Rapid decrease of MgAlO2.5 component in bridgmanite with pressure
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: bridgmanite, oxygen vacancy, pressure, water, argon, slab stagnation, lower mantle
- Share this article





Article views:10,407Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
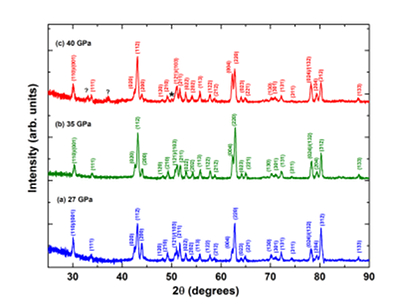 Figure 1 patterns of En90Brm10 subjected to (a) 27, (b) 35 and (c) 40 GPa and at 2000 oK. Number in parentheses represents the index of bridgmanite. Question marks denote unidentified peaks. Asterisk denotes the probable (200) peak of periclase. | 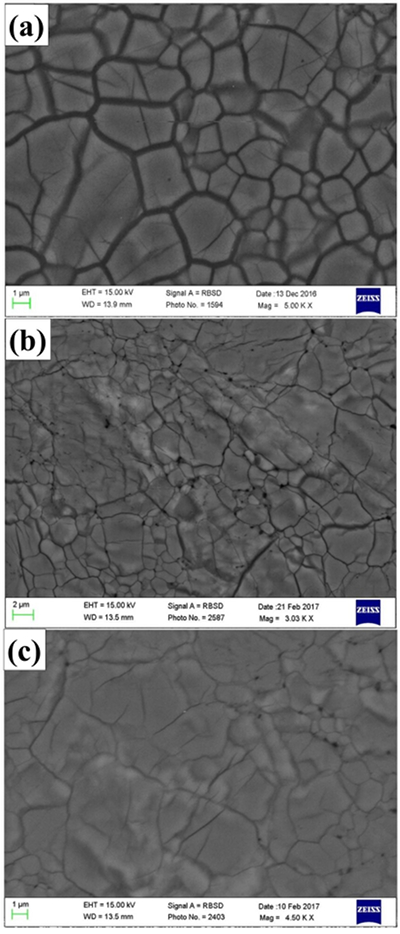 Figure 2 Representative BSE images of En90Brm10 subjected to (a) 27 GPa, (b) 35 and (c) 40 GPa and 2000 oK. |  Table 1 Experimental conditions, run products, and phase compositions. | 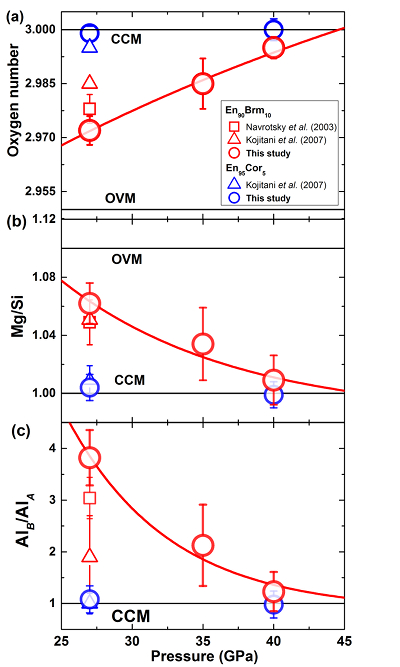 Figure 3 Plots of (a) oxygen number, (b) Mg/Si ratio, and (c) Al ratio in A and B sites (AlB/AlA) in bridgmanite for En90Brm10 and En95Cor5 versus pressure. The black lines represent the compositions associated with the oxygen-vacancy substitution mechanism (OVM) and charge-coupled substitution mechanism (CCM) for bridgmanite. The red line is the least squares fitting (Mg/Si = Aexp(–P/B) + C, where P is pressure) of the present data. |
| Figure 1 | Figure 2 | Table 1 | Figure 3 |
Supplementary Figures and Tables
 Table S-1 Chemical compositions of the starting glass material. | 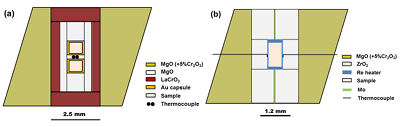 Figure S-1 The schematic illustration of (a) 7/3 and (b) 5.7/1.5 cell assembly for high pressure and high temperature experiments. | 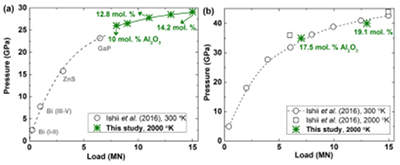 Figure S-2 Pressure calibrations of 7/3 (a) and 5.7/1.5 (b) cell assembly in IRIS-15 at 300 and 2000 oK, respectively. |  Table S-2 The experimental condition and composition of MgSiO3 bridgmanite. |
| Table S-1 | Figure S-1 | Figure S-2 | Table S-2 |
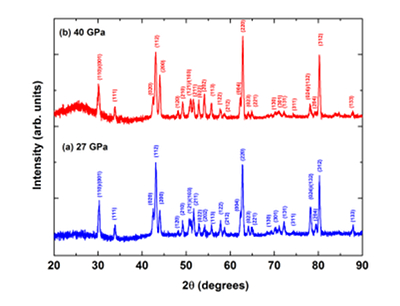 Figure S-3 XRD patterns of the recovered sample for En95Cor5 composition at 27 (a) and 40 GPa (b) under 2000 oK, respectively. Number in parentheses represents the index of bridgmanite. | 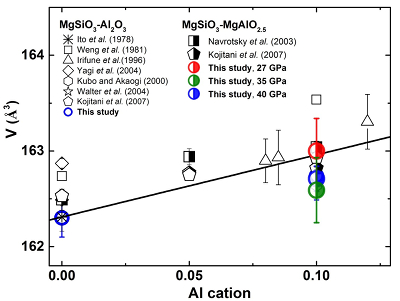 Figure S-4 Unit cell volumes of bridgmanite along the system MgSiO3–Al2O3 and MgSiO3–MgAlO2.5 in the present and previous studies. The solid line presents the linear fitting results on values for bridgmanite along the system MgSiO3-Al2O3 (Liu et al., 2016). |  Table S-3 Elastic properties of minerals related with Eq. S-2 used for thermodynamic calculations. | 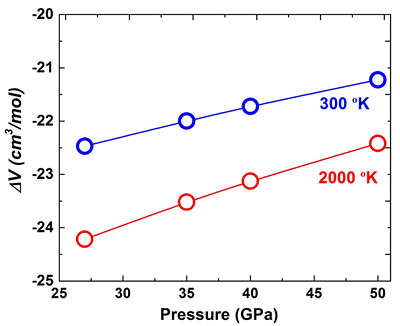 Figure S-5 The calculated molar volume change of Eq. S-2 in the text along 300 and 2000 °K, respectively. |
| Figure S-3 | Figure S-4 | Table S-3 | Figure S-5 |
top
Introduction
Aluminium (Al) is the fifth most abundant element in the Earth’s mantle (McDonough and Sun, 1995
McDonough, W.F., Sun, S.-S. (1995) The composition of the Earth. Chemical Geology 120, 223–253.
). Under the Earth’s lower mantle conditions, Al3+ is mainly accommodated in bridgmanite in a pyrolite mantle (Irifune, 1994Irifune, T. (1994) Absence of an aluminous phase in the upper part of the Earth's lower mantle. Nature 370, 131–133.
). Al substitution in the Mg, Si or both sites modifies the crystal chemistry of bridgmanite, and thereby changes the physical properties of bridgmanite (Zhang and Weidner, 1999Zhang, J., Weidner, D.J. (1999) Thermal equation of state of aluminum–enriched silicate perovskite. Science 284, 782–784.
; Brodholt, 2000Brodholt, J.P. (2000) Pressure-induced changes in the compression mechanism of aluminous perovskite in the Earth's mantle. Nature 407, 620–622.
). Therefore, understanding the Al substitution mechanism is essential to investigating the mineralogy and dynamics of the lower mantle.Two competing Al substitution mechanisms occur in bridgmanite (Hirsch and Shankland, 1991
Hirsch, L.M., Shankland, T.J. (1991) Point defects in silicate perovskite. Geophysical Research Letters 18, 1305–1308.
). One is the charge-coupled substitution (Mg2+ + Si4+ = Al3+ + Al3+; components along the MgSiO3–Al2O3 binary system), where two Al cations substitute for one Mg with the roughly 8-fold coordination (A site) and one Si with the 6-fold coordination (B site). The other mechanism is the oxygen-vacancy substitution (2Si4+ + O2- = 2Al3+ + Vo, where Vo is an oxygen vacancy; components along the MgSiO3–MgAlO2.5 binary system), where two Al cations replace two Si in the B sites and one oxygen vacancy forms to compensate for the excess negative charges. The oxygen vacancies in bridgmanite have special importance for understanding lower mantle processes because oxygen vacancies may induce water (Vo + O2- + H2O = 2OH-1; Navrotsky, 1999Navrotsky, A. (1999) A lesson from ceramics. Science 284, 1788–1789.
) and noble gases into bridgmanite (Shcheka and Keppler, 2012Shcheka, S.S., Keppler, H. (2012) The origin of the terrestrial noble-gas signature. Nature 490, 531–534.
).Earlier theoretical calculations investigated whether oxygen vacancies exist in bridgmanite (Brodholt, 2000
Brodholt, J.P. (2000) Pressure-induced changes in the compression mechanism of aluminous perovskite in the Earth's mantle. Nature 407, 620–622.
; Yamamoto et al.; 2003Yamamoto, T., Yuen, D.A., Ebisuzaki, T. (2003) Substitution mechanism of Al ions in MgSiO3 perovskite under high pressure conditions from first-principles calculations. Earth and Planetary Science Letters 206, 617–625.
; Akber–Knutson and Bukowinski, 2004Akber-Knutson, S., Bukowinski, M.S.T. (2004) The energetics of aluminum solubility into MgSiO3 perovskite at lower mantle conditions. Earth and Planetary Science Letters 220, 317–330.
). Brodholt (2000)Brodholt, J.P. (2000) Pressure-induced changes in the compression mechanism of aluminous perovskite in the Earth's mantle. Nature 407, 620–622.
suggested that oxygen-vacancy substitution should dominate at pressures only up to 30 GPa. Yamamoto et al. (2003)Yamamoto, T., Yuen, D.A., Ebisuzaki, T. (2003) Substitution mechanism of Al ions in MgSiO3 perovskite under high pressure conditions from first-principles calculations. Earth and Planetary Science Letters 206, 617–625.
argued oxygen-vacancy substitution should be unfavourable in the entire lower mantle. Furthermore, energetic calculations by Akber–Knutson and Bukowinski (2004)Akber-Knutson, S., Bukowinski, M.S.T. (2004) The energetics of aluminum solubility into MgSiO3 perovskite at lower mantle conditions. Earth and Planetary Science Letters 220, 317–330.
suggested that oxygen-vacancy substitution could only account for 3–4 % and less than 1 % of Al substitutions in the shallower and deeper parts of the lower mantle, respectively.High pressure and high temperature synthesis (Navrotsky et al., 2003
Navrotsky, A., Schoenitz, M., Kojitani, H., Xu, H., Zhang, J., Weidner, D.J., Jeanloz, R. (2003) Aluminum in magnesium silicate perovskite: Formation, structure, and energetics of magnesium-rich defect solid solutions. Journal of Geophysical Research 108, 2330.
