Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017
Affiliations | Corresponding Author | Cite as | Funding informationLe Merrer, M., Colombani, J. (2017) Comment on "Repulsion between calcite crystals and grain detachment during water-rock interaction" by Levenson and Emmanuel, 2017. Geochem. Persp. Let. 6, 1–2.
n/a
- Share this article





Article views:3,843Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Figures and Tables
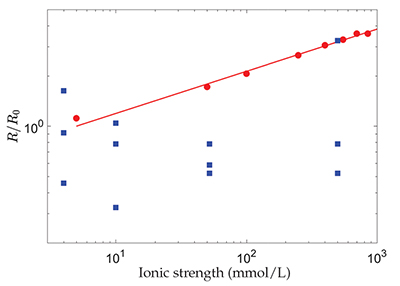 Figure 1 Evolution with the ionic strength, I, of the dissolution rate, R, of polished, polycrystalline calcite, renormalised by its value at I = 5 mmol/L (red dots) (Colombani, 2016) and the chemical dissolution rate, R, measured in the grain detachment experiments renormalised by the average of its values at I = 4 mmol/L (blue squares) (Levenson and Emmanuel, 2017). The straight line corresponds to R ∝ I0.25. |
| Figure 1 |
top
Comment
Levenson and Emmanuel suggested recently that the mechanism of carbonate rock weathering in fluids is not limited to nanoscale processes but that chemico-mechanical processes also take place at the micrometre scale, such as grain detachment from the material surface. This phenomenon was first observed in flowing liquids (Levenson and Emmanuel, 2016
Levenson, Y., Emmanuel, S. (2016) Quantifying micron-scale grain detachment during weathering experiments on limestone. Geochimica et Cosmochimica Acta 173, 86–96.
). In this case, the removal of the grain was understood to be a consequence both of mineral dissolution at grain boundaries and shear stress imposed by the fluid on the grain. Unexpectedly, this grain removal process has been subsequently observed in quiescent liquids. From these experiments, Levenson and Emmanuel (2017)Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
showed atomic force microscopy (AFM) pictures where grains unambiguously disappeared from the surface, even when the rock was left in a solution at rest. The expulsion of the grains was interpreted to result from dissolution and from repulsive forces between the grain surface and the underlying surface. Based on AFM measurements, such repulsion is believed to be caused by interactions between the Debye layers, as well as hydration of the strongly hydrophilic calcite surfaces (Røyne et al., 2015Røyne, A., Dalby, K.N., Hassenkam, T. (2015) Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophysical Research Letters 42, 4786–4794.
).We argue here that the grain expulsion cannot be attributed to these repulsive forces alone. Indeed, in the range of ionic strength investigated by the authors, the actual Debye length, i.e. the thickness of the double layer, cannot exceed a few nanometers (Israelachvili, 2011
Israelachvili, J. (2011) Intermolecular and Surface Forces. Third Edition, Academic Press, London.
). Therefore the force resulting from the repulsion between the Debye layer of a grain and the surface is likely to move the grain a few nanometres away from the surface. This intersurface distance is even smaller for the repulsion forces stemming from the calcite hydrophilicity invoked by Røyne et al. (2015)Røyne, A., Dalby, K.N., Hassenkam, T. (2015) Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophysical Research Letters 42, 4786–4794.
but the ejected grains are micrometre scale, as shown in Figure 1 of Levenson and Emmanuel (2016)Levenson, Y., Emmanuel, S. (2016) Quantifying micron-scale grain detachment during weathering experiments on limestone. Geochimica et Cosmochimica Acta 173, 86–96.
. Therefore to be permanently removed from the surface, one grain has to be lifted, against gravity, a few micrometres, to escape the environment of the surrounding grains. So the interaction range of the repulsive forces is at least 3 orders of magnitude too small to lead to this micrometre scale displacement of the grain and explain its ejection. In other words, although the magnitude of the repulsive potential energy might be comparable to the gravitational potential energy, the total potential energy is minimal when the grain is displaced only a few nanometres from its initial position. To move the grain a micrometre further away from the surface, some work must be done by an external force. Subsequently, we discuss the possible sources of this external force.A first effect, able to overcome this energy barrier and to expel the grain, is thermal agitation. Indeed, the applied pressure necessary to lift a cubic grain of size a is Pgr = (ρcc − ρs)ga, where ρcc and ρs represent the density of calcium carbonate and the solution, (ρcc = 2710 kg m-3 and ρs = 1000 kg m-3) and g represents the acceleration of gravity (g = 9.81 N kg-1). In Figures 1 and S-2 of Levenson and Emmanuel (2017)
Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
, the size of the ejected particles is in the range of 1 to 5 µm so we assume a ~ 1 µm and find Pgr ≈ 2 × 10-2 Pa. Comparatively, the pressure exerted by the thermal energy on the grain scales as Pth = kBT/a3, with kB, the Boltzmann constant, and T, the temperature. For the same grain size, at ambient temperature, we obtain Pth = 4 × 10−3 Pa. The thermal agitation is then almost one order of magnitude lower than the energy necessary to remove a grain, which does not make it a very likely explanation for the experiments presented, except in the case of the smallest grains observed by the authors.Going back to the study of grain detachment in a flowing liquid, we wonder whether the shear imposed by the flow could explain the ejection of grains in this configuration (Levenson and Emmanuel, 2016
Levenson, Y., Emmanuel, S. (2016) Quantifying micron-scale grain detachment during weathering experiments on limestone. Geochimica et Cosmochimica Acta 173, 86–96.
