Reply to comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Le Merrer and Colombani, 2017
Affiliations | Corresponding Author | Cite as | Funding informationLevenson, Y., Emmanuel, S. (2017) Reply to comment on "Repulsion between calcite crystals and grain detachment during water-rock interaction" by Le Merrer and Colombani, 2017. Geochem. Persp. Let. 6, 3–4.
Israel Science Foundation
- Share this article





Article views:3,657Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Figures and Tables
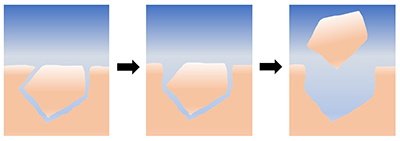 Figure 1 Schematic representation of the proposed mechanism for grain removal under quiescent conditions. At first, the grains are locked together mechanically; as they dissolve and the grains become unlocked, they are able to leave the surface by intergranular repulsion. |
| Figure 1 |
top
Reply
The comment by Le Merrer and Colombani (2017)
Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
focuses on the mechanisms that could account for our experiments, in which we observed the detachment of micrometre scale calcite grains from the surface of micritic limestone during contact with a reactive fluid (Levenson and Emmanuel, 2017Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
). They discuss some of the forces acting on the grains and imply that our observations are likely to be artefacts of the experimental method. Furthermore, they suggest that because our measured calcite dissolution rates do not match exactly the dependence on ionic strength predicted by Colombani (2016)Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
, an “unidentified phenomenon” could be at play in our experiments. While Le Merrer and Colombani (2017)Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
raise some valid points, we think that an alternative interpretation is possible. We are pleased to be able to discuss these aspects of our paper in greater detail.In experiments carried out under a range of flow conditions, we have observed the detachment of micrometre size grains from the surface of micritic limestone (Emmanuel and Levenson, 2014
Emmanuel, S., Levenson, Y. (2014) Limestone weathering rates accelerated by micron-scale grain detachment. Geology 42, 751–754.
; Levenson and Emmanuel, 2016Levenson, Y., Emmanuel, S. (2016) Quantifying micron-scale grain detachment during weathering experiments on limestone. Geochimica et Cosmochimica Acta 173, 86–96.
, 2017Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
). Le Merrer and Colombani (2017)Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
show that under our experimental conditions neither thermal agitation nor fluid shearing are strong enough to overcome gravity and remove grains from the surface. Moreover, they assert that although the repulsive forces related to the electric double layer and hydration are much stronger than the gravitational force, repulsion cannot remove grains from the surface because it only operates over the relatively short range of several nanometres. However, by considering the energy gains and losses involved in removing a grain from the surface, it can be shown that this is not necessarily the case. From the relationship describing the effective weight of a grain, F = (ρcalcite − ρwater)gVgrain, where ρcalcite represents the density of calcite (2710 kg m-3), ρwater, the density of water (1000 kg m-3), g, gravitational acceleration (9.81 m s-2), and Vgrain, the grain volume, it follows that a 1 µm calcite grain surrounded by water would gain ~2 × 10-20 J in gravitational potential energy for every micrometre it is removed from the surface in the vertical direction. According to the experiments carried out by Røyne et al. (2015)Røyne, A., Dalby, K.N., Hassenkam, T. (2015) Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophysical Research Letters 42, 4786–4794.
, calcite surfaces in close proximity in pure water experience only repulsive forces so a calcite grain in contact with another calcite surface would possess a certain potential energy that could be translated into gravitational or kinetic energy. An estimate of this energy can be made by integrating the area under the force-distance curves, reported by Røyne et al. (2015)Røyne, A., Dalby, K.N., Hassenkam, T. (2015) Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophysical Research Letters 42, 4786–4794.
, and then normalising by the surface area of the crystals used in their experiments. The crystal surface areas were reported to be in the range of 300 to 3500 µm2, and accordingly, we estimate the potential energy to be ~0.1–1.7 × 10-20 J µm-2. Using these values, a 1 µm calcite grain with an area of 5 µm2, in contact with another calcite surface, has ~0.5–8 × 10-20 J of potential energy. Significantly, the higher end of this range is great enough to suggest that intergranular repulsion could overcome gravity. However, if a grain is ejected from the surface, viscous drag would act to dissipate the energy, although estimating the rate of dissipation depends on knowledge of the particle velocity, which is entirely unconstrained.As the above discussion highlights, precisely evaluating the tiny forces acting at and near calcite surfaces under our experimental conditions represents a nontrivial challenge. The standard model used to describe the interaction between surfaces, i.e. DLVO theory, takes into account van der Waals forces and electric double layer repulsion. Despite its success, it has been shown to be limited in its description of the forces operating at ranges of <5 nm (Israelachvili, 2011
Israelachvili, J. (2011) Intermolecular and Surface Forces. Third Edition, Academic Press, London.