) and nuclear magnetic resonance (NMR) spectroscopy (Stebbins et al., 2003Stebbins, J.F., Kojitani, H., Akaogi, M., Navrotsky, A. (2003) Aluminum substitution in MgSiO3 perovskite: Investigation of multiple mechanisms by 27Al NMR. American Mineralogist 88, 1161–1164.
) suggested that oxygen-vacancy substitution dominates in bridgmanite with 5–10 mol. % MgAlO2.5 at 27 GPa and 1873 oK. On the other hand, X-ray diffraction measurements in a laser-heated diamond anvil demonstrated that bridgmanite coexisted with periclase from a glass starting material with a composition of 90 mol. % MgSiO3 and 10 mol. % MgAlO2.5 at 40 ± 10 GPa and 2000–2500 oK (Walter et al., 2006Walter, M., Tronnes, R.G., Armstrong, L.S., Lord, O., Caldwell, W.A., Clark, A.M. (2006) Subsolidus phase relations and perovskite compressibility in the system MgO–AlO1.5–SiO2 with implications for Earth's lower mantle. Earth and Planetary Science Letters 248, 77–89.
), indicating that the MgAlO2.5 component was not present in bridgmanite and thereby oxygen-vacancy substitution was unfavoured under these conditions. Note that pressure and temperature uncertainties in this study were relatively large, and the composition of bridgmanite was not determined owing to its very small sample size. Thus, it is still uncertain whether MgAlO2.5 can be a dominant component in bridgmanite in the lower mantle.Here, we investigate the solubility of the MgAlO2.5 component in bridgmanite as a function of pressure from 27 to 40 GPa at a constant temperature of 2000 oK and discuss water and noble gas storage capacities in the lower mantle.
top
Experimental Methods
The starting materials were glasses with En90Brm10 (En: MgSiO3; Brm: MgAlO2.5; mol. %) and En95Cor5 (Cor: Al2O3) compositions (see section S-1 in the Supplementary Information for synthesis details and Table S-1 for their compositions). High pressure and high temperature experiments were conducted using ultra-high pressure multi-anvil technology with carbide anvils (Ishii et al., 2016
Ishii, T., Shi, L., Huang, R., Tsujino, N., Druzhbin, D., Myhill, R., Li, Y., Wang, L., Yamamoto, T., Miyajima, N., Kawazoe, T., Nishiyama, N., Higo, Y., Tange, Y., Katsura, T. (2016) Generation of pressure over 40 GPa using Kawai–type multi–anvil press with tungsten carbide anvils. Review of Scientific Instruments 87, 024501-1–024501-6.
), and detailed techniques can be found in Figures S-1 and S-2 in the Supplementary Information. As shown in Table 1, bridgmanite samples with En90Brm10 and En95Cor5 compositions were first synthesised at 27 GPa and 2000 oK for 3 hours. These samples were annealed at pressures of 35 and 40 GPa and a temperature of 2000 oK for 2 and 3 hours. The recovered samples were analysed by X-ray diffraction (XRD), scanning electron microscopy (SEM), and electron probe microanalysis (EPMA) (see section S-2 in the Supplementary Information for analytical analyses).top
Results
XRD patterns of bridgmanite with the En90Brm10 starting composition synthesised at 27, 35 and 40 GPa are shown in Figure 1, while those for En95Cor5 composition are shown in Figure S-3. All diffraction peaks of the sample for the En90Brm10 composition synthesised at 27 GPa were assigned to those of bridgmanite (Ito and Matsui, 1978
Ito, E., Matsui, Y. (1978) Synthesis and crystal–chemical characterization of MgSiO3 perovskite. Earth and Planetary Science Letters 38, 443–450.
), indicating the formation of a single phase bridgmanite. The diffraction peaks of the En90Brm10 bridgmanite sample annealed at 35 GPa were also assigned to bridgmanite. For the sample annealed at 40 GPa, the majority of its peaks were assigned to bridgmanite, except for three extra weak peaks with d-spacings of 2.11 (2θ = 50.3o), 2.80 (2θ = 37.5o) and 3.15 (2θ = 32.9o) Å. The peak with d-spacing of 3.15 Å was suggested to result from the presence of a superstructure (Navrotsky et al., 2003Navrotsky, A., Schoenitz, M., Kojitani, H., Xu, H., Zhang, J., Weidner, D.J., Jeanloz, R. (2003) Aluminum in magnesium silicate perovskite: Formation, structure, and energetics of magnesium-rich defect solid solutions. Journal of Geophysical Research 108, 2330.
). The weak peak with d-spacing of 2.11 Å may correspond to (200) of MgO (periclase), which indicates the coexistence of a trace amount of periclase with bridgmanite at 40 GPa and 2000 oK as consistent with the observation by Walter et al. (2006)Walter, M., Tronnes, R.G., Armstrong, L.S., Lord, O., Caldwell, W.A., Clark, A.M. (2006) Subsolidus phase relations and perovskite compressibility in the system MgO–AlO1.5–SiO2 with implications for Earth's lower mantle. Earth and Planetary Science Letters 248, 77–89.
. The sample decomposed to periclase and aluminous bridgmanite, suggesting a lower solubility of the MgAlO2.5 component with increasing pressure.
Figure 1 XRD patterns of En90Brm10 subjected to (a) 27, (b) 35 and (c) 40 GPa and at 2000 oK. Number in parentheses represents the index of bridgmanite. Question marks denote unidentified peaks. Asterisk denotes the probable (200) peak of periclase.
Textural observations by SEM showed that all samples consist of a single phase. Although the XRD patterns showed the presence of periclase for the sample annealed at 40 GPa, this phase was not detected by back scattered electron (BSE) observations in Figure 2. Compositions of bridgmanite with grains larger than 3 µm were analysed by EPMA and shown in Table 1, where the total cation numbers were normalised to two to show clearly the variation of oxygen number with pressure.

Figure 2 Representative BSE images of En90Brm10 subjected to (a) 27 GPa, (b) 35 and (c) 40 GPa and 2000 oK.
Table 1 Experimental conditions, run products, and phase compositions.
| Start Comp | Phases | MgO | Al2O3 | SiO2 | Total | O | Mg | Al | Si |
| IRIS333 (27 GPa, 2000 °K; 3 hours) | |||||||||
| En90Brm10 | Brg [n = 40] | 39.82 (23) | 5.13 (15) | 55.92 (24) | 100.86 (14) | 2.973 (4) | 0.979 (5) | 0.100 (3) | 0.922 (4) |
| En95Cor5 | Brg [n = 26] | 37.93 (22) | 5.11 (12) | 56.31 (3) | 99.35 (55) | 2.998 (2) | 0.952 (5) | 0.101 (2) | 0.948 (4) |
| IRIS357 (35 GPa, 2000 °K; 2 hours) | |||||||||
| En90Brm10 | Brg [n = 52] | 39.11 (52) | 5.19(13) | 56.38 (45) | 100.69 (25) | 2.984 (7) | 0.970 (13) | 0.102 (2) | 0.938 (7) |
| IRIS351 (40 GPa, 2000 °K *; 3 hours) | |||||||||
| En90Brm10 | Brg [n = 55] | 38.01 (40) | 5.09 (12) | 56.01 (46) | 99.11 (65) | 2.995 (4) | 0.955 (8) | 0.101 (2) | 0.944 (4) |
| En95Cor5 | Brg [n = 20] | 38.44 (32) | 5.12 (16) | 57.37 (55) | 100.92 (72) | 2.999 (3) | 0.949 (6) | 0.100 (3) | 0.950 (3) |
Oxide analyses are reported in wt. %. n: number of analysis points.
*: temperature was evaluated from a calibrated power curve derived from a lower temperature of 1500 °K.
Number in parentheses represents standard deviation for the last digit(s). Abbreviation: Brg, bridgmanite.
The number of oxygen atoms in bridgmanite from the En90Brm10 and En95Cor5 compositions in the present and previous studies (Navrotsky et al., 2003
Navrotsky, A., Schoenitz, M., Kojitani, H., Xu, H., Zhang, J., Weidner, D.J., Jeanloz, R. (2003) Aluminum in magnesium silicate perovskite: Formation, structure, and energetics of magnesium-rich defect solid solutions. Journal of Geophysical Research 108, 2330.
; Kojitani et al., 2007Kojitani, H., Katsura, T., Akaogi, M. (2007) Aluminum substitution mechanisms in perovskite-type MgSiO3: an investigation by Rietveld analysis. Physics and Chemistry of Minerals 34, 257–267.
) are plotted in Figure 3a. At 27 GPa, the number of oxygens in bridgmanite for the En90Brm10 composition is 2.973 ± 0.004, which is slightly lower than those in Navrotsky et al. (2003)Navrotsky, A., Schoenitz, M., Kojitani, H., Xu, H., Zhang, J., Weidner, D.J., Jeanloz, R. (2003) Aluminum in magnesium silicate perovskite: Formation, structure, and energetics of magnesium-rich defect solid solutions. Journal of Geophysical Research 108, 2330.
and Kojitani et al. (2007)Kojitani, H., Katsura, T., Akaogi, M. (2007) Aluminum substitution mechanisms in perovskite-type MgSiO3: an investigation by Rietveld analysis. Physics and Chemistry of Minerals 34, 257–267.
and substantially lower than that of En95Cor5 bridgmanite. This value shows clearly the presence of oxygen vacancies as consistent with 27Al NMR observations by Stebbins et al. (2003)Stebbins, J.F., Kojitani, H., Akaogi, M., Navrotsky, A. (2003) Aluminum substitution in MgSiO3 perovskite: Investigation of multiple mechanisms by 27Al NMR. American Mineralogist 88, 1161–1164.
. However, the number of oxygens in bridgmanite for the En90Brm10 composition monotonically increases to 2.984 ± 0.007 at 35 GPa, and finally reaches 2.995 ± 0.004 at 40 GPa, which is within the uncertainties of En95Cor5 bridgmanite at the same pressure, indicating almost no oxygen vacancies at 40 GPa.Figure 3b shows the variation in the Mg/Si cation ratio in these two kinds of aluminous bridgmanite against pressures. At 27 GPa, this ratio of the sample from the En90Brm10 composition was 1.06 ± 0.01, which lies between the ideal compositional lines associated with the oxygen-vacancy and charge-coupled substitution. With increasing pressure, it decreases to 1.03 ± 0.02 at 35 GPa, and then to 1.01 ± 0.02 at 40 GPa, which finally lies on the ideal composition line of the charge-coupled substitution for the case of En95Cor5 bridgmanite.