). The viscous shear stress is written τ = ηγ˙, where η represents the viscosity of the fluid (η = 10-3 Pa s) and γ˙, the velocity gradient, which can be estimated using γ˙ ∼ v/h (v, the fluid velocity and h, the height of fluid above the surface). With the values v = 2 mm/s (at most) and h = 0.5 mm given in the article, we obtain a shear stress, τ = 4 × 10-3 Pa. So we obtain a stress imposed by the flux to the grain perfectly comparable with the one exerted by thermal agitation and in both cases, these stresses appear too low to provide a convincing explanation for particle removal.In addition, we would like to emphasise that the frequency of detachment events is quite identical in the presence (Fig. 12 in Levenson and Emmanuel, 2016
Levenson, Y., Emmanuel, S. (2016) Quantifying micron-scale grain detachment during weathering experiments on limestone. Geochimica et Cosmochimica Acta 173, 86–96.
) and in the absence (Fig. 3 in Levenson and Emmanuel, 2017Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
) of flow, ranging mainly between 2 and 5 events/hr, for the same scanned surface area (20 × 20 µm2) and scanning rate. Thus grain removal is likely to have the same origin in both cases, an origin that probably is neither thermal agitation, nor the shear stress imposed by flow, and which cannot be solely a nanometre scale repulsive force — although this force should exist to counteract van der Waals attraction between the surfaces.We do not have a definitive explanation for the grain ejection events observed here but we would like to stress the fact that AFM might disturb surfaces during observations at the nanoscale in sometimes unexpected ways (Pachon-Rodriguez et al., 2011
Pachon-Rodriguez, E.A., Piednoir, A., Colombani, J. (2011) Pressure solution at the molecular scale. Physical Review Letters 107, 146102.
).As underlined by Levenson and Emmanuel (2017)
Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
, the force applied by the tip on the sample surface is F ~ 2 nN in water and the weight of a micrometre sized cubic grain is W = (ρcc − ρs)ga3 ≈ 2 × 10-5 nN. Hence once the grain boundaries have been attacked by the solvent, the motion of the grain by the AFM tip is doubtless. It is also possible, regarding the extremely low weight of the grains, that even residual vibrations induce grain displacement. To evaluate the influence of AFM measurements on grain detachment, the authors present additional experiments based on electron microscopy (ESEM). However, given the low weight of the grains, it is likely that mechanical disturbance during drying and transport of the sample from the AFM to the ESEM could explain the loss of the attacked grains more probably than repulsive forces.Finally, the authors also show that the material dissolution rate is independent of the ionic strength of the solution (fixed by dissolved NaCl). Based on a review of the experimental measurements of the dissolution rate, R, for calcite, it has been shown that the rate scales in alkaline conditions as R = γ-1 kexp(1 − Ω1/2), with γ representing the mean ionic activity coefficient of the Ca2+ and CO32- ions, Ω, the undersaturation and kexp, the dissolution rate constant (kexp = 6 × 10-6 mol m-2 s-1 for polished polycrystalline samples) (Colombani, 2016
Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
). We can consider that this dependence on γ and Ω still holds in the conditions of the experiments of Levenson and Emmanuel, slightly acidic and with a constant concentration of EDTA. If we interpret the far from equilibrium conditions stated by the authors as Ω = 0, and use the values of γ measured for various ionic strengths, I, in NaCl solutions given by Rickard and Sjöberg (1983)Rickard, D., Sjöberg, E.L. (1983) Mixed kinetic control of calcite dissolution rates. American Journal of Science 283, 815–830.
