Unravelling the controls on the molybdenum isotope ratios of river waters
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:1,907Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
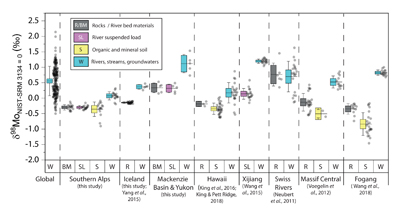 Figure 1 Molybdenum isotope ratios (δ98/95Mo, NIST-SRM3134 = 0 ‰) for this study (Southern Alps, Iceland, Mackenzie Basin and Yukon), alongside published measurements with: R = rocks, BM = river bed materials (grey); SL = suspended load (pink); S = soils (yellow); W = water (blue). Measurements are shown as grey dots, bars show the ±2 s.e. and whiskers ±2 s.d. | 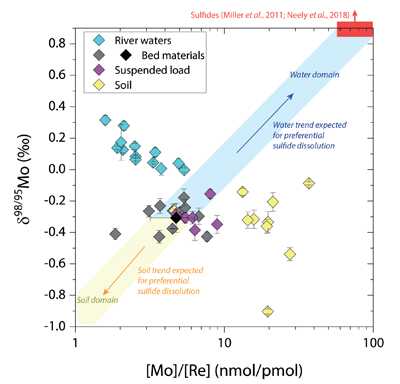 Figure 2 The Mo isotope ratios of materials from the western Southern Alps, New Zealand, versus the Mo to Re concentration ratios for river waters (light blue), river bed materials (grey), suspended load (purple) and soils (yellow). Black diamond is the mean of the bed material samples. Shaded domains show the expected fields of soil and water compositions if preferential dissolution of sulfides was occuring, but data lie perpendicular to this trend implying an alternative mechanism is responsible for fractionation patterns observed. | 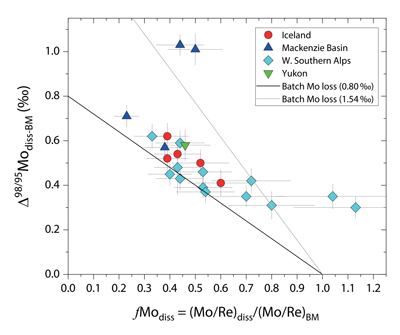 Figure 3 The fraction of Mo remaining in river water, fModiss, estimated using the ratio of Mo to rhenium (Re) in the dissolved load relative to parent materials, versus the difference in δ98/95Mo between river water and river bed materials. Lines are a batch fractionation model using fractionation factors between a solution and secondary mineral phases, based on fractionation factors of -0.8 ‰ (black) to -1.4 ‰ (grey) (Goldberg et al., 2009; Barling and Anbar, 2004). Error bars indicated for fModiss are the propagated 2 s.e. errors on (Mo/Re)BM, which is the main source of uncertainty. Error bars for Δ98/95Modiss-BM incorporate the 2 s.d analytical error on δ98/95Modiss and the 2 s.e. of the mean δ98/95MoBM. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
The cycling of molybdenum (Mo) in Earth’s surface environments holds key information on the weathering and redox reactions that control atmospheric gas concentrations (Arnold et al., 2004
Arnold, G.L., Anbar, A.D., Barling, J., Lyons, T.W. (2004) Molybdenum isotope evidence for widespread anoxia in mid-proterozoic oceans. Science 304, 87-90.
; Dickson, 2017Dickson, A.J. (2017) A molybdenum-isotope perspective on Phanerozoic deoxygenation events. Nature Geoscience 10, 721-726.
). This is because Mo isotopes (reported here as δ98/95Mo = [((98Mo/95Mo)sample/ (98Mo/95Mo)NIST-SRM-3134) - 1 ] × 1000 [‰]) can be fractionated during Mo removal from seawater, depending on the redox conditions of the sediment pore waters and overlying water column, and dissolved Mo speciation (Kerl et al., 2017Kerl, C.F., Lohmayer, R., Bura-Nakić, E., Vance, D., Planer-Friedrich, B. (2017) Experimental confirmation of isotope fractionation in thiomolybdates using ion chromatographic separation and detection by multicollector ICPMS. Analytical Chemistry 89, 3123-3129.
). Reconstructing the δ98/95Mo values of seawater is a recognised method for assessing the extent of past euxinic conditions and is linked to ocean oxygenation (Pearce et al., 2008Pearce, C.R., Cohen, A.S., Coe, A.L., Burton, K.W. (2008) Molybdenum isotope evidence for global ocean anoxia coupled with perturbations to the carbon cycle during the Early Jurassic. Geology 36, 231-234.
