Decoupling of dissolved and bedrock neodymium isotopes during sedimentary cycling
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:3,373Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
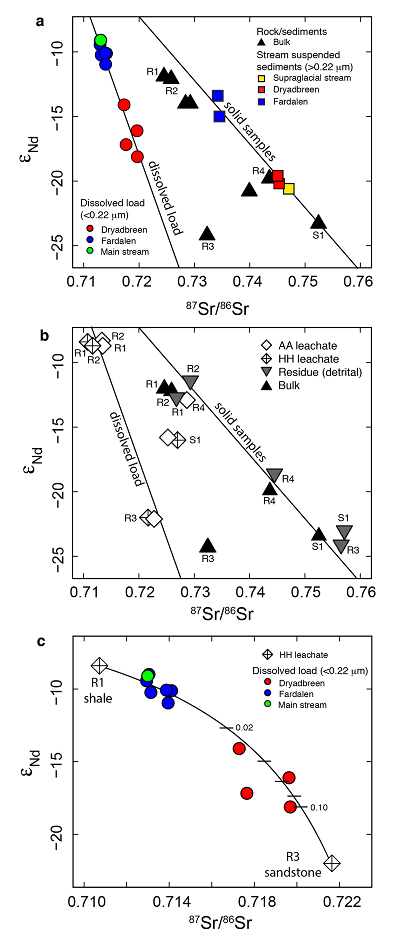 Figure 1 Dissolved (<0.22 µm) and solid samples (rock, sediment and stream suspended sediment) form distinct arrays in Sr-Nd space. (a) A linear regression is fitted for the dissolved samples (r2 = 0.93, p < 0.001). The linear line for the solid samples is the mixing line between a Greenlandic (87Sr/86Sr = 0.78059, εNd = -37.1) and Siberian (87Sr/86Sr = 0.70626, εNd = -0.4) sediment source with identical Sr/Nd mass ratios (Hindshaw et al., 2018). The four rock and one glacial sediment sample subjected to the leaching procedure are labelled. (b) The isotopic compositions of the leachates, residual and bulk samples. The dissolved load array is bound by leachates of the sandstone (R3) at one end and leachates of the shale samples (R1 and R2) at the other end. (c) The dissolved samples can be fitted with a mixing line between the HH leachate end members. The best fit line uses the concentration and isotopic values measured in R3-HH and R1-HH (Table S-3). The numbers on the mixing line refer to the mass fraction of the sandstone end member in the mixture. Error bars are smaller than symbol size. | 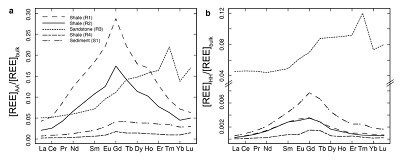 Figure 2 REE concentrations of the leachates normalised to bulk. (a) AA leach. (b) HH leach. REE concentrations are normalised against the bulk REE concentrations of the same sample. Note the scale break in (b). The sandstone sample (R3) has a HREE enriched pattern for both leachates. Shale samples R1 and R2 have MREE enriched patterns for both leachates. REE data is reported in Table S-5. |
| Figure 1 | Figure 2 |
top
Introduction
Radiogenic neodymium isotopes (143Nd/144Nd), commonly reported as εNd (Goldstein and Jacobsen, 1987
Goldstein, S.J., Jacobsen, S.B. (1987) The Nd and Sr isotopic systematics of river-water dissolved material: Implications for the sources of Nd and Sr in seawater. Chemical Geology 66, 245-272.
), have influenced our understanding of geophysical processes, from planetary differentiation to ocean circulation. Continental growth curves are based on εNd values (Taylor and McLennan, 1985Taylor, S.R., McLennan, S.M. (1985) The continental crust: Its composition and evolution. Blackwell Scientific, Boston, MA.
) and changes in ocean dynamics and silicate weathering have been inferred from εNd measurements (Piepgras and Wasserburg, 1980Piepgras, D.J., Wasserburg, G.J. (1980) Neodymium isotopic variations in seawater. Earth and Planetary Science Letters 50, 128-138.
; Bayon et al., 2009Bayon, G., Burton, K.W., Soulet, G., Vigier, N., Dennielou, B., Etoubleau, J., Ponzevera, E., German, C.R., Nesbitt, R.W. (2009) Hf and Nd isotopes in marine sediments: Constraints on global silicate weathering. Earth and Planetary Science Letters 277, 318-326.
).On a finer spatial and temporal scale, variations in the εNd values of seawater recovered from archives, such as foraminifera (e.g., Vance and Burton, 1999
Vance, D., Burton, K. (1999) Neodymium isotopes in planktonic foraminifera: a record of the response of continental weathering and ocean circulation rates to climate change. Earth and Planetary Science Letters 173, 365-379.
) and Fe-Mn (oxyhydr)oxides (e.g., Bayon et al., 2004Bayon, G., German, C.R., Burton, K.W., Nesbitt, R.W., Rogers, N. (2004) Sedimentary Fe-Mn oxyhydroxides as paleoceanographic archives and the role of aeolian flux in regulating oceanic dissolved REE. Earth and Planetary Science Letters 224, 477-492.
), are commonly interpreted as indicating relative contributions of different water masses and associated changes in ocean circulation. The accuracy of conclusions gleaned from these sediment and seawater εNd records is however dependent on understanding the processes affecting the Nd concentration and isotopic composition during transport of Nd from rock to seawater via rivers and during sediment dissolution in the ocean (e.g., Jeandel et al., 2007Jeandel, C., Arsouze, T., Lacan, F., Téchiné, P., Dutay, J.-C. (2007) Isotopic Nd compositions and concentrations of the lithogenic inputs into the ocean: A compilation, with an emphasis on the margins. Chemical Geology 239, 156-164.
).Many studies have assumed that solutes released during dissolution have the same εNd composition as the bulk rocks being dissolved. However, riverine dissolved εNd values can be different from both the suspended load and the bedrock over which the river has flowed (Goldstein and Jacobsen, 1987
Goldstein, S.J., Jacobsen, S.B. (1987) The Nd and Sr isotopic systematics of river-water dissolved material: Implications for the sources of Nd and Sr in seawater. Chemical Geology 66, 245-272.
