Water in alkali feldspar: The effect of rhyolite generation on the lunar hydrogen budget
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: Moon, water, alkali feldspar, felsic magma generation
- Share this article





Article views:10,278Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract

Saal, A.E., Hauri, E.H., Cascio, M.L., Van Orman, J.A., Rutherford, M.C., Cooper, R.F. (2008) Volatile content of lunar volcanic glasses and the presence of water in the Moon’s interior. Nature 454, 192–195.
), melt inclusions (Hauri et al., 2011Hauri, E.H., Weinreich, T., Saal, A.E., Rutherford, M.C., Van Orman, J.A. (2011) High pre-eruptive water contents preserved in lunar melt inclusions. Science 333, 213–215.
), apatite (Boyce et al., 2010Boyce, J.W., Liu, Y., Rossman, G.R., Guan, Y., Eiler, J.M., Stolper, E.M., Taylor, L.A. (2010) Lunar apatite with terrestrial volatile abundances. Nature 466, 466–469.
; McCubbin et al., 2010McCubbin, F.M., Steele, A., Hauri, E.H., Nekvasil, H., Yamashita, S., Hemley, R.J. (2010) Nominally hydrous magmatism on the Moon. Proceedings of the National Academy of Sciences 107, 11223–11228.
), and plagioclase (Hui et al., 2013Hui, H., Peslier, A.H., Zhang, Y., Neal, C.R. (2013) Water in lunar anorthosites and evidence for a wet early Moon. Nature Geoscience 6, 177–180.
) suggests water played a role in the chemical differentiation of the Moon. Water contents measured in plagioclase feldspar, a dominant mineral in the ancient crustal lunar highlands have been used to predict that 320 ppm water initially existed in the lunar magma ocean (Hui et al., 2013Hui, H., Peslier, A.H., Zhang, Y., Neal, C.R. (2013) Water in lunar anorthosites and evidence for a wet early Moon. Nature Geoscience 6, 177–180.
) whereas measurements in apatite, found as a minor mineral in lunar rocks, representing younger potassium-enriched melt predict a bulk Moon with <100 ppm water. Here we show that water in alkali feldspar, a common mineral in potassium-enriched rocks, can have ~20 ppm water, which implies magmatic water contents of ~1 wt. % in chemically evolved rhyolitic magmas. The source for these wet, potassium-rich magmas probably contained ~1000 ppm H2O. Thus, lunar granites with ages from 4.3–3.9 Ga (Meyer et al., 1996Meyer, C., Williams, I.S., Compston, W. (1996) Uranium-lead ages for lunar zircons: Evidence for a prolonged period of granophyre formation from 4.32 to 3.88 Ga. Meteoritics & Planetary Science 31, 370–387.
) likely crystallised from relatively wet melts that degassed upon crystallisation. Geochemical surveys by the Lunar Prospector (Jolliff et al., 2011Jolliff, B.L., Wiseman, S.A., Lawrence, S.L., Tran, T.N., Robinson, M.S., Sato, H., Hawke, B.R., Scholten, F., Oberst, J., Hiesinger, H., van der Bogert, C.H., Greenhagen, B.T., Glotch, T.D., Paige, D.A. (2011) Non-mare silicic volcanism on the lunar farside at Compton-Belkovich. Nature Geoscience 4, 566–571.
) and Diviner Lunar Radiometer Experiment (Glotch et al., 2010Glotch, T.D., Lucey, P.G., Bandfield, J.L., Greenhagen, B.T., Thomas, I.R., Elphic, R.C., Bowles, N., Wyatt, M.B., Allen, C.C., Donaldson Hanna, K., Paige, D.A. (2010) Highly Silicic Compositions on the Moon. Science 329, 1510–1513.
; Jolliff et al., 2011Jolliff, B.L., Wiseman, S.A., Lawrence, S.L., Tran, T.N., Robinson, M.S., Sato, H., Hawke, B.R., Scholten, F., Oberst, J., Hiesinger, H., van der Bogert, C.H., Greenhagen, B.T., Glotch, T.D., Paige, D.A. (2011) Non-mare silicic volcanism on the lunar farside at Compton-Belkovich. Nature Geoscience 4, 566–571.
) indicating the global significance of evolved igneous rocks suggest that the formation of these granites removed water from some mantle source regions, helping to explain the existence of mare basalts with <10 ppm water, but must have left regions of the interior relatively wet as seen by the water content in volcanic glass and melt inclusions. Although these early-formed evolved melts were water-rich, their petrogenesis supports the conclusion that the Moon’s mantle had <100 ppm water for most of its history.Figures and Tables
 Table 1 NanoSIMS data for alkali feldspar and SiO2 phases from 2 clasts found in Apollo sample 15405,78. For alkali feldspar, data from 4 regions of interest (ROI) are presented, taken from 2 different clasts. Raw – Suprasil values are the calculated concentration data for the alkali feldspar minus the baseline calculated from the Suprasil anhydrous glass. Raw – average SiO2 are the calculated concentration data for the alkali feldspar minus the average from the SiO2 phase from the clasts. | 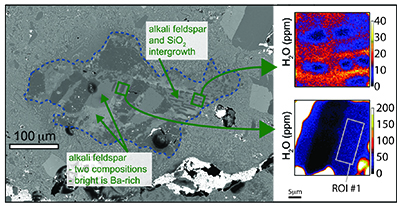 Figure 1 BSE image of granitoid clast (outlined in dashed line) from sample 15405,78, on the left. Clast consists of alkali feldspar and a silica phase. The variation in brightness of the feldspar relates to Ba (i.e. bright has more Ba). On the right are chemical maps obtained by NanoSIMS at DTM. Water correlates with mineralogy. The alkali feldspar consistently has ~20 ppm H2O. The silica phase has similar water contents as the blank obtained on anhydrous glass (2–3 ppm). | 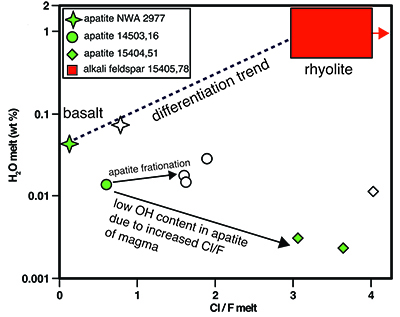 Figure 2 Plot of the estimated Cl/F ratio of magma versus estimated water content for lunar magmas (y-axis in log scale). Apatite data are from McCubbin et al. (2010). Water estimates from apatite assume 95 % crystallisation prior to apatite crystallisation. Cl/F melt estimates from apatite assume DF/DCl is 10 (Webster et al., 2009). Within individual samples, boundary layer crystallisation will create apatites with higher Cl and OH- contents than those initially growing from their host magma, thus the lowest water estimates from each sample (green fill) should be more representative of the initial magma composition (Boyce et al., 2014). Schematic differentiation trend is shown between basalt and rhyolite. For reference, OH- data from apatites from lunar granite 14321,1047 (Robinson et al., 2014) predict H2O melt compositions of 0.001 wt. %, and likely have Cl/F ratios higher than 3. |
| Table 1 | Figure 1 | Figure 2 |
top
Introduction
Understanding the volatile budget of the Moon is important because it can have profound effects on melt viscosity (Dingwell et al., 1985
Dingwell, D.B., Scarfe, C.M., Cronin, D.J. (1985) The effect of fluorine on viscosities in the system Na2O-Al2O3-SiO2: implications for phonolites, trachytes and rhyolites. American Mineralogist 70, 80–87.
; Baker and Vaillancourt, 1995Baker, D.R., Vaillancourt, J. (1995) The low viscosities of F + H2O-bearing granitic melts and implications for melt extraction and transport. Earth and Planetary Science Letters 132, 199–211.
