Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation
Affiliations | Corresponding Author | Cite asKeywords: core formation, planetary differentiation, S in the core, Cu isotopes, terrestrial Pb paradox
- Share this article





-
Article views:20,482Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract

The differentiation of Earth into a metallic core and silicate mantle left its signature on the chemical and isotopic composition of the bulk silicate Earth (BSE). This is seen in the depletion of siderophile (metal-loving) relative to lithophile (rock-loving) elements in Earth’s mantle as well as the silicon isotope offset between primitive meteorites (i.e. bulk Earth) and BSE, which is generally interpreted as a proof that Si is present in Earth’s core. Another putative light element in Earth’s core is sulphur; however, estimates of core S abundance vary significantly and, due to its volatile nature, no unequivocal S isotopic signature for core fractionation has thus far been detected. Here we present new high precision isotopic data for Cu, a chalcophile (sulphur-loving) element, which shows that Earth’s mantle is isotopically fractionated relative to bulk Earth. Results from high pressure equilibration experiments suggest that the sense of Cu isotopic fractionation between BSE and bulk Earth requires that a sulphide-rich liquid segregated from Earth’s mantle during differentiation, which likely entered the core. Such an early-stage removal of a sulphide-rich phase from the mantle presents a possible solution to the long-standing 1st terrestrial lead paradox.
Figures and Tables
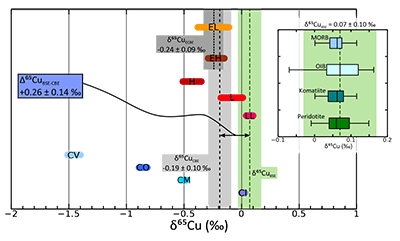 Figure 1 Copper isotope range of the primitive (chondritic) meteorite groups. Inset: Box and whisker plot showing the range of Cu isotope compositions for the terrestrial samples used in constraining the BSE Cu isotope composition. Green box and dotted line represents the composition of BSE, light grey box and long dashes represent the composition of “chondritic bulk Earth” (CBE), dark grey box and short dashes represent the composition of “enstatite chondrite bulk Earth” (ECBE). Errors on the estimates are all 2 s.d. | 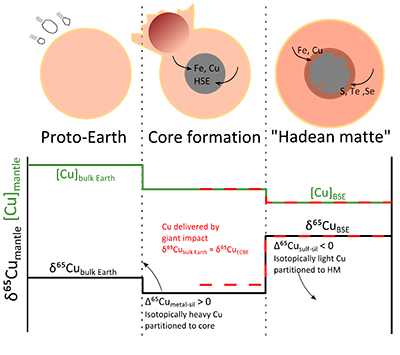 Figure 2 Copper isotope range of the primitive (chondritic) meteorite groups. Inset: Box and whisker plot showing the range of Cu isotope compositions for the terrestrial samples used in constraining the BSE Cu isotope composition. Green box and dotted line represents the composition of BSE, light grey box and long dashes represent the composition of “chondritic bulk Earth” (CBE), dark grey box and short dashes represent the composition of “enstatite chondrite bulk Earth” (ECBE). Errors on the estimates are all 2 s.d. | 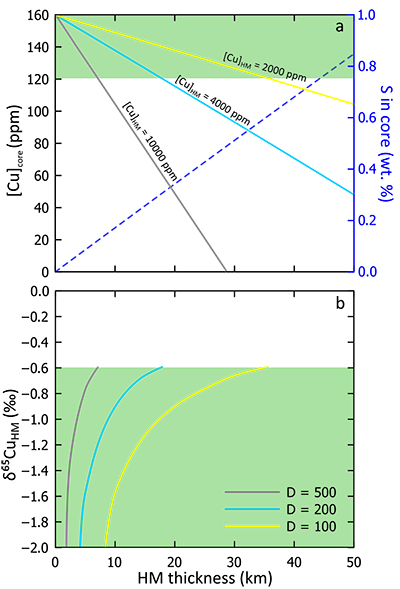 Figure 3 Results of modelled effects of removal of a “Hadean Matte” from the mantle. The effects on (a) Cu concentration of Earth’s core and (b) required Cu isotope composition of the HM to produce a modern-day δ65CuBSE – plotted as a function of the thickness of the HM. D is the sulphide-silicate Cu partition coefficient. (a) Green box defines “allowed” Cu concentrations of Earth’s core; [Cu]core < 120 ppm do not comply with the siderophile nature of Cu during core formation. Blue dotted line describes the amount of S added to the core (in wt.%) in the case of total mixing of the HM composed of a stoichiometric Fe-O-S liquid into the core. (b) Lines plotted here limited to those “allowed” in top panel. | 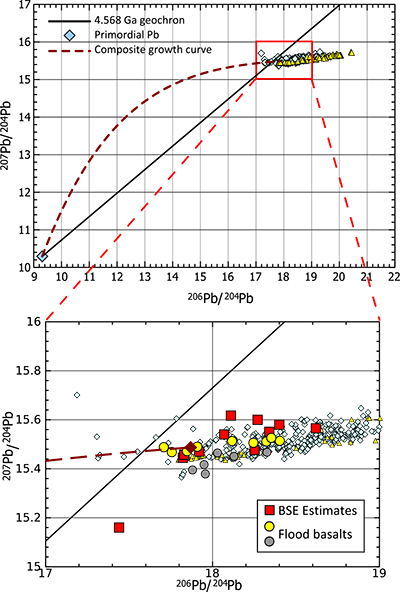 Figure 4 Modelled evolution of mantle Pb isotope composition as a result of Pb partitioning into the core at 50 Ma and the HM at 100 Ma (composite growth curve). Compilation of mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) data taken from PetDB (http://www.earthchem.org/petdb) which all plot to the right (more radiogenic) side of the terrestrial (4.568 Ga) geochron (based on the evolution of Pb from primordial source – based on Canyon Diablo Troilite). Lower figure is a zoom, showing that the composite curve agrees with some of the more unradiogenic estimates for modern day BSE Pb isotope composition (Halliday, 2004), as well as the compositions of certain flood basalt provinces (yellow: Ontong Java Plateau; grey: Baffin Island) thought to best represent ancient primitive mantle composition (Jackson and Carlson, 2011). |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Supplementary Figures and Tables
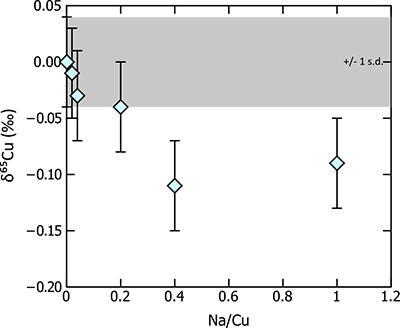 Figure S-1 Results of Na doping tests on Cu isotope measurements – sample is 250 ppb Cu SRM987 doped with various Na concentrations to results in Na/Cu between 0.001 and 1. Measurable isotopic offsets are present in samples with Na/Cu values of >0.2. Error bars are 1 s.d. |  Table S-1 Cu isotope standards. |  Table S-2 Komatiites. |  Table S-3 Peridotites (orogenic lherzolites - literature). |
| Figure S-1 | Table S-1 | Table S-2 | Table S-3 |
 Table S-4 Mid ocean ridge basalts. |  Table S-5 Kilauea Iki. |  Table S-6 Hekla. |  Table S-7 Ocean island basalts. |
| Table S-4 | Table S-5 | Table S-6 | Table S-7 |
 Table S-8 Enstatite chondrites. | 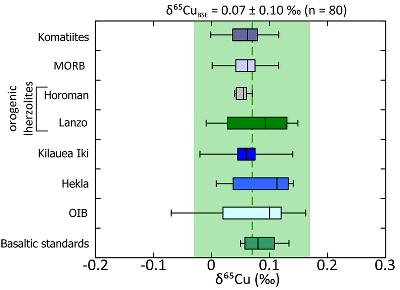 Figure S-2 Box and whisker plot showing the range of Cu isotope compositions for the terrestrial samples used in constraining the BSE Cu isotope composition. Box represents 1st and 3rd quartile, line in box represents the median and “whiskers” show the range of the data (see Tables S-2-S-7, S-9). There is no statistical difference between each group. Calculated BSE value plotted as green dashed line, green box shows 2 s.d. precision of the estimate. |  Table S-9 BSE and bulk Earth averages. | 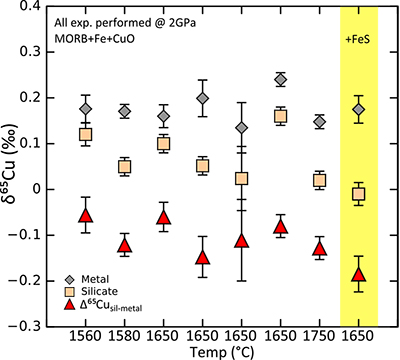 Figure S-3 Summary of metal-silicate Cu isotope equilibration experiments – note that this figure is not a graph (the x axis is discrete, not continuous). All Δ65Cusil-met values are negative but there does not appear to be a strong temperature control on these values. The one experiment with S added gives the largest Δ65Cusil-met value, this makes sense if there is minor amounts of S-hosted Cu that was dissolved with the non-magnetic (“silicate”) phase – as Cu sulphides are shown to be isotopically light w.r.t. silicates and metal. Error bars are 1s.d. |
| Table S-8 | Figure S-2 | Table S-9 | Figure S-3 |
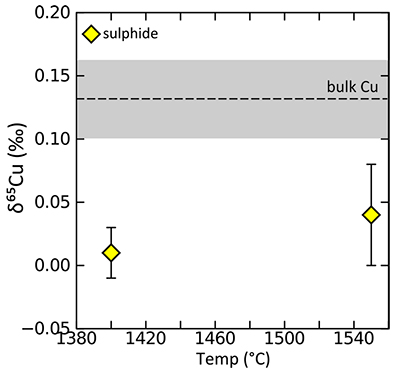 Figure S-4 Summary of sulphide-silicate Cu isotope equilibration experiments – we are so far unable to successfully measure the silicate phase, due to the presence of disseminated sulphides which overwhelm the Cu isotope signature of the silicates. Here we show the Cu isotope composition of the bulk Cu metal as well as sulphide phases from two experiments (wherein only the temperature was varied). In both cases the sulphides are enriched in the light Cu isotope relative to bulk, and there is a small decrease in fractionation magnitude (although within error) as temperature increases. |  Table S-10 Experimental results. | 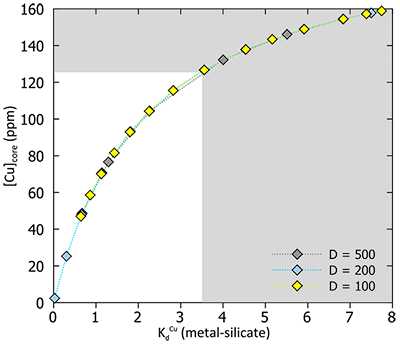 Figure S-5 Relationship between calculated Cu in core (as a result of increasing thickness of HM) and apparent DCu metal silicate values – this value should be at least greater than 3.5, which equates to >120 ppm Cu in the core. D = DCusulfide-silicate therefore, for BSE [Cu] = 20 ppm, D = 500→[Cu]HM = 10000; D = 200→[Cu]HM = 4000; D = 100→[Cu]HM = 2000. | 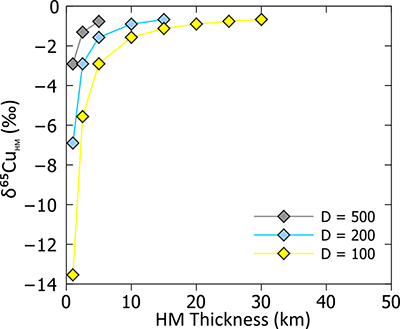 Figure S-6 Relationship between thickness of Hadean Matte and Cu isotope composition, for various [Cu]HM concentrations. As the thickness reduces, the isotope composition of the HM becomes more extreme, toward very light values. |
| Figure S-4 | Table S-10 | Figure S-5 | Figure S-6 |
top
Letter
The budget of light elements in Earth’s core is a long-standing geochemical problem (Poirier, 1994
Poirier, J.-P. (1994) Light elements in the Earth's outer core: A critical review. Physics of the Earth and Planetary Interiors 85, 319-337.
), as constraining such elements and their abundances can tell us much about the physiochemical conditions of Earth’s differentiation. Sulphur is often cited as one such element: cosmochemical estimates suggest that the core contains ~2 wt. % S (Dreibus and Palme, 1996Dreibus, G., Palme, H. (1996) Cosmochemical constraints on the sulphur content in the Earth's core. Geochimica et Cosmochimica Acta 60, 1125-1130.
); sulphur in the core is seemingly necessary to explain mantle W and Mo abundances (Wade et al., 2012Wade, J., Wood, B.J., Tuff, J. (2012) Metal–silicate partitioning of Mo and W at high pressures and temperatures: Evidence for late accretion of sulphur to the Earth. Geochimica et Cosmochimica Acta 85, 58-74.
) and can explain the disparity between the radiometric Pb and W isotope ages of the mantle (Wood and Halliday, 2005Wood, B.J., Halliday, A.N. (2005) Cooling of the Earth and core formation after the giant impact. Nature 437, 1345-1348.
). However, recent molecular dynamics estimates suggest S may not be present at all in the core (Badro et al., 2014Badro, J., Cote, A.S., Brodholt, J.P. (2014) A seismologically consistent compositional model of Earth’s core. Proceedings of the National Academy of Science 111, 7542-7545.
); also, it is unclear as to whether S entered the core as an iron alloy, or as a discrete sulphide phase (O’Neill, 1991O'Neill, H.St.C. (1991) The origin of the moon and the early history of the earth - A chemical model. Part 2: The earth. Geochimica et Cosmochimica Acta 55, 1159-1172.
). Further complications stem from the fact that late addition of extra-terrestrial S to the mantle, post-core formation, should overwhelm any pre-existing S (isotope) signature (the “late veneer”; Holzheid et al., 2000Holzheid, A., Sylvester, P., O’Neill, H.St.C., Rubie, D.C., Palme, H. (2000) Evidence for a late chondritic veneer in the Earth’s mantle from high-pressure partitioning of palladium and platinum. Nature 406, 396-399.
; Wang and Becker, 2013Wang, Z., Becker, H. (2013) Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature 499, 328-331.
). In an effort to investigate the role of S during Earth’s differentiation, we have investigated the Cu isotope compositions of bulk Earth and BSE; this is because Cu is siderophile and strongly chalcophile (~2/3 of Earth’s Cu is thought to be in the core; Palme and O’Neill, 2014Palme, H., O'Neill, H.St.C. (2014) Cosmochemical estimates of mantle composition. In: Carlson, R.W. (Ed) The mantle and core, Treatise on Geochemistry, 2nd edition, 3, 1-39.
, McDonough, 2003McDonough, W.F. (2003) Compositional model for the Earth's core. In: Carlson, R.W. (Ed) The mantle and core, Treatise on Geochemistry,1st edition, 2, 547-568.
) but is less volatile than S, so is abundant enough in Earth’s mantle to have been largely unaffected by a late veneer.To begin with, it was necessary to obtain robust Cu isotope compositions for both BSE and bulk Earth. To this end, we measured the Cu isotope composition of 88 extra-terrestrial and terrestrial samples using high precision multi-collector inductively coupled plasma mass spectrometry (MC-ICPMS; see Supplementary Information for methods and data tables) and combined these results with pre-existing literature data.
Choosing samples to constrain the Cu isotope composition of BSE is not trivial, due to the specific behaviour of Cu during mantle melting. The concentration of Cu in a mantle melt is predominantly controlled by the consumption of sulphide phases by such a melt (Lee et al., 2012). If melt fraction remains below ~25 %, residual sulphides should retain Cu, which could potentially give rise to isotopic fractionation. With this in mind, two lithologies were initially chosen to constrain the copper isotopic composition of BSE. The first were komatiites, mantle-derived ultramafic lavas generated by high degrees (>25 %) of mantle melting and typically found in Archaean terrains (Arndt, 2008
Arndt, N. (2008) Komatiite. First edition, Cambridge University Press, United Kingdom.
). In this study, we analysed komatiite samples from two localities; 2.4 Ga Vetreny Belt (Baltic Shield) and 2.7 Ga Belingwe (South Africa). The second were “fertile” orogenic lherzolites from Lanzo (Italy) and Horoman (Japan); that is, samples of the mantle that appear to have undergone little to no melt depletion. These data were augmented by Cu isotope analyses of mid-ocean ridge basalts (MORB), which are typically formed by fairly high (10-15 %) degrees of melting of upper mantle. We also include data from a variety of ocean island basalt samples to investigate the possibility of Cu isotope mantle heterogeneities (see Supplementary Information for all sample information).The Cu isotope compositions of terrestrial basalts and ultramafic rocks define a limited range (–0.07 ‰ < δ65Cu < +0.16 ‰; Fig. 1, δ65Cu = [(65Cu/63Cusample / 65Cu/63Custd.) – 1] × 1000; where std. is NIST SRM976). Despite the potential for Cu isotope fractionation through sulphide retention, each sample group is statistically identical and the data are normally distributed (Supplementary Information), providing a robust and precise average BSE Cu isotope composition of δ65CuBSE = 0.07 ± 0.10 ‰ (2 s.d.).

Figure 1 Copper isotope range of the primitive (chondritic) meteorite groups. Inset: Box and whisker plot showing the range of Cu isotope compositions for the terrestrial samples used in constraining the BSE Cu isotope composition. Green box and dotted line represents the composition of BSE, light grey box and long dashes represent the composition of “chondritic bulk Earth” (CBE), dark grey box and short dashes represent the composition of “enstatite chondrite bulk Earth” (ECBE). Errors on the estimates are all 2 s.d.
It is of course impossible to obtain a sample of the ‘bulk Earth’ and hence, like many studies before ours, we assume that bulk Earth formed from primitive (chondritic) meteorites (e.g., Palme and O’Neill, 2014
Palme, H., O'Neill, H.St.C. (2014) Cosmochemical estimates of mantle composition. In: Carlson, R.W. (Ed) The mantle and core, Treatise on Geochemistry, 2nd edition, 3, 1-39.
). The issue then is to decide which group(s) of meteorites best represents Earth, in terms of its Cu budget. This is particularly important, as the range of chondrite Cu isotope compositions span a wide range (–1.45 ‰ < δ65Cu < +0.07 ‰, Fig. 1; Luck et al., 2005Luck, J.M., Ben Othman, D., Albarède., F. (2005) Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochimica et Cosmochimica Acta 69, 5351-5363.
), thus we now discuss a number of model-dependent scenarios.a) The Earth’s Cu budget was established early in Earth’s accretion: Like Cu, large isotope variations also exist in systems such as O, Ti, Cr, Ru, Ca etc., suggesting that no single chondrite group represents a perfect match to Earth, and many workers posit a mixture. Based on modelling of Fitoussi and Bourdon (2012)
Fitoussi, C., Bourdon, B. (2012) Silicon isotope evidence against an enstatite chondrite earth. Science 335, 1477-1480.
, we calculate a chondritic bulk Earth (CBE) value of δ65CuCBE = –0.19 ± 0.10 ‰ (2 s.d., Fig. 1). Another approach is to utilise the enstatite chondrites; despite being chemically dissimilar, these meteorites are identical to the Earth for most isotope systems (Dauphas et al., 2014Dauphas, N., Chen, J.H., Zhang, J., Papanastassiou, D.A., Davis, A.M., Travaglio, C. (2014) Calcium-48 isotopic anomalies in bulk chondrites and achondrites: Evidence for a uniform isotopic reservoir in the inner protoplanetary disk. Earth and Planetary Science Letters 407, 96-108.
) and many models suggest that the material that accreted to form the Earth contained a large proportion of enstatite chondrite-like planetesimals (Dauphas et al., 2014Dauphas, N., Chen, J.H., Zhang, J., Papanastassiou, D.A., Davis, A.M., Travaglio, C. (2014) Calcium-48 isotopic anomalies in bulk chondrites and achondrites: Evidence for a uniform isotopic reservoir in the inner protoplanetary disk. Earth and Planetary Science Letters 407, 96-108.
). Both enstatite chondrites groups have similar ranges as well as having identical δ65Cu values (Fig. 1); a mixture of EH and EL chondrites gives an enstatite chondrite bulk Earth (ECBE) of δ65Cu = –0.24 ± 0.09 ‰ (2 s.d., Fig. 1), identical to δ65CuCBE.b) The Earth’s Cu was delivered late, as a result of the Moon-forming giant impact: The mantle budget of Cu and other moderately volatile elements may be dominated by the final 10 % of material accreted to Earth, associated for example with the Moon-forming giant impactor, Theia (Albarède, 2009
Albarède, F. (2009) Volatile accretion history of the terrestrial planets and dynamic implications. Nature 461, 1227-1233.
). The Cu in the mantle, therefore, would have escaped the effects of all but the final stages of planetary differentiation, as Hf-W isotope data suggests that the majority of the core had formed by the time of impact (Kleine et al., 2010Kleine, T., Palme, H., Mezger, K., Halliday, A.N. (2010) Hf-W chronometry of lunar metals and the age and early differentiation of the Moon. Science 310, 1671-1674.
). Work on Ag isotopes by Schönbächler et al. (2010)Schönbächler, M., Carlson, R.W., Horan, M.F., Mock, T.D., Hauri, E.H. (2010) Heterogeneous accretion and the moderately volatile element budget of Earth. Science 328, 884-887.
seemed to indicate that the impactor material was dominated by CI-like material. If this is the case, then our data would seem to support their model; our estimate for BSE is almost identical to the Cu isotope composition of CI chondrites. However, more recent isotope data seems to rule out a CI-like impactor; in particular, precise lunar O isotope data suggests that the impactor had an enstatite chondrite isotope signature (Herwartz et al., 2014Herwartz, D., Pack, A., Friedrichs, B., Bischoff, A. (2014) Identification of the giant impactor Theia in lunar rocks. Science 344, 1146-1150.
). In this instance, again, the enstatite chondrite model would seem most representative of bulk Earth.Accepting either model ‘a’ or ‘b’ above, the bulk Earth Cu isotope composition lies somewhere between δ65Cu = –0.19 ± 0.10 ‰ and –0.24 ± 0.09 ‰. Therefore, in terms of Cu isotopes, BSE is enriched in the heavy Cu isotope compared to bulk Earth, with a minimum offset (taking bulk Earth to be –0.19 ‰) of +0.26 ± 0.14 ‰ (2 s.d., Fig. 1). This suggests that some process related to planetary differentiation and accretion has affected the Cu isotope composition of Earth’s mantle; we now consider the two most likely culprits: volatile loss of Cu, and core formation.