Figure 3c shows the ratios of Al at the A and B sites (AlB/AlA) in bridgmanite. This ratio of the sample for En90Brm10 decreases from 3.82 ± 0.53 at 27 GPa to 1.23 ± 0.38 at 40 GPa, which is finally within the uncertainties of unity, the expected result of the charge-coupled substitution. In contrast, this ratio for En95Cor5 bridgmanite remains almost unchanged with increasing pressure and lies on the line associated with the charge-coupled substitution.

Figure 3 Plots of (a) oxygen number, (b) Mg/Si ratio, and (c) Al ratio in A and B sites (AlB/AlA) in bridgmanite for En90Brm10 and En95Cor5 versus pressure. The black lines represent the compositions associated with the oxygen-vacancy substitution mechanism (OVM) and charge-coupled substitution mechanism (CCM) for bridgmanite. The red line is the least squares fitting (Mg/Si = Aexp(–P/B) + C, where P is pressure) of the present data.
The mass balance calculation implies that 1.2 ± 0.2 and 1.9 ± 0.4 wt. % periclase should coexist with bridgmanite for the En90Brm10 composition at 35 and 40 GPa, respectively. However, XRD patterns exhibited only one peak of periclase from the sample at 40 GPa, and that of the sample at 35 GPa and SEM showed no evidence for the coexistence of periclase. No observation of periclase by SEM could be explained by polishing out tiny interstitial grains.
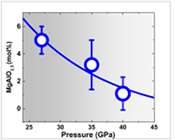
The results of these observations can be summarised such that the proportion of the MgAlO2.5 component in bridgmanite decreases with increasing pressure: 5.7 ± 0.8 mol. % at 27 GPa, 3.2 ± 1.8 mol. % at 35 GPa, and 1.1 ± 1.0 mol. % at 40 GPa (see the detailed calculation in section S-6 in the Supplementary Information). Thus, this component should become negligible at pressures above 40 GPa. Theoretically, the proportion of the MgAlO2.5 component should decrease more rapidly than demonstrated in this study because our staring sample was not ideally saturated with this component (see section S-6 in the Supplementary Information). It is noted that the oxygen fugacity has a negligible effect on the oxygen vacancy for Fe-free minerals (e.g., Smyth and Stocker, 1975
Smyth, D.M., Stocker, R.L. (1975) Point defects and non-stoichiometry in forsterite. Physics of the Earth and Planetary Interiors 10, 183–1932.
; Stocker and Smyth, 1978Stocker, R.L., Smyth, D.M. (1978) Effect of enstatite activity and oxygen partial pressure on the point-defect chemistry of olivine. Physics of the Earth and Planetary Interiors 16, 146–156.
). Compositions of synthetic Al-bearing bridgmanite being consistent with each other by using different metal capsules (such as gold and rhenium) and also comparable to those of the glass starting materials in the present and previous studies (Navrotsky et al., 2003Navrotsky, A., Schoenitz, M., Kojitani, H., Xu, H., Zhang, J., Weidner, D.J., Jeanloz, R. (2003) Aluminum in magnesium silicate perovskite: Formation, structure, and energetics of magnesium-rich defect solid solutions. Journal of Geophysical Research 108, 2330.
; Kojitani et al., 2007Kojitani, H., Katsura, T., Akaogi, M. (2007) Aluminum substitution mechanisms in perovskite-type MgSiO3: an investigation by Rietveld analysis. Physics and Chemistry of Minerals 34, 257–267.
) further confirms this point. Therefore, pressure is the only factor to result in this rapid decrease of the MgAlO2.5 component in bridgmanite.The present conclusion can be understood by a simple volume comparison based on the following reaction:
Eq. 1
2MgAlO2.5 (Brg) = Al2O3 (Brg) + 2MgO (Per)
Liu, Z.D., Irifune, T., Nishi, M., Tange, Y., Arimoto, T., Shinmei, T. (2016) Phase relations in the system MgSiO3–Al2O3 up to 52 GPa and 2000 K. Physics of the Earth and Planetary Interiors 257, 18–27.
. Therefore, the molar volume of the left hand side component of Equation 1 is larger than that of the right hand side components by 1.92 cm3/mole, suggesting that the MgAlO2.5 component becomes unstable with increasing pressure (see Fig. S-5).top
Geochemical Implications
Although the water storage of the lower mantle is uncertain, it has significant impacts on the chemical evolution of the mantle. The oxygen vacancy substitution, one of the most likely mechanisms, in aluminous bridgmanite may provide suitable storage sites to incorporate water in the shallower part of the lower mantle (e.g., Navrotsky, 1999
Navrotsky, A. (1999) A lesson from ceramics. Science 284, 1788–1789.
; Brodholt, 2000Brodholt, J.P. (2000) Pressure-induced changes in the compression mechanism of aluminous perovskite in the Earth's mantle. Nature 407, 620–622.
) according to the following reaction:Eq. 2
2MgAlO2.5 (Brg) + H2O (fluid) = 2MgHAlO3 (Brg)
Hernández, E., Alfè, D., Brodholt, J. (2013) The incorporation of water into lower-mantle perovskites: a first-principles study. Earth and Planetary Science Letters 364, 37–43.
). Hence, H2O is expected to be incorporated in bridgmanite as MgHAlO3 in the lower mantle because of high pressures. Murakami et al. (2002)Murakami, M., Hirose, K., Yurimoto, H., Nakashima, S., Takafuji, N. (2002) Water in Earth's lower mantle. Science 295, 1885–1887.
and Litasov et al. (2003)Litasov, K., Ohtani, E., Langenhorst, F., Yurimoto, H., Kubo, T., Kondo, T. (2003) Water solubility in Mg-perovskites and water storage capacity in the lower mantle. Earth and Planetary Science Letters 211, 189–203.
reported that synthetic Al-Fe-bearing bridgmanite in mantle peridotite can contain 1000–2000 ppm water at 26 GPa and 1500–1923 oK, which is significantly higher than that in synthetic MgSiO3 bridgmanite at 24–25 GPa and 1573–1873 oK (Bolfan-Casanova et al., 2000Bolfan-Casanova, N., Keppler, H., Rubie, D.C. (2000) Water partitioning between nominally anhydrous minerals in MgO-SiO2-H2O system up to 24 GPa: implications for the distribution of water in the Earth’s mantle. Earth and Planetary Science Letters 182, 209–221.
; Litasov et al., 2003Litasov, K., Ohtani, E., Langenhorst, F., Yurimoto, H., Kubo, T., Kondo, T. (2003) Water solubility in Mg-perovskites and water storage capacity in the lower mantle. Earth and Planetary Science Letters 211, 189–203.
). Following Equation 2, the amount of 2.2 mol. % MgAlO2.5 in bridgmanite can explain those reported high water amounts in bridgmanite. In contrast, Bolfan-Casanova et al. (2003)Bolfan-Casanova N., Keppler, H., Rubie, D.C. (2003) Water partitioning at 660 km depth and evidence for very low water solubility in magnesium silicate perovskite. Geophysical Research Letters 30, 1905.
and Panero et al. (2015)Panero, W.R., Pigott, J.S., Reaman, D.M., Kabbes, J.E., Liu, Z. (2015) Dry (Mg,Fe)SiO3 perovskite in the Earth's lower mantle. Journal of Geophysical Research 120, 894–908.
showed that Al-Fe-bearing bridgmanite had a very low water content (<10 ppm) at 24–26 GPa and 1600–2000 oK. Even though bridgmanite contained these amounts of water in their experimental conditions, the water solubility should rapidly decrease with increasing pressure due to a rapid decrease of MgAlO2.5 component in bridgmanite. Since bridgmanite has a negligible MgAlO2.5 component at pressures above 40 GPa, no water could be stored in regions deeper than 1000 km depth, i.e. in the majority of the lower mantle. Furthermore, the ambient lower mantle temperatures (Katsura et al., 2010Katsura, T., Yoneda, A., Yamazaki, D., Yoshino, T., Ito, E. (2010) Adiabatic temperature profile in the mantle. Physics of the Earth and Planetary Interiors 183, 212–218.
) are too high for hydrous minerals (Walter et al., 2015Walter, M.J., Thomson, A.R., Wang, W., Lord, O.T., Ross, J., McMahon, S.C., Baron, M.A., Melekhova, E., Kleppe, A.K., Kohn, S.C. (2015) The stability of hydrous silicates in Earth's lower mantle: Experimental constraints from the systems MgO-SiO2-H2O and MgO-Al2O3-SiO2-H2O. Chemical Geology 418, 16–29.
), therefore, we conclude that the majority of lower mantle is dry.Shcheka and Keppler (2012)
Shcheka, S.S., Keppler, H. (2012) The origin of the terrestrial noble-gas signature. Nature 490, 531–534.
showed that MgSiO3 bridgmanite contained ~1 wt. % argon at 25 GPa and 1873–2073 oK dissolved through oxygen-vacancy substitution. Theoretically, Al-bearing bridgmanite may accommodate larger amounts of argon than MgSiO3 bridgmanite because of the higher proportion of oxygen vacancies in the uppermost part of the lower mantle. With increasing depths, the incorporation capacity of argon quickly diminishes, due to the rapidly decreasing oxygen vacancies in aluminous bridgmanite, and finally vanishes at depths deeper than 1000 km. Therefore, the explanation for the xenon anomaly in the Earth’s atmosphere given by Shcheka and Keppler (2012)Shcheka, S.S., Keppler, H. (2012) The origin of the terrestrial noble-gas signature. Nature 490, 531–534.
needs to be further investigated.Bridgmanite, the most abundant phase in the lower mantle (80 vol. %; Irifune, 1994
Irifune, T. (1994) Absence of an aluminous phase in the upper part of the Earth's lower mantle. Nature 370, 131–133.
), dominates the viscosity of this region (Girard et al., 2016Girard, J., Amulele, G., Farla, R., Mohiuddin, A., Karato, S.-I. (2016) Shear deformation of bridgmanite and magnesiowüstite aggregates at lower mantle conditions. Science 351, 144–147.
). Seismic observation suggests that some slabs stagnate at 600–1000 km depths (Fukao and Obayashi, 2013Fukao, Y., Obayashi, M. (2013) Subducted slabs stagnant above, penetrating through, and trapped below the 660 km discontinuity. Journal of Geophysical Research: Solid Earth 118, 2013JB010466.
). Since there are no phase transitions in this region, some special explanations are required. One explanation is the increase of viscosity with depth in this region. Since the viscosity of minerals is controlled by atomic diffusivity (Karato and Wu, 1993Karato, S., Wu, P. (1993) Rheology of the upper mantle: a synthesis. Science 260, 771–778.
), the decrease of oxygen-vacancies in aluminous bridgmanite will cause a decrease in element diffusivity. Therefore, the expected decrease in diffusivity for aluminous bridgmanite should cause a high viscosity, which may explain this mid-lower mantle slab stagnation.top
Acknowledgements
The authors thank D. Krauße for his technical assistance in electron microprobe analysis. We also thank H. Fei, S. Shcheka and L. Wang for their fruitful discussion and comments. The authors thank S. Redfern for editing this manuscript, and two anonymous reviewers for their constructive comments. Z. L. was financially supported by the Bayerisches Geoinstitut Visitor’s Program. This study is also supported by research grants to T. K. (BMBF: 05K13WC2, 05K13WC2; DFG: KA3434/3-1, KA3434/7-1, KA3434/8-1, KA3434/9-1).