; Tricca et al., 1999Tricca, A., Stille, P., Steinmann, M., Kiefel, B., Samuel, J., Eikenberg, J. (1999) Rare earth elements and Sr and Nd isotopic compositions of dissolved and suspended loads from small river systems in the Vosges mountains (France), the river Rhine and groundwater. Chemical Geology 160, 139-158.
; Andersson et al., 2001Andersson, P.S., Dahlqvist, R., Ingri, J., Gustafsson, Ö. (2001) The isotopic composition of Nd in a boreal river: A reflection of selective weathering and colloidal transport. Geochimica et Cosmochimica Acta 65, 521-527.
; Rickli et al., 2013Rickli, J., Frank, M., Stichel, T., Georg, R.B., Vance, D., Halliday, A.N. (2013) Controls on the incongruent release of hafnium during weathering of metamorphic and sedimentary catchments. Geochimica et Cosmochimica Acta 101, 263-284.
). Additionally the rare earth element (REE) chemistry of sediment/soil leachates indicates that REE (including Sm and Nd) are mobile and can be fractionated during chemical weathering and diagenesis (e.g., Bock et al., 1994Bock, B., McLennan, S.M., Hanson, G.N. (1994) Rare earth element redistribution and its effects on the neodymium isotope system in the Austin Glen Member of the Normanskill Formation, New York, USA. Geochimica et Cosmochimica Acta 58, 5245-5253.
; Viers and Wasserburg, 2004Viers, J., Wasserburg, G.J. (2004) Behavior of Sm and Nd in a lateritic soil profile. Geochimica et Cosmochimica Acta 68, 2043-2054.
). Goldstein and Jacobsen (1987)Goldstein, S.J., Jacobsen, S.B. (1987) The Nd and Sr isotopic systematics of river-water dissolved material: Implications for the sources of Nd and Sr in seawater. Chemical Geology 66, 245-272.
proposed that dissolved load εNd values are controlled by labile phases but direct evidence has been lacking. In this study we present εNd data on the dissolved load, stream suspended sediment and leachates (which access labile phases) of rock and glacial sediment samples in the same catchment to investigate the compositions and decoupling of these reservoirs.We present data for an Arctic catchment (Svalbard, Supplementary Information) where the bedrock εNd and 87Sr/86Sr data were interpreted as a two component mixture between two sources: Proterozoic sediments derived from Greenlandic basement rocks and Carboniferous to Jurassic sediments derived from Siberian basalts (Hindshaw et al., 2018
Hindshaw, R.S., Tosca, N.J., Piotrowski, A.M., Tipper, E.T. (2018) Clay mineralogy, strontium and neodymium isotope ratios in the sediments of two High Arctic catchments (Svalbard). Earth Surface Dynamics 6, 141-161.
). Due to recent glaciation, there has been negligible soil development which along with the large εNd range in the rocks exposed in the catchment (>12 epsilon units), the well-constrained field setting and its geographic location proximal to deep water formation, makes it an ideal site to evaluate the processes affecting εNd during river transport from terrestrial rock sources to the ocean. We find that similar to the well-established behaviour of radiogenic Sr (e.g., Blum et al., 1994Blum, J.D., Erel, Y., Brown, K. (1994) 87Sr/86Sr ratios of Sierra Nevada stream waters: Implications for relative mineral weathering rates. Geochimica et Cosmochimica Acta 58, 5019-5025.
), the dissolved εNd values are distinct from stream suspended sediments. Through sequential extractions of catchment rock and glacial sediment samples we demonstrate that dissolved εNd values are controlled by the most readily dissolved components, which are isotopically distinct from the bulk.top
Decoupling Water Chemistry from Bulk Rock Compositions
The dissolved load (<0.22 µm) has higher εNd (2.0-5.5) and lower 87Sr/86Sr (0.020-0.028) values (Fig. 1a, Table S-2) compared to the corresponding stream suspended sediment samples (>0.22 µm; Hindshaw et al., 2018
Hindshaw, R.S., Tosca, N.J., Piotrowski, A.M., Tipper, E.T. (2018) Clay mineralogy, strontium and neodymium isotope ratios in the sediments of two High Arctic catchments (Svalbard). Earth Surface Dynamics 6, 141-161.
). These differences result in an offset between the dissolved load Sr-Nd array and solid sample array (Fig. 1a), implying that the solid samples contain isotopically distinct phases that are preferentially weathered.To investigate the compositions of the phases which are most labile and therefore likely to contribute to water chemistry, a range of rock and glacial sediment samples were leached (Supplementary Information, Haley et al., 2008
Haley, B.A., Frank, M., Spielhagen, R.F., Eisenhauer, A. (2008) Influence of brine formation on Arctic Ocean circulation over the past 15 million years. Nature Geoscience 1, 68-72.
; Chen et al., 2012Chen, T.-Y., Frank, M., Haley, B. A., Gutjahr, M., Spielhagen, R.F. (2012) Variations of North Atlantic inflow to the central Arctic Ocean over the last 14 million years inferred from hafnium and neodymium isotopes. Earth and Planetary Science Letters 353-354, 82-92.
). The εNd values of hydroxylamine hydrochloride (HH) and acetic acid (AA) leachates are always higher than the εNd values of the bulk sample (Fig. 1b, Table S-3).The dissolved load samples define a linear trend bounded by the leachates from the three rock samples containing >1 % bulk Nd in the leachates (Table S-4). A mixing line can be fitted between HH leachate end members R1 and R3, which passes through the dissolved load samples (Fig. 1c), implying that the dissolved load composition is a mixture of these two labile end members. The chemical extraction procedure, which was developed to extract seawater Nd isotopes from authigenic phases in sediment cores (e.g., Haley et al., 2008
Haley, B.A., Frank, M., Spielhagen, R.F., Eisenhauer, A. (2008) Influence of brine formation on Arctic Ocean circulation over the past 15 million years. Nature Geoscience 1, 68-72.
