Munsch, P., Harmand, M., Prouteau, G., Khodja, H. (2019) Xenon and iodine behaviour in magmas. Earth and Planetary Science Letters 522, 144–154. https://doi.org/10.1016/j.epsl.2019.06.031
). In minerals, the tightly bound nature of Xe leads to the suggestion that either interstitial sites or crystal vacancies are possible sites for the Xe atoms (Hiyagon and Ozima, 1986Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
). Theoretical calculations have shown that Xe can chemically bond to oxygen in quartz (Probert, 2010Probert, M.I.J. (2010) An ab initio study of xenon retention in α-quartz. Journal of Physics: Condensed Matter 22, 025501. https://doi.org/10.1088/0953-8984/22/2/025501
; Crépisson et al., 2019Crépisson, C., Sanloup, C., Blanchard, M., Hudspeth, J., Glazyrin, K., Capitani, F. (2019) The Xe-SiO2 System at Moderate Pressure and High Temperature. Geochemistry, Geophysics, Geosystems 20, 992–1003. https://doi.org/10.1029/2018GC007779
) and olivine (Crépisson et al., 2018Crépisson, C., Blanchard, M., Lazzeri, M., Balan, E., Sanloup, C. (2018) New constraints on Xe incorporation mechanisms in olivine from first-principles calculations. Geochimica et Cosmochimica Acta 222, 146–155. https://doi.org/10.1016/j.gca.2017.10.028
) under modest P by substituting a Si atom. This precludes the use of mineral/melt partitioning models considering Xe as a ‘zero’-charge species (Brooker et al., 2003Brooker, R.A., Du, Z., Blundy, J.D., Kelley, S.P., Allan, N.L., Wood, B.J., Chamorro, E.M., Wartho, J.-A., Purton, J.A. (2003) The ‘zero charge’ partitioning behaviour of noble gases during mantle melting. Nature 423, 738–741. https://doi.org/10.1038/nature01708
). The radius of chemically bonded Xe is, indeed, much smaller than that of inert Xe, with three oxygen atoms located at 2.0 Å in a planar configuration in olivine (Crépisson et al., 2018Crépisson, C., Blanchard, M., Lazzeri, M., Balan, E., Sanloup, C. (2018) New constraints on Xe incorporation mechanisms in olivine from first-principles calculations. Geochimica et Cosmochimica Acta 222, 146–155. https://doi.org/10.1016/j.gca.2017.10.028
), and two nearest oxygen atoms at 2.0 Å, and two further ones at 2.3 Å in quartz (Crépisson et al., 2019Crépisson, C., Sanloup, C., Blanchard, M., Hudspeth, J., Glazyrin, K., Capitani, F. (2019) The Xe-SiO2 System at Moderate Pressure and High Temperature. Geochemistry, Geophysics, Geosystems 20, 992–1003. https://doi.org/10.1029/2018GC007779
). Xenon crystal chemistry has not been reported for pyroxene nor feldspar but for the latter it might be similar to that in quartz, both being tectosilicates. The contrasting DXecrystal/melt values between olivine/basalt and pyroxene-feldspar/basalt may relate to the different local environments of Xe in these minerals.Over the course of Earth’s history, Xe should have been strongly retained in olivines crystallising from magma oceans, and in olivine-rich residues in present day mantle partial melting processes. Preferential release to the atmosphere is instead expected from high T (>1000 °C) crustal processes, turning to a moderate retention in pyroxenes and feldspars equilibrated with lower T melts such as evolved hydrous magmas in continental crust and arc contexts (Chen et al., 2022
Chen, Q., Sanloup, C., Bureau, H., Rzeplinski, I., Glazyrin, K., Farla, R. (2022) Probing the partitioning behaviour of Xe using in situ X-ray synchrotron techniques at high P–T conditions. High Pressure Research 42, 318–335. https://doi.org/10.1080/08957959.2022.2144290
). The petrological behaviour of Xe hence supports the trapping-at-depth scenario (Rzeplinski et al., 2022Rzeplinski, I., Sanloup, C., Gilabert, E., Horlait, D. (2022) Hadean isotopic fractionation of xenon retained in deep silicates. Nature 606, 713–717. https://doi.org/10.1038/s41586-022-04710-4
), whereby a succession of collisions between pre-planetary embryos led to the depletion in terrestrial and Martian Xe light isotopes due to Xe trapping and oxidation in crystallising magma oceans, while over 99 % (Harper and Jacobsen, 1996Harper Jr., C.L., Jacobsen, S.B. (1996) Noble Gases and Earth’s Accretion. Science 273, 1814–1818. https://doi.org/10.1126/science.273.5283.1814
) of the initial budget was expelled from growing planetesimals by exsolution from melt at low P in convecting magma oceans followed by atmospheric losses on a few Myr timescale. The late veneer chondritic input to the atmosphere followed by partial-only Xe degassing concomitantly with the emplacement of the continental crust led to the rising Kr/Xe ratio and progressively heavier Xe observed in Archean atmospheric samples (Broadley et al., 2022Broadley, M.W., Byrne, D.J., Ardoin, L., Almayrac, M.G., Bekaert, D.V., Marty, B. (2022) High precision noble gas measurements of hydrothermal quartz reveal variable loss rate of Xe from the Archean atmosphere. Earth and Planetary Science Letters 588, 117577. https://doi.org/10.1016/j.epsl.2022.117577