Editor: Simon Redfern
top
References
Akber-Knutson, S., Bukowinski, M.S.T. (2004) The energetics of aluminum solubility into MgSiO3 perovskite at lower mantle conditions. Earth and Planetary Science Letters 220, 317–330.
 Show in context
Show in context Earlier theoretical calculations investigated whether oxygen vacancies exist in bridgmanite (Brodholt, 2000; Yamamoto et al.; 2003; Akber–Knutson and Bukowinski, 2004).
View in article
Furthermore, energetic calculations by Akber–Knutson and Bukowinski (2004) suggested that oxygen-vacancy substitution could only account for 3–4 % and less than 1 % of Al substitutions in the shallower and deeper parts of the lower mantle, respectively.
View in article
Bolfan-Casanova, N., Keppler, H., Rubie, D.C. (2000) Water partitioning between nominally anhydrous minerals in MgO-SiO2-H2O system up to 24 GPa: implications for the distribution of water in the Earth’s mantle. Earth and Planetary Science Letters 182, 209–221.
 Show in context
Show in context Murakami et al. (2002) and Litasov et al. (2003) reported that synthetic Al-Fe-bearing bridgmanite in mantle peridotite can contain 1000–2000 ppm water at 26 GPa and 1500–1923 oK, which is significantly higher than that in synthetic MgSiO3 bridgmanite at 24–25 GPa and 1573–1873 oK (Bolfan-Casanova et al., 2000; Litasov et al., 2003).
View in article
Bolfan-Casanova N., Keppler, H., Rubie, D.C. (2003) Water partitioning at 660 km depth and evidence for very low water solubility in magnesium silicate perovskite. Geophysical Research Letters 30, 1905.
 Show in context
Show in context In contrast, Bolfan-Casanova et al. (2003) and Panero et al. (2015) showed that Al-Fe-bearing bridgmanite had a very low water content (<10 ppm) at 24–26 GPa and 1600–2000 oK.
View in article
Brodholt, J.P. (2000) Pressure-induced changes in the compression mechanism of aluminous perovskite in the Earth's mantle. Nature 407, 620–622.
 Show in context
Show in context Al substitution in the Mg, Si or both sites modifies the crystal chemistry of bridgmanite, and thereby changes the physical properties of bridgmanite (Zhang and Weidner, 1999; Brodholt, 2000).
View in article
Earlier theoretical calculations investigated whether oxygen vacancies exist in bridgmanite (Brodholt, 2000; Yamamoto et al.; 2003; Akber–Knutson and Bukowinski, 2004).
View in article
Brodholt (2000) suggested that oxygen-vacancy substitution should dominate at pressures only up to 30 GPa.
View in article
The oxygen vacancy substitution, one of the most likely mechanisms, in aluminous bridgmanite may provide suitable storage sites to incorporate water in the shallower part of the lower mantle (e.g., Navrotsky, 1999; Brodholt, 2000) according to the following reaction: 2MgAlO2.5 (Brg) + H2O (fluid) = 2MgHAlO3 (Brg)
View in article
Fukao, Y., Obayashi, M. (2013) Subducted slabs stagnant above, penetrating through, and trapped below the 660 km discontinuity. Journal of Geophysical Research: Solid Earth 118, 2013JB010466.
 Show in context
Show in context Seismic observation suggests that some slabs stagnate at 600–1000 km depths (Fukao and Obayashi, 2013).
View in article
Girard, J., Amulele, G., Farla, R., Mohiuddin, A., Karato, S.-I. (2016) Shear deformation of bridgmanite and magnesiowüstite aggregates at lower mantle conditions. Science 351, 144–147.
 Show in context
Show in context Bridgmanite, the most abundant phase in the lower mantle (80 vol. %; Irifune, 1994), dominates the viscosity of this region (Girard et al., 2016).
View in article
Hernández, E., Alfè, D., Brodholt, J. (2013) The incorporation of water into lower-mantle perovskites: a first-principles study. Earth and Planetary Science Letters 364, 37–43.
 Show in context
Show in context Although the volume of the MgHAlO3 component in bridgmanite is unknown, we could assume that it has a similar volume with the MgAlO2.5 component because of an actually zero volume of the proton (Hernández et al., 2013).
View in article
Hirsch, L.M., Shankland, T.J. (1991) Point defects in silicate perovskite. Geophysical Research Letters 18, 1305–1308.
 Show in context
Show in context Two competing Al substitution mechanisms occur in bridgmanite (Hirsch and Shankland, 1991).
View in article
Irifune, T. (1994) Absence of an aluminous phase in the upper part of the Earth's lower mantle. Nature 370, 131–133.
 Show in context
Show in context Under the Earth’s lower mantle conditions, Al3+ is mainly accommodated in bridgmanite in a pyrolite mantle (Irifune, 1994).
View in article
Bridgmanite, the most abundant phase in the lower mantle (80 vol. %; Irifune, 1994), dominates the viscosity of this region (Girard et al., 2016).
View in article
Ishii, T., Shi, L., Huang, R., Tsujino, N., Druzhbin, D., Myhill, R., Li, Y., Wang, L., Yamamoto, T., Miyajima, N., Kawazoe, T., Nishiyama, N., Higo, Y., Tange, Y., Katsura, T. (2016) Generation of pressure over 40 GPa using Kawai–type multi–anvil press with tungsten carbide anvils. Review of Scientific Instruments 87, 024501-1–024501-6.
 Show in context
Show in context High pressure and high temperature experiments were conducted using ultra-high pressure multi-anvil technology with carbide anvils (Ishii et al., 2016), and detailed techniques can be found in Figures S-1 and S-2 in the Supplementary Information.
View in article
Ito, E., Matsui, Y. (1978) Synthesis and crystal–chemical characterization of MgSiO3 perovskite. Earth and Planetary Science Letters 38, 443–450.
 Show in context
Show in context All diffraction peaks of the sample for the En90Brm10 composition synthesised at 27 GPa were assigned to those of bridgmanite (Ito and Matsui, 1978), indicating the formation of a single phase bridgmanite.
View in article
Karato, S., Wu, P. (1993) Rheology of the upper mantle: a synthesis. Science 260, 771–778.
 Show in context
Show in context Since the viscosity of minerals is controlled by atomic diffusivity (Karato and Wu, 1993), the decrease of oxygen-vacancies in aluminous bridgmanite will cause a decrease in element diffusivity.
View in article
Katsura, T., Yoneda, A., Yamazaki, D., Yoshino, T., Ito, E. (2010) Adiabatic temperature profile in the mantle. Physics of the Earth and Planetary Interiors 183, 212–218.
 Show in context
Show in context Furthermore, the ambient lower mantle temperatures (Katsura et al., 2010) are too high for hydrous minerals (Walter et al., 2015), therefore, we conclude that the majority of lower mantle is dry.
View in article
Kojitani, H., Katsura, T., Akaogi, M. (2007) Aluminum substitution mechanisms in perovskite-type MgSiO3: an investigation by Rietveld analysis. Physics and Chemistry of Minerals 34, 257–267.
 Show in context
Show in context The number of oxygen atoms in bridgmanite from the En90Brm10 and En95Cor5 compositions in the present and previous studies (Navrotsky et al., 2003; Kojitani et al., 2007) are plotted in Figure 3a.
View in article
At 27 GPa, the number of oxygens in bridgmanite for the En90Brm10 composition is 2.973 ± 0.004, which is slightly lower than those in Navrotsky et al. (2003) and Kojitani et al. (2007) and substantially lower than that of En95Cor5 bridgmanite.
View in article
Compositions of synthetic Al-bearing bridgmanite being consistent with each other by using different metal capsules (such as gold and rhenium) and also comparable to those of the glass starting materials in the present and previous studies (Navrotsky et al., 2003; Kojitani et al., 2007) further confirms this point.
View in article
Litasov, K., Ohtani, E., Langenhorst, F., Yurimoto, H., Kubo, T., Kondo, T. (2003) Water solubility in Mg-perovskites and water storage capacity in the lower mantle. Earth and Planetary Science Letters 211, 189–203.
 Show in context
Show in context Murakami et al. (2002) and Litasov et al. (2003) reported that synthetic Al-Fe-bearing bridgmanite in mantle peridotite can contain 1000–2000 ppm water at 26 GPa and 1500–1923 oK, which is significantly higher than that in synthetic MgSiO3 bridgmanite at 24–25 GPa and 1573–1873 oK (Bolfan-Casanova et al., 2000; Litasov et al., 2003).
View in article
Liu, Z.D., Irifune, T., Nishi, M., Tange, Y., Arimoto, T., Shinmei, T. (2016) Phase relations in the system MgSiO3–Al2O3 up to 52 GPa and 2000 K. Physics of the Earth and Planetary Interiors 257, 18–27.
 Show in context
Show in context The molar volumes of the hypothetical end members of MgAlO2.5 and Al2O3 bridgmanite (for Al cation number of 0.1 per formula unit) under ambient conditions are estimated to be 24.50 and 24.54 cm3/mole, respectively, from the volume composition data in Figure S-4 and Liu et al. (2016).
View in article
McDonough, W.F., Sun, S.-S. (1995) The composition of the Earth. Chemical Geology 120, 223–253.
 Show in context
Show in context Aluminium (Al) is the fifth most abundant element in the Earth’s mantle (McDonough and Sun, 1995).
View in article
Murakami, M., Hirose, K., Yurimoto, H., Nakashima, S., Takafuji, N. (2002) Water in Earth's lower mantle. Science 295, 1885–1887.
 Show in context
Show in context Murakami et al. (2002) and Litasov et al. (2003) reported that synthetic Al-Fe-bearing bridgmanite in mantle peridotite can contain 1000–2000 ppm water at 26 GPa and 1500–1923 oK, which is significantly higher than that in synthetic MgSiO3 bridgmanite at 24–25 GPa and 1573–1873 oK (Bolfan-Casanova et al., 2000; Litasov et al., 2003).
View in article
Navrotsky, A. (1999) A lesson from ceramics. Science 284, 1788–1789.
 Show in context
Show in context Navrotsky, A., Schoenitz, M., Kojitani, H., Xu, H., Zhang, J., Weidner, D.J., Jeanloz, R. (2003) Aluminum in magnesium silicate perovskite: Formation, structure, and energetics of magnesium-rich defect solid solutions. Journal of Geophysical Research 108, 2330.
 Show in context
Show in context Panero, W.R., Pigott, J.S., Reaman, D.M., Kabbes, J.E., Liu, Z. (2015) Dry (Mg,Fe)SiO3 perovskite in the Earth's lower mantle. Journal of Geophysical Research 120, 894–908.
 Show in context
Show in context In contrast, Bolfan-Casanova et al. (2003) and Panero et al. (2015) showed that Al-Fe-bearing bridgmanite had a very low water content (<10 ppm) at 24–26 GPa and 1600–2000 oK.
