Magma dynamics of ancient Mt. Etna inferred from clinopyroxene isotopic and trace element systematics
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: Etna, hafnium, neodymium, lead, isotope, trace elements, peridotite, pyroxenite, mantle, assimilation, Timpe Santa Caterina, thermobarometry, clinopyroxene, basalt
- Share this article





Article views:7,631Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
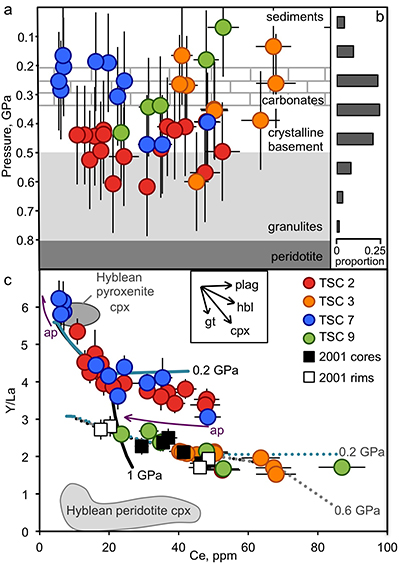 Figure 1 (a) Ce contents of TSC cpx as a function of single-cpx pressure estimates (1σ uncertainty) superimposed on Etna stratigraphy (after Spilliaert et al., 2006). (b) Proportions of ancient Etna barometry from this study and previous work (cf. Supplementary Information, n = 287). (c) TSC cpx and 2001 eruption cpx (Viccaro et al., 2006) shown with Hyblean pyroxenite and peridotite cpx fields (Correale et al., 2012, and references therein). Isobaric cpx fractionation modelling for peridotite melt (solid lines) and pyroxenite melt (dashed lines) at 1.0 (black), 0.6 (grey), and 0.2 (blue) GPa performed using alphaMELTS (Smith and Asimow, 2005); conditions described in Supplementary Information. Fractionation of apatite, well known to incorporate REEs, is modelled in purple using the partitioning of Prowatke and Klemme (2006). Ol+cpx±opx+sp is present at the start of both trends, though olivine drops out at T < ~1100 °C for pyroxenite melt. | 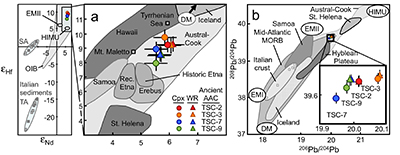 Figure 2 Ancient Etna cpx and WR data. (a) εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005. Hafnium isotopic values for Italian sediments (Conticelli et al., 2002; Brems et al., 2013) are calculated from Nd isotopic data and both cases following the seawater array (SA) and the terrestrial array (TA) of Vervoort et al. (2011) are shown. (b) 208Pb/204Pb vs. 206Pb/204Pb shown with OIB and mid-ocean ridge basalt (MORB) fields, historic Etna (Viccaro and Cristofolini, 2008) and Hyblean Plateau field from Trua et al. (1998). Italian crustal values from Conticelli et al. (2002). External reproducibility is conservatively set at 0.01 for 206Pb/204Pb and 0.02 for 208Pb/204Pb. |
| Figure 1 | Figure 2 |
Supplementary Figures and Tables
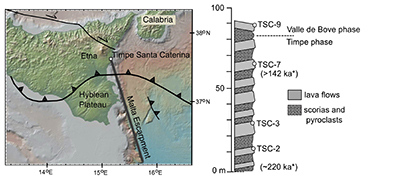 Figure S-1 Location of Timpe Santa Caterina outcrop on base map from GeoMapApp (http://www.geomapapp.org; Ryan et al., 2009). Major geologic features from Rosenbaum and Lister (2004). Stratigraphy based on Corsaro et al. (2002) with section base at sea level (0 m). Dates (*) from Gillot et al. (1994). | 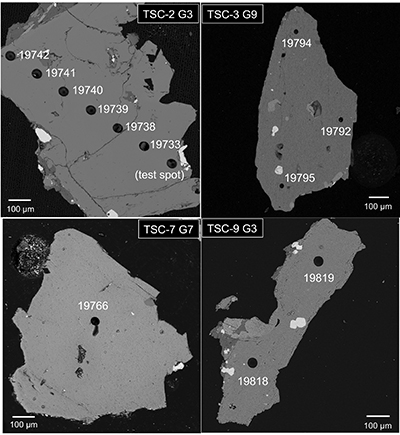 Figure S-2 Back scattered electron images of representative clinopyroxene grains from TSC lavas with laser ablation spots (Alfred University electron microprobe). |  Table S-1 Electron microprobe analyses of TSC* clinopyroxene LA-ICP-MS laser spots. All iron reported as FeO. Operating conditions used at the University of Oregon (UO) and Massachusetts Institute of Technology (MIT) facilities were 15 keV accelerating voltage and 10 nA beam current, with all analyses using a focused beam of ~1 microns and 30 s count times. Data were reduced using the CITZAF correction procedure of Armstrong (1995). The few totals lower than 98 wt. % have been omitted. MIT JEOL JXA-8200 electron microprobe uncertainties (1σ) are calculated from the standard deviation of replicate analyses of the DJ35 diopside-jadeite glass standard and several points inferred from back scattered electron imaging to be from the same clinopyroxene crystal growth zone. |  Table S-2 Trace element data (ppm) collected by LA-ICP-MS at the University of New Hampshire. |  Table S-3 Comparison of LA-ICP-MS repeat analyses of ML3B-G glass standard with reported literature values. |
| Figure S-1 | Figure S-2 | Table S-1 | Table S-2 | Table S-3 |
 Table S-4 TSC clinopyroxene major element compositions (wt. %) along grain transects were analysed on the Massachusetts Institute of Technology (MIT) JEOL-JXA-8200 Superprobe. Uncertainty (2s) has been calculated from the standard deviation of replicate analyses of the DJ35 diopside-jadeite glass and ALP7 aluminous orthopyroxene standards, as well as several points inferred from back scattered electron imaging to be from the same clinopyroxene crystal growth zone: SiO2 (0.31), TiO2 (0.02), Al2O3 (0.07), FeO (0.08), MgO (0.11), MnO (0.01), CaO (0.30), Na2O (0.04), K (0.01), Cr2O3 (0.02). |  Table S-5 Hf-Nd-Pb isotopic data for Timpe Santa Caterina whole rock (WR) and clinopyroxene (cpx) separates*. The uncertainties reported for Nd and Hf isotope ratios are internal 2 s.e. We use the values of external reproducibility as reported in the footnote to identify analytically resolvable WR-cpx disequilibrium discernable above the 2σ level. |  Table S-6 Ranges of whole rock and clinopyroxene major and minor element compositions (wt. %) observed for the Timpe Santa Caterina flows studied. | 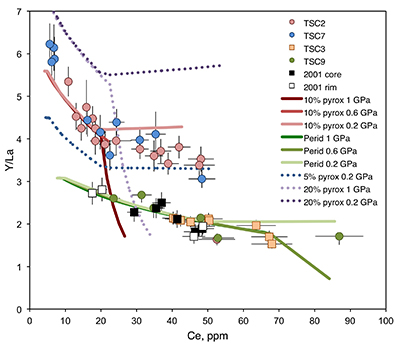 Figure S-3 Clinopyroxene trace element evolution during isobaric fractionation of peridotite melt (green) and 10 % pyroxenite component melts (red) at 1.0, 0.6, and 0.2 GPa. Modelling sensitivity to starting composition is illustrated by including paths for melts with 5 % and 20 % pyroxenite component shown as blue and purple dotted lines, respectively. |
| Table S-4 | Table S-5 | Table S-6 | Figure S-3 |
top
Introduction
Volcanism began at Mount Etna, Europe’s largest and most active volcano, at ~0.5 Ma (Gillot et al., 1994
Gillot, P.Y., Kieffer, G., Romano, R. (1994) The evolution of Mount Etna in the light of potassium-argon dating. Acta Vulcanologica 5, 81–87.
), with ancient lavas now exposed around the perimeter of the modern-day edifice. Tholeiitic lavas were overlain by transitional and alkaline sequences starting at ~230 ka (Gillot et al., 1994Gillot, P.Y., Kieffer, G., Romano, R. (1994) The evolution of Mount Etna in the light of potassium-argon dating. Acta Vulcanologica 5, 81–87.
; Branca and Del Carlo, 2004Branca, S., Del Carlo, P. (2004) Eruptions of Mt. Etna during the past 3,200 Years: A revised compilation integrating the historical and stratigraphic records. In: Bonaccorso, A., Calvari, S., Coltelli, M., Del Negro, C., Falsaperla, S. (Eds.) Mt. Etna: Volcano Laboratory. American Geophysical Union, Washington, D.C., 1–27.
). Mt. Etna sits on the northern edge of the African plate at the European-African collision zone and the western hinge of escarpments dividing it from where the Ionian slab descends beneath the Aeolian arc (Fig. S-1, Supplementary Information). Volcanism has been attributed to the manifestation of mantle upwelling independent of, or in response to, a slab tear (e.g., Gasperini et al., 2002Gasperini, D., Blichert-Toft, J., Bosch, D., Del Moro, A., Macera, P., Albarède, F. (2002) Upwelling of deep mantle material through a plate window; evidence from the geochemistry of Italian basaltic volcanics. Journal of Geophysical Research 107, 2367.
), subduction-related fluid-triggered melting (e.g., Armienti et al., 2007Armienti, P., Tonarini, S., Innocenti, F., D'Orazio, M. (2007) Mount Etna pyroxene as tracer of petrogenetic processes and dynamics of the feeding system. In: Beccaluva, L., Bianchini, G., Wilson, M. (Eds.) Cenazoic volcanism in the Mediterranean Area. Geological Society of America Special Paper 418, 265–276.
and references therein) or enhanced decompression melting resulting from convective anomalies (Gvirtzman and Nur, 1999Gvirtzman, Z., Nur, A. (1999) The formation of Mount Etna as the consequence of slab rollback. Nature 401, 782–785.
; Schellart, 2010Schellart, W.P. (2010) Mount Etna–Iblean volcanism caused by rollback-induced upper mantle upwelling around the Ionian slab edge: An alternative to the plume model. Geology 38, 691–694.
).Magmatic products of the early Etna centres, including those of the ancient alkali centres active at ~200–100 ka, bear mantle-derived isotopic signatures consistent with contributions from both enriched and depleted source components (Marty et al., 1994
Marty, B., Trull, T., Lussiez, P., Basile, I., Tanguy, J.-C. (1994) He, Ar, O, Sr and Nd isotope constraints on the origin and evolution of Mount Etna magmatism. Earth and Planetary Science Letters 126, 23–39.
; Tanguy et al., 1997Tanguy, J.-C., Condomines, M., Kieffer, G. (1997) Evolution of the Mount Etna magma: Constraints on the present feeding system and eruptive mechanism. Journal of Volcanology and Geothermal Research 75, 221–250.
). Though more recent Etna volcanic products exhibit distinctive signs of assimilation in the form of elevated Sr isotopic values and large ion lithophile element enrichments (Tonarini et al., 1995Tonarini, S., Armienti, P., D'Orazio, M., Innocenti, F., Pompilio, M., Petrini, R. (1995) Geochemical and isotopic monitoring of Mt. Etna 1989-1993 eruptive activity: bearing on the shallow feeding system. Journal of Volcanology and Geothermal Research 64, 95–115.
), the degree to which crustal contamination influenced early alkaline products is uncertain. Similarly, magmatic processes between mantle melting regions and shallow reservoirs supplying volcanic activity remain enigmatic. In this study, we combine clinopyroxene (cpx) barometry, trace element concentrations, and Pb, Hf, and Nd mineral-whole rock (WR) isotopic (dis)equilibria to constrain source compositions and differentiation depths of magmas feeding lavas erupted at Timpe Santa Caterina (TSC). The advantage of employing these three isotopic systems together lies in the coupling of the slowly diffusing Hf and Nd with the more rapidly diffusing Pb, thereby providing the potential to infer magma assembly processes prior to eruption during this early period.top
Sample Selection and Analytical Methods
Lavas at TSC encompass the whole ancient alkaline magmatism period at Etna, from 220 ka near sea level to likely <100 ka exposed atop the sea cliff (Gillot et al., 1994
Gillot, P.Y., Kieffer, G., Romano, R. (1994) The evolution of Mount Etna in the light of potassium-argon dating. Acta Vulcanologica 5, 81–87.
). Early trachybasaltic and basaltic flows, TSC-2 and TSC-3, are overlain by more alkalic basanites and phonotephritic lavas (TSC-7 and TSC-9). Flows selected for this study contain the most abundant large cpx from the TSC suite (Fig. S-2, Supplementary Information). Major and trace element and isotopic analytical details and data are provided in Tables S-1 to S-5 (Supplementary Information).top
Results and Discussion
Barometry. Observed cpx phenocrysts (>2 mm, large relative to other TSC lava phases) coupled with theoretical modelling of Etna compositions indicate early cpx crystallisation; hence cpx holds a potential record of pre-eruptive magma assembly processes (Armienti et al., 2009
Armienti, P., Gasperini, D., Perinelli, C., Putirka, K.D. (2009) A new model for estimating deep-level magma ascent rates from thermobarometry: an example from Mt. Etna and implications for deep-seated magma dehydration. Acta Vulcanologica 21, 145–158.
). Crystallisation temperatures and pressures, solved iteratively using a single-cpx thermometer and single-cpx barometer for hydrous systems (respectively, Eqs. 32d and 32b in Putirka, 2008Putirka, K.D. (2008) Thermometers and barometers for volcanic systems. Reviews in Mineralogy and Geochemistry 69, 61–120.
), yielded temperatures of 1060–1175 °C and an average pressure of 0.34 ± 0.16 GPa (Fig. 1a,b). Thermobarometric model accuracy was evaluated using a literature dataset of >100 experimentally coexisting cpx-liquid pairs over a compositional range bracketing TSC lavas and cpx compositions (cf. Supplementary Information Table S-6 for equations, ranges, references, and selection criteria). As noted by Mollo et al. (2010)Mollo, S., Del Gaudio, P., Ventura, G., Iezzi, G., Scarlato, P. (2010) Dependence of clinopyroxene composition on cooling rate in basaltic magmas: Implications for thermobarometry. Lithos 118, 302–312.
, single-cpx barometers can outperform liquid-based models for volatile-rich alkaline compositions. The single-cpx barometer for hydrous systems yields an average uncertainty of 0.17 versus 0.28 GPa for the cpx-liquid model of Putirka et al. (2003)Putirka, K.D., Mikaelian, H., Ryerson, F., Shaw, H. (2003) New clinopyroxene-liquid thermometers for mafic, evolved, and volatile-bearing lava compositions, with applications to lavas from Tibet and the Snake River Plain, Idaho. American Mineralogist 88, 1542–1554.
for the compiled experiments, placing a lower bound of pressures recorded by TSC cpx at below 0.8 GPa, within the uppermost lithospheric mantle.Crystallisation of TSC-2 and TSC-7 cpx generally occurred at depths centred around 0.5 GPa and 0.2–3 GPa, respectively (Fig. 1a), suggesting specific magma reservoir locations near the crystalline basement-granulite boundary and within the carbonate platform beneath Etna. More continuous polybaric crystallisation is apparent in TSC-3 and TSC-9. Combined with previous work on Etna lavas (see Supplementary Information), thermobarometry indicates that the bulk of ancient clinopyroxene phenocrysts crystallised between 0.5 and 0.2 GPa (Fig. 1b).

Figure 1 (a) Ce contents of TSC cpx as a function of single-cpx pressure estimates (1σ uncertainty) superimposed on Etna stratigraphy (after Spilliaert et al., 2006
Spilliaert, N., Allard, P., Métrich, N., Sobolev, A.V. (2006) Melt inclusion record of the conditions of ascent, degassing, and extrusion of volatile-rich alkali basalt during the powerful 2002 flank eruption of Mount Etna (Italy). Journal of Geophysical Research 111, B04203.
). (b) Proportions of ancient Etna barometry from this study and previous work (cf. Supplementary Information, n = 287). (c) TSC cpx and 2001 eruption cpx (Viccaro et al., 2006Viccaro, M., Ferlito, C., Cortesogno, L., Cristofolini, R., Gaggero, L. (2006) Magma mixing during the 2001 event at Mount Etna (Italy): effects on the eruptive dynamics. Journal of Volcanology and Geothermal Research 149, 139–159.
) shown with Hyblean pyroxenite and peridotite cpx fields (Correale et al., 2012Correale, A., Martelli, M., Paonita, A., Rizzo, A., Brusca, L., Scribano, V. (2012) New evidence of mantle heterogeneity beneath the Hyblean Plateau (southeast Sicily, Italy) as inferred from noble gases and geochemistry of ultramafic xenoliths. Lithos 132–133, 70–81.
, and references therein). Isobaric cpx fractionation modelling for peridotite melt (solid lines) and pyroxenite melt (dashed lines) at 1.0 (black), 0.6 (grey), and 0.2 (blue) GPa performed using alphaMELTS (Smith and Asimow, 2005Smith, P.M., Asimow, P.D. (2005) Adiabat_1ph: A new public front-end to the MELTS, pMELTS, and pHMELTS models. Geochemistry Geophysics Geosystems 6, Q02004.
); conditions described in Supplementary Information. Fractionation of apatite, well known to incorporate REEs, is modelled in purple using the partitioning of Prowatke and Klemme (2006)Prowatke, S., Klemme, S. (2006) Trace element partitioning between apatite and silicate melts. Geochimica et Cosmochimica Acta 70, 4513–4527.
. Ol+cpx±opx+sp is present at the start of both trends, though olivine drops out at T < ~1100 °C for pyroxenite melt.Heterogeneous mantle sources for ancient Etna. Clinopyroxene trace element concentrations, when coupled with single-cpx barometry pressure estimates, place constraints on magma source compositions and crustal mixing depths. Cerium, incompatible in all major TSC phases, functions as a fractionation proxy and indicator of magma evolution. Two distinct crystallisation paths are apparent in TSC cpx: trends characterised by high Y/La (TSC-2, TSC-7) and low Y/La (TSC-3, TSC-9) when linked with Ce (Fig. 1c). Clinopyroxene from the 2001 eruption also follow the low-Y/La trend, as do other known historic and recent Etna cpx (Viccaro et al., 2006
Viccaro, M., Ferlito, C., Cortesogno, L., Cristofolini, R., Gaggero, L. (2006) Magma mixing during the 2001 event at Mount Etna (Italy): effects on the eruptive dynamics. Journal of Volcanology and Geothermal Research 149, 139–159.
). Scarlato et al. (2014)Scarlato, P., Mollo, S., Blundy, J.D., Iezzi, G., Tiepolo, M. (2014) The role of natural solidification paths on REE partitioning between clinopyroxene and melt. Bulletin of Volcanology 76, 810, doi: 10.1007/s00445-014-0810-1.
have documented preferential HREE incorporation into cpx relative to LREE as a function of cooling rate, but in TSC phenocrysts, HREE-like Y has either negative or no correlation with major element chemistry associated with elevated cooling rates (e.g., Na, AlIV, and Ti). Accordingly, we interpret the Y/La-Ce trends to reflect source characteristics beneath Etna over time rather than being a feature of crystallisation conditions.Clinopyroxene grains record existence of magmas beneath Etna deriving from melting of both pyroxenitic and peridotitic mantle components. The source characterisation enabled by analysis of Y/La-Ce trends in Etna TSC cpx can also be used to evaluate the composition of cpx in pyroxenite and peridotite xenoliths from the nearby Hyblean Plateau (Fig. 1c). Clinopyroxene from Hyblean pyroxenite xenoliths plot along the high-Y/La TSC trend (Fig. 1c), which is reproduced with a primary melt generated by a heterogeneous source of 10 % dry pyroxenite and 90 % hydrated peridotite in which ~10 % of each lithology melts and mixes at 1.5 GPa. Figure S-3 shows hypothetical source compositions with up to 20 % pyroxenite to constrain model sensitivity (Supplementary Information). These lithologies, similar to those determined by Correale et al. (2014)
Correale, A., Paonita, A., Martelli, M., Rizzo, A., Rotolo, S.G., Corsaro, R.A., Di Renzo, V. (2014) A two-component mantle source feeding Mt.Etna magmatism: Insights from the geochemistry of primitive magmas. Lithos 184–187, 243–258.
modelling trace element systematics in primitive Etna WR samples <15 ka, are distinct from peridotitic cpx from the nearby Hyblean plateau that fall below the low-Y/La trend. Low Y/La in cpx may result from either a hydrated peridotite source or a more evolved melt of the mixed pyroxenite source following apatite saturation.Isotopic (dis)equilibria. Most Etna mineral-WR pair isotopic work has focused on the Sr and Nd systems in recent lavas (e.g., Tonarini et al., 1995
Tonarini, S., Armienti, P., D'Orazio, M., Innocenti, F., Pompilio, M., Petrini, R. (1995) Geochemical and isotopic monitoring of Mt. Etna 1989-1993 eruptive activity: bearing on the shallow feeding system. Journal of Volcanology and Geothermal Research 64, 95–115.
), which generally exhibit more radiogenic Sr and less radiogenic Nd than ancient lavas. Within recent eruptive episodes, marked increases in WR 87Sr/86Sr are often accompanied by 87Sr/86Sr WR-cpx disequilibria (e.g., 0.70348 cpx core values accompanied by 0.70362 WR values in 2001 eruptives; Armienti et al., 2007Armienti, P., Tonarini, S., Innocenti, F., D'Orazio, M. (2007) Mount Etna pyroxene as tracer of petrogenetic processes and dynamics of the feeding system. In: Beccaluva, L., Bianchini, G., Wilson, M. (Eds.) Cenazoic volcanism in the Mediterranean Area. Geological Society of America Special Paper 418, 265–276.
).Our approach employing coupled Hf, Nd, and Pb isotopic signatures in ancient volcanics brings three distinct chemical affinities to bear on determining magma assembly, as recorded in cpx trace elements, at depths constrained by thermobarometry. As refractory elements diffusing slowly in clinopyroxene (cf. Van Orman et al., 2001
Van Orman, J.A., Grove, T.L., Shimizu, N. (2001) Rare earth element diffusion in diopside: influence of temperature, pressure, and ionic radius, and an elastic model for diffusion in silicates. Contributions to Mineralogy and Petrology 141, 687–703.
), Nd and Hf may be expected to retain isotopic signatures from early crystallisation depths and exhibit large isotopic disequilibria with hosting magmas subject to mixing with recharging, or assimilating magmas, carrying isotopically distinctive compositions immediately prior to eruption. In contrast, Pb diffuses relatively rapidly, making Pb isotope systematics an especially promising approach for placing constraints on magma residence times within the crust.Since each separate cpx analysis represents digestion of multiple grains likely crystallised at different depths, reported isotopic values reflect an average over the polybaric cpx crystallisation history. However, sluggish Nd and Hf re-equilibration will manifest itself as WR-cpx disequilibria in cases of late-stage incorporation of any volumetrically significant isotopically distinct magma during the final stages of magma assembly.
Neodymium and Hf isotopic compositions (Fig. 2a) of TSC cpx and WR demonstrate they are insignificantly distinctive at the 2σ level. However, it is notable that all cpx have slightly more enriched Nd isotopic signatures that trend toward those of continental values. This could result from a recharge process of fresher mantle-derived material that drives eruption. Late-stage shallow contamination, by contrast, would impart enriched crustal signatures to the WR, presumably after cpx phenocryst formation. Though Hf and Nd isotopic data for sedimentary units directly beneath Etna are unavailable for comparison with cpx and WR values, Sicilian beach sand εNd derived from the western extension of sedimentary units underlying Etna and crustal rocks of south and central Italy are all considerably more enriched (Fig. 2a; εNd -10.3 to -16.0, Conticelli et al., 2002
Conticelli, S., D'Antonio, M., Pinarelli, L., Civetta, L. (2002) Source contamination and mantle heterogeneity in the genesis of Italian potassic and ultrapotassic volcanic rocks: Sr‚ Nd‚ Pb isotope data from Roman Province and Southern Tuscany. Mineralogy and Petrology 74,189–222.
; Brems et al., 2013Brems, D., Ganio, M., Latruwe, K., Balcaen, L., Carremans, M., Gimeno, D., Silvestri, A., Vanhaecke, F., Muchez, P., Degryse, P. (2013) Isotopes on the beach, part 2: neodymium isotopic analysis for the provenancing of Roman glass-making. Archaeometry 55, 449–464.
). Such large differences make it unlikely that crustal sediments contributed to the Hf and Nd isotopic compositions observed in TSC cpx and WR materials. Rather, we infer that the isotopic signatures of these magmas were locked in at pressures corresponding, at minimum, to early cpx crystallisation at mid-crustal pressures of 0.5–0.2 GPa.
Figure 2 Ancient Etna cpx and WR data. (a) εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003
Lassiter, J.C., Blichert-Toft, J., Hauri, E.H., Barsczus, H.G. (2003) Isotope and trace element variations in lavas from Raivavae and Rapa, Cook‚ Austral islands: constraints on the nature of HIMU- and EM-mantle and the origin of mid-plate volcanism in French Polynesia. Chemical Geology 202, 115–138.
; Stracke et al., 2003Stracke, A., Bizimis, M., Salters, V.J.M. (2003) Recycling oceanic crust: Quantitative constraints. Geochemistry Geophysics Geosystems 4, 8003.
; Gaffney et al., 2004Gaffney, A.M., Nelson, B.K., Blichert-Toft, J. (2004) Geochemical constraints on the role of oceanic lithosphere in intra-volcano heterogeneity at West Maui, Hawaii. Journal of Petrology 45, 1663–1687.
; Huang et al., 2005Huang, S., Frey, F.A., Blichert-Toft, J., Fodor, R.V., Bauer, G.R., Xu, G. (2005) Enriched components in the Hawaiian plume: Evidence from Kahoolawe Volcano, Hawaii. Geochemistry Geophysics Geosystems 6, Q11006.
; Xu et al., 2007Xu, G., Frey, F.A., Clague, D.A., Abouchami, W., Blichert-Toft, J., Cousens, B., Weisler, M. (2007) Geochemical characteristics of West Molokai shield-and postshield-stage lavas: Constraints on Hawaiian plume models. Geochemistry Geophysics Geosystems 8, Q08G21.
; Sims et al., 2008Sims, K.W.W., Blichert-Toft, J., Kyle, P.R., Pichat, S., Gauthier, P.-J., Blusztajn, J., Kelly, P., Ball, L., Layne, G. (2008) A Sr, Nd, Hf, and Pb isotope perspective on the genesis and long-term evolution of alkaline magmas from Erebus volcano, Antarctica. Journal of Volcanology and Geothermal Research 177, 606–618.
; Blichert-Toft and Albarède, 2009Blichert-Toft, J., Albarède, F. (2009) Mixing of isotopic heterogeneities in the Mauna Kea plume conduit. Earth and Planetary Science Letters 282, 190–200.
; Yamasaki et al., 2009Yamasaki, S., Kani, T., Hanan, B.B., Tagami, T. (2009) Isotopic geochemistry of Hualalai shield-stage tholeiitic basalts from submarine North Kona region, Hawaii. Journal of Volcanology and Geothermal Research 185, 223–230.
; Garcia et al., 2010Garcia, M.O., Swinnard, L., Weis, D., Greene, A.R., Tagami, T., Sano, H., Gandy, C.E. (2010) Petrology, geochemistry and geochronology of Kaua ‘i Lavas over 4· 5 Myr: Implications for the origin of rejuvenated volcanism and the evolution of the Hawaiian plume. Journal of Petrology 51, 1507–1540.
; Peate et al., 2010Peate, D.W., Breddam, K., Baker, J.A., Kurz, M.D., Barker, A.K., Prestvik, T., Grassineau, N., Skovgaard, A.C. (2010) Compositional characteristics and spatial distribution of enriched Icelandic mantle components. Journal of Petrology 51, 1447–1475.
; Chekol et al., 2011Chekol, T.A., Kobayashi, K., Yokoyama, T., Sakaguchi, C., Nakamura, E. (2011) Timescales of magma differentiation from basalt to andesite beneath Hekla Volcano, Iceland: Constraints from U-series disequilibria in lavas from the last quarter-millennium flows. Geochimica et Cosmochimica Acta 75, 256–283.
; Salters et al., 2011Salters, V.J.M., Mallick, S., Hart, S.R., Langmuir, C.E., Stracke, A. (2011) Domains of depleted mantle: New evidence from hafnium and neodymium isotopes. Geochemistry Geophysics Geosystems 12, Q08001.
; Viccaro et al., 2011Viccaro, M., Nicotra, E., Millar, I.L., Cristofolini, R. (2011) The magma source at Mount Etna volcano: Perspectives from the Hf isotope composition of historic and recent lavas. Chemical Geology 281, 343–351.
); mantle components from Zindler and Hart, 1986Zindler, A., Hart, S. (1986) Chemical geodynamics. Annual Review of Earth and Planetary Sciences 14, 493–571.
; Salters and White, 1998Salters, V.J.M., White, W.M. (1998) Hf isotope constraints on mantle evolution. Chemical Geology 145, 447–460.
; Workman et al., 2004Workman, R.K., Hart, S.R., Jackson, M., Regelous, M., Farley, K.A., Blusztajn, J., Kurz, M., Staudigel, H. (2004) Recycled metasomatized lithosphere as the origin of the Enriched Mantle II (EM2) end-member: Evidence from the Samoan Volcanic Chain. Geochemistry Geophysics Geosystems 5, Q04008.
; Stracke et al., 2005Stracke, A., Hofmann, A.W., Hart, S.R. (2005) FOZO, HIMU, and the rest of the mantle zoo. Geochemistry Geophysics Geosystems 6, Q05007.
; Workman and Hart, 2005Workman, R.K., Hart, S.R. (2005) Major and trace element composition of the depleted MORB mantle (DMM). Earth and Planetary Science Letters 231, 53–72.
. Hafnium isotopic values for Italian sediments (Conticelli et al., 2002Conticelli, S., D'Antonio, M., Pinarelli, L., Civetta, L. (2002) Source contamination and mantle heterogeneity in the genesis of Italian potassic and ultrapotassic volcanic rocks: Sr‚ Nd‚ Pb isotope data from Roman Province and Southern Tuscany. Mineralogy and Petrology 74,189–222.
; Brems et al., 2013Brems, D., Ganio, M., Latruwe, K., Balcaen, L., Carremans, M., Gimeno, D., Silvestri, A., Vanhaecke, F., Muchez, P., Degryse, P. (2013) Isotopes on the beach, part 2: neodymium isotopic analysis for the provenancing of Roman glass-making. Archaeometry 55, 449–464.
) are calculated from Nd isotopic data and both cases following the seawater array (SA) and the terrestrial array (TA) of Vervoort et al. (2011)Vervoort, J.D., Plank, T., Prytulak, J. (2011) The Hf-Nd isotopic composition of marine sediments. Geochimica et Cosmochimica Acta 75, 5903–5926.
are shown. (b) 208Pb/204Pb vs. 206Pb/204Pb shown with OIB and mid-ocean ridge basalt (MORB) fields, historic Etna (Viccaro and Cristofolini, 2008Viccaro, M., Cristofolini, R. (2008) Nature of mantle heterogeneity and its role in the short-term geochemical and volcanological evolution of Mt. Etna (Italy). Lithos 105, 272–288.
) and Hyblean Plateau field from Trua et al. (1998)Trua, T., Esperança, S., Mazzuoli, R. (1998) The evolution of the lithospheric mantle along the N. African Plate: geochemical and isotopic evidence from the tholeiitic and alkaline volcanic rocks of the Hyblean plateau, Italy. Contributions to Mineralogy and Petrology 131, 307–322.
. Italian crustal values from Conticelli et al. (2002)Conticelli, S., D'Antonio, M., Pinarelli, L., Civetta, L. (2002) Source contamination and mantle heterogeneity in the genesis of Italian potassic and ultrapotassic volcanic rocks: Sr‚ Nd‚ Pb isotope data from Roman Province and Southern Tuscany. Mineralogy and Petrology 74,189–222.
. External reproducibility is conservatively set at 0.01 for 206Pb/204Pb and 0.02 for 208Pb/204Pb.Constraints on mantle mixing processes. In spite of barometric model uncertainties, sites of cpx crystallisation (shallow crust vs. lower crust/mantle) can be readily distinguished by the barometry and thus provide meaningful stratigraphic context for cpx isotopic values. The lack of significant disequilibrium can be explained by magma sources feeding Etna during the period of ancient alkaline eruptive activity being either broadly isotopically homogeneous or well mixed before eruption. The few reported WR-cpx pairs from 15–30 ka (Valle del Bove sequence; D'Orazio et al., 1997
D'Orazio, M., Tonarini, S., Innocenti, F., Pompilio, M. (1997) Northern Valle del Bove volcanic succession (Mt. Etna, Sicily): petrography, geochemistry and Sr-Nd isotope data. Acta Vulcanologica 9, 73–86.
) show corresponding Sr and Nd isotopic equilibria (isotopic differences <0.00002 and <0.00001, respectively) and results here extend this phenomenon back an additional 200 ka.The interpretation of limited mixing is further supported by the observed equilibrium in three of the TSC lavas between cpx and WR Pb isotopic signatures (Fig. 2b). Only one WR-cpx pair (TSC-7) exhibits isotopic disequilibrium in the Pb isotope system just outside the range of external reproducibility. Limited crustal storage time implied by Pb isotopic cpx-WR equilibria also bolsters trace element records of crystallisation from heterogeneously sourced magmas being largely preserved in this system. Trace element modelling of sources is particularly valuable in cases where source isotopic signatures are relatively well homogenised.
The restricted isotopic range of TSC cpx and WR values contrasts sharply with the variety of Pb isotopic signatures observed for plagioclase-rich and magnetic splits of a finer-grained 260 ka Etna tholeiite (SdV-1) reported by Bryce and DePaolo (2004)
Bryce, J.G., DePaolo, D.J. (2004) Pb isotopic heterogeneity in basaltic phenocrysts. Geochimica et Cosmochimica Acta 68, 4453–4468.
and olivine-hosted melt inclusions from recent (2002) eruptions (Rose-Koga et al., 2012Rose-Koga, E.F., Koga, K.T., Schiano, P., Le Voyer, M., Shimizu, N., Whitehouse, M.J., Clocchiatti, R. (2012) Mantle source heterogeneity for South Tyrrhenian magmas revealed by Pb isotopes and halogen contents of olivine-hosted melt inclusions. Chemical Geology 334, 266–279.
). Possible explanations include that these lavas may sample geographically different magma supplies or derive from magmas experiencing additional mixing immediately prior to eruption, as inferred from olivine in recent lavas (Kahl et al., 2011Kahl, M., Chakraborty, S., Costa, F., Pompilio, M. (2011) Dynamic plumbing system beneath volcanoes revealed by kinetic modeling, and the connection to monitoring data: An example from Mt. Etna. Earth and Planetary Science Letters 308, 11–22.