), chemical diffusion (Harrison and Watson, 1983Harrison, T.M., Watson, E.B. (1983) Kinetics of zircon dissolution and zirconium diffusion in granitic melts of variable water content. Contributions to Mineralogy and Petrology 84, 66–72.
), and solidus temperatures (Johannes and Holtz, 1996Johannes, W., Holtz, F. (1996) Petrogenesis and experimental petrology of granitic rocks. Springer-Verlag, Berlin.
). These properties control physiochemical processes of magmas, including degassing, which have significant effects on planetary differentiation. The lack of hydrous silicate phases and hydrothermal alteration in lunar samples returned by the Apollo missions are observations commonly used to suggest that the Moon is depleted in hydrogen (plus other volatile elements) when compared to Earth, and thus water is generally not considered important in the chemical and physical differentiation of the Moon. The relative depletion in volatile elements in the Moon is thought to have occurred during a giant impact between a proto-Earth and a Mars-sized object (Hartman and Davis, 1975Hartman, WK., Davis, D.R. (1975) Satellite-sized planetesimals and lunar origin. Icarus 24, 504–515.
; Canup and Asphaug, 2001Canup, R.M., Asphaug, E. (2001) Origin of the Moon in a giant impact near the end of the Earth’s formation. Nature 412, 708–712.
) resulting in volatile depletion and a lunar magma ocean, although Earth may have received additional input of volatiles by impacts after Moon formation (Owen and Bar-Nun, 1995Owen, T., Bar-Nun, A. (1995) Comets, impact, and atmospheres. Icarus 116, 155–116.
). For these reasons, the recent conflicting evidence for significant lunar water requires further investigation.On Earth, water and halogen contents of igneous rocks generally correlate with other incompatible elements like K, Rb, Th, and U (e.g., Stolper and Newman, 1994
Stolper, E., Newman, S. (1994) The role of water in the petrogenesis of Mariana trough magmas. Earth and Planetary Science Letters 121, 293–325.
). Recent spectroscopic data from the Moon (Klima et al., 2013Klima, R., Cahill, J., Hagerty, J., Lawrence, D. (2013) Remote detection of magmatic water in Bullialdus Crater on the Moon. Nature Geoscience 6, 737–741.
) support this trend, with a positive correlation between water and Th. Modelling of lunar magma ocean crystallisation (Elkins-Tanton and Grove, 2011Elkins-Tanton, L.T., Grove, T.L. (2011) Water (hydrogen) in the lunar mantle: Results from petrology and magma ocean modeling. Earth and Planetary Science Letters 307, 173–179.
) predicts a similar chemical differentiation to that seen in magma systems on Earth, with the highest levels of hydrogen in the evolved melt residuum of the magma ocean (i.e. urKREEP). However, sample-based estimates of water content of KREEP-rich magmas from measurements of OH-, F, and Cl in lunar apatites suggest a low water concentration in the KREEP component with 2 to 140 ppm magmatic water (McCubbin et al., 2010McCubbin, F.M., Steele, A., Hauri, E.H., Nekvasil, H., Yamashita, S., Hemley, R.J. (2010) Nominally hydrous magmatism on the Moon. Proceedings of the National Academy of Sciences 107, 11223–11228.
). Using these data Elkins-Tanton and Grove (2011)Elkins-Tanton, L.T., Grove, T.L. (2011) Water (hydrogen) in the lunar mantle: Results from petrology and magma ocean modeling. Earth and Planetary Science Letters 307, 173–179.
predict that the bulk water content of the magma ocean would have been <10 ppm. In contrast, Hui et al. (2013)Hui, H., Peslier, A.H., Zhang, Y., Neal, C.R. (2013) Water in lunar anorthosites and evidence for a wet early Moon. Nature Geoscience 6, 177–180.
estimate water contents of 320 ppm for the bulk Moon and 1.4 wt. % for urKREEP from water measured in lunar anorthosite.In order to address these discrepant estimates for the water content in KREEP and ultimately the bulk water content of the Moon, we measured water in nominally anhydrous minerals in the KREEP-rich sample 15405,78. We present data for water from alkali feldspar and a silica polymorph from granitoid clasts. The measurements of water in lunar alkali feldspar are the first of their kind. Alkali feldspar and apatite are the two most important mineralogical carriers of the KREEP component; as alkali feldspar is the main K phase and apatite (± whitlockite) are the main REE and P phases on the Moon. These data in conjunction with OH-, F, and Cl data from apatites in rocks with varying amounts of alkali feldspar are used to determine the water distribution in the KREEP reservoir and whether water estimates from KREEP-rich rocks suggest a dry or wet bulk Moon.
top
Results and Discussion
NanoSIMS ion microprobe measurements of water in alkali feldspar and a silica polymorph from sample 15405,78 were employed following the approach of Hauri et al. (2006
Hauri, E.H., Shaw, A.M., Wang, J., Dixon, J.E., King, P.L., Mandeville, C. (2006) Matrix effects in hydrogen isotope analysis of silicate glasses by SIMS. Chemical Geology 235, 353–365.
; see Supplementary Information). In Apollo sample 15405,78 granitoid clast xenoliths are found in the groundmass (Ryder, 1976Ryder, G. (1976) Lunar sample 15405: Remnant of a KREEP basalt-granite differentiated pluton. Earth and Planetary Science Letters 29, 255–268.
) and generally consist of intergrowths of alkali feldspar and a silica polymorph (Fig. 1). In the studied sample plagioclase is largely absent, only occurring as small patches in a few clasts. The water concentration in the alkali feldspar is uniform with values of ~20 ppm H2O (Table 1). Reconnaissance scanning electron microscope and electron microprobe mineral compositional analysis show that the alkali feldspar is two-phase, with some high Ba zones (Fig. 1), but there is no observed variation in water content between the high- and low-Ba feldspar (although the vast majority of the analyses were made on the low-Ba feldspar). The silica polymorph OH- measurements are equivalent to measurements from the anhydrous Suprasil glass standard.Table 1 NanoSIMS data for alkali feldspar and SiO2 phases from 2 clasts found in Apollo sample 15405,78. For alkali feldspar, data from 4 regions of interest (ROI) are presented, taken from 2 different clasts. Raw – Suprasil values are the calculated concentration data for the alkali feldspar minus the baseline calculated from the Suprasil anhydrous glass. Raw – average SiO2 are the calculated concentration data for the alkali feldspar minus the average from the SiO2 phase from the clasts.
| Alkali feldspar (ppm) | ||||||
| ROI 1 | ROI 2 | ROI 3 | ROI 4 | Average | 2 S.E. | |
| Raw | 22.8 | 22.3 | 20 | 21.3 | 21.6 | 1.25 |
| Raw – Suprasil | 20.7 | 20.2 | 17.9 | 19.2 | 19.5 | 1.25 |
| Raw – average SiO2 | 20.1 | 19.6 | 17.3 | 18.6 | 18.9 | 1.25 |
| SiO2 phase (ppm) | ||||||
| ROI 1 | ROI 2 | ROI 3 | Average | 2 S.E. | ||
| Raw | 2.89 | 2.39 | 2.78 | 2.68 | 0.304 | |

Figure 1 BSE image of granitoid clast (outlined in dashed line) from sample 15405,78, on the left. Clast consists of alkali feldspar and a silica phase. The variation in brightness of the feldspar relates to Ba (i.e. bright has more Ba). On the right are chemical maps obtained by NanoSIMS at DTM. Water correlates with mineralogy. The alkali feldspar consistently has ~20 ppm H2O. The silica phase has similar water contents as the blank obtained on anhydrous glass (2–3 ppm).
Hydrogen contained in nominally anhydrous minerals is normally in the form H2O or OH- (Johnson and Rossman, 2004
Johnson, E.A., Rossman, G.R. (2004) A survey of hydrous species and concentrations in igneous feldspars. American Mineralogist 89, 560–600.