View in article
Shcheka, S.S., Keppler, H. (2012) The origin of the terrestrial noble-gas signature. Nature 490, 531–534.
 Show in context
Show in context The oxygen vacancies in bridgmanite have special importance for understanding lower mantle processes because oxygen vacancies may induce water (Vo + O2- + H2O = 2OH-1; Navrotsky, 1999) and noble gases into bridgmanite (Shcheka and Keppler, 2012).
View in article
Shcheka and Keppler (2012) showed that MgSiO3 bridgmanite contained ~1 wt. % argon at 25 GPa and 1873–2073 oK dissolved through oxygen-vacancy substitution.
View in article
Therefore, the explanation for the xenon anomaly in the Earth’s atmosphere given by Shcheka and Keppler (2012) needs to be further investigated.
View in article
Smyth, D.M., Stocker, R.L. (1975) Point defects and non-stoichiometry in forsterite. Physics of the Earth and Planetary Interiors 10, 183–1932.
 Show in context
Show in context It is noted that the oxygen fugacity has a negligible effect on the oxygen vacancy for Fe-free minerals (e.g., Smyth and Stocker, 1975; Stocker and Smyth, 1978).
View in article
Stebbins, J.F., Kojitani, H., Akaogi, M., Navrotsky, A. (2003) Aluminum substitution in MgSiO3 perovskite: Investigation of multiple mechanisms by 27Al NMR. American Mineralogist 88, 1161–1164.
 Show in context
Show in context High pressure and high temperature synthesis (Navrotsky et al., 2003) and nuclear magnetic resonance (NMR) spectroscopy (Stebbins et al., 2003) suggested that oxygen-vacancy substitution dominates in bridgmanite with 5–10 mol. % MgAlO2.5 at 27 GPa and 1873 oK.
View in article
This value shows clearly the presence of oxygen vacancies as consistent with 27Al NMR observations by Stebbins et al. (2003).
View in article
Stocker, R.L., Smyth, D.M. (1978) Effect of enstatite activity and oxygen partial pressure on the point-defect chemistry of olivine. Physics of the Earth and Planetary Interiors 16, 146–156.
 Show in context
Show in context It is noted that the oxygen fugacity has a negligible effect on the oxygen vacancy for Fe-free minerals (e.g., Smyth and Stocker, 1975; Stocker and Smyth, 1978).
View in article
Walter, M., Tronnes, R.G., Armstrong, L.S., Lord, O., Caldwell, W.A., Clark, A.M. (2006) Subsolidus phase relations and perovskite compressibility in the system MgO–AlO1.5–SiO2 with implications for Earth's lower mantle. Earth and Planetary Science Letters 248, 77–89.
 Show in context
Show in context On the other hand, X-ray diffraction measurements in a laser-heated diamond anvil demonstrated that bridgmanite coexisted with periclase from a glass starting material with a composition of 90 mol. % MgSiO3 and 10 mol. % MgAlO2.5 at 40 ± 10 GPa and 2000–2500 oK (Walter et al., 2006), indicating that the MgAlO2.5 component was not present in bridgmanite and thereby oxygen-vacancy substitution was unfavoured under these conditions.
View in article
The weak peak with d-spacing of 2.11 Å may correspond to (200) of MgO (periclase), which indicates the coexistence of a trace amount of periclase with bridgmanite at 40 GPa and 2000 oK as consistent with the observation by Walter et al. (2006).
View in article
Walter, M.J., Thomson, A.R., Wang, W., Lord, O.T., Ross, J., McMahon, S.C., Baron, M.A., Melekhova, E., Kleppe, A.K., Kohn, S.C. (2015) The stability of hydrous silicates in Earth's lower mantle: Experimental constraints from the systems MgO-SiO2-H2O and MgO-Al2O3-SiO2-H2O. Chemical Geology 418, 16–29.
 Show in context
Show in context Furthermore, the ambient lower mantle temperatures (Katsura et al., 2010) are too high for hydrous minerals (Walter et al., 2015), therefore, we conclude that the majority of lower mantle is dry.
View in article
Yamamoto, T., Yuen, D.A., Ebisuzaki, T. (2003) Substitution mechanism of Al ions in MgSiO3 perovskite under high pressure conditions from first-principles calculations. Earth and Planetary Science Letters 206, 617–625.
 Show in context
Show in context Earlier theoretical calculations investigated whether oxygen vacancies exist in bridgmanite (Brodholt, 2000; Yamamoto et al.; 2003; Akber–Knutson and Bukowinski, 2004).
View in article
Yamamoto et al. (2003) argued oxygen-vacancy substitution should be unfavourable in the entire lower mantle.
View in article
Zhang, J., Weidner, D.J. (1999) Thermal equation of state of aluminum–enriched silicate perovskite. Science 284, 782–784.
 Show in context
Show in context Al substitution in the Mg, Si or both sites modifies the crystal chemistry of bridgmanite, and thereby changes the physical properties of bridgmanite (Zhang and Weidner, 1999; Brodholt, 2000).
View in article
top
Supplementary Information
1. Glass Preparation
Besides with En90Brm10 and En95Cor5 glass, En100 glass is also prepared for the standard for composition comparison. All glasses were prepared from oxide mixtures using reagent grade chemicals: MgO, SiO2, and Al2O3, fused and homogenised at least two times at 2000 oK for one hour, and finally quenched into water. After that, the glasses were finely ground into powder, then checked by powder X-ray diffraction to confirm absence of solid phases. They were finally kept in a high vacuum box before experiments. Based on the compositions in Table S-1, the total cation number is normalised to two to see oxygen vacancies clearly in the starting material. Therefore, the composition of the En90Brm10 glass is Mg0.982±0.006Al0.101±0.001Si0.918±0.003O2.969±0.003, which clearly deviates from the Al2O3 stoichiometry and is deficient in oxygen. This glass starting material contains about 6.4 ± 0.9 mol. % MgAlO2.5 and 1.9 ± 0.5 mol. % AlO1.5, which is slightly smaller than that with the nominal MgAlO2.5 component of En90Brm10. Therefore the starting material is not ideally saturated with MgAlO2.5 as En90Brm10. Here, we approximated this non-stoichiometric glass starting material with Mg0.982±0.006Al0.101±0.001Si0.918±0.003O2.969±0.003 as En90Brm10-glass. The En100 and En95Cor5 glasses have the intended compositions within analytical errors.
Table S-1 Chemical compositions of the starting glass material.
| Starting material | MgO | Al2O3 | SiO2 | Total | O | Mg | Al | Si |
| En90Brm10 (n = 25) | 39.49 (18) | 5.11 (5) | 55.08 (37) | 99.68 (40) | 2.969 (3) | 0.982 (6) | 0.101 (1) | 0.918 (3) |
| En95Cor5 (n = 15) | 38.42 (29) | 5.10 (5) | 57.47 (48) | 100.99 (52) | 3.002 (7) | 0.948 (9) | 0.100 (2) | 0.951 (7) |
| En100 (n = 12) | 40.39 (50) | – | 59.39 (60) | 99.78 (36) | 2.994 (7) | 1.006 (14) | – | 0.994 (7) |
Oxide analyses are reported in wt. %. n: number of analysis points.
Number in parentheses represents standard deviation and is placed in the last digit (s).
2. High Pressure Technique in Kawai-type Multi-anvil Apparatus

Figure S-1 The schematic illustration of (a) 7/3 and (b) 5.7/1.5 cell assembly for high pressure and high temperature experiments.
Bridgmanite samples were first synthesised at a pressure of 27 GPa and a temperature of 2000 oK for 3 hours using a 7/3 (OEL/TEL = octahedral edge length of pressure medium/truncated edge length of anvil) cell assembly (Fig. S-1a) in a Kawai-type multi-anvil apparatus (IRIS-15) with a DIA-type guide block and a maximum press load of 15 MN in Bayerisches Geoinstitut, University of Bayreuth, Germany. After that, the bridgmanite samples were cut into two parts for X-ray diffraction, scanning electron microscopy and electron probe microanalysis, respectively.
Furthermore, two annealing high pressure experiments are conducted at pressures of 35 and 40 GPa, respectively, and a temperature of 2000 oK for 2 and 3 hours using a synthetic single phase non-stoichiometric bridgmanite (hereafter as En90Brm10-Brg), which was synthesised from the starting En90Brm10 glass at 27 GPa and 2000 oK, as a starting material in a 5.7/1.5 cell assembly (Fig. S-1b) by means of the ultra-high pressure multi-anvil technology (Ishii et al., 2016). The 7/3 and 5.7/1.5 cell assemblies are shown in Figure S-1, and LaCrO3 and rhenium (Re) were used for the heaters in these two cell assemblies, respectively. Pressure calibrations of these two cell assemblies at room temperature and high temperature were reported by Ishii et al. (2016), and also shown in Figure S-2 based on the Al2O3 solubility in bridgmanite (Liu et al., 2016). The pressure uncertainty of 7/3 cell assembly is ±0.5 GPa (Ishii et al., 2016), while that for 5.7/1.5 is ±1 GPa based on the uncertainties of Al2O3 solubility in bridgmanite. Temperature was measured with a W97Re3–W75Re25 thermocouple adjacent to the sample capsules. In experiments where no direct temperature reading from the thermocouple was obtained, temperatures were estimated using power-temperature relationships established by preceding runs.

Figure S-2 Pressure calibrations of 7/3 (a) and 5.7/1.5 (b) cell assembly in IRIS-15 at 300 and 2000 oK, respectively.
3. Analytical Methods
Phases in recovered samples were identified using a micro-focused X-ray diffractometer with a Co anode operated at 40 kV, 500 µA. MgSiO3 bridgmanite, synthesised in the IRIS333 run (Table S-2), was used as the external standard to calibrate the Bragg angle (2θ) of the instrument. XRD profiles were collected for about one hour. Electron back scattered images were collected by a LEO1530 scanning electron microscope operating at an acceleration voltage of 15 kV and a beam current of 10 nA. Chemical compositions were determined by a JEOL JXA-8200 electron probe microanalyser (EPMA) operating at 15 kV and 5 nA with standards of enstatite for Mg and Si, and pyrope for Al.
Table S-2 The experimental condition and composition of MgSiO3 bridgmanite.
| Starting material | Phases | MgO | Al2O3 | SiO2 | Total | O | Mg | Al | Si |
| IRIS333 (27 GPa, 2000 °K; 3 hours) | |||||||||
| En100 | Brg (n = 25) | 40.39 (50) | – | 59.39 (60) | 99.78 (36) | 2.996 (3) | 1.006 (9) | – | 0.995(7) |
Oxide analyses are reported in wt. %. n: number of analysis points.
Number in parentheses represents standard deviation and is placed in the last digit (s).
Abbreviation: Brg, bridgmanite.
4. XRD of En95Cor5-bridgmanite at 27 and 40 GPa and 2000 oK
Figure S-3a shows XRD patterns for synthetic En95Cor5-bridgmanite at pressures of 27 GPa and 2000 oK, and Figure S-3b shows that for the annealing sample for En95Cor5-bridgmanite at 40 GPa and 2000 oK. Their compositions are almost consistent with that of starting glass, indicating that bridgmanite is the only phase in these recovered samples.