). Variable Pb isotopic compositions in olivine-hosted melt inclusions could signify that minute amounts of isotopically distinct melts are simply insufficiently abundant to change the “deep”, dominant isotopic signal locked into cpx.Lack of Hf-Nd-Pb isotopic disequilibria in ancient TSC lavas between cpx-WR pairs indicates that any mixing of isotopically distinct magmas supplying ancient Etna eruptions occurred at depths preceding cpx crystallisation. Melts then rose to the surface without significant assimilation in (and associated heat exchange with) shallow reservoirs.
top
Conclusions
Thermobarometrically controlled elemental and isotopic analyses of clinopyroxene provide a means to reconstruct ancient magma assembly processes at Mt. Etna. Single-crystal cpx barometry places most phenocryst crystallisation within the mid-crust and permits distinction between deep and shallow processes when coupled with trace element and isotopic data. In situ trace element data from cpx allow for the assessment of pyroxenite vs. peridotite contributions to Etna magmas. Chemical signatures apparent in these ancient lavas as well as in modern products suggest that hydrated peridotite has been an important component of the magma source region over the history of this volcano. The present dataset further supports the interpretation that observed isotopic systematics in ancient Etna lavas resulted from mixing between depleted and enriched mantle sources, with volatile-bearing peridotite and pyroxenite components preferentially melting to generate volatile-rich ancient alkaline volcanism. Hf-Nd-Pb isotopic equilibria between TSC WR and cpx are consistent with a model of an ancient Etna plumbing system wherein melts were homogenised below mid-crustal depths and then rapidly transported to the surface without substantial assimilation of crustal material at pressures lower than 0.5 GPa. More extensive combinations of bulk isotopic stratigraphy with mineral barometric and trace element modelling as applied here are expected to afford opportunities to reconstruct the longevity of magmatic plumbing systems and deconvolve distinctive magma source regions feeding Mt. Etna through time.
top
Acknowledgements
We thank Nilanjan Chatterjee, Julie Chouinard, Philippe Télouk, and Gerald Wynick for technical assistance, the Alfred University Center for Advanced Ceramic Technology (CACT), and Wendy Bohrson for comments on an earlier version of the manuscript. We appreciate thoughtful suggestions of Pietro Armienti, two anonymous reviewers, and the editorial handling by Bruce Watson, all of which improved the quality of our manuscript. Financial support from NSF grant EAR-1057611 to JGB and SAM, the UNH Undergraduate Research Opportunities Program to MM, and the French Agence Nationale de la Recherche (grant ANR-10-BLANC-0603 M&Ms – Mantle Melting – Measurements, Models, Mechanisms) to JBT is gratefully acknowledged.
Editor: Bruce Watson
top
References
Armienti, P., Tonarini, S., Innocenti, F., D'Orazio, M. (2007) Mount Etna pyroxene as tracer of petrogenetic processes and dynamics of the feeding system. In: Beccaluva, L., Bianchini, G., Wilson, M. (Eds.) Cenazoic volcanism in the Mediterranean Area. Geological Society of America Special Paper 418, 265–276.
 Show in context
Show in context Volcanism has been attributed to the manifestation of mantle upwelling independent of, or in response to, a slab tear (e.g., Gasperini et al., 2002), subduction-related fluid-triggered melting (e.g., Armienti et al., 2007 and references therein) or enhanced decompression melting resulting from convective anomalies (Gvirtzman and Nur, 1999; Schellart, 2010).
View in article
Most Etna mineral-WR pair isotopic work has focused on the Sr and Nd systems in recent lavas (e.g., Tonarini et al., 1995; Armienti et al., 2007), which generally exhibit more radiogenic Sr and less radiogenic Nd than ancient lavas.
View in article
Within recent eruptive episodes, marked increases in WR 87Sr/86Sr are often accompanied by 87Sr/86Sr WR-cpx disequilibria (e.g., 0.70348 cpx core values accompanied by 0.70362 WR values in 2001 eruptives; Armienti et al., 2007).
View in article
Armienti, P., Gasperini, D., Perinelli, C., Putirka, K.D. (2009) A new model for estimating deep-level magma ascent rates from thermobarometry: an example from Mt. Etna and implications for deep-seated magma dehydration. Acta Vulcanologica 21, 145–158.
 Show in context
Show in context Observed cpx phenocrysts (>2 mm, large relative to other TSC lava phases) coupled with theoretical modelling of Etna compositions indicate early cpx crystallisation; hence cpx holds a potential record of pre-eruptive magma assembly processes (Armienti et al. 2009).
View in article
Blichert-Toft, J., Albarède, F. (2009) Mixing of isotopic heterogeneities in the Mauna Kea plume conduit. Earth and Planetary Science Letters 282, 190–200.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Branca, S., Del Carlo, P. (2004) Eruptions of Mt. Etna during the past 3,200 Years: A revised compilation integrating the historical and stratigraphic records. In: Bonaccorso, A., Calvari, S., Coltelli, M., Del Negro, C., Falsaperla, S. (Eds.) Mt. Etna: Volcano Laboratory. American Geophysical Union, Washington, D.C., 1–27.
 Show in context
Show in context Tholeiitic lavas were overlain by transitional and alkaline sequences starting at ~230 ka (Gillot et al., 1994; Branca and Del Carlo, 2004).
View in article
Brems, D., Ganio, M., Latruwe, K., Balcaen, L., Carremans, M., Gimeno, D., Silvestri, A., Vanhaecke, F., Muchez, P., Degryse, P. (2013) Isotopes on the beach, part 2: neodymium isotopic analysis for the provenancing of Roman glass-making. Archaeometry 55, 449–464.
 Show in context
Show in contextThough Hf and Nd isotopic data for sedimentary units directly beneath Etna are unavailable for comparison with cpx and WR values, Sicilian beach sand εNd derived from the western extension of sedimentary units underlying Etna and crustal rocks of south and central Italy are all considerably more enriched (Fig. 2a; εNd -10.3 to -16.0, Conticelli et al., 2002; Brems et al., 2013).
View in article
Figure 2 [...] Hafnium isotopic values for Italian sediments (Conticelli et al., 2002; Brems et al., 2013) are calculated from Nd isotopic data and both cases following the seawater array (SA) and the terrestrial array (TA) of Vervoort et al. (2011) are shown.
View in article
Bryce, J.G., DePaolo, D.J. (2004) Pb isotopic heterogeneity in basaltic phenocrysts. Geochimica et Cosmochimica Acta 68, 4453–4468.
 Show in context
Show in context The restricted isotopic range of TSC cpx and WR values contrasts sharply with the variety of Pb isotopic signatures observed for plagioclase-rich and magnetic splits of a finer-grained 260 ka Etna tholeiite (SdV-1) reported by Bryce and DePaolo (2004) and olivine-hosted melt inclusions from recent (2002) eruptions (Rose-Koga et al., 2012).
View in article
Chekol, T.A., Kobayashi, K., Yokoyama, T., Sakaguchi, C., Nakamura, E. (2011) Timescales of magma differentiation from basalt to andesite beneath Hekla Volcano, Iceland: Constraints from U-series disequilibria in lavas from the last quarter-millennium flows. Geochimica et Cosmochimica Acta 75, 256–283.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Conticelli, S., D'Antonio, M., Pinarelli, L., Civetta, L. (2002) Source contamination and mantle heterogeneity in the genesis of Italian potassic and ultrapotassic volcanic rocks: Sr‚ Nd‚ Pb isotope data from Roman Province and Southern Tuscany. Mineralogy and Petrology 74,189–222.
 Show in context
Show in contextThough Hf and Nd isotopic data for sedimentary units directly beneath Etna are unavailable for comparison with cpx and WR values, Sicilian beach sand εNd derived from the western extension of sedimentary units underlying Etna and crustal rocks of south and central Italy are all considerably more enriched (Fig. 2a; εNd -10.3 to -16.0, Conticelli et al., 2002; Brems et al., 2013).
View in article
Figure 2 [...] Hafnium isotopic values for Italian sediments (Conticelli et al., 2002; Brems et al., 2013) are calculated from Nd isotopic data and both cases following the seawater array (SA) and the terrestrial array (TA) of Vervoort et al. (2011) are shown.
View in article
Figure 2 [...] Italian crustal values from Conticelli et al. (2002).
View in article
Correale, A., Martelli, M., Paonita, A., Rizzo, A., Brusca, L., Scribano, V. (2012) New evidence of mantle heterogeneity beneath the Hyblean Plateau (southeast Sicily, Italy) as inferred from noble gases and geochemistry of ultramafic xenoliths. Lithos 132–133, 70–81.
 Show in context
Show in context Figure 1 [...] (c) TSC cpx and 2001 eruption cpx (Viccaro et al., 2006) shown with Hyblean pyroxenite and peridotite cpx fields (Correale et al., 2012, and references therein).
View in article
Correale, A., Paonita, A., Martelli, M., Rizzo, A., Rotolo, S.G., Corsaro, R.A., Di Renzo, V. (2014) A two-component mantle source feeding Mt.Etna magmatism: Insights from the geochemistry of primitive magmas. Lithos 184–187, 243–258.
 Show in context
Show in contextThese lithologies, similar to those determined by Correale et al. (2014) modelling trace element systematics in primitive Etna WR samples <15 ka, are distinct from peridotitic cpx from the nearby Hyblean plateau that fall below the low-Y/La trend.
View in article
D'Orazio, M., Tonarini, S., Innocenti, F., Pompilio, M. (1997) Northern Valle del Bove volcanic succession (Mt. Etna, Sicily): petrography, geochemistry and Sr-Nd isotope data. Acta Vulcanologica 9, 73–86.
 Show in context
Show in context The few reported WR-cpx pairs from 15–30 ka (Valle del Bove sequence; D'Orazio et al., 1997) show corresponding Sr and Nd isotopic equilibria (isotopic differences <0.00002 and <0.00001, respectively) and results here extend this phenomenon back an additional 200 ka.
View in article
Gaffney, A.M., Nelson, B.K., Blichert-Toft, J. (2004) Geochemical constraints on the role of oceanic lithosphere in intra-volcano heterogeneity at West Maui, Hawaii. Journal of Petrology 45, 1663–1687.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Garcia, M.O., Swinnard, L., Weis, D., Greene, A.R., Tagami, T., Sano, H., Gandy, C.E. (2010) Petrology, geochemistry and geochronology of Kaua ‘i Lavas over 4· 5 Myr: Implications for the origin of rejuvenated volcanism and the evolution of the Hawaiian plume. Journal of Petrology 51, 1507–1540.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Gasperini, D., Blichert-Toft, J., Bosch, D., Del Moro, A., Macera, P., Albarède, F. (2002) Upwelling of deep mantle material through a plate window; evidence from the geochemistry of Italian basaltic volcanics. Journal of Geophysical Research 107, 2367.
 Show in context
Show in context Volcanism has been attributed to the manifestation of mantle upwelling independent of, or in response to, a slab tear (e.g., Gasperini et al., 2002), subduction-related fluid-triggered melting (e.g., Armienti et al., 2007 and references therein) or enhanced decompression melting resulting from convective anomalies (Gvirtzman and Nur, 1999; Schellart, 2010).
View in article
Gillot, P.Y., Kieffer, G., Romano, R. (1994) The evolution of Mount Etna in the light of potassium-argon dating. Acta Vulcanologica 5, 81–87.
 Show in context
Show in context Volcanism began at Mount Etna, Europe’s largest and most active volcano, at ~0.5 Ma (Gillot et al., 1994), with ancient lavas now exposed around the perimeter of the modern-day edifice.
View in article
Tholeiitic lavas were overlain by transitional and alkaline sequences starting at ~230 ka (Gillot et al., 1994; Branca and Del Carlo, 2004).
View in article
Lavas at TSC encompass the whole ancient alkaline magmatism period at Etna, from 220 ka near sea level to likely <100 ka exposed atop the sea cliff (Gillot et al., 1994).
View in article
Gvirtzman, Z., Nur, A. (1999) The formation of Mount Etna as the consequence of slab rollback. Nature 401, 782–785.
 Show in context
Show in context Volcanism has been attributed to the manifestation of mantle upwelling independent of, or in response to, a slab tear (e.g., Gasperini et al., 2002), subduction-related fluid-triggered melting (e.g., Armienti et al., 2007 and references therein) or enhanced decompression melting resulting from convective anomalies (Gvirtzman and Nur, 1999; Schellart, 2010).
View in article
Huang, S., Frey, F.A., Blichert-Toft, J., Fodor, R.V., Bauer, G.R., Xu, G. (2005) Enriched components in the Hawaiian plume: Evidence from Kahoolawe Volcano, Hawaii. Geochemistry Geophysics Geosystems 6, Q11006.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Kahl, M., Chakraborty, S., Costa, F., Pompilio, M. (2011) Dynamic plumbing system beneath volcanoes revealed by kinetic modeling, and the connection to monitoring data: An example from Mt. Etna. Earth and Planetary Science Letters 308, 11–22.
 Show in context
Show in context Possible explanations include that these lavas may sample geographically different magma supplies or derive from magmas experiencing additional mixing immediately prior to eruption, as inferred from olivine in recent lavas (Kahl et al., 2011).
View in article
Lassiter, J.C., Blichert-Toft, J., Hauri, E.H., Barsczus, H.G. (2003) Isotope and trace element variations in lavas from Raivavae and Rapa, Cook‚ Austral islands: constraints on the nature of HIMU- and EM-mantle and the origin of mid-plate volcanism in French Polynesia. Chemical Geology 202, 115–138.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Marty, B., Trull, T., Lussiez, P., Basile, I., Tanguy, J.-C. (1994) He, Ar, O, Sr and Nd isotope constraints on the origin and evolution of Mount Etna magmatism. Earth and Planetary Science Letters 126, 23–39.
 Show in context
Show in contextMagmatic products of the early Etna centres, including those of the ancient alkali centres active at ~200–100 ka, bear mantle-derived isotopic signatures consistent with contributions from both enriched and depleted source components (Marty et al., 1994; Tanguy et al., 1997).
View in article
Mollo, S., Del Gaudio, P., Ventura, G., Iezzi, G., Scarlato, P. (2010) Dependence of clinopyroxene composition on cooling rate in basaltic magmas: Implications for thermobarometry. Lithos 118, 302–312.
 Show in context
Show in contextAs noted by Mollo et al. (2010), single-cpx barometers can outperform liquid-based models for volatile-rich alkaline compositions.
View in article
Peate, D.W., Breddam, K., Baker, J.A., Kurz, M.D., Barker, A.K., Prestvik, T., Grassineau, N., Skovgaard, A.C. (2010) Compositional characteristics and spatial distribution of enriched Icelandic mantle components. Journal of Petrology 51, 1447–1475.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Prowatke, S., Klemme, S. (2006) Trace element partitioning between apatite and silicate melts. Geochimica et Cosmochimica Acta 70, 4513–4527.
 Show in context
Show in contextFigure 1 [...] Fractionation of apatite, well known to incorporate REEs, is modelled in purple using the partitioning of Prowatke and Klemme (2006).
View in article
Putirka, K.D. (2008) Thermometers and barometers for volcanic systems. Reviews in Mineralogy and Geochemistry 69, 61–120.
 Show in context
Show in contextCrystallisation temperatures and pressures, solved iteratively using a single-cpx thermometer and single-cpx barometer for hydrous systems (respectively, Eqs. 32d and 32b in Putirka, 2008), yielded temperatures of 1060–1175 °C and an average pressure of 0.34 ± 0.16 GPa (Fig. 1a,b).
View in article
Putirka, K.D., Mikaelian, H., Ryerson, F., Shaw, H. (2003) New clinopyroxene-liquid thermometers for mafic, evolved, and volatile-bearing lava compositions, with applications to lavas from Tibet and the Snake River Plain, Idaho. American Mineralogist 88, 1542–1554.
 Show in context
Show in contextThe single-cpx barometer for hydrous systems yields an average uncertainty of 0.17 versus 0.28 GPa for the cpx-liquid model of Putirka et al. (2003) for the compiled experiments, placing a lower bound of pressures recorded by TSC cpx at below 0.8 GPa, within the uppermost lithospheric mantle.
View in article
Rose-Koga, E.F., Koga, K.T., Schiano, P., Le Voyer, M., Shimizu, N., Whitehouse, M.J., Clocchiatti, R. (2012) Mantle source heterogeneity for South Tyrrhenian magmas revealed by Pb isotopes and halogen contents of olivine-hosted melt inclusions. Chemical Geology 334, 266–279.
 Show in context
Show in context The restricted isotopic range of TSC cpx and WR values contrasts sharply with the variety of Pb isotopic signatures observed for plagioclase-rich and magnetic splits of a finer-grained 260 ka Etna tholeiite (SdV-1) reported by Bryce and DePaolo (2004) and olivine-hosted melt inclusions from recent (2002) eruptions (Rose-Koga et al., 2012).
View in article
Salters, V.J.M., White, W.M. (1998) Hf isotope constraints on mantle evolution. Chemical Geology 145, 447–460.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Salters, V.J.M., Mallick, S., Hart, S.R., Langmuir, C.E., Stracke, A. (2011) Domains of depleted mantle: New evidence from hafnium and neodymium isotopes. Geochemistry Geophysics Geosystems 12, Q08001.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Scarlato, P., Mollo, S., Blundy, J.D., Iezzi, G., Tiepolo, M. (2014) The role of natural solidification paths on REE partitioning between clinopyroxene and melt. Bulletin of Volcanology 76, 810, doi: 10.1007/s00445-014-0810-1.
 Show in context
Show in context Scarlato et al. (2014) have documented preferential HREE incorporation into cpx relative to LREE as a function of cooling rate, but in TSC phenocrysts, HREE-like Y has either negative or no correlation with major element chemistry associated with elevated cooling rates (e.g., Na, AlIV, and Ti).
View in article
Schellart, W.P. (2010) Mount Etna–Iblean volcanism caused by rollback-induced upper mantle upwelling around the Ionian slab edge: An alternative to the plume model. Geology 38, 691–694.
 Show in context
Show in contextVolcanism has been attributed to the manifestation of mantle upwelling independent of, or in response to, a slab tear (e.g., Gasperini et al., 2002), subduction-related fluid-triggered melting (e.g., Armienti et al., 2007 and references therein) or enhanced decompression melting resulting from convective anomalies (Gvirtzman and Nur, 1999; Schellart, 2010).
View in article
Smith, P.M., Asimow, P.D. (2005) Adiabat_1ph: A new public front-end to the MELTS, pMELTS, and pHMELTS models. Geochemistry Geophysics Geosystems 6, Q02004.
 Show in context
Show in context Figure 1 [...] Isobaric cpx fractionation modelling for peridotite melt (solid lines) and pyroxenite melt (dashed lines) at 1.0 (black), 0.6 (grey), and 0.2 (blue) GPa performed using alphaMELTS (Smith and Asimow, 2005); conditions described in Supplementary Information.
View in article
Sims, K.W.W., Blichert-Toft, J., Kyle, P.R., Pichat, S., Gauthier, P.-J., Blusztajn, J., Kelly, P., Ball, L., Layne, G. (2008) A Sr, Nd, Hf, and Pb isotope perspective on the genesis and long-term evolution of alkaline magmas from Erebus volcano, Antarctica. Journal of Volcanology and Geothermal Research 177, 606–618.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Spilliaert, N., Allard, P., Métrich, N., Sobolev, A.V. (2006) Melt inclusion record of the conditions of ascent, degassing, and extrusion of volatile-rich alkali basalt during the powerful 2002 flank eruption of Mount Etna (Italy). Journal of Geophysical Research 111, B04203.
 Show in context
Show in context Figure 1 (a) Ce contents of TSC cpx as a function of single-cpx pressure estimates (1σ uncertainty) superimposed on Etna stratigraphy (after Spilliaert et al., 2006).
View in article
Stracke, A., Bizimis, M., Salters, V.J.M. (2003) Recycling oceanic crust: Quantitative constraints. Geochemistry Geophysics Geosystems 4, 8003.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Stracke, A., Hofmann, A.W., Hart, S.R. (2005) FOZO, HIMU, and the rest of the mantle zoo. Geochemistry Geophysics Geosystems 6, Q05007.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Tanguy, J.-C., Condomines, M., Kieffer, G. (1997) Evolution of the Mount Etna magma: Constraints on the present feeding system and eruptive mechanism. Journal of Volcanology and Geothermal Research 75, 221–250.
 Show in context
Show in context Magmatic products of the early Etna centres, including those of the ancient alkali centres active at ~200–100 ka, bear mantle-derived isotopic signatures consistent with contributions from both enriched and depleted source components (Marty et al., 1994; Tanguy et al., 1997).
View in article
Tonarini, S., Armienti, P., D'Orazio, M., Innocenti, F., Pompilio, M., Petrini, R. (1995) Geochemical and isotopic monitoring of Mt. Etna 1989-1993 eruptive activity: bearing on the shallow feeding system. Journal of Volcanology and Geothermal Research 64, 95–115.
 Show in context
Show in context Though more recent Etna volcanic products exhibit distinctive signs of assimilation in the form of elevated Sr isotopic values and large ion lithophile element enrichments (Tonarini et al., 1995), the degree to which crustal contamination influenced early alkaline products is uncertain.
View in article
Most Etna mineral-WR pair isotopic work has focused on the Sr and Nd systems in recent lavas (e.g., Tonarini et al., 1995; Armienti et al., 2007), which generally exhibit more radiogenic Sr and less radiogenic Nd than ancient lavas.
View in article
Trua, T., Esperança, S., Mazzuoli, R. (1998) The evolution of the lithospheric mantle along the N. African Plate: geochemical and isotopic evidence from the tholeiitic and alkaline volcanic rocks of the Hyblean plateau, Italy. Contributions to Mineralogy and Petrology 131, 307–322.
 Show in context
Show in context Figure 2 [...] (b) 208Pb/204Pb vs. 206Pb/204Pb shown with OIB and mid-ocean ridge basalt (MORB) fields, historic Etna (Viccaro and Cristofolini, 2008) and Hyblean Plateau field from Trua et al. (1998).
View in article
Van Orman, J.A., Grove, T.L., Shimizu, N. (2001) Rare earth element diffusion in diopside: influence of temperature, pressure, and ionic radius, and an elastic model for diffusion in silicates. Contributions to Mineralogy and Petrology 141, 687–703.
 Show in context
Show in context As refractory elements diffusing slowly in clinopyroxene (cf. Van Orman et al., 2001), Nd and Hf may be expected to retain isotopic signatures from early crystallisation depths and exhibit large isotopic disequilibria with hosting magmas subject to mixing with recharging, or assimilating magmas, carrying isotopically distinctive compositions immediately prior to eruption.
View in article
Vervoort, J.D., Plank, T., Prytulak, J. (2011) The Hf-Nd isotopic composition of marine sediments. Geochimica et Cosmochimica Acta 75, 5903–5926.
 Show in context
Show in context Figure 2 [...] Hafnium isotopic values for Italian sediments (Conticelli et al., 2002; Brems et al., 2013) are calculated from Nd isotopic data and both cases following the seawater array (SA) and the terrestrial array (TA) of Vervoort et al. (2011) are shown.
View in article
Viccaro, M., Cristofolini, R. (2008) Nature of mantle heterogeneity and its role in the short-term geochemical and volcanological evolution of Mt. Etna (Italy). Lithos 105, 272–288.
 Show in context
Show in context Figure 2 [...] (b) 208Pb/204Pb vs. 206Pb/204Pb shown with OIB and mid-ocean ridge basalt (MORB) fields, historic Etna (Viccaro and Cristofolini, 2008) and Hyblean Plateau field from Trua et al. (1998).
View in article
Viccaro, M., Ferlito, C., Cortesogno, L., Cristofolini, R., Gaggero, L. (2006) Magma mixing during the 2001 event at Mount Etna (Italy): effects on the eruptive dynamics. Journal of Volcanology and Geothermal Research 149, 139–159.
 Show in context
Show in context Figure 1 [...] (b) Proportions of ancient Etna barometry from this study and previous work (cf. Supplementary Information, n = 287).
View in article
Clinopyroxene from the 2001 eruption also follow the low-Y/La trend, as do other known historic and recent Etna cpx (Viccaro et al., 2006).
View in article
Viccaro, M., Nicotra, E., Millar, I.L., Cristofolini, R. (2011) The magma source at Mount Etna volcano: Perspectives from the Hf isotope composition of historic and recent lavas. Chemical Geology 281, 343–351.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Workman, R.K., Hart, S.R., Jackson, M., Regelous, M., Farley, K.A., Blusztajn, J., Kurz, M., Staudigel, H. (2004) Recycled metasomatized lithosphere as the origin of the Enriched Mantle II (EM2) end-member: Evidence from the Samoan Volcanic Chain. Geochemistry Geophysics Geosystems 5, Q04008.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Workman, R.K., Hart, S.R. (2005) Major and trace element composition of the depleted MORB mantle (DMM). Earth and Planetary Science Letters 231, 53–72.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Xu, G., Frey, F.A., Clague, D.A., Abouchami, W., Blichert-Toft, J., Cousens, B., Weisler, M. (2007) Geochemical characteristics of West Molokai shield-and postshield-stage lavas: Constraints on Hawaiian plume models. Geochemistry Geophysics Geosystems 8, Q08G21.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Yamasaki, S., Kani, T., Hanan, B.B., Tagami, T. (2009) Isotopic geochemistry of Hualalai shield-stage tholeiitic basalts from submarine North Kona region, Hawaii. Journal of Volcanology and Geothermal Research 185, 223–230.
 Show in context
Show in context Figure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
Zindler, A., Hart, S. (1986) Chemical geodynamics. Annual Review of Earth and Planetary Sciences 14, 493–571.
 Show in context
Show in contextFigure 2 [...] εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003; Stracke et al., 2003; Gaffney et al., 2004; Huang et al., 2005; Xu et al., 2007; Sims et al., 2008; Blichert-Toft and Albarède, 2009; Yamasaki et al., 2009; Garcia et al., 2010; Peate et al., 2010; Chekol et al., 2011; Salters et al., 2011; Viccaro et al., 2011); mantle components from Zindler and Hart, 1986; Salters and White, 1998; Workman et al., 2004; Stracke et al., 2005; Workman and Hart, 2005.
View in article
top
Supplementary Information
Geologic Setting, Analytical Details, and Isotopic Measurements

Figure S-1 Location of Timpe Santa Caterina outcrop on base map from GeoMapApp (http://www.geomapapp.org; Ryan et al., 2009). Major geologic features from Rosenbaum and Lister (2004). Stratigraphy based on Corsaro et al. (2002) with section base at sea level (0 m). Dates (*) from Gillot et al. (1994).
Timpe Santa Caterina clinopyroxene phenocrysts (typically at least 1 mm, shortest dimension) were handpicked, mounted in epoxy, and polished to 0.3 µm. Trace element concentrations were collected by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) using a Nu AttoM high resolution spectrometer and New Wave UP213 (213 nm) deep-UV YAG laser ablation system at the University of New Hampshire with a ~40 μm diameter laser spot size. Samples were lightly polished to remove any sputtered debris and major element compositions near the laser pits were then analysed by electron microprobe at the University of Oregon. Major and trace element data, additional analytical details, and uncertainties are reported below (Tables S-1, S-2, and S-3). Additional cpx major element data (Table S-4) not associated with LA-ICP-MS measurements but used for thermobarometry were collected at the Massachusetts Institute of Technology (MIT).

Figure S-2 Back scattered electron images of representative clinopyroxene grains from TSC lavas with laser ablation spots (Alfred University electron microprobe).
Table S-1 Electron microprobe analyses of TSC* clinopyroxene LA-ICP-MS laser spots. All iron reported as FeO. Operating conditions used at the University of Oregon (UO) and Massachusetts Institute of Technology (MIT) facilities were 15 keV accelerating voltage and 10 nA beam current, with all analyses using a focused beam of ~1 microns and 30 s count times. Data were reduced using the CITZAF correction procedure of Armstrong (1995). The few totals lower than 98 wt. % have been omitted. MIT JEOL JXA-8200 electron microprobe uncertainties (1σ) are calculated from the standard deviation of replicate analyses of the DJ35 diopside-jadeite glass standard and several points inferred from back scattered electron imaging to be from the same clinopyroxene crystal growth zone.
Analyses (wt. %) near laser spots TSC2_G1_3, TSC_G3_1, TSC2_G4_2, TSC7_G2_2, and TSC9_G5_2 totalled <98 wt. %. CaO abundances of the nearest same-grain spot were used to calibrate trace element concentrations (from TSC2_G1_2, TSC2_G3_2, TSC2_G4_1, TSC7_G2_1, and TSC9_G5_2, respectively).