, the dissolution rate of the mineral should evolve significantly with I, as shown in Figure 1. Therefore, the absence of a change in chemical dissolution rate with the ionic strength, over almost 3 orders of magnitude, that was reported by the authors from the AFM experiments, could be attributed to the large dispersion of the measurements or to an unidentified phenomenon that fixes the dissolution kinetics (Fig. 1).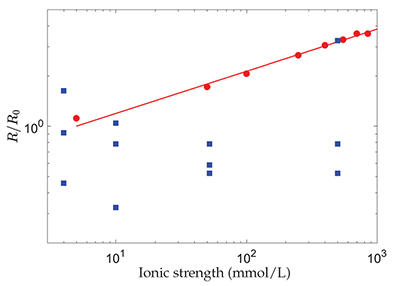
Figure 1 Evolution with the ionic strength, I, of the dissolution rate, R, of polished, polycrystalline calcite, renormalised by its value at I = 5 mmol/L (red dots) (Colombani, 2016
Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
) and the chemical dissolution rate, R, measured in the grain detachment experiments renormalised by the average of its values at I = 4 mmol/L (blue squares) (Levenson and Emmanuel, 2017Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
). The straight line corresponds to R ∝ I0.25.In conclusion, Levenson and Emmanuel (2017)
Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
have shown that the weathering of calcium carbonate rocks involves phenomena at various scales and that mechanisms occurring at the microscale, such as grain detachment, should not be forgotten, for weathering rate estimation, for example. Nevertheless, the phenomena at the base of such mechanisms still need a thorough investigation to be clearly understood.Editor: Susan Stipp
top
References
Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
 Show in context
Show in contextBased on a review of the experimental measurements of the dissolution rate, R, for calcite, it has been shown that rate scales in alkaline conditions as R = γ-1 kexp(1 − Ω1/2), with γ representing the mean ionic activity coefficient of the Ca2+ and CO32- ions, Ω, the undersaturation and kexp, the dissolution rate constant (kexp = 6 × 10-6 mol m-2 s-1 for polished polycrystalline samples) (Colombani, 2016).
View in article
Figure 1 Evolution with the ionic strength, I, of the dissolution rate, R, of polished, polycrystalline calcite, renormalised by its value at I = 5 mmol/L (red dots) (Colombani, 2016) and the chemical dissolution rate, R, measured in the grain detachment experiments renormalised by the average of its values at I = 4 mmol/L (blue squares) (Levenson and Emmanuel, 2017).
View in article
Israelachvili, J. (2011) Intermolecular and Surface Forces. Third Edition, Academic Press, London.
 Show in context
Show in context We argue here that the grain expulsion cannot be attributed to these repulsive forces alone. Indeed, in the range of ionic strength investigated by the authors, the actual Debye length, i.e. the thickness of the double layer, cannot exceed a few nanometers (Israelachvili, 2011).
View in article
Levenson, Y., Emmanuel, S. (2016) Quantifying micron-scale grain detachment during weathering experiments on limestone. Geochimica et Cosmochimica Acta 173, 86–96.
 Show in context
Show in context This phenomenon was first observed in flowing liquids (Levenson and Emmanuel, 2016).
View in article
This intersurface distance is even smaller for the repulsion forces stemming from the calcite hydrophilicity invoked by Røyne et al. (2015) but the ejected grains are micrometre scale, as shown in Figure 1 of Levenson and Emmanuel (2016).
View in article
Going back to the study of grain detachment in a flowing liquid, we wonder whether the shear imposed by the flow could explain the ejection of grains in this configuration (Levenson and Emmanuel, 2016).
View in article
In addition, we would like to emphasise that the frequency of detachment events is quite identical in the presence (Fig. 12 in Levenson and Emmanuel, 2016) and in the absence (Fig. 3 in Levenson and Emmanuel, 2017) of flow, ranging mainly between 2 and 5 events/hr, for the same scanned surface area (20 × 20 µm2) and scanning rate.
View in article
Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
 Show in context
Show in contextUnexpectedly, this grain removal process has been subsequently observed in quiescent liquids. From these experiments, Levenson and Emmanuel (2017) showed atomic force microscopy (AFM) pictures where grains unambiguously disappeared from the surface, even when the rock was left in a solution at rest.
View in article
In Figures 1 and S-2 of Levenson and Emmanuel (2017), the size of the ejected particles is in the range of 1 to 5 µm so we assume a ~ 1 µm and find Pgr ≈ 2 × 10-2 Pa.