Preferential removal of the lighter Cu isotope during volatile loss could lead to enrichment in isotopically heavy Cu in Earth’s mantle. This, however, can be discounted by considering the Zn isotope system. Zinc is more volatile and less siderophile/chalcophile than Cu (Lodders, 2003
Lodders, K. (2003) Solar System abundances and condensation temperatures of the elements. Astrophysical Journal Letters 591, 1220-1247.
); like all moderately volatile, lithophile elements, Earth’s mantle is depleted in Zn compared to most chondrite groups suggesting partial loss or incomplete accretion (Palme and O’Neill, 2014Palme, H., O'Neill, H.St.C. (2014) Cosmochemical estimates of mantle composition. In: Carlson, R.W. (Ed) The mantle and core, Treatise on Geochemistry, 2nd edition, 3, 1-39.
). However, the bulk Earth Zn isotope composition is equal to or lighter than those same meteorites (Albarède, 2009Albarède, F. (2009) Volatile accretion history of the terrestrial planets and dynamic implications. Nature 461, 1227-1233.
; Chen et al., 2013Chen, H., Savage, P.S., Teng, F.-Z., Helz, R.T., Moynier, F. (2013) Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk Earth. Earth and Planetary Science Letters 369-370, 34-42.
), i.e. contrary to the isotope effect predicted by volatile loss. Hence whichever process(es) resulted in the volatility-related depletion of Zn in Earth’s mantle did not affect its isotopes. It is therefore unlikely that such a process can explain the heavy Cu isotope enrichment in BSE.Planetary differentiation is therefore the most likely explanation for the Cu isotope difference between BSE and bulk Earth. To further investigate the behaviour of Cu isotopes during core formation, i.e. metal-silicate and sulphide-silicate equilibration, we performed a preliminary series of high-pressure, high temperature experiments wherein natural basalt rock powder, doped with Cu (as either metal or oxide) was equilibrated with either pure Fe metal or stoichiometric FeS under fully molten conditions (see Supplementary Information). In the metal-silicate experiments, the direction of isotopic fractionation between the two phases (Δ65Cumetal-silicate) was always slightly positive, varying little with temperature (~ +0.1 ‰). Crucially, in the sulphide-silicate experiments the sense of fractionation is negative, opposite and with a larger magnitude to that of metal-silicate equilibration (> –0.5 ‰). These data agree with the sense of Cu isotope fractionation between metal, silicate and sulphide measured in iron meteorites (Williams and Archer, 2011
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal-sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166-3178.
), as well as the extremely light Cu isotope compositions measured in secondary sulphide minerals (Markl et al., 2006Markl, G., Lahaye, Y., Schwinn, G. (2006) Copper isotopes as monitors of redox processes in hydrothermal mineralization. Geochimica et Cosmochimica Acta 70, 4215-4228.
). Therefore, the positive Cu isotope difference between BSE and bulk Earth cannot be due to metal-silicate fractionation, because the isotopic fractionation has the incorrect sense. Instead, Earth’s “missing” light Cu must be stored in a sulphide-rich reservoir.Mantle sulphides are a potential explanation; however, given the relative Cu isotope homogeneity of mantle-derived lithologies (Fig. 1) as well as the fact that a typical peridotite contains >90 % of its Cu in sulphides (Lee et al., 2012), this requires that a relatively significant budget of Cu is stored in sulphides that are never sampled by mantle melting or by tectonic exhumation. This does not seem to be the case: for instance, komatiites, which formed via high degrees of partial melting (such that at least 95 % of the Cu in the mantle source should be transferred to the melt), have Cu isotope compositions equal to that of orogenic lherzolites, which are (arguably) direct samples of the mantle. Komatiites also provide a temporal view on mantle composition and suggest that the Cu isotope composition of BSE was established at least as far back as 2.7 Ga, the age of our oldest sample. Similarly, ocean island basalts, which potentially sample sulphide-rich pyroxenites, show no evidence for significant Cu isotope mantle heterogeneity (Fig. 1). The lower continental crust is also a possible reservoir for isotopically light Cu sulphides – however, even if the bulk Cu concentration in the lower continental crust was three times current estimates, it would only represent ~0.6 wt. % of BSE Cu and so would require an unfeasibly light composition (δ65Cu < –40 ‰). Hence, sulphides in the mantle or crust may host some isotopically light Cu, but are apparently not abundant enough to account for the significant Cu isotope offset between BSE and bulk Earth.
An alternative explanation is the early-stage formation of a sulphide-rich (Fe-O-S) liquid in the mantle, as the final volatile-rich residue after crystallisation of a magma ocean; this is often called the “Hadean Matte” (HM; Fig. 2; O’Neill, 1991
O'Neill, H.St.C. (1991) The origin of the moon and the early history of the earth - A chemical model. Part 2: The earth. Geochimica et Cosmochimica Acta 55, 1159-1172.
). Given its higher density compared to ambient mantle, a HM should pond at the core/mantle boundary and, potentially, admix into the core, isolating it from subsequent re-equilibration. Such a reservoir has been invoked to explain moderately siderophile element abundances in the mantle (O’Neill, 1991O'Neill, H.St.C. (1991) The origin of the moon and the early history of the earth - A chemical model. Part 2: The earth. Geochimica et Cosmochimica Acta 55, 1159-1172.
) and the mismatch of various core formation chronometers (Wood and Halliday, 2005Wood, B.J., Halliday, A.N. (2005) Cooling of the Earth and core formation after the giant impact. Nature 437, 1345-1348.
), and could host significant amounts of Cu. To this end, we have attempted to calculate the mass and composition of a Hadean Matte needed to balance the Cu isotope offset between BSE and bulk Earth. Following previous models, the HM should form after segregation of the majority (99 %) of the core; as such we have used a simple two-stage model, starting from a chondritic (δ65CuCBE) proto-Earth, where Cu is first partitioned into the core (metal-silicate equilibration), then a sulphide phase (Fig. 2). This is based on model ‘a’ above, i.e. Earth’s Cu budget was established early in Earth’s accretion. Assuming instead that Earth’s mantle Cu was delivered by an enstatite chondrite-like Giant impactor (model ‘b’ above) does not significantly change the modelling, except that mantle Cu does not experience major metal-silicate equilibration, only sulphide-silicate equilibration (Fig. 2).
Figure 2 Schematic evolution of Cu concentration and isotopic composition of Earth’s mantle as modelled in this contribution. Earth accretes as a mixture of chondrites such that the bulk Earth Cu isotope composition is δ65CuCBE. Core formation sequesters ~60 % of Earth’s Cu in the metal phase, which is enriched in the heavy isotope, driving Earth’s mantle to a lighter composition. The formation of a Fe-O-S layer, the “Hadean Matte”, sequesters isotopically light Cu, driving Earth’s mantle to its present day composition (δ65CuBSE). Alternatively, Cu is delivered by an enstatite chondrite-like Giant Impactor, and mantle Cu only experiences sulphide-silicate equilibration.
A condition of the model ‘a’ is that the Cu normally assumed to be in Earth’s core is now apportioned between HM and core. Fixing a range of likely Cu concentrations in the HM, we calculated the remaining core [Cu] as a function of HM mass (or thickness, assuming a fixed density). This relationship is shown in Figure 3a, wherein the amount of Cu in the core reduces as the HM thickness and Cu concentration increases. The value of [Cu]core should not fall below 120 ppm because Cu is also siderophile (Siebert et al., 2013
Siebert, J., Badro, J., Antonangeli, D., Ryerson, F.J. (2013) Terrestrial accretion under oxidizing conditions. Science 339, 1194-1197.
), which predicts a maximum HM thickness of ~35 km.We then calculated the Cu isotope composition for each HM scenario required to drive the equilibrating silicate (i.e. the mantle) towards the modern-day BSE value. This is shown in Figure 3b, where a smaller HM results in a more negative HM Cu isotope composition. We calculate a range of HM Cu isotope compositions because our preliminary experiments were simply used to assess the sense, not the magnitude of isotope fractionation; however, because large isotope fractionations (> 2 ‰) are not expected at the temperatures associated with the formation of a HM (3000-4000 K), a minimum thickness of ~2 km is predicted, even for the most Cu-rich HM. The maximum size for each HM as controlled by its Cu concentration corresponds to a minimum Δ65Cusulphide-silicate value of ~ –0.6 ‰. To further constrain this model, further work is required to accurately parameterise Cu isotope fractionation factors, but these model predictions are in general agreement with our experimental data (Supplementary Information).
Admixing a Fe-O-S liquid into Earth’s core will affect core composition, specifically with regard to the light elements S and O. Given the constraints provided above (HM mass ≤ 1.6 % of Earth’s core), addition of a HM to the core will have a small effect on the core O composition (< 0.25 wt. % addition); for S, the effect is more significant – our model suggests that up to ~0.5 wt. % S could be added (Fig. 3a), which is in line with recent estimates based on molecular dynamics (Badro et al., 2014
Badro, J., Cote, A.S., Brodholt, J.P. (2014) A seismologically consistent compositional model of Earth’s core. Proceedings of the National Academy of Science 111, 7542-7545.
) and siderophile element partitioning studies (Siebert et al., 2013Siebert, J., Badro, J., Antonangeli, D., Ryerson, F.J. (2013) Terrestrial accretion under oxidizing conditions. Science 339, 1194-1197.
) – of course, this does not preclude further core addition of S as a metal alloy.
Figure 3 Results of modelled effects of removal of a “Hadean Matte” from the mantle. The effects on (a) Cu concentration of Earth’s core and (b) required Cu isotope composition of the HM to produce a modern-day δ65CuBSE – plotted as a function of the thickness of the HM. D is the sulphide-silicate Cu partition coefficient. (a) Green box defines “allowed” Cu concentrations of Earth’s core; [Cu]core < 120 ppm do not comply with the siderophile nature of Cu during core formation. Blue dotted line describes the amount of S added to the core (in wt.%) in the case of total mixing of the HM composed of a stoichiometric Fe-O-S liquid into the core. (b) Lines plotted here limited to those “allowed” in top panel.
Finally, we estimate the effect that removal of the HM could have on mantle lead isotope composition. Lead can be strongly chalcophile, and an early fractionation of isotopically primitive Pb by a sulphide-rich phase is often cited as a solution to the 1st terrestrial Pb paradox; that is, the observation that, in 206Pb/204Pb vs. 207Pb/204Pb space, most mantle-derived rocks, continental sediments etc. plot to the right of the terrestrial geochron (either the meteoritic, 4.568 Ga geochron or the later Hf-W core formation cessation age of ~4.53 Ga, Fig. 4; Kramers and Tolstikhin, 1997
Kramers, J.D., Tolstikhin, I.N. (1997) Two terrestrial lead isotope paradoxes, forward transport modelling, core formation and the history of the continental crust. Chemical Geology 139, 75-110.
). Modelling the evolution of mantle Pb isotope composition resulting from two fractionation events, metal-silicate equilibration at 50 Ma and sulphide-silicate equilibration at 100 Ma (following Galer and Goldstein, 1996Galer, S.J.G., Goldstein S.L. (1996) Influence of accretion on lead in the Earth. In: Basu, A., Hart, S. (Eds.) Earth Processes: Reading the Isotopic Code, AGU Geophysical Monograph 95, 75-98.
; see Supplementary Information for details), predicts a present-day mantle Pb isotopic composition that is comparable to empirical estimates for BSE, albeit the more unradiogenic ones (Fig. 4; Halliday, 2004Halliday, A.N. (2004) Mixing, volatile loss and compositional change during impact-driven accretion of the Earth. Nature 427, 505-509.
) as well as the Pb composition of the ancient primitive mantle as estimated using flood basalts (Jackson and Carlson, 2011Jackson, M.G., Carlson, R. (2011) An ancient recipe for flood basalt genesis. Nature 476, 316-319.
). Therefore, this does not preclude further unradiogenic Pb reservoirs, such as sulphides in refractory mantle phases (Burton et al., 2012Burton, K.W., Cenki-Tok, B., Mokadem, F., Harvey, J., Gannoun, A., Alard, O., Parkinson, I.J. (2012) Unradiogenic lead in Earth's upper mantle. Nature Geoscience 5, 570-573.
) or late accretion of mantle Pb (Albarède, 2009Albarède, F. (2009) Volatile accretion history of the terrestrial planets and dynamic implications. Nature 461, 1227-1233.
).
Figure 4 Modelled evolution of mantle Pb isotope composition as a result of Pb partitioning into the core at 50 Ma and the HM at 100 Ma (composite growth curve). Compilation of mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) data taken from PetDB (http://www.earthchem.org/petdb) which all plot to the right (more radiogenic) side of the terrestrial (4.568 Ga) geochron (based on the evolution of Pb from primordial source – based on Canyon Diablo Troilite). Lower figure is a zoom, showing that the composite curve agrees with some of the more unradiogenic estimates for modern day BSE Pb isotope composition (Halliday, 2004
Halliday, A.N. (2004) Mixing, volatile loss and compositional change during impact-driven accretion of the Earth. Nature 427, 505-509.
), as well as the compositions of certain flood basalt provinces (yellow: Ontong Java Plateau; grey: Baffin Island) thought to best represent ancient primitive mantle composition (Jackson and Carlson, 2011Jackson, M.G., Carlson, R. (2011) An ancient recipe for flood basalt genesis. Nature 476, 316-319.
).To conclude, the Cu isotope composition of the BSE seems to require that large scale sulphide-silicate equilibration occurred sometime in Earth’s history; here, we have modelled it as the formation of a discreet Fe-O-S reservoir, a “Hadean Matte”, which ponded to the base of the mantle during the final stages of Earth’s differentiation. Such a feature likely admixed into Earth’s core; however, if any of this material remains, such material could account for recently detected non-chondritic S isotope compositions in Earth's mantle (Labidi et al., 2013
Labidi, J., Cartigny, P., Moreira, M. (2013) Non-chondritic sulphur isotope composition of the terrestrial mantle. Nature 501, 208-211.
). Finally, the Martian core is thought to have up to 14 wt. % S (Wänke and Dreibus, 1994Wänke, H., Dreibus, G. (1994) Chemistry and accretion history of Mars. Philosophical Transactions of the Royal Society of London Series A 349, 285-293.
) so FeS-silicate equilibration during core formation could have a significant effect on Mars’ mantle; Cu isotopes have the potential to identify this effect.top
Acknowledgements
This research was supported by the Marie Curie IOF Fellowship “Isovolc”, funding from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement #207467 (DECore) and under the H2020 framework program/ERC grant agreement #637503 (Pristine), as well as the financial support of the UnivEarthS Labex program at Sorbonne Paris Cité (ANR-10-LABX-0023 and ANR-11-IDEX-0005-02). FM thanks the ANR through a chaire d’excellence Sorbonne Paris Cité and the INSU through the PNP program. ISP acknowledges support from NSF EAR0946629. The authors would like to thank Katharina Lodders, Bruce Fegley, Astrid Holzheid and Kevin Burton for discussions, Juliens Foriel and Moureau for analytical support, and Manuel Moreira, Euan Nisbet, Gary Byerly, Tim Elliott, Fang-Zhen Teng, and Rosalind Helz for sample provision. Three anonymous reviewers and executive editor Graham Pearson greatly improved the text.
Editor: Graham Pearson
top
Author Contributions
P.S. and F.M. conceived the study and organised sample acquisition, F.M. and H.C. implemented the Cu isotopic analyses and improved the methods, P.S. and H.C. produced all of the Cu isotope data. P.S. produced all of the tables and figures and wrote the majority of the text. G.S., J.S. and J.B. designed and performed the high pressure experiments, performed electron probe analyses and wrote the experimental methods section. I.P. provided the komatiite samples. All of the authors were involved in critiquing the work during its authorship.
top
References
Albarède, F. (2009) Volatile accretion history of the terrestrial planets and dynamic implications. Nature 461, 1227-1233.
 Show in context
Show in context The mantle budget of Cu and other moderately volatile elements may be dominated by the final 10 % of material accreted to Earth, associated for example with the Moon-forming giant impactor, Theia (Albarède, 2009).
View in article
Zinc is more volatile and less siderophile/chalcophile than Cu (Lodders, 2003); like all moderately volatile, lithophile elements, Earth’s mantle is depleted in Zn compared to most chondrite groups suggesting partial loss or incomplete accretion (Palme and O’Neill, 2014). However, the bulk Earth Zn isotope composition is equal to or lighter than those same meteorites (Albarède, 2009; Chen et al., 2013), i.e. contrary to the isotope effect predicted by volatile loss.
View in article
Therefore, this does not preclude further unradiogenic Pb reservoirs, such as sulphides in refractory mantle phases (Burton et al., 2012) or late accretion of mantle Pb (Albarède, 2009).
View in article
Arndt, N. (2008) Komatiite. First edition, Cambridge University Press, United Kingdom.
 Show in context
Show in context The first were komatiites, mantle-derived ultramafic lavas generated by high degrees (>25 %) of mantle melting and typically found in Archaean terrains (Arndt, 2008).
View in article
Badro, J., Cote, A.S., Brodholt, J.P. (2014) A seismologically consistent compositional model of Earth’s core. Proceedings of the National Academy of Science 111, 7542-7545.
 Show in context
Show in context However, recent molecular dynamics estimates suggest S may not be present at all in the core (Badro et al., 2014); also, it is unclear as to whether S entered the core as an iron alloy, or as a discrete sulphide phase (O'Neill, 1991).
View in article
Given the constraints provided above (HM mass ≤ 1.6 % of Earth’s core), addition of a HM to the core will have a small effect on the core O composition (< 0.25 wt. % addition); for S, the effect is more significant – our model suggests that up to ~0.5 wt. % S could be added (Fig. 3a), which is in line with recent estimates based on molecular dynamics (Badro et al., 2014) and siderophile element partitioning studies (Siebert et al., 2013) – of course, this does not preclude further core addition of S as a metal alloy.
View in article
Burton, K.W., Cenki-Tok, B., Mokadem, F., Harvey, J., Gannoun, A., Alard, O., Parkinson, I.J. (2012) Unradiogenic lead in Earth's upper mantle. Nature Geoscience 5, 570-573.
 Show in context
Show in context Therefore, this does not preclude further unradiogenic Pb reservoirs, such as sulphides in refractory mantle phases (Burton et al., 2012) or late accretion of mantle Pb (Albarède, 2009).
View in article
Chen, H., Savage, P.S., Teng, F.-Z., Helz, R.T., Moynier, F. (2013) Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk Earth. Earth and Planetary Science Letters 369-370, 34-42.
 Show in context
Show in context Zinc is more volatile and less siderophile/chalcophile than Cu (Lodders, 2003); like all moderately volatile, lithophile elements, Earth’s mantle is depleted in Zn compared to most chondrite groups suggesting partial loss or incomplete accretion (Palme and O’Neill, 2014). However, the bulk Earth Zn isotope composition is equal to or lighter than those same meteorites (Albarède, 2009; Chen et al., 2013), i.e. contrary to the isotope effect predicted by volatile loss.
View in article
Dauphas, N., Chen, J.H., Zhang, J., Papanastassiou, D.A., Davis, A.M., Travaglio, C. (2014) Calcium-48 isotopic anomalies in bulk chondrites and achondrites: Evidence for a uniform isotopic reservoir in the inner protoplanetary disk. Earth and Planetary Science Letters 407, 96-108.
 Show in context
Show in context Another approach is to utilise the enstatite chondrites; despite being chemically dissimilar, these meteorites are identical to the Earth for most isotope systems (Dauphas et al., 2014) and many models suggest that the material that accreted to form the Earth contained a large proportion of enstatite chondrite-like planetesimals (Dauphas et al., 2014).
View in article
Dreibus, G., Palme, H. (1996) Cosmochemical constraints on the sulphur content in the Earth's core. Geochimica et Cosmochimica Acta 60, 1125-1130.
 Show in context
Show in context Sulphur is often cited as one such element: cosmochemical estimates suggest that the core contains ~2 wt. % S (Dreibus and Palme, 1996); sulphur in the core is seemingly necessary to explain mantle W and Mo abundances (Wade et al., 2012) and can explain the disparity between the radiometric Pb and W isotope ages of the mantle (Wood and Halliday, 2005).
View in article
Fitoussi, C., Bourdon, B. (2012) Silicon isotope evidence against an enstatite chondrite earth. Science 335, 1477-1480.
 Show in context
Show in context Based on modelling of Fitoussi and Bourdon (2012), we calculate a chondritic bulk Earth (CBE) value of δ65CuCBE = –0.19 ± 0.10 ‰ (2 s.d., Fig. 1).
View in article
Galer, S.J.G., Goldstein S.L. (1996) Influence of accretion on lead in the Earth. In: Basu, A., Hart, S. (Eds.) Earth Processes: Reading the Isotopic Code, AGU Geophysical Monograph 95, 75-98.
 Show in context
Show in context Modelling the evolution of mantle Pb isotope composition resulting from two fractionation events, metal-silicate equilibration at 50 Ma and sulphide-silicate equilibration at 100 Ma (following Galer and Goldstein, 1996; see Supplementary Information for details), predicts a present-day mantle Pb isotopic composition that is comparable to empirical estimates for BSE, albeit the more unradiogenic ones (Fig. 4; Halliday, 2004) as well as the Pb composition of the ancient primitive mantle as estimated using flood basalts (Jackson and Carlson, 2011).
View in article
Halliday, A.N. (2004) Mixing, volatile loss and compositional change during impact-driven accretion of the Earth. Nature 427, 505-509.
 Show in context
Show in context Modelling the evolution of mantle Pb isotope composition resulting from two fractionation events, metal-silicate equilibration at 50 Ma and sulphide-silicate equilibration at 100 Ma (following Galer and Goldstein, 1996; see Supplementary Information for details), predicts a present-day mantle Pb isotopic composition that is comparable to empirical estimates for BSE, albeit the more unradiogenic ones (Fig. 4; Halliday, 2004) as well as the Pb composition of the ancient primitive mantle as estimated using flood basalts (Jackson and Carlson, 2011).
View in article
Figure 4 [...] Lower figure is a zoom, showing that the composite curve agrees with some of the more unradiogenic estimates for modern day BSE Pb isotope composition (Halliday, 2004), as well as the compositions of certain flood basalt provinces (yellow: Ontong Java Plateau; grey: Baffin Island) thought to best represent ancient primitive mantle composition (Jackson and Carlson, 2011).
View in article
Herwartz, D., Pack, A., Friedrichs, B., Bischoff, A. (2014) Identification of the giant impactor Theia in lunar rocks. Science 344, 1146-1150.