| TSC2_G1_1 | 1 σ | TSC2_G1_2 | 1 σ | TSC2_G1_4 | 1 σ | TSC2_G3_2 | 1 σ | TSC2_G3_3 | 1 σ | |||||
| SiO2 | 46.19 | 0.10 | 46.07 | 0.10 | 47.40 | 0.15 | 49.74 | 0.10 | 49.77 | 0.10 | ||||
| TiO2 | 1.78 | 0.03 | 1.96 | 0.03 | 2.02 | 0.01 | 0.95 | 0.02 | 0.94 | 0.02 | ||||
| Al2O3 | 7.08 | 0.04 | 7.46 | 0.04 | 7.67 | 0.03 | 3.78 | 0.03 | 4.06 | 0.03 | ||||
| FeO | 8.70 | 0.16 | 9.22 | 0.16 | 8.99 | 0.04 | 8.23 | 0.15 | 7.85 | 0.15 | ||||
| MnO | 0.13 | 0.01 | 0.15 | 0.01 | 0.17 | 0.01 | 0.17 | 0.01 | 0.18 | 0.01 | ||||
| MgO | 12.76 | 0.05 | 12.53 | 0.05 | 11.96 | 0.05 | 14.71 | 0.06 | 14.43 | 0.06 | ||||
| CaO | 21.19 | 0.07 | 20.93 | 0.07 | 20.87 | 0.15 | 20.98 | 0.07 | 21.32 | 0.07 | ||||
| Na2O | 0.59 | 0.03 | 0.57 | 0.03 | 0.49 | 0.02 | 0.54 | 0.03 | 0.45 | 0.03 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.02 | 0.03 | 0.04 | 0.01 | 0.04 | 0.03 | 0.00 | 0.03 | ||||
| TOTAL | 98.43 | 98.91 | 99.62 | 99.14 | 98.99 | |||||||||
| TSC2_G3_4 | 1 σ | TSC2_G3_5 | 1 σ | TSC2_G3_6 | 1 σ | TSC2_G4_1 | 1 σ | TSC2_G2_1 | 1 σ | |||||
| SiO2 | 48.41 | 0.10 | 48.81 | 0.10 | 48.21 | 0.10 | 48.03 | 0.10 | 47.13 | 0.10 | ||||
| TiO2 | 1.30 | 0.02 | 1.47 | 0.02 | 2.02 | 0.03 | 1.40 | 0.02 | 1.64 | 0.02 | ||||
| Al2O3 | 5.50 | 0.04 | 4.87 | 0.03 | 5.81 | 0.04 | 6.99 | 0.04 | 6.38 | 0.04 | ||||
| FeO | 8.65 | 0.16 | 7.79 | 0.15 | 8.33 | 0.15 | 7.06 | 0.14 | 8.62 | 0.16 | ||||
| MnO | 0.14 | 0.01 | 0.16 | 0.01 | 0.17 | 0.01 | 0.06 | 0.01 | 0.14 | 0.01 | ||||
| MgO | 13.58 | 0.06 | 13.80 | 0.06 | 13.07 | 0.05 | 13.40 | 0.06 | 13.00 | 0.05 | ||||
| CaO | 21.03 | 0.07 | 21.16 | 0.07 | 21.19 | 0.07 | 21.99 | 0.07 | 21.36 | 0.07 | ||||
| Na2O | 0.56 | 0.03 | 0.58 | 0.03 | 0.52 | 0.03 | 0.40 | 0.03 | 0.74 | 0.04 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.01 | 0.03 | 0.00 | 0.00 | 0.14 | 0.03 | 0.01 | 0.03 | ||||
| TOTAL | 99.18 | 98.65 | 99.31 | 99.49 | 99.02 | |||||||||
| TSC2_G2_2 | 1 σ | TSC2_G2_3 | 1 σ | TSC2_G8_1 | 1 σ | TSC2_G8_2 | 1 σ | TSC7_G2_1 | 1 σ | |||||
| SiO2 | 47.51 | 0.10 | 48.04 | 0.10 | 47.34 | 0.10 | 47.47 | 0.10 | 47.37 | 0.10 | ||||
| TiO2 | 1.42 | 0.02 | 1.47 | 0.02 | 1.63 | 0.02 | 1.68 | 0.03 | 1.86 | 0.03 | ||||
| Al2O3 | 6.65 | 0.04 | 6.73 | 0.04 | 6.42 | 0.04 | 6.37 | 0.04 | 7.16 | 0.04 | ||||
| FeO | 8.23 | 0.15 | 8.11 | 0.15 | 8.62 | 0.16 | 8.33 | 0.15 | 7.83 | 0.15 | ||||
| MnO | 0.12 | 0.01 | 0.13 | 0.01 | 0.15 | 0.01 | 0.14 | 0.01 | 0.12 | 0.01 | ||||
| MgO | 13.18 | 0.06 | 13.20 | 0.06 | 12.61 | 0.05 | 12.47 | 0.05 | 12.83 | 0.05 | ||||
| CaO | 21.50 | 0.07 | 21.19 | 0.07 | 21.16 | 0.07 | 21.08 | 0.07 | 21.71 | 0.07 | ||||
| Na2O | 0.58 | 0.03 | 0.59 | 0.03 | 0.56 | 0.03 | 0.62 | 0.03 | 0.55 | 0.03 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.02 | 0.03 | 0.01 | 0.03 | 0.02 | 0.03 | 0.00 | 0.03 | 0.01 | 0.03 | ||||
| TOTAL | 99.22 | 99.47 | 98.51 | 98.15 | 99.43 | |||||||||
| TSC7_G5_1 | 1 σ | TSC7_G7_1 | 1 σ | TSC7_G9_1 | 1 σ | TSC7_G10_1 | 1 σ | TSC7_G10_2 | 1 σ | |||||
| SiO2 | 46.86 | 0.10 | 48.29 | 0.10 | 46.40 | 0.10 | 48.66 | 0.10 | 48.80 | 0.10 | ||||
| TiO2 | 1.87 | 0.03 | 1.73 | 0.03 | 1.75 | 0.03 | 1.78 | 0.03 | 1.17 | 0.02 | ||||
| Al2O3 | 6.31 | 0.04 | 5.37 | 0.04 | 7.34 | 0.04 | 4.81 | 0.03 | 5.54 | 0.04 | ||||
| FeO | 7.83 | 0.15 | 7.95 | 0.15 | 6.65 | 0.14 | 7.84 | 0.15 | 5.69 | 0.13 | ||||
| MnO | 0.13 | 0.01 | 0.14 | 0.01 | 0.10 | 0.01 | 0.14 | 0.01 | 0.07 | 0.01 | ||||
| MgO | 12.90 | 0.05 | 13.29 | 0.06 | 13.14 | 0.05 | 13.24 | 0.06 | 14.41 | 0.06 | ||||
| CaO | 22.19 | 0.07 | 21.70 | 0.07 | 22.78 | 0.07 | 21.89 | 0.07 | 23.04 | 0.07 | ||||
| Na2O | 0.53 | 0.03 | 0.56 | 0.03 | 0.43 | 0.03 | 0.46 | 0.03 | 0.34 | 0.03 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | 0.04 | 0.03 | 0.13 | 0.03 | ||||
| TOTAL | 98.63 | 99.04 | 98.59 | 98.85 | 99.18 | |||||||||
| TSC7_G10_3 | 1 σ | TSC7_G10_4 | 1 σ | TSC7_G10_5 | 1 σ | TSC7_G10_6 | 1 σ | TSC3_G1_1 | 1 σ | |||||
| SiO2 | 48.24 | 0.10 | 49.07 | 0.10 | 49.50 | 0.10 | 49.48 | 0.10 | 48.68 | 0.10 | ||||
| TiO2 | 1.19 | 0.02 | 0.99 | 0.02 | 0.94 | 0.02 | 1.34 | 0.02 | 1.56 | 0.02 | ||||
| Al2O3 | 5.40 | 0.04 | 5.05 | 0.03 | 4.82 | 0.03 | 3.95 | 0.03 | 4.89 | 0.03 | ||||
| FeO | 5.55 | 0.13 | 5.38 | 0.12 | 5.42 | 0.13 | 7.90 | 0.15 | 7.47 | 0.15 | ||||
| MnO | 0.07 | 0.01 | 0.07 | 0.01 | 0.06 | 0.01 | 0.15 | 0.01 | 0.16 | 0.01 | ||||
| MgO | 14.20 | 0.06 | 14.54 | 0.06 | 14.96 | 0.06 | 14.11 | 0.06 | 13.43 | 0.06 | ||||
| CaO | 22.91 | 0.07 | 22.77 | 0.07 | 22.78 | 0.07 | 22.15 | 0.07 | 21.92 | 0.07 | ||||
| Na2O | 0.33 | 0.03 | 0.38 | 0.03 | 0.36 | 0.03 | 0.39 | 0.03 | 0.46 | 0.03 | ||||
| K2O | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.10 | 0.03 | 0.25 | 0.04 | 0.27 | 0.04 | 0.06 | 0.03 | 0.02 | 0.03 | ||||
| TOTAL | 97.99 | 98.50 | 99.12 | 99.52 | 98.59 | |||||||||
| TSC3_G1_2 | 1 σ | TSC3_G1_3 | 1 σ | TSC3_G3_1 | 1 σ | TSC3_G3_2 | 1 σ | TSC3_G3_3 | 1 σ | |||||
| SiO2 | 45.12 | 0.10 | 46.57 | 0.10 | 49.08 | 0.10 | 48.10 | 0.10 | 49.31 | 0.10 | ||||
| TiO2 | 2.51 | 0.03 | 2.19 | 0.03 | 1.52 | 0.02 | 1.56 | 0.02 | 1.41 | 0.02 | ||||
| Al2O3 | 7.87 | 0.04 | 6.86 | 0.04 | 5.45 | 0.04 | 5.15 | 0.03 | 4.26 | 0.03 | ||||
| FeO | 8.34 | 0.15 | 8.19 | 0.15 | 8.53 | 0.16 | 7.71 | 0.15 | 7.61 | 0.15 | ||||
| MnO | 0.14 | 0.01 | 0.15 | 0.01 | 0.19 | 0.01 | 0.17 | 0.01 | 0.17 | 0.01 | ||||
| MgO | 11.79 | 0.05 | 12.35 | 0.05 | 13.37 | 0.06 | 13.58 | 0.06 | 14.03 | 0.06 | ||||
| CaO | 22.01 | 0.07 | 22.16 | 0.07 | 21.03 | 0.07 | 22.24 | 0.07 | 22.18 | 0.07 | ||||
| Na2O | 0.45 | 0.03 | 0.51 | 0.03 | 0.64 | 0.03 | 0.54 | 0.03 | 0.48 | 0.03 | ||||
| K2O | 0.02 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.01 | 0.03 | 0.02 | 0.03 | 0.01 | 0.03 | 0.00 | 0.03 | ||||
| TOTAL | 98.26 | 98.99 | 99.85 | 99.05 | 99.47 | |||||||||
| TSC3_G9_1 | 1 σ | TSC3_G9_3 | 1 σ | TSC3_G9_4 | 1 σ | TSC9_G1_1 | 1 σ | TSC9_G1_2 | 1 σ | |||||
| SiO2 | 49.35 | 0.10 | 49.34 | 0.10 | 47.90 | 0.10 | 48.32 | 0.10 | 47.92 | 0.10 | ||||
| TiO2 | 1.59 | 0.02 | 1.32 | 0.02 | 1.95 | 0.03 | 1.25 | 0.02 | 1.69 | 0.03 | ||||
| Al2O3 | 5.20 | 0.04 | 4.43 | 0.03 | 6.23 | 0.04 | 6.29 | 0.04 | 5.82 | 0.04 | ||||
| FeO | 8.36 | 0.15 | 7.61 | 0.15 | 8.18 | 0.15 | 8.43 | 0.15 | 7.21 | 0.14 | ||||
| MnO | 0.16 | 0.01 | 0.15 | 0.01 | 0.15 | 0.01 | 0.14 | 0.01 | 0.11 | 0.01 | ||||
| MgO | 13.43 | 0.06 | 14.10 | 0.06 | 12.90 | 0.05 | 13.00 | 0.05 | 13.25 | 0.06 | ||||
| CaO | 22.26 | 0.07 | 22.56 | 0.07 | 21.80 | 0.07 | 22.06 | 0.07 | 22.00 | 0.07 | ||||
| Na2O | 0.57 | 0.03 | 0.37 | 0.03 | 0.48 | 0.03 | 0.43 | 0.03 | 0.44 | 0.03 | ||||
| K2O | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| Cr2O3 | 0.05 | 0.03 | 0.00 | 0.03 | 0.02 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | ||||
| TOTAL | 100.96 | 99.87 | 99.62 | 99.93 | 98.45 | |||||||||
| TSC9_G3_1 | 1 σ | TSC9_G3_2 | 1 σ | TSC9_G5_1 | 1 σ | |||||||||
| SiO2 | 47.39 | 0.10 | 48.74 | 0.10 | 47.55 | 0.10 | ||||||||
| TiO2 | 1.97 | 0.03 | 1.63 | 0.02 | 1.63 | 0.03 | ||||||||
| Al2O3 | 5.61 | 0.04 | 5.29 | 0.04 | 4.87 | 0.03 | ||||||||
| FeO | 7.52 | 0.15 | 7.27 | 0.14 | 7.66 | 0.15 | ||||||||
| MnO | 0.14 | 0.01 | 0.13 | 0.01 | 0.17 | 0.01 | ||||||||
| MgO | 13.19 | 0.06 | 13.60 | 0.06 | 13.44 | 0.06 | ||||||||
| CaO | 22.33 | 0.07 | 22.16 | 0.07 | 22.48 | 0.07 | ||||||||
| Na2O | 0.56 | 0.03 | 0.53 | 0.03 | 0.46 | 0.03 | ||||||||
| K2O | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | ||||||||
| Cr2O3 | 0.01 | 0.03 | 0.00 | 0.03 | 0.04 | 0.03 | ||||||||
| TOTAL | 98.74 | 99.36 | 98.31 | |||||||||||
* TSC samples were collected from a cliff below Via Pianetto at 37°36’21” N and 15°10’20” E from the base at sea level to the top, approximately 85 m above, as shown in the cross section of Figure S-1.
Download in ExcelTable S-2 Trace element data (ppm) collected by LA-ICP-MS at the University of New Hampshire.
| TSC2_G1_1 | 1 s.e. | TSC2_G1_2 | 1 s.e. | TSC2_G1_3 | 1 s.e. | TSC2_G1_4 | 1 s.e. | TSC2_G3_1 | 1 s.e. | |||||
| Li | 50 | 7 | 103 | 14 | 55 | 8 | 60 | 8 | 54 | 7 | ||||
| Sc | 105 | 3 | 91 | 3 | 97 | 3 | 97 | 3 | 98 | 3 | ||||
| Ti | 10307 | 233 | 8896 | 154 | 9244 | 187 | 10272 | 294 | 5911 | 98 | ||||
| V | 317 | 13 | 257 | 11 | 318 | 14 | 327 | 15 | 224 | 10 | ||||
| Cr | 66 | 3 | 59 | 2 | 60 | 3 | 51 | 3 | 24 | 2 | ||||
| Ni | 68 | 7 | 58 | 6 | 62 | 6 | 38 | 3 | 51 | 4 | ||||
| Sr | 132 | 7 | 124 | 6 | 116 | 6 | 128 | 8 | 132 | 7 | ||||
| Y | 54 | 1 | 45 | 1 | 42 | 1 | 46 | 2 | 38 | 1 | ||||
| Zr | 128 | 4 | 107 | 4 | 99 | 4 | 114 | 6 | 75 | 3 | ||||
| Nb | 0.9 | 0.3 | 1.6 | 0.2 | 0.89 | 0.2 | 1.0 | 0.2 | 0.6 | 0.1 | ||||
| Sn | 2.3 | 0.3 | 1.0 | 0.3 | 1.8 | 0.2 | 4.2 | 0.3 | 1.6 | 0.4 | ||||
| La | 15 | 1 | 12.0 | 0.9 | 11.2 | 0.7 | 14 | 1 | 11.0 | 0.8 | ||||
| Ce | 48 | 4 | 42 | 3 | 37 | 3 | 48 | 4 | 39 | 3 | ||||
| Pr | 9.3 | 0.9 | 7.9 | 0.8 | 7.2 | 0.7 | 9 | 1 | 7.1 | 0.7 | ||||
| Nd | 49 | 4 | 43 | 3 | 38 | 3 | 45 | 3 | 38 | 3 | ||||
| Sm | 14.0 | 0.7 | 12 | 1 | 11.0 | 0.5 | 12.2 | 0.7 | 9.6 | 0.4 | ||||
| Eu | 4.5 | 0.5 | 4.0 | 0.4 | 3.3 | 0.3 | 4.6 | 0.4 | 3.5 | 0.3 | ||||
| Gd | 15 | 2 | 15 | 1 | 12 | 1 | 13 | 2 | 10.0 | 0.8 | ||||
| Tb | 2.3 | 0.2 | 2.2 | 0.2 | 1.5 | 0.1 | 1.8 | 0.2 | 1.8 | 0.1 | ||||
| Dy | 14.0 | 0.5 | 10 | 1 | 9.7 | 0.6 | 12 | 1 | 10.4 | 0.5 | ||||
| Ho | 2.5 | 0.1 | 2.25 | 0.2 | 2.0 | 0.1 | 1.9 | 0.1 | 1.9 | 0.1 | ||||
| Er | 7.7 | 0.7 | 5.1 | 0.6 | 5.0 | 0.5 | 5.4 | 0.7 | 4.4 | 0.6 | ||||
| Tm | 0.88 | 0.07 | 0.62 | 0.09 | 0.72 | 0.02 | 0.5 | 0.1 | 0.60 | 0.02 | ||||
| Yb | 3.6 | 0.4 | 3.9 | 0.3 | 3.9 | 0.3 | 3.2 | 0.5 | 2.6 | 0.3 | ||||
| Lu | 0.49 | 0.07 | 0.6 | 0.1 | 0.45 | 0.09 | 0.5 | 0.2 | 0.4 | 0.1 | ||||
| Hf | 8.2 | 0.8 | 7.4 | 0.5 | 5.0 | 0.6 | 8 | 1 | 4.7 | 0.5 | ||||
| Pb | 0.14 | 0.03 | 0.17 | 0.03 | 0.19 | 0.03 | 0.4 | 0.3 | 0.21 | 0.05 | ||||
| Th | 0.29 | 0.03 | 0.20 | 0.03 | 0.19 | 0.03 | 0.25 | 0.03 | 0.13 | 0.02 | ||||
| U | 0.025 | 0.009 | 0.029 | 0.008 | 0.038 | 0.010 | 0.002 | 0.009 | 0.008 | 0.008 | ||||
| TSC2_G3_2 | 1 s.e. | TSC2_G3_3 | 1 s.e. | TSC2_G3_4 | 1 s.e. | TSC2_G3_5 | 1 s.e. | TSC2_G3_6 | 1 s.e. | |||||
| Li | 14 | 2 | 5.4 | 0.7 | 14 | 2 | 9 | 1 | 16 | 2 | ||||
| Sc | 113 | 3 | 106 | 3 | 96 | 8 | 90 | 2 | 89 | 3 | ||||
| Ti | 4888 | 85 | 4772 | 102 | 5551 | 390 | 6382 | 173 | 6255 | 125 | ||||
| V | 218 | 9 | 234 | 10 | 249 | 19 | 273 | 14 | 269 | 11 | ||||
| Cr | 24 | 1 | 30 | 1 | 32 | 3 | 46 | 2 | 42 | 2 | ||||
| Ni | 41 | 3 | 37 | 4 | 38 | 3 | 42 | 3 | 39 | 3 | ||||
| Sr | 84 | 4 | 77 | 4 | 74 | 6 | 82 | 4 | 86 | 4 | ||||
| Y | 21.6 | 0.6 | 20.2 | 0.6 | 18 | 2 | 20.4 | 0.7 | 20 | 0.6 | ||||
| Zr | 47 | 2 | 40 | 2 | 38 | 2 | 44 | 2 | 44 | 2 | ||||
| Nb | 0.36 | 0.06 | 0.41 | 0.08 | 0.29 | 0.08 | 0.59 | 0.09 | 0.8 | 0.1 | ||||
| Sn | 0.5 | 0.1 | 0.5 | 0.2 | 0.7 | 0.2 | 0.7 | 0.2 | 0.7 | 0.1 | ||||
| La | 5.1 | 0.4 | 4.3 | 0.3 | 4.2 | 0.4 | 4.6 | 0.3 | 5.1 | 0.3 | ||||
| Ce | 18 | 1 | 16 | 1 | 14 | 2 | 18 | 1 | 18 | 1 | ||||
| Pr | 3.6 | 0.4 | 3.3 | 0.3 | 3 | 0.4 | 3.3 | 0.3 | 3.7 | 0.4 | ||||
| Nd | 21 | 2 | 16 | 2 | 16 | 1 | 16 | 1 | 20 | 1 | ||||
| Sm | 5.1 | 0.3 | 4.3 | 0.2 | 4.2 | 0.7 | 3.9 | 0.1 | 3.8 | 0.2 | ||||
| Eu | 1.4 | 0.2 | 1.4 | 0.1 | 1.2 | 0.1 | 1.2 | 0.1 | 1.4 | 0.2 | ||||
| Gd | 6.7 | 0.8 | 4.8 | 0.4 | 4 | 0.8 | 4.9 | 0.4 | 5.8 | 0.5 | ||||
| Tb | 1.1 | 0.1 | 0.9 | 0.1 | 1 | 0.1 | 0.96 | 0.06 | 1 | 0.1 | ||||
| Dy | 4.9 | 0.2 | 4.4 | 0.2 | 3.4 | 0.6 | 3.9 | 0.3 | 4.7 | 0.4 | ||||
| Ho | 0.74 | 0.06 | 0.69 | 0.07 | 0.63 | 0.02 | 0.75 | 0.05 | 0.59 | 0.03 | ||||
| Er | 2.3 | 0.3 | 1.9 | 0.2 | 1.6 | 0.4 | 1.6 | 0.2 | 1.9 | 0.2 | ||||
| Tm | 0.29 | 0.05 | 0.28 | 0.02 | 0.28 | 0.06 | 0.34 | 0.05 | 0.33 | 0.04 | ||||
| Yb | 1.8 | 0.1 | 1.4 | 0.2 | 1.8 | 0.2 | 1.1 | 0.2 | 1 | 0.2 | ||||
| Lu | 0.22 | 0.05 | 0.23 | 0.04 | 0.19 | 0.03 | 0.19 | 0.03 | 0.22 | 0.03 | ||||
| Hf | 1.6 | 0.2 | 1.4 | 0.1 | 1.4 | 0.2 | 1.4 | 0.2 | 1.4 | 0.2 | ||||
| Pb | 0.068 | 0.008 | 0.13 | 0.03 | 0.08 | 0.02 | 0.13 | 0.02 | 0.14 | 0.04 | ||||
| Th | 0.050 | 0.008 | 0.045 | 0.006 | 0.05 | 0.01 | 0.05 | 0.01 | 0.11 | 0.01 | ||||
| U | 0.007 | 0.003 | 0.007 | 0.002 | 0.013 | 0.002 | 0.009 | 0.002 | 0.016 | 0.002 | ||||
| TSC2_G4_1 | 1 s.e. | TSC2_G4_2 | 1 s.e. | TSC2_G2_1 | 1 s.e. | TSC2_G2_2 | 1 s.e. | TSC2_G2_3 | 1 s.e. | |||||
| Li | 53 | 7 | 59 | 8 | 18 | 3 | 46 | 6 | 28 | 4 | ||||
| Sc | 131 | 4 | 129 | 5 | 117 | 3 | 105 | 3 | 139 | 6 | ||||
| Ti | 6293 | 115 | 6055 | 128 | 8882 | 153 | 6910 | 140 | 8867 | 314 | ||||
| V | 275 | 11 | 279 | 12 | 314 | 14 | 288 | 12 | 332 | 17 | ||||
| Cr | 642 | 23 | 640 | 27 | 42 | 3 | 54 | 3 | 44 | 3 | ||||
| Ni | 106 | 7 | 107 | 7 | 52 | 4 | 68 | 4 | 39 | 3 | ||||
| Sr | 99 | 5 | 84 | 4 | 112 | 6 | 95 | 5 | 89 | 5 | ||||
| Y | 18 | 1 | 17 | 0.6 | 41 | 1 | 36 | 2 | 38 | 2 | ||||
| Zr | 34 | 2 | 30 | 1 | 84 | 3 | 102 | 7 | 81 | 4 | ||||
| Nb | 0.42 | 0.08 | 0.1 | 0.1 | 0.6 | 0.1 | 4.7 | 0.5 | 1.1 | 0.2 | ||||
| Sn | 0.5 | 0.2 | 0.5 | 0.2 | 1.6 | 0.2 | 0.8 | 0.2 | 1.2 | 0.3 | ||||
| La | 4.1 | 0.3 | 3.2 | 0.2 | 11.4 | 0.7 | 22 | 2 | 10.1 | 0.8 | ||||
| Ce | 13 | 1 | 10.9 | 0.9 | 35 | 3 | 53 | 5 | 31 | 3 | ||||
| Pr | 2.5 | 0.3 | 2.2 | 0.3 | 6.6 | 0.7 | 8 | 1 | 6 | 0.6 | ||||
| Nd | 13 | 1 | 12 | 1 | 38 | 3 | 37 | 4 | 32 | 2 | ||||
| Sm | 3.6 | 0.5 | 3.1 | 0.2 | 10.4 | 0.9 | 8.8 | 0.8 | 9.5 | 0.2 | ||||
| Eu | 1.5 | 0.2 | 1 | 0.1 | 3.1 | 0.3 | 2.4 | 0.3 | 2.7 | 0.3 | ||||
| Gd | 5 | 0.7 | 5.3 | 0.8 | 13 | 1 | 10 | 1 | 10 | 1 | ||||
| Tb | 0.7 | 0.1 | 0.56 | 0.09 | 1.6 | 0.1 | 1.4 | 0.1 | 1.3 | 0.1 | ||||
| Dy | 3.3 | 0.1 | 4 | 0.4 | 8.2 | 0.6 | 7.9 | 0.3 | 7.5 | 0.4 | ||||
| Ho | 1 | 0.04 | 0.61 | 0.03 | 1.5 | 0.1 | 1.4 | 0.1 | 1.4 | 0.1 | ||||
| Er | 1.1 | 0.3 | 1.7 | 0.3 | 4.8 | 0.4 | 4.3 | 0.5 | 3.3 | 0.4 | ||||
| Tm | 0.27 | 0.07 | 0.23 | 0.07 | 0.55 | 0.06 | 0.6 | 0.1 | 0.34 | 0.04 | ||||
| Yb | 1.3 | 0.2 | 0.6 | 0.3 | 3.5 | 0.2 | 3.1 | 0.3 | 2.4 | 0.4 | ||||
| Lu | 0.32 | 0.04 | 0.21 | 0.09 | 0.54 | 0.09 | 0.7 | 0.1 | 0.35 | 0.06 | ||||
| Hf | 1.1 | 0.2 | 1.3 | 0.1 | 4.2 | 0.4 | 3.3 | 0.5 | 3.4 | 0.3 | ||||
| Pb | 0.03 | 0.02 | 0.09 | 0.03 | 0.08 | 0.02 | 1.4 | 0.19 | 0.11 | 0.04 | ||||
| Th | 0.062 | 0.005 | 0.04 | 0.01 | 0.2 | 0.01 | 1.8 | 0.2 | 0.19 | 0.02 | ||||
| U | 0.007 | 0.002 | 0.006 | 0.003 | 0.018 | 0.004 | 0.19 | 0.03 | 0.024 | 0.004 | ||||
| TSC2_G8_1 | 1 s.e. | TSC2_G8_2 | 1 s.e. | TSC7_G2_1 | 1 s.e. | TSC7_G2_2 | 1 s.e. | TSC7_G5_1 | 1 s.e. | |||||
| Li | 10 | 1 | 34 | 5 | 21 | 3 | 5.8 | 0.8 | 2.7 | 0.4 | ||||
| Sc | 101 | 3 | 107 | 4 | 107 | 3 | 100 | 3 | 127 | 5 | ||||
| Ti | 8210 | 151 | 8450 | 374 | 10814 | 216 | 9617 | 196 | 9624 | 290 | ||||
| V | 302 | 13 | 306 | 18 | 314 | 14 | 321 | 13 | 265 | 12 | ||||
| Cr | 52 | 2 | 48 | 2 | 76 | 6 | 68 | 3 | 360 | 39 | ||||
| Ni | 46 | 4 | 44 | 3 | 57 | 4 | 46 | 3 | 56 | 6 | ||||
| Sr | 85 | 5 | 76 | 5 | 138 | 7 | 115 | 6 | 113 | 7 | ||||
| Y | 27 | 1 | 25 | 2 | 36 | 1 | 37 | 2 | 28 | 1 | ||||
| Zr | 62 | 3 | 62 | 3 | 94 | 3 | 95 | 3 | 79 | 5 | ||||
| Nb | 0.4 | 0.1 | 0.7 | 0.1 | 1.4 | 0.2 | 0.6 | 0.1 | 0.7 | 0.2 | ||||
| Sn | 0.8 | 0.2 | 0.6 | 0.2 | 1.6 | 0.3 | 1.2 | 0.1 | 2.7 | 0.6 | ||||
| La | 6.9 | 0.4 | 6.5 | 0.5 | 9.2 | 0.7 | 9.1 | 0.6 | 6.3 | 0.7 | ||||
| Ce | 24 | 5 | 21 | 2 | 31 | 2 | 35 | 3 | 24 | 2 | ||||
| Pr | 4.5 | 0.5 | 4.5 | 0.5 | 6 | 0.6 | 6.8 | 0.7 | 4.6 | 0.5 | ||||
| Nd | 21 | 2 | 21 | 2 | 32 | 3 | 36 | 3 | 28 | 2 | ||||
| Sm | 7.3 | 0.5 | 6.1 | 0.4 | 9.2 | 0.9 | 9.2 | 0.6 | 6.3 | 0.3 | ||||
| Eu | 2.1 | 0.2 | 1.6 | 0.2 | 3 | 0.5 | 3.6 | 0.4 | 2.7 | 0.3 | ||||
| Gd | 6.9 | 0.9 | 5.9 | 0.7 | 10.7 | 0.8 | 8.4 | 0.7 | 6.4 | 0.8 | ||||
| Tb | 1 | 0.1 | 0.91 | 0.06 | 1.6 | 0.1 | 1.5 | 0.1 | 1.23 | 0.09 | ||||
| Dy | 5.9 | 0.5 | 5.6 | 0.2 | 6.5 | 0.4 | 7.2 | 0.4 | 6.2 | 0.3 | ||||
| Ho | 0.83 | 0.08 | 0.83 | 0.08 | 1.7 | 0.2 | 1.7 | 0.1 | 1.2 | 0.1 | ||||
| Er | 3.3 | 0.6 | 3.6 | 0.5 | 4.4 | 0.8 | 3.4 | 0.4 | 2.6 | 0.5 | ||||
| Tm | 0.24 | 0.03 | 0.24 | 0.03 | 0.46 | 0.09 | 0.51 | 0.03 | 0.27 | 0.08 | ||||
| Yb | 2 | 0.2 | 1.9 | 0.2 | 2.8 | 0.2 | 2.5 | 0.3 | 1.5 | 0.3 | ||||
| Lu | 0.27 | 0.08 | 0.24 | 0.05 | 0.4 | 0.1 | 0.32 | 0.05 | 0.29 | 0.06 | ||||
| Hf | 2.7 | 0.2 | 3.1 | 0.3 | 3.4 | 0.4 | 3.9 | 0.3 | 3.9 | 0.4 | ||||
| Pb | 0.08 | 0.02 | 0.05 | 0.02 | 0.12 | 0.05 | 0.13 | 0.02 | 0.1 | 0.03 | ||||
| Th | 0.12 | 0.02 | 0.09 | 0.01 | 0.15 | 0.03 | 0.15 | 0.02 | 0.079 | 0.008 | ||||
| U | 0.018 | 0.006 | 0.017 | 0.006 | 0.019 | 0.006 | 0.016 | 0.006 | 0.007 | 0.003 | ||||
| TSC7_G7_1 | 1 s.e. | TSC7_G9_1 | 1 s.e. | TSC7_G10_1 | 1 s.e. | TSC7_G10_2 | 1 s.e. | TSC7_G10_3 | 1 s.e. | |||||
| Li | 5.1 | 0.7 | 3.8 | 0.6 | 6.9 | 0.9 | 4.3 | 0.6 | 5.4 | 0.8 | ||||
| Sc | 145 | 5 | 125 | 4 | 133 | 3 | 134 | 3 | 143 | 4 | ||||
| Ti | 11294 | 172 | 9592 | 192 | 8341 | 236 | 6522 | 169 | 6252 | 113 | ||||
| V | 343 | 14 | 298 | 13 | 325 | 15 | 240 | 11 | 239 | 10 | ||||
| Cr | 44 | 2 | 109 | 4 | 751 | 38 | 1005 | 46 | 824 | 39 | ||||
| Ni | 49 | 4 | 88 | 6 | 50 | 6 | 90 | 5 | 87 | 5 | ||||
| Sr | 136 | 7 | 137 | 8 | 80 | 4 | 68 | 3 | 68 | 4 | ||||
| Y | 47 | 1 | 23 | 1 | 23 | 1 | 10 | 0 | 10 | 0 | ||||
| Zr | 137 | 5 | 56 | 3 | 73 | 3 | 24 | 1 | 23 | 1 | ||||
| Nb | 1.5 | 0.1 | 0.53 | 0.09 | 0.7 | 0.1 | 0.09 | 0.07 | 0.13 | 0.04 | ||||
| Sn | 2.1 | 0.4 | 2.4 | 0.4 | 0.5 | 0.2 | 0.6 | 0.2 | 0.54 | 0.07 | ||||
| La | 15 | 1 | 5.5 | 0.4 | 6.2 | 0.5 | 1.8 | 0.2 | 1.6 | 0.1 | ||||
| Ce | 48 | 4 | 20 | 2 | 23 | 2 | 7 | 0.6 | 6.8 | 0.5 | ||||
| Pr | 9.1 | 0.9 | 3.9 | 0.4 | 4.3 | 0.4 | 1.5 | 0.2 | 1.4 | 0.1 | ||||
| Nd | 48 | 4 | 23 | 2 | 23 | 2 | 7.8 | 0.8 | 8.1 | 0.6 | ||||
| Sm | 12.5 | 0.4 | 5.6 | 0.4 | 5.6 | 0.6 | 2.6 | 0.3 | 2.7 | 0.3 | ||||
| Eu | 4.3 | 0.4 | 2 | 0.3 | 1.6 | 0.1 | 0.81 | 0.09 | 0.8 | 0.07 | ||||
| Gd | 11.5 | 0.9 | 5.9 | 0.8 | 6.8 | 0.6 | 2.5 | 0.3 | 2.4 | 0.3 | ||||
| Tb | 1.9 | 0.2 | 1.11 | 0.09 | 1 | 0.1 | 0.51 | 0.05 | 0.48 | 0.07 | ||||
| Dy | 10.2 | 0.7 | 4.9 | 0.6 | 4.1 | 0.2 | 2.5 | 0.3 | 1.88 | 0.06 | ||||
| Ho | 2.6 | 0.3 | 1.2 | 0.1 | 0.8 | 0.03 | 0.31 | 0.02 | 0.35 | 0.03 | ||||
| Er | 4.3 | 0.5 | 2.7 | 0.3 | 2.5 | 0.3 | 0.81 | 0.09 | 0.9 | 0.1 | ||||
| Tm | 0.76 | 0.06 | 0.18 | 0.04 | 0.25 | 0.04 | 0.12 | 0.03 | 0.12 | 0.01 | ||||
| Yb | 3.3 | 0.5 | 1.4 | 0.1 | 1.9 | 0.3 | 0.7 | 0.2 | 0.9 | 0.1 | ||||
| Lu | 0.49 | 0.14 | 0.33 | 0.06 | 0.33 | 0.04 | 0.05 | 0.04 | 0.13 | 0.04 | ||||
| Hf | 5.9 | 0.6 | 2.5 | 0.3 | 3.9 | 0.3 | 1.6 | 0.2 | 1.5 | 0.1 | ||||
| Pb | 0.16 | 0.03 | 0.06 | 0.01 | 0.08 | 0.02 | 0.025 | 0.003 | 0.05 | 0.02 | ||||
| Th | 0.25 | 0.03 | 0.08 | 0.02 | 0.08 | 0.01 | 0.02 | 0.01 | 0.03 | 0.008 | ||||
| U | 0.021 | 0.003 | 0.017 | 0.006 | 0.011 | 0.004 | nd | 0.004 | 0.003 | |||||
| TSC7_G10_4 | 1 s.e. | TSC7_G10_5 | 1 s.e. | TSC7_G10_6 | 1 s.e. | TSC3_G1_1 | 1 s.e. | TSC3_G1_2 | 1 s.e. | |||||
| Li | 3.3 | 0.5 | 2.4 | 0.4 | 3 | 0.4 | 2.6 | 0.6 | 1.3 | 0.3 | ||||
| Sc | 131 | 4 | 131 | 5 | 144 | 5 | 81 | 2 | 91 | 3 | ||||
| Ti | 5276 | 143 | 5415 | 101 | 7483 | 175 | 7430 | 159 | 10948 | 178 | ||||
| V | 210 | 12 | 221 | 9 | 282 | 12 | 173 | 8 | 218 | 9 | ||||
| Cr | 2332 | 116 | 1363 | 59 | 69 | 3 | 19 | 1 | 15 | 1 | ||||
| Ni | 93 | 5 | 89 | 5 | 36 | 2 | 24 | 4 | 26 | 2 | ||||
| Sr | 64 | 3 | 62 | 3 | 76 | 4 | 188 | 10 | 196 | 10 | ||||
| Y | 9 | 1 | 9 | 0 | 19 | 1 | 36 | 1 | 40 | 1 | ||||
| Zr | 19 | 1 | 20 | 1 | 52 | 2 | 135 | 4 | 193 | 5 | ||||
| Nb | 0.18 | 0.02 | 0.15 | 0.05 | 0.34 | 0.03 | 1 | 0.1 | 2.1 | 0.2 | ||||
| Sn | 0.42 | 0.05 | 0.36 | 0.06 | 0.8 | 0.1 | 1.7 | 0.3 | 2.2 | 0.4 | ||||
| La | 1.6 | 0.1 | 1.47 | 0.09 | 4.4 | 0.3 | 17 | 1 | 24 | 1 | ||||
| Ce | 6.2 | 0.5 | 5.6 | 0.5 | 16 | 1 | 50 | 4 | 67 | 5 | ||||
| Pr | 1.3 | 0.2 | 1.2 | 0.1 | 3.2 | 0.3 | 11 | 1 | 14 | 1 | ||||
| Nd | 7.2 | 0.6 | 7.1 | 0.7 | 19 | 1 | 57 | 5 | 72 | 5 | ||||
| Sm | 2.4 | 0.6 | 2.4 | 0.2 | 4.4 | 0.3 | 10.6 | 0.5 | 14 | 0.7 | ||||
| Eu | 0.77 | 0.06 | 0.7 | 0.1 | 1.5 | 0.2 | 4.2 | 0.4 | 5.1 | 0.6 | ||||
| Gd | 1.9 | 0.2 | 2.8 | 0.2 | 5.5 | 0.6 | 16 | 1 | 18 | 2 | ||||
| Tb | 0.24 | 0.03 | 0.46 | 0.03 | 0.71 | 0.07 | 1.9 | 0.2 | 1.8 | 0.2 | ||||
| Dy | 1.7 | 0.1 | 1.81 | 0.3 | 4.2 | 0.1 | 9.8 | 0.4 | 10 | 0.6 | ||||
| Ho | 0.29 | 0.04 | 0.29 | 0.03 | 0.76 | 0.04 | 1.7 | 0.2 | 1.72 | 0.08 | ||||
| Er | 1 | 0.1 | 0.7 | 0.1 | 2.3 | 0.2 | 2.9 | 0.3 | 3.7 | 0.5 | ||||
| Tm | 0.09 | 0.02 | 0.1 | 0.04 | 0.18 | 0.03 | 0.53 | 0.05 | 0.6 | 0.1 | ||||
| Yb | 0.49 | 0.08 | 0.5 | 0.03 | 1.8 | 0.3 | 3.1 | 0.4 | 2.2 | 0.6 | ||||
| Lu | 0.08 | 0.01 | 0.07 | 0.02 | 0.19 | 0.05 | 0.4 | 0.06 | 0.5 | 0.1 | ||||
| Hf | 0.9 | 0.1 | 1.3 | 0.2 | 2.3 | 0.3 | 5.4 | 0.4 | 7.4 | 0.6 | ||||
| Pb | 0.03 | 0.01 | 0.03 | 0.01 | 0.04 | 0.01 | 0.11 | 0.02 | 0.08 | 0.01 | ||||
| Th | 0.015 | 0.004 | 0.027 | 0.006 | 0.053 | 0.006 | 0.21 | 0.01 | 0.42 | 0.06 | ||||
| U | 0.003 | 0.002 | 0.003 | 0.001 | 0.008 | 0.001 | 0.021 | 0.005 | 0.045 | 0.006 | ||||
| TSC3_G1_3 | 1 s.e. | TSC3_G3_1 | 1 s.e. | TSC3_G3_2 | 1 s.e. | TSC3_G3_3 | 1 s.e. | TSC3_G9_1 | 1 s.e. | |||||
| Li | 3.4 | 0.6 | 4.5 | 0.7 | 1 | 0.3 | 0.9 | 0.2 | 6.6 | 1 | ||||
| Sc | 93 | 3 | 60 | 2 | 84 | 3 | 101 | 3 | 113 | 4 | ||||
| Ti | 10122 | 201 | 7869 | 133 | 7534 | 145 | 6712 | 123 | 8850 | 302 | ||||
| V | 213 | 18 | 195 | 8 | 186 | 8 | 194 | 9 | 268 | 12 | ||||
| Cr | 9.5 | 0.8 | 11.6 | 0.5 | 10.3 | 0.8 | 10 | 1 | 11.5 | 0.7 | ||||
| Ni | 21 | 3 | 21 | 2 | 17 | 1 | 14 | 2 | 18 | 3 | ||||
| Sr | 255 | 19 | 163 | 8 | 157 | 8 | 149 | 8 | 181 | 12 | ||||
| Y | 40 | 1 | 31.7 | 0.8 | 29.6 | 0.7 | 29 | 1 | 37.9 | 0.9 | ||||
| Zr | 186 | 6 | 114 | 4 | 109 | 3 | 114 | 4 | 183 | 6 | ||||
| Nb | 5.4 | 0.8 | 1.1 | 0.1 | 0.76 | 0.07 | 0.9 | 0.1 | 1.8 | 0.1 | ||||
| Sn | 1.7 | 0.4 | 0.9 | 0.2 | 0.7 | 0.3 | 0.6 | 0.2 | 0.8 | 0.2 | ||||
| La | 26 | 2 | 15.5 | 0.9 | 14.2 | 0.9 | 13.5 | 0.8 | 19 | 1 | ||||
| Ce | 68 | 6 | 45 | 3 | 42 | 3 | 40 | 3 | 64 | 6 | ||||
| Pr | 13 | 1 | 9 | 1 | 9 | 1 | 8.3 | 0.8 | 12 | 1 | ||||
| Nd | 66 | 5 | 48 | 4 | 43 | 3 | 38 | 3 | 53 | 4 | ||||
| Sm | 13.7 | 0.7 | 11 | 1 | 10 | 1 | 9.7 | 0.6 | 12.8 | 0.6 | ||||
| Eu | 3.9 | 0.3 | 4 | 0 | 3 | 0 | 3.1 | 0.3 | 3.4 | 0.3 | ||||
