Abiotic syntheses of pyrite: clues to assess the biogenicity of pyrite spherules
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:321Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
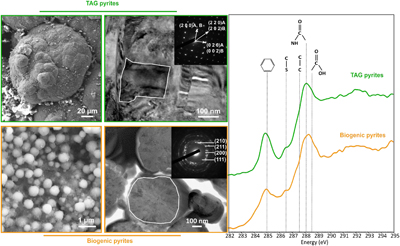 Figure 1 SEM, TEM (left) and STXM characterisation (right) of pyrite aggregates isolated from TAG hydrothermal field (in green) compared to biogenic pyrites produced by living T. kodakarensis KOD1 cells (in orange) (Truong et al., 2023). |  Figure 2 SEM investigations of pyrites produced at 85 °C for 96 hr in a colloidal sulfur and Fe2+-rich medium compared to (a) pyrites isolated from TAG hydrothermal field, and (b) biogenic pyrite spherules produced in presence of KOD1 cells (Truong et al., 2023). (c) No pyrite produced in absence of organic matter. (d) Pyrites produced in presence of yeast extract and tryptone. (e–f) Micrometric pyrite crystals produced in presence of graphitic carbon. (g) Massive rounded pyrites measuring 10 μm produced in presence of KOD1 envelopes. (h) Rounded pyrites produced in presence of KOD1 lysates. (i) No pyrite produced in presence of both KOD1 supernatant and intracellular material but a predominance of colloidal phosphates as evidence by (j) associated EDXS spectrum. | 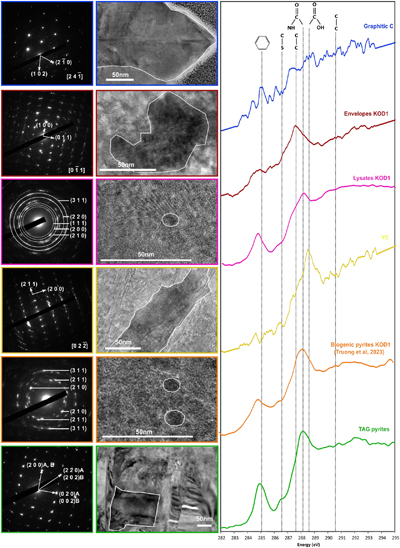 Figure 3 TEM, associated SAED patterns and STXM characterisation of TAG rounded pyrites (in green), biogenic pyrites (in orange) and abiotic counterparts produced at 85 °C for 96 hr in a colloidal sulfur and Fe2+-rich medium: yeast extract and tryptone (in yellow), KOD1 lysates (in pink), KOD1 envelopes (in red) and graphitic carbon (in blue). Of note, spots (100) and (011) are not allowed by the Pa3 space group of pyrite but are here enabled by double diffraction. The white outines correspond to the crystalline domain diffracted in the associated SAED pattern (TAG pyrites; YE; envelopes KOD1; Graphitic C), or to a putative crystalline domain for powder SAED pattern (biogenic pyrite KOD1; lysates KOD1). | 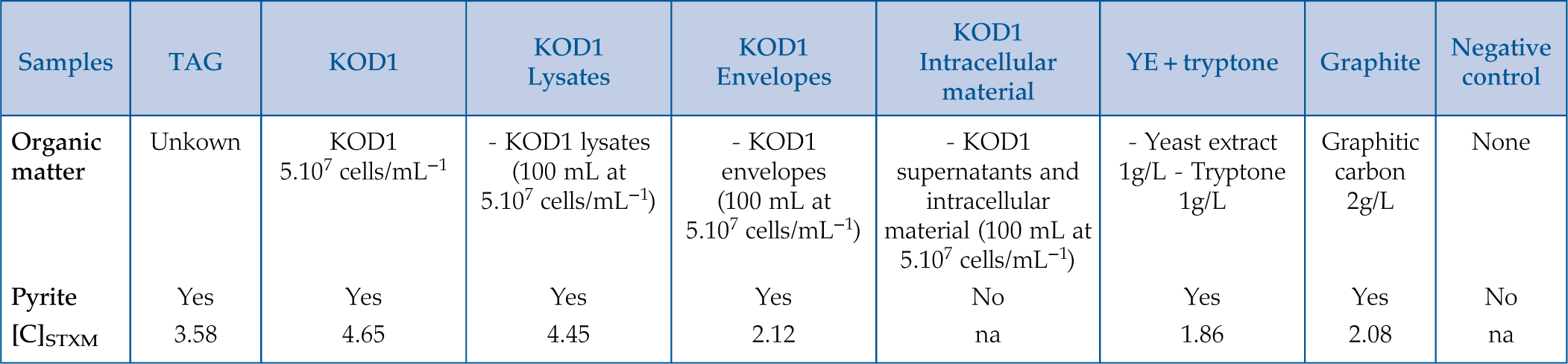 Table 1 Summary of the production of pyrites and their carbon content for abiotic synthesis made with different types of organic matter (85 °C – 96 hr), compared to biogenic pyrites (i.e. KOD1) and TAG pyrites. |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
top
Letter
In 50 years of research, the known thermal limit of life has been extended several times, rising from 70 °C, to over 100 °C, to 113 °C and finally to 122 °C (Brock and Freeze, 1969
Brock, T. D., Freeze, H. (1969) Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. Journal of bacteriology 98, 289–297. https://doi.org/10.1128/jb.98.1.289-297.1969
; Blöch et al., 1997Blöchl, E., Rachel, R., Burggraf, S., Hafenbradl, D., Jannasch, H.W., Stetter, K.O. (1997) Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113 C. Extremophiles 1, 14–21. https://doi.org/10.1007/s007920050010
; Kashefi and Lovley, 2003Kashefi, K., Lovley, D.R. (2003) Extending the upper temperature limit for life. Science 301, 934–934. https://doi.org/10.1126/science.1086823
; Takai et al., 2008Takai, K., Nakamura, K., Toki, T., Tsunogai, U., Miyazaki, M., Miyazaki, J., Horikoshi, K. (2008) Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proceedings of the National Academy of Sciences, 105, 10949–10954. https://doi.org/10.1073/pnas.0712334105
). Current estimates set the maximum temperature at which life could exist between 113 °C and 150 °C (Stetter, 1999Stetter, K.O. (1999) Extremophiles and their adaptation to hot environments. FEBS Letters, 452, 22–25. https://doi.org/10.1016/S0014-5793(99)00663-8
; Cowan and Tow, 2004Cowan, D.A., Tow, L.A. (2004) Endangered Antarctic environments. Annual Reviews in Microbiology 58, 649–690. https://doi.org/10.1146/annurev.micro.57.030502.090811
; Merino et al., 2019Merino, N., Aronson, H.S., Bojanova, D.P., Feyhl-Buska, J., Wong, M.L., Zhang, S., Giovannelli, D. (2019) Living at the extremes: extremophiles and the limits of life in a planetary context. Frontiers in microbiology 10, 780. https://doi.org/10.3389/fmicb.2019.00780
). Life could therefore withstand the temperature conditions inside the external parts of chimneys of black smokers, whose pore spaces could harbour microhabitats for hyperthermophilic microorganisms.The incubation of chimney pieces enables the isolation of various microbial strains (e.g., Takai et al., 2001
Takai, K., Komatsu, T., Inagaki, F., Horikoshi, K. (2001) Distribution of Archaea in a black smoker chimney structure. Applied Environmental Microbiology 67, 3618–3629. https://doi.org/10.1128/AEM.67.8.3618-3629.2001
; Nercessian et al., 2003Nercessian, O., Reysenbach, A.L., Prieur, D., Jeanthon, C. (2003) Archaeal diversity associated with in situ samplers deployed on hydrothermal vents on the East Pacific Rise (13 degrees N). Environmental Microbiology 5, 492–502. https://doi.org/10.1046/j.1462-2920.2003.00437.x
). The detection of biomarkers in chimney samples, such as the gene coding for 16S ribosomal RNA (Schrenk et al., 2003Schrenk, M., Kelley, D., Delaney, J., Baross, J. (2003) Incidence and diversity of microorganisms within the walls of an active deep-sea sulfide chimney. Applied and Environmental Microbiology 69, 3580–3592. https://doi.org/10.1128/AEM.69.6.3580-3592.2003
) or bacterial and archaeal lipids (Blumenberg, 2007Blumenberg, M., Seifert, R., Petersen, S., Michaelis, W. (2007) Biosignatures present in a hydrothermal massive sulfide from the Mid-Atlantic Ridge. Geobiology 5, 435–450. https://doi.org/10.1111/j.1472-4669.2007.00126.x
), has led some authors to suggest that chimneys are, or have been, colonised by (hyper)thermophilic microorganisms. However, biomarkers may have been transported from the surrounding fluids within the chimney wall after their production. To date, there is still no direct observation of the presence of (hyper)thermophilic microorganisms in the walls of black smoker chimneys. The abrupt fluctuations in temperature, pH and redox conditions of sulfur-rich hydrothermal vents (Tivey et al., 2002Tivey, M.A., Bradley, A. M., Joyce, T. M., Kadko, D. (2002) Insights into tide-related variability at seafloor hydrothermal vents from time-series temperature measurements. Earth and Planetary Science Letters 202, 693–707. https://doi.org/10.1016/S0012-821X(02)00801-4
; Schrenk et al., 2003Schrenk, M., Kelley, D., Delaney, J., Baross, J. (2003) Incidence and diversity of microorganisms within the walls of an active deep-sea sulfide chimney. Applied and Environmental Microbiology 69, 3580–3592. https://doi.org/10.1128/AEM.69.6.3580-3592.2003
; Lin et al., 2016Lin, T., Ver Eecke, H., Breves, E., Dyar, M., Jamieson, J., Hannington, M., Dahle, H., Bishop, J., Lane, M., Butterfield, D., Kelley, D., Lilley, M., Baross, J., Holden, J. (2016) Linkages between mineralogy, fluid chemistry, and microbial communities within hydrothermal chimneys from the Endeavour segment, Juan de Fuca ridge. Geochemistry Geophysics Geosystems 17, 300–323. https://doi.org/10.1002/2015GC006091