) until the late veneer got overprinted, while lighter Xe in the mantle (Peron and Moreira, 2018Péron, S., Moreira, M. (2018) Onset of volatile recycling into the mantle determined by xenon anomalies. Geochemical Perspectives Letters 9, 21–25. https://doi.org/10.7185/geochemlet.1833
) would result from mixing mass fractionated Xe with subducted Archean atmospheric Xe. This Archean evolution is not observed on Mars where such events did not occur. That the retention of Xe differs in minerals while its impact on isotopic fractionating is similar (Rzeplinski et al., 2022Rzeplinski, I., Sanloup, C., Gilabert, E., Horlait, D. (2022) Hadean isotopic fractionation of xenon retained in deep silicates. Nature 606, 713–717. https://doi.org/10.1038/s41586-022-04710-4
) implies that Xe elemental and isotopic evolution cannot be modelled by the same Rayleigh distillation law. Indeed, Rayleigh-predicted Kr/Xe ratios for the Archean atmosphere disagree (Broadley et al., 2022Broadley, M.W., Byrne, D.J., Ardoin, L., Almayrac, M.G., Bekaert, D.V., Marty, B. (2022) High precision noble gas measurements of hydrothermal quartz reveal variable loss rate of Xe from the Archean atmosphere. Earth and Planetary Science Letters 588, 117577. https://doi.org/10.1016/j.epsl.2022.117577
), a mismatch attributed to variable Xe loss over time which is challenging to reconcile with a continuous isotopic evolution.top
Acknowledgements
We acknowledge K. Curtis-Benson for providing parts for cell-assemblies and arranging shipments before and after experiments, O. Boudouma for SEM measurement, N. Rivodini for EPMA analyses. Funding: Q. Chen is funded by CSC scholarship (#201806340094). HPCAT operations are supported by DOE-NNSA’s Office of Experimental Sciences. The Advanced Photon Source is a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Editor: Anat Shahar
top
References
Anders, E., Owen, T. (1977) Mars and Earth: Origin and Abundance of Volatiles. Science 198, 453–465. https://doi.org/10.1126/science.198.4316.453
 Show in context
Show in context Xenon is also unique for its enigmatic atmospheric depletion relative to lighter noble gases (Anders and Owen, 1977), known as the ‘Xe paradox’, and its strong depletion in light isotopes (Krummenacher et al., 1962).
View in article
Ardoin, L., Broadley, M.W, Almayrac, M., Avice, G., Byrne, D.J., Tarantola, A., Lepland, A., Saito, T., Komiya, T., Shibuya, T., Marty, B. (2022) The end of the isotopic evolution of atmospheric xenon. Geochemical Perspective Letters 20, 43–47. https://doi.org/10.7185/geochemlet.2207
 Show in context
Show in context Atmospheric escape and trapping-at-depth scenarios have been proposed to explain both observations (Ardoin et al., 2022; Broadley et al., 2022; Rzeplinski et al., 2022 and references therein), and are not exclusive.
View in article
Batiza, R., Bernatowicz, T.J., Hohenberg, C.M., Podosek, F.A. (1979) Relations of noble gas abundances to petrogenesis and magmatic evolution of some oceanic basalts and related differentiated volcanic rocks. Contributions to Mineralogy and Petrology 69, 301–313. https://doi.org/10.1007/BF00372332
 Show in context
Show in context Consistently, natural measurements of Xe mineral/basalt partitioning obtained by analysing parent and partially crystallised magmas (Batiza et al., 1979), or coexisting magma and olivine crystals (Kaneoka et al., 1983), show Xe compatibility with DXecrystal/basalt a few-fold above unity. Natural measurements must, nonetheless, be considered with caution (Carroll and Draper, 1994) due to potential magma degassing processes if minerals and melt did not re-equilibrate, and/or if crystals contain vapour inclusions (Kaneoka et al., 1983), which as for experiments are difficult to interpret.
View in article
Natural rocks dataset: residual rock/basalt partition coefficients from Batiza et al. (1979).
View in article
Considered together, the present high P-T clinopyroxene-feldspar/melt and olivine/melt data are consistent with the natural reports of bulk peridotite/melt partitioning coefficient a few-fold above unity from oceanic island basalts (Batiza et al., 1979; Kaneoka et al., 1983).
View in article
Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
 Show in context
Show in context Noble gases partitioning between major minerals and melt is indeed a debated issue, with Xe spanning the largest range amongst noble gases crystal/melt partitioning data (noted as DXecrystal/melt), with up to 6 orders of magnitude from 6 × 10−4 to 351 for DXeolivine/basalt (Hiyagon and Ozima, 1986; Broadhurst et al., 1992; Heber et al., 2007).