| Gd | 17 | 2 | 12 | 1 | 12 | 1 | 9.9 | 0.8 | 13 | 1 | ||||
| Tb | 1.6 | 0.2 | 1.6 | 0.2 | 1.4 | 0.2 | 1.3 | 0.1 | 1.8 | 0.1 | ||||
| Dy | 9.6 | 0.7 | 7.4 | 0.4 | 6.1 | 0.3 | 5.5 | 0.4 | 9 | 0.5 | ||||
| Ho | 1.4 | 0.05 | 1.48 | 0.08 | 1.07 | 0.07 | 1.1 | 0.08 | 1.4 | 0.1 | ||||
| Er | 3.1 | 0.5 | 2.9 | 0.4 | 2.7 | 0.4 | 3.2 | 0.4 | 4.3 | 0.6 | ||||
| Tm | 0.54 | 0.05 | 0.39 | 0.08 | 0.29 | 0.04 | 0.4 | 0.06 | 0.38 | 0.07 | ||||
| Yb | 2.5 | 0.5 | 2.8 | 0.5 | 2.2 | 0.3 | 2.8 | 0.6 | 2.3 | 0.1 | ||||
| Lu | 0.29 | 0.07 | 0.34 | 0.07 | 0.38 | 0.08 | 0.31 | 0.08 | 0.4 | 0.06 | ||||
| Hf | 7.3 | 0.7 | 4.2 | 0.3 | 4.4 | 0.4 | 4.3 | 0.3 | 6.8 | 0.7 | ||||
| Pb | 0.9 | 0.2 | 0.07 | 0.01 | 0.11 | 0.01 | 0.04 | 0.01 | 0.16 | 0.03 | ||||
| Th | 1.2 | 0.2 | 0.23 | 0.03 | 0.15 | 0.01 | 0.17 | 0.02 | 0.35 | 0.04 | ||||
| U | 0.32 | 0.06 | 0.021 | 0.004 | 0.011 | 0.003 | 0.023 | 0.003 | 0.062 | 0.008 | ||||
| TSC3_G9_3 | 1 s.e. | TSC3_G9_4 | 1 s.e. | TSC9_G1_1 | 1 s.e. | TSC9_G1_2 | 1 s.e. | TSC9_G3_1 | 1 s.e. | |||||
| Li | 0.3 | 0.3 | 1.3 | 0.2 | 36 | 5 | 33 | 5 | 28 | 4 | ||||
| Sc | 105 | 4 | 88 | 2 | 53 | 1 | 78 | 2 | 114 | 3 | ||||
| Ti | 9521 | 375 | 9280 | 198 | 5875 | 95 | 8005 | 159 | 10648 | 166 | ||||
| V | 278 | 14 | 242 | 10 | 227 | 10 | 263 | 13 | 216 | 9 | ||||
| Cr | 21 | 2 | 13.9 | 0.7 | 36 | 3 | 42 | 2 | 23 | 1 | ||||
| Ni | 32 | 2 | 23 | 3 | 37 | 2 | 35 | 4 | 22 | 2 | ||||
| Sr | 154 | 8 | 168 | 9 | 123 | 8 | 116 | 7 | 150 | 7 | ||||
| Y | 25.8 | 0.8 | 30.8 | 0.9 | 15.6 | 0.4 | 21.4 | 0.8 | 31.1 | 0.9 | ||||
| Zr | 108 | 4 | 130 | 4 | 49 | 1 | 67 | 2 | 142 | 4 | ||||
| Nb | 1.4 | 0.2 | 1.2 | 0.2 | 0.5 | 0.1 | 0.61 | 0.07 | 1.1 | 0.1 | ||||
| Sn | 1.1 | 0.2 | 0.8 | 0.2 | 0.53 | 0.06 | 1 | 0.2 | 1.6 | 0.2 | ||||
| La | 12.2 | 0.8 | 14.8 | 0.9 | 6 | 0.4 | 8 | 0.5 | 14.5 | 0.9 | ||||
| Ce | 41 | 3 | 50 | 4 | 24 | 2 | 31 | 3 | 48 | 4 | ||||
| Pr | 8.1 | 0.8 | 10 | 1 | 4.2 | 0.4 | 5.6 | 0.6 | 8.7 | 0.9 | ||||
| Nd | 36 | 3 | 44 | 3 | 21 | 2 | 28 | 2 | 46 | 3 | ||||
| Sm | 9.2 | 0.4 | 11 | 1 | 5.8 | 0.5 | 7.6 | 0.5 | 11.6 | 0.4 | ||||
| Eu | 2.9 | 0.2 | 3.4 | 0.3 | 1.7 | 0.2 | 2.6 | 0.4 | 3.2 | 0.3 | ||||
| Gd | 8.7 | 0.9 | 9.8 | 0.8 | 4.6 | 0.5 | 6.7 | 0.9 | 10.7 | 0.9 | ||||
| Tb | 1.17 | 0.08 | 1.3 | 0.1 | 0.68 | 0.09 | 0.91 | 0.06 | 1.3 | 0.1 | ||||
| Dy | 5 | 0.1 | 6.6 | 0.3 | 3.7 | 0.5 | 5.6 | 0.4 | 7.4 | 0.4 | ||||
| Ho | 0.99 | 0.06 | 1.1 | 0.1 | 0.83 | 0.02 | 1.13 | 0.09 | 1.6 | 0.1 | ||||
| Er | 3.3 | 0.3 | 2.8 | 0.5 | 1.7 | 0.3 | 2.3 | 0.2 | 3.6 | 0.4 | ||||
| Tm | 0.39 | 0.06 | 0.42 | 0.03 | 0.21 | 0.02 | 0.24 | 0.03 | 0.28 | 0.02 | ||||
| Yb | 1.8 | 0.3 | 2.5 | 0.2 | 1.2 | 0.2 | 1.25 | 0.05 | 2 | 0.4 | ||||
| Lu | 0.29 | 0.08 | 0.37 | 0.05 | 0.07 | 0.02 | 0.18 | 0.02 | 0.29 | 0.04 | ||||
| Hf | 3.7 | 0.2 | 4.4 | 0.4 | 2.2 | 0.2 | 3 | 0.2 | 6.3 | 0.4 | ||||
| Pb | 0.04 | 0.01 | 0.05 | 0.01 | 0.04 | 0.02 | 0.04 | 0.02 | 0.1 | 0.01 | ||||
| Th | 0.19 | 0.01 | 0.19 | 0.02 | 0.088 | 0.006 | 0.1 | 0.02 | 0.2 | 0.01 | ||||
| U | 0.017 | 0.007 | 0.021 | 0.005 | 0.017 | 0.004 | 0.019 | 0.005 | 0.025 | 0.003 | ||||
| TSC9_G3_2 | 1 s.e. | TSC9_G5_1 | 1 s.e. | TSC9_G5_2 | 1 s.e. | |||||||||
| Li | 24 | 3 | 18 | 3 | 10 | 1 | ||||||||
| Sc | 99 | 2 | 99 | 5 | 114 | 3 | ||||||||
| Ti | 8647 | 135 | 9205 | 538 | 10315 | 165 | ||||||||
| V | 184 | 8 | 255 | 14 | 271 | 12 | ||||||||
| Cr | 21 | 1 | 24 | 3 | 7 | 1 | ||||||||
| Ni | 26 | 2 | 27 | 4 | 18 | 2 | ||||||||
| Sr | 146 | 7 | 151 | 14 | 184 | 9 | ||||||||
| Y | 24.5 | 0.9 | 24 | 1 | 40 | 1 | ||||||||
| Zr | 94 | 3 | 98 | 7 | 158 | 6 | ||||||||
| Nb | 0.82 | 0.07 | 0.8 | 0.1 | 1.8 | 0.2 | ||||||||
| Sn | 1.2 | 0.2 | 1.1 | 0.2 | 1.4 | 0.4 | ||||||||
| La | 10.3 | 0.6 | 14 | 1 | 24 | 1 | ||||||||
| Ce | 35 | 3 | 53 | 5 | 87 | 7 | ||||||||
| Pr | 6.3 | 0.6 | 8 | 0.8 | 14 | 1 | ||||||||
| Nd | 35 | 3 | 39 | 3 | 64 | 5 | ||||||||
| Sm | 9.2 | 0.4 | 9 | 1 | 16 | 1 | ||||||||
| Eu | 2.3 | 0.2 | 2.6 | 0.2 | 5 | 0 | ||||||||
| Gd | 8.4 | 0.9 | 8 | 1 | 15 | 1 | ||||||||
| Tb | 1 | 0.1 | 1.04 | 0.07 | 2 | 0 | ||||||||
| Dy | 6.1 | 0.3 | 5.5 | 0.4 | 10.3 | 0.5 | ||||||||
| Ho | 0.9 | 0.1 | 0.99 | 0.08 | 1.5 | 0.07 | ||||||||
| Er | 2.4 | 0.3 | 2.5 | 0.4 | 3.7 | 0.3 | ||||||||
| Tm | 0.3 | 0.05 | 0.29 | 0.06 | 0.39 | 0.04 | ||||||||
| Yb | 1.3 | 0.2 | 1.7 | 0.2 | 3.2 | 0.2 | ||||||||
| Lu | 0.26 | 0.04 | 0.16 | 0.03 | 0.44 | 0.06 | ||||||||
| Hf | 3.8 | 0.2 | 4.5 | 0.5 | 6.8 | 0.4 | ||||||||
| Pb | 0.05 | 0.01 | 0.22 | 0.05 | 0.14 | 0.03 | ||||||||
| Th | 0.117 | 0.008 | 0.18 | 0.03 | 0.23 | 0.02 | ||||||||
| U | 0.034 | 0.005 | 0.033 | 0.008 | 0.04 | 0.005 | ||||||||
Trace element analytical methods
Trace element concentrations of TSC clinopyroxenes were collected by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) using a Nu AttoM high resolution spectrometer and New Wave UP213 (213 nm) deep-UV YAG laser ablation system at the University of New Hampshire. Laser spots on sample material were ~40 μm in diameter.
The concentration of trace element i in the clinopyroxene sample, (Cisample), reported in ppm, was calculated using the following relationship:
Eq. S-1
Calcium concentrations (CCasample), measured near each laser ablation pit by electron microprobe, served as the internal reference element. I represents the background corrected signal intensity of a material and R denotes the reference standard glass, ML3B-G. Trace element signal intensities were calculated from LA-ICP-MS measurements via the following processing procedure: an ablation interval of typically 12–15 peak count cycles was corrected for background by subtracting a background count average (typically of 24 background cycles, with half taken before sample count collection and half after) from each cycle collected within the ablation interval (the sample peak). The median of either four or five sub-intervals within the ablation interval was used to represent the background corrected sample count value (Iisample). ML3B-G glass served as the reference standard and two ML3B-G points were measured between every set of five unknowns. Background corrected count values for the ML3B-G glass standard (IiR) were collected in the same manner. Concentrations at each sample location were calculated using the reference standard signals collected closest in time to the sample measurement over the course of the analyses.
The error propagated for each analysis consisted of the standard error (s.e.) of the mean for the subintervals of sample peak selected, the uncertainty of internal standard electron microprobe Ca measurement, and the uncertainty of the calibration standard ML3B-G measurements, which was taken as the difference between the reported ML3B-G concentrations (Jochum et al., 2006) and the average of repeat analyses of ML3B-G over the course of the TSC clinopyroxene measurements reported in this study.
Table S-3 Comparison of LA-ICP-MS repeat analyses of ML3B-G glass standard with reported literature values.
| ML3B-G* | LA-ICP-MS | |||||
| literature value | average | |||||
| ppm | ppm | Δ lit-avg | Δ as % | 1 σ* | 1 σ as % | |
| Li | 4.50 | 5.20 | 0.70 | 15.5 | 0.93 | 21 |
| Sc | 31.60 | 31.01 | -0.59 | -1.9 | 1.30 | 4 |
| Ti | 12769.78 | 12823.95 | 54.16 | 0.4 | 368.34 | 3 |
| V | 268.00 | 278.64 | 10.64 | 4.0 | 17.92 | 7 |
| Cr | 177.00 | 182.94 | 5.94 | 3.4 | 9.81 | 6 |
| Ni | 107.00 | 112.63 | 5.63 | 5.3 | 6.76 | 6 |
| Sr | 312.00 | 327.32 | 15.32 | 4.9 | 21.96 | 7 |
| Y | 23.90 | 24.29 | 0.39 | 1.6 | 1.85 | 8 |
| Zr | 122.00 | 124.98 | 2.98 | 2.4 | 8.76 | 7 |
| Nb | 8.61 | 9.17 | 0.56 | 6.5 | 0.93 | 11 |
| Sn | 1.14 | 1.16 | 0.02 | 1.4 | 0.38 | 33 |
| La | 8.99 | 9.53 | 0.54 | 6.0 | 0.97 | 11 |
| Ce | 23.10 | 24.95 | 1.85 | 8.0 | 2.69 | 12 |
| Pr | 3.43 | 3.80 | 0.37 | 10.7 | 0.49 | 14 |
| Nd | 16.70 | 17.95 | 1.25 | 7.5 | 2.22 | 13 |
| Sm | 4.75 | 4.79 | 0.04 | 0.8 | 0.73 | 15 |
| Eu | 1.67 | 1.81 | 0.14 | 8.6 | 0.21 | 12 |
| Gd | 5.26 | 5.67 | 0.41 | 7.8 | 0.92 | 17 |
| Tb | 0.80 | 0.84 | 0.04 | 5.5 | 0.18 | 23 |
| Dy | 4.84 | 4.85 | 0.01 | 0.2 | 0.58 | 12 |
| Ho | 0.91 | 0.91 | 0.00 | 0.5 | 0.13 | 14 |
| Er | 2.44 | 2.64 | 0.20 | 8.4 | 0.41 | 17 |
| Tm | 0.32 | 0.33 | 0.00 | 1.5 | 0.06 | 20 |
| Yb | 2.06 | 2.00 | -0.06 | -2.7 | 0.45 | 22 |
| Lu | 0.29 | 0.32 | 0.04 | 13.2 | 0.08 | 28 |
| Hf | 3.22 | 3.42 | 0.20 | 6.2 | 0.76 | 24 |
| Pb | 1.38 | 1.53 | 0.15 | 10.5 | 0.23 | 17 |
| Th | 0.55 | 0.57 | 0.02 | 4.5 | 0.06 | 11 |
| U | 0.44 | 0.50 | 0.05 | 12.2 | 0.09 | 20 |
* ML3B-G was analysed twice between every 5 unknown samples, totalling 24 standard analyses during collection of the TSC data reported in this study. Analytical uncertainty is determined by having the first of each two ML3B-G analyses serve as the 'standard' for calculating the concentration of the second ML3B-G analysis, which is run as an unknown sample. The average value of the 12 ML3B-G 'unknowns' is compared here with the literature values for these trace elements (Jochum et al., 2006) along with the standard deviation of these ML3B-G runs.
Download in ExcelTable S-4 TSC clinopyroxene major element compositions (wt. %) along grain transects were analysed on the Massachusetts Institute of Technology (MIT) JEOL-JXA-8200 Superprobe. Uncertainty (2s) has been calculated from the standard deviation of replicate analyses of the DJ35 diopside-jadeite glass and ALP7 aluminous orthopyroxene standards, as well as several points inferred from back scattered electron imaging to be from the same clinopyroxene crystal growth zone: SiO2 (0.31), TiO2 (0.02), Al2O3 (0.07), FeO (0.08), MgO (0.11), MnO (0.01), CaO (0.30), Na2O (0.04), K (0.01), Cr2O3 (0.02).
| SiO2 | TiO2 | Al2O3 | FeOt† | MnO | MgO | CaO | Na2O | K2O | Cr2O3 | Total | |
| TSC-2A-2 | 47.74 | 1.41 | 6.8 | 7.17 | 0.11 | 13.09 | 21.8 | 0.3 | 0.01 | 0.01 | 98.44 |
| TSC-2A-3 | 48.57 | 1.3 | 6.49 | 7.95 | 0.13 | 12.82 | 21.42 | 0.4 | 0 | 0.04 | 99.12 |
| TSC-2A-4 | 47.88 | 1.29 | 6.4 | 7.71 | 0.13 | 13.22 | 21.28 | 0.36 | 0 | 0.04 | 98.31 |
| TSC-2A-5 | 48.71 | 1.23 | 6.17 | 7.94 | 0.15 | 13.44 | 21.44 | 0.44 | 0 | 0.03 | 99.54 |
| TSC-2A-6 | 49.17 | 1.12 | 5.33 | 7.71 | 0.14 | 13.85 | 21.37 | 0.46 | 0 | 0.03 | 99.18 |
| TSC-2A-7 | 48.69 | 1.24 | 5.59 | 6.35 | 0.09 | 14.23 | 22.09 | 0.42 | 0.01 | 0.04 | 98.74 |
| TSC-2A-8 | 49.57 | 1.18 | 5.64 | 6.44 | 0.1 | 14.25 | 22.38 | 0.32 | 0 | 0.03 | 99.9 |
| TSC-2A-9 | 49.47 | 1.14 | 5.59 | 6.65 | 0.1 | 14.17 | 22.14 | 0.42 | 0 | 0.01 | 99.69 |
| TSC-2A-10 | 48.87 | 1.19 | 6.58 | 7.25 | 0.09 | 13.54 | 22.2 | 0.48 | 0.02 | 0.04 | 100.26 |
| TSC-2A-11 | 48.63 | 1.2 | 6.49 | 7.26 | 0.12 | 13.75 | 21.79 | 0.44 | 0 | 0.05 | 99.71 |
| TSC-2A-12 | 48.94 | 1.22 | 6.33 | 7.15 | 0.11 | 13.77 | 21.93 | 0.4 | 0.01 | 0.03 | 99.89 |
| TSC-2A-13 | 49.2 | 1.03 | 5.96 | 7.03 | 0.14 | 13.95 | 21.88 | 0.35 | 0.01 | 0.07 | 99.62 |
| TSC-2B-2 | 49.45 | 1.28 | 3.84 | 8.31 | 0.19 | 14.56 | 20.99 | 0.54 | 0 | 0.05 | 99.21 |
| TSC-2B-3 | 48.12 | 1.51 | 4.98 | 7.97 | 0.14 | 14.27 | 20.91 | 0.55 | 0.01 | 0.01 | 98.47 |
| TSC-2B-4 | 49.78 | 0.99 | 4.17 | 7.77 | 0.16 | 14.93 | 21.22 | 0.5 | 0 | 0.01 | 99.53 |
| TSC-2B-5 | 50.21 | 0.92 | 3.64 | 7.77 | 0.17 | 15.17 | 20.93 | 0.48 | 0 | 0.01 | 99.31 |
| TSC-2B-6 | 50.43 | 0.92 | 3.78 | 7.79 | 0.18 | 15.24 | 21.38 | 0.43 | 0 | 0 | 100.16 |
| TSC-2B-7 | 50.43 | 1.13 | 3.86 | 7.8 | 0.17 | 14.89 | 21.34 | 0.37 | 0 | 0.01 | 100 |
| TSC-2B-8 | 50.83 | 0.93 | 3.54 | 7.93 | 0.18 | 14.99 | 20.85 | 0.41 | 0 | 0.01 | 99.67 |
| TSC-2B-9 | 50.96 | 0.99 | 3.69 | 7.94 | 0.18 | 14.95 | 21.13 | 0.38 | 0 | 0 | 100.21 |
| TSC-2B-10 | 50.38 | 0.99 | 3.76 | 7.85 | 0.19 | 15.22 | 21.33 | 0.47 | 0 | 0.002 | 100.18 |
| TSC-2C-1 | 47.4 | 2.02 | 7.67 | 8.99 | 0.17 | 11.96 | 20.87 | 0.49 | 0.01 | 0.04 | 99.62 |
| TSC-2C-2 | 46.54 | 1.93 | 7.23 | 8.73 | 0.17 | 12.4 | 20.59 | 0.53 | 0.01 | 0.06 | 98.19 |
| TSC-2C-3 | 46.51 | 1.96 | 7.23 | 8.69 | 0.15 | 12.74 | 20.78 | 0.48 | 0 | 0.04 | 98.59 |
| TSC-2C-4 | 46.47 | 1.94 | 7.26 | 9 | 0.18 | 13.1 | 20.74 | 0.58 | 0 | 0.01 | 99.29 |
| TSC-2C-5 | 46.71 | 1.87 | 7.03 | 8.84 | 0.18 | 12.99 | 20.79 | 0.58 | 0 | 0.02 | 99.01 |
| TSC-2C-6 | 46.93 | 1.84 | 7.02 | 8.76 | 0.15 | 13.09 | 20.81 | 0.58 | 0.01 | 0.02 | 99.21 |
| TSC-2C-7 | 47.07 | 1.83 | 6.91 | 8.67 | 0.17 | 13.21 | 20.95 | 0.53 | 0 | 0.03 | 99.37 |
| TSC-2C-8 | 46.79 | 1.87 | 7.13 | 8.99 | 0.17 | 13.24 | 20.88 | 0.52 | 0 | 0.01 | 99.6 |
| TSC-2C-9 | 46.53 | 1.89 | 6.98 | 8.91 | 0.17 | 13.37 | 21.2 | 0.56 | 0 | 0.02 | 99.63 |
| TSC-2C-10 | 49.14 | 0.88 | 3.97 | 7.75 | 0.17 | 15.58 | 21.38 | 0.58 | 0.01 | 0 | 99.45 |
| TSC-2D-2 | 48.9 | 1.42 | 3.93 | 7.77 | 0.16 | 13.93 | 21.67 | 0.35 | 0 | 0.04 | 98.17 |
| TSC-2D-3 | 49.1 | 1.4 | 3.85 | 7.72 | 0.14 | 14.23 | 21.63 | 0.41 | 0.01 | 0.04 | 98.53 |
| TSC-2D-4 | 50.42 | 1.24 | 3.51 | 7.62 | 0.13 | 14.55 | 21.87 | 0.43 | 0 | 0.03 | 99.79 |
| TSC-2D-5 | 49.85 | 1.38 | 4.01 | 7.96 | 0.14 | 14.33 | 21.96 | 0.4 | 0 | 0.04 | 100.06 |
| TSC-2D-6 | 50.45 | 1.32 | 4.01 | 7.45 | 0.15 | 14.28 | 21.73 | 0.53 | 0.01 | 0.02 | 99.95 |
| TSC-2D-7 | 50.01 | 1.2 | 3.5 | 7.9 | 0.16 | 14.81 | 21.75 | 0.47 | 0.01 | 0.01 | 99.82 |
| TSC-2D-8 | 49.81 | 1.42 | 3.84 | 7.6 | 0.17 | 14.68 | 22.12 | 0.45 | 0 | 0.02 | 100.13 |
| TSC-2D-9 | 49.32 | 1.58 | 4.19 | 8.11 | 0.16 | 14.35 | 21.76 | 0.4 | 0 | 0.03 | 99.9 |
| TSC-2D-10 | 46.77 | 1.55 | 4.53 | 8.3 | 0.17 | 14.79 | 21.51 | 0.52 | 0 | 0.01 | 98.15 |
| TSC-3A-1 | 49.14 | 1.61 | 4.81 | 7.46 | 0 | 13.71 | 22.47 | 0.51 | 0 | 0 | 99.72 |
| TSC-3A-2 | 49.09 | 1.67 | 4.73 | 7.42 | 0 | 13.61 | 22.52 | 0.46 | 0 | 0 | 99.5 |
| TSC-3A-3 | 49.01 | 1.65 | 4.99 | 8.19 | 0 | 13.31 | 21.66 | 0.61 | 0 | 0 | 99.43 |
| TSC-3A-4 | 48 | 2.19 | 5.63 | 8.61 | 0 | 12.67 | 22.03 | 0.61 | 0 | 0 | 99.74 |
| TSC-3A-5 | 48.68 | 2.02 | 4.91 | 8.23 | 0 | 12.96 | 22.16 | 0.68 | 0 | 0 | 99.64 |
| TSC-3A-6 | 49.22 | 2.04 | 4.13 | 9.22 | 0 | 12.67 | 22.13 | 0.66 | 0.01 | 0 | 100.09 |
| TSC-3A-7 | 47.9 | 2.31 | 5.09 | 8.67 | 0.02 | 12.56 | 22.05 | 0.63 | 0 | 0 | 99.23 |
| TSC-3A-8 | 50.42 | 1.51 | 2.96 | 9.24 | 0.02 | 12.83 | 22.01 | 0.7 | 0 | 0 | 99.69 |
| TSC-3A-9 | 45.8 | 2.74 | 7.3 | 8.89 | 0.02 | 12.1 | 21.9 | 0.54 | 0 | 0 | 99.29 |
| TSC-3A-10 | 49.91 | 1.39 | 4.11 | 7.92 | 0.01 | 13.96 | 21.64 | 0.57 | 0 | 0 | 99.51 |
| TSC-3A-11 | 49.49 | 1.47 | 4.72 | 7.33 | 0.01 | 13.89 | 22.09 | 0.55 | 0 | 0 | 99.55 |
| TSC-3A-12 | 49.74 | 1.46 | 4.61 | 8.23 | 0 | 13.71 | 21.49 | 0.57 | 0 | 0 | 99.81 |
| TSC-3B-1 | 50.23 | 0.95 | 4.59 | 5.24 | 0 | 14.97 | 23.05 | 0.39 | 0 | 0.18 | 99.6 |
| TSC-3B-2 | 50.09 | 0.96 | 4.77 | 5.4 | 0 | 14.92 | 22.93 | 0.43 | 0 | 0.17 | 99.67 |
| TSC-3B-3 | 49.47 | 1.14 | 5.28 | 5.47 | 0 | 14.88 | 22.82 | 0.46 | 0 | 0.21 | 99.73 |
| TSC-3B-4 | 49.35 | 1.1 | 5.43 | 5.64 | 0 | 14.62 | 22.72 | 0.43 | 0 | 0.15 | 99.44 |
| TSC-3B-5 | 50.12 | 0.89 | 4.79 | 5.38 | 0 | 14.98 | 22.8 | 0.4 | 0 | 0.19 | 99.55 |
| TSC-3B-6 | 49.28 | 1.14 | 5.4 | 5.56 | 0 | 14.46 | 22.84 | 0.41 | 0 | 0.11 | 99.21 |
| TSC-3B-7 | 49.33 | 1.12 | 5.26 | 5.4 | 0.02 | 14.49 | 22.85 | 0.47 | 0.01 | 0.24 | 99.18 |
| TSC-3B-8 | 49.29 | 1.01 | 5.02 | 5.34 | 0.02 | 14.68 | 22.64 | 0.5 | 0 | 0.22 | 98.72 |
| TSC-3B-9 | 49.1 | 1.56 | 4.38 | 7.87 | 0 | 13.91 | 22.15 | 0.47 | 0 | 0 | 99.45 |
| TSC-3B-10 | 49.88 | 1.52 | 3.86 | 7.93 | 0.01 | 14.08 | 22.05 | 0.41 | 0 | 0 | 99.74 |
| TSC-3B-11 | 49.51 | 1.17 | 5.4 | 5.54 | 0 | 14.63 | 22.98 | 0.38 | 0 | 0.17 | 99.77 |
| TSC-3B-12 | 49.41 | 1.11 | 5.33 | 5.48 | 0 | 14.49 | 22.83 | 0.42 | 0 | 0.18 | 99.26 |
| TSC-3C-1 | 48.55 | 2 | 4.96 | 8.44 | 0 | 13.06 | 22.2 | 0.54 | 0 | 0.02 | 99.76 |
| TSC-3C-2 | 48.06 | 2.15 | 5.26 | 8.53 | 0 | 12.91 | 22.2 | 0.56 | 0 | 0 | 99.67 |
| TSC-3C-3 | 48.19 | 2 | 4.96 | 8.52 | 0 | 12.97 | 22.38 | 0.59 | 0 | 0.05 | 99.66 |
| TSC-3C-4 | 48 | 2.12 | 5.14 | 8.52 | 0 | 12.68 | 21.96 | 0.62 | 0 | 0 | 99.04 |
| TSC-3C-5 | 48.28 | 2.02 | 4.93 | 8.33 | 0 | 12.75 | 22.13 | 0.64 | 0 | 0.02 | 99.09 |
| TSC-3C-6 | 47.43 | 2.46 | 5.48 | 8.89 | 0 | 12.43 | 21.93 | 0.62 | 0 | 0 | 99.24 |
| TSC-3C-7 | 49.68 | 1.66 | 3.69 | 8.22 | 0 | 13.46 | 21.62 | 0.61 | 0 | 0 | 98.95 |
| TSC-3C-8 | 48.78 | 1.88 | 4.65 | 8.46 | 0 | 12.98 | 22.01 | 0.58 | 0.01 | 0.04 | 99.38 |
| TSC-3C-9 | 47.95 | 1.89 | 5.44 | 8.35 | 0 | 13.23 | 22.19 | 0.46 | 0 | 0.01 | 99.52 |
| TSC-3C-10 | 48.32 | 2.08 | 4.94 | 8.66 | 0 | 12.89 | 21.85 | 0.64 | 0 | 0.02 | 99.4 |
| TSC-3C-11 | 47.71 | 2.36 | 5.48 | 8.73 | 0 | 12.57 | 22.07 | 0.58 | 0 | 0 | 99.5 |
| TSC-3D-1 | 47.72 | 2.05 | 5.72 | 8.19 | 0 | 12.82 | 22.3 | 0.56 | 0 | 0 | 99.36 |
| TSC-3D-2 | 47.94 | 2.13 | 5.51 | 8.46 | 0 | 12.97 | 22.13 | 0.54 | 0.01 | 0 | 99.69 |
| TSC-3D-3 | 48.27 | 1.8 | 5.25 | 8.24 | 0 | 12.97 | 22.26 | 0.58 | 0.01 | 0 | 99.38 |
| TSC-3D-4 | 48.21 | 1.99 | 5.27 | 7.86 | 0.03 | 13.06 | 22.31 | 0.52 | 0 | 0.01 | 99.26 |
| TSC-3D-5 | 47.37 | 2.27 | 6.03 | 8.16 | 0 | 12.63 | 22.38 | 0.56 | 0.01 | 0 | 99.4 |
| TSC-3D-6 | 47.61 | 2.18 | 5.82 | 8.37 | 0 | 12.55 | 22.14 | 0.65 | 0 | 0 | 99.32 |
| TSC-3D-7 | 48.35 | 2.16 | 4.88 | 8.61 | 0 | 12.99 | 21.72 | 0.67 | 0 | 0 | 99.38 |
| TSC-3D-8 | 48.83 | 1.64 | 5.04 | 8.16 | 0.03 | 13.2 | 22.07 | 0.63 | 0.01 | 0 | 99.61 |
| TSC-3D-9 | 48.05 | 1.47 | 6.25 | 7.53 | 0 | 13.51 | 22.24 | 0.51 | 0 | 0 | 99.56 |
| TSC-3D-10 | 48.65 | 1.39 | 5.93 | 7.52 | 0 | 13.72 | 22.17 | 0.52 | 0 | 0.01 | 99.92 |
| TSC-7A-1 | 47.74 | 1.59 | 5.46 | 7.83 | 0.18 | 14.04 | 21.51 | 0.77 | 0 | 0 | 99.13 |
| TSC-7A-2 | 48.72 | 1.4 | 4.85 | 7.68 | 0.18 | 14.12 | 21.69 | 0.64 | 0 | 0.02 | 99.3 |
| TSC-7A-3 | 48.55 | 1.48 | 4.77 | 7.7 | 0.17 | 14.09 | 21.71 | 0.47 | 0 | 0.04 | 98.97 |
| TSC-7A-4 | 49.07 | 1.4 | 4.37 | 7.7 | 0.16 | 14.2 | 21.73 | 0.56 | 0.01 | 0.03 | 99.23 |
| TSC-7A-5 | 49.59 | 1.3 | 3.93 | 7.5 | 0.16 | 14.26 | 21.91 | 0.58 | 0 | 0.05 | 99.28 |
| TSC-7A-6 | 48.73 | 1.59 | 4.54 | 7.64 | 0.15 | 13.73 | 22.17 | 0.48 | 0.02 | 0.03 | 99.09 |
| TSC-7A-7 | 49.01 | 1.5 | 4.37 | 7.73 | 0.17 | 13.68 | 22.06 | 0.51 | 0 | 0.02 | 99.04 |
| TSC-7A-8 | 49.31 | 1.38 | 4.36 | 7.52 | 0.16 | 13.93 | 21.93 | 0.47 | 0 | 0.02 | 99.08 |
| TSC-7A-9 | 49.92 | 1.37 | 4.24 | 7.5 | 0.15 | 13.96 | 21.86 | 0.51 | 0 | 0.04 | 99.55 |
| TSC-7A-10 | 50.13 | 1.32 | 4.27 | 7.46 | 0.16 | 13.63 | 21.72 | 0.41 | 0 | 0.02 | 99.13 |
| TSC-7B-2 | 49.94 | 1.26 | 3.98 | 7.38 | 0.17 | 14.23 | 21.94 | 0.47 | 0.01 | 0 | 99.38 |
| TSC-7B-3 | 48.58 | 1.46 | 4.77 | 7.28 | 0.18 | 13.95 | 21.97 | 0.38 | 0.01 | 0 | 98.59 |
| TSC-7B-4 | 49.14 | 1.29 | 4.48 | 7.49 | 0.15 | 14.27 | 21.66 | 0.49 | 0 | 0.03 | 98.99 |
| TSC-7B-5 | 48.32 | 1.28 | 4.08 | 7.23 | 0.16 | 14.87 | 21.8 | 0.37 | 0 | 0.02 | 98.12 |
| TSC-7B-6 | 50.09 | 1.26 | 3.76 | 7.52 | 0.16 | 14.03 | 21.56 | 0.46 | 0.01 | 0.01 | 98.86 |
| TSC-7B-7 | 49.82 | 1.22 | 3.74 | 7.58 | 0.19 | 14.16 | 21.45 | 0.47 | 0 | 0.02 | 98.65 |
| TSC-7B-8 | 46.93 | 2.04 | 5.91 | 7.77 | 0.14 | 13.11 | 21.94 | 0.46 | 0 | 0.05 | 98.35 |
| TSC-7B-9 | 47.13 | 2.09 | 5.68 | 8.23 | 0.15 | 12.87 | 21.81 | 0.49 | 0 | 0.01 | 98.46 |
| TSC-7B-10 | 47.35 | 1.87 | 5.81 | 7.75 | 0.15 | 13.49 | 21.87 | 0.6 | 0.01 | 0 | 98.9 |
| TSC-9A-1 | 47.95 | 2.58 | 5.02 | 9.03 | 0 | 12.47 | 22.06 | 0.7 | 0 | 0.01 | 99.82 |
| TSC-9A-2 | 48.04 | 1.98 | 5.81 | 7.42 | 0 | 13.23 | 22.82 | 0.52 | 0 | 0 | 99.82 |
| TSC-9A-3 | 48.18 | 2.22 | 5.45 | 8.29 | 0.05 | 13.07 | 22.44 | 0.72 | 0 | 0.03 | 100.44 |
| TSC-9A-4 | 47.85 | 2.09 | 5.62 | 7.89 | 0.03 | 13.02 | 22.3 | 0.58 | 0 | 0 | 99.38 |
| TSC-9A-5 | 47.06 | 2.37 | 6.66 | 8.02 | 0.03 | 12.82 | 22.89 | 0.51 | 0 | 0 | 100.37 |
| TSC-9A-6 | 47.76 | 2.33 | 5.93 | 8.06 | 0.01 | 12.82 | 22.29 | 0.62 | 0 | 0.05 | 99.86 |
| TSC-9A-7 | 48.56 | 1.7 | 5.27 | 7.49 | 0.02 | 13.54 | 22.81 | 0.56 | 0 | 0 | 99.95 |
| TSC-9A-8 | 49.44 | 1.68 | 4.51 | 7.07 | 0.01 | 14.02 | 22.46 | 0.48 | 0 | 0.01 | 99.67 |
| TSC-9A-9 | 49.62 | 1.67 | 4.07 | 7.6 | 0.02 | 13.6 | 21.95 | 0.68 | 0 | 0 | 99.21 |
| TSC-9A-10 | 47.12 | 2.06 | 6.59 | 8.45 | 0 | 12.7 | 22.08 | 0.52 | 0 | 0 | 99.52 |
| TSC-9B-1 | 47.85 | 2.13 | 5.79 | 7.71 | 0 | 13.05 | 22.35 | 0.56 | 0 | 0.02 | 99.47 |
| TSC-9B-2 | 49.76 | 1.33 | 4.17 | 7.54 | 0.01 | 14.03 | 21.87 | 0.62 | 0 | 0.01 | 99.34 |
| TSC-9B-3 | 48.41 | 1.73 | 5.12 | 7.63 | 0.03 | 13.38 | 22.2 | 0.51 | 0.01 | 0 | 99.02 |
| TSC-9B-4 | 49.42 | 1.43 | 4.35 | 7.07 | 0.03 | 14.18 | 22.46 | 0.53 | 0.01 | 0.01 | 99.49 |
| TSC-9B-5 | 49.63 | 1.51 | 4.49 | 7.38 | 0.02 | 13.89 | 21.81 | 0.62 | 0.01 | 0 | 99.36 |
| TSC-9B-6 | 50.17 | 1.42 | 3.73 | 7.24 | 0.02 | 14.16 | 22.26 | 0.5 | 0 | 0 | 99.5 |
| TSC-9B-7 | 49.73 | 1.5 | 4.24 | 7.51 | 0.02 | 13.8 | 22.1 | 0.59 | 0 | 0 | 99.48 |
| TSC-9B-8 | 49.37 | 1.67 | 4.44 | 7.61 | 0 | 13.92 | 22.38 | 0.62 | 0 | 0.04 | 100.05 |
| TSC-9B-9 | 49.63 | 1.46 | 4.39 | 7.06 | 0 | 14.17 | 22.66 | 0.46 | 0 | 0 | 99.83 |
| TSC-9B-10 | 50.25 | 1.3 | 3.71 | 7.21 | 0 | 14.58 | 22.58 | 0.44 | 0 | 0.01 | 100.09 |
| TSC-9C-1 | 46.86 | 2.55 | 6.22 | 8.38 | 0.02 | 12.71 | 22.49 | 0.59 | 0 | 0 | 99.82 |
| TSC-9C-2 | 48.48 | 1.88 | 5.02 | 7.65 | 0.03 | 13.32 | 22.52 | 0.59 | 0 | 0 | 99.49 |
| TSC-9C-3 | 50.56 | 1.55 | 3.26 | 7.69 | 0.01 | 14.3 | 21.37 | 0.62 | 0.01 | 0 | 99.37 |
| TSC-9C-4 | 49.8 | 1.42 | 3.97 | 7.39 | 0 | 14.12 | 21.85 | 0.49 | 0.01 | 0 | 99.04 |
| TSC-9C-5 | 49.99 | 1.41 | 3.88 | 7.15 | 0 | 14.29 | 21.91 | 0.52 | 0 | 0 | 99.15 |
| TSC-9C-6 | 49.34 | 1.67 | 4.39 | 7.5 | 0 | 13.84 | 21.67 | 0.58 | 0.01 | 0 | 98.99 |
| TSC-9C-7 | 49.24 | 1.63 | 4.31 | 7.56 | 0.01 | 13.7 | 21.99 | 0.53 | 0 | 0 | 98.96 |
| TSC-9C-8 | 48.81 | 1.78 | 5.01 | 7.59 | 0 | 13.48 | 22.48 | 0.64 | 0.01 | 0 | 99.8 |
| TSC-9C-9 | 48.87 | 1.63 | 5.16 | 7.15 | 0 | 13.66 | 22.7 | 0.53 | 0.01 | 0 | 99.71 |
| TSC-9C-10 | 46.41 | 2.54 | 6.99 | 8.16 | 0 | 12.51 | 22.64 | 0.56 | 0.01 | 0 | 99.82 |
*Operating conditions of the MIT JXA-8200 consisted of a 15 keV accelerating voltage and 10 nA beam current, with all analyses using a focused beam of ~1 μm and 30 s count times. Data were reduced using the CITZAF correction procedure of Armstrong (1995). The few totals lower than 98 wt. % have been omitted.