) make in situ observations difficult.A recent study of chimney samples from a black smoker at the Trans-Atlantic Geotraverse (TAG) site on the Mid-Atlantic Ridge revealed the presence of pyrite (FeS2) spherules, whose morphologies and chemical characteristics suggested that they constitute a biosignature (Truong et al., 2024
Truong, C., Bernard, S., Baudin, F., Gorlas, A., Guyot, F. (2024) Carbon-containing pyrite spherules: mineral biosignature in black smokers? European Journal of Mineralogy 36, 813–83. https://doi.org/10.5194/ejm-36-813-2024
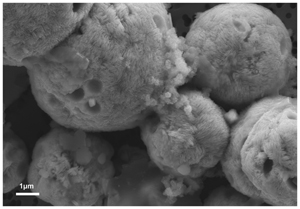
). These polycrystalline pyrite spherules exhibit a rounded shape, with a diameter ranging between 2 μm and 100 μm (Fig. 1 and Fig. 2a) showing some similarities with pyrite spherules made in mineralised cultures of the hyperthermophilic Thermococcus kodakarensis KOD1 archaeon (Truong et al., 2023Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
) and with pyrite spherules found in culture experiments at ambient temperature (Berg et al., 2020Berg, J.S., Duverger, A., Cordier, L., Laberty-Robert, C., Guyot, F., Miot, J. (2020) Rapid pyritization in the presence of a sulfur/sulfate-reducing bacterial consortium. Scientific Reports 10, 8264. https://doi.org/10.1038/s41598-020-64990-6
; Duverger et al., 2020Duverger, A., Berg, J.S., Busigny, V., Guyot, F., Bernard, S., Miot, J. (2020) Mechanisms of pyrite formation promoted by sulfate-reducing bacteria in pure culture. Frontiers in Earth Science 8, 588310. https://doi.org/10.3389/feart.2020.588310
). Moreover, these pyrite spherules contain organic compounds displaying a spectroscopic signature similar to that of a living cell, with a predominance of amide groups (Fig. 1). The similarities between the pyrite spherules produced in the presence of Thermococcales and those observed in the chimneys at the TAG site now call for a better understanding of pyrite production, the possible abiotic production of such pyrite spherules needs to be excluded to consider them biosignatures. In other words, a number of questions remain to be addressed. Can pyrite spherules be produced abiotically? Is organic matter necessary for their production? Does the nature of organic material present influence the morphology and microtexture of these spherules? Do the pyrites record information from the initially present organic matter? Providing answers to these questions requires conducting abiotic syntheses of pyrites under conditions mimicking those existing within chimneys of black smokers.
Figure 1 SEM, TEM (left) and STXM characterisation (right) of pyrite aggregates isolated from TAG hydrothermal field (in green) compared to biogenic pyrites produced by living T. kodakarensis KOD1 cells (in orange) (Truong et al., 2023
Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
).
Figure 2 SEM investigations of pyrites produced at 85 °C for 96 hr in a colloidal sulfur and Fe2+-rich medium compared to (a) pyrites isolated from TAG hydrothermal field, and (b) biogenic pyrite spherules produced in presence of KOD1 cells (Truong et al., 2023
Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
). (c) No pyrite produced in absence of organic matter. (d) Pyrites produced in presence of yeast extract and tryptone. (e–f) Micrometric pyrite crystals produced in presence of graphitic carbon. (g) Massive rounded pyrites measuring 10 μm produced in presence of KOD1 envelopes. (h) Rounded pyrites produced in presence of KOD1 lysates. (i) No pyrite produced in presence of both KOD1 supernatant and intracellular material but a predominance of colloidal phosphates as evidence by (j) associated EDXS spectrum.Here, we report the results of abiotic syntheses carried out under experimental conditions reproducing those of black smokers., i.e. in a medium rich in reactive Fe2+ and reduced sulfur compounds (Gartman et al., 2011
Gartman, A., Yücel, M., Madison, A., Chu, D., Ma, S., Janzen, C., Becker, E., Beinart, R., Girguis, P., Luther, G. (2011) Sulfide oxidation across diffuse flow zones of hydrothermal vents. Aquatic Geochemistry 17, 583–601. https://doi.org/10.1007/s10498-011-9136-1
; Findlay et al., 2016Findlay, A.J. (2016) Microbial impact on polysulfide dynamics in the environment, FEMS Microbiology Letters 363. https://doi.org/10.1093/femsle/fnw103
). Solutions containing colloidal sulfur (20 mM), ferrous iron Fe2+ (FeSO4 5 mM) and sulfide (Na2S 1.3 mM) were submitted to 85 °C for 96 hr in the presence (or not) of various carbon compounds (Table 1). First, a control experiment was carried out in the absence of any organic material. Then, a series of two syntheses were conducted in the presence of organic compounds unrelated to archaea cells: (1) in the presence of poorly soluble and poorly reactive graphitic carbon flakes, and (2) in the presence of the yeast extract and tryptone often used for the growth of cultures of Thermococcales (e.g., Gorlas et al., 2015Gorlas, A., Marguet, E., Gill, S., Geslin, C., Guigner, J.-M., Guyot, F., Forterre, P. (2015) Sulfur vesicles from Thermococcales: A possible role in sulfur detoxifying mechanisms. Biochimie 118, 356–364. https://doi.org/10.1016/j.biochi.2015.07.026
, 2018Gorlas, A., Jacquemot, P., Guigner, J.-M., Gill, S., Forterre, P., Guyot, F. (2018) Greigite nanocrystals produced by hyperthermophilic archaea of Thermococcales order. PLoS One 13, e0201549. https://doi.org/10.1371/journal.pone.0201549
, 2022Gorlas, A., Mariotte, T., Morey, L., Truong, C., Bernard, S., Guigner, J.-M., Oberto, J., Baudin, F., Landrot, G., Baya, C., Le Pape, P., Morin, G., Forterre, P., Guyot, F. (2022) Precipitation of greigite and pyrite induced by Thermococcales: an advantage to live in Fe- and S-rich environments? Environmental Microbiology 24, 626–642. https://doi.org/10.1111/1462-2920.15915
). Another series of three syntheses were also conducted in the presence of organic compounds produced by hyperthermophilic T. kodakarensis KOD1 cells: (1) in the presence of cell envelopes consisting of lipid-rich cell membranes and S-layer proteins, recovered from cell lysis, named hereafter KOD1 envelopes, (2) in the presence of the supernatant and intracellular material, the last being recovered from cell lysis, hereafter named KOD1 supernatant + intracellular material, and (3) in the presence of whole cell lysates, i.e. containing the envelopes and the intracellular material of KOD1, hereafter named KOD1 lysates (for each fraction, see experimental protocol in Supplementary Information). The products of these syntheses were then characterised using scanning electron microscopy (SEM), transmission electron microscopy (TEM) and scanning transmission X-ray microscopy coupled to X-ray absorption near edge structure spectroscopy (STXM-XANES) to document the nature, the morphology and the microtexture of the pyrite produced as well as the nature of their potential organic content.Table 1 Summary of the production of pyrites and their carbon content for abiotic synthesis made with different types of organic matter (85 °C – 96 hr), compared to biogenic pyrites (i.e. KOD1) and TAG pyrites.