) and water contents in alkali feldspars from Earth vary from 10s to 1000s of ppm (Johnson and Rossman, 2004Johnson, E.A., Rossman, G.R. (2004) A survey of hydrous species and concentrations in igneous feldspars. American Mineralogist 89, 560–600.
). In alkali feldspar in plutonic rocks on Earth the hydrogen is mostly found as H2O and in volcanic rocks it is generally OH- (Johnson and Rossman, 2004Johnson, E.A., Rossman, G.R. (2004) A survey of hydrous species and concentrations in igneous feldspars. American Mineralogist 89, 560–600.
). Johnson and Rossman (2004)Johnson, E.A., Rossman, G.R. (2004) A survey of hydrous species and concentrations in igneous feldspars. American Mineralogist 89, 560–600.
hypothesise that the speciation of hydrogen in the feldspar is a function of the species in the liquid in equilibrium with the crystal. Thus, the H2O in plutonic rocks on Earth might be due to late stage hydrous-fluid/rock interactions in the crust. Due to the lack of evidence of post crystallisation hydous-fluid/mineral interactions in sample 15405,78, we suggest water was incorporated into the feldspar during crystallisation from the silicate melt. However, future work to understand whether hydrogen in the alkali feldspar studied here is in the form OH- or H2O would help clarify the origin of the hydrogen. In addition, D/H isotopic analyses on the feldspar would help assess if there is an exodemic component.Estimates for the water distribution coefficient (D) between alkali feldspar and a representative range of felsic host melts can be determined from the Bishop Tuff of California, a well-studied, compositionally zoned rhyolite. Johnson and Rossman (2004)
Johnson, E.A., Rossman, G.R. (2004) A survey of hydrous species and concentrations in igneous feldspars. American Mineralogist 89, 560–600.
measured 90 ppm OH- in sanidines from the tuff; however, recalculation using a new molar absorption coefficient for sanidine (Mosenfelder et al., 2015Mosenfelder, J.L., Rossman, G.R., Johnson, E.A. (2015) Hydrous species in feldspars: A reassessment based on FTIR and SIMS. American Mineralogist 100, 1209–1221.
) produces a value of 65 ppm. Water contents from melt inclusions from the Bishop Tuff show a range from 2.3 to 6.0 wt. % (Schmitt and Simon, 2004Schmitt, A.K., Simon, J.I. (2004) Boron isotopic variations in hydrous rhyolitic melts: a case study from Long Valley, California. Contributions to Mineralogy and Petrology 146, 590–608.
). Thus the range of D for water in alkali feldspar from the Bishop Tuff is 0.001 – 0.004 using the full range in values for both the sanidine and the melt inclusions. These values are similar to a D obtained for plagioclase feldspar in felsic melt of 0.004 (Johnson, 2006Johnson, E.A. (2006) Water in nominally anhydrous crustal minerals: Speciation, concentration, and geologic significance. Reviews in Mineralogy and Geochemistry 62, 117–154.
). It should be noted that because the Moon formed under rather reducing conditions the effect of oxygen fugacity on H solubility in feldspar may be important. Experiments by Yang et al. (2012)Yang, X. (2012) An experimental study of H solubility in feldspars: Effect of composition, oxygen fugacity, temperature and pressure and implications for crustal processes. Geochimica et Cosmochimica Acta 97, 46–57.
show that the H solubility is higher (2–3x) at very reducing conditions, but because the oxygen fugacity during the crystallisation of the granitoids is unknown we cautiously use the range calculated from the Bishop Tuff. Using the range in D of 0.001 – 0.004 we obtain an estimate of 0.5 to 2 wt. % water in the felsic lunar melt (Fig. 2) at the time of alkali feldspar crystallisation for the granitoid clasts from sample 15405,78. Given that alkali feldspar and the silica polymorph make up the vast majority of these granitoid clasts, the water estimate at the time of alkali feldspar crystallisation closely approximates the magmatic value. Because the melt was initially not water saturated (Johannes and Holtz, 1996Johannes, W., Holtz, F. (1996) Petrogenesis and experimental petrology of granitic rocks. Springer-Verlag, Berlin.
) little water should have been lost prior to crystallisation. Despite the fact that 0.5 to 2 wt. % water is the wettest magma inferred for the Moon, it still would likely not stabilise hydrous phases (Merzbacher and Eggler, 1984Merzbacher, C., Eggler, D.H. (1984) A magmatic geohygrometer – Application to mount St. Helens and other dacitic magmas. Geology 12, 587–590.
). Instead, the water and halogens likely left the system as a highly water-rich fluid.
Figure 2 Plot of the estimated Cl/F ratio of magma versus estimated water content for lunar magmas (y-axis in log scale). Apatite data are from McCubbin et al. (2010)
McCubbin, F.M., Steele, A., Hauri, E.H., Nekvasil, H., Yamashita, S., Hemley, R.J. (2010) Nominally hydrous magmatism on the Moon. Proceedings of the National Academy of Sciences 107, 11223–11228.
. Water estimates from apatite assume 95 % crystallisation prior to apatite crystallisation. Cl/F melt estimates from apatite assume DF/DCl is 10 (Webster et al., 2009Webster, J.D., Tappen, C.M., Mandeville, C.W. (2009) Partitioning behavior of chlorine and fluorine in the system apatite-melt-fluid. II: Felsic silicate systems at 200 MPa. Geochimica et Cosmochimica Acta 73, 559–581.
). Within individual samples, boundary layer crystallisation will create apatites with higher Cl and OH- contents than those initially growing from their host magma, thus the lowest water estimates from each sample (green fill) should be more representative of the initial magma composition (Boyce et al., 2014Boyce, J.W., Tomlinson, S.M., McCubbin, F.M., Greenwood, J.P., Treiman, A.H. (2014) The lunar apatite paradox. Science 344, 400–402.
). Schematic differentiation trend is shown between basalt and rhyolite. For reference, OH- data from apatites from lunar granite 14321,1047 (Robinson et al., 2014Robinson, K.L., Barnes, J.J., Tartèse, R., Nagashima, K., Hallis, L.J., Franchi, I.A., Amand, M., Taylor, G.J. (2014) Primitive lunar water in evolved rocks? In 45th Lunar and Planetary Science Conference. 1607.
) predict H2O melt compositions of 0.001 wt. %, and likely have Cl/F ratios higher than 3.Volatile data from apatites in lunar basalts and alkali-suite clasts from McCubbin et al. (2010)
McCubbin, F.M., Steele, A., Hauri, E.H., Nekvasil, H., Yamashita, S., Hemley, R.J. (2010) Nominally hydrous magmatism on the Moon. Proceedings of the National Academy of Sciences 107, 11223–11228.
show a conflicting trend with lower OH- and higher Cl/F in rocks that have more alkali feldspar (i.e. more K-rich). Magmatic water estimates based on apatite measurements from an alkali-rich clast from Apollo sample 15404,51 range from as low as 0.001 to 0.014 wt. % H2O (Fig. 2). As calculated by McCubbin et al. (2010)McCubbin, F.M., Steele, A., Hauri, E.H., Nekvasil, H., Yamashita, S., Hemley, R.J. (2010) Nominally hydrous magmatism on the Moon. Proceedings of the National Academy of Sciences 107, 11223–11228.
these estimates assume 95 % crystallisation of the host magma prior to formation of the apatite. Although alkali feldspar is in clasts found in sample 15404,51, it is not a major phase. To compare our magma water estimates for lunar granitoids with apatite estimates from a similar rock type we use the published NanoSIMS OH- data of apatites from evolved lunar rocks (Barnes et al., 2014Barnes, J.J., Tartèse, R., Anand, M., McCubbin, F.M., Franchi, I.A., Starkey, N.A., Russell, S.S. (2014) The origin of water in the primitive Moon as revealed by the lunar highlands samples. Earth and Planetary Science Letters 390, 244–252.