View in article
Indeed, while noble gas content in melts is homogeneous, such is not the case in minerals, with almost systematic reports of heterogeneous distribution of heavy noble gases, often at the micron or sub-micron scale (Hiyagon and Ozima, 1986; Broadhurst et al., 1992; Heber et al., 2007).
View in article
Analytical techniques: UV laser ablation (Heber et al., 2007), step heating (Hiyagon and Ozima, 1986; Broadhurst et al., 1992).
View in article
Clinopyroxene-feldspar/melt data are broadly consistent with the T-trend reported for pyroxene/felsic melt under similar P (Chen et al., 2022), and intermediate within the range of literature values at ambient or near ambient P (Broadhurst et al., 1992; Heber et al., 2007).
View in article
Olivine/melt data (Fig. 2) confirm the compatible nature of Xe in olivine predicted from ab initio calculations (Crépisson et al., 2018), are similar to ambient P data from Broadhurst et al. (1992), and correspond to the higher range from Heber et al. (2007).
View in article
Summary of Xe crystal/melt partition coefficients. Data abbreviations: H1986, Hiyagon and Ozima (1986); B1992, Broadhurst et al. (1992); H2007, Heber et al. (2007); C2022, Chen et al. (2022); PC265, Cell2, and Cell3 are data from this study (Table 2).
View in article
Broadley, M.W., Byrne, D.J., Ardoin, L., Almayrac, M.G., Bekaert, D.V., Marty, B. (2022) High precision noble gas measurements of hydrothermal quartz reveal variable loss rate of Xe from the Archean atmosphere. Earth and Planetary Science Letters 588, 117577. https://doi.org/10.1016/j.epsl.2022.117577
 Show in context
Show in context Atmospheric escape and trapping-at-depth scenarios have been proposed to explain both observations (Ardoin et al., 2022; Broadley et al., 2022; Rzeplinski et al., 2022 and references therein), and are not exclusive.
View in article
The late veneer chondritic input to the atmosphere followed by partial-only Xe degassing concomitantly with the emplacement of the continental crust led to the rising Kr/Xe ratio and progressively heavier Xe observed in Archean atmospheric samples (Broadley et al., 2022) until the late veneer got overprinted, while lighter Xe in the mantle (Peron and Moreira, 2018) would result from mixing mass fractionated Xe with subducted Archean atmospheric Xe.
View in article
That the retention of Xe differs in minerals while its impact on isotopic fractionating is similar (Rzeplinski et al., 2022) implies that Xe elemental and isotopic evolution cannot be modelled by the same Rayleigh distillation law. Indeed, Rayleigh-predicted Kr/Xe ratios for the Archean atmosphere disagree (Broadley et al., 2022), a mismatch attributed to variable Xe loss over time which is challenging to reconcile with a continuous isotopic evolution.
View in article
Brooker, R.A., Du, Z., Blundy, J.D., Kelley, S.P., Allan, N.L., Wood, B.J., Chamorro, E.M., Wartho, J.-A., Purton, J.A. (2003) The ‘zero charge’ partitioning behaviour of noble gases during mantle melting. Nature 423, 738–741. https://doi.org/10.1038/nature01708
 Show in context
Show in context This precludes the use of mineral/melt partitioning models considering Xe as a ‘zero’-charge species (Brooker et al., 2003).
View in article
Carroll, M.R., Stolper, E.M. (1993) Noble gas solubilities in silicate melts and glasses: New experimental results for argon and the relationship between solubility and ionic porosity. Geochimica et Cosmochimica Acta 57, 5039–5051. https://doi.org/10.1016/0016-7037(93)90606-W
 Show in context
Show in context It is, nonetheless, established that melt composition strongly affects trace element partitioning (Schmidt et al., 2006), with one order of magnitude difference between gabbroic and granitic melts, an effect also expected to be strong for noble gases based on solubility values in melts (Carroll and Stolper, 1993; Schmidt and Keppler, 2002).
View in article
In magmas, noble gases enter the ring structure of silicate melts (Carroll and Stolper, 1993), eventually leading to oxidation in the case of Xe in compressed silica-rich melt (Leroy et al., 2019).
View in article
Carroll, M.R., Draper, D.S. (1994) Noble gases as trace elements in magmatic processes. Chemical Geology 117, 37–56. https://doi.org/10.1016/0009-2541(94)90120-1
 Show in context
Show in context Consistently, natural measurements of Xe mineral/basalt partitioning obtained by analysing parent and partially crystallised magmas (Batiza et al., 1979), or coexisting magma and olivine crystals (Kaneoka et al., 1983), show Xe compatibility with DXecrystal/basalt a few-fold above unity. Natural measurements must, nonetheless, be considered with caution (Carroll and Draper, 1994) due to potential magma degassing processes if minerals and melt did not re-equilibrate, and/or if crystals contain vapour inclusions (Kaneoka et al., 1983), which as for experiments are difficult to interpret.