), appears to target the same labile, end member phases as natural chemical weathering conditions.The first end member is defined by the leachates from the shale samples R1 and R2 (Fig. 1b). Given that these shales were deposited in a deep water marine environment (Hindshaw et al., 2018
Hindshaw, R.S., Tosca, N.J., Piotrowski, A.M., Tipper, E.T. (2018) Clay mineralogy, strontium and neodymium isotope ratios in the sediments of two High Arctic catchments (Svalbard). Earth Surface Dynamics 6, 141-161.
), this labile phase is likely an authigenic phase precipitated from seawater. To aid in identifying the source of Nd, we utilise rare earth element (REE) patterns (e.g., Haley et al., 2004Haley, B.A., Klinkhammer, G.P., McManus, J. (2004) Rare earth elements in pore waters of marine sediments. Geochimica et Cosmochimica Acta 68, 1265-1279.
; Supplementary Information). When normalised to the bulk REE pattern, the AA and HH leachates of R1 and R2 have a middle REE (MREE) enrichment (Fig. 2, Table S-5), which indicates the REE are hosted in authigenic phosphate minerals and/or Fe-Mn (oxyhydr)oxides (e.g., Goldberg et al., 1963Goldberg, E.D., Koide, M., Schmitt, R.A., Smith, R.H. (1963) Rare-earth distributions in the marine environment. Journal of Geophysical Research 68, 4209-4217.
; Sholkovitz et al., 1999Sholkovitz, E.R., Elderfield, H., Szymczak, R., Casey, K. (1999) Island weathering: river sources of rare earth elements to the Western Pacific Ocean. Marine Chemistry 68, 39-57.
, Supplementary Information). The Sm/Nd ratios in the leachate samples (AA: 0.27-0.30; HH: 0.37-0.39) are higher than those of the bulk rock (0.19, typical for shale; McCulloch and Wasserburg, 1978McCulloch, M.T., Wasserburg, G.J. (1978) Sm-Nd and Rb-Sr chronology of continental crust formation. Science 200, 1003-1011.
) consistent with leaching of marine precipitates, as these phases preferentially incorporate Sm (Goldstein et al., 1984Goldstein, S.L., O'Nions, R.K., Hamilton, P.J. (1984) A Sm-Nd isotopic study of atmospheric dusts and particulates from major river systems. Earth and Planetary Science Letters 70, 221-236.
).The second end member is defined by the leachates from the sandstone sample (R3, Fig. 1b). The AA and HH leachates removed 91 % Ca and 88 % Sr respectively (Table S-4), with a Ca/Sr mass ratio of 325 (Veizer, 1983
Veizer, J. (1983) Chemical diagenesis of carbonates: theory and application of trace element technique. In: Arthur, M.A., Anderson, T.F., Kaplan, I.R., Veizer, J., Land, L.S. (Eds.) Stable isotopes in sedimentary geology. Society of Economic Paleontologists and Mineralogists Short Course Notes Vol. 10. SEPM Society for Sedimentary Geology, Dallas, USA, 3-1-3-100.
), strongly suggesting the presence of a carbonate phase. The sandstone leachates have a Sm/Nd mass ratio (0.20-0.21) typical for carbonates (~0.20, Hua et al., 2013Hua, G., Yuansheng, D., Lian, Z., Jianghai, Y., Hu, H. (2013) Trace and rare earth elemental geochemistry of carbonate succession in the Middle Gaoyuzhuang Formation, Pingquan Section: Implications for Early Mesoproterozoic ocean redox conditions. Journal of Palaeogeography 2, 209-221.
), and a high REE (HREE) enrichment (Fig. 2), typical for complexation with carbonate (e.g., Byrne and Sholkovitz, 1996Byrne, R.H., Sholkovitz, E.R. (1996) Marine chemistry and geochemistry of the lanthanides. In: Gschneidner, Jr., K.A., Eyring, L. (Eds.) Handbook on the Physics and Chemistry of Rare Earths. Vol. 23. Elsevier, Oxford, 497-593.
). This implies that Nd, like Sr, is hosted in the carbonate phase of this rock sample.In summary, εNd values of the dissolved load are distinct from those of stream suspended sediment sampled at the same time. We attribute this to the preferential leaching of two components found in sedimentary rocks in the catchment; 1) authigenic phosphates and/or Fe-Mn (oxyhydr)oxides, and 2) carbonate. Both phases have chemical and isotopic compositions distinct from the detrital silicate fraction (Fig. 1b).

Figure 1 Dissolved (<0.22 µm) and solid samples (rock, sediment and stream suspended sediment) form distinct arrays in Sr-Nd space. (a) A linear regression is fitted for the dissolved samples (r2 = 0.93, p < 0.001). The linear line for the solid samples is the mixing line between a Greenlandic (87Sr/86Sr = 0.78059, εNd = -37.1) and Siberian (87Sr/86Sr = 0.70626, εNd = -0.4) sediment source with identical Sr/Nd mass ratios (Hindshaw et al., 2018
Hindshaw, R.S., Tosca, N.J., Piotrowski, A.M., Tipper, E.T. (2018) Clay mineralogy, strontium and neodymium isotope ratios in the sediments of two High Arctic catchments (Svalbard). Earth Surface Dynamics 6, 141-161.
). The four rock and one glacial sediment sample subjected to the leaching procedure are labelled. (b) The isotopic compositions of the leachates, residual and bulk samples. The dissolved load array is bound by leachates of the sandstone (R3) at one end and leachates of the shale samples (R1 and R2) at the other end. (c) The dissolved samples can be fitted with a mixing line between the HH leachate end members. The best fit line uses the concentration and isotopic values measured in R3-HH and R1-HH (Table S-3). The numbers on the mixing line refer to the mass fraction of the sandstone end member in the mixture. Error bars are smaller than symbol size.