† All iron reported as FeO
Neodymium, Hf, and Pb isotopic compositions were measured on the Nu Plasma 500 HR multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) at the Ecole Normale Supérieure de Lyon.
Table S-5 Hf-Nd-Pb isotopic data for Timpe Santa Caterina whole rock (WR) and clinopyroxene (cpx) separates*. The uncertainties reported for Nd and Hf isotope ratios are internal 2 s.e. We use the values of external reproducibility as reported in the footnote to identify analytically resolvable WR-cpx disequilibrium discernable above the 2σ level.
| 143Nd/144Nd | εNd † | 176Hf/177Hf † | εHf § | 206Pb/204Pb § | 207Pb/204Pb § | 208Pb/204Pb # | |
| TSC-2 | |||||||
| WR | 0.512952(2) | 6.1 | 0.283035(5) | 9.3 | 20.015 | 15.668 | 39.645 |
| cpx | 0.512942(3) | 5.9 | 0.283033(4) | 9.2 | 20.014 | 15.670 | 39.645 |
| TSC-3 | |||||||
| WR | 0.512948(3) | 6 | 0.283032(4) | 9.2 | 20.078 | 15.670 | 39.666 |
| cpx | 0.512937(2) | 5.8 | 0.283047(6) | 9.7 | 20.072 | 15.668 | 39.655 |
| TSC-7 | |||||||
| WR | 0.512929(4) | 5.7 | 0.283012(4) | 8.5 | 19.990 | 15.676 | 39.648 |
| cpx | 0.512921(2) | 5.5 | 0.283024(6) | 8.9 | 19.946 | 15.668 | 39.587 |
| TSC-9 | |||||||
| WR | 0.512930(3) | 5.7 | 0.283014(4) | 8.6 | 19.987 | 15.675 | 39.647 |
| cpx | 0.512922(3) | 5.5 | 0.282996(4) | 7.9 | 19.978 | 15.669 | 39.620 |
* WR samples (italicised) are from Bryce et al. (2011) and are reported here for convenience. For the clinopyroxene samples, aliquots of 0.5 to 2 mm clinopyroxene handpicked from the TSC lavas were first leached in hot (~120° C) 6 N HCl to remove any Pb surface contamination following techniques outlined in Blichert-Toft and Albarède (2009). The resulting residues were subsequently digested in a mixture of concentrated HF-HNO3. Lead was separated prior to Hf and Nd separation using techniques described in Bryce and DePaolo (2004). Total Pb procedural blanks were <40 pg. Hafnium was separated from the Pb column eluent using the three column procedure described for high magnesium samples by Blichert-Toft (2001). Neodymium was from the Pb column eluent, separated from the residue of the first Hf column using a three column procedure starting with a small (0.5 mL) cation exchange resin (AG50x8) to strip off Fe and other major ions. The REE-rich elutions were subsequently passed through a 0.5 mL column filled with TRU-Spec resin to concentrate further the REEs, where the 2 M HNO3 was used to elute other ions and the REEs were collected with water. Nd was finally separated from Sm using a 1.6 mL LN-Spec column and a 0.25 M HCl elution. Total Nd procedural blanks were <30 pg and total Hf procedural blanks were <20 pg.
† For the Nd isotopic measurements, instrument performance was monitored with a laboratory solution, and accuracy was assessed through repeated (n = 9) analyses of BCR-1 which yielded 0.512638 (with external 2σ = 0.000020). εNd was calculated using a CHUR value of 143Nd/144Nd = 0.512638.
§ Hf isotopic analyses were obtained following the techniques described in Blichert-Toft et al. (1997). The 100 ppb JMC 475 Hf standard, run throughout the analytical session (n = 21) to monitor instrument performance, yielded 176Hf/177Hf = 0.282160 (with external 2σ = 0.000015). εHf was calculated using a CHUR value of 176Hf/177Hf = 0.282772 (Blichert-Toft and Albarède, 1997).
# Mass fractionation in Pb isotope analyses was corrected via Tl normalisation as described in White et al. (2000), and ratios were additionally adjusted for drift using the standard bracketing technique outlined in Albarède et al. (2004) using the NIST SRM values reported in Eisele et al. (2003). Four NIST SRM 981 samples, run as “blind” amongst the 17 bracketing standards analysed, yielded averages (with 2σ external precision) of 208Pb/204Pb = 36.7271 (0.0019), 207Pb/204Pb = 15.4978 (0.0009) and 206Pb/204Pb = 16.9408 (0.0012).
Clinopyroxene Thermobarometry Model Evaluation
Experimental dataset
Although cpx-liquid thermobarometry (Putirka et al., 2003) has been used successfully with recent Etna lavas (e.g., Armienti et al., 2007), single-cpx models avoid the additional uncertainty inherent in back-calculating plausible equilibrium liquids. Crystallisation temperatures and pressures were solved iteratively using a single-clinopyroxene thermometer, Eq. 32d in Putirka (2008) but referred to here as Eq. S-2, and single-clinopyroxene barometer for hydrous systems (Eq. 32b of that work, here as Eq. S-3).
Eq. S-2
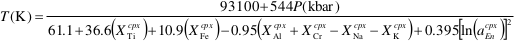
Eq. S-3
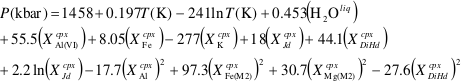
This section details the selection criteria for the experimental clinopyroxene-liquid pairs bracketing the Timpe Santa Caterina whole rock (Bryce, 1998) and clinopyroxene compositions (Table S-4) in order to evaluate the accuracy of published clinopyroxene thermobarometric models for alkaline magmas. Experiments containing coexisting clinopyroxene and liquid phases at 0-1.0 GPa were culled from the Library of Experimental Phase Relations (LEPR) database (Hirschmann et al., 2008). Liquid composition constraints consisted of SiO2 = 45–52 wt. %, Al2O3 = 15–20 wt. %, and MgO < 8.1 wt.%, ranges designed to span the potential TSC liquid compositions coexisting with clinopyroxene. Experiments were further culled by limiting clinopyroxene Al2O3 content to <9 wt. % and CaO wt. % to within 2 wt. % of the upper and lower concentrations observed in TSC clinopyroxene (range 18–25 wt. %). This produced a clinopyroxene-liquid dataset of >100 pairs from experimental studies (Hess et al., 1978; Mahood and Baker, 1986; Baker et al., 1987; Sack et al., 1987; Tormey et al., 1987; Gee and Sack, 1988; Kelemen et al., 1990; Kennedy et al., 1990; Sisson and Grove, 1993; Kawamoto, 1996; Métrich and Rutherford, 1998; Wood and Trigila, 2001; Pichavant et al., 2002; Grove et al., 2003; Barclay and Carmichael, 2004; Nekvasil et al., 2004; Di Carlo et al., 2006; Feig et al., 2006; Scoates et al., 2006; Almeev et al., 2007; Villiger et al., 2007; Mercer and Johnston, 2008; Conte et al., 2009).
Table S-6 Ranges of whole rock and clinopyroxene major and minor element compositions (wt. %) observed for the Timpe Santa Caterina flows studied.
| Lava | Clinopyroxene | |||
| Low | High | Low | High | |
| SiO2 | 46.6 | 50.7 | 45.8 | 51 |
| TiO2 | 1.6 | 2.1 | 0.9 | 2.7 |
| Al2O3 | 16.8 | 19.8 | 3 | 7.7 |
| FeO* | 8.2 | 10.9 | 5.2 | 9.2 |
| MnO | 0.15 | 0.19 | 0 | 0.19 |
| MgO | 3.4 | 6.3 | 12 | 15.6 |
| CaO | 8.7 | 10.9 | 20.3 | 23.1 |
| Na2O | 3.7 | 5.5 | 0.3 | 0.8 |
| K2O | 1 | 2.1 | 0 | 0.02 |
| P2O5 | 0.5 | 1.4 | 0 | 0.24 |
P-T estimates for TSC clinopyroxene crystallisation based on these models indicate they record a wide range of crustal pressures, from just above the current Moho to the near-surface, with a corresponding temperature range of 1175–1060 °C. Because the barometer requires temperature as an input, sensitivity to temperature was evaluated. Varying barometer input temperatures (obtained from single-cpx thermometer Eq. 32d of Putirka, 2008) by ±50 ºC yields calculated pressures on average 0.12 GPa lower and 0.18 GPa higher, respectively, for these compositions. Undegassed melt H2O content was assumed to be 3 wt. %, after Métrich et al. (2004). However, adopting a H2O input of 2 wt. % generates pressure estimates averaging ~0.05 GPa lower and temperatures ~5 ºC lower than for 3 wt. % H2O.
Figure 1b shows proportions of calculated pressures (n = 287) from single-clinopyroxene thermobarometry of ancient (>80 ka) Etna clinopyroxene compositions of those reported here and previous workers (Tanguy, 1978; Nazzareni et al., 2003; Lopez et al., 2006; Ferlito et al., 2010; Giacomoni et al., 2016).
alphaMELTS v. 1.4 modelling of clinopyroxene trace element content (Fig. S-3)
TSC clinopyroxene trace elements are compared with the evolution of clinopyroxene compositions during cooling of magmas at 1.0, 0.6, and 0.2 GPa. Clinopyroxene fractionating from two mantle melts are shown in Figure S-2: a dry pyroxenite/hydrated (3 wt. % H2O) peridotite melt mixture (solid lines) and a less hydrated (1 wt. % H2O) peridotite melt (dashed lines).
Pyroxenite/peridotite mix composition. A hypothetical melt composition, ‘10pyrper03’, containing 10:90 pyroxenite/peridotite melt was calculated from liquids generated using the alphaMELTS software interface to access the (pH)MELTS family of modelling algorithms. The pyroxenite component was obtained from batch decompression melting of a dry pyroxenite with an average composition from Hyblean pyroxenite xenoliths XIP-4 and XIP-14 reported in Correale et al. (2012). The same melting conditions were applied to a Hyblean peridotite composition (average of xenoliths XIH-1 and XIH-2, Correale et al., 2012) hydrated with 3 wt. % H2O. The pyroxenite and hydrated peridotite both generated 10 % melting at ~1.5 GPa at a pHMELTS input temperature in the 1350–1360 °C range and initial fO2 of NNO+1. The 10:90 ratio was chosen because calculated Y/La concentrations of clinopyroxene coexisting with this melt approximately match the most primitive clinopyroxene in the TSC suite.
Peridotite composition. The hypothetical peridotite melt ‘perid01_20kb’ is the liquid phase produced by 5 % batch decompression melting at 2.0 GPa and 1435 °C using pHMELTS, which is calibrated for pressures between 4 and 1 GPa. It was also chosen for its ability to fractionate clinopyroxene with calculated Y/La similar to TSC clinopyroxene.
To better approximate the likely complex transport, magma replenishment, and crystallisation processes beneath ancient Etna, clinopyroxene compositional evolution in Y/La space was calculated separately as what would fractionate from batch liquids at every decreasing 20 °C temperature step in MELTS. MELTS has been calibrated for pressures at or less than 1.0 GPa and all modelled clinopyroxene compositional paths shown are based on MELTS simulations of magma differentiation (Ghiorso et al., 1995). The alphaMELTS (Smith and Asimow, 2005) default partition coefficients (Kd) of McKenzie and O’Nions (1991) were used for all trace elements in both generating the initial melts and for the isobaric fractionation runs.
Open system behaviour was approximated via a two-step process. First, MELTS batch liquids were generated using the McKenzie and O’Nions Kd values likely relevant to mantle and lower crustal conditions. Second, at each temperature step a hypothetical trace element composition of clinopyroxene instantaneously crystallising from the liquid was calculated using Kds more representative of low pressure partitioning behaviour. Models reported here use the Kds of Hauri et al. (1994) for La and Ce (0.0515 and 0.108 respectively) and Dorais and Tubrett (2008) for Y (0.575), which was calculated for use with the Hauri et al. (1994) Kd values from the partitioning model for clinopyroxene Kds of Wood and Blundy (1997). These are similar to other low pressure Kd datasets reported in Hill et al. (2000) and Laubier et al. (2014), though the choice of a somewhat low value for La is needed to reproduce the highest Y/La of more primitive TSC clinopyroxenes. Generally, the primary difference in the Kd datasets used in these models is the Y partitioning behaviour between clinopyroxene and melt, which is taken to be 0.20 at high pressure and 0.575 at low pressure. Attempts to use the hydrous system model for clinopyroxene Kd values of Sun and Liang (2012), which calculates comparatively low Kd values relative to previously mentioned models and datasets, produced poor fits to TSC clinopyroxene compositions.

Figure S-3 Clinopyroxene trace element evolution during isobaric fractionation of peridotite melt (green) and 10 % pyroxenite component melts (red) at 1.0, 0.6, and 0.2 GPa. Modelling sensitivity to starting composition is illustrated by including paths for melts with 5 % and 20 % pyroxenite component shown as blue and purple dotted lines, respectively.
The effects of apatite saturation are considered, as whole rock CIPW norm abundances of apatite range from ~1 to 3 % in TSC lavas. Middle rare earth elements (MREE) partition more strongly into apatite than light (L-) and heavy (H-) REE, with Y behaviour being similar to that of HREE. The dominant influence of apatite would be to shift modelled clinopyroxene trace element evolution paths toward lower Ce values. Simple fractional crystallisation modelling of apatite fractionation using the apatite/liquid Kds of Prowatke and Klemme (2006) early from hypothetical hydrated peridotite and mixed component source melts would extend possible compositions directly away from the observed TSC clinopyroxene trends in Y/La vs. Ce space. However, the effects of apatite fractionation on liquids in equilibrium with the most evolved TSC-2 and TSC-7 clinopyroxenes could significantly drive liquids towards lower Ce that could reproduce the TSC-3, TSC-9, and modern Etna clinopyroxene trend. In that scenario, a hydrated peridotite source is not needed and TSC and modern Etna clinopyroxene can be successfully modelled as the products of a mixed hydrated peridotite/10 % pyroxenite source with varying degrees of apatite fractionation during storage and ascent prior to eruption.
Supplementary Information References
Almeev, R.R., Holtz, F., Koepke, J., Parat, F., Botcharnikov, R.E. (2007) The effect of H2O on olivine crystallization in MORB: Experimental calibration at 200 MPa. American Mineralogist 92, 670–674.
Armienti, P., Tonarini, S., Innocenti, F., D'Orazio, M. (2007) Mount Etna pyroxene as tracer of petrogenetic processes and dynamics of the feeding system, in Beccaluva, L., Bianchini, G., and Wilson, M., ed., Cenazoic volcanism in the Mediterranean Area. Geological Society of America Special Paper 418, 265–276.
Armstrong, J.T. (1995) Citzaf—a package of correction programs for the quantitative electron microbeam X-ray analysis of thick polished materials, thin-films, and particles. Microbeam Analysis 4, 177–200.
Baker, D.R., Eggler, D.H. (1987) Compositions of anhydrous and hydrous melts coexisting with plagioclase, augite, and olivine or low-Ca pyroxene from 1 atm to 8 kbar; application to the Aleutian volcanic center of Atka. American Mineralogist 72, 12–28.
Barclay, J., Carmichael, I.S.E. (2004) A hornblende basalt from western Mexico: water-saturated phase relations constrain a pressure–temperature window of eruptibility. Journal of Petrology 45, 485–506.
Blichert-Toft, J. (2001) On the Lu-Hf isotope geochemistry of silicate rocks. Geostandards Newsletter 25, 41–56.
Blichert-Toft, J., Albarède, F. (1997) The Lu-Hf isotope geochemistry of chondrites and the evolution of the mantle-crust system. Earth and Planetary Science Letters 148, 243-258. Erratum: Earth and Planetary Science Letters 154 (1998), 349.
Blichert-Toft, J., Albarède, F. (2009) Mixing of isotopic heterogeneities in the Mauna Kea plume conduit. Earth and Planetary Science Letters 282, 190–200.
Blichert-Toft, J., Chauvel, C., Albarède, F. (1997) Separation of Hf and Lu for high-precision isotope analysis of rock samples by magnetic sector-multiple collector ICP-MS. Contributions to Mineralogy and Petrology 127, 248–260.
Bryce, J.G. (1998) Aspects of alkaline and basaltic magmagenesis: Department of Geological Sciences, University of California-Santa Barbara, Ph.D. thesis, 178 pp.
Bryce, J.G., DePaolo, D.J. (2004) Pb isotopic heterogeneity in basaltic phenocrysts. Geochimica et Cosmochimica Acta 68, 4453–4468.
Bryce, J.G., Graham, D., Blichert-Toft, J., Hanan, B.B., Miller, S., Barkman, J., Spera, F.J., Tilton, G.R. (2011) Rollback-enhanced decompression melting of a volatile-rich mantle: the ancient lavas of Mt. Etna. Mineralogical Magazine 75, 590.
Conte, A.M., Dolfi, D., Gaeta, M., Misiti, V., Mollo, S., Perinelli, C. (2009) Experimental constraints on evolution of leucite-basanite magma at 1 and 10− 4 GPa: implications for parental compositions of Roman high-potassium magmas. European Journal of Mineralogy 21, 763–782.
Correale, A., Martelli, M., Paonita, A., Rizzo, A., Brusca, L., Scribano, V. (2012) New evidence of mantle heterogeneity beneath the Hyblean Plateau (southeast Sicily, Italy) as inferred from noble gases and geochemistry of ultramafic xenoliths. Lithos 132–133, 70–81.
Corsaro, R.A., Neri, M., Pompilio, M. (2002) Paleo-environmental and volcano-tectonic evolution of the southeastern flank of Mt. Etna during the last 225 ka inferred from the volcanic succession of the ‘Timpe’, Acireale, Sicily. Journal of Volcanology and Geothermal Research 113, 289–306.
Di Carlo, I., Pichavant, M., Rotolo, S.G., Scaillet, B. (2006) Experimental crystallization of a high-K arc basalt: the golden pumice, Stromboli volcano (Italy). Journal of Petrology 47, 1317–1343.
Dorais, M.J., Tubrett, M. (2008) Identification of a subduction zone component in the Higganum dike, Central Atlantic Magmatic Province: a LA-ICPMS study of clinopyroxene with implications for flood basalt petrogenesis. Geochemistry Geophysics Geosystems 9, Q10005, doi:10.1029/2008GC002079.
Eisele, J., Abouchami, W., Galer, S.J., Hofmann, A.W. (2003) The 320 kyr Pb isotope evolution of Mauna Kea lavas recorded in the HSDP-2 drill core. Geochemistry Geophysics Geosystems 4, 8710.
Feig, S.T., Koepke, J., Snow, J.E. (2006) Effect of water on tholeiitic basalt phase equilibria: an experimental study under oxidizing conditions. Contributions to Mineralogy and Petrology 152, 611–638.
Ferlito, C., Nicotra, E. (2010) The dyke swarm of Mount Calanna (Etna, Italy): An example of the uppermost portion of a volcanic plumbing system. Bulletin of Volcanology 72, 1191-1207.
Gee, L.L., Sack, R.O. (1988) Experimental petrology of melilite nephelinites. Journal of Petrology 29, 1233–1255.
Ghiorso, M.S., Sack, R.O. (1995) Chemical mass transfer in magmatic processes IV. A revised and internally consistent thermodynamic model for the interpolation and extrapolation of liquid-solid equilibria in magmatic systems at elevated temperatures and pressures. Contributions to Mineralogy and Petrology 119, 197–212.
Giacomoni, P.P., Coltorti, M., Bryce, J.G., Fahnenstock, M.F., Guitreau, M. (2016) Mt. Etna plumbing system revealed by combined textural, compositional, and thermobarometric studies in clinopyroxenes. Contributions to Mineralogy and Petrology 171, 34, doi: 10.1007/s00410-016-1247-7.
Grove, T.L., Elkins-Tanton, L.T., Parman, S.W., Chatterjee, N., Müntener, O., Gaetani, G.A. (2003) Fractional crystallization and mantle-melting controls on calc-alkaline differentiation trends. Contributions to Mineralogy and Petrology 145, 515–533.
Hauri, E.H., Wagner, T.P., Grove, T.L. (1994) Experimental and natural partitioning of Th, U, Pb, and other trace elements between garnet, clinopyroxene, and basaltic melts. Chemical Geology 117, 149–166.
Hess, P.C., Rutherford, M.J., Campbell, H.W. (1978) Ilmenite crystallization in nonmare basalt-Genesis of KREEP and high-Ti mare basalt: In Lunar and Planetary Science Conference Proceedings 9, 705–724.
Hill, E., Wood, B.J., Blundy, J.D. (2000) The effect of Ca-Tschermaks component on trace-element partitioning between clinopyroxene and silicate melt. Lithos 53, 203–215.
Hirschmann, M.M., Ghiorso, M.S., Davis, F.A., Gordon, S.M., Mukherjee, S., Grove, T.L., Krawczynski, M., Medard, E., Till, C.B. (2008) Library of Experimental Phase Relations (LEPR): A database and Web portal for experimental magmatic phase equilibria data. Geochemistry Geophysics Geosystems 9, Q03011.
Jochum, K.., Stoll, B., Herwig, K., Willbold, M., Hofmann, A. W., Amini, M., Aarburg, S., Abouchami, W., Hellebrand, E., Mocek, B., Raczek, I., Stracke, A., Alard, O., Bouman, C., Becker, S., Ducking, M., Bratz, H., Klemd, R., de Bruin, D., Canil, D., Cornell, D., de Hoog, C.J., Dalpe, C., Danyushevsky, L., Eisenhauer, A., Gao, Y.J., Snow, J.E., Goschopf, N., Gunther, D., Latkoczy, C., Guillong, M., Hauri, E.H., Hofer, H.E., Lahaye, Y., Horz, K., Jacob, D.E., Kassemann, S.A., Kent, A.J.R., Ludwig, T., Zack, T., Mason, P.R.D., Meixner, A., Rosner, M., Misawa, K. J., Nash, B.P., Pfander, J., Premo, W.R., Sun, W.D., Tiepolo, M., Vannucci, R., Vennemann, T., Wayne, D., Woodhead, J.D. (2006). MPI-DING reference glasses for in situ microanalysis: New reference values for element concentrations and isotope ratios. Geochemistry Geophysics Geosystems 7, Q02008.
Kawamoto, T. (1996) Experimental constraints on differentiation and H2O abundance of calc-alkaline magmas. Earth and Planetary Science Letters 144, 577–589.
Kelemen, P.B., Joyce, D.B., Webster, J.D., Holloway, J.R. (1990) Reaction between ultramafic rock and fractionating basaltic magma II. Experimental investigation of reaction between olivine tholeiite and harzburgite at 1150–1050 C and 5 kb. Journal of Petrology 31, 99–134.
Kennedy, A.K., Grove, T.L., Johnson, R.W. (1990) Experimental and major element constraints on the evolution of lavas from Lihir Island, Papua New Guinea. Contributions to Mineralogy and Petrology 104, 722–734.
Laubier, M., Grove, T.L., Langmuir, C.H. (2014) Trace element mineral/melt partitioning for basaltic and basaltic andesitic melts: An experimental and laser ICP-MS study with application to the oxidation state of mantle source regions. Earth and Planet Science Letters 392, 265–278.
Lopez, M., Pompilio, M., Rotolo, S.G. (2006) Petrology of some amphibole-bearing volcanics of the pre-Ellittico period (102-80 ka), Mt. Etna. Periodico di Mineralogia 75, 151–166.
Mahood, G.A., Baker, D.R. (1986) Experimental constraints on depths of fractionation of mildly alkalic basalts and associated felsic rocks: Pantelleria, Strait of Sicily. Contributions to Mineralogy and Petrology 93, 251–264.
McKenzie, D., O'Nions, R.K., (1991) Partial Melt Distributions from Inversion of Rare-Earth Element Concentrations. Journal of Petrology 32, 1021–1091.
Mercer, C.N., Johnston, A.D. (2008) Experimental studies of the P–T–H2O near-liquidus phase relations of basaltic andesite from North Sister Volcano, High Oregon Cascades: constraints on lower-crustal mineral assemblages. Contributions to Mineralogy and Petrology 155, 571–592.
Métrich, N., Allard, P., Spilliaert, N., Andronico, D., Burton, M. (2004) 2001 flank eruption of the alkali- and volatile-rich primitive basalt responsible for Mount Etna’s evolution in the last three decades. Earth and Planetary Science Letters 228, 1–17.
Métrich, N., Rutherford, M.J. (1998) Low pressure crystallization paths of H2O-saturated basaltic-hawaiitic melts from Mt Etna: Implications for open-system degassing of basaltic volcanoes. Geochimica et Cosmochimica Acta 62, 1195–1205.
Nazzareni, S., Busa, T., Cristofolini, R. (2003) Magmatic crystallization of Cr-Al diopside and Al-Fe3+ diopside from the ancient alkaline basalts (Mt. Etna, Sicily) European Journal of Mineralogy 15, 81–93.
Nekvasil, H., Dondolini, A., Horn, J., Filiberto, J., Long, H., Lindsley, D.H. (2004) The origin and evolution of silica-saturated alkalic suites: an experimental study. Journal of Petrology 45, 693–721.
Pichavant, M., Martel, C., Bourdier, J.L., Scaillet, B. (2002) Physical conditions, structure and dynamics of a zoned magma chamber: Mount Pelée (Martinique, Lesser Antilles arc). Journal of Geophysical Research B: Solid Earth 107, 101029–101055.
Prowatke, S., Klemme, S. (2006) Trace element partitioning between apatite and silicate melts. Geochimica et Cosmochimica Acta 70, 4513–4527.
Putirka, K.D. (2008) Thermometers and barometers for volcanic systems. Reviews in Mineralogy and Geochemistry 69, 61–120.
Putirka, K.D., Mikaelian, H., Ryerson, F., Shaw, H. (2003) New clinopyroxene-liquid thermometers for mafic, evolved, and volatile-bearing lava compositions, with applications to lavas from Tibet and the Snake River Plain, Idaho. American Mineralogist 88, 1542-1554.
Rosenbaum, G., Lister, G.S. (2004) Neogene and Quaternary rollback evolution of the Tyrrhenian Sea, the Apennines, and the Sicilian Maghrebides. Tectonics 23, TC1013, doi:10.1029/2003TC001518.
Ryan, W.B.F., Carbotte, S.M., Coplan, J.O., O'Hara, S., Melkonian, A., Arko, R., Weissel, R.A., Ferrini, V., Goodwillie, A., Nitsche, F., Bonczkowski, J., Zemsky, R. (2009) Global Multi-Resolution Topography synthesis. Geochemistry Geophysics Geosystems 10, Q03014, doi:10.1029/2008GC002332.
Sack, R.O., Walker, D., Carmichael, I.S. (1987) Experimental petrology of alkalic lavas: constraints on cotectics of multiple saturation in natural basic liquids. Contributions to Mineralogy and Petrology 96, 1–23.
Scoates, J.S., Lo Cascio, M., Weis, D., Lindsley, D.H. (2006) Experimental constraints on the origin and evolution of mildly alkalic basalts from the Kerguelen Archipelago, Southeast Indian Ocean. Contributions to Mineralogy and Petrology 151, 582–599.
Sisson, T.W., Grove, T.L. (1993) Experimental investigations of the role of H2O in calc-alkaline differentiation and subduction zone magmatism. Contributions to Mineralogy and Petrology 113, 143–166.
Smith, P.M., Asimow, P.D. (2005) Adiabat_1ph: A new public front-end to the MELTS, pMELTS, and pHMELTS models. Geochemistry Geophysics Geosystems 6, Q02004.
Sun, C., Liang, Y. (2012) Distribution of REE between clinopyroxene and basaltic melt along a mantle adiabat: effects of major element composition, water, and temperature. Contributions to Mineralogy and Petrology 163, 807–823.
Tanguy, J.-C. (1978) Tholeiitic basalt magmatism of Mount Etna and its relations with the alkaline series. Contributions to Mineralogy and Petrology 66, 51–67.
Tormey, D.R., Grove, T.L., Bryan, W.B. (1987) Experimental petrology of normal MORB near the Kane Fracture Zone: 22–25 N, mid-Atlantic ridge. Contributions to Mineralogy and Petrology 96, 121–139.
Villiger, S., Ulmer, P., Müntener, O. (2007) Equilibrium and fractional crystallization experiments at 0-7 GPa; the effect of pressure on phase relations and liquid compositions of tholeiitic magmas. Journal of Petrology 48, 159–184.