View in article
Chen, Q., Sanloup, C., Bureau, H., Rzeplinski, I., Glazyrin, K., Farla, R. (2022) Probing the partitioning behaviour of Xe using in situ X-ray synchrotron techniques at high P–T conditions. High Pressure Research 42, 318–335. https://doi.org/10.1080/08957959.2022.2144290
 Show in context
Show in context To circumvent these problems, we have recently developed a new method combining synchrotron X-ray fluorescence and diffraction techniques with large volume presses (Chen et al., 2022).
View in article
Nonetheless, due to the low global D value, Xe had to be incompatible with both pyroxene and feldspar at our experimental conditions, consistent with the reported T-dependence of D for clinopyroxene/felsic melt (Chen et al., 2022).
View in article
Clinopyroxene-feldspar/melt data are broadly consistent with the T-trend reported for pyroxene/felsic melt under similar P (Chen et al., 2022), and intermediate within the range of literature values at ambient or near ambient P (Broadhurst et al., 1992; Heber et al., 2007).
View in article
Summary of Xe crystal/melt partition coefficients. Data abbreviations: H1986, Hiyagon and Ozima (1986); B1992, Broadhurst et al. (1992); H2007, Heber et al. (2007); C2022, Chen et al. (2022); PC265, Cell2, and Cell3 are data from this study (Table 2).
View in article
Preferential release to the atmosphere is instead expected from high T (>1000 °C) crustal processes, turning to a moderate retention in pyroxenes and feldspars equilibrated with lower T melts such as evolved hydrous magmas in continental crust and arc contexts (Chen et al., 2022).
View in article
Crépisson, C., Blanchard, M., Lazzeri, M., Balan, E., Sanloup, C. (2018) New constraints on Xe incorporation mechanisms in olivine from first-principles calculations. Geochimica et Cosmochimica Acta 222, 146–155. https://doi.org/10.1016/j.gca.2017.10.028
 Show in context
Show in context Olivine/melt data (Fig. 2) confirm the compatible nature of Xe in olivine predicted from ab initio calculations (Crépisson et al., 2018), are similar to ambient P data from Broadhurst et al. (1992), and correspond to the higher range from Heber et al. (2007).
View in article
Theoretical calculations have shown that Xe can chemically bond to oxygen in quartz (Probert, 2010; Crépisson et al., 2019) and olivine (Crépisson et al., 2018) under modest P by substituting a Si atom.
View in article
The radius of chemically bonded Xe is, indeed, much smaller than that of inert Xe, with three oxygen atoms located at 2.0 Å in a planar configuration in olivine (Crépisson et al., 2018), and two nearest oxygen atoms at 2.0 Å, and two further ones at 2.3 Å in quartz (Crépisson et al., 2019).
View in article
Crépisson, C., Sanloup, C., Blanchard, M., Hudspeth, J., Glazyrin, K., Capitani, F. (2019) The Xe-SiO2 System at Moderate Pressure and High Temperature. Geochemistry, Geophysics, Geosystems 20, 992–1003. https://doi.org/10.1029/2018GC007779
 Show in context
Show in context Theoretical calculations have shown that Xe can chemically bond to oxygen in quartz (Probert, 2010; Crépisson et al., 2019) and olivine (Crépisson et al., 2018) under modest P by substituting a Si atom.
View in article
The radius of chemically bonded Xe is, indeed, much smaller than that of inert Xe, with three oxygen atoms located at 2.0 Å in a planar configuration in olivine (Crépisson et al., 2018), and two nearest oxygen atoms at 2.0 Å, and two further ones at 2.3 Å in quartz (Crépisson et al., 2019).
View in article
Czuppon, G., Matsumoto, T., Handler, M.R., Matsuda, J.-I. (2009) Noble gases in spinel peridotite xenoliths from Mt Quincan, North Queensland, Australia: Undisturbed MORB-type noble gases in the subcontinental lithospheric mantle. Chemical Geology 266, 19–28. https://doi.org/10.1016/j.chemgeo.2009.03.029
 Show in context
Show in context Facing these experimental controversies, the peridotitic database shows an enrichment in Xe over other noble gases in xenoliths (Hennecke and Manuel, 1975; Poreda and Farley, 1992; Czuppon et al., 2009).
View in article
Harper Jr., C.L., Jacobsen, S.B. (1996) Noble Gases and Earth’s Accretion. Science 273, 1814–1818. https://doi.org/10.1126/science.273.5283.1814
 Show in context
Show in context The petrological behaviour of Xe hence supports the trapping-at-depth scenario (Rzeplinski et al., 2022), whereby a succession of collisions between pre-planetary embryos led to the depletion in terrestrial and Martian Xe light isotopes due to Xe trapping and oxidation in crystallising magma oceans, while over 99 % (Harper and Jacobsen, 1996) of the initial budget was expelled from growing planetesimals by exsolution from melt at low P in convecting magma oceans followed by atmospheric losses on a few Myr timescale.