Figure 2 REE concentrations of the leachates normalised to bulk. (a) AA leach. (b) HH leach. REE concentrations are normalised against the bulk REE concentrations of the same sample. Note the scale break in (b). The sandstone sample (R3) has a HREE enriched pattern for both leachates. Shale samples R1 and R2 have MREE enriched patterns for both leachates. REE data is reported in Table S-5.
top
Implications for Leaching an Isotopically Distinct Labile Phase
Sedimentary rocks contain a mixture of authigenic, detrital and biological components. The results from this study imply that in areas with extensive sedimentary rock cover, weathering of labile phases may dominate the riverine Nd flux, with two important implications. First, εNd measurements of bulk rock or the detrital silicate fraction will not represent the continental εNd input to ocean water masses. Second, analogous to 87Sr/86Sr, εNd released to rivers and seawater will be controlled by the availability of (often) isotopically distinct labile phases e.g., oxides. In weathering-limited regimes, where the availability of labile phases is not limited, εNd will be subject to environmental factors, such as discharge and temperature, which determine whether the dissolution of labile phases is promoted or suppressed (West et al., 2005
West, A.J., Galy, A., Bickle, M. (2005) Tectonic and climatic controls on silicate weathering, Earth and Planetary Science Letters 235, 211-228.
).This has important implications for studies calculating budgets of seawater Nd isotopes relative to lithological sources, suggesting greater temporal and spatial variability. Using the Arctic Ocean as an example, where 49 % of the land drains shales (Amiotte Suchet et al., 2003
Amiotte Suchet, P., Probst, J.-L., Ludwig, W. (2003) Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Global Biogeochemical Cycles 17, 1038.
), we predict (Supplementary Information) that if a change in climatic or erosional conditions resulted in the weathering regime evolving from transport-limited, where the contribution of labile phases is negligible, to weathering-limited, where the dissolution of labile phases is dominant, then this would shift the εNd value of the ocean by 1 epsilon unit in just over 1τ (Nd residence time; Fig. S-2). This basic calculation only considers the dissolved load. However, if the proportion of sedimentary rock exposed to chemical weathering and the flux of stream suspended sediment were greater immediately after periods of glaciation (Vance et al., 2009Vance, D., Teagle, D.A.H., Foster, G.L. (2009) Variable Quaternary chemical weathering fluxes and imbalances in marine geochemical budgets. Nature 458, 493-496.
), then increased preferential weathering of labile phases from that sediment could occur in the marine environment, providing a climate-linked mechanism to alter the end member composition of water masses, independent of the source of Nd isotopes to the site of deep water formation, or of the location of deep water formation.On long geological timescales, we suggest that if a fraction of the dissolved load was derived from a labile phase with a marine origin, then it could act as a “buffer” on seawater compositions. This would result in the geochemical decoupling between a labile sedimentary reservoir, which more readily exchanges with seawater due to rapid dissolution kinetics, and a silicate-bound reservoir, which maintains its composition set by crystallisation, plus radiogenic ingrowth through time. The leachates measured in this study have Sm/Nd ratios >20 % higher than the silicate fraction in the same sample (Table S-3), in agreement with literature values (Shaw and Wasserburg, 1985
Shaw, H.F., Wasserburg, G.J. (1985) Sm-Nd in marine carbonates and phosphates: Implications for Nd isotopes in seawater and crustal ages. Geochimica et Cosmochimica Acta 49, 503-518.
; Charbonnier et al., 2012Charbonnier, G., Pucéat, E., Bayon, G., Desmares, D., Dera, G., Durlet, C., Deconinck, J.-F., Amédro, F., Gourlan, A.T., Pellenard, P., Bomou, B. (2012) Reconstruction of the Nd isotope composition of seawater on epicontinental seas: Testing the potential of Fe-Mn oxyhydroxide coatings on foraminifera tests for deep-time investigations. Geochimica et Cosmochimica Acta 99, 39-56.
). If allowed to evolve 500 Myr, the εNd composition of the labile reservoir would become 1.5 epsilon units more radiogenic than the detrital silicate fraction due to ingrowth of 143Nd (Supplementary Information). The exchange of this labile reservoir with seawater would result in seawater with a more radiogenic composition than the geological terranes that surround it, even in the absence of a volcanic fraction within marine sediment, which tends to contribute Nd preferentially to seawater (Pearce et al., 2013Pearce, C.R., Jones, M.T., Oelkers, E.H., Pradoux, C., Jeandel, C. (2013) The effect of particulate dissolution on the neodymium (Nd) isotope and Rare Earth Element (REE) composition of seawater. Earth and Planetary Science Letters 369-370, 138-147.
; Wilson et al., 2013Wilson, D.J., Piotrowski, A.M., Galy, A., Clegg, J.A. (2013) Reactivity of neodymium carriers in deep sea sediments: Implications for boundary exchange and paleoceanography. Geochimica et Cosmochimica Acta 109, 197-221.
). Exchange of εNd between seawater and labile phases during sediment recycling would cause the composition of terrestrial fine sediment to shift progressively further from bulk silicate rock towards higher εNd values over long geological timescales.top
Acknowledgements
This project was funded by a Swiss National Science Foundation Fellowship for prospective researchers (PBEZP2-137335), a Marie Curie Intra-European Fellowship (PIEF-GA-2012-331501), and NERC Standard Grant NE/M001865/1. Fieldwork was supported by an Arctic Field Grant (219165/E10, The Research Council of Norway). RSH thanks the fieldwork team, Christina Larkin and Tom Williams for assistance with the leaching procedure and Greg de Souza for advice on oceanic box models. We wish to thank Catherine Jeandel, two anonymous reviewers and editor Liane Benning for their constructive comments that helped improve this manuscript.
Editor: Liane G. Benning
top
References
Amiotte Suchet, P., Probst, J.-L., Ludwig, W. (2003) Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Global Biogeochemical Cycles 17, 1038.
 Show in context
Show in contextUsing the Arctic Ocean as an example, where 49 % of the land drains shales (Amiotte Suchet et al., 2003), we predict (Supplementary Information) that if a change in climatic or erosional conditions resulted in the weathering regime evolving from transport-limited, where the contribution of labile phases is negligible, to weathering-limited, where the dissolution of labile phases is dominant, then this would shift the εNd value of the ocean by 1 epsilon unit in just over 1τ (Nd residence time; Fig. S-2).