 Show in context
Show in context However, more recent isotope data seems to rule out a CI-like impactor; in particular, precise lunar O isotope data suggests that the impactor had an enstatite chondrite isotope signature (Herwartz et al., 2014).
View in article
Holzheid, A., Sylvester, P., O’Neill, H.St.C., Rubie, D.C., Palme, H. (2000) Evidence for a late chondritic veneer in the Earth’s mantle from high-pressure partitioning of palladium and platinum. Nature 406, 396-399.
 Show in context
Show in context Further complications stem from the fact that late addition of extra-terrestrial S to the mantle, post-core formation, should overwhelm any pre-existing S (isotope) signature (the “late veneer”; Holzheid et al., 2000; Wang and Becker, 2013).
View in article
Jackson, M.G., Carlson, R. (2011) An ancient recipe for flood basalt genesis. Nature 476, 316-319.
 Show in context
Show in context Modelling the evolution of mantle Pb isotope composition resulting from two fractionation events, metal-silicate equilibration at 50 Ma and sulphide-silicate equilibration at 100 Ma (following Galer and Goldstein, 1996; see Supplementary Information for details), predicts a present-day mantle Pb isotopic composition that is comparable to empirical estimates for BSE, albeit the more unradiogenic ones (Fig. 4; Halliday, 2004) as well as the Pb composition of the ancient primitive mantle as estimated using flood basalts (Jackson and Carlson, 2011).
View in article
Figure 4 [...] Lower figure is a zoom, showing that the composite curve agrees with some of the more unradiogenic estimates for modern day BSE Pb isotope composition (Halliday, 2004), as well as the compositions of certain flood basalt provinces (yellow: Ontong Java Plateau; grey: Baffin Island) thought to best represent ancient primitive mantle composition (Jackson and Carlson, 2011).
View in article
Kleine, T., Palme, H., Mezger, K., Halliday, A.N. (2010) Hf-W chronometry of lunar metals and the age and early differentiation of the Moon. Science 310, 1671-1674.
 Show in context
Show in context The Cu in the mantle, therefore, would have escaped the effects of all but the final stages of planetary differentiation, as Hf-W isotope data suggests that the majority of the core had formed by the time of impact (Kleine et al., 2010).
View in article
Kramers, J.D., Tolstikhin, I.N. (1997) Two terrestrial lead isotope paradoxes, forward transport modelling, core formation and the history of the continental crust. Chemical Geology 139, 75-110.
 Show in context
Show in context Lead can be strongly chalcophile, and an early fractionation of isotopically primitive Pb by a sulphide-rich phase is often cited as a solution to the 1st terrestrial Pb paradox; that is, the observation that, in 206Pb/204Pb vs. 207Pb/204Pb space, most mantle-derived rocks, continental sediments etc. plot to the right of the terrestrial geochron (either the meteoritic, 4.568 Ga geochron or the later Hf-W core formation cessation age of ~4.53 Ga, Fig. 4; Kramers and Tolstikhin, 1997).
View in article
Labidi, J., Cartigny, P., Moreira, M. (2013) Non-chondritic sulphur isotope composition of the terrestrial mantle. Nature 501, 208-211.
 Show in context
Show in context Such a feature likely admixed into Earth’s core; however, if any of this material remains, such material could account for recently detected non-chondritic S isotope compositions in Earth's mantle (Labidi et al., 2013).
View in article
Lee, C.-T.A., Luffi, P., Chin, E.J., Bouchet, R., Dasgupta, R., Morton, D.M., Le Roux, V., Yin, Q.-Z., Jin, D. (2012) Copper systematics in arc magmas and implications for crust-mantle differentiation. Science 335, 64-66.
 Show in context
Show in context Lodders, K. (2003) Solar System abundances and condensation temperatures of the elements. Astrophysical Journal Letters 591, 1220-1247.
 Show in context
Show in context Zinc is more volatile and less siderophile/chalcophile than Cu (Lodders, 2003); like all moderately volatile, lithophile elements, Earth’s mantle is depleted in Zn compared to most chondrite groups suggesting partial loss or incomplete accretion (Palme and O’Neill, 2014).
View in article
Luck, J.M., Ben Othman, D., Albarède., F. (2005) Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochimica et Cosmochimica Acta 69, 5351-5363.
 Show in context
Show in context This is particularly important, as the range of chondrite Cu isotope compositions span a wide range (–1.45 ‰ < δ65Cu < +0.07 ‰, Fig. 1; Luck et al., 2005), thus we now discuss a number of model-dependent scenarios.
View in article
Markl, G., Lahaye, Y., Schwinn, G. (2006) Copper isotopes as monitors of redox processes in hydrothermal mineralization. Geochimica et Cosmochimica Acta 70, 4215-4228.
 Show in context
Show in context These data agree with the sense of Cu isotope fractionation between metal, silicate and sulphide measured in iron meteorites (Williams and Archer, 2011), as well as the extremely light Cu isotope compositions measured in secondary sulphide minerals (Markl et al., 2006).
View in article
McDonough, W.F. (2003) Compositional model for the Earth's core. In: Carlson, R.W. (Ed) The mantle and core, Treatise on Geochemistry,1st edition, 2, 547-568.
 Show in context
Show in context In an effort to investigate the role of S during Earth’s differentiation, we have investigated the Cu isotope compositions of bulk Earth and BSE; this is because Cu is siderophile and strongly chalcophile (~2/3 of Earth’s Cu is thought to be in the core; Palme and O’Neill, 2014, McDonough, 2003) but is less volatile than S, so is abundant enough in Earth’s mantle to have been largely unaffected by a late veneer.
View in article
O'Neill, H.St.C. (1991) The origin of the moon and the early history of the earth - A chemical model. Part 2: The earth. Geochimica et Cosmochimica Acta 55, 1159-1172.
 Show in context
Show in contextHowever, recent molecular dynamics estimates suggest S may not be present at all in the core (Badro et al., 2014); also, it is unclear as to whether S entered the core as an iron alloy, or as a discrete sulphide phase (O'Neill, 1991).
View in article
An alternative explanation is the early-stage formation of a sulphide-rich (Fe-O-S) liquid in the mantle, as the final volatile-rich residue after crystallisation of a magma ocean; this is often called the “Hadean Matte” (HM; Fig. 2; O’Neill, 1991).
View in article
Such a reservoir has been invoked to explain moderately siderophile element abundances in the mantle (O’Neill, 1991) and the mismatch of various core formation chronometers (Wood and Halliday, 2005), and could host significant amounts of Cu.
View in article
Palme, H., O'Neill, H.St.C. (2014) Cosmochemical estimates of mantle composition. In: Carlson, R.W. (Ed) The mantle and core, Treatise on Geochemistry, 2nd edition, 3, 1-39.
 Show in context
Show in context In an effort to investigate the role of S during Earth’s differentiation, we have investigated the Cu isotope compositions of bulk Earth and BSE; this is because Cu is siderophile and strongly chalcophile (~2/3 of Earth’s Cu is thought to be in the core; Palme and O’Neill, 2014, McDonough, 2003) but is less volatile than S, so is abundant enough in Earth’s mantle to have been largely unaffected by a late veneer.
View in article
It is of course impossible to obtain a sample of the ‘bulk Earth’ and hence, like many studies before ours, we assume that bulk Earth formed from primitive (chondritic) meteorites (e.g., Palme and O’Neill, 2014).
View in article
Zinc is more volatile and less siderophile/chalcophile than Cu (Lodders, 2003); like all moderately volatile, lithophile elements, Earth’s mantle is depleted in Zn compared to most chondrite groups suggesting partial loss or incomplete accretion (Palme and O’Neill, 2014).
View in article
Poirier, J.-P. (1994) Light elements in the Earth's outer core: A critical review. Physics of the Earth and Planetary Interiors 85, 319-337.
 Show in context
Show in context The budget of light elements in Earth’s core is a long-standing geochemical problem (Poirier, 1994), as constraining such elements and their abundances can tell us much about the physiochemical conditions of Earth’s differentiation.
View in article
Schönbächler, M., Carlson, R.W., Horan, M.F., Mock, T.D., Hauri, E.H. (2010) Heterogeneous accretion and the moderately volatile element budget of Earth. Science 328, 884-887.
 Show in context
Show in context Work on Ag isotopes by Schönbächler et al. (2010) seemed to indicate that the impactor material was dominated by CI-like material.
View in article
Siebert, J., Badro, J., Antonangeli, D., Ryerson, F.J. (2013) Terrestrial accretion under oxidizing conditions. Science 339, 1194-1197.
 Show in context
Show in context The value of [Cu]core should not fall below 120 ppm because Cu is also siderophile (Siebert et al., 2013), which predicts a maximum HM thickness of ~35 km.
View in article
Given the constraints provided above (HM mass ≤ 1.6 % of Earth’s core), addition of a HM to the core will have a small effect on the core O composition (< 0.25 wt. % addition); for S, the effect is more significant – our model suggests that up to ~0.5 wt. % S could be added (Fig. 3a), which is in line with recent estimates based on molecular dynamics (Badro et al., 2014) and siderophile element partitioning studies (Siebert et al., 2013) – of course, this does not preclude further core addition of S as a metal alloy.
View in article
Wade, J., Wood, B.J., Tuff, J. (2012) Metal–silicate partitioning of Mo and W at high pressures and temperatures: Evidence for late accretion of sulphur to the Earth. Geochimica et Cosmochimica Acta 85, 58-74.
 Show in context
Show in context Sulphur is often cited as one such element: cosmochemical estimates suggest that the core contains ~2 wt. % S (Dreibus and Palme, 1996); sulphur in the core is seemingly necessary to explain mantle W and Mo abundances (Wade et al., 2012) and can explain the disparity between the radiometric Pb and W isotope ages of the mantle (Wood and Halliday, 2005).
View in article
Wang, Z., Becker, H. (2013) Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature 499, 328-331.
 Show in context
Show in context Further complications stem from the fact that late addition of extra-terrestrial S to the mantle, post-core formation, should overwhelm any pre-existing S (isotope) signature (the “late veneer”; Holzheid et al., 2000; Wang and Becker, 2013).
View in article
Wänke, H., Dreibus, G. (1994) Chemistry and accretion history of Mars. Philosophical Transactions of the Royal Society of London Series A 349, 285-293.
 Show in context
Show in context Finally, the Martian core is thought to have up to 14 wt. % S (Wänke and Dreibus, 1994) so FeS-silicate equilibration during core formation could have a significant effect on Mars’ mantle; Cu isotopes have the potential to identify this effect.
View in article
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal-sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166-3178.
 Show in context
Show in context These data agree with the sense of Cu isotope fractionation between metal, silicate and sulphide measured in iron meteorites (Williams and Archer, 2011), as well as the extremely light Cu isotope compositions measured in secondary sulphide minerals (Markl et al., 2006).
View in article
Wood, B.J., Halliday, A.N. (2005) Cooling of the Earth and core formation after the giant impact. Nature 437, 1345-1348.
 Show in context
Show in context Sulphur is often cited as one such element: cosmochemical estimates suggest that the core contains ~2 wt. % S (Dreibus and Palme, 1996); sulphur in the core is seemingly necessary to explain mantle W and Mo abundances (Wade et al., 2012) and can explain the disparity between the radiometric Pb and W isotope ages of the mantle (Wood and Halliday, 2005).
View in article
Such a reservoir has been invoked to explain moderately siderophile element abundances in the mantle (O’Neill, 1991) and the mismatch of various core formation chronometers (Wood and Halliday, 2005), and could host significant amounts of Cu.
View in article
top
Supplementary Information
1. Samples
Choosing samples to constrain the Cu isotope composition of BSE is not trivial, due to the specific behaviour of Cu during mantle melting (Lee et al., 2012
Lee, C.-T.A., Luffi, P., Chin, E.J., Bouchet, R., Dasgupta, R., Morton, D.M., Le Roux, V., Yin, Q.-Z., Jin, D. (2012) Copper systematics in arc magmas and implications for crust-mantle differentiation. Science 335, 64-66.
). Any remaining residual sulphides will retain Cu, and partial melting with a degree typical for generating MORB or OIB magmas will not remove all Cu from a fertile peridotitic source. Such elemental fractionation could potentially give rise to isotopic fractionation. A predicted melt fraction of over 25 % is required before Cu is quantitatively transferred to the melt phase.With this in mind, two lithologies appear suitable as prospective samples for ascertaining the copper isotopic composition of BSE. The first are komatiites, which are mantle-derived ultramafic lavas, generated by high degrees (>25 %) of mantle melting and typically found in Archaean terrains. In this study, we analysed komatiite samples from two localities; 2.4 Ga Vetreny Belt (Baltic Shield, Puchtel et al., 1996
Puchtel, I.S., Hofmann, A.W., Mezger, K., Shchipansky, A.A., Kulikov, V.S., Kulikova, V.V. (1996) Petrology of a 2.41 Ga remarkably fresh komatiitic basalt lava lake in Lion Hills, central Vetreny Belt, Baltic Shield. Contributions to Mineralogy and Petrology 124, 273-290.
) and 2.7 Ga Belingwe (South Africa; Puchtel et al., 2009Puchtel, I.S., Walker, R.J., Brandon, A.D., Nisbet, E.G. (2009) Pt–Re–Os and Sm–Nd isotope and HSE and REE systematics of the 2.7 Ga Belingwe and Abitibi komatiites. Geochimica et Cosmochimica Acta 73, 6367-6389.
). The second are “fertile” orogenic lherzolites. Again, melting will deplete a mantle source, but not qualitatively so for Cu, which may give rise to isotopic fractionation. There are already Cu isotope data for peridotites, although many remain unpublished – we have used orogenic lherzolite data from such studies to use in our BSE average; specifically Lanzo (Italy; Ben Othman et al., 2006Ben Othman, D., Luck, J.M., Bodinier, J.L., Arndt, N.T., Albarède, F. (2006) Cu–Zn isotopic variations in the Earth’s mantle. Geochimica et Cosmochimica Acta 70, A46.
) and Horoman (Japan; Ikehata and Hirata, 2012Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497.
).We analysed a number of mid-ocean ridge basalts (MORB), which are typically formed by fairly high (10-15 %) degrees of melting of upper mantle. The samples represent the major ocean basins, from the Mid-Atlantic Ridge, East Pacific Rise and Pacific Antarctic Rise, and the Central and Southwest Indian Ridges. We also include data from a variety of ocean island basalt samples to investigate the possibility of Cu isotope mantle heterogeneities. Samples were taken from a range of localities which represent a range of the posited mantle end-members (e.g., Stracke et al., 2005
Stracke, A., Hofmann, A.W., Hart, S.R. (2005) FOZO, HIMU and the rest of the mantle zoo. Geochemistry, Geophysics, Geosystems 6.
). These include Hawaii (Kilauea Iki lava lake, DMM), Iceland (Hekla volcano, DMM), Tubuaii (South Pacific, HIMU), Fogo (Cape Verde, EM-1), Santo Antao (Cape Verde, “young” HIMU), Sao Miguel (Azores, EM-2-HIMU mixture) and La Palma (Canary Islands, HIMU).To assist in constraining a value for bulk Earth, we analysed a representative selection of enstatite chondrites (Keil, 1989
Keil, K. (1989) Enstatite meteorites and their parent bodies. Meteoritics and Planetary Science 24, 195-208.
). The sample set consisted of four samples from the high iron group, EH (Qingzhen EH3, Kota Kota EH3, Abee EH4 and Indarch EH4) and four from the low iron group, EL (MAC88184 EL3, LON94100 EL6, Hvittis EL6 and Eagle EL6).2. Methods for Cu isotope analysis
All samples were received as powders, processed variously by alumina jaw crusher and disk mills, agate ball mills and mortar and pestle. Approximately 25 – 100 mg of each sample powder was dissolved in pre-cleaned PFA beakers under heat lamps, using a 4:1 mixture of concentrated, triple-distilled HF-HNO3, followed by 6 N HCl to destroy fluoride complexes. The HCl dissolution/dry down step was repeated, and the residues were taken up in 1 ml 7 N HCl + 0.001 % H2O2 for ion chromatography.
All samples and external standards were purified for Cu isotope analysis using a single column ion chromatographic technique (modified after Maréchal et al., 1999
Maréchal, C.N., Télouk, P., Albarède, F. (1999) Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chemical Geology 156, 251-273.
). The procedure utilises Bio-Rad AG MP-1 anion resin, which, at low pH in chloride form, has a high partition coefficient for Cu. Typically, 100 % of each sample aliquot was loaded onto the column, apart from when column yield/chemistry was being tested whereby the aliquot was split 50:50 between two separate columns (see below). After sample loading, matrix elements were eluted in 7 ml of 7 N HCl + 0.001 % H2O2. The Cu was then eluted in a further 20 ml of 7 N HCl + 0.001 % H2O2. The samples were evaporated to dryness and the whole procedure was repeated to further purify the Cu. The final samples were taken up in 0.1 N HNO3 for analysis.Copper isotope analysis was performed on Neptune Plus Multi-Collector Inductively-Coupled-Plasma Mass-Spectrometers (MC-ICP-MS) at Washington University in St. Louis and the Institut de Physique du Globe, Paris. Samples were introduced into the instrument using an ESI PFA microflow nebuliser (100 μl min-1 flow rate) running into a glass spray chamber. The instrument was operated at low resolution (m/Δm ~ 450, where Δm is defined at 5 % and 95 % of peak height), with the 65Cu and 63Cu isotope beams collected in the C (central) and L2 Faraday cups respectively. Matrix elements were monitored using 62Ni and 64Zn beams in L3 and L1 in the same cup setup. Under typical running conditions, a 0.5 ppm Cu solution generated a 0.06-0.08 nA ion beam (6-8 V total signal using 10-11 Ω resistors). Instrument background signal (typically <10 mV total Cu) was measured at the beginning of each analytical session and the subsequent sample measurements were corrected for using these data. The total procedural blank contained ~4 ng Cu, which equates to <1 % of the Cu sample analyte.
Isotope ratios were measured in static mode, with each measurement consisting of 25 cycles of 8.4 second integrations, with a 3 second idle time. Ratios were calculated in the Thermo Neptune Data Evaluation software, which discarded any outliers at the 2 sigma confidence level. To correct for instrumental mass bias, isotope measurements were calculated using the standard-sample bracketing protocol relative to the NIST SRM976 standard, whereby variations in Cu ratios are defined using the delta notation δ65Cu as follows: δ65Cu = [(65Cu/63Cusample / 65Cu/63CuSRM976) – 1] × 1000. Each sample δ65Cu is an average of at least 3 analyses and a typical measurement session lasted around 12 hours. Instrumental drift over this time period was always quasi-linear. Calculating the δ65Cu value of SRM976 using adjacent bracketing standard measurements for two typical analysis session gives δ65Cu = –0.01 ± 0.13 ‰ (2 s.d., n = 33) and δ65Cu = 0.00 ± 0.05 ‰ (2 s.d., n = 44).
To assess method accuracy and ensure inter-laboratory comparison, we routinely analysed USGS standards BHVO-2, BIR-1, BCR-2 and AGV-1/-2. Our data for these standards (Table S-1) are in excellent agreement with the accepted values. To assess method reproducibility, a number of the sample aliquots were split 50:50; each split was separately processed through the chemistry and analysed on different days – these “repeats” are listed in Tables S-6 and S-7, and are identical to the initial measurements. A number of samples were repeated by dissolving a new aliquot of powder; again, these redissolutions have identical Cu isotope compositions to their original measurements (Tables S-1 and S-4).
Residual Na in a sample aliquot that has not been removed via the purification process can affect the accuracy of Cu isotope measurements (Larner et al., 2011
Larner, F., Rehkämper, M., Coles, B.J., Kreissig, K., Weiss, D.J., Sampson, B., Unsworth, C., Strekopytov, S. (2011) A new separation procedure for Cu prior to stable isotope analysis by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 26, 1627–1632.
). Specifically, Na forms 23Na40Ar+ in the plasma of the mass spectrometer, which is nominally the same mass as 63Cu+ and can therefore result in an overestimate of this isotope, driving the measured Cu isotope ratio to artificially lighter values. The ion chromatography procedure described above should quantitatively remove Na; nevertheless, we tested the magnitude of such an effect by doping aliquots of the Cu bracketing standard (SRM987, 250 ppb Cu) with various quantities of Na, resulting in Na/Cu values in the standards ranging from 0.001 to 1. Analysis of these aliquots shows that increasing Na/Cu results in an isotopic shift to lighter values, as predicted (Table S-1, Fig. S-1), with an offset of ~0.1 ‰ at high (>0.4) Na/Cu values. However, at lower Na/Cu this effect is minor, and does not result in an isotopic shift within error, from the standard. As a matter of course, we monitored the Na/Cu of each sample before analysis – if greater than 0.1, further sample purification was performed. With most of the mafic and ultramafic samples analysed for this study, low Na contents means that this is rarely an issue. Nevertheless, the presence of Na (coupled with low Cu contents) in a sample has the potential to bias Cu isotope data, and we were very careful to monitor sample [Na].
Figure S-1 Results of Na doping tests on Cu isotope measurements – sample is 250 ppb Cu SRM976 doped with various Na concentrations to results in Na/Cu between 0.001 and 1. Measurable isotopic offsets are present in samples with Na/Cu values of >0.2. Error bars are 1 s.d.