View in article
Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
 Show in context
Show in context Noble gases partitioning between major minerals and melt is indeed a debated issue, with Xe spanning the largest range amongst noble gases crystal/melt partitioning data (noted as DXecrystal/melt), with up to 6 orders of magnitude from 6 × 10−4 to 351 for DXeolivine/basalt (Hiyagon and Ozima, 1986; Broadhurst et al., 1992; Heber et al., 2007).
View in article
Indeed, while noble gas content in melts is homogeneous, such is not the case in minerals, with almost systematic reports of heterogeneous distribution of heavy noble gases, often at the micron or sub-micron scale (Hiyagon and Ozima, 1986; Broadhurst et al., 1992; Heber et al., 2007).
View in article
For instance, olivine-melt experiment compositions range from synthetic Fe-free forsterite/61 % SiO2-rich melt (Heber et al., 2007) to natural olivine/basalt (Hiyagon and Ozima, 1986).
View in article
Analytical techniques: UV laser ablation (Heber et al., 2007), step heating (Hiyagon and Ozima, 1986; Broadhurst et al., 1992).
View in article
Clinopyroxene-feldspar/melt data are broadly consistent with the T-trend reported for pyroxene/felsic melt under similar P (Chen et al., 2022), and intermediate within the range of literature values at ambient or near ambient P (Broadhurst et al., 1992; Heber et al., 2007).
View in article
Olivine/melt data (Fig. 2) confirm the compatible nature of Xe in olivine predicted from ab initio calculations (Crépisson et al., 2018), are similar to ambient P data from Broadhurst et al. (1992), and correspond to the higher range from Heber et al. (2007).
View in article
Summary of Xe crystal/melt partition coefficients. Data abbreviations: H1986, Hiyagon and Ozima (1986); B1992, Broadhurst et al. (1992); H2007, Heber et al. (2007); C2022, Chen et al. (2022); PC265, Cell2, and Cell3 are data from this study (Table 2).
View in article
Note that Heber et al. (2007) discarded all values above unity due to the observation of gas bubbles in crystals, while at least some might form from exsolution upon quenching.
View in article
Hennecke, E.W., Manuel, O.K. (1975) Noble gases in an Hawaiian xenolith. Nature 257, 778–780. https://doi.org/10.1038/257778b0
 Show in context
Show in context Facing these experimental controversies, the peridotitic database shows an enrichment in Xe over other noble gases in xenoliths (Hennecke and Manuel, 1975; Poreda and Farley, 1992; Czuppon et al., 2009).
View in article
Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
 Show in context
Show in context Noble gases partitioning between major minerals and melt is indeed a debated issue, with Xe spanning the largest range amongst noble gases crystal/melt partitioning data (noted as DXecrystal/melt), with up to 6 orders of magnitude from 6 × 10−4 to 351 for DXeolivine/basalt (Hiyagon and Ozima, 1986; Broadhurst et al., 1992; Heber et al., 2007).
View in article
A few experiments were carried out at higher P up to 1.5 GPa (Hiyagon and Ozima, 1986) but with only adsorbed air on starting sample as noble gas source.
View in article
Indeed, while noble gas content in melts is homogeneous, such is not the case in minerals, with almost systematic reports of heterogeneous distribution of heavy noble gases, often at the micron or sub-micron scale (Hiyagon and Ozima, 1986; Broadhurst et al., 1992; Heber et al., 2007).
View in article
Some data were discarded on this ground, despite sometimes clear elemental fractionation from the original gas (Hiyagon and Ozima, 1986), which is not expected for passively trapped gas. Indeed, Xe was observed to retro-diffuse out of olivine in high P experiments upon T-quenching (Sanloup et al., 2011), an exsolution process that could explain at least part of the bubbles observed on quenched samples.
View in article
Step heating experiments show that heavy noble gases are tightly bound in minerals, with largest fractions being released above 1000 °C (Hiyagon and Ozima, 1986), arguing against physical adsorption.
View in article
For instance, olivine-melt experiment compositions range from synthetic Fe-free forsterite/61 % SiO2-rich melt (Heber et al., 2007) to natural olivine/basalt (Hiyagon and Ozima, 1986).
View in article
Analytical techniques: UV laser ablation (Heber et al., 2007), step heating (Hiyagon and Ozima, 1986; Broadhurst et al., 1992).
View in article
Data discarded by authors due to contamination of crystals with bubbles are given in italics. For Hiyagon and Ozima (1986), glass composition is bulk sample starting composition.
View in article
Summary of Xe crystal/melt partition coefficients. Data abbreviations: H1986, Hiyagon and Ozima (1986); B1992, Broadhurst et al. (1992); H2007, Heber et al. (2007); C2022, Chen et al. (2022); PC265, Cell2, and Cell3 are data from this study (Table 2).