). Røyne et al. (2015)Røyne, A., Dalby, K.N., Hassenkam, T. (2015) Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophysical Research Letters 42, 4786–4794.
attribute the high repulsive forces they measure to additional hydration forces. Steric effects, hydrophobicity and the presence of asperities have also been suggested as mechanisms to account for disagreement between DLVO theory and measurements (Israelachvili, 2011Israelachvili, J. (2011) Intermolecular and Surface Forces. Third Edition, Academic Press, London.
). Most important, all of these models assume that the surfaces are homogeneous and that they are in chemical equilbrium with the fluid. This was certainly not the case for our experiments, which were carried out far from equilibrium, so the surfaces of the calcite grains would be subject to dissolution. The highly heterogeneous nature of dissolution at this scale (Fischer et al., 2012Fischer C., Arvidson R.S., Lüttge A. (2012) How predictable are dissolution rates of crystalline material? Geochimica et Cosmochimica Acta 98, 177–185.
; Levenson and Emmanuel, 2013Levenson, Y., Emmanuel S. (2013) Pore-scale heterogeneous reaction rates on a dissolving limestone surface. Geochimica et Cosmochimica Acta 119, 188–197.
) is also likely to have a strong impact on charge distribution on the grain surfaces. Thus, the use of standard models to evaluate interactions betweens calcite grains in our experiments is unlikely to yield accurate results.In their comment, Le Merrer and Colombani (2017)
Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
imply that the most likely mechanism for grain removal during the experiments is plucking by the atomic force microscope (AFM) tip, that we used to image the surface. Such an effect is possible and in an effort to determine if the tip was responsible for removing grains, we reported on a series of experiments in which the surface was imaged once only with AFM at the beginning of a period of zero flow (Levenson and Emmanuel, 2017Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
). The experiment was then allowed to run for an additional hour, after which the sample was dried and imaged using scanning electron microscopy. In 9 of the 13 imaged regions, we found instances of grain removal. Le Merrer and Colombani (2017)Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
do acknowledge that the grains in these experiments were not removed by the AFM tip; instead they suggest that drying and transport could be responsible for their removal. This explanation cannot be excluded.In addition to discussing the mechanisms of grain removal, Le Merrer and Colombani (2017)
Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
explore an apparent discrepancy between the theoretical dependence of calcite dissolution rates on ionic strength predicted by Colombani (2016)Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
. The small spatial scale measured in atomic force microscopy measurements and the high variability of reaction rates at the micrometre scale (Fischer et al., 2012Fischer C., Arvidson R.S., Lüttge A. (2012) How predictable are dissolution rates of crystalline material? Geochimica et Cosmochimica Acta 98, 177–185.
; Levenson and Emmanuel, 2013Levenson, Y., Emmanuel S. (2013) Pore-scale heterogeneous reaction rates on a dissolving limestone surface. Geochimica et Cosmochimica Acta 119, 188–197.
) make a comparison of AFM derived rates with bulk rates problematic (Morse et al., 2007Morse J.W., Arvidson R.S., Lüttge A. (2007) Calcium carbonate formation and dissolution. Chemical Reviews 107, 342–381.
). High variability is evident in the spread of rates that we observe in our experiments, although some of the measurements do in fact fall remarkably close to the model rate (Le Merrer and Colombani, 2017Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
; Fig. 1). Thus, the lack of apparent dependence of the reaction rate on ionic strength could simply result from the high variance in our measured rates and there is no justification for the suggestion made by Le Merrer and Colombani (2017)Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
that an unidentified phenomenon is controlling the reaction rates in our experiments.In the mechanism that we propose to account for grain detachment (Fig. 1), calcite grains are mechanically locked together prior to interaction with the fluid; as the edges of the grains dissolve, the grains become unlocked, allowing intergranular repulsion to eject them from the surface. To further explore the precise mechanism by which grains are removed from the surface, we would ideally carry out a series of experiments using completely noncontact imaging, such as the relatively new method of in situ digital holographic microscopy (Brand et al., 2017
Brand, A.S., Feng, P., Bullard, J.W. (2017) Calcite dissolution rate spectra measured by in situ digital holographic microscopy. Geochimica et Cosmochimica Acta 213, 317–329.