Table S-1 Cu isotope standards.
| Sample | δ65Cu (‰) | 2 s.d. | n | RefSI |
| BHVO-2 | 0.14 | 0.05 | 5 | |
| 0.06 | 0.01 | 3 | ||
| 0.11 | 0.02 | 3 | ||
| Average | 0.10 | 0.08 | 3 | |
| 0.10 | 0.04 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| 0.10 | 0.07 | Moynier et al., 2010 Moynier, F., Koeberl, C., Beck, P., Jourdan, F., Telouk, P. (2010) Isotopic fractionation of Cu in tektites. Geochimica et Cosmochimica Acta 74, 799-807. | ||
| 0.15 | 0.05 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| AGV-1 | -0.01 | 0.03 | 4 | |
| -0.01 | 0.09 | 3 | ||
| AGV-2 | 0.01 | 0.11 | 3 | |
| Average | 0.00 | 0.02 | 3 | |
| 0.11 | 0.04 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| 0.10 | 0.11 | Moynier et al., 2010 Moynier, F., Koeberl, C., Beck, P., Jourdan, F., Telouk, P. (2010) Isotopic fractionation of Cu in tektites. Geochimica et Cosmochimica Acta 74, 799-807. | ||
| 0.05 | 0.04 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| BIR-1 | 0.09 | 0.01 | 2 | |
| 0.08 | 0.07 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| -0.02 | 0.10 | Li et al., 2009 Li, W., Jackson, S.E., Pearson, N.J., Alard, O., Chappell, B.W. (2009) Cu isotopic signature of granites from the Lachlan Fold Belt, SE Australia. Chemical Geology 258, 38-49. | ||
| -0.01 | 0.04 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| BCR-2 | 0.19 | 0.07 | 2 | |
| 0.14 | 0.05 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| 0.07 | 0.08 | Archer and Vance. 2004 Archer, C., Vance, D. (2004) Mass discrimination correction in multiple-collector plasma source mass spectrometry: an example using Cu and Zn isotopes. Journal of Analytical Atomic Spectrometry 19, 656-665. | ||
| 0.19 | 0.08 | Bigalke et al., 2010 Bigalke, M., Kobza, J., Weyer, S., Wilcke, W. (2010) Stable Cu and Zn isotope ratios as tracers of sources and transport of Cu and Zn in contaminated soil. Geochimica et Cosmochimica Acta 74, 6801-6813. | ||
| 0.22 | 0.04 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| Na doping tests | ||||
| Sample | δ65Cu (‰) | 2 s.d. | Na/Cu | |
| Cu SRM976 (250 ppb Cu) | 0.00 | 0.08 | 0.001 | |
| SRM976 + 5 ppb Na | -0.01 | 0.08 | 0.02 | |
| SRM976 + 10 ppb Na | -0.03 | 0.08 | 0.04 | |
| SRM976 + 50 ppb Na | -0.04 | 0.08 | 0.2 | |
| SRM976 + 100 ppb Na | -0.11 | 0.08 | 0.4 | |
| SRM976 + 250 ppb Na | -0.09 | 0.08 | 1 | |
3. Cu isotope data
Terrestrial sample Cu isotope data used in calculating δ65CuBSE are presented in Tables S-2 thru S-7, and the enstatite chondrite data are given in Table S-8.
Komatiite Cu isotope data are presented in Table S-2. The Vetreny and Belingwe komatiites have Cu contents equal or greater than those predicted by melting of a fertile mantle source (Lee et al., 2012
Lee, C.-T.A., Luffi, P., Chin, E.J., Bouchet, R., Dasgupta, R., Morton, D.M., Le Roux, V., Yin, Q.-Z., Jin, D. (2012) Copper systematics in arc magmas and implications for crust-mantle differentiation. Science 335, 64-66.
), hence we use these sample data to provide a komatiitic Cu isotope average of δ65Cu = 0.06 ± 0.06 ‰ (2 s.d., n = 14).We use the lherzolite literature data from two sources (Ben Othman et al., 2006
Ben Othman, D., Luck, J.M., Bodinier, J.L., Arndt, N.T., Albarède, F. (2006) Cu–Zn isotopic variations in the Earth’s mantle. Geochimica et Cosmochimica Acta 70, A46.
; Ikehata and Hirata, 2012Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497.
). Ben Othman et al., 2006Ben Othman, D., Luck, J.M., Bodinier, J.L., Arndt, N.T., Albarède, F. (2006) Cu–Zn isotopic variations in the Earth’s mantle. Geochimica et Cosmochimica Acta 70, A46.
analysed the Cu isotope ratio of peridotites from two massifs, Beni-Bousera and Lanzo and three xenolith suites, Kilbourne Hole, San Carlos and Kaapvaal. All data describe a fairly wide spread (δ65Cu = –0.22 to 0.15 ‰), however, only the Lanzo suite were samples of orogenic lherzolites, and these define a much narrower isotopic range. It is these data we utilise in our BSE average (Table S-3); average δ65Cu = 0.08 ± 0.12 ‰ (2 s.d., n = 9).Ikehata and Hirata (2012)
Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497.
utilised laser ablation MC-ICP-MS to analyse native Cu grains from orogenic lherzolites from the Horoman massif, Japan. Morphologically, the Cu grains were identified as either primary (magmatic) or secondary (metasomatic). The primary Cu grains have a very limited Cu isotope range (δ65Cu = 0.04 to 0.07 ‰, Table S-3), and are identical to a plagioclase lherzolite the same study analysed by solution MC-ICPMS. These data give an average value of δ65Cu = 0.05 ± 0.02 ‰ (2 s.d., n = 7).To investigate possible Cu isotope heterogeneities in the mantle, as well as the possibility of Cu isotope fractionation during different degrees of partial melting, a comprehensive selection of mid-ocean ridge basalts (MORBs) and ocean island basalts (OIB) with varying chemical affinities were analysed (see SI Section 1). The MORB data are given in Table S-4. The data span a very narrow range (δ65Cu = 0.00 to 0.12 ‰) and there are no variations between ocean basins, hence we take a global MORB average of δ65Cu = 0.06 ± 0.06 ‰ (2 s.d., n = 17).
The OIB data are given in Tables S-5 thru S-7. These included two suites of basalts from “depleted mantle”-type plumes (Kilauea Iki lava lake, Hawaii and Hekla volcano, Iceland), as well as more isotopically “enriched” end-member plumes: Tubuaii, South Pacific, (HIMU), Fogo, Cape Verde (EM-1), Santo Antao, Cape Verde (“young” HIMU), Sao Miguel, Azores (EM-2-HIMU mixture) and La Palma, Canary Islands (HIMU).
Cu isotope analysis of 2 eruption samples and 8 Kilauea Iki lava lake samples reveal little variation (Table S-4). The average composition of Kilauea Iki lavas is δ65Cu = 0.04 ± 0.09 ‰ (2 s.d., n = 10; Table S-5). The average value for Hekla basalts, δ65Cu = 0.08 ± 0.13 ‰ (2 s.d., n = 5; Table S-6) is identical to Kilauea Iki (as well as the komatiite, MORB and lherzolite values). The final ocean island basalts (OIBs) representing various mantle isotopic “end-members” (Stracke et al., 2005
Stracke, A., Hofmann, A.W., Hart, S.R. (2005) FOZO, HIMU and the rest of the mantle zoo. Geochemistry, Geophysics, Geosystems 6.
) are less well-characterised than the others described above, with scant elemental data available in the literature. Nevertheless, the range of Cu isotope compositions defined by the OIBs is slightly larger, but generally comparable to the other datasets (δ65Cu range = –0.07 to 0.16 ‰, Table S-7) and the average value (δ65Cu = 0.07 ± 0.14 ‰ 2 s.d., n = 15) is identical to all the others described above.Our new data for the enstatite chondrites are given in Table S-8. The group averages for EH and EL chondrites (EH δ65Cu = –0.23 ± 0.06 ‰; EL δ65Cu = –0.26 ± 0.13 ‰) are notable in that they are isotopically indistinguishable from each other but are distinct from all other chondrite groups (see Fig. 1 in main text).
Table S-2 Komatiites.
| Sample | δ65Cu (‰) | 2 s.d.1 | n | Age (Ga) | Cu (ppm) |
| Vetreny | |||||
| 91105/1 | 0.10 | 0.05 | 3 | 2.4 | 63 |
| 91105 | 0.04 | 0.07 | 3 | 2.4 | 54 |
| 91104 | 0.04 | 0.09 | 3 | 2.4 | 67 |
| 91106 | 0.04 | 0.01 | 3 | 2.4 | 62 |
| 12101 | 0.05 | 0.01 | 5 | 2.4 | 95 |
| 12111 | 0.10 | 0.02 | 5 | 2.4 | 94 |
| 12117 | 0.00 | 0.07 | 5 | 2.4 | 92 |
| 12124 | 0.09 | 0.02 | 5 | 2.4 | 95 |
| Belingwe | |||||
| TN1 | 0.06 | 0.01 | 5 | 2.69 | 67 |
| TN3 | 0.03 | 0.02 | 5 | 2.69 | 75 |
| TN16 | 0.06 | 0.02 | 5 | 2.69 | 47 |
| TN17 | 0.03 | 0.02 | 3 | 2.69 | 512 |
| TN21 | 0.11 | 0.02 | 5 | 2.69 | 452 |
| ZV10 | 0.07 | 0.02 | 3 | 2.69 | 52 |
| Average | 0.06 | 0.06 | 14 | ||
1 2 standard deviations based on either the repeat measurements or, in the case where there is only 1 measurement, based on the 2 s.d. of external standard analysis that day.
2 Cu concentrations measured on MC-ICP-MS at Washington University in St. Louis.
Table S-3 Peridotites (orogenic lherzolites - literature).
| Lanzo | δ65Cu (‰) | 2 s.d. | n |
| Ben Othman et al., 2006 Ben Othman, D., Luck, J.M., Bodinier, J.L., Arndt, N.T., Albarède, F. (2006) Cu–Zn isotopic variations in the Earth’s mantle. Geochimica et Cosmochimica Acta 70, A46. | -0.01 | ||
| 0.13 | |||
| 0.01 | |||
| 0.03 | |||
| 0.09 | |||
| 0.09 | |||
| 0.15 | |||
| 0.10 | |||
| 0.15 | |||
| Average | 0.08 | 0.12 | 9 |
| Horoman | |||
| Type 1 (magmatic native Cu). Ikehata and Hirata, 2012 Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497. | 0.04 | ||
| 0.06 | |||
| 0.07 | |||
| 0.06 | |||
| 0.04 | |||
| 0.05 | |||
| Plagioclase lherzolite. Ikehata and Hirata, 2012 Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497. | 0.05 | ||
| Average | 0.05 | 0.02 | 7 |
Table S-4 Mid ocean ridge basalts.
| Sample | δ65Cu (‰) | 2 s.d. | n |
| Mid Atlantic Ridge | |||
| DIVA1 15-5 | 0.09 | 0.03 | 3 |
| DIVA1 13-3 | 0.09 | 0.03 | 3 |
| EW9309 27D-1g | 0.12 | 0.05 | 3 |
| EW9309 4D-3g | 0.06 | 0.01 | 3 |
| EW9309 10D-3g | 0.07 | 0.04 | 3 |
| RD87 DR29-101 | 0.06 | 0.05 | 3 |
| RD87 DR24 | 0.02 | 0.02 | 3 |
| RD87 DR18-102 | 0.06 | 0.03 | 3 |
| Central Indian Ridge | |||
| MD57 D7-5 | 0.04 | 0.01 | 3 |
| MD57 D2-8 | 0.04 | 0.09 | 3 |
| Pacific Antarctic Ridge | |||
| PAC2 DR37-3g | 0.08 | 0.01 | 3 |
| PAC2 DR32-1g | 0.08 | 0.02 | 3 |
| East Pacific Rise | |||
| SEARISE2 DR3 | 0.04 | 0.00 | 3 |
| redissolution | 0.05 | 0.03 | 3 |
| CYP78 12-34 | 0.07 | 0.04 | 3 |
| Southwest Indian Ridge | |||
| SWIFT DR32-1-3g | 0.10 | 0.02 | 3 |
| SWIFT DR06-3-6g | 0.06 | 0.05 | 3 |
| SWIFT DR04-2-3g | 0.00 | 0.03 | 3 |
| Average | 0.06 | 0.06 | 17 |
Table S-5 Kilauea Iki.
| Sample | δ65Cu (‰) | 2 s.d.1 | n | Cu (ppm) | MgO (wt.%) |
| Eruption samples | |||||
| Iki-22 | 0.06 | 0.08 | 1 | 120 | 19.52 |
| Iki-58 | 0.06 | 0.08 | 1 | 124 | 8.08 |
| Lava lake | |||||
| KI67-3-6.8 | 0.08 | 0.08 | 1 | 60 | 25.83 |
| KI79-3-150.4 | -0.02 | 0.08 | 1 | 102 | 13.51 |
| KI67-3-27.5 | 0.04 | 0.08 | 1 | 86 | 12.01 |
| KI75-1-121.5 | 0.14 | 0.08 | 1 | 138 | 7.77 |
| KI75-1-75.2 | -0.01 | 0.08 | 1 | 168 | 5.77 |
| KI79-1R1-170.9 | 0.00 | 0.08 | 1 | 365 | 3.48 |
| KI67-2-85.7 | 0.05 | 0.08 | 1 | 450 | 2.6 |
| KI81-2-88.6 | 0.04 | 0.08 | 1 | 345 | 2.37 |
| Average | 0.04 | 0.09 | 10 | ||
1 Errors based on the 2 s.d. of external standard analysis that day.
Download in ExcelTable S-6 Hekla.
| Sample | δ65Cu (‰) | 2 s.d.1 | n | Cu (ppm) | SiO2 (wt.%) |
| Basalts | |||||
| HEK04-09 | 0.14 | 0.08 | 1 | 39.8 | 46.97 |
| HEK05-09 | 0.00 | 0.08 | 1 | 47.1 | 46.47 |
| repeat | 0.01 | 0.21 | 2 | ||
| HEK09-09 | 0.06 | 0.08 | 1 | 47.3 | 46.64 |
| repeat | -0.02 | 0.10 | 3 | ||
| HEK07-09 | 0.11 | 0.08 | 1 | 25.4 | 47.01 |
| HEK12-09 | 0.13 | 0.08 | 1 | 26 | 46.42 |
| Average | 0.08 | 0.13 | 5 | ||
1 2 standard deviations based on either the repeat measurements or. in the case where there is only 1 measurement, based on the 2 s.d. of external standard analysis that day.
Download in ExcelTable S-7 Ocean island basalts.
| Sample | δ65Cu (‰) | 2 s.d.1 | n |
| Santo Antao | |||
| SA-1 | 0.12 | 0.09 | 1 |
| SA-5 | 0.00 | 0.05 | 2 |
| repeat | -0.06 | 0.03 | 3 |
| SA-6 | 0.10 | 0.07 | 2 |
| SA-8 | 0.12 | 0.09 | 1 |
| SA-11 | -0.07 | 0.05 | 2 |
| repeat | -0.07 | 0.07 | 3 |
| La Palma | |||
| W-1949 48c | 0.16 | 0.10 | 2 |
| 1585 45d | 0.13 | 0.09 | 1 |
| LP69d | 0.08 | 0.03 | 2 |
| repeat | 0.03 | 0.04 | 3 |
| Sao Miguel | |||
| SM-7 | 0.08 | 0.09 | 1 |
| repeat | 0.03 | 0.11 | 3 |
| SM-33 | 0.02 | 0.07 | 2 |
| Fogo | |||
| FG-2 | 0.11 | 0.04 | 2 |
| FG-6 | 0.12 | 0.10 | 2 |
| repeat | 0.08 | 0.06 | 3 |
| FG-12 | 0.14 | 0.09 | 1 |
| Tubuaii | |||
| TBA-C29 | 0.02 | 0.07 | 2 |
| Iceland | |||
| RHY01-09 | -0.03 | 0.09 | 1 |
| Average | 0.07 | 0.14 | 15 |
“Repeat” signifies reanalysis of the same sample aliquot on a different day.
1 2 standard deviations based on either the repeat measurements or. in the case where there is only 1 measurement, based on the 2 s.d. of external standard analysis that day.
Table S-8 Enstatite chondrites.
| Sample | Type | δ65Cu (‰) | 2 s.d. | n |
| EH | ||||
| Qingzhen | EH3 | -0.29 | 0.03 | 3 |
| Kota Kota | EH3 | -0.15 | 0.03 | 3 |
| Abee | EH4 | -0.24 | 0.06 | 3 |
| Indarch | EH4 | -0.25 | 0.05 | 3 |
| Average EH | -0.23 | 0.06 | 4 | |
| EL | ||||
| MAC88184 | EL3 | -0.11 | 0.06 | 3 |
| LON94100 | EL6 | -0.21 | 0.03 | 3 |
| Hvittis | EL6 | -0.38 | 0.03 | 3 |
| Eagle | EL6 | -0.35 | 0.05 | 3 |
| Average EL | -0.26 | 0.13 | 4 | |
4. Bulk Silicate Earth and Bulk Earth Cu isotope averages
The average Cu isotope compositions for all the terrestrial samples analysed in this study, as well as the literature data for peridotites, are summarised in Table S-9 and in Figure 1 and Figure S-2. Also listed is the average value of various basaltic standards given by the literature data (see Table S-1). As all averages are statistically identical and the distribution of all data is normal, a simple arithmetic mean is taken for the BSE value, which gives a composition of δ65Cu = 0.07 ± 0.10 ‰ (2 s.d.).
The Cu isotope compositions of all chondrite groups are presented in Table S-9, as well as the average Cu content for each group (Luck et al., 2003
Luck, J.-M., Ben Othman, D., Barrat, J.A., Albarède, F. (2003) Coupled 63Cu and 16O excesses in chondrites. Geochimica et Cosmochimica Acta 67, 143-151.
, 2005Luck, J.-M., Ben Othman, D., Albarède, F. (2005) Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochimica et Cosmochimica Acta 69, 5351-5363.
; Barrat et al., 2012Barrat, J.A., Zanda, B., Moynier, F., Bollinger, C., Liorzou, C., Bayon, G. (2012) Geochemistry of CI chondrites: Major and trace elements, and Cu and Zn Isotopes. Geochimica et Cosmochimica Acta 83, 79-92.
). The Chondritic Bulk Earth (CBE) was calculated by using the “best” mixture of ordinary and carbonaceous chondrites estimated in Fitoussi and Bourdon (2012)Fitoussi, C., Bourdon, B. (2012) Silicon isotope evidence against an enstatite chondrite earth. Science 335, 1477-1480.
for the Earth, using O and Cr isotopes (0.16 CI: 0.21 CO: 0.63 LL), which, when weighted using [Cu], gives a value of δ65CuCBE = –0.19 ± 0.10 ‰, 2 s.d. Another estimate, made using a chondritic mixture based on O isotopes as well as major element considerations (given by Lodders, 2000Lodders, K. (2000) An oxygen isotope mixing model for the accretion and composition of rocky planets. Space Science Reviews 92, 341-354.
, as 0.70:0.21:0.05:0.04 EH:H:CV:CI), results in a bulk Earth composition of δ65Cu = –0.28 ± 0.07 ‰, 2 s.d.Using a mixture of 50:50 EH:EL chondrites gives an Enstatite Chondrite Bulk Earth (ECBE) value of δ65CuECBE = –0.24 ± 0.09 ‰, 2 s.d., statistically identical to that of δ65CuCBE. This would also be the value for BSE assuming that as Cu is a moderately volatile element, the majority of Cu in the mantle was delivered late on in Earth’s accretion, mostly from the giant impactor. This is because the most recent estimate of the impactor composition, based on high precision O isotope measurements, suggests that it was, isotopically, closest to enstatite chondrites (Herwartz et al., 2014
Herwartz, D., Pack, A., Friedrichs, B., Bischoff, A. (2014) Identification of the giant impactor Theia in lunar rocks. Science 344, 1146-1150.
). Of all the estimates for bulk Earth calculated from chondrites, we conservatively utilise the closest value to that of the mantle in the following modelling (δ65CuCBE = –0.19 ± 0.10 ‰).
Figure S-2 Box and whisker plot showing the range of Cu isotope compositions for the terrestrial samples used in constraining the BSE Cu isotope composition. Box represents 1st and 3rd quartile, line in box represents the median and “whiskers” show the range of the data (see Tables S-2-S-7, S-9). There is no statistical difference between each group. Calculated BSE value plotted as green dashed line, green box shows 2 s.d. precision of the estimate.
Table S-9 BSE and bulk Earth averages.
| BSE average | ||||
| Group | Average δ65Cu (‰) | 2 s.d. | n | |
| Komatiites | 0.06 | 0.06 | 14 | |
| Orogenic lherzolites | 0.07 | 0.09 | 16 | |
| MORBs | 0.06 | 0.06 | 17 | |
| Kilauea Iki basalts | 0.04 | 0.09 | 10 | |
| Hekla basalts | 0.08 | 0.13 | 5 | |
| OIBs | 0.07 | 0.14 | 15 | |
| Basaltic standards | 0.06 | 0.11 | 3 | |
| BSE average | 0.07 | 0.10 | 80 | |
| Bulk Earth average | ||||
| Meteorite group | Average δ65Cu (‰) | 2 s.d. | n | Average Cu (ppm) |
| CI | 0.02 | 0.16 | 3 | 122 |
| CM | -0.5 | 0.06 | 2 | 115 |
| CO | -0.86 | 0.07 | 2 | 122 |
| CV | -1.45 | 0.08 | 2 | 100 |
| H | -0.44 | 0.11 | 3 | 81 |
| L | -0.08 | 0.13 | 5 | 88 |
| LL | 0.07 | 0.06 | 2 | 80 |
| EH | -0.23 | 0.06 | 4 | 210 |
| EL | -0.26 | 0.13 | 4 | 120 |
| CBE average | -0.19 | 0.10 | ||
| ECBE average | -0.24 | 0.09 | ||
| ACBE average | -0.28 | 0.07 | ||
5. Experimental determination of Cu isotope fractionation between metal, sulphide and silicate
The following represents a pilot study to confirm the sense and approximate magnitude of equilibrium Cu isotope fractionation that results from high temperature, high pressure equilibration of Cu between metal, silicate, and sulphide phases. Only temperature was varied.
Experiments were performed at 2 GPa at temperatures between 1400 and 1750 °C using an end-loaded piston cylinder apparatus at IPG, Paris. All experiments were conducted using a standard talc/Pyrex (below 1550 °C) or BaCO3 (above 1550 °C) pressure cell, graphite furnace, inner MgO spacers, and 8 mm diameter MgO capsules with 3 mm diameter sample chambers (Siebert et al., 2011
Siebert, J., Corgne, A., Ryerson, F.J. (2011) Systematics of metal-silicate partitioning for many siderophile elements applied to Earth's core formation. Geochimica et Cosmochimica Acta 75, 1451-1489.
). Oxygen fugacity was keptExperiments were quenched by turning off the electrical power to the furnace. Run duration was kept short (~1 min for metal-silicate experiments, ~5 min for sulphide-silicate, Table S-10) to avoid percolation of the charge through the polycrystalline MgO capsules and limit MgO contamination (it has been shown that run durations as short as few tens of second, at superliquidus conditions, were sufficient to reach chemical equilibrium at similar P–T conditions; Thibault and Walter, 1995
Thibault, Y., Walter, M.J. (1995) The influence of pressure and temperature on the metal-silicate partition coefficients of nickel and cobalt in a model C1 chondrite and implications for metal segregation in a deep magma ocean. Geochimica et Cosmochimica Acta 59, 991-1002.
). Moreover, similar run durations were found sufficient to reach Fe isotope equilibrium (Poitrasson et al., 2013Poitrasson, F., Roskosz, M., Corgne, A. (2013) No iron isotope fractionation between molten alloys and silicate melt to 2000 °C and 7.7 GPa: Experimental evidence and implications for planetary differentiation and accretion. Earth and Planetary Science Letters 278, 376-385.