White, W.M., Albarède, F., Telouk, P. (2000) High-precision analysis of Pb isotope ratios by multi-collector ICP-MS. Chemical Geology 167, 257–270.
Wood, B.J., Blundy, J.D. (1997) A predictive model for rare earth element partitioning between clinopyroxene and anhydrous silicate melt. Contributions to Mineralogy and Petrology 129, 166–181.
Wood, B.J., Trigila, R. (2001) Experimental determination of aluminous clinopyroxene–melt partition coefficients for potassic liquids, with application to the evolution of the Roman province potassic magmas. Chemical Geology 172, 213–223.
Figures and Tables
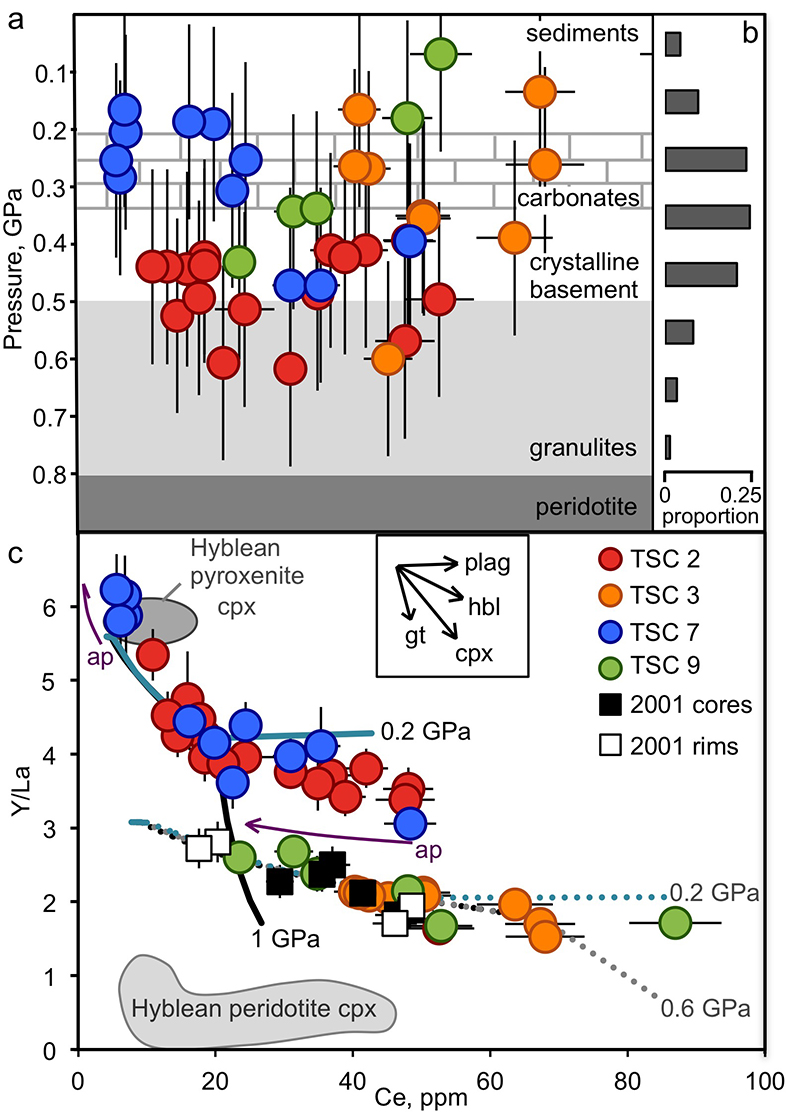
Figure 1 (a) Ce contents of TSC cpx as a function of single-cpx pressure estimates (1σ uncertainty) superimposed on Etna stratigraphy (after Spilliaert et al., 2006
Spilliaert, N., Allard, P., Métrich, N., Sobolev, A.V. (2006) Melt inclusion record of the conditions of ascent, degassing, and extrusion of volatile-rich alkali basalt during the powerful 2002 flank eruption of Mount Etna (Italy). Journal of Geophysical Research 111, B04203.
). (b) Proportions of ancient Etna barometry from this study and previous work (cf. Supplementary Information, n = 287). (c) TSC cpx and 2001 eruption cpx (Viccaro et al., 2006Viccaro, M., Ferlito, C., Cortesogno, L., Cristofolini, R., Gaggero, L. (2006) Magma mixing during the 2001 event at Mount Etna (Italy): effects on the eruptive dynamics. Journal of Volcanology and Geothermal Research 149, 139–159.
) shown with Hyblean pyroxenite and peridotite cpx fields (Correale et al., 2012Correale, A., Martelli, M., Paonita, A., Rizzo, A., Brusca, L., Scribano, V. (2012) New evidence of mantle heterogeneity beneath the Hyblean Plateau (southeast Sicily, Italy) as inferred from noble gases and geochemistry of ultramafic xenoliths. Lithos 132–133, 70–81.
, and references therein). Isobaric cpx fractionation modelling for peridotite melt (solid lines) and pyroxenite melt (dashed lines) at 1.0 (black), 0.6 (grey), and 0.2 (blue) GPa performed using alphaMELTS (Smith and Asimow, 2005Smith, P.M., Asimow, P.D. (2005) Adiabat_1ph: A new public front-end to the MELTS, pMELTS, and pHMELTS models. Geochemistry Geophysics Geosystems 6, Q02004.
); conditions described in Supplementary Information. Fractionation of apatite, well known to incorporate REEs, is modelled in purple using the partitioning of Prowatke and Klemme (2006)Prowatke, S., Klemme, S. (2006) Trace element partitioning between apatite and silicate melts. Geochimica et Cosmochimica Acta 70, 4513–4527.
. Ol+cpx±opx+sp is present at the start of both trends, though olivine drops out at T < ~1100 °C for pyroxenite melt.
Figure 2 Ancient Etna cpx and WR data. (a) εHf vs. εNd for recent and historic Etna and the Mediterranean region. Historic Etna, mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) fields from Lassiter et al., 2003
Lassiter, J.C., Blichert-Toft, J., Hauri, E.H., Barsczus, H.G. (2003) Isotope and trace element variations in lavas from Raivavae and Rapa, Cook‚ Austral islands: constraints on the nature of HIMU- and EM-mantle and the origin of mid-plate volcanism in French Polynesia. Chemical Geology 202, 115–138.
; Stracke et al., 2003Stracke, A., Bizimis, M., Salters, V.J.M. (2003) Recycling oceanic crust: Quantitative constraints. Geochemistry Geophysics Geosystems 4, 8003.
; Gaffney et al., 2004Gaffney, A.M., Nelson, B.K., Blichert-Toft, J. (2004) Geochemical constraints on the role of oceanic lithosphere in intra-volcano heterogeneity at West Maui, Hawaii. Journal of Petrology 45, 1663–1687.
; Huang et al., 2005Huang, S., Frey, F.A., Blichert-Toft, J., Fodor, R.V., Bauer, G.R., Xu, G. (2005) Enriched components in the Hawaiian plume: Evidence from Kahoolawe Volcano, Hawaii. Geochemistry Geophysics Geosystems 6, Q11006.
; Xu et al., 2007Xu, G., Frey, F.A., Clague, D.A., Abouchami, W., Blichert-Toft, J., Cousens, B., Weisler, M. (2007) Geochemical characteristics of West Molokai shield-and postshield-stage lavas: Constraints on Hawaiian plume models. Geochemistry Geophysics Geosystems 8, Q08G21.
; Sims et al., 2008Sims, K.W.W., Blichert-Toft, J., Kyle, P.R., Pichat, S., Gauthier, P.-J., Blusztajn, J., Kelly, P., Ball, L., Layne, G. (2008) A Sr, Nd, Hf, and Pb isotope perspective on the genesis and long-term evolution of alkaline magmas from Erebus volcano, Antarctica. Journal of Volcanology and Geothermal Research 177, 606–618.
; Blichert-Toft and Albarède, 2009Blichert-Toft, J., Albarède, F. (2009) Mixing of isotopic heterogeneities in the Mauna Kea plume conduit. Earth and Planetary Science Letters 282, 190–200.
; Yamasaki et al., 2009Yamasaki, S., Kani, T., Hanan, B.B., Tagami, T. (2009) Isotopic geochemistry of Hualalai shield-stage tholeiitic basalts from submarine North Kona region, Hawaii. Journal of Volcanology and Geothermal Research 185, 223–230.
; Garcia et al., 2010Garcia, M.O., Swinnard, L., Weis, D., Greene, A.R., Tagami, T., Sano, H., Gandy, C.E. (2010) Petrology, geochemistry and geochronology of Kaua ‘i Lavas over 4· 5 Myr: Implications for the origin of rejuvenated volcanism and the evolution of the Hawaiian plume. Journal of Petrology 51, 1507–1540.
; Peate et al., 2010Peate, D.W., Breddam, K., Baker, J.A., Kurz, M.D., Barker, A.K., Prestvik, T., Grassineau, N., Skovgaard, A.C. (2010) Compositional characteristics and spatial distribution of enriched Icelandic mantle components. Journal of Petrology 51, 1447–1475.
; Chekol et al., 2011Chekol, T.A., Kobayashi, K., Yokoyama, T., Sakaguchi, C., Nakamura, E. (2011) Timescales of magma differentiation from basalt to andesite beneath Hekla Volcano, Iceland: Constraints from U-series disequilibria in lavas from the last quarter-millennium flows. Geochimica et Cosmochimica Acta 75, 256–283.
; Salters et al., 2011Salters, V.J.M., Mallick, S., Hart, S.R., Langmuir, C.E., Stracke, A. (2011) Domains of depleted mantle: New evidence from hafnium and neodymium isotopes. Geochemistry Geophysics Geosystems 12, Q08001.
; Viccaro et al., 2011Viccaro, M., Nicotra, E., Millar, I.L., Cristofolini, R. (2011) The magma source at Mount Etna volcano: Perspectives from the Hf isotope composition of historic and recent lavas. Chemical Geology 281, 343–351.
); mantle components from Zindler and Hart, 1986Zindler, A., Hart, S. (1986) Chemical geodynamics. Annual Review of Earth and Planetary Sciences 14, 493–571.
; Salters and White, 1998Salters, V.J.M., White, W.M. (1998) Hf isotope constraints on mantle evolution. Chemical Geology 145, 447–460.
; Workman et al., 2004Workman, R.K., Hart, S.R., Jackson, M., Regelous, M., Farley, K.A., Blusztajn, J., Kurz, M., Staudigel, H. (2004) Recycled metasomatized lithosphere as the origin of the Enriched Mantle II (EM2) end-member: Evidence from the Samoan Volcanic Chain. Geochemistry Geophysics Geosystems 5, Q04008.
; Stracke et al., 2005Stracke, A., Hofmann, A.W., Hart, S.R. (2005) FOZO, HIMU, and the rest of the mantle zoo. Geochemistry Geophysics Geosystems 6, Q05007.
; Workman and Hart, 2005Workman, R.K., Hart, S.R. (2005) Major and trace element composition of the depleted MORB mantle (DMM). Earth and Planetary Science Letters 231, 53–72.
. Hafnium isotopic values for Italian sediments (Conticelli et al., 2002Conticelli, S., D'Antonio, M., Pinarelli, L., Civetta, L. (2002) Source contamination and mantle heterogeneity in the genesis of Italian potassic and ultrapotassic volcanic rocks: Sr‚ Nd‚ Pb isotope data from Roman Province and Southern Tuscany. Mineralogy and Petrology 74,189–222.
; Brems et al., 2013Brems, D., Ganio, M., Latruwe, K., Balcaen, L., Carremans, M., Gimeno, D., Silvestri, A., Vanhaecke, F., Muchez, P., Degryse, P. (2013) Isotopes on the beach, part 2: neodymium isotopic analysis for the provenancing of Roman glass-making. Archaeometry 55, 449–464.
) are calculated from Nd isotopic data and both cases following the seawater array (SA) and the terrestrial array (TA) of Vervoort et al. (2011)Vervoort, J.D., Plank, T., Prytulak, J. (2011) The Hf-Nd isotopic composition of marine sediments. Geochimica et Cosmochimica Acta 75, 5903–5926.
are shown. (b) 208Pb/204Pb vs. 206Pb/204Pb shown with OIB and mid-ocean ridge basalt (MORB) fields, historic Etna (Viccaro and Cristofolini, 2008Viccaro, M., Cristofolini, R. (2008) Nature of mantle heterogeneity and its role in the short-term geochemical and volcanological evolution of Mt. Etna (Italy). Lithos 105, 272–288.
) and Hyblean Plateau field from Trua et al. (1998)Trua, T., Esperança, S., Mazzuoli, R. (1998) The evolution of the lithospheric mantle along the N. African Plate: geochemical and isotopic evidence from the tholeiitic and alkaline volcanic rocks of the Hyblean plateau, Italy. Contributions to Mineralogy and Petrology 131, 307–322.
. Italian crustal values from Conticelli et al. (2002)Conticelli, S., D'Antonio, M., Pinarelli, L., Civetta, L. (2002) Source contamination and mantle heterogeneity in the genesis of Italian potassic and ultrapotassic volcanic rocks: Sr‚ Nd‚ Pb isotope data from Roman Province and Southern Tuscany. Mineralogy and Petrology 74,189–222.
. External reproducibility is conservatively set at 0.01 for 206Pb/204Pb and 0.02 for 208Pb/204Pb.Back to article
Supplementary Figures and Tables
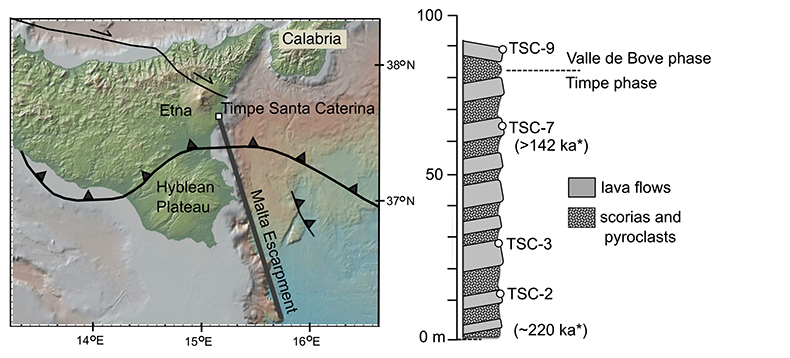
Figure S-1 Location of Timpe Santa Caterina outcrop on base map from GeoMapApp (http://www.geomapapp.org; Ryan et al., 2009). Major geologic features from Rosenbaum and Lister (2004). Stratigraphy based on Corsaro et al. (2002) with section base at sea level (0 m). Dates (*) from Gillot et al. (1994).
Figure S-2 Back scattered electron images of representative clinopyroxene grains from TSC lavas with laser ablation spots (Alfred University electron microprobe).
Table S-1 Electron microprobe analyses of TSC* clinopyroxene LA-ICP-MS laser spots. All iron reported as FeO. Operating conditions used at the University of Oregon (UO) and Massachusetts Institute of Technology (MIT) facilities were 15 keV accelerating voltage and 10 nA beam current, with all analyses using a focused beam of ~1 microns and 30 s count times. Data were reduced using the CITZAF correction procedure of Armstrong (1995). The few totals lower than 98 wt. % have been omitted. MIT JEOL JXA-8200 electron microprobe uncertainties (1σ) are calculated from the standard deviation of replicate analyses of the DJ35 diopside-jadeite glass standard and several points inferred from back scattered electron imaging to be from the same clinopyroxene crystal growth zone.
Analyses (wt. %) near laser spots TSC2_G1_3, TSC_G3_1, TSC2_G4_2, TSC7_G2_2, and TSC9_G5_2 totalled <98 wt. %. CaO abundances of the nearest same-grain spot were used to calibrate trace element concentrations (from TSC2_G1_2, TSC2_G3_2, TSC2_G4_1, TSC7_G2_1, and TSC9_G5_2, respectively).
| TSC2_G1_1 | 1 σ | TSC2_G1_2 | 1 σ | TSC2_G1_4 | 1 σ | TSC2_G3_2 | 1 σ | TSC2_G3_3 | 1 σ | |||||
| SiO2 | 46.19 | 0.10 | 46.07 | 0.10 | 47.40 | 0.15 | 49.74 | 0.10 | 49.77 | 0.10 | ||||
| TiO2 | 1.78 | 0.03 | 1.96 | 0.03 | 2.02 | 0.01 | 0.95 | 0.02 | 0.94 | 0.02 | ||||
| Al2O3 | 7.08 | 0.04 | 7.46 | 0.04 | 7.67 | 0.03 | 3.78 | 0.03 | 4.06 | 0.03 | ||||
| FeO | 8.70 | 0.16 | 9.22 | 0.16 | 8.99 | 0.04 | 8.23 | 0.15 | 7.85 | 0.15 | ||||
| MnO | 0.13 | 0.01 | 0.15 | 0.01 | 0.17 | 0.01 | 0.17 | 0.01 | 0.18 | 0.01 | ||||
| MgO | 12.76 | 0.05 | 12.53 | 0.05 | 11.96 | 0.05 | 14.71 | 0.06 | 14.43 | 0.06 | ||||
| CaO | 21.19 | 0.07 | 20.93 | 0.07 | 20.87 | 0.15 | 20.98 | 0.07 | 21.32 | 0.07 | ||||
| Na2O | 0.59 | 0.03 | 0.57 | 0.03 | 0.49 | 0.02 | 0.54 | 0.03 | 0.45 | 0.03 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.02 | 0.03 | 0.04 | 0.01 | 0.04 | 0.03 | 0.00 | 0.03 | ||||
| TOTAL | 98.43 | 98.91 | 99.62 | 99.14 | 98.99 | |||||||||
| TSC2_G3_4 | 1 σ | TSC2_G3_5 | 1 σ | TSC2_G3_6 | 1 σ | TSC2_G4_1 | 1 σ | TSC2_G2_1 | 1 σ | |||||
| SiO2 | 48.41 | 0.10 | 48.81 | 0.10 | 48.21 | 0.10 | 48.03 | 0.10 | 47.13 | 0.10 | ||||
| TiO2 | 1.30 | 0.02 | 1.47 | 0.02 | 2.02 | 0.03 | 1.40 | 0.02 | 1.64 | 0.02 | ||||
| Al2O3 | 5.50 | 0.04 | 4.87 | 0.03 | 5.81 | 0.04 | 6.99 | 0.04 | 6.38 | 0.04 | ||||
| FeO | 8.65 | 0.16 | 7.79 | 0.15 | 8.33 | 0.15 | 7.06 | 0.14 | 8.62 | 0.16 | ||||
| MnO | 0.14 | 0.01 | 0.16 | 0.01 | 0.17 | 0.01 | 0.06 | 0.01 | 0.14 | 0.01 | ||||
| MgO | 13.58 | 0.06 | 13.80 | 0.06 | 13.07 | 0.05 | 13.40 | 0.06 | 13.00 | 0.05 | ||||
| CaO | 21.03 | 0.07 | 21.16 | 0.07 | 21.19 | 0.07 | 21.99 | 0.07 | 21.36 | 0.07 | ||||
| Na2O | 0.56 | 0.03 | 0.58 | 0.03 | 0.52 | 0.03 | 0.40 | 0.03 | 0.74 | 0.04 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.01 | 0.03 | 0.00 | 0.00 | 0.14 | 0.03 | 0.01 | 0.03 | ||||
| TOTAL | 99.18 | 98.65 | 99.31 | 99.49 | 99.02 | |||||||||
| TSC2_G2_2 | 1 σ | TSC2_G2_3 | 1 σ | TSC2_G8_1 | 1 σ | TSC2_G8_2 | 1 σ | TSC7_G2_1 | 1 σ | |||||
| SiO2 | 47.51 | 0.10 | 48.04 | 0.10 | 47.34 | 0.10 | 47.47 | 0.10 | 47.37 | 0.10 | ||||
| TiO2 | 1.42 | 0.02 | 1.47 | 0.02 | 1.63 | 0.02 | 1.68 | 0.03 | 1.86 | 0.03 | ||||
| Al2O3 | 6.65 | 0.04 | 6.73 | 0.04 | 6.42 | 0.04 | 6.37 | 0.04 | 7.16 | 0.04 | ||||
| FeO | 8.23 | 0.15 | 8.11 | 0.15 | 8.62 | 0.16 | 8.33 | 0.15 | 7.83 | 0.15 | ||||
| MnO | 0.12 | 0.01 | 0.13 | 0.01 | 0.15 | 0.01 | 0.14 | 0.01 | 0.12 | 0.01 | ||||
| MgO | 13.18 | 0.06 | 13.20 | 0.06 | 12.61 | 0.05 | 12.47 | 0.05 | 12.83 | 0.05 | ||||
| CaO | 21.50 | 0.07 | 21.19 | 0.07 | 21.16 | 0.07 | 21.08 | 0.07 | 21.71 | 0.07 | ||||
| Na2O | 0.58 | 0.03 | 0.59 | 0.03 | 0.56 | 0.03 | 0.62 | 0.03 | 0.55 | 0.03 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.02 | 0.03 | 0.01 | 0.03 | 0.02 | 0.03 | 0.00 | 0.03 | 0.01 | 0.03 | ||||
| TOTAL | 99.22 | 99.47 | 98.51 | 98.15 | 99.43 | |||||||||
| TSC7_G5_1 | 1 σ | TSC7_G7_1 | 1 σ | TSC7_G9_1 | 1 σ | TSC7_G10_1 | 1 σ | TSC7_G10_2 | 1 σ | |||||
| SiO2 | 46.86 | 0.10 | 48.29 | 0.10 | 46.40 | 0.10 | 48.66 | 0.10 | 48.80 | 0.10 | ||||
| TiO2 | 1.87 | 0.03 | 1.73 | 0.03 | 1.75 | 0.03 | 1.78 | 0.03 | 1.17 | 0.02 | ||||
| Al2O3 | 6.31 | 0.04 | 5.37 | 0.04 | 7.34 | 0.04 | 4.81 | 0.03 | 5.54 | 0.04 | ||||
| FeO | 7.83 | 0.15 | 7.95 | 0.15 | 6.65 | 0.14 | 7.84 | 0.15 | 5.69 | 0.13 | ||||
| MnO | 0.13 | 0.01 | 0.14 | 0.01 | 0.10 | 0.01 | 0.14 | 0.01 | 0.07 | 0.01 | ||||
| MgO | 12.90 | 0.05 | 13.29 | 0.06 | 13.14 | 0.05 | 13.24 | 0.06 | 14.41 | 0.06 | ||||
| CaO | 22.19 | 0.07 | 21.70 | 0.07 | 22.78 | 0.07 | 21.89 | 0.07 | 23.04 | 0.07 | ||||
| Na2O | 0.53 | 0.03 | 0.56 | 0.03 | 0.43 | 0.03 | 0.46 | 0.03 | 0.34 | 0.03 | ||||
| K2O | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | 0.04 | 0.03 | 0.13 | 0.03 | ||||
| TOTAL | 98.63 | 99.04 | 98.59 | 98.85 | 99.18 | |||||||||
| TSC7_G10_3 | 1 σ | TSC7_G10_4 | 1 σ | TSC7_G10_5 | 1 σ | TSC7_G10_6 | 1 σ | TSC3_G1_1 | 1 σ | |||||
| SiO2 | 48.24 | 0.10 | 49.07 | 0.10 | 49.50 | 0.10 | 49.48 | 0.10 | 48.68 | 0.10 | ||||
| TiO2 | 1.19 | 0.02 | 0.99 | 0.02 | 0.94 | 0.02 | 1.34 | 0.02 | 1.56 | 0.02 | ||||
| Al2O3 | 5.40 | 0.04 | 5.05 | 0.03 | 4.82 | 0.03 | 3.95 | 0.03 | 4.89 | 0.03 | ||||
| FeO | 5.55 | 0.13 | 5.38 | 0.12 | 5.42 | 0.13 | 7.90 | 0.15 | 7.47 | 0.15 | ||||
| MnO | 0.07 | 0.01 | 0.07 | 0.01 | 0.06 | 0.01 | 0.15 | 0.01 | 0.16 | 0.01 | ||||
| MgO | 14.20 | 0.06 | 14.54 | 0.06 | 14.96 | 0.06 | 14.11 | 0.06 | 13.43 | 0.06 | ||||
| CaO | 22.91 | 0.07 | 22.77 | 0.07 | 22.78 | 0.07 | 22.15 | 0.07 | 21.92 | 0.07 | ||||
| Na2O | 0.33 | 0.03 | 0.38 | 0.03 | 0.36 | 0.03 | 0.39 | 0.03 | 0.46 | 0.03 | ||||
| K2O | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.10 | 0.03 | 0.25 | 0.04 | 0.27 | 0.04 | 0.06 | 0.03 | 0.02 | 0.03 | ||||
| TOTAL | 97.99 | 98.50 | 99.12 | 99.52 | 98.59 | |||||||||
| TSC3_G1_2 | 1 σ | TSC3_G1_3 | 1 σ | TSC3_G3_1 | 1 σ | TSC3_G3_2 | 1 σ | TSC3_G3_3 | 1 σ | |||||
| SiO2 | 45.12 | 0.10 | 46.57 | 0.10 | 49.08 | 0.10 | 48.10 | 0.10 | 49.31 | 0.10 | ||||
| TiO2 | 2.51 | 0.03 | 2.19 | 0.03 | 1.52 | 0.02 | 1.56 | 0.02 | 1.41 | 0.02 | ||||
| Al2O3 | 7.87 | 0.04 | 6.86 | 0.04 | 5.45 | 0.04 | 5.15 | 0.03 | 4.26 | 0.03 | ||||
| FeO | 8.34 | 0.15 | 8.19 | 0.15 | 8.53 | 0.16 | 7.71 | 0.15 | 7.61 | 0.15 | ||||
| MnO | 0.14 | 0.01 | 0.15 | 0.01 | 0.19 | 0.01 | 0.17 | 0.01 | 0.17 | 0.01 | ||||
| MgO | 11.79 | 0.05 | 12.35 | 0.05 | 13.37 | 0.06 | 13.58 | 0.06 | 14.03 | 0.06 | ||||
| CaO | 22.01 | 0.07 | 22.16 | 0.07 | 21.03 | 0.07 | 22.24 | 0.07 | 22.18 | 0.07 | ||||
| Na2O | 0.45 | 0.03 | 0.51 | 0.03 | 0.64 | 0.03 | 0.54 | 0.03 | 0.48 | 0.03 | ||||
| K2O | 0.02 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | ||||
| Cr2O3 | 0.00 | 0.03 | 0.01 | 0.03 | 0.02 | 0.03 | 0.01 | 0.03 | 0.00 | 0.03 | ||||
| TOTAL | 98.26 | 98.99 | 99.85 | 99.05 | 99.47 | |||||||||
| TSC3_G9_1 | 1 σ | TSC3_G9_3 | 1 σ | TSC3_G9_4 | 1 σ | TSC9_G1_1 | 1 σ | TSC9_G1_2 | 1 σ | |||||
| SiO2 | 49.35 | 0.10 | 49.34 | 0.10 | 47.90 | 0.10 | 48.32 | 0.10 | 47.92 | 0.10 | ||||
| TiO2 | 1.59 | 0.02 | 1.32 | 0.02 | 1.95 | 0.03 | 1.25 | 0.02 | 1.69 | 0.03 | ||||
| Al2O3 | 5.20 | 0.04 | 4.43 | 0.03 | 6.23 | 0.04 | 6.29 | 0.04 | 5.82 | 0.04 | ||||
| FeO | 8.36 | 0.15 | 7.61 | 0.15 | 8.18 | 0.15 | 8.43 | 0.15 | 7.21 | 0.14 | ||||
| MnO | 0.16 | 0.01 | 0.15 | 0.01 | 0.15 | 0.01 | 0.14 | 0.01 | 0.11 | 0.01 | ||||
| MgO | 13.43 | 0.06 | 14.10 | 0.06 | 12.90 | 0.05 | 13.00 | 0.05 | 13.25 | 0.06 | ||||
| CaO | 22.26 | 0.07 | 22.56 | 0.07 | 21.80 | 0.07 | 22.06 | 0.07 | 22.00 | 0.07 | ||||
| Na2O | 0.57 | 0.03 | 0.37 | 0.03 | 0.48 | 0.03 | 0.43 | 0.03 | 0.44 | 0.03 | ||||
| K2O | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| Cr2O3 | 0.05 | 0.03 | 0.00 | 0.03 | 0.02 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | ||||
| TOTAL | 100.96 | 99.87 | 99.62 | 99.93 | 98.45 | |||||||||
| TSC9_G3_1 | 1 σ | TSC9_G3_2 | 1 σ | TSC9_G5_1 | 1 σ | |||||||||
| SiO2 | 47.39 | 0.10 | 48.74 | 0.10 | 47.55 | 0.10 | ||||||||
| TiO2 | 1.97 | 0.03 | 1.63 | 0.02 | 1.63 | 0.03 | ||||||||
| Al2O3 | 5.61 | 0.04 | 5.29 | 0.04 | 4.87 | 0.03 | ||||||||
| FeO | 7.52 | 0.15 | 7.27 | 0.14 | 7.66 | 0.15 | ||||||||
| MnO | 0.14 | 0.01 | 0.13 | 0.01 | 0.17 | 0.01 | ||||||||
| MgO | 13.19 | 0.06 | 13.60 | 0.06 | 13.44 | 0.06 | ||||||||
| CaO | 22.33 | 0.07 | 22.16 | 0.07 | 22.48 | 0.07 | ||||||||
| Na2O | 0.56 | 0.03 | 0.53 | 0.03 | 0.46 | 0.03 | ||||||||
| K2O | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | ||||||||
| Cr2O3 | 0.01 | 0.03 | 0.00 | 0.03 | 0.04 | 0.03 | ||||||||
| TOTAL | 98.74 | 99.36 | 98.31 | |||||||||||
* TSC samples were collected from a cliff below Via Pianetto at 37°36’21” N and 15°10’20” E from the base at sea level to the top, approximately 85 m above, as shown in the cross section of Figure S-1.