View in article
Andersson, P.S., Dahlqvist, R., Ingri, J., Gustafsson, Ö. (2001) The isotopic composition of Nd in a boreal river: A reflection of selective weathering and colloidal transport. Geochimica et Cosmochimica Acta 65, 521-527.
 Show in context
Show in contextHowever, riverine dissolved εNd values can be different from both the suspended load and the bedrock over which the river has flowed (Goldstein and Jacobsen, 1987; Tricca et al., 1999; Andersson et al., 2001; Rickli et al., 2013).
View in article
Bayon, G., German, C.R., Burton, K.W., Nesbitt, R.W., Rogers, N. (2004) Sedimentary Fe-Mn oxyhydroxides as paleoceanographic archives and the role of aeolian flux in regulating oceanic dissolved REE. Earth and Planetary Science Letters 224, 477-492.
 Show in context
Show in context On a finer spatial and temporal scale, variations in the εNd values of seawater recovered from archives, such as foraminifera (e.g., Vance and Burton, 1999) and Fe-Mn (oxyhydr)oxides (e.g., Bayon et al., 2004), are commonly interpreted as indicating relative contributions of different water masses and associated changes in ocean circulation.
View in article
Bayon, G., Burton, K.W., Soulet, G., Vigier, N., Dennielou, B., Etoubleau, J., Ponzevera, E., German, C.R., Nesbitt, R.W. (2009) Hf and Nd isotopes in marine sediments: Constraints on global silicate weathering. Earth and Planetary Science Letters 277, 318-326.
 Show in context
Show in contextContinental growth curves are based on εNd values (Taylor and McLennan, 1985) and changes in ocean dynamics and silicate weathering have been inferred from εNd measurements (Piepgras and Wasserburg, 1980; Bayon et al., 2009).
View in article
Blum, J.D., Erel, Y., Brown, K. (1994) 87Sr/86Sr ratios of Sierra Nevada stream waters: Implications for relative mineral weathering rates. Geochimica et Cosmochimica Acta 58, 5019-5025.
 Show in context
Show in context We find that similar to the well-established behaviour of radiogenic Sr (e.g., Blum et al., 1994), the dissolved εNd values are distinct from stream suspended sediments.
View in article
Bock, B., McLennan, S.M., Hanson, G.N. (1994) Rare earth element redistribution and its effects on the neodymium isotope system in the Austin Glen Member of the Normanskill Formation, New York, USA. Geochimica et Cosmochimica Acta 58, 5245-5253.
 Show in context
Show in context Additionally the rare earth element (REE) chemistry of sediment/soil leachates indicates that REE (including Sm and Nd) are mobile and can be fractionated during chemical weathering and diagenesis (e.g., Bock et al., 1994; Viers and Wasserburg, 2004).
View in article
Byrne, R.H., Sholkovitz, E.R. (1996) Marine chemistry and geochemistry of the lanthanides. In: Gschneidner, Jr., K.A., Eyring, L. (Eds.) Handbook on the Physics and Chemistry of Rare Earths. Vol. 23. Elsevier, Oxford, 497-593.
 Show in context
Show in contextThe sandstone leachates have a Sm/Nd mass ratio (0.20-0.21) typical for carbonates (~0.20, Hua et al., 2013), and a high REE (HREE) enrichment (Fig. 2), typical for complexation with carbonate (e.g., Byrne and Sholkovitz, 1996).
View in article
Charbonnier, G., Pucéat, E., Bayon, G., Desmares, D., Dera, G., Durlet, C., Deconinck, J.-F., Amédro, F., Gourlan, A.T., Pellenard, P., Bomou, B. (2012) Reconstruction of the Nd isotope composition of seawater on epicontinental seas: Testing the potential of Fe-Mn oxyhydroxide coatings on foraminifera tests for deep-time investigations. Geochimica et Cosmochimica Acta 99, 39-56.
 Show in context
Show in context The leachates measured in this study have Sm/Nd ratios >20 % higher than the silicate fraction in the same sample (Table S-3), in agreement with literature values (Shaw and Wasserburg, 1985; Charbonnier et al., 2012).
View in article
Chen, T.-Y., Frank, M., Haley, B. A., Gutjahr, M., Spielhagen, R.F. (2012) Variations of North Atlantic inflow to the central Arctic Ocean over the last 14 million years inferred from hafnium and neodymium isotopes. Earth and Planetary Science Letters 353-354, 82-92.
 Show in context
Show in context To investigate the compositions of the phases which are most labile and therefore likely to contribute to water chemistry, a range of rock and glacial sediment samples were leached (Supplementary Information, Haley et al., 2008; Chen et al., 2012).
View in article
Goldberg, E.D., Koide, M., Schmitt, R.A., Smith, R.H. (1963) Rare-earth distributions in the marine environment. Journal of Geophysical Research 68, 4209-4217.
 Show in context
Show in contextWhen normalised to the bulk REE pattern, the AA and HH leachates of R1 and R2 have a middle REE (MREE) enrichment (Fig. 2, Table S-5), which indicates the REE are hosted in authigenic phosphate minerals and/or Fe-Mn (oxyhydr)oxides (e.g., Goldberg et al., 1963; Sholkovitz et al., 1999, Supplementary Information).
View in article
Goldstein, S.L., O'Nions, R.K., Hamilton, P.J. (1984) A Sm-Nd isotopic study of atmospheric dusts and particulates from major river systems. Earth and Planetary Science Letters 70, 221-236.
 Show in context
Show in contextThe Sm/Nd ratios in the leachate samples (AA: 0.27-0.30; HH: 0.37-0.39) are higher than those of the bulk rock (0.19, typical for shale; McCulloch and Wasserburg, 1978) consistent with leaching of marine precipitates, as these phases preferentially incorporate Sm (Goldstein et al., 1984).
View in article
Goldstein, S.J., Jacobsen, S.B. (1987) The Nd and Sr isotopic systematics of river-water dissolved material: Implications for the sources of Nd and Sr in seawater. Chemical Geology 66, 245-272.