), albeit at higher temperatures. Starting materials were prepared by mixing powders of metallic Fe or FeS with ground natural MORB. Relative proportions of metal/sulphide and silicate were 1/3 and 2/3 by mass respectively for all experiments. Cu was added at the 1 wt. % level as high purity CuO oxide, Cu metal or a 50:50 mixture of the two to assess isotopic equilibration with “reversal experiments” (see Table S-10 for details).Elemental data was acquired via electron microprobe, carried out with a Cameca SX100. Due to the quench textures, analyses with a 20 μm defocused beam for both metal and silicate melts were used to determine the bulk compositions. Operating conditions were 15 kV accelerating voltage, 10 nA beam current and counting times of 10–20 s on peak and background for major elements in silicate (Si, Mg, Fe, Al, Ca, Na, Ti) and metal (Fe, S, Cu, O). Multiple analyses of all phases revealed only minor chemical variations, indicating homogeneous phase composition and confirming that chemical equilibrium had been reached.
Experimental charges were separated from their capsule and, in the case of the metal-silicate experiments, ground to a fine powder in an agate pestle and mortar. The magnetic phase was separated using a hand magnet and dissolved in conc. HCl, which further avoided digestion of silicate material. The remaining silicate powder was dissolved as for the rest of the silicate samples. Note that this will not guarantee quantitative separation, so the fractionation factors listed below are likely to be minimum values.
For the sulphide-silicate experiments, much of the sulphide coalesced into a ball, which was picked from the charge (given the sulphide started as a disseminated powder, this is further evidence that the charges were well equilibrated – a similar fact is true for the metal-silicate experiments whereby all the Cu was added as CuO – and no CuO was detected after the experiment had run). This was then digested in aqua regia overnight. Imaging of these charges revealed a significant amount of disseminated sulphides in the silicate phase; presence of such in the silicate dissolution will overwhelm the silicate Cu isotope signature, given the extremely high Cu content in all sulphides. To date, efforts to remove the sulphides by leaching have been unsuccessful. Therefore, we analysed the sulphide bleb against the starting Cu metal which, assuming no Cu loss (see below), gives a qualitative sense of Cu isotope fractionation – sufficient for our modelling. Efforts to further constrain Cu isotope behaviour in this respect are ongoing. All experiment dissolutions were purified by column chemistry and analysed as for natural samples (see SI Section 2).
The results of the piston cylinder experiments are given in Table S-10. For the metal-silicate experiments, using the partition coefficient parameterisation given in (Corgne et al., 2008
Corgne, A., Keshav, S., Wood, B.J., McDonough, W.F., Fei Y. (2008) Metal–silicate partitioning and constraints on core composition and oxygen fugacity during Earth accretion. Geochimica et Cosmochimica Acta 72, 574-589.
) predicts DCumetal-silicate values of between 35 and 45. The predicted and measured DCumetal-silicate values are within the same magnitude and the experimental values show a general increase with increasing temperature – an exception being experiment 61. This was repeated (experiment 73) and a more realistic DCumetal-silicate value was measured – the high D value measured in experiment 61 is thought to be a result of BaCO3 contamination in the charge. To confirm equilibrium was reached in the experiments, a reversal experiment (71, 72 and 73) was performed – the DCumetal-silicate values and Δ65Cu values are identical, implying equilibrium was reached. Further evidence for the lack of Cu loss during these experiments comes from calculating the bulk Cu isotope composition of the charge based on phase compositions (i.e. metal + silicate) and comparing it to the composition of the bulk experiment, i.e. that of the CuO (or Cu metal) added to the charge, which are equal (calculated av. δ65Cu = 0.16 ± 0.02 ‰ vs. CuO δ65Cu = 0.15 ± 0.02 ‰; when Cu was added as Cu metal: calculated δ65Cu = 0.13 ± 0.05 ‰ vs. Cu metal δ65Cu = 0.13 ± 0.03 ‰; see Table S-10). Note that there was one aliquot of CuO powder used during the experiments that was exhausted before we could analyse its bulk composition (exp. 71 and 73) and these bulk compositions are slightly different to the others. Although we could not perform such tests with our sulphide experiments, we are confident that, given the similar experimental conditions, lower experimental temperatures, homogeneous phase compositions etc., no Cu was lost from those charges either.For the sulphide experiments, Cu contents in the silicates were typically below regular detection limits of the instrument (<200 ppm Cu); however, these concentrations can give us order of magnitude estimates on DCusulfide-silicate ranging between 500 and 900. These are in line with estimates based on more detailed studies (Gaetani and Grove, 1997
Gaetani, G.A., Grove, T.L. (1997) Partitioning of moderately siderophile elements among olivine, silicate melt, and sulphide melt: Constraints on core formation in the Earth and Mars. Geochimica et Cosmochimica Acta 61, 1829-1846.
; Fellows and Canil, 2012Fellows, S.A., Canil, D. (2012) Experimental study of the partitioning of Cu during partial melting of Earth’s mantle. Earth and Planetary Science Letters 337-338, 133-143.
); this, along with lack of elemental zoning seen in the electron probe analyses, indicates that chemical equilibrium was met. In all piston-cylinder experiments, there is some reaction of the charge with the capsule material (in our case, MgO). Typically, Fe will react with MgO to form ferro-periclase; in this case, Fe is removed from the charge. If large amounts of Fe are lost, then FeS will not be stable, and other non-stoichiometric sulphide phases may form, potentially affecting Cu (isotope) partitioning. In our case, we kept the experimental run times as short as possible to avoid this, nevertheless, the MgO content of our charge increased slightly, indicating capsule interaction. To check for this, we measured sulphide Fe and S concentrations by electron microprobe; in all cases, the sulphide has equal molar amounts of Fe and S (1:1) indicating that the sulphide phase was FeS, and suggesting that the degree of Fe loss did not affect S activity.All metal-silicate experiments give positive Δ65Cumet-sil (where Δ65Cux-y = δ65Cux – δ65Cuy, Table S-10) in that the metal phase is consistently enriched in the heavier Cu isotope. The data agree with the sense and general magnitude of the Δ65Cumet-sil value measured in Williams and Archer (2011)
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal-sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75,
, viz. +0.48 ‰, on the basis of one analysis of a silicate-bearing iron meteorite. There does not appear to be a strong control of temperature over Δ65Cumet-sil (Fig. S-3) over the T range used in the experiments. As a result, we cannot parameterise the fractionation factor as a function of temperature to predict the Δ65Cumet-sil value at temperatures associated with core formation, except to say that, as temperatures increase, equilibrium isotope fractionation factors approach unity. Hence, these are certainly maximum values in terms of temperature dependence; however, they are also potentially minimum values given that we could not be sure that complete phase separation was achieved during processing. Also we do not have any data on how parameters such as pressure, oxygen fugacity and composition affect the fractionation factor, which could equally cause the Δ65Cusil-met to increase in magnitude. Clearly, further studies are necessary.As noted above, we were unable to quantitatively remove sulphides from the “silicate” phases of the experiments. Instead we have analysed the sulphide bleb as well as the Cu starting material, in an effort to constrain the sense (and order of magnitude) of Cu isotope fractionation during sulphide-silicate equilibrium – this is assuming minimal Cu loss, which we believe is valid (see above). In both experiments, the sulphide was enriched in the lighter Cu isotope relative to the bulk and, also, isotopic difference is smaller at higher temperatures (Table S-10, Fig. S-4), indicating that Δ65Cusulfide-sil is negative and reduces with temperature, as would be predicted. Using mass balance to estimate order-of-magnitude Δ65Cusulfide-sil values gives a wide range, from ~ –0.5 to –20‰, due to the large effect of error propagation. In light of the higher temperatures of core formation relative to these experiments, as well as the degree of fractionation observed previously in secondary sulphide minerals (viz. > –3.0 ‰; Markl et al., 2006
Markl, G., Lahaye, Y., Schwinn, G. (2006) Copper isotopes as monitors of redox processes in hydrothermal mineralization. Geochimica et Cosmochimica Acta 70, 4215-4228.
), we tentatively suggest that lower magnitudes in the range predicted by our experiments (e.g., Δ65Cusulfide-sil > –1.0 ‰) are more likely.
Figure S-3 Summary of metal-silicate Cu isotope equilibration experiments – note that this figure is not a graph (the x axis is discrete, not continuous). All Δ65Cusil-met values are negative but there does not appear to be a strong temperature control on these values. The one experiment with S added gives the largest Δ65Cusil-met value, this makes sense if there is minor amounts of S-hosted Cu that was dissolved with the non-magnetic (“silicate”) phase – as Cu sulphides are shown to be isotopically light w.r.t. silicates and metal. Error bars are 1 s.d.

Figure S-4 Summary of sulphide-silicate Cu isotope equilibration experiments – we are so far unable to successfully measure the silicate phase, due to the presence of disseminated sulphides which overwhelm the Cu isotope signature of the silicates. Here we show the Cu isotope composition of the bulk Cu metal as well as sulphide phases from two experiments (wherein only the temperature was varied). In both cases the sulphides are enriched in the light Cu isotope relative to bulk, and there is a small decrease in fractionation magnitude (although within error) as temperature increases.
Table S-10 Experimental results.
| Metal-silicate experiments | All @ 2 GPa | |||||||||
| Initial components: MORB + Fe + CuO | ||||||||||
| Experiment | Phase | δ65Cu (‰) | 2 s.d. | Cu conc. | D [Cu]m/[Cu]sil | Δm-sil (‰) | 2 s.d. | T °C | t @max T | |
| 61 | Silicate | 0.10 | 0.04 | 238 ppm | 160 | 0.06 | 0.06 | 1650 | 1 min | |
| Metal | 0.16 | 0.05 | 3.80 % | |||||||
| 67 | Silicate | 0.05 | 0.04 | 484 ppm | 38 | 0.12 | 0.05 | 1580 | 1 m 20 s | |
| Metal | 0.17 | 0.03 | 1.90 % | |||||||
| 68 | Silicate | 0.12 | 0.05 | 441 ppm | 49 | 0.06 | 0.08 | 1560 | 30 s | |
| Metal | 0.18 | 0.06 | 2.10 % | |||||||
| 69 | Silicate | 0.02 | 0.04 | 432 ppm | 58 | 0.13 | 0.05 | 1750 | 30 s | |
| Metal | 0.15 | 0.03 | 2.50 % | |||||||
| Reversal experiments | ||||||||||
| Initial components: MORB + Fe + Cu + CuO | ||||||||||
| 71 | Silicate | 0.05 | 0.04 | 668 ppm | 64 | 0.15 | 0.09 | 1650 | 35 s | |
| Metal | 0.2 | 0.08 | 4.30 % | |||||||
| Initial components: MORB + Fe + Cu | ||||||||||
| 72 | Silicate | 0.02 | 0.04 | 478 ppm | 69 | 0.11 | 0.07 | 1650 | 30 s | |
| Metal | 0.13 | 0.06 | 3.30 % | |||||||
| Initial components: MORB + Fe + CuO | ||||||||||
| 73 | Silicate | 0.16 | 0.04 | 507 ppm | 54 | 0.07 | 0.05 | 1650 | 30 s | |
| Metal | 0.24 | 0.03 | 2.80 % | |||||||
| Initial components: MORB + Fe + CuO + FeS | ||||||||||
| 64 | Silicate | -0.01 | 0.05 | 46 | 0.18 | 0.08 | 1650 | 30 s | ||
| Metal | 0.17 | 0.06 | ||||||||
| Initial components: MORB + FeS + Cu | D | Δsul-bulk (‰) | ||||||||
| [Cu]sul/[Cu]sil | ||||||||||
| ELMO113 | Silicate | n/a | ~50 ppm | ~500 | -0.12 | 0.04 | 1400 | 6 min | ||
| Sulphide | 0.01 | 0.02 | 2.60 % | |||||||
| ELMO114 | Silicate | n/a | ~60 ppm | ~900 | -0.09 | 0.05 | 1550 | 3 min | ||
| Sulphide | 0.04 | 0.04 | 5.40 % | |||||||
| Bulk Cu | Metal | 0.13 | 0.03 | 99.99 % | ||||||
| Bulk CuO | Oxide (CuO) | 0.15 | 0.02 | ~80 % | ||||||
Experiments 71 and 72 are reversal experiments (i.e. all Cu in 72 was reduced, all Cu in 71 was CuO). Experiment 73: repeat of Experiment 61 – high D value due to BaCO3?
Download in Excel6. Copper isotope constraints on the thickness of the Hadean Matte
Our model for the formation of a Fe-O-S layer at the base of Earth’s mantle, i.e. the Hadean Matte, is based on O’Neill (1991)
O'Neill, H.St.C. (1991) The origin of the moon and the early history of the earth - A chemical model. Part 2: The earth. Geochimica et Cosmochimica Acta 55, 1159-1172.
and Lee et al., (2007)Lee, C.-T.A., Yin, Q.-Z., Lenardic. A., Agranier, A., O’Neill, C.J., Thiagarajan, N. (2007) Trace-element composition of Fe-rich residual liquids formed by fractional crystallization: Implications for the Hadean magma ocean. Geochimica et Cosmochimica Acta 71, 3601-3615.
, whereby such a layer forms as the last liquid remaining after crystallisation of a magma ocean. At that time, the core was 99 % of its present size. The Fe-O-S layer is denser than the silicate mantle, and so it sinks to the core-mantle boundary. There is no limit as to whether it is subsequently admixed into the core, but re-equilibration with the mantle should be limited. In the model, we do not take into account any additional Cu added to Earth via late veneer, as it should be volumetrically insignificant relative to the amount of Cu left in the mantle. In terms of Cu, this means that the current BSE evolved over a two stage process, where first Cu was sequestered into the core, and then into the Fe-O-S layer – with the attendant isotopic fractionation associated with each phase equilibration.The first issue when attempting a Cu isotope mass balance is to fix the abundances of Cu in the BSE, the bulk Earth, and the core. McDonough (2003)
McDonough, W.F. (2003) Compositional model for the Earth's core. In: Carlson, R.W. (Ed.) The mantle and core, Treatise on Geochemistry, 1st edition, 2, 547-568.
gives 30 ppm as an estimate for BSE, based on the Cu vs. MgO correlation of massif peridotites. However, Palme and O’Neill (2014)Palme, H., O'Neill, H.St.C. (2014) Cosmochemical estimates of mantle composition. In: Carlson, R.W. (Ed.) The mantle and core, Treatise on Geochemistry, 2nd edition, 3, 1-39.
suggest that 30 ppm Cu in the BSE is too high, and use the average composition of xenolith peridotites (20 ppm Cu) as a more realistic estimate. Corgne et al. (2008)Corgne, A., Keshav, S., Wood, B.J., McDonough, W.F., Fei Y. (2008) Metal–silicate partitioning and constraints on core composition and oxygen fugacity during Earth accretion. Geochimica et Cosmochimica Acta 72, 574-589.
also suggested that 20 ppm BSE Cu is a more realistic estimate, based on partitioning experiments. These data suggest that the core should have ~ 160 ppm Cu, which translates into 64 ppm Cu in the bulk Earth. Hence, we take these estimates (BSE = 20 ppm, bulk Earth = 64 ppm, core = 160 ppm) as working abundances for our mass balance.Assuming the Fe-O-S layer (hereafter referred to as “HM”, Hadean Matte) forms as a constant thickness mantling the core, then its mass (mHM in kg) can be related to its thickness (xHM in m) by:
Eq. S-1
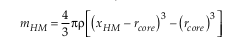
where ρ is the assumed density of a Fe-O-S liquid (7000 kg m-3) and rcore is the radius of the core (3.47 x 106 m). The mass of Cu in the HM (mHMCu) can then be estimated using the partition coefficient (DCuS-Sil) of Cu between sulphide and silicate liquid such that:
Eq. S-2

where [Cu]BSE is the current Cu concentration of Bulk Silicate Earth (20 ppm). Values of DCuS-Sil between silicate and sulphide liquids have been experimentally determined, and can exceed 1000 (Gaetani and Grove, 1997
Gaetani, G.A., Grove, T.L. (1997) Partitioning of moderately siderophile elements among olivine, silicate melt, and sulphide melt: Constraints on core formation in the Earth and Mars. Geochimica et Cosmochimica Acta 61, 1829-1846.
; Fellows and Canil, 2012Fellows, S.A., Canil, D. (2012) Experimental study of the partitioning of Cu during partial melting of Earth’s mantle. Earth and Planetary Science Letters 337-338, 133-143.
; Kiseeva and Wood, 2013Kiseeva, E.S., Wood, B.J. (2013) A simple model for chalcophile element partitioning between sulphide and silicate liquids with geochemical applications. Earth and Planetary Science Letters 383, 68-81.
). However, the presence of O in a FeS liquid will reduce the chalcophile nature of Cu (Kiseeva and Wood, 2013Kiseeva, E.S., Wood, B.J. (2013) A simple model for chalcophile element partitioning between sulphide and silicate liquids with geochemical applications. Earth and Planetary Science Letters 383, 68-81.
), and recent work suggests that Cu is more compatible in silicate phases than earlier estimates predicted. As such, we have limited the DCuS-Sil values in our model to between 100 and 500.We then use the estimated concentration of Cu in the core (160 ppm) and apportion this reservoir between the core and HM. This model thus provides an adjusted Cu composition of the core by:
Eq. S-3
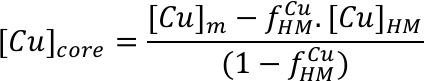
where [Cu]m is the total Cu (160 ppm) and fCuHM is the fraction of Cu in the Hadean Matte, where fCuHM = mCuHM / (mCuHM + mCucore). Equations S-1, S-2 and S-3 can be combined to relate [Cu]core to xHM, the results for DCuS-Sil values of 100, 200, and 500 are shown in Figure 3a in the main text.
Further constraints can be placed on this model. Although Cu becomes less siderophile at high pressures associated with magma ocean fractionation, the metal phase should be enriched by at least a factor of ~3.5 in Cu relative to the silicate (Corgne et al., 2008
Corgne, A., Keshav, S., Wood, B.J., McDonough, W.F., Fei Y. (2008) Metal–silicate partitioning and constraints on core composition and oxygen fugacity during Earth accretion. Geochimica et Cosmochimica Acta 72, 574-589.
). In our model, the core forms first, and sequesters Cu, hence [Cu]core should be at least 3.5x greater than that of the mantle before removal of the HM. Figure S-5 shows the relationship between [Cu]core and effective DCumetal-Sil for all the models – the curve suggests that, to be above 3.5, the core should contain at least ~120 ppm Cu, limiting the scenarios (see Fig. 3a).Once [Cu] for core and HM are fixed, the Cu isotope evolution of the mantle can be modelled, again following two stage model. The Cu isotope mass balance between the primordial mantle (PM; that is, the mantle after core formation but before Hadean Matte segregation) and core is related to the bulk Earth composition as such:
Eq. S-4
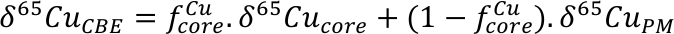
where CBE is Chondritic Bulk Earth, as estimated in SI Section 4 (δ65CuCBE = –0.19 ± 0.10 ‰). Although we do not know the absolute Cu isotope composition of the core, we can replace this term with the metal-silicate fractionation factor, Δ65Cum-s, estimated using the experiments (here we assume a slightly positive Δ65Cum-s of +0.10 ‰, Table S-10), where:
Eq. S-5
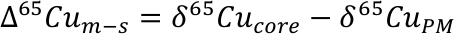
Eq. S-6
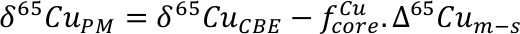
As Δ65Cum-s is positive, this results in a primordial mantle that has a lighter Cu isotope composition than CBE (δ65Cu = –0.26 ‰; Figure 2 in main text). If we were to instead assume that all Cu was delivered post metal-silicate equilibration by the giant impact, this is where the story starts. Assuming an enstatite chondrite-like impactor, this would set the composition of Earth’s mantle to δ65CuECBE = -0.24 ± 0.09 ‰, 2 s.d. This is also slightly lighter than CBE and, in fact, identical to our predicted primordial (post-core formation) Cu isotope composition.
To reach the current mantle (BSE) Cu isotope composition, the subsequent removal of Cu into the HM should preferentially remove the lighter isotopes. The composition of the HM needed to balance BSE vs. PM can therefore be calculated:
Eq. S-7
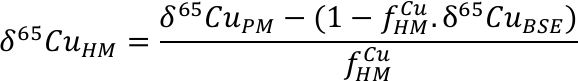
The range of δ65CuHM values for the various allowed scenarios in Figure 3a are shown in Figure S-6. These can be converted into Δ65Cusil-s values. Although we have no constraints on this value except that it will be negative relative to BSE, at mantle temperatures, Δ65Cusil-s > 3 ‰ may be unrealistic. Therefore, only those solutions where Δ65Cusil-s < 2 ‰ are shown in the main text (Fig. 3b).

Figure S-5 Relationship between calculated Cu in core (as a result of increasing thickness of HM) and apparent DCu metal silicate values – this value should be at least greater than 3.5, which equates to >120 ppm Cu in the core. D = DCusulfide-silicate therefore, for BSE [Cu] = 20 ppm, D = 500→[Cu]HM = 10000; D = 200→[Cu]HM = 4000; D = 100→[Cu]HM = 2000.

Figure S-6 Relationship between thickness of Hadean Matte and Cu isotope composition, for various [Cu]HM concentrations. As the thickness reduces, the isotope composition of the HM becomes more extreme, toward very light values.
7. Chemical consequences of forming the Hadean Matte on Earth’s mantle and core
We choose a relatively thick layer for the HM (30 km) predicted by our models, in order to constrain the maximum elemental and isotopic effects. In terms of major element composition of the HM, we assume a stoichiometric Fe-O-S mixture, whereby the HM is composed of ~54 wt. % Fe, ~15 wt. % O and ~31 % wt. % S.
Iron is a major element in the mantle; hence, a 30 km thick HM layer, composed of 54 % Fe by mass, would only represent <1 % of Earth’s Fe budget, and ~7 % of the non-metallic terrestrial Fe. The effect on mantle O abundance is smaller (<<1 % of mantle O). In terms of assessing the impact of admixing the HM into the core, obviously, the effect on core Fe content is insignificant. With respect to O, admixing of a 30 km thick HM would add 0.25 wt. % O to the core.