). Irrespective of whether the grains are removed by intergranular repulsion, by interaction with the AFM tip, or by drying, our experiments demonstrate that tiny purturbations in the system can cause grains to be detached from the surfaces of reacting limestone. In addition to highlighting the potential for new experiments to understand these effects, this discussion also emphasises the inadequacy of our current theoretical frameworks in describing the forces acting between nonideal mineral surfaces during dissolution and precipitation. The development of models that can bridge this gap will undoubtedly provide insight into the mechanical and chemical behaviour of rocks in a range of natural systems.
Figure 1 Schematic representation of the proposed mechanism for grain removal under quiescent conditions. At first, the grains are locked together mechanically; as they dissolve and the grains become unlocked, they are able to leave the surface by intergranular repulsion.
Editor: Susan Stipp
top
References
Brand, A.S., Feng, P., Bullard, J.W. (2017) Calcite dissolution rate spectra measured by in situ digital holographic microscopy. Geochimica et Cosmochimica Acta 213, 317–329.
 Show in context
Show in context To further explore the precise mechanism by which grains are removed from the surface, we would ideally carry out a series of experiments using completely noncontact imaging, such as the relatively new method of in situ digital holographic microscopy (Brand et al., 2017).
View in article
Colombani, J. (2016) The alkaline dissolution rate of calcite. Journal of Physical Chemistry Letters 7, 2376–2380.
 Show in context
Show in context Furthermore, they suggest that because our measured calcite dissolution rates do not match exactly the dependence on ionic strength predicted by Colombani (2016), an “unidentified phenomenon” could be at play in our experiments.
View in article
In addition to discussing the mechanisms of grain removal, Le Merrer and Colombani (2017) explore an apparent discrepancy between the theoretical dependence of calcite dissolution rates on ionic strength predicted by Colombani (2016).
View in article
Emmanuel, S., Levenson, Y. (2014) Limestone weathering rates accelerated by micron-scale grain detachment. Geology 42, 751–754.
 Show in context
Show in context In experiments carried out under a range of flow conditions, we have observed the detachment of micrometre size grains from the surface of micritic limestone (Emmanuel and Levenson, 2014; Levenson and Emmanuel, 2016, 2017).
View in article
Fischer C., Arvidson R.S., Lüttge A. (2012) How predictable are dissolution rates of crystalline material? Geochimica et Cosmochimica Acta 98, 177–185.
 Show in context
Show in contextThe highly heterogeneous nature of dissolution at this scale (Fischer et al., 2012; Levenson and Emmanuel, 2013) is also likely to have a strong impact on charge distribution on the grain surfaces.
View in article
The small spatial scale measured in atomic force microscopy measurements and the high variability of reaction rates at the micrometre scale (Fischer et al., 2012; Levenson and Emmanuel, 2013) make a comparison of AFM derived rates with bulk rates problematic (Morse et al., 2007).
View in article
Israelachvili, J. (2011) Intermolecular and Surface Forces. Third Edition, Academic Press, London.
 Show in context
Show in contextDespite its success, it has been shown to be limited in its description of the forces operating at ranges of <5 nm (Israelachvili, 2011).
View in article
Steric effects, hydrophobicity and the presence of asperities have also been suggested as mechanisms to account for disagreement between DLVO theory and measurements (Israelachvili, 2011).
View in article
Le Merrer, M., Colombani, J. (2017) Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017. Geochemical Perspectives Letters 6, 1–2.
 Show in context
Show in contextThe comment by Le Merrer and Colombani (2017) focuses on the mechanisms that could account for our experiments, in which we observed the detachment of micrometre scale calcite grains from the surface of micritic limestone during contact with a reactive fluid (Levenson and Emmanuel, 2017).
View in article
While Le Merrer and Colombani (2017) raise some valid points, we think that an alternative interpretation is possible.
View in article
Le Merrer and Colombani (2017) show that under our experimental conditions neither thermal agitation nor fluid shearing are strong enough to overcome gravity and remove grains from the surface.
View in article
In their comment, Le Merrer and Colombani (2017) imply that the most likely mechanism for grain removal during the experiments is plucking by the atomic force microscope (AFM) tip, that we used to image the surface.
View in article
Le Merrer and Colombani (2017) do acknowledge that the grains in these experiments were not removed by the AFM tip; instead they suggest that drying and transport could be responsible for their removal.
View in article
In addition to discussing the mechanisms of grain removal, Le Merrer and Colombani (2017) explore an apparent discrepancy between the theoretical dependence of calcite dissolution rates on ionic strength predicted by Colombani (2016).