| Samples | TAG | KOD1 | KOD1 Lysates | KOD1 Envelopes | KOD1 Intracellular material | YE + tryptone | Graphite | Negative control |
| Organic matter | Unkown | KOD1 5.107 cells/mL−1 | - KOD1 lysates (100 mL at 5.107 cells/mL−1) | - KOD1 envelopes (100 mL at 5.107 cells/mL−1) | - KOD1 supernatants and intracellular material (100 mL at 5.107 cells/mL−1) | - Yeast extract 1g/L - Tryptone 1g/L | Graphitic carbon 2g/L | None |
| Pyrite | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| [C] STXM | 3.58 | 4.65 | 4.45 | 2.12 | na | 1.86 | 2.08 | na |
No pyrite was produced in the absence of organic material (Fig. 2c), highlighting that the presence of colloidal sulfur, ferrous iron and sulfide is not sufficient to produce pyrite under the experimental conditions chosen in this study. Indeed, it is known that higher concentrations of sulfides (typically 180 mM of Na2S instead of the 1.3 mM used in the present study) are needed to allow the H2S pathway of abiotic pyrite precipitation (e.g., Mansor and Fantle, 2019
Mansor, M., Fantle, M.S. (2019) A novel framework for interpreting pyrite-based Fe isotope records of the past. Geochimica et Cosmochimica Acta 253, 39–62. https://doi.org/10.1016/j.gca.2019.03.017
). On the other hand, pyrites were produced in almost all the syntheses conducted in the presence of organic matter, with sizes, morphologies and microtextures depending on the organic matter used for synthesis. Pyrites produced in the presence of graphitic carbon flakes are sub-micrometric (around 1 μm or less) and can be observed on the surface of graphitic carbon flakes as sand-rose-like crystals (Fig. 2e,f). Similarly, the pyrites produced in the presence of yeast extract and tryptone display a sand-rose shape, but measure 2 to 10 μm in diameter (Fig. 2d). In the presence of KOD1 envelopes, pyrites appear as spherules measuring 10 μm in diameter, with no crystallites visible at the SEM scale (Fig. 2h). This is in contrast to the pyrite spherules measuring 2 to 5 μm produced in the presence of KOD1 lysates (Fig. 2i). Of note, no pyrite was produced in the presence of KOD1 intracellular material only (Fig. 2j). One explanation could be the need for reactive surface areas available for pyrite nucleation and growth, which are absent without organic matter and possibly insufficient in the synthesis with KOD1 intracellular matter alone. As yeast extract and tryptone also are very soluble organic compounds, another explanation could involve the concentration of soluble organic matter which has been demonstrated to influence organomineralisation (Cosmidis et al., 2019Cosmidis, J., Nims, C.W., Diercks, D., Templeton, A.S. (2019) Formation and stabilization of elemental sulfur through organomineralization. Geochimica et Cosmochimica Acta 247, 59–82. https://doi.org/10.1016/j.gca.2018.12.025
).Overall, considering the sizes and morphologies at the micrometre scale, only pyrites produced in the presence of KOD1 envelopes or KOD1 lysates show similarities with pyrite spherules produced in the presence of living T. kodakarensis KOD1 (Truong et al., 2023
Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
) and with pyrite spherules reported in the chimney walls of black smokers (Truong et al., 2024Truong, C., Bernard, S., Baudin, F., Gorlas, A., Guyot, F. (2024) Carbon-containing pyrite spherules: mineral biosignature in black smokers? European Journal of Mineralogy 36, 813–83. https://doi.org/10.5194/ejm-36-813-2024
).In line with previous observations, TEM and STXM analyses revealed that pyrites produced in the presence of graphitic carbon flakes exhibit significant differences from spherules produced in the presence of T. kodakarensis cells. The crystallographic domains are between 200 and 300 nm in size, and the low [C]STXM value of 2.08 suggests that these pyrites associated with graphite have trapped a very small amount of carbon, preventing a proper XANES spectrum to be collected (Fig. 3, Table 1). Pyrites produced in the presence of yeast extract and tryptone also display specific characteristics: they have crystallographic domains of several hundred nanometres and have trapped only a small amount of carbon, as evidenced by a very low [C]STXM value of 1.86. The XANES spectrum of this organic material is dominated by an absorption feature at 288.6 eV (attributed to COOH groups; Le Guillou et al., 2018
Le Guillou, C., Bernard, S., De la Peña, F., Le Brech, Y. (2018) XANES-Based Quantification of Carbon Functional Group Concentrations. Analytical Chemistry 90, 8379–8386. https://doi.org/10.1021/acs.analchem.8b00689
) (Fig. 3). Finally, pyrites produced in the presence of KOD1 envelopes do not display chemical characteristics similar to those of pyrite spherules produced in the presence of living cells: the microtexture itself is not very different, with crystalline domains between 50 and 100 nm in size, but they contain less organic matter (the [C]STXM value is only 2.12). The XANES spectrum of this organic carbon is consistent with lipids, with a small absorption feature at 285.1 eV (attributed to C=C bonds; Le Guillou et al., 2018Le Guillou, C., Bernard, S., De la Peña, F., Le Brech, Y. (2018) XANES-Based Quantification of Carbon Functional Group Concentrations. Analytical Chemistry 90, 8379–8386. https://doi.org/10.1021/acs.analchem.8b00689
) and a main absorption feature at 287.6 eV (attributed to C-H bonds; Le Guillou et al., 2018Le Guillou, C., Bernard, S., De la Peña, F., Le Brech, Y. (2018) XANES-Based Quantification of Carbon Functional Group Concentrations. Analytical Chemistry 90, 8379–8386. https://doi.org/10.1021/acs.analchem.8b00689
) (Fig. 3).
Figure 3 TEM, associated SAED patterns and STXM characterisation of TAG rounded pyrites (in green), biogenic pyrites (in orange) and abiotic counterparts produced at 85 °C for 96 hr in a colloidal sulfur and Fe2+-rich medium: yeast extract and tryptone (in yellow), KOD1 lysates (in pink), KOD1 envelopes (in red) and graphitic carbon (in blue). Of note, spots (100) and (011) are not allowed by the Pa3 space group of pyrite but are here enabled by double diffraction. The white outines correspond to the crystalline domain diffracted in the associated SAED pattern (TAG pyrites; YE; envelopes KOD1; Graphitic C), or to a putative crystalline domain for powder SAED pattern (biogenic pyrite KOD1; lysates KOD1).