The current elemental and isotopic S composition of the mantle is compatible with the formation of the HM. This is because experiments suggest that most, if not all, of primordial S was removed from the mantle during planetary differentiation, into either the metal or sulphide phase (O’Neill, 1991
O'Neill, H.St.C. (1991) The origin of the moon and the early history of the earth - A chemical model. Part 2: The earth. Geochimica et Cosmochimica Acta 55, 1159-1172.
). The current (relatively low) S content of the mantle is thought to have been delivered during late accretion by volatile-rich impactors, based on the chondritic S isotope composition of most mantle-derived rocks (Chaussidon et al., 1991Chaussidon, M., Sheppard, S., Michard, A. (1991) Hydrogen, sulphur and neodymium isotope variations in the mantle beneath the EPR at 12°50'N. In: Taylor, H.P., O’Neil, J.R., Kaplan, I.R. (Eds.) Stable Isotope Geochemistry: a Tribute to Samuel Epstein. Geochemical Society Special Publication 3, 325–337.
). Any S remaining in the primordial mantle, post core formation, would be isotopically light relative to chondrites, as metal-silicate S fractionation preferentially enriches the metal in the heavier isotopes (Shahar et al., 2009Shahar, A., Fei, Y., Liu, M.C., Wang, J. (2009) Sulfur isotopic fractionation during the differentiation of Mars. Geochimica et Cosmochimica Acta 73, A1201.
). This would mean that, if remaining primordial S was removed from the mantle by a Fe-O-S layer, it would have an isotopically light composition. Admixing the HM into the core is predicted to add up to 0.6 wt. % S to the core.The effect on mantle Pb isotope composition
There is a long-standing conundrum regarding the Pb isotope composition of the BSE, in that, in 206Pb/204Pb vs. 207Pb/204Pb space, most mantle-derived rocks, continental sediments, etc. plot to the right of the terrestrial geochron (either the meteorite 4.568 Ga geochron or the later Hf-W core formation cessation age of ~4.53 Ga, Fig. 4 in main text). This means that most terrestrial mantle-derived samples have an overabundance of radiogenic 206Pb. Assuming that Earth has an identical primordial Pb (and U) isotope budget to meteorites, there must be some complementary unradiogenic reservoir. However, there is no consensus over what this reservoir could be. This is known as the first terrestrial Pb paradox (Allègre, 1969
Allègre, C.J. (1969) Comportement des systèmes U-Th-Pb dans le manteau supérieur et modèle d'évolution de ce dernier au cours des temps géologiques. Earth and Planetary Science Letters 5, 261-269.
).Regardless of the existence of the Pb paradox, the silicate Earth has to have evolved with a higher μ (μ = 238U/204Pb) than that predicted for a chondritic bulk Earth (μ ~ 1, Allègre et al., 1995
Allègre, C.J., Manhes, G., Göpel, C. (1995) The age of the Earth. Geochimica et Cosmochimica Acta 59, 1445-1456.
) to explain the overall high modern day 206Pb/204Pb and 207Pb/204Pb ratios in silicate samples. To explain the predicted modern day BSE μ values of between 8 and 10, the silicate Earth has to have roughly 60× less 204Pb than bulk Earth. One way to fractionate U and Pb is via core formation – this is because Pb is both siderophile and chalcophile at conditions relevant to core formation (Bouhifd et al., 2013Bouhifd, M.A., Andrault, D., Bolfan-Casanova, N., Hammouda, T., Devidal, J.L. (2013) Metal–silicate partitioning of Pb and U: Effects of metal composition and oxygen fugacity. Geochimica et Cosmochimica Acta 114, 13–28.
) whereas U is lithophile.The possibility of U/Pb fractionation as a result of core formation led to a possible solution to the Pb paradox (Allègre et al., 1982
Allègre, C.J., Dupré, B., Brévart, O. (1982) Chemical aspects of the formation of the core. Philosophical Transactions of the Royal Society of London Series A 306, 49-59.
, Galer and Goldstein, 1996Galer, S.J.G., Goldstein S.L. (1996) Influence of accretion on lead in the Earth. In: Basu, A., Hart, S. (Eds.) Earth Processes: Reading the Isotopic Code, AGU Geophysical Monograph 95, 75-98.
). Specifically, a younger age for the Pb isotope system, e.g., 4.468 Ga, is invoked - in this case, the Pb geochron is less steep and passes through the centre of the MORB/OIB arrays. The younger “Pb age” of the silicate Earth is often cited to represent the cessation of core formation. The main issue with this interpretation is that the Hf-W age of core formation and the Pb age of core formation do not agree – with the Pb age consistently younger (~100 Ma after the Solar System formation) than the Hf-W age (~35 Ma). To explain this, some workers have posited that the Hf-W age reflects the bulk of core segregation, whereas the Pb age reflects a late stage addition of S-rich material into the core (Wood and Halliday, 2010Wood, B.J., Halliday, A.N. (2010) The lead isotopic age of the Earth can be explained by core formation alone. Nature 465, 767-770.
). This fits well with the Hadean Matte hypothesis. Here, we model the Pb isotope evolution of the mantle with 2 fractionation events (as with our mantle Cu isotope model).Our model follows that of Galer and Goldstein (1996)
Galer, S.J.G., Goldstein S.L. (1996) Influence of accretion on lead in the Earth. In: Basu, A., Hart, S. (Eds.) Earth Processes: Reading the Isotopic Code, AGU Geophysical Monograph 95, 75-98.
, and calculates ingrowth of uranogenic Pb as a result of increasing μ values as 204Pb is removed first into the core at 50 Ma, then into the 30 km-thick HM at 100 Ma. We take a bulk Earth 204Pb concentration of 700 ppb and that of the present day mantle of 104 ppb. Using this latter value, we can use previously determined values of DPbS-sil to predict the 204Pb concentration in the HM, and then by mass balance calculate the amount of 204Pb needed to reside in the core before HM formation. Values for DPbS-sil are all >1, but vary somewhat; here, we choose a value of 50, based on two more recent studies (Li and Audetat, 2012Li, Y., Audétat, A. (2012) Partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulphide phases and hydrous basanite melt at upper mantle conditions. Earth and Planetary Science Letters 355-356, 327–340.
; Wood et al., 2008Wood, B.J., Nielsen, S.G., Rehkämper, M., Halliday, A.N. (2008) The effects of core formation on the Pb- and Tl- isotopic composition of the silicate Earth. Earth and Planetary Science Letters 269, 326–336.
). This gives 204Pb = 5200 ppb in the HM, and ~ 1900 ppb in the core. Calculating the apparent metal-silicate DPb from these concentrations gives a value of ~13, which is in agreement with the recent estimates (Wood et al., 2008Wood, B.J., Nielsen, S.G., Rehkämper, M., Halliday, A.N. (2008) The effects of core formation on the Pb- and Tl- isotopic composition of the silicate Earth. Earth and Planetary Science Letters 269, 326–336.
). Starting with a bulk Earth μ of ~0.9, core formation would increase mantle μ to ~6.1, then HM would further increase this to ~8.5.Figure 4 in the main text shows the evolution of mantle Pb isotope composition for this 2-stage fractionation model. The insert shows the growth curve of mantle Pb at the intersection with the terrestrial geochron, as well as a selection of Pb isotope BSE estimates (Halliday, 2004
Halliday, A.N. (2004) Mixing, volatile loss and compositional change during impact-driven accretion of the Earth. Nature 427, 505-509.
) and flood basalt compositions, which are thought to best represent the ancient primitive mantle (Jackson and Carlson, 2011Jackson, M.G., Carlson, R. (2011) An ancient recipe for flood basalt genesis. Nature 476, 316-319.
). The figure shows that our model matches fairly well some of the more unradiogenic estimates of Pb isotope mantle composition, which indicates that formation of a 30 km thick HM could explain the first Pb paradox. However, if the true Pb isotope composition of the mantle is more radiogenic (i.e., the bulk of the estimates plotted in Fig. 4), this leaves the door open for further unradiogenic Pb reservoirs.Supplementary Information References
Allègre, C.J. (1969) Comportement des systèmes U-Th-Pb dans le manteau supérieur et modèle d'évolution de ce dernier au cours des temps géologiques. Earth and Planetary Science Letters 5, 261-269.
 Show in context
Show in context This is known as the first terrestrial Pb paradox (Allègre, 1969).
View in Supplementary Information
Allègre, C.J., Dupré, B., Brévart, O. (1982) Chemical aspects of the formation of the core. Philosophical Transactions of the Royal Society of London Series A 306, 49-59.
 Show in context
Show in context The possibility of U/Pb fractionation as a result of core formation led to a possible solution to the Pb paradox (Allègre et al., 1982, Galer and Goldstein, 1996).
View in Supplementary Information
Allègre, C.J., Manhes, G., Göpel, C. (1995) The age of the Earth. Geochimica et Cosmochimica Acta 59, 1445-1456.
 Show in context
Show in context Regardless of the existence of the Pb paradox, the silicate Earth has to have evolved with a higher μ (μ = 238U/204Pb) than that predicted for a chondritic bulk Earth (μ ~ 1, Allègre et al., 1995) to explain the overall high modern day 206Pb/204Pb and 207Pb/204Pb ratios in silicate samples.
View in Supplementary Information
Archer, C., Vance, D. (2004) Mass discrimination correction in multiple-collector plasma source mass spectrometry: an example using Cu and Zn isotopes. Journal of Analytical Atomic Spectrometry 19, 656-665.
 Show in context
Show in context In Table S-1
View in Supplementary Information
Barrat, J.A., Zanda, B., Moynier, F., Bollinger, C., Liorzou, C., Bayon, G. (2012) Geochemistry of CI chondrites: Major and trace elements, and Cu and Zn Isotopes. Geochimica et Cosmochimica Acta 83, 79-92.
 Show in context
Show in context The Cu isotope compositions of all chondrite groups are presented in Table S-9, as well as the average Cu content for each group (Luck et al., 2003, 2005; Barrat et al., 2012).
View in Supplementary Information
Ben Othman, D., Luck, J.M., Bodinier, J.L., Arndt, N.T., Albarède, F. (2006) Cu–Zn isotopic variations in the Earth’s mantle. Geochimica et Cosmochimica Acta 70, A46.
 Show in context
Show in context There are already Cu isotope data for peridotites, although many remain unpublished – we have used orogenic lherzolite data from such studies to use in our BSE average; specifically Lanzo (Italy; Ben Othman et al., 2006) and Horoman (Japan; Ikehata and Hirata, 2012).
View in Supplementary Information
We use the lherzolite literature data from two sources (Ben Othman et al., 2006; Ikehata and Hirata, 2012).
View in Supplementary Information
Ben Othman et al. (2006) analysed the Cu isotope ratio of peridotites from two massifs, Beni-Bousera and Lanzo and three xenolith suites, Kilbourne Hole, San Carlos and Kaapvaal.
View in Supplementary Information
In Table S-3
View in Supplementary Information
Bigalke, M., Kobza, J., Weyer, S., Wilcke, W. (2010) Stable Cu and Zn isotope ratios as tracers of sources and transport of Cu and Zn in contaminated soil. Geochimica et Cosmochimica Acta 74, 6801-6813.
 Show in context
Show in context In Table S-1
View in Supplementary Information
Bouhifd, M.A., Andrault, D., Bolfan-Casanova, N., Hammouda, T., Devidal, J.L. (2013) Metal–silicate partitioning of Pb and U: Effects of metal composition and oxygen fugacity. Geochimica et Cosmochimica Acta 114, 13–28.
 Show in context
Show in context To explain the predicted modern day BSE μ values of between 8 and 10, the silicate Earth has to have roughly 60× less 204Pb than bulk Earth. One way to fractionate U and Pb is via core formation – this is because Pb is both siderophile and chalcophile at conditions relevant to core formation (Bouhifd et al., 2013) whereas U is lithophile.
View in Supplementary Information
Chaussidon, M., Sheppard, S., Michard, A. (1991) Hydrogen, sulphur and neodymium isotope variations in the mantle beneath the EPR at 12°50'N. In: Taylor, H.P., O’Neil, J.R., Kaplan, I.R. (Eds.) Stable Isotope Geochemistry: a Tribute to Samuel Epstein. Geochemical Society Special Publication 3, 325–337.
 Show in context
Show in context The current (relatively low) S content of the mantle is thought to have been delivered during late accretion by volatile-rich impactors, based on the chondritic S isotope composition of most mantle-derived rocks (Chaussidon et al., 1991).
View in Supplementary Information
Corgne, A., Keshav, S., Wood, B.J., McDonough, W.F., Fei Y. (2008) Metal–silicate partitioning and constraints on core composition and oxygen fugacity during Earth accretion. Geochimica et Cosmochimica Acta 72, 574-589.
 Show in context
Show in context For the metal-silicate experiments, using the partition coefficient parameterisation given in (Corgne et al., 2008) predicts DCumetal-silicate values of between 35 and 45.
View in Supplementary Information
Corgne et al. (2008) also suggested that 20 ppm BSE Cu is a more realistic estimate, based on partitioning experiments.
View in Supplementary Information
Although Cu becomes less siderophile at high pressures associated with magma ocean fractionation, the metal phase should be enriched by at least a factor of ~3.5 in Cu relative to the silicate (Corgne et al., 2008).
View in Supplementary Information
Fellows, S.A., Canil, D. (2012) Experimental study of the partitioning of Cu during partial melting of Earth’s mantle. Earth and Planetary Science Letters 337-338, 133-143.
 Show in context
Show in context These are in line with estimates based on more detailed studies (Gaetani and Grove, 1997; Fellows and Canil, 2012); this, along with lack of elemental zoning seen in the electron probe analyses, indicates that chemical equilibrium was met. In all piston-cylinder experiments, there is some reaction of the charge with the capsule material (in our case, MgO).
View in Supplementary Information
where [Cu]BSE is the current Cu concentration of Bulk Silicate Earth (20 ppm). Values of DCuS-Sil between silicate and sulphide liquids have been experimentally determined, and can exceed 1000 (Gaetani and Grove, 1997; Fellows and Canil, 2012; Kiseeva and Wood, 2013).
View in Supplementary Information
Fitoussi, C., Bourdon, B. (2012) Silicon isotope evidence against an enstatite chondrite earth. Science 335, 1477-1480.
 Show in context
Show in context The Chondritic Bulk Earth (CBE) was calculated by using the “best” mixture of ordinary and carbonaceous chondrites estimated in Fitoussi and Bourdon (2012) for the Earth, using O and Cr isotopes (0.16 CI: 0.21 CO: 0.63 LL), which, when weighted using [Cu], gives a value of δ65CuCBE = –0.19 ± 0.10 ‰, 2 s.d.
View in Supplementary Information
Gaetani, G.A., Grove, T.L. (1997) Partitioning of moderately siderophile elements among olivine, silicate melt, and sulphide melt: Constraints on core formation in the Earth and Mars. Geochimica et Cosmochimica Acta 61, 1829-1846.
 Show in context
Show in contextThese are in line with estimates based on more detailed studies (Gaetani and Grove, 1997; Fellows and Canil, 2012); this, along with lack of elemental zoning seen in the electron probe analyses, indicates that chemical equilibrium was met. In all piston-cylinder experiments, there is some reaction of the charge with the capsule material (in our case, MgO).
View in Supplementary Information
where [Cu]BSE is the current Cu concentration of Bulk Silicate Earth (20 ppm). Values of DCuS-Sil between silicate and sulphide liquids have been experimentally determined, and can exceed 1000 (Gaetani and Grove, 1997; Fellows and Canil, 2012; Kiseeva and Wood, 2013).
View in Supplementary Information
Galer, S.J.G., Goldstein S.L. (1996) Influence of accretion on lead in the Earth. In: Basu, A., Hart, S. (Eds.) Earth Processes: Reading the Isotopic Code, AGU Geophysical Monograph 95, 75-98.
 Show in context
Show in context The possibility of U/Pb fractionation as a result of core formation led to a possible solution to the Pb paradox (Allègre et al., 1982, Galer and Goldstein, 1996).
View in Supplementary Information
Our model follows that of Galer and Goldstein (1996), and calculates ingrowth of uranogenic Pb as a result of increasing μ values as 204Pb is removed first into the core at 50 Ma, then into the 30 km-thick HM at 100 Ma. We take a bulk Earth 204Pb concentration of 700 ppb and that of the present day mantle of 104 ppb.
View in Supplementary Information
Halliday, A.N. (2004) Mixing, volatile loss and compositional change during impact-driven accretion of the Earth. Nature 427, 505-509.
 Show in context
Show in context The insert shows the growth curve of mantle Pb at the intersection with the terrestrial geochron, as well as a selection of Pb isotope BSE estimates (Halliday, 2004) and flood basalt compositions, which are thought to best represent the ancient primitive mantle (Jackson and Carlson, 2011).
View in Supplementary Information
Herwartz, D., Pack, A., Friedrichs, B., Bischoff, A. (2014) Identification of the giant impactor Theia in lunar rocks. Science 344, 1146-1150.
 Show in context
Show in context This is because the most recent estimate of the impactor composition, based on high precision O isotope measurements, suggests that it was, isotopically, closest to enstatite chondrites (Herwartz et al., 2014).
View in Supplementary Information
Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497.
 Show in context
Show in context There are already Cu isotope data for peridotites, although many remain unpublished – we have used orogenic lherzolite data from such studies to use in our BSE average; specifically Lanzo (Italy; Ben Othman et al., 2006) and Horoman (Japan; Ikehata and Hirata, 2012).
View in Supplementary Information
We use the lherzolite literature data from two sources (Ben Othman et al., 2006; Ikehata and Hirata, 2012).
View in Supplementary Information
Ikehata and Hirata (2012) utilised laser ablation MC-ICP-MS to analyse native Cu grains from orogenic lherzolites from the Horoman massif, Japan.
View in Supplementary Information
In Table S-3
View in Supplementary Information
Jackson, M.G., Carlson, R. (2011) An ancient recipe for flood basalt genesis. Nature 476, 316-319.
 Show in context
Show in context The insert shows the growth curve of mantle Pb at the intersection with the terrestrial geochron, as well as a selection of Pb isotope BSE estimates (Halliday, 2004) and flood basalt compositions, which are thought to best represent the ancient primitive mantle (Jackson and Carlson, 2011).
View in Supplementary Information
Keil, K. (1989) Enstatite meteorites and their parent bodies. Meteoritics and Planetary Science 24, 195-208.
 Show in context
Show in context To assist in constraining a value for bulk Earth, we analysed a representative selection of enstatite chondrites (Keil, 1989).
View in Supplementary Information
Kiseeva, E.S., Wood, B.J. (2013) A simple model for chalcophile element partitioning between sulphide and silicate liquids with geochemical applications. Earth and Planetary Science Letters 383, 68-81.
 Show in context
Show in context where [Cu]BSE is the current Cu concentration of Bulk Silicate Earth (20 ppm). Values of DCuS-Sil between silicate and sulphide liquids have been experimentally determined, and can exceed 1000 (Gaetani and Grove, 1997; Fellows and Canil, 2012; Kiseeva and Wood, 2013).
View in Supplementary Information
However, the presence of O in a FeS liquid will reduce the chalcophile nature of Cu (Kiseeva and Wood, 2013), and recent work suggests that Cu is more compatible in silicate phases than earlier estimates predicted. As such, we have limited the DCuS-Sil values in our model to between 100 and 500.
View in Supplementary Information
Larner, F., Rehkämper, M., Coles, B.J., Kreissig, K., Weiss, D.J., Sampson, B., Unsworth, C., Strekopytov, S. (2011) A new separation procedure for Cu prior to stable isotope analysis by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 26, 1627–1632.
 Show in context
Show in context Residual Na in a sample aliquot that has not been removed via the purification process can affect the accuracy of Cu isotope measurements (Larner et al., 2011).
View in Supplementary Information
Lee, C.-T.A., Yin, Q.-Z., Lenardic. A., Agranier, A., O’Neill, C.J., Thiagarajan, N. (2007) Trace-element composition of Fe-rich residual liquids formed by fractional crystallization: Implications for the Hadean magma ocean. Geochimica et Cosmochimica Acta 71, 3601-3615.
 Show in context
Show in context Our model for the formation of a Fe-O-S layer at the base of Earth’s mantle, i.e. the Hadean Matte, is based on O’Neill (1991) and Lee et al., (2007), whereby such a layer forms as the last liquid remaining after crystallisation of a magma ocean.
View in Supplementary Information
Lee, C.-T.A., Luffi, P., Chin, E.J., Bouchet, R., Dasgupta, R., Morton, D.M., Le Roux, V., Yin, Q.-Z., Jin, D. (2012) Copper systematics in arc magmas and implications for crust-mantle differentiation. Science 335, 64-66.
 Show in context
Show in context Choosing samples to constrain the Cu isotope composition of BSE is not trivial, due to the specific behaviour of Cu during mantle melting (Lee et al., 2012).
View in Supplementary Information
The Vetreny and Belingwe komatiites have Cu contents equal or greater than those predicted by melting of a fertile mantle source (Lee et al., 2012), hence we use these sample data to provide a komatiitic Cu isotope average of δ65Cu = 0.06 ± 0.06 ‰ (2 s.d., n = 14).
View in Supplementary Information
Li, W., Jackson, S.E., Pearson, N.J., Alard, O., Chappell, B.W. (2009) Cu isotopic signature of granites from the Lachlan Fold Belt, SE Australia. Chemical Geology 258, 38-49.
 Show in context
Show in context In Table S-1
View in Supplementary Information
Li, Y., Audétat, A. (2012) Partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulphide phases and hydrous basanite melt at upper mantle conditions. Earth and Planetary Science Letters 355-356, 327–340.
 Show in context
Show in context Values for DPbS-sil are all >1, but vary somewhat; here, we choose a value of 50, based on two more recent studies (Li and Audetat, 2012; Wood et al., 2008).
View in Supplementary Information
Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133.
 Show in context
Show in contextIn Table S-1
View in Supplementary Information
Lodders, K. (2000) An oxygen isotope mixing model for the accretion and composition of rocky planets. Space Science Reviews 92, 341-354.
 Show in context
Show in context Another estimate, made using a chondritic mixture based on O isotopes as well as major element considerations (given by Lodders, 2000, as 0.70:0.21:0.05:0.04 EH:H:CV:CI), results in a bulk Earth composition of δ65Cu = –0.28 ± 0.07 ‰, 2 s.d.
View in Supplementary Information
Luck, J.-M., Ben Othman, D., Barrat, J.A., Albarède, F. (2003) Coupled 63Cu and 16O excesses in chondrites. Geochimica et Cosmochimica Acta 67, 143-151.