View in article
Additionally, the only high P previous dataset for olivine/melt partitioning was obtained with only air adsorbed on samples as a source of noble gases (Hiyagon and Ozima, 1986).
View in article
In minerals, the tightly bound nature of Xe leads to the suggestion that either interstitial sites or crystal vacancies are possible sites for the Xe atoms (Hiyagon and Ozima, 1986).
View in article
Kaneoka, I., Takaoka, N., Clague, D.A. (1983) Noble gas systematics for coexisting glass and olivine crystals in basalts and dunite xenoliths from Loihi Seamount. Earth and Planetary Science Letters 66, 427–437. https://doi.org/10.1016/0012-821X(83)90156-5
 Show in context
Show in context Consistently, natural measurements of Xe mineral/basalt partitioning obtained by analysing parent and partially crystallised magmas (Batiza et al., 1979), or coexisting magma and olivine crystals (Kaneoka et al., 1983), show Xe compatibility with DXecrystal/basalt a few-fold above unity. Natural measurements must, nonetheless, be considered with caution (Carroll and Draper, 1994) due to potential magma degassing processes if minerals and melt did not re-equilibrate, and/or if crystals contain vapour inclusions (Kaneoka et al., 1983), which as for experiments are difficult to interpret.
View in article
Considered together, the present high P-T clinopyroxene-feldspar/melt and olivine/melt data are consistent with the natural reports of bulk peridotite/melt partitioning coefficient a few-fold above unity from oceanic island basalts (Batiza et al., 1979; Kaneoka et al., 1983).
View in article
Krummenacher, D., Merrihue, C.M., Pepin, R.O., Reynolds, J.H. (1962) Meteoritic krypton and barium versus the general isotopic anomalies in xenon. Geochimica et Cosmochimica Acta 26, 231–249. https://doi.org/10.1016/0016-7037(62)90014-5
 Show in context
Show in context Xenon is also unique for its enigmatic atmospheric depletion relative to lighter noble gases (Anders and Owen, 1977), known as the ‘Xe paradox’, and its strong depletion in light isotopes (Krummenacher et al., 1962).
View in article
Leroy, C., Bureau, H., Sanloup, C., Raepsaet, C., Glazyrin, K., Munsch, P., Harmand, M., Prouteau, G., Khodja, H. (2019) Xenon and iodine behaviour in magmas. Earth and Planetary Science Letters 522, 144–154. https://doi.org/10.1016/j.epsl.2019.06.031
 Show in context
Show in context The latter study may underestimate DXeolivine/basalt as 1) the melt was enriched in silica (Table 1) while Xe solubility increases by a factor of five, for instance, between a MORB and a haplogranite (Leroy et al., 2019), and 2) crystals with bubbles were discarded on the basis of potential contamination while, at least, some of the bubbles are expected to form upon T-quenching, although this effect could be restricted to high P conditions.
View in article
In magmas, noble gases enter the ring structure of silicate melts (Carroll and Stolper, 1993), eventually leading to oxidation in the case of Xe in compressed silica-rich melt (Leroy et al., 2019).
View in article
Ozima, M., Miura, Y., Podosek, F. (2002) Revisiting I-Xe systematics, an early solar system chronometer. Geochimica et Cosmochimica Acta 66, A576.
 Show in context
Show in context Noble gases provide unique clues to unravel the geochemical evolution of volatile elements upon Earth’s formation to present day geodynamics (Ozima et al., 2002 and references therein), assuming their inertness and volatile behaviour.
View in article
Parai, R. (2022) A dry ancient plume mantle from noble gas isotopes. Proceedings of the National Academy of Sciences 119, e2201815119. https://doi.org/10.1073/pnas.2201815119
 Show in context
Show in context Importantly, Xe retention does not extend to lower mantle silicates (Shcheka and Keppler, 2012), consistent with the plume source being depleted in Xe compared to MORB source (Parai, 2022).
View in article
Péron, S., Moreira, M. (2018) Onset of volatile recycling into the mantle determined by xenon anomalies. Geochemical Perspectives Letters 9, 21–25. https://doi.org/10.7185/geochemlet.1833
 Show in context
Show in context The late veneer chondritic input to the atmosphere followed by partial-only Xe degassing concomitantly with the emplacement of the continental crust led to the rising Kr/Xe ratio and progressively heavier Xe observed in Archean atmospheric samples (Broadley et al., 2022) until the late veneer got overprinted, while lighter Xe in the mantle (Peron and Moreira, 2018) would result from mixing mass fractionated Xe with subducted Archean atmospheric Xe.
View in article
Poreda, R.J., Farley, K.A. (1992) Rare gases in Samoan xenoliths. Earth and Planetary Science Letters 113, 129–144. https://doi.org/10.1016/0012-821X(92)90215-H
 Show in context
Show in context Facing these experimental controversies, the peridotitic database shows an enrichment in Xe over other noble gases in xenoliths (Hennecke and Manuel, 1975; Poreda and Farley, 1992; Czuppon et al., 2009).