; Robinson et al., 2014Robinson, K.L., Barnes, J.J., Tartèse, R., Nagashima, K., Hallis, L.J., Franchi, I.A., Amand, M., Taylor, G.J. (2014) Primitive lunar water in evolved rocks? In 45th Lunar and Planetary Science Conference. 1607.
) including the largest specimen of lunar granite, 14321,1047. Following the same approach as above yields apatite water estimates for the rhyolitic magmas at <0.04 wt. % H2O. Excluding two outlier OH- measurements from Barnes et al. (2014)Barnes, J.J., Tartèse, R., Anand, M., McCubbin, F.M., Franchi, I.A., Starkey, N.A., Russell, S.S. (2014) The origin of water in the primitive Moon as revealed by the lunar highlands samples. Earth and Planetary Science Letters 390, 244–252.
lowers the magmatic water estimate to 0.004 wt. %. Thus, magmatic water estimates from alkali feldspar and apatite in petrologically similar lunar granitoid clasts differ by approximately 2 to 3 orders of magnitude.Notably, the inferred magmatic water contents based on OH- in lunar apatite have recently been shown to be unreliable due to the co-dependent compatibilities of F, Cl, and OH- (Boyce et al., 2014
Boyce, J.W., Tomlinson, S.M., McCubbin, F.M., Greenwood, J.P., Treiman, A.H. (2014) The lunar apatite paradox. Science 344, 400–402.
). Specifically, fractionation of apatite within a single magma can remove F, leading to later forming apatites with high OH- concentrations that produce inaccurate, high magmatic water estimates (Fig. 2). This likely explains the two highest OH- values from Robinson et al. (2014)Robinson, K.L., Barnes, J.J., Tartèse, R., Nagashima, K., Hallis, L.J., Franchi, I.A., Amand, M., Taylor, G.J. (2014) Primitive lunar water in evolved rocks? In 45th Lunar and Planetary Science Conference. 1607.
and implies that apatite with the lowest OH- content in an individual sample (Fig. 2) should give the best estimate of magmatic water. More important is the fact that evolved magmas with high Cl/F can crystallise apatite that generally excludes OH-, regardless of the water content of the magma (McCubbin et al., 2011McCubbin, F.M., Jolliff, B.L., Nekvasil, H., Carpenter, P.K., Zeigler, R.A., Steele, A., Elardo, S.M., Lindsley, D.H. (2011) Fluorine and chlorine abundances in lunar apatite: Implications for heterogeneous distributions of magmatic volatiles in the lunar interior. Geochimica et Cosmochimica Acta 75, 5073–5093.
; Boyce et al., 2014Boyce, J.W., Tomlinson, S.M., McCubbin, F.M., Greenwood, J.P., Treiman, A.H. (2014) The lunar apatite paradox. Science 344, 400–402.
). Estimated Cl/F of magmas from mare basalts, alkali suite, and KREEP-rich rocks show a progression toward higher Cl/F as magma compositions become more K-rich (Fig. 2). Associated with the increase in magma Cl/F is a decrease in OH- in apatite, and thus an apparent decrease in magmatic water (Fig. 2).top
Implications
Zircon ages from lunar granitic rocks (e.g., Meyer et al., 1996
Meyer, C., Williams, I.S., Compston, W. (1996) Uranium-lead ages for lunar zircons: Evidence for a prolonged period of granophyre formation from 4.32 to 3.88 Ga. Meteoritics & Planetary Science 31, 370–387.
) span from 4.3 to 3.9 Ga, with an age of 4.3 Ga for 15405. Thus, granitoids analysed here either reflect the end of extensive magma ocean solidification, or the beginning of secondary crust formation through partial melting, i.e. very small-scale partial melts during initiation of density driven stratification (i.e. local mantle overturn). Regardless of whether the granitoids represent the final liquids or the first partial melts of lunar magma ocean materials, the new data imply that high water content rhyolitic magmas existed locally in the lunar crust around 4.3 Ga (Meyer et al., 1996Meyer, C., Williams, I.S., Compston, W. (1996) Uranium-lead ages for lunar zircons: Evidence for a prolonged period of granophyre formation from 4.32 to 3.88 Ga. Meteoritics & Planetary Science 31, 370–387.
). The reduced viscosity of these rhyolitic melts appear to have allowed them to easily ascend through the crust and possibly degas. The removal of water and chlorine from the lunar interior by the generation of these magmas would likely deplete some of the source regions of later magmatic activity (e.g., mare volcanism). Whether younger granites with different trace element compositions (e.g., 14321,1027; Warren et al., 1983Warren, P. H., Taylor, G.J. Keil, K., Shirley, D.N., Wasson, J.T. (1983) Petrology and chemistry of two “large” granite clasts from the Moon: Earth and Planetary Science Letters 64, 175–185.
) crystallised from similarly hydrous magmas is unknown.The rhyolitic magmas are believed to represent a distilled component of the urKREEP reservoir, reflecting a 10–100x enrichment in water. If true, our data suggest urKREEP had between 50 and 2000 ppm water; consistent with a dry bulk Moon (1–50 ppm water; Elkins-Tanton and Grove, 2011
Elkins-Tanton, L.T., Grove, T.L. (2011) Water (hydrogen) in the lunar mantle: Results from petrology and magma ocean modeling. Earth and Planetary Science Letters 307, 173–179.
) and much less than the 1 to 2 wt. % predicted for urKREEP if the bulk Moon was as wet as suggested by Hui et al. (2013)Hui, H., Peslier, A.H., Zhang, Y., Neal, C.R. (2013) Water in lunar anorthosites and evidence for a wet early Moon. Nature Geoscience 6, 177–180.
from plagioclase in ferroan anorthosite. The discrepancy between the results and interpretations of Hui et al. (2013)Hui, H., Peslier, A.H., Zhang, Y., Neal, C.R. (2013) Water in lunar anorthosites and evidence for a wet early Moon. Nature Geoscience 6, 177–180.
and this study could be due to the hypotheses used to understand how each formed or the physical processes that affected the plagioclase from the primary crust differently than the later granitoid rocks (e.g., degassing).If the granitoid rocks on the Moon were generally produced by hydrous magmas with 0.5–2 wt. % water (~1 wt. %), then the amount of granitic material on the Moon can be used to estimate the amount of water removed from the bulk Moon by felsic magmatism. Using the K2O concentration estimated for the silicate portion of the Moon (Longhi, 2006
Longhi, J. (2006) Petrogenesis of picritic mare magmas: Constraints on the extent of early lunar differentiation. Geochimica et Cosmochimica Acta 70, 5919–5934.
) there would be ~0.02 % alkali feldspar. Assuming that this alkali feldspar makes up 25–50 % of the granitic rock of the Moon, then the bulk silicate Moon would be ~0.1 % granite. Thus, the bulk Moon water content would have been decreased by ~10 ppm by felsic magmatism between 4.3 and 3.9 Ga. Globally, this early period of dehydration would have had a significant effect on the water content of the already dry bulk Moon, removing between a 1/4 to mostly all of the water. The water depletion events were likely distributed heterogeneously within the mantle, depending on the K distribution. Some regions could have retained 1000s of ppm water that contributed to later magmatism, as seen in water contents in volcanic glass and melt inclusions from mare basalts.top
Acknowledgements
NASA Curation is thanked for skillful processing of the Apollo sample used in the study. CAPTEM is thanked for sample allocations. The image used for the graphical abstract is credited to NASA/JSC/Arizona State University. We are grateful to Editor Bruce Watson and reviewers Rebecca Lange, Jed Mosenfelder, and one anonymous individual who helped improve this contribution. This work was supported by NASA LASER NNH10ZDA001N-0077 and SSW NH15ZDA001N-0411 grants to JIS, an Astrobiology Institute grant to CIW for CA and JW, and a NASA Postdoctoral Fellowship that supported RDM.