). Rivers are the largest input flux of Mo to oceans (~3.1 × 108 mol yr-1). Consequently, the isotope ratios of dissolved Mo in rivers (δ98/95Modiss) control the δ98/95Mo values of seawater and estimations of the extent of past seawater euxinia from geochemical records (Archer and Vance, 2008Archer, C., Vance, D. (2008) The isotopic signature of the global riverine molybdenum flux and anoxia in the ancient oceans. Nature Geoscience 1, 597-600.
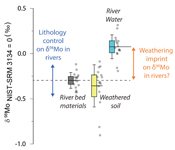
).The measured range of δ98/95Modiss values in rivers is >2 ‰ (Fig. 1). Some of this variability has been linked to the Mo isotope fractionation occurring during chemical weathering and the formation of secondary minerals, such as iron (Fe) and manganese (Mn) (oxyhydr)oxides (Pearce et al., 2010
Pearce, C.R., Burton, K.W., von Strandmann, P.A.P., James, R.H., Gíslason, S.R. (2010) Molybdenum isotope behaviour accompanying weathering and riverine transport in a basaltic terrain. Earth and Planetary Science Letters 295, 104-114.
; Wang et al., 2015Wang, Z., Ma, J., Li, J., Wei, G., Chen, X., Deng, W., Xie, L., Lu, W., Zou, L. (2015) Chemical weathering controls on variations in the molybdenum isotopic composition of river water: Evidence from large rivers in China. Chemical Geology 410, 201-212.
, 2018Wang, Z., Ma, J., Li, J., Wei, G., Zeng, T., Li, L., Zhang, L., Deng, W., Xie, L., Liu, Z. (2018) Fe (hydro) oxide controls Mo isotope fractionation during the weathering of granite. Geochimica et Cosmochimica Acta 226, 1-17.
). However, other studies have emphasised the role of lithology and weathering of labile phases, such as sulfide minerals, in setting the δ98/95Modiss of rivers (Voegelin et al., 2012Voegelin, A.R., Nägler, T.F., Pettke, T., Neubert, N., Steinmann, M., Pourret, O., Villa, I.M. (2012) The impact of igneous bedrock weathering on the Mo isotopic composition of stream waters: Natural samples and laboratory experiments. Geochimica et Cosmochimica Acta 86, 150-165.
; Neely et al., 2018Neely, R.A., Gislason, S.R., Ólafsson, M., McCoy-West, A.J., Pearce, C.R., Burton, K.W. (2018) Molybdenum isotope behaviour in groundwaters and terrestrial hydrothermal systems, Iceland. Earth and Planetary Science Letters 486, 108-118.
). It is important to constrain their relative importance to understand how and why δ98/95Modiss values of rivers might change. For instance, changes in the extent of primary and secondary weathering could lead to changes in the δ98/95Modiss of rivers over geological timescales, which may leave an imprint on seawater chemistry (Dickson, 2017Dickson, A.J. (2017) A molybdenum-isotope perspective on Phanerozoic deoxygenation events. Nature Geoscience 10, 721-726.
). Untangling the dual controls of source and process on river δ98/95Modiss values is challenging (King and Pett-Ridge, 2018King, E.K., Pett-Ridge, J.C. (2018) Reassessing the dissolved molybdenum isotopic composition of ocean inputs: The effect of chemical weathering and groundwater. Geology 46, 955-958.
). This is primarily because we lack information on the δ98/95Mo values of rocks and soils in many river catchments (Archer and Vance, 2008Archer, C., Vance, D. (2008) The isotopic signature of the global riverine molybdenum flux and anoxia in the ancient oceans. Nature Geoscience 1, 597-600.
).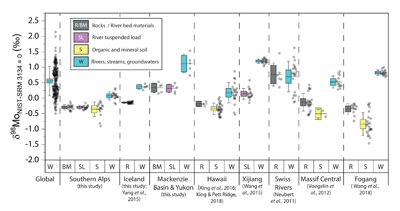
Figure 1 Molybdenum isotope ratios (δ98/95Mo, NIST-SRM3134 = 0 ‰) for this study (Southern Alps, Iceland, Mackenzie Basin and Yukon), alongside published measurements with: R = rocks, BM = river bed materials (grey); SL = suspended load (pink); S = soils (yellow); W = water (blue). Measurements are shown as grey dots, bars show the ±2 s.e. and whiskers ±2 s.d.