Back to article | Download in ExcelTable S-2 Trace element data (ppm) collected by LA-ICP-MS at the University of New Hampshire.
| TSC2_G1_1 | 1 s.e. | TSC2_G1_2 | 1 s.e. | TSC2_G1_3 | 1 s.e. | TSC2_G1_4 | 1 s.e. | TSC2_G3_1 | 1 s.e. | |||||
| Li | 50 | 7 | 103 | 14 | 55 | 8 | 60 | 8 | 54 | 7 | ||||
| Sc | 105 | 3 | 91 | 3 | 97 | 3 | 97 | 3 | 98 | 3 | ||||
| Ti | 10307 | 233 | 8896 | 154 | 9244 | 187 | 10272 | 294 | 5911 | 98 | ||||
| V | 317 | 13 | 257 | 11 | 318 | 14 | 327 | 15 | 224 | 10 | ||||
| Cr | 66 | 3 | 59 | 2 | 60 | 3 | 51 | 3 | 24 | 2 | ||||
| Ni | 68 | 7 | 58 | 6 | 62 | 6 | 38 | 3 | 51 | 4 | ||||
| Sr | 132 | 7 | 124 | 6 | 116 | 6 | 128 | 8 | 132 | 7 | ||||
| Y | 54 | 1 | 45 | 1 | 42 | 1 | 46 | 2 | 38 | 1 | ||||
| Zr | 128 | 4 | 107 | 4 | 99 | 4 | 114 | 6 | 75 | 3 | ||||
| Nb | 0.9 | 0.3 | 1.6 | 0.2 | 0.89 | 0.2 | 1.0 | 0.2 | 0.6 | 0.1 | ||||
| Sn | 2.3 | 0.3 | 1.0 | 0.3 | 1.8 | 0.2 | 4.2 | 0.3 | 1.6 | 0.4 | ||||
| La | 15 | 1 | 12.0 | 0.9 | 11.2 | 0.7 | 14 | 1 | 11.0 | 0.8 | ||||
| Ce | 48 | 4 | 42 | 3 | 37 | 3 | 48 | 4 | 39 | 3 | ||||
| Pr | 9.3 | 0.9 | 7.9 | 0.8 | 7.2 | 0.7 | 9 | 1 | 7.1 | 0.7 | ||||
| Nd | 49 | 4 | 43 | 3 | 38 | 3 | 45 | 3 | 38 | 3 | ||||
| Sm | 14.0 | 0.7 | 12 | 1 | 11.0 | 0.5 | 12.2 | 0.7 | 9.6 | 0.4 | ||||
| Eu | 4.5 | 0.5 | 4.0 | 0.4 | 3.3 | 0.3 | 4.6 | 0.4 | 3.5 | 0.3 | ||||
| Gd | 15 | 2 | 15 | 1 | 12 | 1 | 13 | 2 | 10.0 | 0.8 | ||||
| Tb | 2.3 | 0.2 | 2.2 | 0.2 | 1.5 | 0.1 | 1.8 | 0.2 | 1.8 | 0.1 | ||||
| Dy | 14.0 | 0.5 | 10 | 1 | 9.7 | 0.6 | 12 | 1 | 10.4 | 0.5 | ||||
| Ho | 2.5 | 0.1 | 2.25 | 0.2 | 2.0 | 0.1 | 1.9 | 0.1 | 1.9 | 0.1 | ||||
| Er | 7.7 | 0.7 | 5.1 | 0.6 | 5.0 | 0.5 | 5.4 | 0.7 | 4.4 | 0.6 | ||||
| Tm | 0.88 | 0.07 | 0.62 | 0.09 | 0.72 | 0.02 | 0.5 | 0.1 | 0.60 | 0.02 | ||||
| Yb | 3.6 | 0.4 | 3.9 | 0.3 | 3.9 | 0.3 | 3.2 | 0.5 | 2.6 | 0.3 | ||||
| Lu | 0.49 | 0.07 | 0.6 | 0.1 | 0.45 | 0.09 | 0.5 | 0.2 | 0.4 | 0.1 | ||||
| Hf | 8.2 | 0.8 | 7.4 | 0.5 | 5.0 | 0.6 | 8 | 1 | 4.7 | 0.5 | ||||
| Pb | 0.14 | 0.03 | 0.17 | 0.03 | 0.19 | 0.03 | 0.4 | 0.3 | 0.21 | 0.05 | ||||
| Th | 0.29 | 0.03 | 0.20 | 0.03 | 0.19 | 0.03 | 0.25 | 0.03 | 0.13 | 0.02 | ||||
| U | 0.025 | 0.009 | 0.029 | 0.008 | 0.038 | 0.010 | 0.002 | 0.009 | 0.008 | 0.008 | ||||
| TSC2_G3_2 | 1 s.e. | TSC2_G3_3 | 1 s.e. | TSC2_G3_4 | 1 s.e. | TSC2_G3_5 | 1 s.e. | TSC2_G3_6 | 1 s.e. | |||||
| Li | 14 | 2 | 5.4 | 0.7 | 14 | 2 | 9 | 1 | 16 | 2 | ||||
| Sc | 113 | 3 | 106 | 3 | 96 | 8 | 90 | 2 | 89 | 3 | ||||
| Ti | 4888 | 85 | 4772 | 102 | 5551 | 390 | 6382 | 173 | 6255 | 125 | ||||
| V | 218 | 9 | 234 | 10 | 249 | 19 | 273 | 14 | 269 | 11 | ||||
| Cr | 24 | 1 | 30 | 1 | 32 | 3 | 46 | 2 | 42 | 2 | ||||
| Ni | 41 | 3 | 37 | 4 | 38 | 3 | 42 | 3 | 39 | 3 | ||||
| Sr | 84 | 4 | 77 | 4 | 74 | 6 | 82 | 4 | 86 | 4 | ||||
| Y | 21.6 | 0.6 | 20.2 | 0.6 | 18 | 2 | 20.4 | 0.7 | 20 | 0.6 | ||||
| Zr | 47 | 2 | 40 | 2 | 38 | 2 | 44 | 2 | 44 | 2 | ||||
| Nb | 0.36 | 0.06 | 0.41 | 0.08 | 0.29 | 0.08 | 0.59 | 0.09 | 0.8 | 0.1 | ||||
| Sn | 0.5 | 0.1 | 0.5 | 0.2 | 0.7 | 0.2 | 0.7 | 0.2 | 0.7 | 0.1 | ||||
| La | 5.1 | 0.4 | 4.3 | 0.3 | 4.2 | 0.4 | 4.6 | 0.3 | 5.1 | 0.3 | ||||
| Ce | 18 | 1 | 16 | 1 | 14 | 2 | 18 | 1 | 18 | 1 | ||||
| Pr | 3.6 | 0.4 | 3.3 | 0.3 | 3 | 0.4 | 3.3 | 0.3 | 3.7 | 0.4 | ||||
| Nd | 21 | 2 | 16 | 2 | 16 | 1 | 16 | 1 | 20 | 1 | ||||
| Sm | 5.1 | 0.3 | 4.3 | 0.2 | 4.2 | 0.7 | 3.9 | 0.1 | 3.8 | 0.2 | ||||
| Eu | 1.4 | 0.2 | 1.4 | 0.1 | 1.2 | 0.1 | 1.2 | 0.1 | 1.4 | 0.2 | ||||
| Gd | 6.7 | 0.8 | 4.8 | 0.4 | 4 | 0.8 | 4.9 | 0.4 | 5.8 | 0.5 | ||||
| Tb | 1.1 | 0.1 | 0.9 | 0.1 | 1 | 0.1 | 0.96 | 0.06 | 1 | 0.1 | ||||
| Dy | 4.9 | 0.2 | 4.4 | 0.2 | 3.4 | 0.6 | 3.9 | 0.3 | 4.7 | 0.4 | ||||
| Ho | 0.74 | 0.06 | 0.69 | 0.07 | 0.63 | 0.02 | 0.75 | 0.05 | 0.59 | 0.03 | ||||
| Er | 2.3 | 0.3 | 1.9 | 0.2 | 1.6 | 0.4 | 1.6 | 0.2 | 1.9 | 0.2 | ||||
| Tm | 0.29 | 0.05 | 0.28 | 0.02 | 0.28 | 0.06 | 0.34 | 0.05 | 0.33 | 0.04 | ||||
| Yb | 1.8 | 0.1 | 1.4 | 0.2 | 1.8 | 0.2 | 1.1 | 0.2 | 1 | 0.2 | ||||
| Lu | 0.22 | 0.05 | 0.23 | 0.04 | 0.19 | 0.03 | 0.19 | 0.03 | 0.22 | 0.03 | ||||
| Hf | 1.6 | 0.2 | 1.4 | 0.1 | 1.4 | 0.2 | 1.4 | 0.2 | 1.4 | 0.2 | ||||
| Pb | 0.068 | 0.008 | 0.13 | 0.03 | 0.08 | 0.02 | 0.13 | 0.02 | 0.14 | 0.04 | ||||
| Th | 0.050 | 0.008 | 0.045 | 0.006 | 0.05 | 0.01 | 0.05 | 0.01 | 0.11 | 0.01 | ||||
| U | 0.007 | 0.003 | 0.007 | 0.002 | 0.013 | 0.002 | 0.009 | 0.002 | 0.016 | 0.002 | ||||
| TSC2_G4_1 | 1 s.e. | TSC2_G4_2 | 1 s.e. | TSC2_G2_1 | 1 s.e. | TSC2_G2_2 | 1 s.e. | TSC2_G2_3 | 1 s.e. | |||||
| Li | 53 | 7 | 59 | 8 | 18 | 3 | 46 | 6 | 28 | 4 | ||||
| Sc | 131 | 4 | 129 | 5 | 117 | 3 | 105 | 3 | 139 | 6 | ||||
| Ti | 6293 | 115 | 6055 | 128 | 8882 | 153 | 6910 | 140 | 8867 | 314 | ||||
| V | 275 | 11 | 279 | 12 | 314 | 14 | 288 | 12 | 332 | 17 | ||||
| Cr | 642 | 23 | 640 | 27 | 42 | 3 | 54 | 3 | 44 | 3 | ||||
| Ni | 106 | 7 | 107 | 7 | 52 | 4 | 68 | 4 | 39 | 3 | ||||
| Sr | 99 | 5 | 84 | 4 | 112 | 6 | 95 | 5 | 89 | 5 | ||||
| Y | 18 | 1 | 17 | 0.6 | 41 | 1 | 36 | 2 | 38 | 2 | ||||
| Zr | 34 | 2 | 30 | 1 | 84 | 3 | 102 | 7 | 81 | 4 | ||||
| Nb | 0.42 | 0.08 | 0.1 | 0.1 | 0.6 | 0.1 | 4.7 | 0.5 | 1.1 | 0.2 | ||||
| Sn | 0.5 | 0.2 | 0.5 | 0.2 | 1.6 | 0.2 | 0.8 | 0.2 | 1.2 | 0.3 | ||||
| La | 4.1 | 0.3 | 3.2 | 0.2 | 11.4 | 0.7 | 22 | 2 | 10.1 | 0.8 | ||||
| Ce | 13 | 1 | 10.9 | 0.9 | 35 | 3 | 53 | 5 | 31 | 3 | ||||
| Pr | 2.5 | 0.3 | 2.2 | 0.3 | 6.6 | 0.7 | 8 | 1 | 6 | 0.6 | ||||
| Nd | 13 | 1 | 12 | 1 | 38 | 3 | 37 | 4 | 32 | 2 | ||||
| Sm | 3.6 | 0.5 | 3.1 | 0.2 | 10.4 | 0.9 | 8.8 | 0.8 | 9.5 | 0.2 | ||||
| Eu | 1.5 | 0.2 | 1 | 0.1 | 3.1 | 0.3 | 2.4 | 0.3 | 2.7 | 0.3 | ||||
| Gd | 5 | 0.7 | 5.3 | 0.8 | 13 | 1 | 10 | 1 | 10 | 1 | ||||
| Tb | 0.7 | 0.1 | 0.56 | 0.09 | 1.6 | 0.1 | 1.4 | 0.1 | 1.3 | 0.1 | ||||
| Dy | 3.3 | 0.1 | 4 | 0.4 | 8.2 | 0.6 | 7.9 | 0.3 | 7.5 | 0.4 | ||||
| Ho | 1 | 0.04 | 0.61 | 0.03 | 1.5 | 0.1 | 1.4 | 0.1 | 1.4 | 0.1 | ||||
| Er | 1.1 | 0.3 | 1.7 | 0.3 | 4.8 | 0.4 | 4.3 | 0.5 | 3.3 | 0.4 | ||||
| Tm | 0.27 | 0.07 | 0.23 | 0.07 | 0.55 | 0.06 | 0.6 | 0.1 | 0.34 | 0.04 | ||||
| Yb | 1.3 | 0.2 | 0.6 | 0.3 | 3.5 | 0.2 | 3.1 | 0.3 | 2.4 | 0.4 | ||||
| Lu | 0.32 | 0.04 | 0.21 | 0.09 | 0.54 | 0.09 | 0.7 | 0.1 | 0.35 | 0.06 | ||||
| Hf | 1.1 | 0.2 | 1.3 | 0.1 | 4.2 | 0.4 | 3.3 | 0.5 | 3.4 | 0.3 | ||||
| Pb | 0.03 | 0.02 | 0.09 | 0.03 | 0.08 | 0.02 | 1.4 | 0.19 | 0.11 | 0.04 | ||||
| Th | 0.062 | 0.005 | 0.04 | 0.01 | 0.2 | 0.01 | 1.8 | 0.2 | 0.19 | 0.02 | ||||
| U | 0.007 | 0.002 | 0.006 | 0.003 | 0.018 | 0.004 | 0.19 | 0.03 | 0.024 | 0.004 | ||||
| TSC2_G8_1 | 1 s.e. | TSC2_G8_2 | 1 s.e. | TSC7_G2_1 | 1 s.e. | TSC7_G2_2 | 1 s.e. | TSC7_G5_1 | 1 s.e. | |||||
| Li | 10 | 1 | 34 | 5 | 21 | 3 | 5.8 | 0.8 | 2.7 | 0.4 | ||||
| Sc | 101 | 3 | 107 | 4 | 107 | 3 | 100 | 3 | 127 | 5 | ||||
| Ti | 8210 | 151 | 8450 | 374 | 10814 | 216 | 9617 | 196 | 9624 | 290 | ||||
| V | 302 | 13 | 306 | 18 | 314 | 14 | 321 | 13 | 265 | 12 | ||||
| Cr | 52 | 2 | 48 | 2 | 76 | 6 | 68 | 3 | 360 | 39 | ||||
| Ni | 46 | 4 | 44 | 3 | 57 | 4 | 46 | 3 | 56 | 6 | ||||
| Sr | 85 | 5 | 76 | 5 | 138 | 7 | 115 | 6 | 113 | 7 | ||||
| Y | 27 | 1 | 25 | 2 | 36 | 1 | 37 | 2 | 28 | 1 | ||||
| Zr | 62 | 3 | 62 | 3 | 94 | 3 | 95 | 3 | 79 | 5 | ||||
| Nb | 0.4 | 0.1 | 0.7 | 0.1 | 1.4 | 0.2 | 0.6 | 0.1 | 0.7 | 0.2 | ||||
| Sn | 0.8 | 0.2 | 0.6 | 0.2 | 1.6 | 0.3 | 1.2 | 0.1 | 2.7 | 0.6 | ||||
| La | 6.9 | 0.4 | 6.5 | 0.5 | 9.2 | 0.7 | 9.1 | 0.6 | 6.3 | 0.7 | ||||
| Ce | 24 | 5 | 21 | 2 | 31 | 2 | 35 | 3 | 24 | 2 | ||||
| Pr | 4.5 | 0.5 | 4.5 | 0.5 | 6 | 0.6 | 6.8 | 0.7 | 4.6 | 0.5 | ||||
| Nd | 21 | 2 | 21 | 2 | 32 | 3 | 36 | 3 | 28 | 2 | ||||
| Sm | 7.3 | 0.5 | 6.1 | 0.4 | 9.2 | 0.9 | 9.2 | 0.6 | 6.3 | 0.3 | ||||
| Eu | 2.1 | 0.2 | 1.6 | 0.2 | 3 | 0.5 | 3.6 | 0.4 | 2.7 | 0.3 | ||||
| Gd | 6.9 | 0.9 | 5.9 | 0.7 | 10.7 | 0.8 | 8.4 | 0.7 | 6.4 | 0.8 | ||||
| Tb | 1 | 0.1 | 0.91 | 0.06 | 1.6 | 0.1 | 1.5 | 0.1 | 1.23 | 0.09 | ||||
| Dy | 5.9 | 0.5 | 5.6 | 0.2 | 6.5 | 0.4 | 7.2 | 0.4 | 6.2 | 0.3 | ||||
| Ho | 0.83 | 0.08 | 0.83 | 0.08 | 1.7 | 0.2 | 1.7 | 0.1 | 1.2 | 0.1 | ||||
| Er | 3.3 | 0.6 | 3.6 | 0.5 | 4.4 | 0.8 | 3.4 | 0.4 | 2.6 | 0.5 | ||||
| Tm | 0.24 | 0.03 | 0.24 | 0.03 | 0.46 | 0.09 | 0.51 | 0.03 | 0.27 | 0.08 | ||||
| Yb | 2 | 0.2 | 1.9 | 0.2 | 2.8 | 0.2 | 2.5 | 0.3 | 1.5 | 0.3 | ||||
| Lu | 0.27 | 0.08 | 0.24 | 0.05 | 0.4 | 0.1 | 0.32 | 0.05 | 0.29 | 0.06 | ||||
| Hf | 2.7 | 0.2 | 3.1 | 0.3 | 3.4 | 0.4 | 3.9 | 0.3 | 3.9 | 0.4 | ||||
| Pb | 0.08 | 0.02 | 0.05 | 0.02 | 0.12 | 0.05 | 0.13 | 0.02 | 0.1 | 0.03 | ||||
| Th | 0.12 | 0.02 | 0.09 | 0.01 | 0.15 | 0.03 | 0.15 | 0.02 | 0.079 | 0.008 | ||||
| U | 0.018 | 0.006 | 0.017 | 0.006 | 0.019 | 0.006 | 0.016 | 0.006 | 0.007 | 0.003 | ||||
| TSC7_G7_1 | 1 s.e. | TSC7_G9_1 | 1 s.e. | TSC7_G10_1 | 1 s.e. | TSC7_G10_2 | 1 s.e. | TSC7_G10_3 | 1 s.e. | |||||
| Li | 5.1 | 0.7 | 3.8 | 0.6 | 6.9 | 0.9 | 4.3 | 0.6 | 5.4 | 0.8 | ||||
| Sc | 145 | 5 | 125 | 4 | 133 | 3 | 134 | 3 | 143 | 4 | ||||
| Ti | 11294 | 172 | 9592 | 192 | 8341 | 236 | 6522 | 169 | 6252 | 113 | ||||
| V | 343 | 14 | 298 | 13 | 325 | 15 | 240 | 11 | 239 | 10 | ||||
| Cr | 44 | 2 | 109 | 4 | 751 | 38 | 1005 | 46 | 824 | 39 | ||||
| Ni | 49 | 4 | 88 | 6 | 50 | 6 | 90 | 5 | 87 | 5 | ||||
| Sr | 136 | 7 | 137 | 8 | 80 | 4 | 68 | 3 | 68 | 4 | ||||
| Y | 47 | 1 | 23 | 1 | 23 | 1 | 10 | 0 | 10 | 0 | ||||
| Zr | 137 | 5 | 56 | 3 | 73 | 3 | 24 | 1 | 23 | 1 | ||||
| Nb | 1.5 | 0.1 | 0.53 | 0.09 | 0.7 | 0.1 | 0.09 | 0.07 | 0.13 | 0.04 | ||||
| Sn | 2.1 | 0.4 | 2.4 | 0.4 | 0.5 | 0.2 | 0.6 | 0.2 | 0.54 | 0.07 | ||||
| La | 15 | 1 | 5.5 | 0.4 | 6.2 | 0.5 | 1.8 | 0.2 | 1.6 | 0.1 | ||||
| Ce | 48 | 4 | 20 | 2 | 23 | 2 | 7 | 0.6 | 6.8 | 0.5 | ||||
| Pr | 9.1 | 0.9 | 3.9 | 0.4 | 4.3 | 0.4 | 1.5 | 0.2 | 1.4 | 0.1 | ||||
| Nd | 48 | 4 | 23 | 2 | 23 | 2 | 7.8 | 0.8 | 8.1 | 0.6 | ||||
| Sm | 12.5 | 0.4 | 5.6 | 0.4 | 5.6 | 0.6 | 2.6 | 0.3 | 2.7 | 0.3 | ||||
| Eu | 4.3 | 0.4 | 2 | 0.3 | 1.6 | 0.1 | 0.81 | 0.09 | 0.8 | 0.07 | ||||
| Gd | 11.5 | 0.9 | 5.9 | 0.8 | 6.8 | 0.6 | 2.5 | 0.3 | 2.4 | 0.3 | ||||
| Tb | 1.9 | 0.2 | 1.11 | 0.09 | 1 | 0.1 | 0.51 | 0.05 | 0.48 | 0.07 | ||||
| Dy | 10.2 | 0.7 | 4.9 | 0.6 | 4.1 | 0.2 | 2.5 | 0.3 | 1.88 | 0.06 | ||||
| Ho | 2.6 | 0.3 | 1.2 | 0.1 | 0.8 | 0.03 | 0.31 | 0.02 | 0.35 | 0.03 | ||||
| Er | 4.3 | 0.5 | 2.7 | 0.3 | 2.5 | 0.3 | 0.81 | 0.09 | 0.9 | 0.1 | ||||
| Tm | 0.76 | 0.06 | 0.18 | 0.04 | 0.25 | 0.04 | 0.12 | 0.03 | 0.12 | 0.01 | ||||
| Yb | 3.3 | 0.5 | 1.4 | 0.1 | 1.9 | 0.3 | 0.7 | 0.2 | 0.9 | 0.1 | ||||
| Lu | 0.49 | 0.14 | 0.33 | 0.06 | 0.33 | 0.04 | 0.05 | 0.04 | 0.13 | 0.04 | ||||
| Hf | 5.9 | 0.6 | 2.5 | 0.3 | 3.9 | 0.3 | 1.6 | 0.2 | 1.5 | 0.1 | ||||
| Pb | 0.16 | 0.03 | 0.06 | 0.01 | 0.08 | 0.02 | 0.025 | 0.003 | 0.05 | 0.02 | ||||
| Th | 0.25 | 0.03 | 0.08 | 0.02 | 0.08 | 0.01 | 0.02 | 0.01 | 0.03 | 0.008 | ||||
| U | 0.021 | 0.003 | 0.017 | 0.006 | 0.011 | 0.004 | nd | 0.004 | 0.003 | |||||
| TSC7_G10_4 | 1 s.e. | TSC7_G10_5 | 1 s.e. | TSC7_G10_6 | 1 s.e. | TSC3_G1_1 | 1 s.e. | TSC3_G1_2 | 1 s.e. | |||||
| Li | 3.3 | 0.5 | 2.4 | 0.4 | 3 | 0.4 | 2.6 | 0.6 | 1.3 | 0.3 | ||||
| Sc | 131 | 4 | 131 | 5 | 144 | 5 | 81 | 2 | 91 | 3 | ||||
| Ti | 5276 | 143 | 5415 | 101 | 7483 | 175 | 7430 | 159 | 10948 | 178 | ||||
| V | 210 | 12 | 221 | 9 | 282 | 12 | 173 | 8 | 218 | 9 | ||||
| Cr | 2332 | 116 | 1363 | 59 | 69 | 3 | 19 | 1 | 15 | 1 | ||||
| Ni | 93 | 5 | 89 | 5 | 36 | 2 | 24 | 4 | 26 | 2 | ||||
| Sr | 64 | 3 | 62 | 3 | 76 | 4 | 188 | 10 | 196 | 10 | ||||
| Y | 9 | 1 | 9 | 0 | 19 | 1 | 36 | 1 | 40 | 1 | ||||
| Zr | 19 | 1 | 20 | 1 | 52 | 2 | 135 | 4 | 193 | 5 | ||||
| Nb | 0.18 | 0.02 | 0.15 | 0.05 | 0.34 | 0.03 | 1 | 0.1 | 2.1 | 0.2 | ||||
| Sn | 0.42 | 0.05 | 0.36 | 0.06 | 0.8 | 0.1 | 1.7 | 0.3 | 2.2 | 0.4 | ||||
| La | 1.6 | 0.1 | 1.47 | 0.09 | 4.4 | 0.3 | 17 | 1 | 24 | 1 | ||||
| Ce | 6.2 | 0.5 | 5.6 | 0.5 | 16 | 1 | 50 | 4 | 67 | 5 | ||||
| Pr | 1.3 | 0.2 | 1.2 | 0.1 | 3.2 | 0.3 | 11 | 1 | 14 | 1 | ||||
| Nd | 7.2 | 0.6 | 7.1 | 0.7 | 19 | 1 | 57 | 5 | 72 | 5 | ||||
| Sm | 2.4 | 0.6 | 2.4 | 0.2 | 4.4 | 0.3 | 10.6 | 0.5 | 14 | 0.7 | ||||
| Eu | 0.77 | 0.06 | 0.7 | 0.1 | 1.5 | 0.2 | 4.2 | 0.4 | 5.1 | 0.6 | ||||
| Gd | 1.9 | 0.2 | 2.8 | 0.2 | 5.5 | 0.6 | 16 | 1 | 18 | 2 | ||||
| Tb | 0.24 | 0.03 | 0.46 | 0.03 | 0.71 | 0.07 | 1.9 | 0.2 | 1.8 | 0.2 | ||||
| Dy | 1.7 | 0.1 | 1.81 | 0.3 | 4.2 | 0.1 | 9.8 | 0.4 | 10 | 0.6 | ||||
| Ho | 0.29 | 0.04 | 0.29 | 0.03 | 0.76 | 0.04 | 1.7 | 0.2 | 1.72 | 0.08 | ||||
| Er | 1 | 0.1 | 0.7 | 0.1 | 2.3 | 0.2 | 2.9 | 0.3 | 3.7 | 0.5 | ||||
| Tm | 0.09 | 0.02 | 0.1 | 0.04 | 0.18 | 0.03 | 0.53 | 0.05 | 0.6 | 0.1 | ||||
| Yb | 0.49 | 0.08 | 0.5 | 0.03 | 1.8 | 0.3 | 3.1 | 0.4 | 2.2 | 0.6 | ||||
| Lu | 0.08 | 0.01 | 0.07 | 0.02 | 0.19 | 0.05 | 0.4 | 0.06 | 0.5 | 0.1 | ||||
| Hf | 0.9 | 0.1 | 1.3 | 0.2 | 2.3 | 0.3 | 5.4 | 0.4 | 7.4 | 0.6 | ||||
| Pb | 0.03 | 0.01 | 0.03 | 0.01 | 0.04 | 0.01 | 0.11 | 0.02 | 0.08 | 0.01 | ||||
| Th | 0.015 | 0.004 | 0.027 | 0.006 | 0.053 | 0.006 | 0.21 | 0.01 | 0.42 | 0.06 | ||||
| U | 0.003 | 0.002 | 0.003 | 0.001 | 0.008 | 0.001 | 0.021 | 0.005 | 0.045 | 0.006 | ||||
| TSC3_G1_3 | 1 s.e. | TSC3_G3_1 | 1 s.e. | TSC3_G3_2 | 1 s.e. | TSC3_G3_3 | 1 s.e. | TSC3_G9_1 | 1 s.e. | |||||
| Li | 3.4 | 0.6 | 4.5 | 0.7 | 1 | 0.3 | 0.9 | 0.2 | 6.6 | 1 | ||||
| Sc | 93 | 3 | 60 | 2 | 84 | 3 | 101 | 3 | 113 | 4 | ||||
| Ti | 10122 | 201 | 7869 | 133 | 7534 | 145 | 6712 | 123 | 8850 | 302 | ||||
| V | 213 | 18 | 195 | 8 | 186 | 8 | 194 | 9 | 268 | 12 | ||||
| Cr | 9.5 | 0.8 | 11.6 | 0.5 | 10.3 | 0.8 | 10 | 1 | 11.5 | 0.7 | ||||
| Ni | 21 | 3 | 21 | 2 | 17 | 1 | 14 | 2 | 18 | 3 | ||||
| Sr | 255 | 19 | 163 | 8 | 157 | 8 | 149 | 8 | 181 | 12 | ||||
| Y | 40 | 1 | 31.7 | 0.8 | 29.6 | 0.7 | 29 | 1 | 37.9 | 0.9 | ||||
| Zr | 186 | 6 | 114 | 4 | 109 | 3 | 114 | 4 | 183 | 6 | ||||
| Nb | 5.4 | 0.8 | 1.1 | 0.1 | 0.76 | 0.07 | 0.9 | 0.1 | 1.8 | 0.1 | ||||
| Sn | 1.7 | 0.4 | 0.9 | 0.2 | 0.7 | 0.3 | 0.6 | 0.2 | 0.8 | 0.2 | ||||
| La | 26 | 2 | 15.5 | 0.9 | 14.2 | 0.9 | 13.5 | 0.8 | 19 | 1 | ||||
| Ce | 68 | 6 | 45 | 3 | 42 | 3 | 40 | 3 | 64 | 6 | ||||
| Pr | 13 | 1 | 9 | 1 | 9 | 1 | 8.3 | 0.8 | 12 | 1 | ||||
| Nd | 66 | 5 | 48 | 4 | 43 | 3 | 38 | 3 | 53 | 4 | ||||
| Sm | 13.7 | 0.7 | 11 | 1 | 10 | 1 | 9.7 | 0.6 | 12.8 | 0.6 | ||||
| Eu | 3.9 | 0.3 | 4 | 0 | 3 | 0 | 3.1 | 0.3 | 3.4 | 0.3 | ||||
| Gd | 17 | 2 | 12 | 1 | 12 | 1 | 9.9 | 0.8 | 13 | 1 | ||||
| Tb | 1.6 | 0.2 | 1.6 | 0.2 | 1.4 | 0.2 | 1.3 | 0.1 | 1.8 | 0.1 | ||||
| Dy | 9.6 | 0.7 | 7.4 | 0.4 | 6.1 | 0.3 | 5.5 | 0.4 | 9 | 0.5 | ||||
| Ho | 1.4 | 0.05 | 1.48 | 0.08 | 1.07 | 0.07 | 1.1 | 0.08 | 1.4 | 0.1 | ||||
| Er | 3.1 | 0.5 | 2.9 | 0.4 | 2.7 | 0.4 | 3.2 | 0.4 | 4.3 | 0.6 | ||||
| Tm | 0.54 | 0.05 | 0.39 | 0.08 | 0.29 | 0.04 | 0.4 | 0.06 | 0.38 | 0.07 | ||||
| Yb | 2.5 | 0.5 | 2.8 | 0.5 | 2.2 | 0.3 | 2.8 | 0.6 | 2.3 | 0.1 | ||||
| Lu | 0.29 | 0.07 | 0.34 | 0.07 | 0.38 | 0.08 | 0.31 | 0.08 | 0.4 | 0.06 | ||||
| Hf | 7.3 | 0.7 | 4.2 | 0.3 | 4.4 | 0.4 | 4.3 | 0.3 | 6.8 | 0.7 | ||||
| Pb | 0.9 | 0.2 | 0.07 | 0.01 | 0.11 | 0.01 | 0.04 | 0.01 | 0.16 | 0.03 | ||||
| Th | 1.2 | 0.2 | 0.23 | 0.03 | 0.15 | 0.01 | 0.17 | 0.02 | 0.35 | 0.04 | ||||
| U | 0.32 | 0.06 | 0.021 | 0.004 | 0.011 | 0.003 | 0.023 | 0.003 | 0.062 | 0.008 | ||||
| TSC3_G9_3 | 1 s.e. | TSC3_G9_4 | 1 s.e. | TSC9_G1_1 | 1 s.e. | TSC9_G1_2 | 1 s.e. | TSC9_G3_1 | 1 s.e. | |||||
| Li | 0.3 | 0.3 | 1.3 | 0.2 | 36 | 5 | 33 | 5 | 28 | 4 | ||||
| Sc | 105 | 4 | 88 | 2 | 53 | 1 | 78 | 2 | 114 | 3 | ||||
| Ti | 9521 | 375 | 9280 | 198 | 5875 | 95 | 8005 | 159 | 10648 | 166 | ||||
| V | 278 | 14 | 242 | 10 | 227 | 10 | 263 | 13 | 216 | 9 | ||||
| Cr | 21 | 2 | 13.9 | 0.7 | 36 | 3 | 42 | 2 | 23 | 1 | ||||
| Ni | 32 | 2 | 23 | 3 | 37 | 2 | 35 | 4 | 22 | 2 | ||||
| Sr | 154 | 8 | 168 | 9 | 123 | 8 | 116 | 7 | 150 | 7 | ||||
| Y | 25.8 | 0.8 | 30.8 | 0.9 | 15.6 | 0.4 | 21.4 | 0.8 | 31.1 | 0.9 | ||||
| Zr | 108 | 4 | 130 | 4 | 49 | 1 | 67 | 2 | 142 | 4 | ||||
| Nb | 1.4 | 0.2 | 1.2 | 0.2 | 0.5 | 0.1 | 0.61 | 0.07 | 1.1 | 0.1 | ||||
| Sn | 1.1 | 0.2 | 0.8 | 0.2 | 0.53 | 0.06 | 1 | 0.2 | 1.6 | 0.2 | ||||
| La | 12.2 | 0.8 | 14.8 | 0.9 | 6 | 0.4 | 8 | 0.5 | 14.5 | 0.9 | ||||
| Ce | 41 | 3 | 50 | 4 | 24 | 2 | 31 | 3 | 48 | 4 | ||||
| Pr | 8.1 | 0.8 | 10 | 1 | 4.2 | 0.4 | 5.6 | 0.6 | 8.7 | 0.9 | ||||
| Nd | 36 | 3 | 44 | 3 | 21 | 2 | 28 | 2 | 46 | 3 | ||||
| Sm | 9.2 | 0.4 | 11 | 1 | 5.8 | 0.5 | 7.6 | 0.5 | 11.6 | 0.4 | ||||
| Eu | 2.9 | 0.2 | 3.4 | 0.3 | 1.7 | 0.2 | 2.6 | 0.4 | 3.2 | 0.3 | ||||
| Gd | 8.7 | 0.9 | 9.8 | 0.8 | 4.6 | 0.5 | 6.7 | 0.9 | 10.7 | 0.9 | ||||
| Tb | 1.17 | 0.08 | 1.3 | 0.1 | 0.68 | 0.09 | 0.91 | 0.06 | 1.3 | 0.1 | ||||
| Dy | 5 | 0.1 | 6.6 | 0.3 | 3.7 | 0.5 | 5.6 | 0.4 | 7.4 | 0.4 | ||||
| Ho | 0.99 | 0.06 | 1.1 | 0.1 | 0.83 | 0.02 | 1.13 | 0.09 | 1.6 | 0.1 | ||||
| Er | 3.3 | 0.3 | 2.8 | 0.5 | 1.7 | 0.3 | 2.3 | 0.2 | 3.6 | 0.4 | ||||
| Tm | 0.39 | 0.06 | 0.42 | 0.03 | 0.21 | 0.02 | 0.24 | 0.03 | 0.28 | 0.02 | ||||
| Yb | 1.8 | 0.3 | 2.5 | 0.2 | 1.2 | 0.2 | 1.25 | 0.05 | 2 | 0.4 | ||||
| Lu | 0.29 | 0.08 | 0.37 | 0.05 | 0.07 | 0.02 | 0.18 | 0.02 | 0.29 | 0.04 | ||||
| Hf | 3.7 | 0.2 | 4.4 | 0.4 | 2.2 | 0.2 | 3 | 0.2 | 6.3 | 0.4 | ||||
| Pb | 0.04 | 0.01 | 0.05 | 0.01 | 0.04 | 0.02 | 0.04 | 0.02 | 0.1 | 0.01 | ||||
| Th | 0.19 | 0.01 | 0.19 | 0.02 | 0.088 | 0.006 | 0.1 | 0.02 | 0.2 | 0.01 | ||||
| U | 0.017 | 0.007 | 0.021 | 0.005 | 0.017 | 0.004 | 0.019 | 0.005 | 0.025 | 0.003 | ||||
| TSC9_G3_2 | 1 s.e. | TSC9_G5_1 | 1 s.e. | TSC9_G5_2 | 1 s.e. | |||||||||
| Li | 24 | 3 | 18 | 3 | 10 | 1 | ||||||||
| Sc | 99 | 2 | 99 | 5 | 114 | 3 | ||||||||
| Ti | 8647 | 135 | 9205 | 538 | 10315 | 165 | ||||||||
| V | 184 | 8 | 255 | 14 | 271 | 12 | ||||||||
| Cr | 21 | 1 | 24 | 3 | 7 | 1 | ||||||||
| Ni | 26 | 2 | 27 | 4 | 18 | 2 | ||||||||
| Sr | 146 | 7 | 151 | 14 | 184 | 9 | ||||||||
| Y | 24.5 | 0.9 | 24 | 1 | 40 | 1 | ||||||||
| Zr | 94 | 3 | 98 | 7 | 158 | 6 | ||||||||
| Nb | 0.82 | 0.07 | 0.8 | 0.1 | 1.8 | 0.2 | ||||||||
| Sn | 1.2 | 0.2 | 1.1 | 0.2 | 1.4 | 0.4 | ||||||||
| La | 10.3 | 0.6 | 14 | 1 | 24 | 1 | ||||||||
| Ce | 35 | 3 | 53 | 5 | 87 | 7 | ||||||||
| Pr | 6.3 | 0.6 | 8 | 0.8 | 14 | 1 | ||||||||
| Nd | 35 | 3 | 39 | 3 | 64 | 5 | ||||||||
| Sm | 9.2 | 0.4 | 9 | 1 | 16 | 1 | ||||||||
| Eu | 2.3 | 0.2 | 2.6 | 0.2 | 5 | 0 | ||||||||
| Gd | 8.4 | 0.9 | 8 | 1 | 15 | 1 | ||||||||
| Tb | 1 | 0.1 | 1.04 | 0.07 | 2 | 0 | ||||||||
| Dy | 6.1 | 0.3 | 5.5 | 0.4 | 10.3 | 0.5 | ||||||||
| Ho | 0.9 | 0.1 | 0.99 | 0.08 | 1.5 | 0.07 | ||||||||
| Er | 2.4 | 0.3 | 2.5 | 0.4 | 3.7 | 0.3 | ||||||||
| Tm | 0.3 | 0.05 | 0.29 | 0.06 | 0.39 | 0.04 | ||||||||
| Yb | 1.3 | 0.2 | 1.7 | 0.2 | 3.2 | 0.2 | ||||||||
| Lu | 0.26 | 0.04 | 0.16 | 0.03 | 0.44 | 0.06 | ||||||||
| Hf | 3.8 | 0.2 | 4.5 | 0.5 | 6.8 | 0.4 | ||||||||
| Pb | 0.05 | 0.01 | 0.22 | 0.05 | 0.14 | 0.03 | ||||||||
| Th | 0.117 | 0.008 | 0.18 | 0.03 | 0.23 | 0.02 | ||||||||
| U | 0.034 | 0.005 | 0.033 | 0.008 | 0.04 | 0.005 | ||||||||
Table S-3 Comparison of LA-ICP-MS repeat analyses of ML3B-G glass standard with reported literature values.
| ML3B-G* | LA-ICP-MS | |||||
| literature value | average | |||||
| ppm | ppm | Δ lit-avg | Δ as % | 1 σ* | 1 σ as % | |
| Li | 4.50 | 5.20 | 0.70 | 15.5 | 0.93 | 21 |
| Sc | 31.60 | 31.01 | -0.59 | -1.9 | 1.30 | 4 |
| Ti | 12769.78 | 12823.95 | 54.16 | 0.4 | 368.34 | 3 |
| V | 268.00 | 278.64 | 10.64 | 4.0 | 17.92 | 7 |
| Cr | 177.00 | 182.94 | 5.94 | 3.4 | 9.81 | 6 |
| Ni | 107.00 | 112.63 | 5.63 | 5.3 | 6.76 | 6 |
| Sr | 312.00 | 327.32 | 15.32 | 4.9 | 21.96 | 7 |
| Y | 23.90 | 24.29 | 0.39 | 1.6 | 1.85 | 8 |
| Zr | 122.00 | 124.98 | 2.98 | 2.4 | 8.76 | 7 |
| Nb | 8.61 | 9.17 | 0.56 | 6.5 | 0.93 | 11 |
| Sn | 1.14 | 1.16 | 0.02 | 1.4 | 0.38 | 33 |
| La | 8.99 | 9.53 | 0.54 | 6.0 | 0.97 | 11 |
| Ce | 23.10 | 24.95 | 1.85 | 8.0 | 2.69 | 12 |
| Pr | 3.43 | 3.80 | 0.37 | 10.7 | 0.49 | 14 |
| Nd | 16.70 | 17.95 | 1.25 | 7.5 | 2.22 | 13 |
| Sm | 4.75 | 4.79 | 0.04 | 0.8 | 0.73 | 15 |
| Eu | 1.67 | 1.81 | 0.14 | 8.6 | 0.21 | 12 |
| Gd | 5.26 | 5.67 | 0.41 | 7.8 | 0.92 | 17 |
| Tb | 0.80 | 0.84 | 0.04 | 5.5 | 0.18 | 23 |
| Dy | 4.84 | 4.85 | 0.01 | 0.2 | 0.58 | 12 |
| Ho | 0.91 | 0.91 | 0.00 | 0.5 | 0.13 | 14 |
| Er | 2.44 | 2.64 | 0.20 | 8.4 | 0.41 | 17 |
| Tm | 0.32 | 0.33 | 0.00 | 1.5 | 0.06 | 20 |
| Yb | 2.06 | 2.00 | -0.06 | -2.7 | 0.45 | 22 |
| Lu | 0.29 | 0.32 | 0.04 | 13.2 | 0.08 | 28 |
| Hf | 3.22 | 3.42 | 0.20 | 6.2 | 0.76 | 24 |
| Pb | 1.38 | 1.53 | 0.15 | 10.5 | 0.23 | 17 |
| Th | 0.55 | 0.57 | 0.02 | 4.5 | 0.06 | 11 |
| U | 0.44 | 0.50 | 0.05 | 12.2 | 0.09 | 20 |
* ML3B-G was analysed twice between every 5 unknown samples, totalling 24 standard analyses during collection of the TSC data reported in this study. Analytical uncertainty is determined by having the first of each two ML3B-G analyses serve as the 'standard' for calculating the concentration of the second ML3B-G analysis, which is run as an unknown sample. The average value of the 12 ML3B-G 'unknowns' is compared here with the literature values for these trace elements (Jochum et al., 2006) along with the standard deviation of these ML3B-G runs.