In contrast, the pyrite spherules produced in the presence of KOD1 lysates closely resemble the pyrite spherules produced in the presence of living T. kodakarensis cells: the microtexture is identical with crystalline domains smaller than 10 nm, and the carbon content is similar, with a [C]STXM value of 4.45. The XANES spectrum of this organic material shows similar features to the spectra of living cells (Benzerara et al., 2006
Benzerara, K., Menguy, N., Lo´pez-García, P., Brown, G. Jr. (2006) Nanoscale detection of organic signatures in carbonate microbialites. Proceedings of the National Academy of Sciences 103, 9440–9445. https://doi.org/10.1073/pnas.0603255103
; Miot et al., 2009Miot, J., Benzerara, K., Obst, M., Kappler, A., Hegler, F., Schädler, S., Bouchez, C., Guyot, F., Morin, G. (2009) Extracellular iron biomineralization by photoautotrophic iron-oxidizing bacteria. Applied Environmental Microbiology 75, 5586–5591. https://doi.org/10.1128/AEM.00490-09
; Li et al., 2014Li, J.H., Bernard, S., Benzerara, K., Beyssac, O., Allard, T., Cosmidis, J., Moussou, J. (2014) Impact of biomineralization on the preservation of microorganisms during fossilization: An experimental perspective. Earth and Planetary Science Letters 400, 113–122. https://doi.org/10.1016/j.epsl.2014.05.031
; Picard et al., 2021Picard, A., Gartman, A., Girguis, P.R. (2021) Interactions Between Iron Sulfide Minerals and Organic Carbon: Implications for Biosignature Preservation and Detection. Astrobiology 21, 587–604. https://doi.org/10.1089/ast.2020.2276
; Truong et al., 2023Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
) and to the spectrum of organic material trapped within the pyrite spherules produced in the presence of living T. kodakarensis cells (Truong et al., 2023Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
), with absorption features at 285.1, 286.5, 287.6, 288.1 and 288.6 eV, attributed to C=C bonds, C-S bonds, C-H bonds, amide groups and carboxylic groups, respectively (Le Guillou et al., 2018Le Guillou, C., Bernard, S., De la Peña, F., Le Brech, Y. (2018) XANES-Based Quantification of Carbon Functional Group Concentrations. Analytical Chemistry 90, 8379–8386. https://doi.org/10.1021/acs.analchem.8b00689
) (Fig. 3). Indeed, these pyrites can be considered to have been produced according to the same recipe (with the same ingredients) as the pyrite spherules produced in the presence of living T. kodakarensis cells, the only difference being that here the cells were dead.Altogether, the results of these syntheses strongly suggest that the chemical nature of the initial organic matter controls the final morphology, microtexture and organic content of the pyrite produced. They also evidence that the production of pyrite spherules is – except for the spherule size distribution – not controlled by live T. kodakarensis cells but that it is rather a production induced by their biomass. An explanation for the production of pyrite spherules may reside in the highly reactive surfaces of microbial cells, either living or dead. Microbial surfaces exhibit organic ligands, which are indeed characterised by a net negative charge, thus conferring upon them the capability of creating strong bonds with Fe2+ (Beveridge, 1989
Beveridge, T. J. (1989) Role of Cellular Design in Bacterial Metal Accumulation and Mineralization. Annual Review of Microbiology 43, 147–171. https://doi.org/10.1146/annurev.mi.43.100189.001051
). Nevertheless, it cannot be ruled out that complex negatively charged abiotic compounds may also play this role. Another important factor may be that organic matter acts as a redox-active component that can promote the formation of intermediate sulfur species, i.e. polysulfides. The presence or enrichment of certain functional groups can have a direct impact on sulfide production, notably via the presence of surface oxygen groups.The amount of carbon sequestered inside pyrite spherules appears to be important for their potential use as biosignatures. In a recent experimental study, Nabeh et al. (2022)
Nabeh, N., Brokaw, C., Picard, A. (2022) Quantification of Organic Carbon Sequestered by Biogenic Iron Sulfide Minerals in Long-Term Anoxic Laboratory Incubations. Frontiers in Microbiology 13, 662219. https://doi.org/10.3389/fmicb.2022.662219
investigated the precipitation of iron sulfides in the presence of various organic compounds (amino acids, tryptone, yeast extract and microbial cells). Their results demonstrated, consistent with the present results, that the sequestration of organic material by iron sulfides in the presence of amino acids, tryptone and yeast extract is low compared to the sequestration of organic material by iron sulfides in the presence of microbial cells (Nabeh et al., 2022Nabeh, N., Brokaw, C., Picard, A. (2022) Quantification of Organic Carbon Sequestered by Biogenic Iron Sulfide Minerals in Long-Term Anoxic Laboratory Incubations. Frontiers in Microbiology 13, 662219. https://doi.org/10.3389/fmicb.2022.662219
). Their explanation involved the binding of iron sulfides and organic molecules on the surface of cells and the formation of organo-mineral aggregates (Nabeh et al., 2022Nabeh, N., Brokaw, C., Picard, A. (2022) Quantification of Organic Carbon Sequestered by Biogenic Iron Sulfide Minerals in Long-Term Anoxic Laboratory Incubations. Frontiers in Microbiology 13, 662219. https://doi.org/10.3389/fmicb.2022.662219
). According to the present results, another important factor would be, in addition to surface availability, the solubility of the organic material present and (maybe most importantly), its oxido-reduction potential. On the one hand, organic compounds locally control sulfide saturation conditions via their redox potential, which influences the size of the crystalline domains (Nývlt, 1968Nývlt, J. (1968) Kinetics of nucleation in solutions. Journal of Crystal Growth 3, 377–383. https://doi.org/10.1016/0022-0248(68)90179-6
). On the other hand, if very soluble, they may be sequestrated within the pyrites at the grain sub-joints, thus stabilising the ultra-small domains and protecting them from recrystallisation.Regarding the size range, the pyrite spherules found within the chimney walls of TAG mound’s black smoker (Fig. 1) (Truong et al., 2024
Truong, C., Bernard, S., Baudin, F., Gorlas, A., Guyot, F. (2024) Carbon-containing pyrite spherules: mineral biosignature in black smokers? European Journal of Mineralogy 36, 813–83. https://doi.org/10.5194/ejm-36-813-2024
) are more similar to the pyrite spherules produced in the presence of KOD1 lysates than to the pyrites produced in the presence of living T. kodakarensis cells. Still, the pyrite spherules from the TAG mound display a larger variability in size and larger crystallographic domains (∼200 nm; Figs. 1 and 3). Growth and/or coalescence of pre-existing spherules as well as the constant, or at least unlimited and non-limiting, supply of Fe and S in natural hydrothermal environments for a duration obviously longer than the duration of the present syntheses may explain bigger sizes in the natural environment. Other parameters need to be better mimicked, especially the pressure and temperature conditions: pressure conditions are higher at the TAG mound (located at a depth of 3650 m, i.e. a hydrostatic pressure of 35 MPa) and temperatures may vary to values significantly higher than 85 °C, eventually leading to the recrystallisation and growth of pyrite produced at lower temperature. There is no doubt that additional abiotic syntheses as well as experimental fossilisation of biogenic pyrite spherules should be conducted to further investigate the possibility of unambiguously determining the origin (i.e. biogenic or abiotic) of natural pyrite spherules.A trail to follow could be the molecular structure of the organic material trapped within the pyrites. As demonstrated here, the chemical nature of the organic material detected within the pyrite spherules may inform the nature of organic compounds initially present in the system. Yet, hydrothermal vents are the location of extensive mixing between seawater (possibly rich in biogenic organic materials) and hydrothermal fluids (possibly rich in abiotic organic materials) (e.g., Maruyama et al., 1993
Maruyama, A., Mita, N., Higashihara, T. (1993) Particulate materials and microbial assemblages around the Izena black smoking vent in the Okinawa Trough. Journal of Oceanography 49, 353–367. https://doi.org/10.1007/BF02269570
; Dick et al., 2010Dick, G.J., Tebo, B.M. (2010) Microbial diversity and biogeochemistry of the Guaymas Basin deep-sea hydrothermal plume. Environmental Microbiology 12, 1334–1347. https://doi.org/10.1111/j.1462-2920.2010.02177.x
), making it difficult to identify sources. Organic compounds can be transported, allowing their presence within walls of chimney even though they have not been produced there. In such a case, one could expect some chemical selection (different organic compounds may not be transported similarly), which does not seem to be the case according to XANES data (Truong et al., 2024Truong, C., Bernard, S., Baudin, F., Gorlas, A., Guyot, F. (2024) Carbon-containing pyrite spherules: mineral biosignature in black smokers? European Journal of Mineralogy 36, 813–83. https://doi.org/10.5194/ejm-36-813-2024
). Given the thermal maturation that such mineral structures have inevitably experienced, it remains difficult to expect to detect pristine biogenic organic materials: amino acids are for instance irreversibly destroyed above 240 °C (Bada et al., 1995Bada, J.L., Miller, S.L., Zhao, M. (1995) The stability of amino acids at submarine hydrothermal vent temperatures. Origins of Life and Evolution of the Biosphere 25, 111–118. https://doi.org/10.1007/BF01581577
), which is a temperature well below that of the hydrothermal fluid circulating through a chimney (e.g,. Tivey et al., 2002Tivey, M.A., Bradley, A. M., Joyce, T. M., Kadko, D. (2002) Insights into tide-related variability at seafloor hydrothermal vents from time-series temperature measurements. Earth and Planetary Science Letters 202, 693–707. https://doi.org/10.1016/S0012-821X(02)00801-4
).All in all, can we consider the pyrite spherules as biosignatures? By definition, a biosignature provides irrefutable proof of biogenicity. The conformity of a biosignature does not only depend on its production by living organisms, but also on the inability of abiotic processes to produce it. There are no irrefutable criteria on which to base the claim that a mineral is biogenic: mineral biosignatures may thus not exist as such. To move forward, we need to rethink the concept of biosignature in terms of probability rather than irrefutability (McMahon and Jordan, 2022
McMahon, S., Jordan, S.F. (2022) A fundamental limit to the search for the oldest fossils. Nature Ecology & Evolution 6, 832–834. https://doi.org/10.1038/s41559-022-01777-0
; Malaterre et al., 2023Malaterre, C., Ten Kate, I. L., Baqué, M., Debaille, V., Grenfell, J.L., Javaux, E. J., Klenner, F., Lara, Y.J., McMahon, S., Moore, K., Noack, L., Patty L., Postberg, F. (2023) Is there such a thing as a biosignature? Astrobiology 23, 1213–1227. https://doi.org/10.1089/ast.2023.0042
). Given the environmental conditions, the morphology, microtexture and organic content (both its value and its nature), is it more likely that the mineral structure investigated was produced by (or in the presence of) living microorganisms or by abiotic processes? Here, the production of pyrite spherules involves the presence of ferrous iron, reactive sulfur in the form of colloidal sulfur as well as the presence of reactive surface and soluble cellular organic material. Within hydrothermal vents, colloidal sulfur can be produced by both living microorganisms and by abiotic processes (e.g., Findlay, 2016Findlay, A.J. (2016) Microbial impact on polysulfide dynamics in the environment, FEMS Microbiology Letters 363. https://doi.org/10.1093/femsle/fnw103
). In contrast, cellular organic material is exclusively produced by living organisms, or at least, a mixture of organic molecules similar in composition to a mixture that life produces is likely to have been produced by life.In such a scheme, it could be concluded that it is more likely that living organisms were involved in the production of the pyrite spherules from the TAG site given their size, morphology, microtexture and organic content (both its quantity and nature). However, an abiotic production of similar pyrite spherules in the presence of abiotic macromolecular organic molecules cannot be ruled out at this stage without further experimental research, using complex abiotic organic molecules while deploying additional characterisation (including isotopic measurements). Plus, given that temperature and/or supersaturation may influence pyrite production, experiments exploring the influence of both temperature and hydrodynamic conditions remain to be conducted.