Figure S-3 XRD patterns of the recovered sample for En95Cor5 composition at 27 (a) and 40 GPa (b) under 2000 oK, respectively. Number in parentheses represents the index of bridgmanite.
5. Unit Cell Volume of Bridgmanite
Figure S-4 shows unit cell volumes of bridgmanite along the system MgSiO3–Al2O3 and MgSiO3–MgAlO2.5 in the present and previous studies (Ito and Matsui, 1978; Weng et al., 1981; Irifune et al., 1996; Kubo and Akaogi, 2000; Navrotsky et al., 2003; Walter et al., 2004, 2006; Kojitani et al., 2007). In the present study, unit cell volumes of the recovered bridgmanite for the En90Brm10 composition at 35 and 40 GPa are slightly lower than those at 27 GPa under the same temperature of 2000 oK, but comparable with those of bridgmanite for En95Cor5 composition within analytical uncertainties in the present study. This may be caused by the slight change of the crystal chemistry for bridgmanite or the stress in these recovered samples at different pressures. Moreover, these volumes of the recovered bridgmanite for the En90Brm10 composition in the present study are comparable to those of previous studies on the same composition within analytical uncertainties (Navrotsky et al., 2003; Walter et al., 2006; Kojitani et al., 2007), which is also within uncertainties of bridgmanite for En95Cor5 composition in previous studies (Weng et al., 1981; Irifune et al., 1996; Kubo and Akaogi, 2000; Walter et al., 2004). The largely scattered value for bridgmanite with the same composition may be caused by the different synthesis conditions, quench history, and analytical methods.

Figure S-4 Unit cell volumes of bridgmanite along the system MgSiO3–Al2O3 and MgSiO3–MgAlO2.5 in the present and previous studies. The solid line presents the linear fitting results on values for bridgmanite along the system MgSiO3-Al2O3 (Liu et al., 2016).
6. Calculation of the Solubility of the MgAlO5 Component in Bridgmanite
It is known that both MgAlO2.5 (the oxygen-vacancy substitution) and AlO1.5 (the charge-coupled substitution) are competing with each other in aluminous bridgmanite in both systems MgSiO3–MgAlO2.5 and –AlO1.5, therefore, the composition of bridgmanite can be represented by the following reaction:
Eq. S-1
MgxAlzSiyOx+1.5z+2y = y MgSiO3 + (x-y) MgAlO2.5 + (z-x+y) AlO1.5
It is noted that the equilibrated proportion at 27 GPa could be higher because our sample at this condition was not ideally saturated with the MgAlO2.5 component for the glass starting material. On the other hand, the equilibrated proportions at 35 and 40 GPa could be lower, because the starting material was bridgmanite that had more MgAlO2.5 component than the present run products. Therefore the proportion of the MgAlO2.5 component should decrease more rapidly than demonstrated in this study.
7. The Variation of Molar Volume for Equation 1
Considering Al cation number of 0.1 for the Equation 1 in the main text based on the present and previous studies, the Eq. 1 can be represented by the following chemical equation:
Eq. S-2
MgAl0.1Si0.9O2.95(Brg) + 0.05 MgSiO3(Brg) = Mg0.95Al0.1Si0.95O3(Brg) + 0.1 MgO (Per)
Eq. S-3
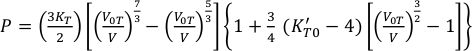
where KT , V0T , V, and K'T0 are the isothermal bulk modulus, zero pressure molar volume, high pressure molar volume and pressure derivative of bulk modulus. The temperature effects for KT and V0T are given by:
Eq. S-4
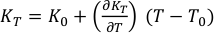
Eq. S-5
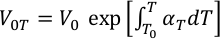
where (∂KT/∂T)P, T0, V0 and α are the temperature derivatives of the bulk modulus, reference temperature, molar volume and volumetric thermal expansion at ambient pressure, respectively. Thermal expansion α is empirically represented by
Eq. S-6
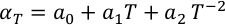
The experimental thermoelastic parameters of these mantle minerals used in Equation S-2 are shown in Table S-3.
The calculated ΔV as a function of pressure is shown in Figure S-5 under 300 and 2000 oK, respectively. It is seen that the values of ΔV are minus at both temperatures at 27–50 GPa, suggesting the pressure would enhance both Equations 1 and S-2 from the left to right direction. Therefore, the pressure will decrease the component of MgAlO2.5 in bridgmanite.
Table S-3 Elastic properties of minerals related with Eq. S-2 used for thermodynamic calculations.
| Phase | MgSiO3-Brg | Mg0.95Al0.1Si0.95O3-Brg | MgAl0.1Si0.9O2.95-Brg | MgO |
| V0 (cm3/mol) | 24.44 (1)a | 24.54 (1)e | 24.50 (1)f | 11.27 (1)h |
| ΔH0(KJ/mol) | 10.79 (196)b | 16.44 (88)b | 10.24 (428)f | 36.48 (50)i |
| ΔS0(KJ/mol) | 0.063b | 0.058b | 0.073f | 0.026i |
| KT298 (GPa) | 256.7 (15)a | 239 (1)e | 259 (8)g | 160.9h |
| KT' | 4.09 (6)a | 4e | 4g | 4.35 (10)h |
| (δKT/δT)P (GPa/°K) | 0.035 (2)c | -0.057 (1)e | -0.057 (1)* | -0.0272h |
| αT= a0+a1T+a2T2 (°K-1) | ||||
| a0 ×105 | 1.982d | 2.08 (26)e | 2.08 (26)* | 3.38i |
| a1 ×108 | 0.818d | 2.21 (67)e | 2.21 (67)* | 12.52i |
| a2 ×10 | -4.740d | 0 | 0 | -19.13i |
| CP= A+BT+CT-2 (°K-1) | ||||
| A×10-2 | 1.769d | 1.769# | 1.769* | 0.66i |
| B×10-3 | -1.565d | -1.565# | -1.565* | -0.36i |
| C ×10-6 | 0 | 0 | 0 | -0.61i |
a: Tange et al. (2012)
b: Akaogi and Ito (1999)
c: Katsura et al. (2009)
d: Funamori et al. (1996)
e: Zhand and Wiedner (1999)
f: Navrotsky et al. (2003)
g: Walter et al. (2006)
h: Kono et al. (2010)
i: Kojitani et al. (2012)
#: the same value with that of MgSiO3-Brg.
*: the same value with that of Mg0.95Al0.1Si0.95O3-Brg.
Abbreviation: Brg: bridgmanite.
Number in parentheses represents standard deviation and is placed in the last digit (s).

Figure S-5 The calculated molar volume change of Eq. S-2 in the text along 300 and 2000 °K, respectively.
Supplementary Information References
Funamori, N., Yagi, T., Utsumi, W., Kondo, T., Uchida, T., Funamori, M. (1996) Thermoelastic properties of MgSiO3perovskite determined by in situ X ray observations up to 30 GPa and 2000 K. Journal of Geophysical Research 101, 8257–8269.
Irifune, T., Koizumi, T., Ando, J. (1996) An experimental study of the garnet-perovskite transformation in the system MgSiO3-Mg3Al2Si3O12. Physics of the Earth and Planetary Interiors 96, 147–157.
Ito, E., Matsui, Y. (1978) Synthesis and crystal–chemical characterization of MgSiO3 perovskite. Earth and Planetary Science Letters 38, 443–450.
Katsura, T., Yokoshi, S., Kawabe, K., Shatskiy, A., Manthilake, M., Zhai, S. M., Fukui, H., Hegoda, H., Yoshino, T., Yamazaki, D., Matsuzaki, T., Yoneda, A., Ito, E., Sugita, M., Tomioka, N., Hagiya, K., Nozawa, A., Funakoshi, K. (2009) P-V-T relations of MgSiO3 perovskite determined by in situ X-ray diffraction using a large-volume high-pressure apparatus. Geophysical Research Letters 36, L01305.
Kojitani, H., Katsura, T., Akaogi, M. (2007) Aluminum substitution mechanisms in perovskite-type MgSiO3: An investigation by Rietveld analysis. Physics and Chemistry of Minerals 34, 257–267.
Kojitani, H., Ishii, T., Akaogi, M. (2012) Thermodynamic investigation of phase equilibrium boundary between calcium ferrite-type MgAl2O4 and MgO + Al2O3. Physics of the Earth and Planetary Interiors 212–213, 100–105.
Kono, Y., Irifune, T., Higo, Y., Inoue, T., Barnhoorn, A. (2010) PVT relation of MgO derived by simultaneous elastic wave velocity and in situ X-ray measurements: A new pressure scale for the mantle transition region. Physics of the Earth and Planetary Interiors 183, 196–211.
Kubo, A., Akaogi, M. (2000) Post-garnet transitions in the system Mg4Si4O12-Mg3Al2Si3O12 up to 28 GPa: phase relations of garnet, ilmenite and perovskite. Physics of the Earth and Planetary Interiors 121, 85–102.
Liu. Z.D., Irifune, T., Nishi. M., Tange, Y., Arimoto, T., Shinmei, T. (2016) Phase relations in the system MgSiO3–Al2O3 up to 52 GPa and 2000 K. Physics of the Earth and Planetary Interiors 257, 18–27.
Navrotsky, A., Schoenitz, M., Kojitani, H., Xu, H., Zhang, J., Weidner, D.J., Jeanloz, R. (2003) Aluminum in magnesium silicate perovskite: Formation, structure, and energetics of magnesium-rich defect solid solutions. Journal of Geophysical Research 108, 2330.
Tange, Y., Kuwayama, Y., Irifune, T., Funakoshi, K.-i., Ohishi, Y. (2012) P-V-T equation of state of MgSiO3 perovskite based on the MgO pressure scale: A comprehensive reference for mineralogy of the lower mantle. Journal of Geophysical Research 117, B06201.
Walter, M., Kubo, A., Yoshino, T., Brodholt, J., Koga, K.T., Ohishi, Y. (2004) Phase relations and equation-of-state of aluminous Mg-silicate perovskite and implications for Earth's lower mantle. Earth and Planetary Science Letters 222, 501–516.
Walter, M., Tronnes, R.G., Armstrong, L.S., Lord, O., Caldwell, W.A., Clark, A.M. (2006) Subsolidus phase relations and perovskite compressibility in the system MgO–AlO1.5–SiO2 with implications for Earth's lower mantle. Earth and Planetary Science Letters 248, 77–89.
Weng, K., Mao, H.K., Bell, P.M. (1981) Lattice parameters of the perovskite phase in the system MgSiO3–CaSiO3–Al2O3. Carnegie Institute Washington, Year Book 81, 273–277.
Yagi, T., Okabe, K., Nishiyama, N., Kubo, A., Kikegawa, T. (2004) Complicated effects of aluminum on the compressibility of silicate perovskite. Physics of the Earth and Planetary Interiors 143–144, 81–89.
Zhang, J., Weidner, D.J. (1999) Thermal equation of state of aluminum–enriched silicate perovskite. Science 284, 782–784.