Back to article | Download in ExcelTable S-4 TSC clinopyroxene major element compositions (wt. %) along grain transects were analysed on the Massachusetts Institute of Technology (MIT) JEOL-JXA-8200 Superprobe. Uncertainty (2s) has been calculated from the standard deviation of replicate analyses of the DJ35 diopside-jadeite glass and ALP7 aluminous orthopyroxene standards, as well as several points inferred from back scattered electron imaging to be from the same clinopyroxene crystal growth zone: SiO2 (0.31), TiO2 (0.02), Al2O3 (0.07), FeO (0.08), MgO (0.11), MnO (0.01), CaO (0.30), Na2O (0.04), K (0.01), Cr2O3 (0.02).
| SiO2 | TiO2 | Al2O3 | FeOt† | MnO | MgO | CaO | Na2O | K2O | Cr2O3 | Total | |
| TSC-2A-2 | 47.74 | 1.41 | 6.8 | 7.17 | 0.11 | 13.09 | 21.8 | 0.3 | 0.01 | 0.01 | 98.44 |
| TSC-2A-3 | 48.57 | 1.3 | 6.49 | 7.95 | 0.13 | 12.82 | 21.42 | 0.4 | 0 | 0.04 | 99.12 |
| TSC-2A-4 | 47.88 | 1.29 | 6.4 | 7.71 | 0.13 | 13.22 | 21.28 | 0.36 | 0 | 0.04 | 98.31 |
| TSC-2A-5 | 48.71 | 1.23 | 6.17 | 7.94 | 0.15 | 13.44 | 21.44 | 0.44 | 0 | 0.03 | 99.54 |
| TSC-2A-6 | 49.17 | 1.12 | 5.33 | 7.71 | 0.14 | 13.85 | 21.37 | 0.46 | 0 | 0.03 | 99.18 |
| TSC-2A-7 | 48.69 | 1.24 | 5.59 | 6.35 | 0.09 | 14.23 | 22.09 | 0.42 | 0.01 | 0.04 | 98.74 |
| TSC-2A-8 | 49.57 | 1.18 | 5.64 | 6.44 | 0.1 | 14.25 | 22.38 | 0.32 | 0 | 0.03 | 99.9 |
| TSC-2A-9 | 49.47 | 1.14 | 5.59 | 6.65 | 0.1 | 14.17 | 22.14 | 0.42 | 0 | 0.01 | 99.69 |
| TSC-2A-10 | 48.87 | 1.19 | 6.58 | 7.25 | 0.09 | 13.54 | 22.2 | 0.48 | 0.02 | 0.04 | 100.26 |
| TSC-2A-11 | 48.63 | 1.2 | 6.49 | 7.26 | 0.12 | 13.75 | 21.79 | 0.44 | 0 | 0.05 | 99.71 |
| TSC-2A-12 | 48.94 | 1.22 | 6.33 | 7.15 | 0.11 | 13.77 | 21.93 | 0.4 | 0.01 | 0.03 | 99.89 |
| TSC-2A-13 | 49.2 | 1.03 | 5.96 | 7.03 | 0.14 | 13.95 | 21.88 | 0.35 | 0.01 | 0.07 | 99.62 |
| TSC-2B-2 | 49.45 | 1.28 | 3.84 | 8.31 | 0.19 | 14.56 | 20.99 | 0.54 | 0 | 0.05 | 99.21 |
| TSC-2B-3 | 48.12 | 1.51 | 4.98 | 7.97 | 0.14 | 14.27 | 20.91 | 0.55 | 0.01 | 0.01 | 98.47 |
| TSC-2B-4 | 49.78 | 0.99 | 4.17 | 7.77 | 0.16 | 14.93 | 21.22 | 0.5 | 0 | 0.01 | 99.53 |
| TSC-2B-5 | 50.21 | 0.92 | 3.64 | 7.77 | 0.17 | 15.17 | 20.93 | 0.48 | 0 | 0.01 | 99.31 |
| TSC-2B-6 | 50.43 | 0.92 | 3.78 | 7.79 | 0.18 | 15.24 | 21.38 | 0.43 | 0 | 0 | 100.16 |
| TSC-2B-7 | 50.43 | 1.13 | 3.86 | 7.8 | 0.17 | 14.89 | 21.34 | 0.37 | 0 | 0.01 | 100 |
| TSC-2B-8 | 50.83 | 0.93 | 3.54 | 7.93 | 0.18 | 14.99 | 20.85 | 0.41 | 0 | 0.01 | 99.67 |
| TSC-2B-9 | 50.96 | 0.99 | 3.69 | 7.94 | 0.18 | 14.95 | 21.13 | 0.38 | 0 | 0 | 100.21 |
| TSC-2B-10 | 50.38 | 0.99 | 3.76 | 7.85 | 0.19 | 15.22 | 21.33 | 0.47 | 0 | 0.002 | 100.18 |
| TSC-2C-1 | 47.4 | 2.02 | 7.67 | 8.99 | 0.17 | 11.96 | 20.87 | 0.49 | 0.01 | 0.04 | 99.62 |
| TSC-2C-2 | 46.54 | 1.93 | 7.23 | 8.73 | 0.17 | 12.4 | 20.59 | 0.53 | 0.01 | 0.06 | 98.19 |
| TSC-2C-3 | 46.51 | 1.96 | 7.23 | 8.69 | 0.15 | 12.74 | 20.78 | 0.48 | 0 | 0.04 | 98.59 |
| TSC-2C-4 | 46.47 | 1.94 | 7.26 | 9 | 0.18 | 13.1 | 20.74 | 0.58 | 0 | 0.01 | 99.29 |
| TSC-2C-5 | 46.71 | 1.87 | 7.03 | 8.84 | 0.18 | 12.99 | 20.79 | 0.58 | 0 | 0.02 | 99.01 |
| TSC-2C-6 | 46.93 | 1.84 | 7.02 | 8.76 | 0.15 | 13.09 | 20.81 | 0.58 | 0.01 | 0.02 | 99.21 |
| TSC-2C-7 | 47.07 | 1.83 | 6.91 | 8.67 | 0.17 | 13.21 | 20.95 | 0.53 | 0 | 0.03 | 99.37 |
| TSC-2C-8 | 46.79 | 1.87 | 7.13 | 8.99 | 0.17 | 13.24 | 20.88 | 0.52 | 0 | 0.01 | 99.6 |
| TSC-2C-9 | 46.53 | 1.89 | 6.98 | 8.91 | 0.17 | 13.37 | 21.2 | 0.56 | 0 | 0.02 | 99.63 |
| TSC-2C-10 | 49.14 | 0.88 | 3.97 | 7.75 | 0.17 | 15.58 | 21.38 | 0.58 | 0.01 | 0 | 99.45 |
| TSC-2D-2 | 48.9 | 1.42 | 3.93 | 7.77 | 0.16 | 13.93 | 21.67 | 0.35 | 0 | 0.04 | 98.17 |
| TSC-2D-3 | 49.1 | 1.4 | 3.85 | 7.72 | 0.14 | 14.23 | 21.63 | 0.41 | 0.01 | 0.04 | 98.53 |
| TSC-2D-4 | 50.42 | 1.24 | 3.51 | 7.62 | 0.13 | 14.55 | 21.87 | 0.43 | 0 | 0.03 | 99.79 |
| TSC-2D-5 | 49.85 | 1.38 | 4.01 | 7.96 | 0.14 | 14.33 | 21.96 | 0.4 | 0 | 0.04 | 100.06 |
| TSC-2D-6 | 50.45 | 1.32 | 4.01 | 7.45 | 0.15 | 14.28 | 21.73 | 0.53 | 0.01 | 0.02 | 99.95 |
| TSC-2D-7 | 50.01 | 1.2 | 3.5 | 7.9 | 0.16 | 14.81 | 21.75 | 0.47 | 0.01 | 0.01 | 99.82 |
| TSC-2D-8 | 49.81 | 1.42 | 3.84 | 7.6 | 0.17 | 14.68 | 22.12 | 0.45 | 0 | 0.02 | 100.13 |
| TSC-2D-9 | 49.32 | 1.58 | 4.19 | 8.11 | 0.16 | 14.35 | 21.76 | 0.4 | 0 | 0.03 | 99.9 |
| TSC-2D-10 | 46.77 | 1.55 | 4.53 | 8.3 | 0.17 | 14.79 | 21.51 | 0.52 | 0 | 0.01 | 98.15 |
| TSC-3A-1 | 49.14 | 1.61 | 4.81 | 7.46 | 0 | 13.71 | 22.47 | 0.51 | 0 | 0 | 99.72 |
| TSC-3A-2 | 49.09 | 1.67 | 4.73 | 7.42 | 0 | 13.61 | 22.52 | 0.46 | 0 | 0 | 99.5 |
| TSC-3A-3 | 49.01 | 1.65 | 4.99 | 8.19 | 0 | 13.31 | 21.66 | 0.61 | 0 | 0 | 99.43 |
| TSC-3A-4 | 48 | 2.19 | 5.63 | 8.61 | 0 | 12.67 | 22.03 | 0.61 | 0 | 0 | 99.74 |
| TSC-3A-5 | 48.68 | 2.02 | 4.91 | 8.23 | 0 | 12.96 | 22.16 | 0.68 | 0 | 0 | 99.64 |
| TSC-3A-6 | 49.22 | 2.04 | 4.13 | 9.22 | 0 | 12.67 | 22.13 | 0.66 | 0.01 | 0 | 100.09 |
| TSC-3A-7 | 47.9 | 2.31 | 5.09 | 8.67 | 0.02 | 12.56 | 22.05 | 0.63 | 0 | 0 | 99.23 |
| TSC-3A-8 | 50.42 | 1.51 | 2.96 | 9.24 | 0.02 | 12.83 | 22.01 | 0.7 | 0 | 0 | 99.69 |
| TSC-3A-9 | 45.8 | 2.74 | 7.3 | 8.89 | 0.02 | 12.1 | 21.9 | 0.54 | 0 | 0 | 99.29 |
| TSC-3A-10 | 49.91 | 1.39 | 4.11 | 7.92 | 0.01 | 13.96 | 21.64 | 0.57 | 0 | 0 | 99.51 |
| TSC-3A-11 | 49.49 | 1.47 | 4.72 | 7.33 | 0.01 | 13.89 | 22.09 | 0.55 | 0 | 0 | 99.55 |
| TSC-3A-12 | 49.74 | 1.46 | 4.61 | 8.23 | 0 | 13.71 | 21.49 | 0.57 | 0 | 0 | 99.81 |
| TSC-3B-1 | 50.23 | 0.95 | 4.59 | 5.24 | 0 | 14.97 | 23.05 | 0.39 | 0 | 0.18 | 99.6 |
| TSC-3B-2 | 50.09 | 0.96 | 4.77 | 5.4 | 0 | 14.92 | 22.93 | 0.43 | 0 | 0.17 | 99.67 |
| TSC-3B-3 | 49.47 | 1.14 | 5.28 | 5.47 | 0 | 14.88 | 22.82 | 0.46 | 0 | 0.21 | 99.73 |
| TSC-3B-4 | 49.35 | 1.1 | 5.43 | 5.64 | 0 | 14.62 | 22.72 | 0.43 | 0 | 0.15 | 99.44 |
| TSC-3B-5 | 50.12 | 0.89 | 4.79 | 5.38 | 0 | 14.98 | 22.8 | 0.4 | 0 | 0.19 | 99.55 |
| TSC-3B-6 | 49.28 | 1.14 | 5.4 | 5.56 | 0 | 14.46 | 22.84 | 0.41 | 0 | 0.11 | 99.21 |
| TSC-3B-7 | 49.33 | 1.12 | 5.26 | 5.4 | 0.02 | 14.49 | 22.85 | 0.47 | 0.01 | 0.24 | 99.18 |
| TSC-3B-8 | 49.29 | 1.01 | 5.02 | 5.34 | 0.02 | 14.68 | 22.64 | 0.5 | 0 | 0.22 | 98.72 |
| TSC-3B-9 | 49.1 | 1.56 | 4.38 | 7.87 | 0 | 13.91 | 22.15 | 0.47 | 0 | 0 | 99.45 |
| TSC-3B-10 | 49.88 | 1.52 | 3.86 | 7.93 | 0.01 | 14.08 | 22.05 | 0.41 | 0 | 0 | 99.74 |
| TSC-3B-11 | 49.51 | 1.17 | 5.4 | 5.54 | 0 | 14.63 | 22.98 | 0.38 | 0 | 0.17 | 99.77 |
| TSC-3B-12 | 49.41 | 1.11 | 5.33 | 5.48 | 0 | 14.49 | 22.83 | 0.42 | 0 | 0.18 | 99.26 |
| TSC-3C-1 | 48.55 | 2 | 4.96 | 8.44 | 0 | 13.06 | 22.2 | 0.54 | 0 | 0.02 | 99.76 |
| TSC-3C-2 | 48.06 | 2.15 | 5.26 | 8.53 | 0 | 12.91 | 22.2 | 0.56 | 0 | 0 | 99.67 |
| TSC-3C-3 | 48.19 | 2 | 4.96 | 8.52 | 0 | 12.97 | 22.38 | 0.59 | 0 | 0.05 | 99.66 |
| TSC-3C-4 | 48 | 2.12 | 5.14 | 8.52 | 0 | 12.68 | 21.96 | 0.62 | 0 | 0 | 99.04 |
| TSC-3C-5 | 48.28 | 2.02 | 4.93 | 8.33 | 0 | 12.75 | 22.13 | 0.64 | 0 | 0.02 | 99.09 |
| TSC-3C-6 | 47.43 | 2.46 | 5.48 | 8.89 | 0 | 12.43 | 21.93 | 0.62 | 0 | 0 | 99.24 |
| TSC-3C-7 | 49.68 | 1.66 | 3.69 | 8.22 | 0 | 13.46 | 21.62 | 0.61 | 0 | 0 | 98.95 |
| TSC-3C-8 | 48.78 | 1.88 | 4.65 | 8.46 | 0 | 12.98 | 22.01 | 0.58 | 0.01 | 0.04 | 99.38 |
| TSC-3C-9 | 47.95 | 1.89 | 5.44 | 8.35 | 0 | 13.23 | 22.19 | 0.46 | 0 | 0.01 | 99.52 |
| TSC-3C-10 | 48.32 | 2.08 | 4.94 | 8.66 | 0 | 12.89 | 21.85 | 0.64 | 0 | 0.02 | 99.4 |
| TSC-3C-11 | 47.71 | 2.36 | 5.48 | 8.73 | 0 | 12.57 | 22.07 | 0.58 | 0 | 0 | 99.5 |
| TSC-3D-1 | 47.72 | 2.05 | 5.72 | 8.19 | 0 | 12.82 | 22.3 | 0.56 | 0 | 0 | 99.36 |
| TSC-3D-2 | 47.94 | 2.13 | 5.51 | 8.46 | 0 | 12.97 | 22.13 | 0.54 | 0.01 | 0 | 99.69 |
| TSC-3D-3 | 48.27 | 1.8 | 5.25 | 8.24 | 0 | 12.97 | 22.26 | 0.58 | 0.01 | 0 | 99.38 |
| TSC-3D-4 | 48.21 | 1.99 | 5.27 | 7.86 | 0.03 | 13.06 | 22.31 | 0.52 | 0 | 0.01 | 99.26 |
| TSC-3D-5 | 47.37 | 2.27 | 6.03 | 8.16 | 0 | 12.63 | 22.38 | 0.56 | 0.01 | 0 | 99.4 |
| TSC-3D-6 | 47.61 | 2.18 | 5.82 | 8.37 | 0 | 12.55 | 22.14 | 0.65 | 0 | 0 | 99.32 |
| TSC-3D-7 | 48.35 | 2.16 | 4.88 | 8.61 | 0 | 12.99 | 21.72 | 0.67 | 0 | 0 | 99.38 |
| TSC-3D-8 | 48.83 | 1.64 | 5.04 | 8.16 | 0.03 | 13.2 | 22.07 | 0.63 | 0.01 | 0 | 99.61 |
| TSC-3D-9 | 48.05 | 1.47 | 6.25 | 7.53 | 0 | 13.51 | 22.24 | 0.51 | 0 | 0 | 99.56 |
| TSC-3D-10 | 48.65 | 1.39 | 5.93 | 7.52 | 0 | 13.72 | 22.17 | 0.52 | 0 | 0.01 | 99.92 |
| TSC-7A-1 | 47.74 | 1.59 | 5.46 | 7.83 | 0.18 | 14.04 | 21.51 | 0.77 | 0 | 0 | 99.13 |
| TSC-7A-2 | 48.72 | 1.4 | 4.85 | 7.68 | 0.18 | 14.12 | 21.69 | 0.64 | 0 | 0.02 | 99.3 |
| TSC-7A-3 | 48.55 | 1.48 | 4.77 | 7.7 | 0.17 | 14.09 | 21.71 | 0.47 | 0 | 0.04 | 98.97 |
| TSC-7A-4 | 49.07 | 1.4 | 4.37 | 7.7 | 0.16 | 14.2 | 21.73 | 0.56 | 0.01 | 0.03 | 99.23 |
| TSC-7A-5 | 49.59 | 1.3 | 3.93 | 7.5 | 0.16 | 14.26 | 21.91 | 0.58 | 0 | 0.05 | 99.28 |
| TSC-7A-6 | 48.73 | 1.59 | 4.54 | 7.64 | 0.15 | 13.73 | 22.17 | 0.48 | 0.02 | 0.03 | 99.09 |
| TSC-7A-7 | 49.01 | 1.5 | 4.37 | 7.73 | 0.17 | 13.68 | 22.06 | 0.51 | 0 | 0.02 | 99.04 |
| TSC-7A-8 | 49.31 | 1.38 | 4.36 | 7.52 | 0.16 | 13.93 | 21.93 | 0.47 | 0 | 0.02 | 99.08 |
| TSC-7A-9 | 49.92 | 1.37 | 4.24 | 7.5 | 0.15 | 13.96 | 21.86 | 0.51 | 0 | 0.04 | 99.55 |
| TSC-7A-10 | 50.13 | 1.32 | 4.27 | 7.46 | 0.16 | 13.63 | 21.72 | 0.41 | 0 | 0.02 | 99.13 |
| TSC-7B-2 | 49.94 | 1.26 | 3.98 | 7.38 | 0.17 | 14.23 | 21.94 | 0.47 | 0.01 | 0 | 99.38 |
| TSC-7B-3 | 48.58 | 1.46 | 4.77 | 7.28 | 0.18 | 13.95 | 21.97 | 0.38 | 0.01 | 0 | 98.59 |
| TSC-7B-4 | 49.14 | 1.29 | 4.48 | 7.49 | 0.15 | 14.27 | 21.66 | 0.49 | 0 | 0.03 | 98.99 |
| TSC-7B-5 | 48.32 | 1.28 | 4.08 | 7.23 | 0.16 | 14.87 | 21.8 | 0.37 | 0 | 0.02 | 98.12 |
| TSC-7B-6 | 50.09 | 1.26 | 3.76 | 7.52 | 0.16 | 14.03 | 21.56 | 0.46 | 0.01 | 0.01 | 98.86 |
| TSC-7B-7 | 49.82 | 1.22 | 3.74 | 7.58 | 0.19 | 14.16 | 21.45 | 0.47 | 0 | 0.02 | 98.65 |
| TSC-7B-8 | 46.93 | 2.04 | 5.91 | 7.77 | 0.14 | 13.11 | 21.94 | 0.46 | 0 | 0.05 | 98.35 |
| TSC-7B-9 | 47.13 | 2.09 | 5.68 | 8.23 | 0.15 | 12.87 | 21.81 | 0.49 | 0 | 0.01 | 98.46 |
| TSC-7B-10 | 47.35 | 1.87 | 5.81 | 7.75 | 0.15 | 13.49 | 21.87 | 0.6 | 0.01 | 0 | 98.9 |
| TSC-9A-1 | 47.95 | 2.58 | 5.02 | 9.03 | 0 | 12.47 | 22.06 | 0.7 | 0 | 0.01 | 99.82 |
| TSC-9A-2 | 48.04 | 1.98 | 5.81 | 7.42 | 0 | 13.23 | 22.82 | 0.52 | 0 | 0 | 99.82 |
| TSC-9A-3 | 48.18 | 2.22 | 5.45 | 8.29 | 0.05 | 13.07 | 22.44 | 0.72 | 0 | 0.03 | 100.44 |
| TSC-9A-4 | 47.85 | 2.09 | 5.62 | 7.89 | 0.03 | 13.02 | 22.3 | 0.58 | 0 | 0 | 99.38 |
| TSC-9A-5 | 47.06 | 2.37 | 6.66 | 8.02 | 0.03 | 12.82 | 22.89 | 0.51 | 0 | 0 | 100.37 |
| TSC-9A-6 | 47.76 | 2.33 | 5.93 | 8.06 | 0.01 | 12.82 | 22.29 | 0.62 | 0 | 0.05 | 99.86 |
| TSC-9A-7 | 48.56 | 1.7 | 5.27 | 7.49 | 0.02 | 13.54 | 22.81 | 0.56 | 0 | 0 | 99.95 |
| TSC-9A-8 | 49.44 | 1.68 | 4.51 | 7.07 | 0.01 | 14.02 | 22.46 | 0.48 | 0 | 0.01 | 99.67 |
| TSC-9A-9 | 49.62 | 1.67 | 4.07 | 7.6 | 0.02 | 13.6 | 21.95 | 0.68 | 0 | 0 | 99.21 |
| TSC-9A-10 | 47.12 | 2.06 | 6.59 | 8.45 | 0 | 12.7 | 22.08 | 0.52 | 0 | 0 | 99.52 |
| TSC-9B-1 | 47.85 | 2.13 | 5.79 | 7.71 | 0 | 13.05 | 22.35 | 0.56 | 0 | 0.02 | 99.47 |
| TSC-9B-2 | 49.76 | 1.33 | 4.17 | 7.54 | 0.01 | 14.03 | 21.87 | 0.62 | 0 | 0.01 | 99.34 |
| TSC-9B-3 | 48.41 | 1.73 | 5.12 | 7.63 | 0.03 | 13.38 | 22.2 | 0.51 | 0.01 | 0 | 99.02 |
| TSC-9B-4 | 49.42 | 1.43 | 4.35 | 7.07 | 0.03 | 14.18 | 22.46 | 0.53 | 0.01 | 0.01 | 99.49 |
| TSC-9B-5 | 49.63 | 1.51 | 4.49 | 7.38 | 0.02 | 13.89 | 21.81 | 0.62 | 0.01 | 0 | 99.36 |
| TSC-9B-6 | 50.17 | 1.42 | 3.73 | 7.24 | 0.02 | 14.16 | 22.26 | 0.5 | 0 | 0 | 99.5 |
| TSC-9B-7 | 49.73 | 1.5 | 4.24 | 7.51 | 0.02 | 13.8 | 22.1 | 0.59 | 0 | 0 | 99.48 |
| TSC-9B-8 | 49.37 | 1.67 | 4.44 | 7.61 | 0 | 13.92 | 22.38 | 0.62 | 0 | 0.04 | 100.05 |
| TSC-9B-9 | 49.63 | 1.46 | 4.39 | 7.06 | 0 | 14.17 | 22.66 | 0.46 | 0 | 0 | 99.83 |
| TSC-9B-10 | 50.25 | 1.3 | 3.71 | 7.21 | 0 | 14.58 | 22.58 | 0.44 | 0 | 0.01 | 100.09 |
| TSC-9C-1 | 46.86 | 2.55 | 6.22 | 8.38 | 0.02 | 12.71 | 22.49 | 0.59 | 0 | 0 | 99.82 |
| TSC-9C-2 | 48.48 | 1.88 | 5.02 | 7.65 | 0.03 | 13.32 | 22.52 | 0.59 | 0 | 0 | 99.49 |
| TSC-9C-3 | 50.56 | 1.55 | 3.26 | 7.69 | 0.01 | 14.3 | 21.37 | 0.62 | 0.01 | 0 | 99.37 |
| TSC-9C-4 | 49.8 | 1.42 | 3.97 | 7.39 | 0 | 14.12 | 21.85 | 0.49 | 0.01 | 0 | 99.04 |
| TSC-9C-5 | 49.99 | 1.41 | 3.88 | 7.15 | 0 | 14.29 | 21.91 | 0.52 | 0 | 0 | 99.15 |
| TSC-9C-6 | 49.34 | 1.67 | 4.39 | 7.5 | 0 | 13.84 | 21.67 | 0.58 | 0.01 | 0 | 98.99 |
| TSC-9C-7 | 49.24 | 1.63 | 4.31 | 7.56 | 0.01 | 13.7 | 21.99 | 0.53 | 0 | 0 | 98.96 |
| TSC-9C-8 | 48.81 | 1.78 | 5.01 | 7.59 | 0 | 13.48 | 22.48 | 0.64 | 0.01 | 0 | 99.8 |
| TSC-9C-9 | 48.87 | 1.63 | 5.16 | 7.15 | 0 | 13.66 | 22.7 | 0.53 | 0.01 | 0 | 99.71 |
| TSC-9C-10 | 46.41 | 2.54 | 6.99 | 8.16 | 0 | 12.51 | 22.64 | 0.56 | 0.01 | 0 | 99.82 |
*Operating conditions of the MIT JXA-8200 consisted of a 15 keV accelerating voltage and 10 nA beam current, with all analyses using a focused beam of ~1 μm and 30 s count times. Data were reduced using the CITZAF correction procedure of Armstrong (1995). The few totals lower than 98 wt. % have been omitted.
† All iron reported as FeO
Table S-5 Hf-Nd-Pb isotopic data for Timpe Santa Caterina whole rock (WR) and clinopyroxene (cpx) separates*. The uncertainties reported for Nd and Hf isotope ratios are internal 2 s.e. We use the values of external reproducibility as reported in the footnote to identify analytically resolvable WR-cpx disequilibrium discernable above the 2σ level.
| 143Nd/144Nd | εNd † | 176Hf/177Hf † | εHf § | 206Pb/204Pb § | 207Pb/204Pb § | 208Pb/204Pb # | |
| TSC-2 | |||||||
| WR | 0.512952(2) | 6.1 | 0.283035(5) | 9.3 | 20.015 | 15.668 | 39.645 |
| cpx | 0.512942(3) | 5.9 | 0.283033(4) | 9.2 | 20.014 | 15.670 | 39.645 |
| TSC-3 | |||||||
| WR | 0.512948(3) | 6 | 0.283032(4) | 9.2 | 20.078 | 15.670 | 39.666 |
| cpx | 0.512937(2) | 5.8 | 0.283047(6) | 9.7 | 20.072 | 15.668 | 39.655 |
| TSC-7 | |||||||
| WR | 0.512929(4) | 5.7 | 0.283012(4) | 8.5 | 19.990 | 15.676 | 39.648 |
| cpx | 0.512921(2) | 5.5 | 0.283024(6) | 8.9 | 19.946 | 15.668 | 39.587 |
| TSC-9 | |||||||
| WR | 0.512930(3) | 5.7 | 0.283014(4) | 8.6 | 19.987 | 15.675 | 39.647 |
| cpx | 0.512922(3) | 5.5 | 0.282996(4) | 7.9 | 19.978 | 15.669 | 39.620 |
* WR samples (italicised) are from Bryce et al. (2011) and are reported here for convenience. For the clinopyroxene samples, aliquots of 0.5 to 2 mm clinopyroxene handpicked from the TSC lavas were first leached in hot (~120° C) 6 N HCl to remove any Pb surface contamination following techniques outlined in Blichert-Toft and Albarède (2009). The resulting residues were subsequently digested in a mixture of concentrated HF-HNO3. Lead was separated prior to Hf and Nd separation using techniques described in Bryce and DePaolo (2004). Total Pb procedural blanks were <40 pg. Hafnium was separated from the Pb column eluent using the three column procedure described for high magnesium samples by Blichert-Toft (2001). Neodymium was from the Pb column eluent, separated from the residue of the first Hf column using a three column procedure starting with a small (0.5 mL) cation exchange resin (AG50x8) to strip off Fe and other major ions. The REE-rich elutions were subsequently passed through a 0.5 mL column filled with TRU-Spec resin to concentrate further the REEs, where the 2 M HNO3 was used to elute other ions and the REEs were collected with water. Nd was finally separated from Sm using a 1.6 mL LN-Spec column and a 0.25 M HCl elution. Total Nd procedural blanks were <30 pg and total Hf procedural blanks were <20 pg.
† For the Nd isotopic measurements, instrument performance was monitored with a laboratory solution, and accuracy was assessed through repeated (n = 9) analyses of BCR-1 which yielded 0.512638 (with external 2σ = 0.000020). εNd was calculated using a CHUR value of 143Nd/144Nd = 0.512638.
§ Hf isotopic analyses were obtained following the techniques described in Blichert-Toft et al. (1997). The 100 ppb JMC 475 Hf standard, run throughout the analytical session (n = 21) to monitor instrument performance, yielded 176Hf/177Hf = 0.282160 (with external 2σ = 0.000015). εHf was calculated using a CHUR value of 176Hf/177Hf = 0.282772 (Blichert-Toft and Albarède, 1997).
# Mass fractionation in Pb isotope analyses was corrected via Tl normalisation as described in White et al. (2000), and ratios were additionally adjusted for drift using the standard bracketing technique outlined in Albarède et al. (2004) using the NIST SRM values reported in Eisele et al. (2003). Four NIST SRM 981 samples, run as “blind” amongst the 17 bracketing standards analysed, yielded averages (with 2σ external precision) of 208Pb/204Pb = 36.7271 (0.0019), 207Pb/204Pb = 15.4978 (0.0009) and 206Pb/204Pb = 16.9408 (0.0012).
Table S-6 Ranges of whole rock and clinopyroxene major and minor element compositions (wt. %) observed for the Timpe Santa Caterina flows studied.
| Lava | Clinopyroxene | |||
| Low | High | Low | High | |
| SiO2 | 46.6 | 50.7 | 45.8 | 51 |
| TiO2 | 1.6 | 2.1 | 0.9 | 2.7 |
| Al2O3 | 16.8 | 19.8 | 3 | 7.7 |
| FeO* | 8.2 | 10.9 | 5.2 | 9.2 |
| MnO | 0.15 | 0.19 | 0 | 0.19 |
| MgO | 3.4 | 6.3 | 12 | 15.6 |
| CaO | 8.7 | 10.9 | 20.3 | 23.1 |
| Na2O | 3.7 | 5.5 | 0.3 | 0.8 |
| K2O | 1 | 2.1 | 0 | 0.02 |
| P2O5 | 0.5 | 1.4 | 0 | 0.24 |
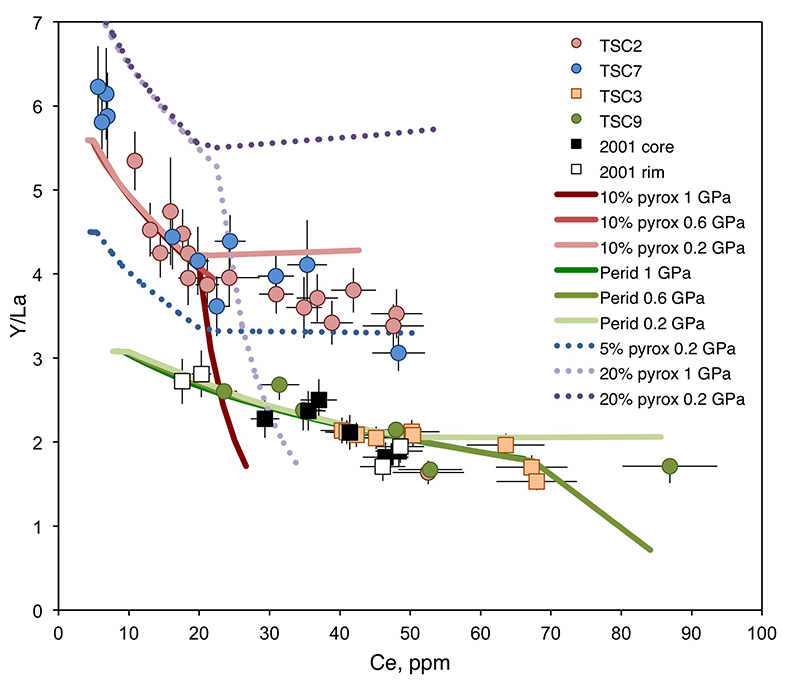
Figure S-3 Clinopyroxene trace element evolution during isobaric fractionation of peridotite melt (green) and 10 % pyroxenite component melts (red) at 1.0, 0.6, and 0.2 GPa. Modelling sensitivity to starting composition is illustrated by including paths for melts with 5 % and 20 % pyroxenite component shown as blue and purple dotted lines, respectively.