Editor: Bruce Watson
top
References
Baker, D.R., Vaillancourt, J. (1995) The low viscosities of F + H2O-bearing granitic melts and implications for melt extraction and transport. Earth and Planetary Science Letters 132, 199–211.
 Show in context
Show in context Understanding the volatile budget of the Moon is important because it can have profound effects on melt viscosity (Dingwell et al., 1985; Baker and Vaillancourt, 1995), chemical diffusion (Harrison and Watson, 1983), and solidus temperatures (Johannes and Holtz, 1996).
View in article
Barnes, J.J., Tartèse, R., Anand, M., McCubbin, F.M., Franchi, I.A., Starkey, N.A., Russell, S.S. (2014) The origin of water in the primitive Moon as revealed by the lunar highlands samples. Earth and Planetary Science Letters 390, 244–252.
 Show in context
Show in context To compare our magma water estimates for lunar granitoids with apatite estimates from a similar rock type we use the published NanoSIMS OH- data of apatites from evolved lunar rocks (Barnes et al., 2014; Robinson, et al., 2014) including the largest specimen of lunar granite, 14321,1047.
View in article
Excluding two outlier OH- measurements from Barnes et al. (2014) lowers the magmatic water estimate to 0.004 wt. %.
View in article
Boyce, J.W., Liu, Y., Rossman, G.R., Guan, Y., Eiler, J.M., Stolper, E.M., Taylor, L.A. (2010) Lunar apatite with terrestrial volatile abundances. Nature 466, 466–469.
 Show in context
Show in context Recent detection of indigenous hydrogen in a diversity of lunar materials, including volcanic glass (Saal et al., 2008), melt inclusions (Hauri et al., 2011), apatite (Boyce et al., 2010; McCubbin et al., 2010), and plagioclase (Hui et al., 2013) suggests water played a role in the chemical differentiation of the Moon.
View in article
Boyce, J.W., Tomlinson, S.M., McCubbin, F.M., Greenwood, J.P., Treiman, A.H. (2014) The lunar apatite paradox. Science 344, 400–402.
 Show in context
Show in context Figure 2 [...] Within individual samples, boundary layer crystallisation will create apatites with higher Cl and OH- contents than those initially growing from their host magma, thus the lowest water estimates from each sample (green fill) should be more representative of the initial magma composition (Boyce et al., 2014).
View in article
Notably, the inferred magmatic water contents based on OH- in lunar apatite have recently been shown to be unreliable due to the co-dependent compatibilities of F, Cl, and OH- (Boyce et al., 2014).
View in article
More important is the fact that evolved magmas with high Cl/F can crystallise apatite that generally excludes OH-, regardless of the water content of the magma (McCubbin et al., 2011; Boyce et al., 2014).
View in article
Canup, R.M., Asphaug, E. (2001) Origin of the Moon in a giant impact near the end of the Earth’s formation. Nature 412, 708–712.
 Show in context
Show in context The relative depletion in volatile elements in the Moon is thought to have occurred during a giant impact between a proto-Earth and a Mars-sized object (Hartman and Davis, 1975; Canup and Asphaug, 2001) resulting in volatile depletion and a lunar magma ocean, although Earth may have received additional input of volatiles by impacts after Moon formation (Owen and Bar-Nun, 1995).
View in article
Dingwell, D.B., Scarfe, C.M., Cronin, D.J. (1985) The effect of fluorine on viscosities in the system Na2O-Al2O3-SiO2: implications for phonolites, trachytes and rhyolites. American Mineralogist 70, 80–87.
 Show in context
Show in context Understanding the volatile budget of the Moon is important because it can have profound effects on melt viscosity (Dingwell et al., 1985; Baker and Vaillancourt, 1995), chemical diffusion (Harrison and Watson, 1983), and solidus temperatures (Johannes and Holtz, 1996).
View in article
Elkins-Tanton, L.T., Grove, T.L. (2011) Water (hydrogen) in the lunar mantle: Results from petrology and magma ocean modeling. Earth and Planetary Science Letters 307, 173–179.
 Show in context
Show in context Modelling of lunar magma ocean crystallisation (Elkins-Tanton and Grove, 2011) predicts a similar chemical differentiation to that seen in magma systems on Earth, with the highest levels of hydrogen in the evolved melt residuum of the magma ocean (i.e. urKREEP).
View in article
Using these data Elkins-Tanton and Grove (2011) predict that the bulk water content of the magma ocean would have been <10 ppm.
View in article
If true, our data suggest urKREEP had between 50 and 2000 ppm water; consistent with a dry bulk Moon (1–50 ppm water; Elkins-Tanton and Grove, 2011) and much less than the 1 to 2 wt. % predicted for urKREEP if the bulk Moon was as wet as suggested by Hui et al. (2013) from plagioclase in ferroan anorthosite.
View in article
Glotch, T.D., Lucey, P.G., Bandfield, J.L., Greenhagen, B.T., Thomas, I.R., Elphic, R.C., Bowles, N., Wyatt, M.B., Allen, C.C., Donaldson Hanna, K., Paige, D.A. (2010) Highly Silicic Compositions on the Moon. Science 329, 1510–1513.
 Show in context
Show in context Geochemical surveys by the Lunar Prospector (Jolliff et al., 2011) and Diviner Lunar Radiometer Experiment (Glotch et al., 2010; Jolliff et al., 2011) indicating the global significance of evolved igneous rocks suggest that the formation of these granites removed water from some mantle source regions, helping to explain the existence of mare basalts with <10 ppm water, but must have left regions of the interior relatively wet as seen by the water content in volcanic glass and melt inclusions.
View in article
Harrison, T.M., Watson, E.B. (1983) Kinetics of zircon dissolution and zirconium diffusion in granitic melts of variable water content. Contributions to Mineralogy and Petrology 84, 66–72.
 Show in context
Show in context Understanding the volatile budget of the Moon is important because it can have profound effects on melt viscosity (Dingwell et al., 1985; Baker and Vaillancourt, 1995), chemical diffusion (Harrison and Watson, 1983), and solidus temperatures (Johannes and Holtz, 1996).
View in article
Hartman, WK., Davis, D.R. (1975) Satellite-sized planetesimals and lunar origin. Icarus 24, 504–515.
 Show in context
Show in context The relative depletion in volatile elements in the Moon is thought to have occurred during a giant impact between a proto-Earth and a Mars-sized object (Hartman and Davis, 1975; Canup and Asphaug, 2001) resulting in volatile depletion and a lunar magma ocean, although Earth may have received additional input of volatiles by impacts after Moon formation (Owen and Bar-Nun, 1995).
View in article
Hauri, E.H., Shaw, A.M., Wang, J., Dixon, J.E., King, P.L., Mandeville, C. (2006) Matrix effects in hydrogen isotope analysis of silicate glasses by SIMS. Chemical Geology 235, 353–365.
 Show in context
Show in context NanoSIMS ion microprobe measurements of water in alkali feldspar and a silica polymorph from sample 15405,78 were employed following the approach of Hauri et al. (2006; see Supplementary Information).
View in article
Hauri, E.H., Weinreich, T., Saal, A.E., Rutherford, M.C., Van Orman, J.A. (2011) High pre-eruptive water contents preserved in lunar melt inclusions. Science 333, 213–215.
 Show in context
Show in context Recent detection of indigenous hydrogen in a diversity of lunar materials, including volcanic glass (Saal et al., 2008), melt inclusions (Hauri et al., 2011), apatite (Boyce et al., 2010; McCubbin et al., 2010), and plagioclase (Hui et al., 2013) suggests water played a role in the chemical differentiation of the Moon.
View in article
Hui, H., Peslier, A.H., Zhang, Y., Neal, C.R. (2013) Water in lunar anorthosites and evidence for a wet early Moon. Nature Geoscience 6, 177–180.