View in article
In addition, we would like to emphasise that the frequency of detachment events is quite identical in the presence (Fig. 12 in Levenson and Emmanuel, 2016) and in the absence (Fig. 3 in Levenson and Emmanuel, 2017) of flow, ranging mainly between 2 and 5 events/hr, for the same scanned surface area (20 × 20 µm2) and scanning rate.
View in article
As underlined by Levenson and Emmanuel (2017), the force applied by the tip on the sample surface is F ~ 2 nN in water and the weight of a micrometre sized cubic grain is W = (ρcc − ρs)ga3 ≈ 2 × 10-5 nN.
View in article
Figure 1 Evolution with the ionic strength, I, of the dissolution rate, R, of polished, polycrystalline calcite, renormalised by its value at I = 5 mmol/L (red dots) (Colombani, 2016) and the chemical dissolution rate, R, measured in the grain detachment experiments renormalised by the average of its values at I = 4 mmol/L (blue squares) (Levenson and Emmanuel, 2017).
View in article
In conclusion, Levenson and Emmanuel (2017) have shown that the weathering of calcium carbonate rocks involves phenomena at various scales and that mechanisms occurring at the microscale, such as grain detachment, should not be forgotten, for weathering rate estimation, for example.
View in article
Pachon-Rodriguez, E.A., Piednoir, A., Colombani, J. (2011) Pressure solution at the molecular scale. Physical Review Letters 107, 146102.
 Show in context
Show in context We do not have a definitive explanation for the grain ejection events observed here but we would like to stress the fact that AFM might disturb surfaces during observations at the nanoscale in sometimes unexpected ways (Pachon-Rodriguez et al., 2011).
View in article
Rickard, D., Sjöberg, E.L. (1983) Mixed kinetic control of calcite dissolution rates. American Journal of Science 283, 815–830.
 Show in context
Show in context If we interpret the far from equilibrium conditions stated by the authors as Ω = 0, and use the values of γ measured for various ionic strengths, I, in NaCl solutions given by Rickard and Sjöberg (1983), the dissolution rate of the mineral should evolve significantly with I, as shown in Figure 1.
View in article
Røyne, A., Dalby, K.N., Hassenkam, T. (2015) Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophysical Research Letters 42, 4786–4794.
 Show in context
Show in contextBased on AFM measurements, such repulsion is believed to be caused by interactions between the Debye layers, as well as hydration of the strongly hydrophilic calcite surfaces (Røyne et al., 2015).
View in article
This intersurface distance is even smaller for the repulsion forces stemming from the calcite hydrophilicity invoked by Røyne et al. (2015) but the ejected grains are micrometre scale, as shown in Figure 1 of Levenson and Emmanuel (2016).
View in article
Figures and Tables
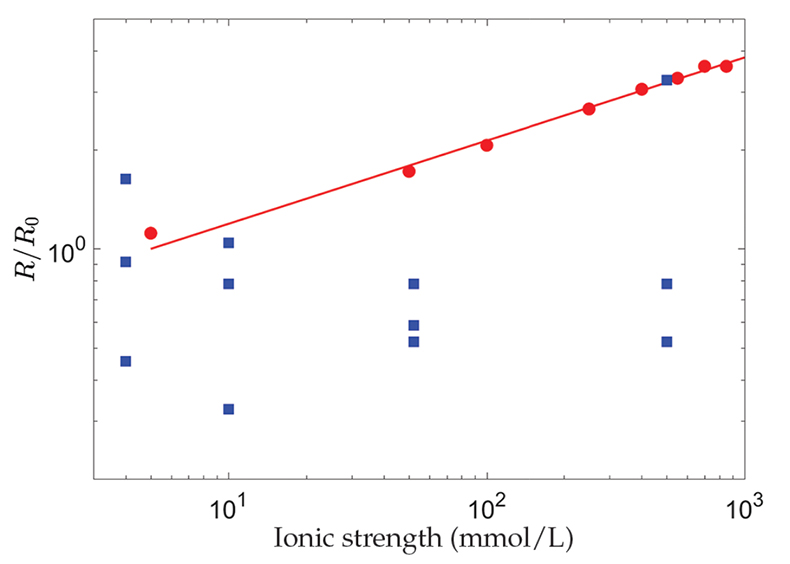
Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
) and the chemical dissolution rate, R, measured in the grain detachment experiments renormalised by the average of its values at I = 4 mmol/L (blue squares) (Levenson and Emmanuel, 2017Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
). The straight line corresponds to R ∝ I0.25.
Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
) and the chemical dissolution rate, R, measured in the grain detachment experiments renormalised by the average of its values at I = 4 mmol/L (blue squares) (Levenson and Emmanuel, 2017Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
). The straight line corresponds to R ∝ I0.25.