View in article
High variability is evident in the spread of rates that we observe in our experiments, although some of the measurements do in fact fall remarkably close to the -rate (Le Merrer and Colombani, 2017; Fig. 1).
View in article
Thus, the lack of apparent dependence of the reaction rate on ionic strength could simply result from the high variance in our measured rates and there is no justification for the suggestion made by Le Merrer and Colombani (2017) that an unidentified phenomenon is controlling the reaction rates in our experiments.
View in article
Levenson, Y., Emmanuel S. (2013) Pore-scale heterogeneous reaction rates on a dissolving limestone surface. Geochimica et Cosmochimica Acta 119, 188–197.
 Show in context
Show in context The highly heterogeneous nature of dissolution at this scale (Fischer et al., 2012; Levenson and Emmanuel, 2013) is also likely to have a strong impact on charge distribution on the grain surfaces.
View in article
The small spatial scale measured in atomic force microscopy measurements and the high variability of reaction rates at the micrometre scale (Fischer et al., 2012; Levenson and Emmanuel, 2013) make a comparison of AFM derived rates with bulk rates problematic (Morse et al., 2007).
View in article
Levenson, Y., Emmanuel, S. (2016) Quantifying micron-scale grain detachment during weathering experiments on limestone. Geochimica et Cosmochimica Acta 173, 86–96.
 Show in context
Show in contextIn experiments carried out under a range of flow conditions, we have observed the detachment of micrometre size grains from the surface of micritic limestone (Emmanuel and Levenson, 2014; Levenson and Emmanuel, 2016, 2017).
View in article
Levenson, Y., Emmanuel, S. (2017) Repulsion between calcite crystals and grain detachment during water-rock interaction. Geochemical Perspectives Letters 3, 133–141.
 Show in context
Show in context The comment by Le Merrer and Colombani (2017) focuses on the mechanisms that could account for our experiments, in which we observed the detachment of micrometre scale calcite grains from the surface of micritic limestone during contact with a reactive fluid (Levenson and Emmanuel, 2017).
View in article
In experiments carried out under a range of flow conditions, we have observed the detachment of micrometre size grains from the surface of micritic limestone (Emmanuel and Levenson, 2014; Levenson and Emmanuel, 2016, 2017).
View in article
Such an effect is possible and in an effort to determine if the tip was responsible for removing grains, we reported on a series of experiments in which the surface was imaged once only with AFM at the beginning of a period of zero flow (Levenson and Emmanuel, 2017).
View in article
Morse J.W., Arvidson R.S., Lüttge A. (2007) Calcium carbonate formation and dissolution. Chemical Reviews 107, 342–381.
 Show in context
Show in context The small spatial scale measured in atomic force microscopy measurements and the high variability of reaction rates at the micrometre scale (Fischer et al., 2012; Levenson and Emmanuel, 2013) make a comparison of AFM derived rates with bulk rates problematic (Morse et al., 2007).
View in article
Røyne, A., Dalby, K.N., Hassenkam, T. (2015) Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophysical Research Letters 42, 4786–4794.
 Show in context
Show in context According to the experiments carried out by Røyne et al. (2015), calcite surfaces in close proximity in pure water experience only repulsive forces so a calcite grain in contact with another calcite surface would possess a certain potential energy that could be translated into gravitational or kinetic energy.
View in article
An estimate of this energy can be made by integrating the area under the force-distance curves, reported by Røyne et al. (2015), and then normalising by the surface area of the crystals used in their experiments.
View in article
Røyne et al. (2015) attribute the high repulsive forces they measure to additional hydration forces.
View in article
Figures and Tables
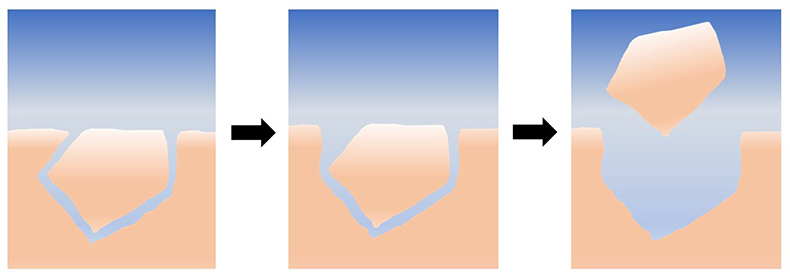
Figure 1 Schematic representation of the proposed mechanism for grain removal under quiescent conditions. At first, the grains are locked together mechanically; as they dissolve and the grains become unlocked, they are able to leave the surface by intergranular repulsion.