top
Author Contributions
CT, SB, AG and FG contributed to the conception and design of the study. AG and CT conducted the Thermococcales cultures and the preparation of the lysates. CT conducted the mineralisation process in anoxic conditions. FG and CT conducted the electron microscopy analyses. SB and CT conducted the STXM analyses. CT wrote the first draft of the manuscript. CT, SB, AG and FG wrote the sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
top
Acknowledgements
We acknowledge the support of the support of the Bicose 2 campaign (IFREMER), IMPMC-MNHN microscopy platform and of the SOLEIL HERMES beamline. We thank Erwan Roussel and Marie-Anne Cambon-Bonavita (IFREMER) for providing us the valuable samples from the Bicose 2 campaign, Elisabeth Malassis (IMPMC) for her administrative support, Sylvain Pont (IMPMC) for his help in SEM-EDXS, Jean-Michel Guigner (IMPMC) for his help in TEM, David Troadec (IEMN) for the preparation of the FIB sections, Corentin Le Guillou (UMET) for his help with STXM and Stefan Stanescu for his expert support of HERMES beamline at SOLEIL. AG was supported by the Agence Nationale de la Recherche, project HYPERBIOMIN (ANR-20-CE02-0001-01). FG was supported by Institut Universitaire de France.
Editor: Andreas Kappler
top
References
Bada, J.L., Miller, S.L., Zhao, M. (1995) The stability of amino acids at submarine hydrothermal vent temperatures. Origins of Life and Evolution of the Biosphere 25, 111–118. https://doi.org/10.1007/BF01581577
 Show in context
Show in context Given the thermal maturation that such mineral structures have inevitably experienced, it remains difficult to expect to detect pristine biogenic organic materials: amino acids are for instance irreversibly destroyed above 240 °C (Bada et al., 1995), which is a temperature well below that of the hydrothermal fluid circulating through a chimney (e.g,. Tivey et al., 2002).
View in article
Berg, J.S., Duverger, A., Cordier, L., Laberty-Robert, C., Guyot, F., Miot, J. (2020) Rapid pyritization in the presence of a sulfur/sulfate-reducing bacterial consortium. Scientific Reports 10, 8264. https://doi.org/10.1038/s41598-020-64990-6
 Show in context
Show in context These polycrystalline pyrite spherules exhibit a rounded shape, with a diameter ranging between 2 μm and 100 μm (Fig. 1 and Fig. 2a) showing some similarities with pyrite spherules made in mineralised cultures of the hyperthermophilic Thermococcus kodakarensis KOD1 archaeon (Truong et al., 2023) and with pyrite spherules found in culture experiments at ambient temperature (Berg et al., 2020; Duverger et al., 2020).
View in article
Benzerara, K., Menguy, N., López-García, P., Brown, G. Jr. (2006) Nanoscale detection of organic signatures in carbonate microbialites. Proceedings of the National Academy of Sciences 103, 9440–9445. https://doi.org/10.1073/pnas.0603255103
 Show in context
Show in context The XANES spectrum of this organic material shows similar features to the spectra of living cells (Benzerara et al., 2006; Miot et al., 2009; Li et al., 2014; Picard et al., 2021; Truong et al., 2023) and to the spectrum of organic material trapped within the pyrite spherules produced in the presence of living T. kodakarensis cells (Truong et al., 2023), with absorption features at 285.1, 286.5, 287.6, 288.1 and 288.6 eV, attributed to C=C bonds, C-S bonds, C-H bonds, amide groups and carboxylic groups, respectively (Le Guillou et al., 2018) (Fig. 3).
View in article
Beveridge, T. J. (1989) Role of Cellular Design in Bacterial Metal Accumulation and Mineralization. Annual Review of Microbiology 43, 147–171. https://doi.org/10.1146/annurev.mi.43.100189.001051
 Show in context
Show in context Microbial surfaces exhibit organic ligands, which are indeed characterised by a net negative charge, thus conferring upon them the capability of creating strong bonds with Fe2+ (Beveridge, 1989).
View in article
Blöchl, E., Rachel, R., Burggraf, S., Hafenbradl, D., Jannasch, H.W., Stetter, K.O. (1997) Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113°C. Extremophiles 1, 14–21. https://doi.org/10.1007/s007920050010
 Show in context
Show in context In 50 years of research, the known thermal limit of life has been extended several times, rising from 70 °C, to over 100 °C, to 113 °C and finally to 122 °C (Brock and Freeze, 1969; Blöch et al., 1997; Kashefi and Lovley, 2003; Takai et al., 2008).
View in article
Blumenberg, M., Seifert, R., Petersen, S., Michaelis, W. (2007) Biosignatures present in a hydrothermal massive sulfide from the Mid-Atlantic Ridge. Geobiology 5, 435–450. https://doi.org/10.1111/j.1472-4669.2007.00126.x
 Show in context
Show in context The detection of biomarkers in chimney samples, such as the gene coding for 16S ribosomal RNA (Schrenk et al., 2003) or bacterial and archaeal lipids (Blumenberg, 2007), has led some authors to suggest that chimneys are, or have been, colonised by (hyper)thermophilic microorganisms.
View in article
Brock, T.D., Freeze, H. (1969) Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. Journal of Bacteriology 98, 289–297. https://doi.org/10.1128/jb.98.1.289-297.1969
 Show in context
Show in context In 50 years of research, the known thermal limit of life has been extended several times, rising from 70 °C, to over 100 °C, to 113 °C and finally to 122 °C (Brock and Freeze, 1969; Blöch et al., 1997; Kashefi and Lovley, 2003; Takai et al., 2008).
View in article
Cosmidis, J., Nims, C.W., Diercks, D., Templeton, A.S. (2019) Formation and stabilization of elemental sulfur through organomineralization. Geochimica et Cosmochimica Acta 247, 59–82. https://doi.org/10.1016/j.gca.2018.12.025
 Show in context
Show in context As yeast extract and tryptone also are very soluble organic compounds, another explanation could involve the concentration of soluble organic matter which has been demonstrated to influence organomineralisation (Cosmidis et al., 2019).
View in article
Cowan, D.A., Tow, L.A. (2004) Endangered Antarctic environments. Annual Reviews in Microbiology 58, 649–690. https://doi.org/10.1146/annurev.micro.57.030502.090811
 Show in context
Show in context Current estimates set the maximum temperature at which life could exist between 113 °C and 150 °C (Stetter, 1999; Cowan and Tow, 2004; Merino et al., 2019).
View in article
Dick, G.J., Tebo, B.M. (2010) Microbial diversity and biogeochemistry of the Guaymas Basin deep-sea hydrothermal plume. Environmental Microbiology 12, 1334–1347. https://doi.org/10.1111/j.1462-2920.2010.02177.x
 Show in context
Show in context Yet, hydrothermal vents are the location of extensive mixing between seawater (possibly rich in biogenic organic materials) and hydrothermal fluids (possibly rich in abiotic organic materials) (e.g., Maruyama et al., 1993; Dick et al., 2010), making it difficult to identify sources.
View in article
Duverger, A., Berg, J.S., Busigny, V., Guyot, F., Bernard, S., Miot, J. (2020) Mechanisms of pyrite formation promoted by sulfate-reducing bacteria in pure culture. Frontiers in Earth Science 8, 588310. https://doi.org/10.3389/feart.2020.588310
 Show in context
Show in context These polycrystalline pyrite spherules exhibit a rounded shape, with a diameter ranging between 2 μm and 100 μm (Fig. 1 and Fig. 2a) showing some similarities with pyrite spherules made in mineralised cultures of the hyperthermophilic Thermococcus kodakarensis KOD1 archaeon (Truong et al., 2023) and with pyrite spherules found in culture experiments at ambient temperature (Berg et al., 2020; Duverger et al., 2020).