Figures and Tables
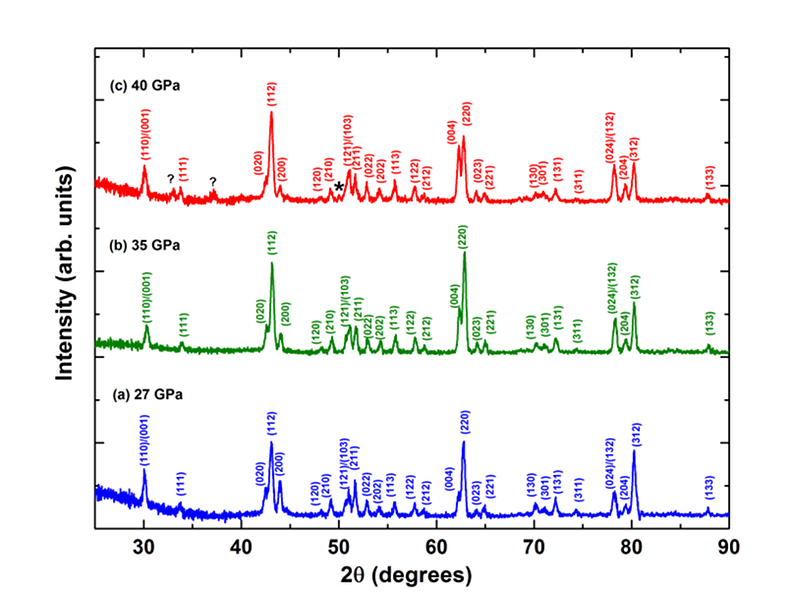
Figure 1 XRD patterns of En90Brm10 subjected to (a) 27, (b) 35 and (c) 40 GPa and at 2000 oK. Number in parentheses represents the index of bridgmanite. Question marks denote unidentified peaks. Asterisk denotes the probable (200) peak of periclase.
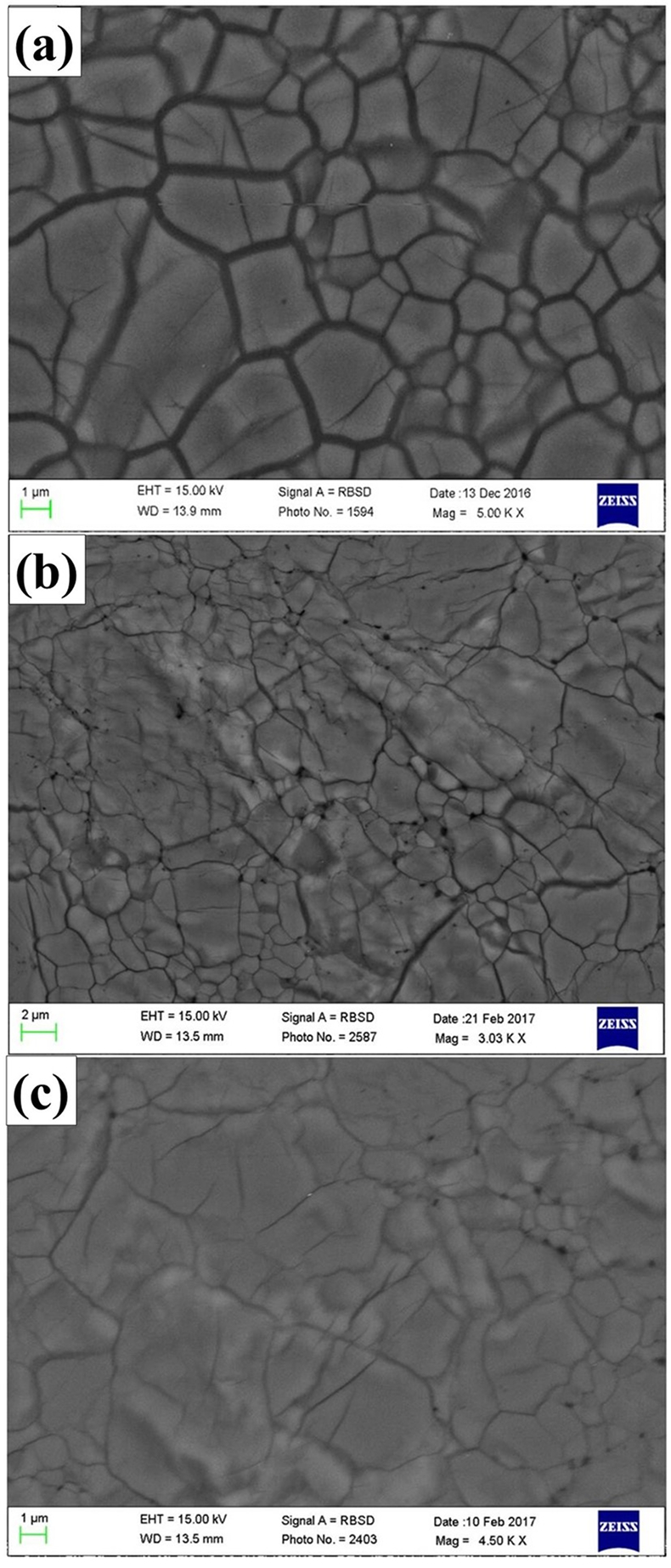
Figure 2 Representative BSE images of En90Brm10 subjected to (a) 27 GPa, (b) 35 and (c) 40 GPa and 2000 oK.
Table 1 Experimental conditions, run products, and phase compositions.
| Start Comp | Phases | MgO | Al2O3 | SiO2 | Total | O | Mg | Al | Si |
| IRIS333 (27 GPa, 2000 °K; 3 hours) | |||||||||
| En90Brm10 | Brg [n = 40] | 39.82 (23) | 5.13 (15) | 55.92 (24) | 100.86 (14) | 2.973 (4) | 0.979 (5) | 0.100 (3) | 0.922 (4) |
| En95Cor5 | Brg [n = 26] | 37.93 (22) | 5.11 (12) | 56.31 (3) | 99.35 (55) | 2.998 (2) | 0.952 (5) | 0.101 (2) | 0.948 (4) |
| IRIS357 (35 GPa, 2000 °K; 2 hours) | |||||||||
| En90Brm10 | Brg [n = 52] | 39.11 (52) | 5.19(13) | 56.38 (45) | 100.69 (25) | 2.984 (7) | 0.970 (13) | 0.102 (2) | 0.938 (7) |
| IRIS351 (40 GPa, 2000 °K *; 3 hours) | |||||||||
| En90Brm10 | Brg [n = 55] | 38.01 (40) | 5.09 (12) | 56.01 (46) | 99.11 (65) | 2.995 (4) | 0.955 (8) | 0.101 (2) | 0.944 (4) |
| En95Cor5 | Brg [n = 20] | 38.44 (32) | 5.12 (16) | 57.37 (55) | 100.92 (72) | 2.999 (3) | 0.949 (6) | 0.100 (3) | 0.950 (3) |
Oxide analyses are reported in wt. %. n: number of analysis points.
*: temperature was evaluated from a calibrated power curve derived from a lower temperature of 1500 °K.
Number in parentheses represents standard deviation for the last digit(s). Abbreviation: Brg, bridgmanite.
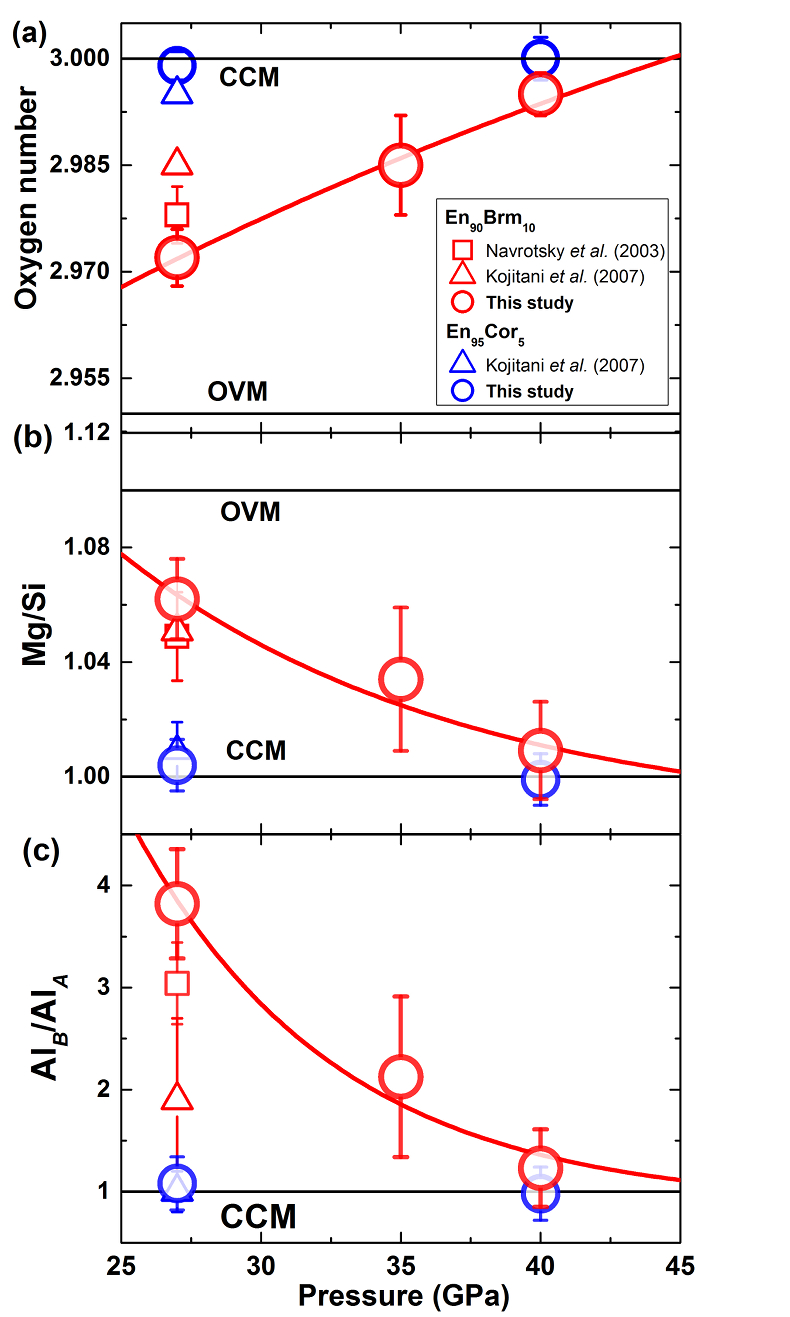
Figure 3 Plots of (a) oxygen number, (b) Mg/Si ratio, and (c) Al ratio in A and B sites (AlB/AlA) in bridgmanite for En90Brm10 and En95Cor5 versus pressure. The black lines represent the compositions associated with the oxygen-vacancy substitution mechanism (OVM) and charge-coupled substitution mechanism (CCM) for bridgmanite. The red line is the least squares fitting (Mg/Si = Aexp(–P/B) + C, where P is pressure) of the present data.