View in article
Probert, M.I.J. (2010) An ab initio study of xenon retention in α-quartz. Journal of Physics: Condensed Matter 22, 025501. https://doi.org/10.1088/0953-8984/22/2/025501
 Show in context
Show in context Theoretical calculations have shown that Xe can chemically bond to oxygen in quartz (Probert, 2010; Crépisson et al., 2019) and olivine (Crépisson et al., 2018) under modest P by substituting a Si atom.
View in article
Rzeplinski, I., Sanloup, C., Gilabert, E., Horlait, D. (2022) Hadean isotopic fractionation of xenon retained in deep silicates. Nature 606, 713–717. https://doi.org/10.1038/s41586-022-04710-4
 Show in context
Show in context Atmospheric escape and trapping-at-depth scenarios have been proposed to explain both observations (Ardoin et al., 2022; Broadley et al., 2022; Rzeplinski et al., 2022 and references therein), and are not exclusive.
View in article
The petrological behaviour of Xe hence supports the trapping-at-depth scenario (Rzeplinski et al., 2022), whereby a succession of collisions between pre-planetary embryos led to the depletion in terrestrial and Martian Xe light isotopes due to Xe trapping and oxidation in crystallising magma oceans, while over 99 % (Harper and Jacobsen, 1996) of the initial budget was expelled from growing planetesimals by exsolution from melt at low P in convecting magma oceans followed by atmospheric losses on a few Myr timescale.
View in article
That the retention of Xe differs in minerals while its impact on isotopic fractionating is similar (Rzeplinski et al., 2022) implies that Xe elemental and isotopic evolution cannot be modelled by the same Rayleigh distillation law. Indeed, Rayleigh-predicted Kr/Xe ratios for the Archean atmosphere disagree (Broadley et al., 2022), a mismatch attributed to variable Xe loss over time which is challenging to reconcile with a continuous isotopic evolution.
View in article
Sanloup, C., Schmidt, B.C., Gudfinnsson, G., Dewaele, A., Mezouar, M. (2011) Xenon and Argon: A contrasting behavior in olivine at depth. Geochimica et Cosmochimica Acta 75, 6271–6284. https://doi.org/10.1016/j.gca.2011.08.023
 Show in context
Show in context Some data were discarded on this ground, despite sometimes clear elemental fractionation from the original gas (Hiyagon and Ozima, 1986), which is not expected for passively trapped gas. Indeed, Xe was observed to retro-diffuse out of olivine in high P experiments upon T-quenching (Sanloup et al., 2011), an exsolution process that could explain at least part of the bubbles observed on quenched samples.
View in article
Xenon X-ray fluorescence signal in olivine crystals is strongly affected by quenching T at high P, with a 2.9 factor decrease in intensity (Fig. 1a), indicating important—although incomplete—Xe exsolution back to room T, consistent with previous observation from in situ X-ray diffraction (Sanloup et al., 2011).
View in article
Schmidt, B.C., Keppler, H. (2002) Experimental evidence for high noble gas solubilities in silicate melts under mantle pressures. Earth and Planetary Science Letters 195, 277–290. https://doi.org/10.1016/S0012-821X(01)00584-2
 Show in context
Show in context It is, nonetheless, established that melt composition strongly affects trace element partitioning (Schmidt et al., 2006), with one order of magnitude difference between gabbroic and granitic melts, an effect also expected to be strong for noble gases based on solubility values in melts (Carroll and Stolper, 1993; Schmidt and Keppler, 2002).
View in article
The starting sample (Supplementary Information) is a synthetic glass relevant for lunar-like magma ocean at the stage of anorthite crystallisation, doped by high P-T synthesis with 0.05 wt. % Xe (Table S-1), i.e. well below Xe solubility in tholeiitic melt (Schmidt and Keppler, 2002) of 0.41 wt. % at 2 GPa to avoid supersaturation, while being high enough for the Xe fluorescence signal to be significantly above noise level.
View in article
Schmidt, M.W., Connolly, J.A.D., Günther, D., Bogaerts, M. (2006) Element Partitioning: The Role of Melt Structure and Composition. Science 312, 1646–1650. https://doi.org/10.1126/science.1126690
 Show in context
Show in context It is, nonetheless, established that melt composition strongly affects trace element partitioning (Schmidt et al., 2006), with one order of magnitude difference between gabbroic and granitic melts, an effect also expected to be strong for noble gases based on solubility values in melts (Carroll and Stolper, 1993; Schmidt and Keppler, 2002).
View in article
Shcheka, S.S., Keppler, H. (2012) The origin of the terrestrial noble-gas signature. Nature 490, 531–534. https://doi.org/10.1038/nature11506
 Show in context
Show in context Importantly, Xe retention does not extend to lower mantle silicates (Shcheka and Keppler, 2012), consistent with the plume source being depleted in Xe compared to MORB source (Parai, 2022).