 Show in context
Show in context Recent detection of indigenous hydrogen in a diversity of lunar materials, including volcanic glass (Saal et al., 2008), melt inclusions (Hauri et al., 2011), apatite (Boyce et al., 2010; McCubbin et al., 2010), and plagioclase (Hui et al., 2013) suggests water played a role in the chemical differentiation of the Moon.
View in article
Water contents measured in plagioclase feldspar, a dominant mineral in the ancient crustal lunar highlands have been used to predict that 320 ppm water initially existed in the lunar magma ocean (Hui et al., 2013) whereas measurements in apatite, found as a minor mineral in lunar rocks, representing younger potassium-enriched melt predict a bulk Moon with <100 ppm water.
View in article
In contrast, Hui et al. (2013) estimate water contents of 320 ppm for the bulk Moon and 1.4 wt. % for urKREEP from water measured in lunar anorthosite.
View in article
If true, our data suggest urKREEP had between 50 and 2000 ppm water; consistent with a dry bulk Moon (1–50 ppm water; Elkins-Tanton and Grove, 2011) and much less than the 1 to 2 wt. % predicted for urKREEP if the bulk Moon was as wet as suggested by Hui et al. (2013) from plagioclase in ferroan anorthosite.
View in article
The discrepancy between the results and interpretations of Hui et al. (2013) and this study could be due to the hypotheses used to understand how each formed or the physical processes that affected the plagioclase from the primary crust differently than the later granitoid rocks (e.g., degassing).
View in article
Johannes, W., Holtz, F. (1996) Petrogenesis and experimental petrology of granitic rocks. Springer-Verlag, Berlin.
 Show in context
Show in context Understanding the volatile budget of the Moon is important because it can have profound effects on melt viscosity (Dingwell et al., 1985; Baker and Vaillancourt, 1995), chemical diffusion (Harrison and Watson, 1983), and solidus temperatures (Johannes and Holtz, 1996).
View in article
Because the melt was initially not water saturated (Johannes and Holtz, 1996) little water should have been lost prior to crystallisation.
View in article
Johnson, E.A. (2006) Water in nominally anhydrous crustal minerals: Speciation, concentration, and geologic significance. Reviews in Mineralogy and Geochemistry 62, 117–154.
 Show in context
Show in context These values are similar to a D obtained for plagioclase feldspar in felsic melt of 0.004 (Johnson, 2006).
View in article
Johnson, E.A., Rossman, G.R. (2004) A survey of hydrous species and concentrations in igneous feldspars. American Mineralogist 89, 560–600.
 Show in context
Show in context Hydrogen contained in nominally anhydrous minerals is normally in the form H2O or OH- (Johnson and Rossman, 2004) and water contents in alkali feldspars from Earth vary from 10s to 1000s of ppm (Johnson and Rossman, 2004).
View in article
In alkali feldspar in plutonic rocks on Earth the hydrogen is mostly found as H2O and in volcanic rocks it is generally OH- (Johnson and Rossman, 2004).
View in article
Johnson and Rossman (2004) hypothesise that the speciation of hydrogen in the feldspar is a function of the species in the liquid in equilibrium with the crystal.
View in article
Johnson and Rossman (2004) measured 90 ppm OH- in sanidines from the tuff; however, recalculation using a new molar absorption coefficient for sanidine (Mosenfelder et al., 2015) produces a value of 65 ppm.
View in article
Jolliff, B.L., Wiseman, S.A., Lawrence, S.L., Tran, T.N., Robinson, M.S., Sato, H., Hawke, B.R., Scholten, F., Oberst, J., Hiesinger, H., van der Bogert, C.H., Greenhagen, B.T., Glotch, T.D., Paige, D.A. (2011) Non-mare silicic volcanism on the lunar farside at Compton-Belkovich. Nature Geoscience 4, 566–571.
 Show in context
Show in context Geochemical surveys by the Lunar Prospector (Jolliff et al., 2011) and Diviner Lunar Radiometer Experiment (Glotch et al., 2010; Jolliff et al., 2011) indicating the global significance of evolved igneous rocks suggest that the formation of these granites removed water from some mantle source regions, helping to explain the existence of mare basalts with <10 ppm water, but must have left regions of the interior relatively wet as seen by the water content in volcanic glass and melt inclusions.
View in article
Klima, R., Cahill, J., Hagerty, J., Lawrence, D. (2013) Remote detection of magmatic water in Bullialdus Crater on the Moon. Nature Geoscience 6, 737–741.
 Show in context
Show in context Recent spectroscopic data from the Moon (Klima et al., 2013) support this trend, with a positive correlation between water and Th.
View in article
Longhi, J. (2006) Petrogenesis of picritic mare magmas: Constraints on the extent of early lunar differentiation. Geochimica et Cosmochimica Acta 70, 5919–5934.
 Show in context
Show in context Using the K2O concentration estimated for the silicate portion of the Moon (Longhi, 2006) there would be ~0.02 % alkali feldspar.
View in article
McCubbin, F.M., Steele, A., Hauri, E.H., Nekvasil, H., Yamashita, S., Hemley, R.J. (2010) Nominally hydrous magmatism on the Moon. Proceedings of the National Academy of Sciences 107, 11223–11228.
 Show in context
Show in context Recent detection of indigenous hydrogen in a diversity of lunar materials, including volcanic glass (Saal et al., 2008), melt inclusions (Hauri et al., 2011), apatite (Boyce et al., 2010; McCubbin et al., 2010), and plagioclase (Hui et al., 2013) suggests water played a role in the chemical differentiation of the Moon.
View in article
However, sample-based estimates of water content of KREEP-rich magmas from measurements of OH-, F, and Cl in lunar apatites suggest a low water concentration in the KREEP component with 2 to 140 ppm magmatic water (McCubbin et al., 2010).
View in article
Figure 2 [...] Apatite data are from McCubbin et al. (2010).
View in article
Volatile data from apatites in lunar basalts and alkali-suite clasts from McCubbin et al. (2010) show a conflicting trend with lower OH- and higher Cl/F in rocks that have more alkali feldspar (i.e. more K-rich).
View in article
As calculated by McCubbin et al. (2010) these estimates assume 95 % crystallisation of the host magma prior to formation of the apatite.
View in article
McCubbin, F.M., Jolliff, B.L., Nekvasil, H., Carpenter, P.K., Zeigler, R.A., Steele, A., Elardo, S.M., Lindsley, D.H. (2011) Fluorine and chlorine abundances in lunar apatite: Implications for heterogeneous distributions of magmatic volatiles in the lunar interior. Geochimica et Cosmochimica Acta 75, 5073–5093.
 Show in context
Show in context More important is the fact that evolved magmas with high Cl/F can crystallise apatite that generally excludes OH-, regardless of the water content of the magma (McCubbin et al., 2011; Boyce et al., 2014).
View in article
Merzbacher, C., Eggler, D.H. (1984) A magmatic geohygrometer – Application to mount St. Helens and other dacitic magmas. Geology 12, 587–590.
 Show in context
Show in context Despite the fact that 0.5 to 2 wt. % water is the wettest magma inferred for the Moon, it still would likely not stabilise hydrous phases (Merzbacher and Eggler, 1984).
View in article
Meyer, C., Williams, I.S., Compston, W. (1996) Uranium-lead ages for lunar zircons: Evidence for a prolonged period of granophyre formation from 4.32 to 3.88 Ga. Meteoritics & Planetary Science 31, 370–387.
 Show in context
Show in context Thus, lunar granites with ages from 4.3–3.9 Ga (Meyer et al., 1996) likely crystallised from relatively wet melts that degassed upon crystallisation.
View in article
Zircon ages from lunar granitic rocks (e.g., Meyer et al., 1996) span from 4.3 to 3.9 Ga, with an age of 4.3 Ga for 15405.