View in article
Gartman, A., Yücel, M., Madison, A., Chu, D., Ma, S., Janzen, C., Becker, E., Beinart, R., Girguis, P., Luther, G. (2011) Sulfide oxidation across diffuse flow zones of hydrothermal vents. Aquatic Geochemistry 17, 583–601. https://doi.org/10.1007/s10498-011-9136-1
 Show in context
Show in context Here, we report the results of abiotic syntheses carried out under experimental conditions reproducing those of black smokers., i.e. in a medium rich in reactive Fe2+ and reduced sulfur compounds (Gartman et al., 2011; Findlay et al., 2016).
View in article
Gorlas, A., Marguet, E., Gill, S., Geslin, C., Guigner, J.-M., Guyot, F., Forterre, P. (2015) Sulfur vesicles from Thermococcales: A possible role in sulfur detoxifying mechanisms. Biochimie 118, 356–364. https://doi.org/10.1016/j.biochi.2015.07.026
 Show in context
Show in context Then, a series of two syntheses were conducted in the presence of organic compounds unrelated to archaea cells: (1) in the presence of poorly soluble and poorly reactive graphitic carbon flakes, and (2) in the presence of the yeast extract and tryptone often used for the growth of cultures of Thermococcales (e.g., Gorlas et al., 2015, 2018, 2022).
View in article
Gorlas, A., Jacquemot, P., Guigner, J.-M., Gill, S., Forterre, P., Guyot, F. (2018) Greigite nanocrystals produced by hyperthermophilic archaea of Thermococcales order. PLoS One 13, e0201549. https://doi.org/10.1371/journal.pone.0201549
 Show in context
Show in context Then, a series of two syntheses were conducted in the presence of organic compounds unrelated to archaea cells: (1) in the presence of poorly soluble and poorly reactive graphitic carbon flakes, and (2) in the presence of the yeast extract and tryptone often used for the growth of cultures of Thermococcales (e.g., Gorlas et al., 2015, 2018, 2022).
View in article
Gorlas, A., Mariotte, T., Morey, L., Truong, C., Bernard, S., Guigner, J.-M., Oberto, J., Baudin, F., Landrot, G., Baya, C., Le Pape, P., Morin, G., Forterre, P., Guyot, F. (2022) Precipitation of greigite and pyrite induced by Thermococcales: an advantage to live in Fe- and S-rich environments? Environmental Microbiology 24, 626–642. https://doi.org/10.1111/1462-2920.15915
 Show in context
Show in context Then, a series of two syntheses were conducted in the presence of organic compounds unrelated to archaea cells: (1) in the presence of poorly soluble and poorly reactive graphitic carbon flakes, and (2) in the presence of the yeast extract and tryptone often used for the growth of cultures of Thermococcales (e.g., Gorlas et al., 2015, 2018, 2022).
View in article
Findlay, A.J. (2016) Microbial impact on polysulfide dynamics in the environment. FEMS Microbiology Letters 363, fnw103. https://doi.org/10.1093/femsle/fnw103
 Show in context
Show in context Here, we report the results of abiotic syntheses carried out under experimental conditions reproducing those of black smokers., i.e. in a medium rich in reactive Fe2+ and reduced sulfur compounds (Gartman et al., 2011; Findlay et al., 2016).
View in article
Within hydrothermal vents, colloidal sulfur can be produced by both living microorganisms and by abiotic processes (e.g., Findlay, 2016).
View in article
Kashefi, K., Lovley, D.R. (2003) Extending the upper temperature limit for life. Science 301, 934–934. https://doi.org/10.1126/science.1086823
 Show in context
Show in context In 50 years of research, the known thermal limit of life has been extended several times, rising from 70 °C, to over 100 °C, to 113 °C and finally to 122 °C (Brock and Freeze, 1969; Blöch et al., 1997; Kashefi and Lovley, 2003; Takai et al., 2008).
View in article
Le Guillou, C., Bernard, S., De la Peña, F., Le Brech, Y. (2018) XANES-Based Quantification of Carbon Functional Group Concentrations. Analytical Chemistry 90, 8379–8386. https://doi.org/10.1021/acs.analchem.8b00689
 Show in context
Show in context The XANES spectrum of this organic material is dominated by an absorption feature at 288.6 eV (attributed to COOH groups; Le Guillou et al., 2018) (Fig. 3).
View in article
The XANES spectrum of this organic carbon is consistent with lipids, with a small absorption feature at 285.1 eV (attributed to C=C bonds; Le Guillou et al., 2018) and a main absorption feature at 287.6 eV (attributed to C-H bonds; Le Guillou et al., 2018) (Fig. 3).
View in article
The XANES spectrum of this organic material shows similar features to the spectra of living cells (Benzerara et al., 2006; Miot et al., 2009; Li et al., 2014; Picard et al., 2021; Truong et al., 2023) and to the spectrum of organic material trapped within the pyrite spherules produced in the presence of living T. kodakarensis cells (Truong et al., 2023), with absorption features at 285.1, 286.5, 287.6, 288.1 and 288.6 eV, attributed to C=C bonds, C-S bonds, C-H bonds, amide groups and carboxylic groups, respectively (Le Guillou et al., 2018) (Fig. 3).
View in article
Li, J.H., Bernard, S., Benzerara, K., Beyssac, O., Allard, T., Cosmidis, J., Moussou, J. (2014) Impact of biomineralization on the preservation of microorganisms during fossilization: An experimental perspective. Earth and Planetary Science Letters 400, 113–122. https://doi.org/10.1016/j.epsl.2014.05.031
 Show in context
Show in context The XANES spectrum of this organic material shows similar features to the spectra of living cells (Benzerara et al., 2006; Miot et al., 2009; Li et al., 2014; Picard et al., 2021; Truong et al., 2023) and to the spectrum of organic material trapped within the pyrite spherules produced in the presence of living T. kodakarensis cells (Truong et al., 2023), with absorption features at 285.1, 286.5, 287.6, 288.1 and 288.6 eV, attributed to C=C bonds, C-S bonds, C-H bonds, amide groups and carboxylic groups, respectively (Le Guillou et al., 2018) (Fig. 3).
View in article
Lin, T., Ver Eecke, H., Breves, E., Dyar, M., Jamieson, J., Hannington, M., Dahle, H., Bishop, J., Lane, M., Butterfield, D., Kelley, D., Lilley, M., Baross, J., Holden, J. (2016) Linkages between mineralogy, fluid chemistry, and microbial communities within hydrothermal chimneys from the Endeavour segment, Juan de Fuca ridge. Geochemistry Geophysics Geosystems 17, 300–323. https://doi.org/10.1002/2015GC006091
 Show in context
Show in context The abrupt fluctuations in temperature, pH and redox conditions of sulfur-rich hydrothermal vents (Tivey et al., 2002; Schrenk et al., 2003; Lin et al., 2016) make in situ observations difficult.
View in article
Malaterre, C., Ten Kate, I.L., Baqué, M., Debaille, V., Grenfell, J.L., Javaux, E.J., Klenner, F., Lara, Y.J., McMahon, S., Moore, K., Noack, L., Patty L., Postberg, F. (2023) Is there such a thing as a biosignature? Astrobiology 23, 1213–1227. https://doi.org/10.1089/ast.2023.0042
 Show in context
Show in context To move forward, we need to rethink the concept of biosignature in terms of probability rather than irrefutability (McMahon and Jordan, 2022; Malaterre et al., 2023).
View in article
Mansor, M., Fantle, M.S. (2019) A novel framework for interpreting pyrite-based Fe isotope records of the past. Geochimica et Cosmochimica Acta 253, 39–62. https://doi.org/10.1016/j.gca.2019.03.017
 Show in context
Show in context Indeed, it is known that higher concentrations of sulfides (typically 180 mM of Na2S instead of the 1.3 mM used in the present study) are needed to allow the H2S pathway of abiotic pyrite precipitation (e.g., Mansor and Fantle, 2019).
View in article
Maruyama, A., Mita, N., Higashihara, T. (1993) Particulate materials and microbial assemblages around the Izena black smoking vent in the Okinawa Trough. Journal of Oceanography 49, 353–367. https://doi.org/10.1007/BF02269570
 Show in context
Show in context Yet, hydrothermal vents are the location of extensive mixing between seawater (possibly rich in biogenic organic materials) and hydrothermal fluids (possibly rich in abiotic organic materials) (e.g., Maruyama et al., 1993; Dick et al., 2010), making it difficult to identify sources.