View in article
Zahnle, K.J., Gacesa, M., Catling, D.C. (2019) Strange messenger: A new history of hydrogen on Earth, as told by Xenon. Geochimica et Cosmochimica Acta 244, 56–85. https://doi.org/10.1016/j.gca.2018.09.017
 Show in context
Show in context However, the main challenge is how to lift the heavy Xe through the atmosphere up to levels where it can be lost (Zahnle et al., 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Methods
- Tables S-1 and S-2
- Figures S-1 to S-5
- MS Datasheets S-1 to S-7
- Supplementary Information References
Download the Supplementary Information (PDF)
Figures
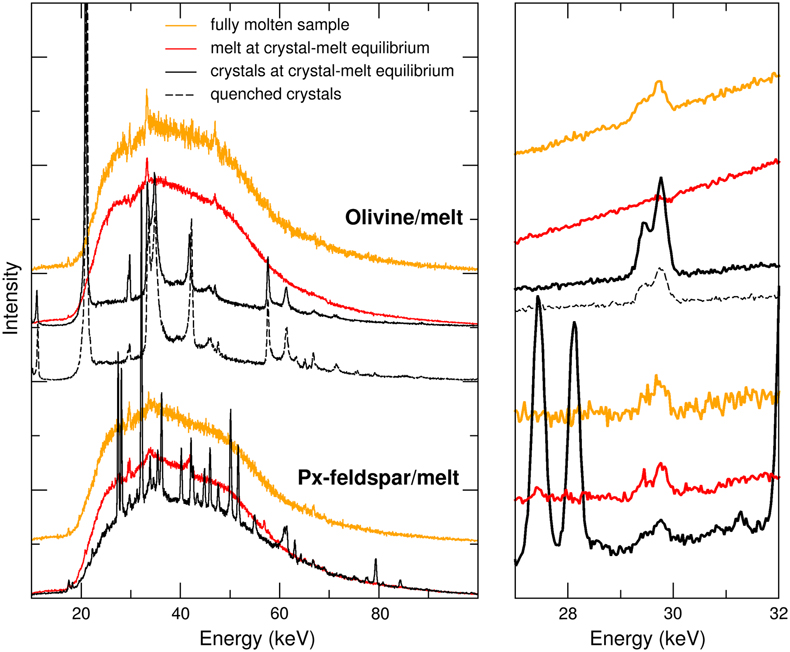
Figure 1 Energy-dispersive X-ray data sets. (Left) Full data sets collected at 10.031°. For each crystal/melt system, data collected at different T are vertically spaced for clarity: fully molten sample (orange), partially molten sample (red for molten zone, black for crystalline or crystal-rich zone), and quenched crystals (black dashed). Peaks at 33 keV and 47 keV are MgO diffraction peaks from the cell-assembly. (Right) Zoom on the Xe Kα1 and Kα2 fluorescence lines (29.4 keV and 29.7 keV). For olivine-melt experiments, data sets in the zoomed panel were collected at 4.0285° to avoid diffraction peaks from crystals overlapping with Xe fluorescence lines.

Figure 2 Summary of Xe crystal/melt partition coefficients. Data abbreviations: H1986, Hiyagon and Ozima (1986)
Hiyagon, H., Ozima, M. (1986) Partition of noble gases between olivine and basalt melt. Geochimica et Cosmochimica Acta 50, 2045–2057. https://doi.org/10.1016/0016-7037(86)90258-9
; B1992, Broadhurst et al. (1992)Broadhurst, C.L., Drake, M.J., Hagee, B.E., Bernatowicz, T.J. (1992) Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochimica et Cosmochimica Acta 56, 709–723. https://doi.org/10.1016/0016-7037(92)90092-W
; H2007, Heber et al. (2007)Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
; C2022, Chen et al. (2022)Chen, Q., Sanloup, C., Bureau, H., Rzeplinski, I., Glazyrin, K., Farla, R. (2022) Probing the partitioning behaviour of Xe using in situ X-ray synchrotron techniques at high P–T conditions. High Pressure Research 42, 318–335. https://doi.org/10.1080/08957959.2022.2144290
; PC265, Cell2, and Cell3 are data from this study (Table 2). Empty symbols indicate room P data; filled symbols indicate data collected between 1.0 and 2.0 GPa (cf. Table 1). Note that Heber et al. (2007)Heber, V.S., Brooker, R.A., Kelley, S.P., Wood, B.J. (2007) Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71, 1041–1061. https://doi.org/10.1016/j.gca.2006.11.010
discarded all values above unity due to the observation of gas bubbles in crystals, while at least some might form from exsolution upon quenching. Natural rocks dataset: residual rock/basalt partition coefficients from Batiza et al. (1979)Batiza, R., Bernatowicz, T.J., Hohenberg, C.M., Podosek, F.A. (1979) Relations of noble gas abundances to petrogenesis and magmatic evolution of some oceanic basalts and related differentiated volcanic rocks. Contributions to Mineralogy and Petrology 69, 301–313. https://doi.org/10.1007/BF00372332
.