View in article
Regardless of whether the granitoids represent the final liquids or the first partial melts of lunar magma ocean materials, the new data imply that high water content rhyolitic magmas existed locally in the lunar crust around 4.3 Ga (Meyer et al., 1996).
View in article
Mosenfelder, J.L., Rossman, G.R., Johnson, E.A. (2015) Hydrous species in feldspars: A reassessment based on FTIR and SIMS. American Mineralogist 100, 1209–1221.
 Show in context
Show in context Johnson and Rossman (2004) measured 90 ppm OH- in sanidines from the tuff; however, recalculation using a new molar absorption coefficient for sanidine (Mosenfelder et al., 2015) produces a value of 65 ppm.
View in article
Owen, T., Bar-Nun, A. (1995) Comets, impact, and atmospheres. Icarus 116, 155–116.
 Show in context
Show in context The relative depletion in volatile elements in the Moon is thought to have occurred during a giant impact between a proto-Earth and a Mars-sized object (Hartman and Davis, 1975; Canup and Asphaug, 2001) resulting in volatile depletion and a lunar magma ocean, although Earth may have received additional input of volatiles by impacts after Moon formation (Owen and Bar-Nun, 1995).
View in article
Robinson, K.L., Barnes, J.J., Tartèse, R., Nagashima, K., Hallis, L.J., Franchi, I.A., Amand, M., Taylor, G.J. (2014) Primitive lunar water in evolved rocks? In 45th Lunar and Planetary Science Conference. 1607.
 Show in context
Show in context Figure 2 [...] For reference, OH- data from apatites from lunar granite 14321,1047 (Robinson et al., 2014) predict H2O melt compositions of 0.001 wt. %, and likely have Cl/F ratios higher than 3.
View in article
To compare our magma water estimates for lunar granitoids with apatite estimates from a similar rock type we use the published NanoSIMS OH- data of apatites from evolved lunar rocks (Barnes et al., 2014; Robinson, et al., 2014) including the largest specimen of lunar granite, 14321,1047.
View in article
This likely explains the two highest OH- values from Robinson et al. (2014) and implies that apatite with the lowest OH- content in an individual sample (Fig. 2) should give the best estimate of magmatic water.
View in article
Ryder, G. (1976) Lunar sample 15405: Remnant of a KREEP basalt-granite differentiated pluton. Earth and Planetary Science Letters 29, 255–268.
 Show in context
Show in context In Apollo sample 15405,78 granitoid clast xenoliths are found in the groundmass (Ryder, 1976) and generally consist of intergrowths of alkali feldspar and a silica polymorph (Fig. 1).
View in article
Saal, A.E., Hauri, E.H., Cascio, M.L., Van Orman, J.A., Rutherford, M.C., Cooper, R.F. (2008) Volatile content of lunar volcanic glasses and the presence of water in the Moon’s interior. Nature 454, 192–195.
 Show in context
Show in context Recent detection of indigenous hydrogen in a diversity of lunar materials, including volcanic glass (Saal et al., 2008), melt inclusions (Hauri et al., 2011), apatite (Boyce et al., 2010; McCubbin et al., 2010), and plagioclase (Hui et al., 2013) suggests water played a role in the chemical differentiation of the Moon.
View in article
Schmitt, A.K., Simon, J.I. (2004) Boron isotopic variations in hydrous rhyolitic melts: a case study from Long Valley, California. Contributions to Mineralogy and Petrology 146, 590–608.
 Show in context
Show in context Water contents from melt inclusions from the Bishop Tuff show a range from 2.3 to 6.0 wt. % (Schmitt and Simon, 2004).
View in article
Stolper, E., Newman, S. (1994) The role of water in the petrogenesis of Mariana trough magmas. Earth and Planetary Science Letters 121, 293–325.
 Show in context
Show in context On Earth, water and halogen contents of igneous rocks generally correlate with other incompatible elements like K, Rb, Th, and U (e.g., Stolper and Newman, 1994).
View in article
Warren, P. H., Taylor, G.J. Keil, K., Shirley, D.N., Wasson, J.T. (1983) Petrology and chemistry of two “large” granite clasts from the Moon: Earth and Planetary Science Letters 64, 175–185.
 Show in context
Show in context Whether younger granites with different trace element compositions (e.g., 14321,1027; Warren et al., 1983) crystallised from similarly hydrous magmas is unknown.
View in article
Webster, J.D., Tappen, C.M., Mandeville, C.W. (2009) Partitioning behavior of chlorine and fluorine in the system apatite-melt-fluid. II: Felsic silicate systems at 200 MPa. Geochimica et Cosmochimica Acta 73, 559–581.
 Show in context
Show in context Figure 2 [...] Water estimates from apatite assume 95 % crystallisation prior to apatite crystallisation. Cl/F melt estimates from apatite assume DF/DCl is 10 (Webster et al., 2009).
View in article
Yang, X. (2012) An experimental study of H solubility in feldspars: Effect of composition, oxygen fugacity, temperature and pressure and implications for crustal processes. Geochimica et Cosmochimica Acta 97, 46–57.
 Show in context
Show in context Experiments by Yang et al. (2012) show that the H solubility is higher (2–3x) at very reducing conditions, but because the oxygen fugacity during the crystallisation of the granitoids is unknown we cautiously use the range calculated from the Bishop Tuff.
View in article
top
Supplementary Information
Supplementary Methods
H2O abundance analyses by NanoSIMS
The abundance of H2O was measured in nominally anhydrous minerals (feldspar and a SiO2- phase) using the Cameca NanoSIMS 50L scanning ion microprobe at the Carnegie Institution of Washington’s Department of Terrestrial Magnetism, following the techniques of Hauri et al. (2006; 2011). A primary ion beam of Cs was used to raster over an area of 25x25 pm, and negatively charged ions of the isotopes 12C, 16OH, 19F, 30Si, 32S and 35Cl were measured simultaneously on six electron multipliers using ion counting. For every analysis location, we first performed a pre-sputtering step on a 30x30 pm area for 2 minutes with a 10 nA primary beam, then switched to a primary beam of 1 nA on a 25x25 pm area for data acquisition. Data were recorded as scanning ion images obtained simultaneously on each mass (12C, 16OH, F, Si, S and C); in this mode, acquisition of a single frame of 256x256 pixel takes 32.768 seconds, and 5 frames were acquired at each analysis location, resulting in a total acquisition time of ~3 minutes. We used basaltic glass ALV519-4-1 as a standard to determine relative sensitivity factors for C, OH-, F, S and Cl, and we used the anhydrous Suprasil standard to monitor the detection limits of these elements, as described in Koga et al. (2003) and Hauri et al. (2006). Mass resolving power of the instrument was set to ~6000 in order to separate 16OH- from 17O as well as other isobaric interferences; average 30Si count rates were on the order of 200,000 cps. For standardisation and detection limit determinations, we obtained data in two different modes of operation: standard Dynamic SIMS, and Scanning Ion Imaging SIMS. For the measurements of lunar nominally anhydrous minerals, only Scanning Ion Imaging SIMS was used.
Data were extracted and processed from the ion images using the L’Image software package for PV Wave written by Larry Nittler (DTM-CIW). Data were corrected for system deadtime, and the five image frames of individual species were drift-corrected and summed; images were ratioed to produce scanning isotope ratio images (12C/30Si, 16OH/30Si, 19F/30Si, 32S/30Si and 35Cl/30Si), and data were extracted from specific user-defined regions of interest (ROIs: feldspar and a SiO2-phase) located within the isotope ratio images. We used a synthetic silica glass (the anhydrous Suprasil glass standard) to establish the H2O detection limit of ≤3 ppm, as determined by multiple separate measurements interspersed with the analyses of the lunar phases. The reported uncertainties based on these count rates follow a Poisson distribution. The reported precision of the feldspar analyses is negligibly affected by the reproducibility of the detection limit (2.7 ± 0.3 2 S.E.). The reported volatile concentrations are obtained by simply subtracting the detection limit from the measured concentrations (Table 1). For the uncertainties, we assigned a conservative 7 % (2 S.E.) for H2O on all the measured regions of interest, which represent the uncertainty calculated by propagating the errors in the detection limit and the counting statistics obtained with the NanoSIMS.