 Show in context
Show in context The Cu isotope compositions of all chondrite groups are presented in Table S-9, as well as the average Cu content for each group (Luck et al., 2003, 2005; Barrat et al., 2012).
View in Supplementary Information
Luck, J.-M., Ben Othman, D., Albarède, F. (2005) Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochimica et Cosmochimica Acta 69, 5351-5363.
 Show in context
Show in context The Cu isotope compositions of all chondrite groups are presented in Table S-9, as well as the average Cu content for each group (Luck et al., 2003, 2005; Barrat et al., 2012).
View in Supplementary Information
Maréchal, C.N., Télouk, P., Albarède, F. (1999) Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chemical Geology 156, 251-273.
 Show in context
Show in context All samples and external standards were purified for Cu isotope analysis using a single column ion chromatographic technique (modified after Maréchal et al., 1999).
View in Supplementary Information
Markl, G., Lahaye, Y., Schwinn, G. (2006) Copper isotopes as monitors of redox processes in hydrothermal mineralization. Geochimica et Cosmochimica Acta 70, 4215-4228.
 Show in context
Show in context In light of the higher temperatures of core formation relative to these experiments, as well as the degree of fractionation observed previously in secondary sulphide minerals (viz. > –3.0 ‰; Markl et al., 2006), we tentatively suggest that lower magnitudes in the range predicted by our experiments (e.g., Δ65Cusulfide-sil > –1.0 ‰) are more likely.
View in Supplementary Information
McDonough, W.F. (2003) Compositional model for the Earth's core. In: Carlson, R.W. (Ed.) The mantle and core, Treatise on Geochemistry, 1st edition, 2, 547-568.
 Show in context
Show in context The first issue when attempting a Cu isotope mass balance is to fix the abundances of Cu in the BSE, the bulk Earth, and the core. McDonough (2003) gives 30 ppm as an estimate for BSE, based on the Cu vs. MgO correlation of massif peridotites.
View in Supplementary Information
Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199.
 Show in context
Show in context In Table S-1
View in Supplementary Information
Moynier, F., Koeberl, C., Beck, P., Jourdan, F., Telouk, P. (2010) Isotopic fractionation of Cu in tektites. Geochimica et Cosmochimica Acta 74, 799-807.
 Show in context
Show in contextIn Table S-1
View in Supplementary Information
O'Neill, H.St.C. (1991) The origin of the moon and the early history of the earth - A chemical model. Part 2: The earth. Geochimica et Cosmochimica Acta 55, 1159-1172.
 Show in context
Show in context Our model for the formation of a Fe-O-S layer at the base of Earth’s mantle, i.e. the Hadean Matte, is based on O’Neill (1991) and Lee et al., (2007), whereby such a layer forms as the last liquid remaining after crystallisation of a magma ocean.
View in Supplementary Information
This is because experiments suggest that most, if not all, of primordial S was removed from the mantle during planetary differentiation, into either the metal or sulphide phase (O’Neill, 1991).
View in Supplementary Information
Palme, H., O'Neill, H.St.C. (2014) Cosmochemical estimates of mantle composition. In: Carlson, R.W. (Ed.) The mantle and core, Treatise on Geochemistry, 2nd edition, 3, 1-39.
 Show in context
Show in context However, Palme and O’Neill (2014) suggest that 30 ppm Cu in the BSE is too high, and use the average composition of xenolith peridotites (20 ppm Cu) as a more realistic estimate.
View in Supplementary Information
Poitrasson, F., Roskosz, M., Corgne, A. (2013) No iron isotope fractionation between molten alloys and silicate melt to 2000 °C and 7.7 GPa: Experimental evidence and implications for planetary differentiation and accretion. Earth and Planetary Science Letters 278, 376-385.
 Show in context
Show in context Moreover, similar run durations were found sufficient to reach Fe isotope equilibrium (Poitrasson et al., 2013), albeit at higher temperatures.
View in Supplementary Information
Puchtel, I.S., Hofmann, A.W., Mezger, K., Shchipansky, A.A., Kulikov, V.S., Kulikova, V.V. (1996) Petrology of a 2.41 Ga remarkably fresh komatiitic basalt lava lake in Lion Hills, central Vetreny Belt, Baltic Shield. Contributions to Mineralogy and Petrology 124, 273-290.
 Show in context
Show in context The first were komatiites, which are mantle-derived ultramafic lavas, generated by high degrees (>25 %) of mantle melting and typically found in Archaean terrains. In this study, we analysed komatiite samples from two localities; 2.4 Ga Vetreny Belt (Baltic Shield, Puchtel et al., 1996) and 2.7 Ga Belingwe (South Africa; Puchtel et al., 2009).
View in Supplementary Information
Puchtel, I.S., Walker, R.J., Brandon, A.D., Nisbet, E.G. (2009) Pt–Re–Os and Sm–Nd isotope and HSE and REE systematics of the 2.7 Ga Belingwe and Abitibi komatiites. Geochimica et Cosmochimica Acta 73, 6367-6389.
 Show in context
Show in context The first were komatiites, which are mantle-derived ultramafic lavas, generated by high degrees (>25 %) of mantle melting and typically found in Archaean terrains. In this study, we analysed komatiite samples from two localities; 2.4 Ga Vetreny Belt (Baltic Shield, Puchtel et al., 1996) and 2.7 Ga Belingwe (South Africa; Puchtel et al., 2009).
View in Supplementary Information
Shahar, A., Fei, Y., Liu, M.C., Wang, J. (2009) Sulfur isotopic fractionation during the differentiation of Mars. Geochimica et Cosmochimica Acta 73, A1201.
 Show in context
Show in context Any S remaining in the primordial mantle, post core formation, would be isotopically light relative to chondrites, as metal-silicate S fractionation preferentially enriches the metal in the heavier isotopes (Shahar et al., 2009).
View in Supplementary Information
Siebert, J., Corgne, A., Ryerson, F.J. (2011) Systematics of metal-silicate partitioning for many siderophile elements applied to Earth's core formation. Geochimica et Cosmochimica Acta 75, 1451-1489.
 Show in context
Show in context All experiments were conducted using a standard talc/Pyrex (below 1550 °C) or BaCO3 (above 1550 °C) pressure cell, graphite furnace, inner MgO spacers, and 8 mm diameter MgO capsules with 3 mm diameter sample chambers (Siebert et al., 2011).
View in Supplementary Information
Stracke, A., Hofmann, A.W., Hart, S.R. (2005) FOZO, HIMU and the rest of the mantle zoo. Geochemistry, Geophysics, Geosystems 6.
 Show in context
Show in context Samples were taken from a range of localities which represent a range of the posited mantle end-members (e.g., Stracke et al., 2005).
View in Supplementary Information
The final ocean island basalts (OIBs) representing various mantle isotopic “end-members” (Stracke et al., 2005) are less well-characterised than the others described above, with scant elemental data available in the literature.
View in Supplementary Information
Thibault, Y., Walter, M.J. (1995) The influence of pressure and temperature on the metal-silicate partition coefficients of nickel and cobalt in a model C1 chondrite and implications for metal segregation in a deep magma ocean. Geochimica et Cosmochimica Acta 59, 991-1002.
 Show in context
Show in context Run duration was kept short (~1 min for metal-silicate experiments, ~5 min for sulphide-silicate, Table S-10) to avoid percolation of the charge through the polycrystalline MgO capsules and limit MgO contamination (it has been shown that run durations as short as few tens of second, at superliquidus conditions, were sufficient to reach chemical equilibrium at similar P–T conditions; Thibault and Walter, 1995).
View in Supplementary Information
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal-sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166-3178.
 Show in context
Show in context All metal-silicate experiments give positive Δ65Cumet-sil (where Δ65Cux-y = δ65Cux – δ65Cuy, Table S-10) in that the metal phase is consistently enriched in the heavier Cu isotope. The data agree with the sense and general magnitude of the Δ65Cumet-sil value measured in Williams and Archer (2011), viz. +0.48 ‰, on the basis of one measurement of a silicate bearing iron meteorite.
View in Supplementary Information
Wood, B.J., Halliday, A.N. (2010) The lead isotopic age of the Earth can be explained by core formation alone. Nature 465, 767-770.
 Show in context
Show in context To explain this, some workers have posited that the Hf-W age reflects the bulk of core segregation, whereas the Pb age reflects a late stage addition of S-rich material into the core (Wood and Halliday, 2010).
View in Supplementary Information
Wood, B.J., Nielsen, S.G., Rehkämper, M., Halliday, A.N. (2008) The effects of core formation on the Pb- and Tl- isotopic composition of the silicate Earth. Earth and Planetary Science Letters 269, 326–336.
 Show in context
Show in context Values for DPbS-sil are all >1, but vary somewhat; here, we choose a value of 50, based on two more recent studies (Li and Audetat, 2012; Wood et al., 2008).
View in Supplementary Information
This gives 204Pb = 5200 ppb in the HM, and ~ 1900 ppb in the core. Calculating the apparent metal-silicate DPb from these concentrations gives a value of ~13, which is in agreement with the recent estimates (Wood et al., 2008).
View in Supplementary Information
Figures and Tables
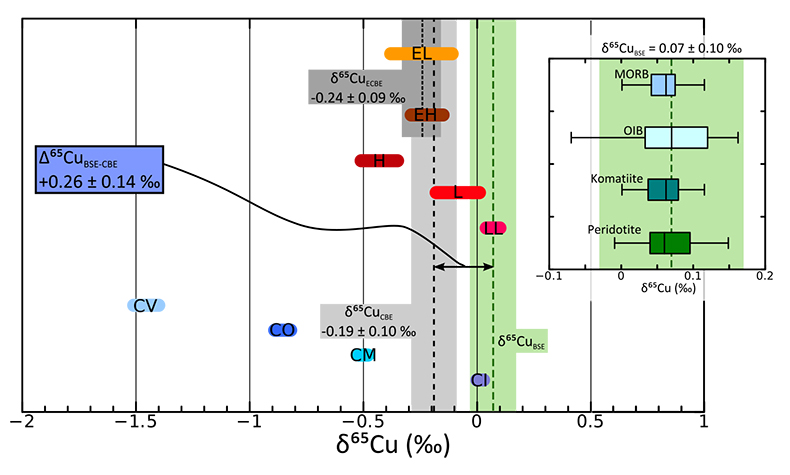
Figure 1 Copper isotope range of the primitive (chondritic) meteorite groups. Inset: Box and whisker plot showing the range of Cu isotope compositions for the terrestrial samples used in constraining the BSE Cu isotope composition. Green box and dotted line represents the composition of BSE, light grey box and long dashes represent the composition of “chondritic bulk Earth” (CBE), dark grey box and short dashes represent the composition of “enstatite chondrite bulk Earth” (ECBE). Errors on the estimates are all 2 s.d.
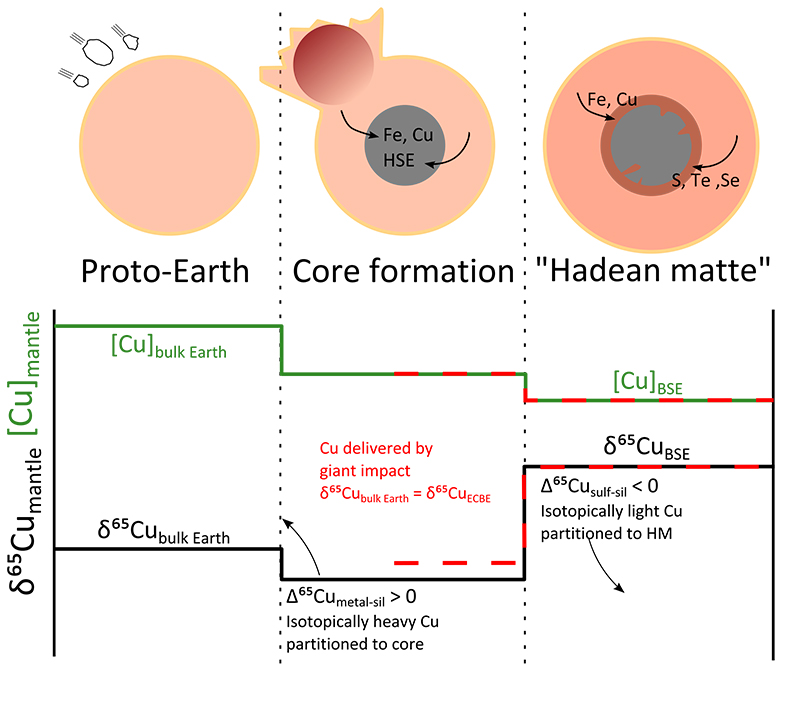
Figure 2 Schematic evolution of Cu concentration and isotopic composition of Earth’s mantle as modelled in this contribution. Earth accretes as a mixture of chondrites such that the bulk Earth Cu isotope composition is δ65CuCBE. Core formation sequesters ~60 % of Earth’s Cu in the metal phase, which is enriched in the heavy isotope, driving Earth’s mantle to a lighter composition. The formation of a Fe-O-S layer, the “Hadean Matte”, sequesters isotopically light Cu, driving Earth’s mantle to its present day composition (δ65CuBSE). Alternatively, Cu is delivered by an enstatite chondrite-like Giant Impactor, and mantle Cu only experiences sulphide-silicate equilibration.
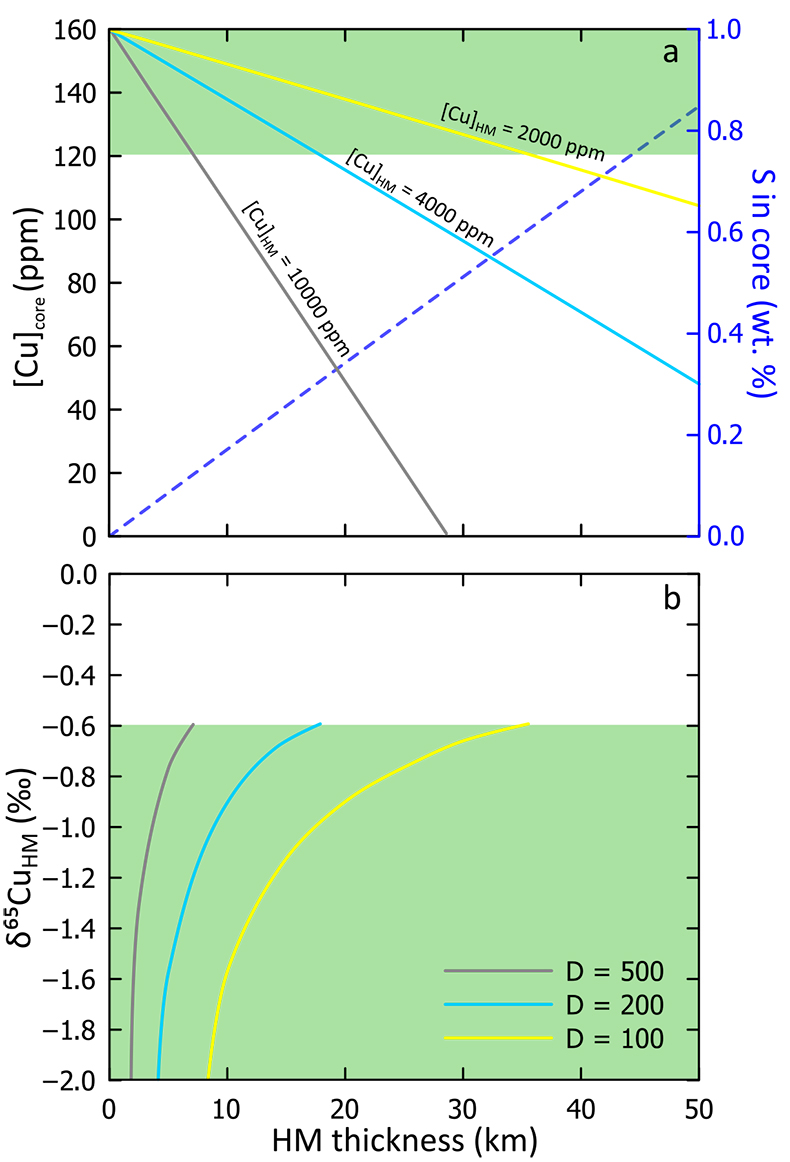
Figure 3 Results of modelled effects of removal of a “Hadean Matte” from the mantle. The effects on (a) Cu concentration of Earth’s core and (b) required Cu isotope composition of the HM to produce a modern-day δ65CuBSE – plotted as a function of the thickness of the HM. D is the sulphide-silicate Cu partition coefficient. (a) Green box defines “allowed” Cu concentrations of Earth’s core; [Cu]core < 120 ppm do not comply with the siderophile nature of Cu during core formation. Blue dotted line describes the amount of S added to the core (in wt.%) in the case of total mixing of the HM composed of a stoichiometric Fe-O-S liquid into the core. (b) Lines plotted here limited to those “allowed” in top panel.
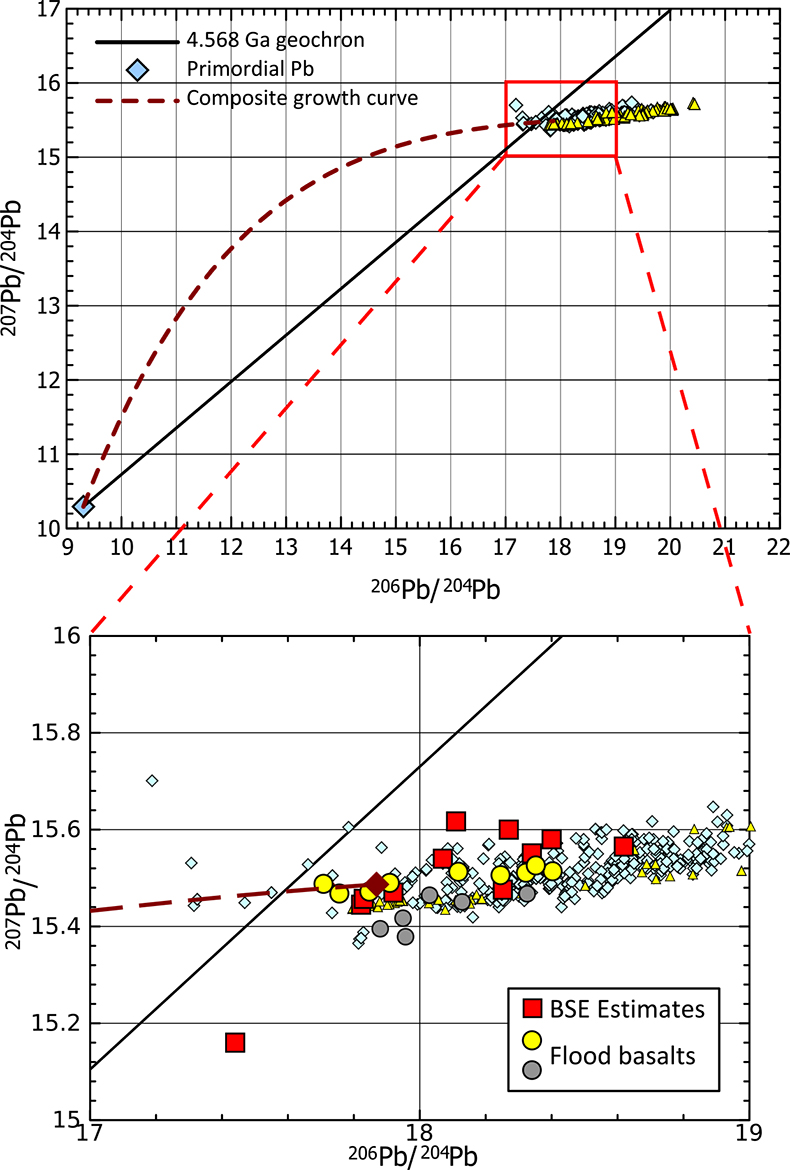
Figure 4 Modelled evolution of mantle Pb isotope composition as a result of Pb partitioning into the core at 50 Ma and the HM at 100 Ma (composite growth curve). Compilation of mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) data taken from PetDB (http://www.earthchem.org/petdb) which all plot to the right (more radiogenic) side of the terrestrial (4.568 Ga) geochron (based on the evolution of Pb from primordial source – based on Canyon Diablo Troilite). Lower figure is a zoom, showing that the composite curve agrees with some of the more unradiogenic estimates for modern day BSE Pb isotope composition (Halliday, 2004), as well as the compositions of certain flood basalt provinces (yellow: Ontong Java Plateau; grey: Baffin Island) thought to best represent ancient primitive mantle composition (Jackson and Carlson, 2011).
Back to article
Supplementary Figures and Tables
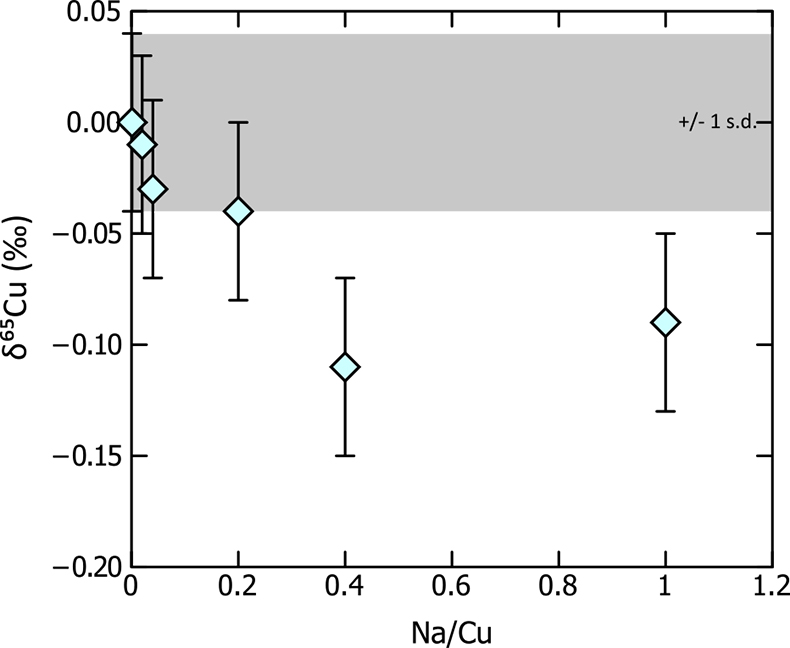
Figure S-1 Results of Na doping tests on Cu isotope measurements – sample is 250 ppb Cu SRM976 doped with various Na concentrations to results in Na/Cu between 0.001 and 1. Measurable isotopic offsets are present in samples with Na/Cu values of >0.2. Error bars are 1 s.d.