 Show in context
Show in context Radiogenic neodymium isotopes (143Nd/144Nd), commonly reported as εNd (Goldstein and Jacobsen, 1987), have influenced our understanding of geophysical processes, from planetary differentiation to ocean circulation.
View in article
However, riverine dissolved εNd values can be different from both the suspended load and the bedrock over which the river has flowed (Goldstein and Jacobsen, 1987; Tricca et al., 1999; Andersson et al., 2001; Rickli et al., 2013).
View in article
Goldstein and Jacobsen (1987) proposed that dissolved load εNd values are controlled by labile phases but direct evidence has been lacking. In this study we present εNd data on the dissolved load, stream suspended sediment and leachates (which access labile phases) of rock and glacial sediment samples in the same catchment to investigate the compositions and decoupling of these reservoirs.
View in article
Haley, B.A., Klinkhammer, G.P., McManus, J. (2004) Rare earth elements in pore waters of marine sediments. Geochimica et Cosmochimica Acta 68, 1265-1279.
 Show in context
Show in context To aid in identifying the source of Nd, we utilise rare earth element (REE) patterns (e.g., Haley et al., 2004; Supplementary Information).
View in article
Haley, B.A., Frank, M., Spielhagen, R.F., Eisenhauer, A. (2008) Influence of brine formation on Arctic Ocean circulation over the past 15 million years. Nature Geoscience 1, 68-72.
 Show in context
Show in contextTo investigate the compositions of the phases which are most labile and therefore likely to contribute to water chemistry, a range of rock and glacial sediment samples were leached (Supplementary Information, Haley et al., 2008; Chen et al., 2012).
View in article
The chemical extraction procedure, which was developed to extract seawater Nd isotopes from authigenic phases in sediment cores (e.g., Haley et al., 2008), appears to target the same labile, end member phases as natural chemical weathering conditions.
View in article
Hindshaw, R.S., Tosca, N.J., Piotrowski, A.M., Tipper, E.T. (2018) Clay mineralogy, strontium and neodymium isotope ratios in the sediments of two High Arctic catchments (Svalbard). Earth Surface Dynamics 6, 141-161.
 Show in context
Show in context We present data for an Arctic catchment (Svalbard, Supplementary Information) where the bedrock εNd and 87Sr/86Sr data were interpreted as a two component mixture between two sources: Proterozoic sediments derived from Greenlandic basement rocks and Carboniferous to Jurassic sediments derived from Siberian basalts (Hindshaw et al., 2018).
View in article
The dissolved load (<0.22 µm) has higher εNd (2.0-5.5) and lower 87Sr/86Sr (0.020-0.028) values (Fig. 1a, Table S-2) compared to the corresponding stream suspended sediment samples (>0.22 µm; Hindshaw et al., 2018).
View in article
Given that these shales were deposited in a deep water marine environment (Hindshaw et al., 2018), this labile phase is likely an authigenic phase precipitated from seawater.
View in article
Figure 1 [...] The linear line for the solid samples is the mixing line between a Greenlandic (87Sr/86Sr = 0.78059, εNd = -37.1) and Siberian (87Sr/86Sr = 0.70626, εNd = -0.4) sediment source with identical Sr/Nd mass ratios (Hindshaw et al., 2018).
View in article
Hua, G., Yuansheng, D., Lian, Z., Jianghai, Y., Hu, H. (2013) Trace and rare earth elemental geochemistry of carbonate succession in the Middle Gaoyuzhuang Formation, Pingquan Section: Implications for Early Mesoproterozoic ocean redox conditions. Journal of Palaeogeography 2, 209-221.
 Show in context
Show in contextThe sandstone leachates have a Sm/Nd mass ratio (0.20-0.21) typical for carbonates (~0.20, Hua et al., 2013), and a high REE (HREE) enrichment (Fig. 2), typical for complexation with carbonate (e.g., Byrne and Sholkovitz, 1996).
View in article
Jeandel, C., Arsouze, T., Lacan, F., Téchiné, P., Dutay, J.-C. (2007) Isotopic Nd compositions and concentrations of the lithogenic inputs into the ocean: A compilation, with an emphasis on the margins. Chemical Geology 239, 156-164.
 Show in context
Show in contextThe accuracy of conclusions gleaned from these sediment and seawater εNd records is however dependent on understanding the processes affecting the Nd concentration and isotopic composition during transport of Nd from rock to seawater via rivers and during sediment dissolution in the ocean (e.g., Jeandel et al., 2007).
View in article
McCulloch, M.T., Wasserburg, G.J. (1978) Sm-Nd and Rb-Sr chronology of continental crust formation. Science 200, 1003-1011.
 Show in context
Show in contextThe Sm/Nd ratios in the leachate samples (AA: 0.27-0.30; HH: 0.37-0.39) are higher than those of the bulk rock (0.19, typical for shale; McCulloch and Wasserburg, 1978) consistent with leaching of marine precipitates, as these phases preferentially incorporate Sm (Goldstein et al., 1984).
View in article
Pearce, C.R., Jones, M.T., Oelkers, E.H., Pradoux, C., Jeandel, C. (2013) The effect of particulate dissolution on the neodymium (Nd) isotope and Rare Earth Element (REE) composition of seawater. Earth and Planetary Science Letters 369-370, 138-147.
 Show in context
Show in context The exchange of this labile reservoir with seawater would result in seawater with a more radiogenic composition than the geological terranes that surround it, even in the absence of a volcanic fraction within marine sediment, which tends to contribute Nd preferentially to seawater (Pearce et al., 2013; Wilson et al., 2013).
View in article
Piepgras, D.J., Wasserburg, G.J. (1980) Neodymium isotopic variations in seawater. Earth and Planetary Science Letters 50, 128-138.
 Show in context
Show in contextContinental growth curves are based on εNd values (Taylor and McLennan, 1985) and changes in ocean dynamics and silicate weathering have been inferred from εNd measurements (Piepgras and Wasserburg, 1980; Bayon et al., 2009).