X-ray mapping and mineral compositions by scanning electron microscope and microprobe
Mineral compositions, X-ray maps and backscattered electron (BSE) maps were obtained at NASA Johnson Space Center (JSC) following standard methods to study the petrography of the clasts, guide NanoSIMS traverses, and verify the mineralogy of the analysis spots. High-resolution BSE images and digital X-ray maps were obtained with a JEOL JSM-7600F SEM at JSC. X-ray maps were obtained with a ThermoElectron SDD X-ray detector and ThermoElectron software using a 15 kV beam and 30 nA beam current.
Supplementary Information References
Hauri, E.H., Weinreich, T., Saal, A.E., Rutherford, M.C., Van Orman, J.A. (2011) High pre-eruptive water contents preserved in lunar melt inclusions. Science 333, 213–215.
Koga, K., Hauri, E.H., Hirschmann, M., Bell, D. (2003) Hydrogen concentration analyses using SIMS and FTIR: Comparision and calibration for nominally anhydrous minerals. Geochemistry, Geophysics, Geosystems 4, doi: 10.1029/2002GC000378.
Figures and Tables
Table 1 NanoSIMS data for alkali feldspar and SiO2 phases from 2 clasts found in Apollo sample 15405,78. For alkali feldspar, data from 4 regions of interest (ROI) are presented, taken from 2 different clasts. Raw – Suprasil values are the calculated concentration data for the alkali feldspar minus the baseline calculated from the Suprasil anhydrous glass. Raw – average SiO2 are the calculated concentration data for the alkali feldspar minus the average from the SiO2 phase from the clasts.
| Alkali feldspar (ppm) | ||||||
| ROI 1 | ROI 2 | ROI 3 | ROI 4 | Average | 2 S.E. | |
| Raw | 22.8 | 22.3 | 20 | 21.3 | 21.6 | 1.25 |
| Raw – Suprasil | 20.7 | 20.2 | 17.9 | 19.2 | 19.5 | 1.25 |
| Raw – average SiO2 | 20.1 | 19.6 | 17.3 | 18.6 | 18.9 | 1.25 |
| SiO2 phase (ppm) | ||||||
| ROI 1 | ROI 2 | ROI 3 | Average | 2 S.E. | ||
| Raw | 2.89 | 2.39 | 2.78 | 2.68 | 0.304 | |
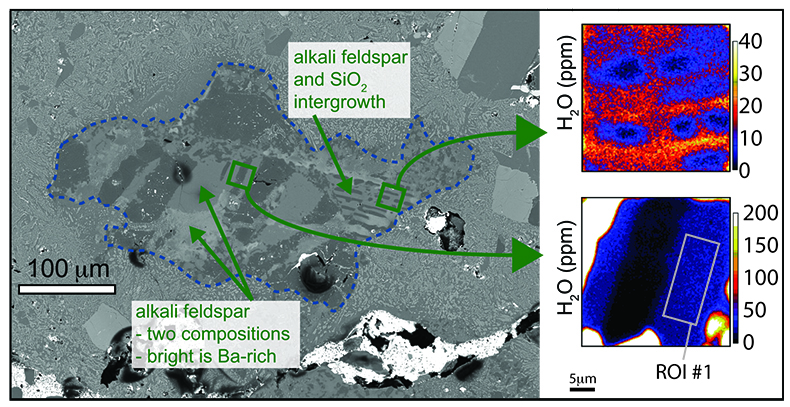
Figure 1 BSE image of granitoid clast (outlined in dashed line) from sample 15405,78, on the left. Clast consists of alkali feldspar and a silica phase. The variation in brightness of the feldspar relates to Ba (i.e. bright has more Ba). On the right are chemical maps obtained by NanoSIMS at DTM. Water correlates with mineralogy. The alkali feldspar consistently has ~20 ppm H2O. The silica phase has similar water contents as the blank obtained on anhydrous glass (2–3 ppm).
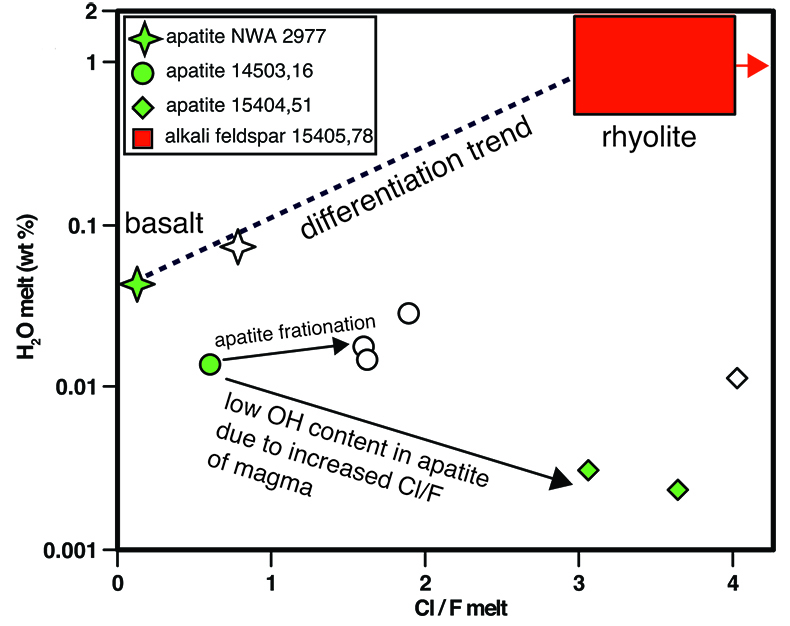
Figure 2 Plot of the estimated Cl/F ratio of magma versus estimated water content for lunar magmas (y-axis in log scale). Apatite data are from McCubbin et al. (2010)
McCubbin, F.M., Steele, A., Hauri, E.H., Nekvasil, H., Yamashita, S., Hemley, R.J. (2010) Nominally hydrous magmatism on the Moon. Proceedings of the National Academy of Sciences 107, 11223–11228.
. Water estimates from apatite assume 95 % crystallisation prior to apatite crystallisation. Cl/F melt estimates from apatite assume DF/DCl is 10 (Webster et al., 2009Webster, J.D., Tappen, C.M., Mandeville, C.W. (2009) Partitioning behavior of chlorine and fluorine in the system apatite-melt-fluid. II: Felsic silicate systems at 200 MPa. Geochimica et Cosmochimica Acta 73, 559–581.
). Within individual samples, boundary layer crystallisation will create apatites with higher Cl and OH- contents than those initially growing from their host magma, thus the lowest water estimates from each sample (green fill) should be more representative of the initial magma composition (Boyce et al., 2014Boyce, J.W., Tomlinson, S.M., McCubbin, F.M., Greenwood, J.P., Treiman, A.H. (2014) The lunar apatite paradox. Science 344, 400–402.
). Schematic differentiation trend is shown between basalt and rhyolite. For reference, OH- data from apatites from lunar granite 14321,1047 (Robinson et al., 2014Robinson, K.L., Barnes, J.J., Tartèse, R., Nagashima, K., Hallis, L.J., Franchi, I.A., Amand, M., Taylor, G.J. (2014) Primitive lunar water in evolved rocks? In 45th Lunar and Planetary Science Conference. 1607.
) predict H2O melt compositions of 0.001 wt. %, and likely have Cl/F ratios higher than 3.