View in article
McMahon, S., Jordan, S.F. (2022) A fundamental limit to the search for the oldest fossils. Nature Ecology & Evolution 6, 832–834. https://doi.org/10.1038/s41559-022-01777-0
 Show in context
Show in context To move forward, we need to rethink the concept of biosignature in terms of probability rather than irrefutability (McMahon and Jordan, 2022; Malaterre et al., 2023).
View in article
Merino, N., Aronson, H.S., Bojanova, D.P., Feyhl-Buska, J., Wong, M.L., Zhang, S., Giovannelli, D. (2019) Living at the extremes: extremophiles and the limits of life in a planetary context. Frontiers in Microbiology 10, 780. https://doi.org/10.3389/fmicb.2019.00780
 Show in context
Show in context Current estimates set the maximum temperature at which life could exist between 113 °C and 150 °C (Stetter, 1999; Cowan and Tow, 2004; Merino et al., 2019).
View in article
Miot, J., Benzerara, K., Obst, M., Kappler, A., Hegler, F., Schädler, S., Bouchez, C., Guyot, F., Morin, G. (2009) Extracellular iron biomineralization by photoautotrophic iron-oxidizing bacteria. Applied Environmental Microbiology 75, 5586–5591. https://doi.org/10.1128/AEM.00490-09
 Show in context
Show in context The XANES spectrum of this organic material shows similar features to the spectra of living cells (Benzerara et al., 2006; Miot et al., 2009; Li et al., 2014; Picard et al., 2021; Truong et al., 2023) and to the spectrum of organic material trapped within the pyrite spherules produced in the presence of living T. kodakarensis cells (Truong et al., 2023), with absorption features at 285.1, 286.5, 287.6, 288.1 and 288.6 eV, attributed to C=C bonds, C-S bonds, C-H bonds, amide groups and carboxylic groups, respectively (Le Guillou et al., 2018) (Fig. 3).
View in article
Nabeh, N., Brokaw, C., Picard, A. (2022) Quantification of Organic Carbon Sequestered by Biogenic Iron Sulfide Minerals in Long-Term Anoxic Laboratory Incubations. Frontiers in Microbiology 13, 662219. https://doi.org/10.3389/fmicb.2022.662219
 Show in context
Show in context In a recent experimental study, Nabeh et al. (2022) investigated the precipitation of iron sulfides in the presence of various organic compounds (amino acids, tryptone, yeast extract and microbial cells).
View in article
Their results demonstrated, consistent with the present results, that the sequestration of organic material by iron sulfides in the presence of amino acids, tryptone and yeast extract is low compared to the sequestration of organic material by iron sulfides in the presence of microbial cells (Nabeh et al., 2022).
View in article
Their explanation involved the binding of iron sulfides and organic molecules on the surface of cells and the formation of organo-mineral aggregates (Nabeh et al., 2022).
View in article
Nercessian, O., Reysenbach, A.L., Prieur, D., Jeanthon, C. (2003) Archaeal diversity associated with in situ samplers deployed on hydrothermal vents on the East Pacific Rise (13°N). Environmental Microbiology 5, 492–502. https://doi.org/10.1046/j.1462-2920.2003.00437.x
 Show in context
Show in contextThe incubation of chimney pieces enables the isolation of various microbial strains (e.g., Takai et al., 2001; Nercessian et al., 2003).
View in article
Nývlt, J. (1968) Kinetics of nucleation in solutions. Journal of Crystal Growth 3, 377–383. https://doi.org/10.1016/0022-0248(68)90179-6
 Show in context
Show in context On the one hand, organic compounds locally control sulfide saturation conditions via their redox potential, which influences the size of the crystalline domains (Nývlt, 1968).
View in article
Picard, A., Gartman, A., Girguis, P.R. (2021) Interactions Between Iron Sulfide Minerals and Organic Carbon: Implications for Biosignature Preservation and Detection. Astrobiology 21, 587–604. https://doi.org/10.1089/ast.2020.2276
 Show in context
Show in context The XANES spectrum of this organic material shows similar features to the spectra of living cells (Benzerara et al., 2006; Miot et al., 2009; Li et al., 2014; Picard et al., 2021; Truong et al., 2023) and to the spectrum of organic material trapped within the pyrite spherules produced in the presence of living T. kodakarensis cells (Truong et al., 2023), with absorption features at 285.1, 286.5, 287.6, 288.1 and 288.6 eV, attributed to C=C bonds, C-S bonds, C-H bonds, amide groups and carboxylic groups, respectively (Le Guillou et al., 2018) (Fig. 3).
View in article
Schrenk, M., Kelley, D., Delaney, J., Baross, J. (2003) Incidence and diversity of microorganisms within the walls of an active deep-sea sulfide chimney. Applied and Environmental Microbiology 69, 3580–3592. https://doi.org/10.1128/AEM.69.6.3580-3592.2003
 Show in context
Show in context The detection of biomarkers in chimney samples, such as the gene coding for 16S ribosomal RNA (Schrenk et al., 2003) or bacterial and archaeal lipids (Blumenberg, 2007), has led some authors to suggest that chimneys are, or have been, colonised by (hyper)thermophilic microorganisms.
View in article
The abrupt fluctuations in temperature, pH and redox conditions of sulfur-rich hydrothermal vents (Tivey et al., 2002; Schrenk et al., 2003; Lin et al., 2016) make in situ observations difficult.
View in article
Stetter, K.O. (1999) Extremophiles and their adaptation to hot environments. FEBS Letters 452, 22–25. https://doi.org/10.1016/S0014-5793(99)00663-8
 Show in context
Show in context Current estimates set the maximum temperature at which life could exist between 113 °C and 150 °C (Stetter, 1999; Cowan and Tow, 2004; Merino et al., 2019).
View in article
Takai, K., Komatsu, T., Inagaki, F., Horikoshi, K. (2001) Distribution of Archaea in a black smoker chimney structure. Applied Environmental Microbiology 67, 3618–3629. https://doi.org/10.1128/AEM.67.8.3618-3629.2001
 Show in context
Show in context The incubation of chimney pieces enables the isolation of various microbial strains (e.g., Takai et al., 2001; Nercessian et al., 2003).
View in article
Takai, K., Nakamura, K., Toki, T., Tsunogai, U., Miyazaki, M., Miyazaki, J., Horikoshi, K. (2008) Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proceedings of the National Academy of Sciences 105, 10949–10954. https://doi.org/10.1073/pnas.0712334105
 Show in context
Show in context In 50 years of research, the known thermal limit of life has been extended several times, rising from 70 °C, to over 100 °C, to 113 °C and finally to 122 °C (Brock and Freeze, 1969; Blöch et al., 1997; Kashefi and Lovley, 2003; Takai et al., 2008).
View in article
Tivey, M.A., Bradley, A.M., Joyce, T.M., Kadko, D. (2002) Insights into tide-related variability at seafloor hydrothermal vents from time-series temperature measurements. Earth and Planetary Science Letters 202, 693–707. https://doi.org/10.1016/S0012-821X(02)00801-4
 Show in context
Show in contextThe abrupt fluctuations in temperature, pH and redox conditions of sulfur-rich hydrothermal vents (Tivey et al., 2002; Schrenk et al., 2003; Lin et al., 2016) make in situ observations difficult.
View in article
Given the thermal maturation that such mineral structures have inevitably experienced, it remains difficult to expect to detect pristine biogenic organic materials: amino acids are for instance irreversibly destroyed above 240 °C (Bada et al., 1995), which is a temperature well below that of the hydrothermal fluid circulating through a chimney (e.g,. Tivey et al., 2002).
View in article
Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14, 1145781. https://doi.org/10.3389/fmicb.2023.1145781
 Show in context
Show in context These polycrystalline pyrite spherules exhibit a rounded shape, with a diameter ranging between 2 μm and 100 μm (Fig. 1 and Fig. 2a) showing some similarities with pyrite spherules made in mineralised cultures of the hyperthermophilic Thermococcus kodakarensis KOD1 archaeon (Truong et al., 2023) and with pyrite spherules found in culture experiments at ambient temperature (Berg et al., 2020; Duverger et al., 2020).
View in article
SEM, TEM (left) and STXM characterisation (right) of pyrite aggregates isolated from TAG hydrothermal field (in green) compared to biogenic pyrites produced by living T. kodakarensis KOD1 cells (in orange) (Truong et al., 2023).
View in article
SEM investigations of pyrites produced at 85 °C for 96 hr in a colloidal sulfur and Fe2+-rich medium compared to (a) pyrites isolated from TAG hydrothermal field, and (b) biogenic pyrite spherules produced in presence of KOD1 cells (Truong et al., 2023).