Back to article
Supplementary Figures and Tables
Table S-1 Chemical compositions of the starting glass material.
| Starting material | MgO | Al2O3 | SiO2 | Total | O | Mg | Al | Si |
| En90Brm10 (n = 25) | 39.49 (18) | 5.11 (5) | 55.08 (37) | 99.68 (40) | 2.969 (3) | 0.982 (6) | 0.101 (1) | 0.918 (3) |
| En95Cor5 (n = 15) | 38.42 (29) | 5.10 (5) | 57.47 (48) | 100.99 (52) | 3.002 (7) | 0.948 (9) | 0.100 (2) | 0.951 (7) |
| En100 (n = 12) | 40.39 (50) | – | 59.39 (60) | 99.78 (36) | 2.994 (7) | 1.006 (14) | – | 0.994 (7) |
Oxide analyses are reported in wt. %. n: number of analysis points.
Number in parentheses represents standard deviation and is placed in the last digit (s).
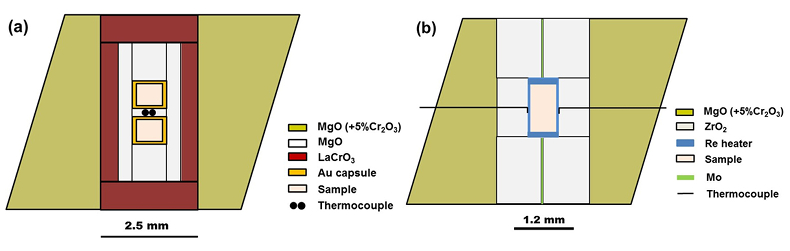
Figure S-1 The schematic illustration of (a) 7/3 and (b) 5.7/1.5 cell assembly for high pressure and high temperature experiments.
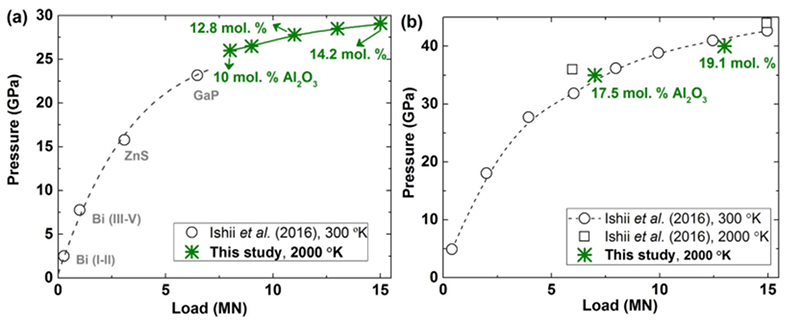
Figure S-2 Pressure calibrations of 7/3 (a) and 5.7/1.5 (b) cell assembly in IRIS-15 at 300 and 2000 oK, respectively.
Table S-2 The experimental condition and composition of MgSiO3 bridgmanite.
| Starting material | Phases | MgO | Al2O3 | SiO2 | Total | O | Mg | Al | Si |
| IRIS333 (27 GPa, 2000 °K; 3 hours) | |||||||||
| En100 | Brg (n = 25) | 40.39 (50) | – | 59.39 (60) | 99.78 (36) | 2.996 (3) | 1.006 (9) | – | 0.995(7) |
Oxide analyses are reported in wt. %. n: number of analysis points.
Number in parentheses represents standard deviation and is placed in the last digit (s).
Abbreviation: Brg, bridgmanite.
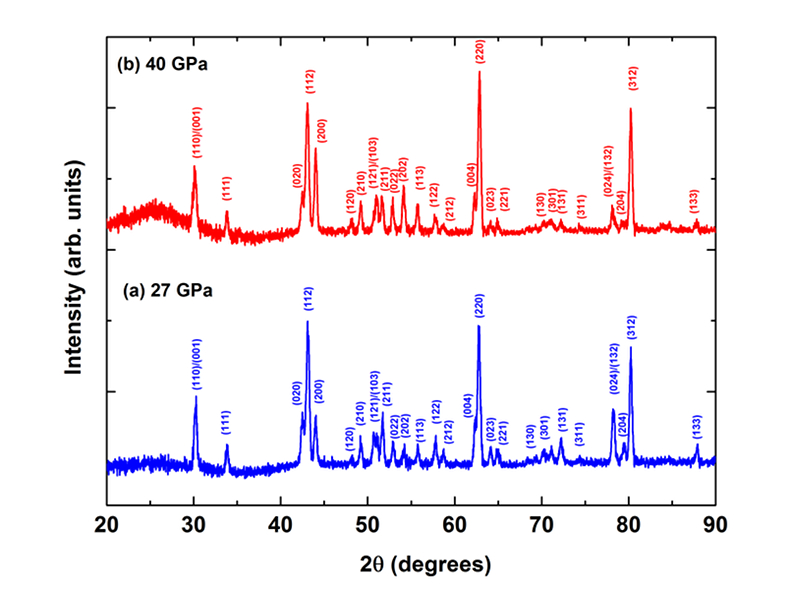
Figure S-3 XRD patterns of the recovered sample for En95Cor5 composition at 27 (a) and 40 GPa (b) under 2000 oK, respectively. Number in parentheses represents the index of bridgmanite.
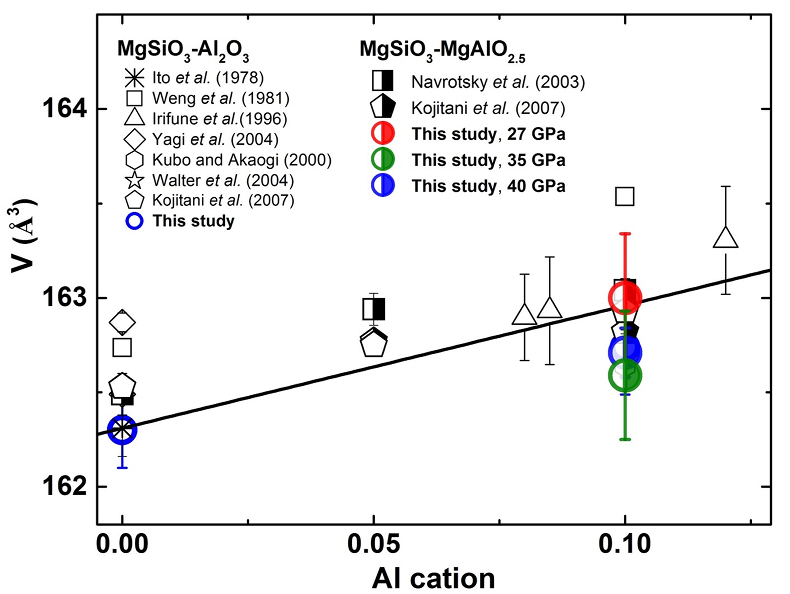
Figure S-4 Unit cell volumes of bridgmanite along the system MgSiO3–Al2O3 and MgSiO3–MgAlO2.5 in the present and previous studies. The solid line presents the linear fitting results on values for bridgmanite along the system MgSiO3-Al2O3 (Liu et al., 2016).
Table S-3 Elastic properties of minerals related with Eq. S-2 used for thermodynamic calculations.
| Phase | MgSiO3-Brg | Mg0.95Al0.1Si0.95O3-Brg | MgAl0.1Si0.9O2.95-Brg | MgO |
| V0 (cm3/mol) | 24.44 (1)a | 24.54 (1)e | 24.50 (1)f | 11.27 (1)h |
| ΔH0(KJ/mol) | 10.79 (196)b | 16.44 (88)b | 10.24 (428)f | 36.48 (50)i |
| ΔS0(KJ/mol) | 0.063b | 0.058b | 0.073f | 0.026i |
| KT298 (GPa) | 256.7 (15)a | 239 (1)e | 259 (8)g | 160.9h |
| KT' | 4.09 (6)a | 4e | 4g | 4.35 (10)h |
| (δKT/δT)P (GPa/°K) | 0.035 (2)c | -0.057 (1)e | -0.057 (1)* | -0.0272h |
| αT= a0+a1T+a2T2 (°K-1) | ||||
| a0 ×105 | 1.982d | 2.08 (26)e | 2.08 (26)* | 3.38i |
| a1 ×108 | 0.818d | 2.21 (67)e | 2.21 (67)* | 12.52i |
| a2 ×10 | -4.740d | 0 | 0 | -19.13i |
| CP= A+BT+CT-2 (°K-1) | ||||
| A×10-2 | 1.769d | 1.769# | 1.769* | 0.66i |
| B×10-3 | -1.565d | -1.565# | -1.565* | -0.36i |
| C ×10-6 | 0 | 0 | 0 | -0.61i |
a: Tange et al. (2012)
b: Akaogi and Ito (1999)
c: Katsura et al. (2009)
d: Funamori et al. (1996)
e: Zhand and Wiedner (1999)
f: Navrotsky et al. (2003)
g: Walter et al. (2006)
h: Kono et al. (2010)
i: Kojitani et al. (2012)
#: the same value with that of MgSiO3-Brg.
*: the same value with that of Mg0.95Al0.1Si0.95O3-Brg.
Abbreviation: Brg: bridgmanite.
Number in parentheses represents standard deviation and is placed in the last digit (s).
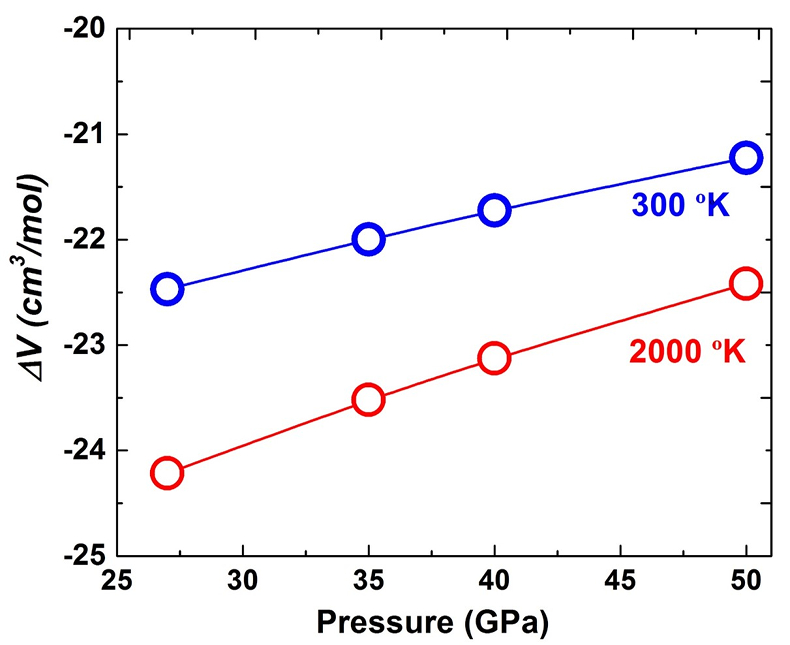
Figure S-5 The calculated molar volume change of Eq. S-2 in the text along 300 and 2000 °K, respectively.