Table S-1 Cu isotope standards.
| Sample | δ65Cu (‰) | 2 s.d. | n | RefSI |
| BHVO-2 | 0.14 | 0.05 | 5 | |
| 0.06 | 0.01 | 3 | ||
| 0.11 | 0.02 | 3 | ||
| Average | 0.10 | 0.08 | 3 | |
| 0.10 | 0.04 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| 0.10 | 0.07 | Moynier et al., 2010 Moynier, F., Koeberl, C., Beck, P., Jourdan, F., Telouk, P. (2010) Isotopic fractionation of Cu in tektites. Geochimica et Cosmochimica Acta 74, 799-807. | ||
| 0.15 | 0.05 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| AGV-1 | -0.01 | 0.03 | 4 | |
| -0.01 | 0.09 | 3 | ||
| AGV-2 | 0.01 | 0.11 | 3 | |
| Average | 0.00 | 0.02 | 3 | |
| 0.11 | 0.04 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| 0.10 | 0.11 | Moynier et al., 2010 Moynier, F., Koeberl, C., Beck, P., Jourdan, F., Telouk, P. (2010) Isotopic fractionation of Cu in tektites. Geochimica et Cosmochimica Acta 74, 799-807. | ||
| 0.05 | 0.04 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| BIR-1 | 0.09 | 0.01 | 2 | |
| 0.08 | 0.07 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| -0.02 | 0.10 | Li et al., 2009 Li, W., Jackson, S.E., Pearson, N.J., Alard, O., Chappell, B.W. (2009) Cu isotopic signature of granites from the Lachlan Fold Belt, SE Australia. Chemical Geology 258, 38-49. | ||
| -0.01 | 0.04 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| BCR-2 | 0.19 | 0.07 | 2 | |
| 0.14 | 0.05 | Moeller et al., 2012 Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177-199. | ||
| 0.07 | 0.08 | Archer and Vance. 2004 Archer, C., Vance, D. (2004) Mass discrimination correction in multiple-collector plasma source mass spectrometry: an example using Cu and Zn isotopes. Journal of Analytical Atomic Spectrometry 19, 656-665. | ||
| 0.19 | 0.08 | Bigalke et al., 2010 Bigalke, M., Kobza, J., Weyer, S., Wilcke, W. (2010) Stable Cu and Zn isotope ratios as tracers of sources and transport of Cu and Zn in contaminated soil. Geochimica et Cosmochimica Acta 74, 6801-6813. | ||
| 0.22 | 0.04 | Liu et al., 2014 Liu, S.-A., Li, D., Li, S., Teng, F.-Z., Ke, S., He, Y., Lu, Y. (2014) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry 29, 122-133. | ||
| Na doping tests | ||||
| Sample | δ65Cu (‰) | 2 s.d. | Na/Cu | |
| Cu SRM976 (250 ppb Cu) | 0.00 | 0.08 | 0.001 | |
| SRM976 + 5 ppb Na | -0.01 | 0.08 | 0.02 | |
| SRM976 + 10 ppb Na | -0.03 | 0.08 | 0.04 | |
| SRM976 + 50 ppb Na | -0.04 | 0.08 | 0.2 | |
| SRM976 + 100 ppb Na | -0.11 | 0.08 | 0.4 | |
| SRM976 + 250 ppb Na | -0.09 | 0.08 | 1 | |
Table S-2 Komatiites.
| Sample | δ65Cu (‰) | 2 s.d.1 | n | Age (Ga) | Cu (ppm) |
| Vetreny | |||||
| 91105/1 | 0.10 | 0.05 | 3 | 2.4 | 63 |
| 91105 | 0.04 | 0.07 | 3 | 2.4 | 54 |
| 91104 | 0.04 | 0.09 | 3 | 2.4 | 67 |
| 91106 | 0.04 | 0.01 | 3 | 2.4 | 62 |
| 12101 | 0.05 | 0.01 | 5 | 2.4 | 95 |
| 12111 | 0.10 | 0.02 | 5 | 2.4 | 94 |
| 12117 | 0.00 | 0.07 | 5 | 2.4 | 92 |
| 12124 | 0.09 | 0.02 | 5 | 2.4 | 95 |
| Belingwe | |||||
| TN1 | 0.06 | 0.01 | 5 | 2.69 | 67 |
| TN3 | 0.03 | 0.02 | 5 | 2.69 | 75 |
| TN16 | 0.06 | 0.02 | 5 | 2.69 | 47 |
| TN17 | 0.03 | 0.02 | 3 | 2.69 | 512 |
| TN21 | 0.11 | 0.02 | 5 | 2.69 | 452 |
| ZV10 | 0.07 | 0.02 | 3 | 2.69 | 52 |
| Average | 0.06 | 0.06 | 14 | ||
1 2 standard deviations based on either the repeat measurements or, in the case where there is only 1 measurement, based on the 2 s.d. of external standard analysis that day.
2 Cu concentrations measured on MC-ICP-MS at Washington University in St. Louis.
Table S-3 Peridotites (orogenic lherzolites - literature).
| Lanzo | δ65Cu (‰) | 2 s.d. | n |
| Ben Othman et al., 2006 Ben Othman, D., Luck, J.M., Bodinier, J.L., Arndt, N.T., Albarède, F. (2006) Cu–Zn isotopic variations in the Earth’s mantle. Geochimica et Cosmochimica Acta 70, A46. | -0.01 | ||
| 0.13 | |||
| 0.01 | |||
| 0.03 | |||
| 0.09 | |||
| 0.09 | |||
| 0.15 | |||
| 0.10 | |||
| 0.15 | |||
| Average | 0.08 | 0.12 | 9 |
| Horoman | |||
| Type 1 (magmatic native Cu). Ikehata and Hirata, 2012 Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497. | 0.04 | ||
| 0.06 | |||
| 0.07 | |||
| 0.06 | |||
| 0.04 | |||
| 0.05 | |||
| Plagioclase lherzolite. Ikehata and Hirata, 2012 Ikehata, K., Hirata, T. (2012) Copper Isotope Characteristics of Copper-Rich Minerals from the Horoman Peridotite Complex, Hokkaido, Northern Japan. Economic Geology 107, 1489-1497. | 0.05 | ||
| Average | 0.05 | 0.02 | 7 |
Table S-4 Mid ocean ridge basalts.
| Sample | δ65Cu (‰) | 2 s.d. | n |
| Mid Atlantic Ridge | |||
| DIVA1 15-5 | 0.09 | 0.03 | 3 |
| DIVA1 13-3 | 0.09 | 0.03 | 3 |
| EW9309 27D-1g | 0.12 | 0.05 | 3 |
| EW9309 4D-3g | 0.06 | 0.01 | 3 |
| EW9309 10D-3g | 0.07 | 0.04 | 3 |
| RD87 DR29-101 | 0.06 | 0.05 | 3 |
| RD87 DR24 | 0.02 | 0.02 | 3 |
| RD87 DR18-102 | 0.06 | 0.03 | 3 |
| Central Indian Ridge | |||
| MD57 D7-5 | 0.04 | 0.01 | 3 |
| MD57 D2-8 | 0.04 | 0.09 | 3 |
| Pacific Antarctic Ridge | |||
| PAC2 DR37-3g | 0.08 | 0.01 | 3 |
| PAC2 DR32-1g | 0.08 | 0.02 | 3 |
| East Pacific Rise | |||
| SEARISE2 DR3 | 0.04 | 0.00 | 3 |
| redissolution | 0.05 | 0.03 | 3 |
| CYP78 12-34 | 0.07 | 0.04 | 3 |
| Southwest Indian Ridge | |||
| SWIFT DR32-1-3g | 0.10 | 0.02 | 3 |
| SWIFT DR06-3-6g | 0.06 | 0.05 | 3 |
| SWIFT DR04-2-3g | 0.00 | 0.03 | 3 |
| Average | 0.06 | 0.06 | 17 |
Table S-5 Kilauea Iki.
| Sample | δ65Cu (‰) | 2 s.d.1 | n | Cu (ppm) | MgO (wt.%) |
| Eruption samples | |||||
| Iki-22 | 0.06 | 0.08 | 1 | 120 | 19.52 |
| Iki-58 | 0.06 | 0.08 | 1 | 124 | 8.08 |
| Lava lake | |||||
| KI67-3-6.8 | 0.08 | 0.08 | 1 | 60 | 25.83 |
| KI79-3-150.4 | -0.02 | 0.08 | 1 | 102 | 13.51 |
| KI67-3-27.5 | 0.04 | 0.08 | 1 | 86 | 12.01 |
| KI75-1-121.5 | 0.14 | 0.08 | 1 | 138 | 7.77 |
| KI75-1-75.2 | -0.01 | 0.08 | 1 | 168 | 5.77 |
| KI79-1R1-170.9 | 0.00 | 0.08 | 1 | 365 | 3.48 |
| KI67-2-85.7 | 0.05 | 0.08 | 1 | 450 | 2.6 |
| KI81-2-88.6 | 0.04 | 0.08 | 1 | 345 | 2.37 |
| Average | 0.04 | 0.09 | 10 | ||
1 Errors based on the 2 s.d. of external standard analysis that day.
Back to article | Download in ExcelTable S-6 Hekla.
| Sample | δ65Cu (‰) | 2 s.d.1 | n | Cu (ppm) | SiO2 (wt.%) |
| Basalts | |||||
| HEK04-09 | 0.14 | 0.08 | 1 | 39.8 | 46.97 |
| HEK05-09 | 0.00 | 0.08 | 1 | 47.1 | 46.47 |
| repeat | 0.01 | 0.21 | 2 | ||
| HEK09-09 | 0.06 | 0.08 | 1 | 47.3 | 46.64 |
| repeat | -0.02 | 0.10 | 3 | ||
| HEK07-09 | 0.11 | 0.08 | 1 | 25.4 | 47.01 |
| HEK12-09 | 0.13 | 0.08 | 1 | 26 | 46.42 |
| Average | 0.08 | 0.13 | 5 | ||
1 2 standard deviations based on either the repeat measurements or. in the case where there is only 1 measurement, based on the 2 s.d. of external standard analysis that day.
Back to article | Download in ExcelTable S-7 Ocean island basalts.
| Sample | δ65Cu (‰) | 2 s.d.1 | n |
| Santo Antao | |||
| SA-1 | 0.12 | 0.09 | 1 |
| SA-5 | 0.00 | 0.05 | 2 |
| repeat | -0.06 | 0.03 | 3 |
| SA-6 | 0.10 | 0.07 | 2 |
| SA-8 | 0.12 | 0.09 | 1 |
| SA-11 | -0.07 | 0.05 | 2 |
| repeat | -0.07 | 0.07 | 3 |
| La Palma | |||
| W-1949 48c | 0.16 | 0.10 | 2 |
| 1585 45d | 0.13 | 0.09 | 1 |
| LP69d | 0.08 | 0.03 | 2 |
| repeat | 0.03 | 0.04 | 3 |
| Sao Miguel | |||
| SM-7 | 0.08 | 0.09 | 1 |
| repeat | 0.03 | 0.11 | 3 |
| SM-33 | 0.02 | 0.07 | 2 |
| Fogo | |||
| FG-2 | 0.11 | 0.04 | 2 |
| FG-6 | 0.12 | 0.10 | 2 |
| repeat | 0.08 | 0.06 | 3 |
| FG-12 | 0.14 | 0.09 | 1 |
| Tubuaii | |||
| TBA-C29 | 0.02 | 0.07 | 2 |
| Iceland | |||
| RHY01-09 | -0.03 | 0.09 | 1 |
| Average | 0.07 | 0.14 | 15 |
“Repeat” signifies reanalysis of the same sample aliquot on a different day.
1 2 standard deviations based on either the repeat measurements or. in the case where there is only 1 measurement, based on the 2 s.d. of external standard analysis that day.
Table S-8 Enstatite chondrites.
| Sample | Type | δ65Cu (‰) | 2 s.d. | n |
| EH | ||||
| Qingzhen | EH3 | -0.29 | 0.03 | 3 |
| Kota Kota | EH3 | -0.15 | 0.03 | 3 |
| Abee | EH4 | -0.24 | 0.06 | 3 |
| Indarch | EH4 | -0.25 | 0.05 | 3 |
| Average EH | -0.23 | 0.06 | 4 | |
| EL | ||||
| MAC88184 | EL3 | -0.11 | 0.06 | 3 |
| LON94100 | EL6 | -0.21 | 0.03 | 3 |
| Hvittis | EL6 | -0.38 | 0.03 | 3 |
| Eagle | EL6 | -0.35 | 0.05 | 3 |
| Average EL | -0.26 | 0.13 | 4 | |
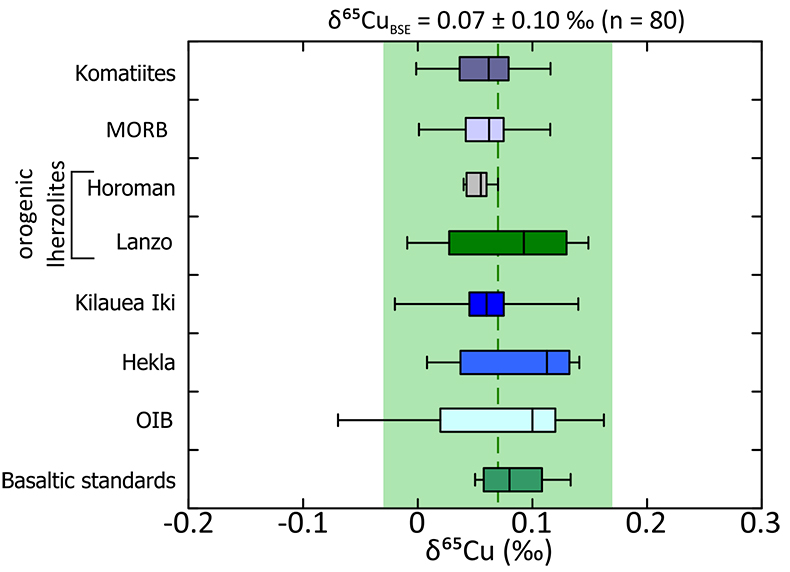
Figure S-2 Box and whisker plot showing the range of Cu isotope compositions for the terrestrial samples used in constraining the BSE Cu isotope composition. Box represents 1st and 3rd quartile, line in box represents the median and “whiskers” show the range of the data (see Tables S-2-S-7, S-9). There is no statistical difference between each group. Calculated BSE value plotted as green dashed line, green box shows 2 s.d. precision of the estimate.
Table S-9 BSE and bulk Earth averages.
| BSE average | ||||
| Group | Average δ65Cu (‰) | 2 s.d. | n | |
| Komatiites | 0.06 | 0.06 | 14 | |
| Orogenic lherzolites | 0.07 | 0.09 | 16 | |
| MORBs | 0.06 | 0.06 | 17 | |
| Kilauea Iki basalts | 0.04 | 0.09 | 10 | |
| Hekla basalts | 0.08 | 0.13 | 5 | |
| OIBs | 0.07 | 0.14 | 15 | |
| Basaltic standards | 0.06 | 0.11 | 3 | |
| BSE average | 0.07 | 0.10 | 80 | |
| Bulk Earth average | ||||
| Meteorite group | Average δ65Cu (‰) | 2 s.d. | n | Average Cu (ppm) |
| CI | 0.02 | 0.16 | 3 | 122 |
| CM | -0.5 | 0.06 | 2 | 115 |
| CO | -0.86 | 0.07 | 2 | 122 |
| CV | -1.45 | 0.08 | 2 | 100 |
| H | -0.44 | 0.11 | 3 | 81 |
| L | -0.08 | 0.13 | 5 | 88 |
| LL | 0.07 | 0.06 | 2 | 80 |
| EH | -0.23 | 0.06 | 4 | 210 |
| EL | -0.26 | 0.13 | 4 | 120 |
| CBE average | -0.19 | 0.10 | ||
| ECBE average | -0.24 | 0.09 | ||
| ACBE average | -0.28 | 0.07 | ||
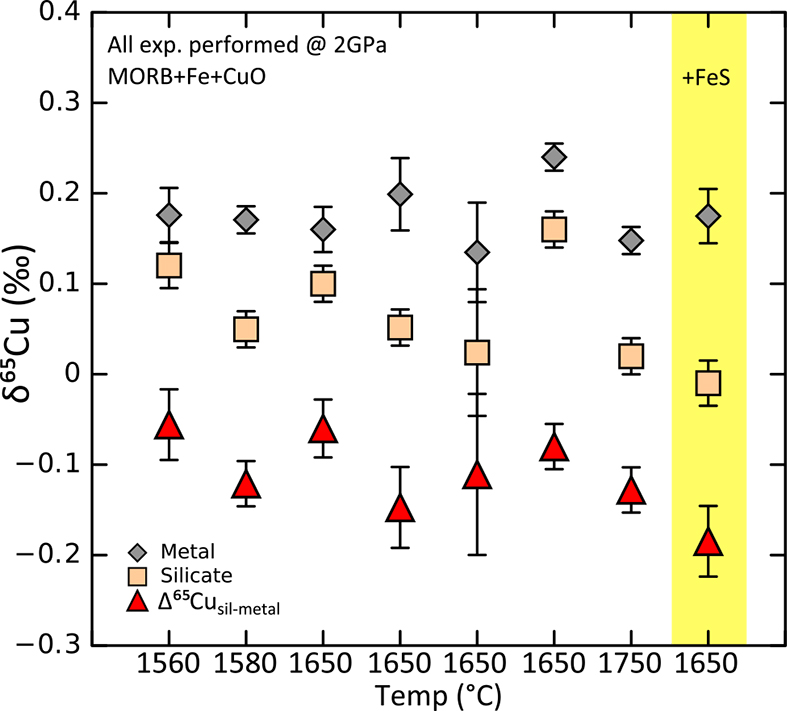
Figure S-3 Summary of metal-silicate Cu isotope equilibration experiments – note that this figure is not a graph (the x axis is discrete, not continuous). All Δ65Cusil-met values are negative but there does not appear to be a strong temperature control on these values. The one experiment with S added gives the largest Δ65Cusil-met value, this makes sense if there is minor amounts of S-hosted Cu that was dissolved with the non-magnetic (“silicate”) phase – as Cu sulphides are shown to be isotopically light w.r.t. silicates and metal. Error bars are 1s.d.

Figure S-4 Summary of sulphide-silicate Cu isotope equilibration experiments – we are so far unable to successfully measure the silicate phase, due to the presence of disseminated sulphides which overwhelm the Cu isotope signature of the silicates. Here we show the Cu isotope composition of the bulk Cu metal as well as sulphide phases from two experiments (wherein only the temperature was varied). In both cases the sulphides are enriched in the light Cu isotope relative to bulk, and there is a small decrease in fractionation magnitude (although within error) as temperature increases.
Table S-10 Experimental results.
| Metal-silicate experiments | All @ 2 GPa | |||||||||
| Initial components: MORB + Fe + CuO | ||||||||||
| Experiment | Phase | δ65Cu (‰) | 2 s.d. | Cu conc. | D [Cu]m/[Cu]sil | Δm-sil (‰) | 2 s.d. | T °C | t @max T | |
| 61 | Silicate | 0.10 | 0.04 | 238 ppm | 160 | 0.06 | 0.06 | 1650 | 1 min | |
| Metal | 0.16 | 0.05 | 3.80 % | |||||||
| 67 | Silicate | 0.05 | 0.04 | 484 ppm | 38 | 0.12 | 0.05 | 1580 | 1 m 20 s | |
| Metal | 0.17 | 0.03 | 1.90 % | |||||||
| 68 | Silicate | 0.12 | 0.05 | 441 ppm | 49 | 0.06 | 0.08 | 1560 | 30 s | |
| Metal | 0.18 | 0.06 | 2.10 % | |||||||
| 69 | Silicate | 0.02 | 0.04 | 432 ppm | 58 | 0.13 | 0.05 | 1750 | 30 s | |
| Metal | 0.15 | 0.03 | 2.50 % | |||||||
| Reversal experiments | ||||||||||
| Initial components: MORB + Fe + Cu + CuO | ||||||||||
| 71 | Silicate | 0.05 | 0.04 | 668 ppm | 64 | 0.15 | 0.09 | 1650 | 35 s | |
| Metal | 0.2 | 0.08 | 4.30 % | |||||||
| Initial components: MORB + Fe + Cu | ||||||||||
| 72 | Silicate | 0.02 | 0.04 | 478 ppm | 69 | 0.11 | 0.07 | 1650 | 30 s | |
| Metal | 0.13 | 0.06 | 3.30 % | |||||||
| Initial components: MORB + Fe + CuO | ||||||||||
| 73 | Silicate | 0.16 | 0.04 | 507 ppm | 54 | 0.07 | 0.05 | 1650 | 30 s | |
| Metal | 0.24 | 0.03 | 2.80 % | |||||||
| Initial components: MORB + Fe + CuO + FeS | ||||||||||
| 64 | Silicate | -0.01 | 0.05 | 46 | 0.18 | 0.08 | 1650 | 30 s | ||
| Metal | 0.17 | 0.06 | ||||||||
| Initial components: MORB + FeS + Cu | D | Δsul-bulk (‰) | ||||||||
| [Cu]sul/[Cu]sil | ||||||||||
| ELMO113 | Silicate | n/a | ~50 ppm | ~500 | -0.12 | 0.04 | 1400 | 6 min | ||
| Sulphide | 0.01 | 0.02 | 2.60 % | |||||||
| ELMO114 | Silicate | n/a | ~60 ppm | ~900 | -0.09 | 0.05 | 1550 | 3 min | ||
| Sulphide | 0.04 | 0.04 | 5.40 % | |||||||
| Bulk Cu | Metal | 0.13 | 0.03 | 99.99 % | ||||||
| Bulk CuO | Oxide (CuO) | 0.15 | 0.02 | ~80 % | ||||||
Experiments 71 and 72 are reversal experiments (i.e. all Cu in 72 was reduced, all Cu in 71 was CuO). Experiment 73: repeat of Experiment 61 – high D value due to BaCO3?
Back to article | Download in Excel
Figure S-5 Relationship between calculated Cu in core (as a result of increasing thickness of HM) and apparent DCu metal silicate values – this value should be at least greater than 3.5, which equates to >120 ppm Cu in the core. D = DCusulfide-silicate therefore, for BSE [Cu] = 20 ppm, D = 500→[Cu]HM = 10000; D = 200→[Cu]HM = 4000; D = 100→[Cu]HM = 2000.

Figure S-6 Relationship between thickness of Hadean Matte and Cu isotope composition, for various [Cu]HM concentrations. As the thickness reduces, the isotope composition of the HM becomes more extreme, toward very light values.