View in article
Overall, considering the sizes and morphologies at the micrometre scale, only pyrites produced in the presence of KOD1 envelopes or KOD1 lysates show similarities with pyrite spherules produced in the presence of living T. kodakarensis KOD1 (Truong et al., 2023) and with pyrite spherules reported in the chimney walls of black smokers (Truong et al., 2024).
View in article
The XANES spectrum of this organic material shows similar features to the spectra of living cells (Benzerara et al., 2006; Miot et al., 2009; Li et al., 2014; Picard et al., 2021; Truong et al., 2023) and to the spectrum of organic material trapped within the pyrite spherules produced in the presence of living T. kodakarensis cells (Truong et al., 2023), with absorption features at 285.1, 286.5, 287.6, 288.1 and 288.6 eV, attributed to C=C bonds, C-S bonds, C-H bonds, amide groups and carboxylic groups, respectively (Le Guillou et al., 2018) (Fig. 3).
View in article
Truong, C., Bernard, S., Baudin, F., Gorlas, A., Guyot, F. (2024) Carbon-containing pyrite spherules: mineral biosignature in black smokers? European Journal of Mineralogy 36, 813–830. https://doi.org/10.5194/ejm-36-813-2024
 Show in context
Show in context A recent study of chimney samples from a black smoker at the Trans-Atlantic Geotraverse (TAG) site on the Mid-Atlantic Ridge revealed the presence of pyrite (FeS2) spherules, whose morphologies and chemical characteristics suggested that they constitute a biosignature (Truong et al., 2024).
View in article
Overall, considering the sizes and morphologies at the micrometre scale, only pyrites produced in the presence of KOD1 envelopes or KOD1 lysates show similarities with pyrite spherules produced in the presence of living T. kodakarensis KOD1 (Truong et al., 2023) and with pyrite spherules reported in the chimney walls of black smokers (Truong et al., 2024).
View in article
Regarding the size range, the pyrite spherules found within the chimney walls of TAG mound’s black smoker (Fig. 1) (Truong et al., 2024) are more similar to the pyrite spherules produced in the presence of KOD1 lysates than to the pyrites produced in the presence of living T. kodakarensis cells.
View in article
In such a case, one could expect some chemical selection (different organic compounds may not be transported similarly), which does not seem to be the case according to XANES data (Truong et al., 2024).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Methods
- Figures S-1 to S-3
- Supplementary Information References
Download the Supplementary Information (PDF)
Figures
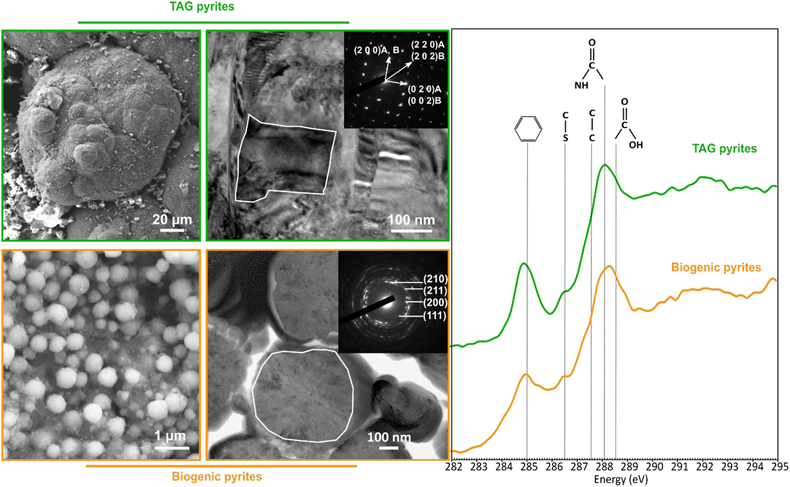
Figure 1 SEM, TEM (left) and STXM characterisation (right) of pyrite aggregates isolated from TAG hydrothermal field (in green) compared to biogenic pyrites produced by living T. kodakarensis KOD1 cells (in orange) (Truong et al., 2023
Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
).
Figure 2 SEM investigations of pyrites produced at 85 °C for 96 hr in a colloidal sulfur and Fe2+-rich medium compared to (a) pyrites isolated from TAG hydrothermal field, and (b) biogenic pyrite spherules produced in presence of KOD1 cells (Truong et al., 2023
Truong, C., Bernard, S., Le Pape, P., Morin, G., Baya, C., Merrot, P., Gorlas, A., Guyot, F. (2023) Production of carbon-containing pyrite spherules induced by hyperthermophilic Thermococcales: a biosignature? Frontiers in Microbiology 14. https://doi.org/10.3389/fmicb.2023.1145781
). (c) No pyrite produced in absence of organic matter. (d) Pyrites produced in presence of yeast extract and tryptone. (e–f) Micrometric pyrite crystals produced in presence of graphitic carbon. (g) Massive rounded pyrites measuring 10 μm produced in presence of KOD1 envelopes. (h) Rounded pyrites produced in presence of KOD1 lysates. (i) No pyrite produced in presence of both KOD1 supernatant and intracellular material but a predominance of colloidal phosphates as evidence by (j) associated EDXS spectrum.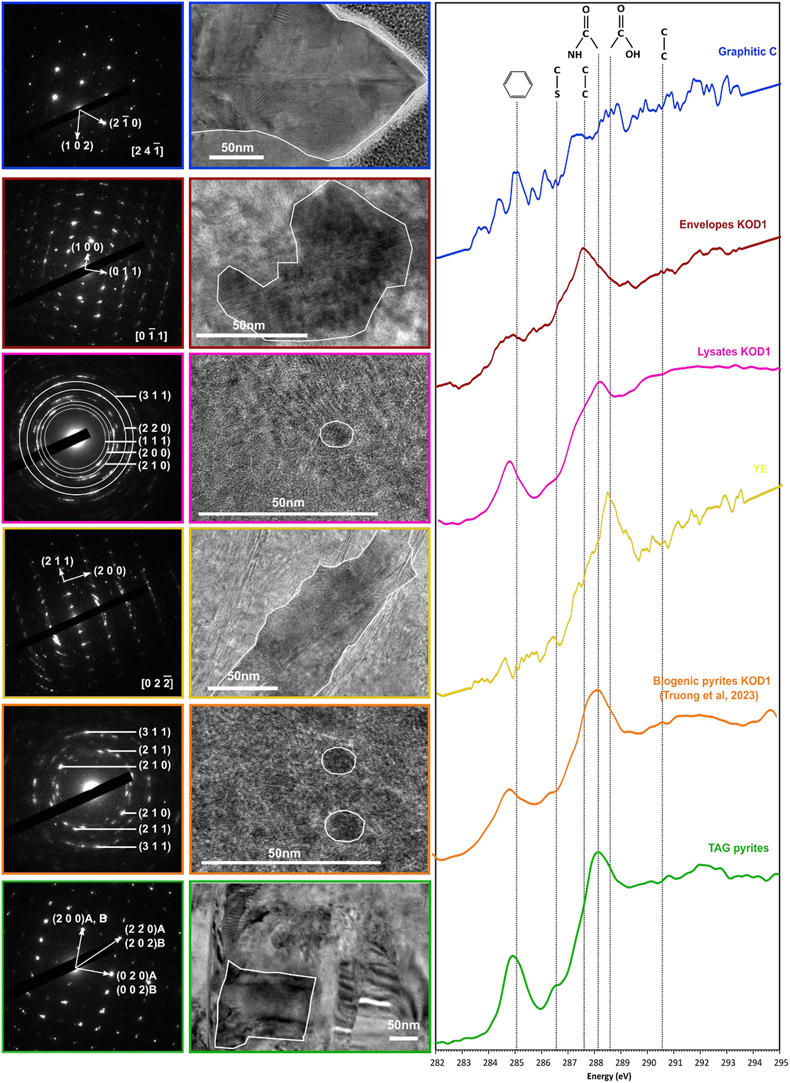
Figure 3 TEM, associated SAED patterns and STXM characterisation of TAG rounded pyrites (in green), biogenic pyrites (in orange) and abiotic counterparts produced at 85 °C for 96 hr in a colloidal sulfur and Fe2+-rich medium: yeast extract and tryptone (in yellow), KOD1 lysates (in pink), KOD1 envelopes (in red) and graphitic carbon (in blue). Of note, spots (100) and (011) are not allowed by the Pa3 space group of pyrite but are here enabled by double diffraction. The white outines correspond to the crystalline domain diffracted in the associated SAED pattern (TAG pyrites; YE; envelopes KOD1; Graphitic C), or to a putative crystalline domain for powder SAED pattern (biogenic pyrite KOD1; lysates KOD1).