View in article
Rickli, J., Frank, M., Stichel, T., Georg, R.B., Vance, D., Halliday, A.N. (2013) Controls on the incongruent release of hafnium during weathering of metamorphic and sedimentary catchments. Geochimica et Cosmochimica Acta 101, 263-284.
 Show in context
Show in contextHowever, riverine dissolved εNd values can be different from both the suspended load and the bedrock over which the river has flowed (Goldstein and Jacobsen, 1987; Tricca et al., 1999; Andersson et al., 2001; Rickli et al., 2013).
View in article
Shaw, H.F., Wasserburg, G.J. (1985) Sm-Nd in marine carbonates and phosphates: Implications for Nd isotopes in seawater and crustal ages. Geochimica et Cosmochimica Acta 49, 503-518.
 Show in context
Show in context The leachates measured in this study have Sm/Nd ratios >20 % higher than the silicate fraction in the same sample (Table S-3), in agreement with literature values (Shaw and Wasserburg, 1985; Charbonnier et al., 2012).
View in article
Sholkovitz, E.R., Elderfield, H., Szymczak, R., Casey, K. (1999) Island weathering: river sources of rare earth elements to the Western Pacific Ocean. Marine Chemistry 68, 39-57.
 Show in context
Show in context When normalised to the bulk REE pattern, the AA and HH leachates of R1 and R2 have a middle REE (MREE) enrichment (Fig. 2, Table S-5), which indicates the REE are hosted in authigenic phosphate minerals and/or Fe-Mn (oxyhydr)oxides (e.g., Goldberg et al., 1963; Sholkovitz et al., 1999, Supplementary Information).
View in article
Taylor, S.R., McLennan, S.M. (1985) The continental crust: Its composition and evolution. Blackwell Scientific, Boston, MA.
 Show in context
Show in contextContinental growth curves are based on εNd values (Taylor and McLennan, 1985) and changes in ocean dynamics and silicate weathering have been inferred from εNd measurements (Piepgras and Wasserburg, 1980; Bayon et al., 2009).
View in article
Tricca, A., Stille, P., Steinmann, M., Kiefel, B., Samuel, J., Eikenberg, J. (1999) Rare earth elements and Sr and Nd isotopic compositions of dissolved and suspended loads from small river systems in the Vosges mountains (France), the river Rhine and groundwater. Chemical Geology 160, 139-158.
 Show in context
Show in contextHowever, riverine dissolved εNd values can be different from both the suspended load and the bedrock over which the river has flowed (Goldstein and Jacobsen, 1987; Tricca et al., 1999; Andersson et al., 2001; Rickli et al., 2013).
View in article
Vance, D., Burton, K. (1999) Neodymium isotopes in planktonic foraminifera: a record of the response of continental weathering and ocean circulation rates to climate change. Earth and Planetary Science Letters 173, 365-379.
 Show in context
Show in context On a finer spatial and temporal scale, variations in the εNd values of seawater recovered from archives, such as foraminifera (e.g., Vance and Burton, 1999) and Fe-Mn (oxyhydr)oxides (e.g., Bayon et al., 2004), are commonly interpreted as indicating relative contributions of different water masses and associated changes in ocean circulation.
View in article
Vance, D., Teagle, D.A.H., Foster, G.L. (2009) Variable Quaternary chemical weathering fluxes and imbalances in marine geochemical budgets. Nature 458, 493-496.
 Show in context
Show in contextHowever, if the proportion of sedimentary rock exposed to chemical weathering and the flux of stream suspended sediment were greater immediately after periods of glaciation (Vance et al., 2009), then increased preferential weathering of labile phases from that sediment could occur in the marine environment, providing a climate-linked mechanism to alter the end member composition of water masses, independent of the source of Nd isotopes to the site of deep water formation, or of the location of deep water formation.
View in article
Veizer, J. (1983) Chemical diagenesis of carbonates: theory and application of trace element technique. In: Arthur, M.A., Anderson, T.F., Kaplan, I.R., Veizer, J., Land, L.S. (Eds.) Stable isotopes in sedimentary geology. Society of Economic Paleontologists and Mineralogists Short Course Notes Vol. 10. SEPM Society for Sedimentary Geology, Dallas, USA, 3-1-3-100.
 Show in context
Show in contextThe AA and HH leachates removed 91 % Ca and 88 % Sr respectively (Table S-4), with a Ca/Sr mass ratio of 325 (Veizer, 1983), strongly suggesting the presence of a carbonate phase.
View in article
Viers, J., Wasserburg, G.J. (2004) Behavior of Sm and Nd in a lateritic soil profile. Geochimica et Cosmochimica Acta 68, 2043-2054.
 Show in context
Show in context Additionally the rare earth element (REE) chemistry of sediment/soil leachates indicates that REE (including Sm and Nd) are mobile and can be fractionated during chemical weathering and diagenesis (e.g., Bock et al., 1994; Viers and Wasserburg, 2004).
View in article
West, A.J., Galy, A., Bickle, M. (2005) Tectonic and climatic controls on silicate weathering, Earth and Planetary Science Letters 235, 211-228.
 Show in context
Show in contextIn weathering-limited regimes, where the availability of labile phases is not limited, εNd will be subject to environmental factors, such as discharge and temperature, which determine whether the dissolution of labile phases is promoted or suppressed (West et al., 2005).
View in article
Wilson, D.J., Piotrowski, A.M., Galy, A., Clegg, J.A. (2013) Reactivity of neodymium carriers in deep sea sediments: Implications for boundary exchange and paleoceanography. Geochimica et Cosmochimica Acta 109, 197-221.
 Show in context
Show in contextThe exchange of this labile reservoir with seawater would result in seawater with a more radiogenic composition than the geological terranes that surround it, even in the absence of a volcanic fraction within marine sediment, which tends to contribute Nd preferentially to seawater (Pearce et al., 2013; Wilson et al., 2013).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Sampling and Analytical Methods
- Precipitation Correction
- Further Details on the Identification of the Source of Labile Sr and Nd in Rocks R1, R2 and R3
- Rough Estimate of the Potential Magnitude of εNd Variation by Changing the Weathering Regime
- Estimate of the Magnitude of Divergence of εNd in Detrital and Labile pools
- Tables S-1 to S-5
- Figures S-1 to S-4
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
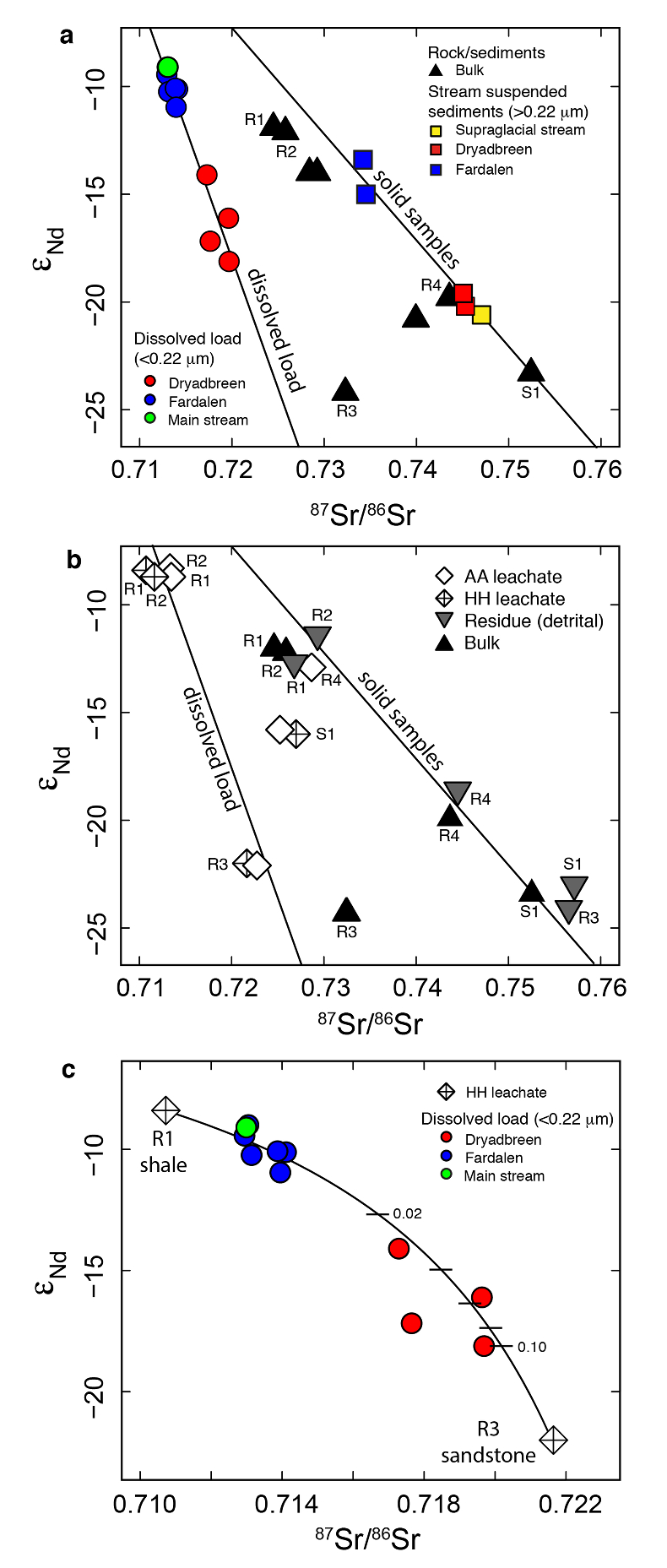
Figure 1 Dissolved (<0.22 µm) and solid samples (rock, sediment and stream suspended sediment) form distinct arrays in Sr-Nd space. (a) A linear regression is fitted for the dissolved samples (r2 = 0.93, p < 0.001). The linear line for the solid samples is the mixing line between a Greenlandic (87Sr/86Sr = 0.78059, εNd = -37.1) and Siberian (87Sr/86Sr = 0.70626, εNd = -0.4) sediment source with identical Sr/Nd mass ratios (Hindshaw et al., 2018
Hindshaw, R.S., Tosca, N.J., Piotrowski, A.M., Tipper, E.T. (2018) Clay mineralogy, strontium and neodymium isotope ratios in the sediments of two High Arctic catchments (Svalbard). Earth Surface Dynamics 6, 141-161.
). The four rock and one glacial sediment sample subjected to the leaching procedure are labelled. (b) The isotopic compositions of the leachates, residual and bulk samples. The dissolved load array is bound by leachates of the sandstone (R3) at one end and leachates of the shale samples (R1 and R2) at the other end. (c) The dissolved samples can be fitted with a mixing line between the HH leachate end members. The best fit line uses the concentration and isotopic values measured in R3-HH and R1-HH (Table S-3). The numbers on the mixing line refer to the mass fraction of the sandstone end member in the mixture. Error bars are smaller than symbol size.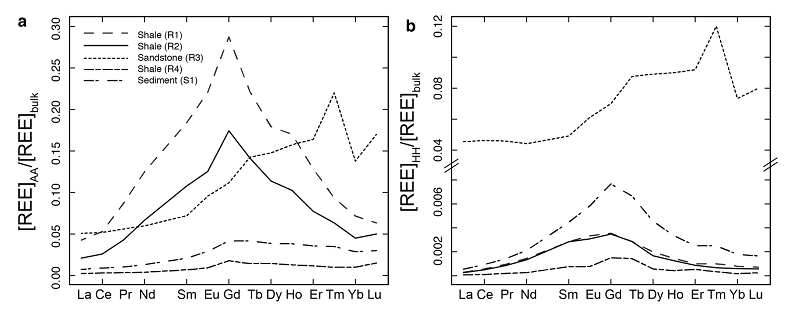
Figure 2 REE concentrations of the leachates normalised to bulk. (a) AA leach. (b) HH leach. REE concentrations are normalised against the bulk REE concentrations of the same sample. Note the scale break in (b). The sandstone sample (R3) has a HREE enriched pattern for both leachates. Shale samples R1 and R2 have MREE enriched patterns for both leachates. REE data is reported in Table S-5.