Here, we measure δ98/95Modiss in river water alongside solid products of erosion and weathering found in river bed materials, suspended sediments and soils (Tables S-1–S-4). We focus on three sets of rivers that have contrasting bedrock geology (albeit with heterogeneities in each location): 13 rivers from the Southern Alps, New Zealand (metasedimentary); the Skaftá River, Iceland (volcanic); and the Mackenzie River and Yukon Rivers, Canada (sedimentary dominated) (Supplementary Information). We use the trace element rhenium (Re), which is hosted in similar phases as Mo but is not susceptible to uptake during Fe-Mn-(oxyhydr)oxide formation (Miller et al., 2011
Miller, C.A., Peucker-Ehrenbrink, B., Walker, B.D., Marcantonio, F. (2011) Re-assessing the surface cycling of molybdenum and rhenium. Geochimica et Cosmochimica Acta 75, 7146-7179.
), to help track the imprint of Mo isotope fractionation during chemical weathering.top
Lithological Imprint on River Water δ98/95Mo
Chemical weathering can oxidise Mo in rocks to the soluble MoO42- anion, which can be leached from soils and delivered as dissolved Mo to rivers (Miller et al., 2011
Miller, C.A., Peucker-Ehrenbrink, B., Walker, B.D., Marcantonio, F. (2011) Re-assessing the surface cycling of molybdenum and rhenium. Geochimica et Cosmochimica Acta 75, 7146-7179.
). The starting isotope ratios of Mo-bearing phases can vary, with contrasts between igneous and sedimentary rocks, where the δ98/95Mo values of the latter depend on the redox state of the depositional environment, and can vary by ~2 ‰ at the outcrop scale (Pearce et al., 2010Pearce, C.R., Burton, K.W., von Strandmann, P.A.P., James, R.H., Gíslason, S.R. (2010) Molybdenum isotope behaviour accompanying weathering and riverine transport in a basaltic terrain. Earth and Planetary Science Letters 295, 104-114.
; Yang et al., 2015Yang, J., Siebert, C., Barling, J., Savage, P., Liang, Y.H., Halliday, A.N. (2015) Absence of molybdenum isotope fractionation during magmatic differentiation at Hekla volcano, Iceland. Geochimica et Cosmochimica Acta 162, 126-136.
; Kendall et al., 2017Kendall, B., Dahl, T.W. Anbar, A.D. (2017) The stable isotope geochemistry of molybdenum. Reviews in Mineralogy and Geochemistry 82, 683-732.
; Neely et al., 2018Neely, R.A., Gislason, S.R., Ólafsson, M., McCoy-West, A.J., Pearce, C.R., Burton, K.W. (2018) Molybdenum isotope behaviour in groundwaters and terrestrial hydrothermal systems, Iceland. Earth and Planetary Science Letters 486, 108-118.
; Li et al., 2019Li, Y., McCoy-West, A.J., Zhang, S., Selby, D., Burton, K.W., Horan, K. (2019) Controlling mechanisms for molybdenum isotope fractionation in porphyry deposits: the Qulong example. Economic Geology 114, 981-992.
).To constrain the composition of the rocks undergoing weathering, the most unweathered parts of river sediment loads can be used; these are typically found in the sand and silts of river bed materials in erosive settings (e.g., Hilton et al., 2010
Hilton, R.G., Galy, A., Hovius, N., Horng, M.J. and Chen, H. (2010) The isotopic composition of particulate organic carbon in mountain rivers of Taiwan. Geochimica et Cosmochimica Acta 74, 3164-3181.
). In the western Southern Alps, bulk river bed material samples across 11 catchments have relatively homogenous isotope ratios, with a mean δ98/95Mo value (NIST-3134 = 0 ‰; Supplementary Information) of -0.30 ± 0.05 ‰ (n = 11, mean ± 2 s.e. unless otherwise stated). These contrast with published rocks from Iceland (-0.15 ± 0.01 ‰; Yang et al., 2015Yang, J., Siebert, C., Barling, J., Savage, P., Liang, Y.H., Halliday, A.N. (2015) Absence of molybdenum isotope fractionation during magmatic differentiation at Hekla volcano, Iceland. Geochimica et Cosmochimica Acta 162, 126-136.
) and our river bed materials from the Mackenzie Basin (0.38 ± 0.14 ‰, n = 4) (Table S-1). The differences are consistent with the relatively organic carbon and sulfide poor greywacke of the Southern Alps (Roser and Cooper, 1990Roser, B.P., Cooper, A.F. (1990) Geochemistry and terrane affiliation of Haast Schist from the western Southern Alps, New Zealand. New Zealand Journal of Geology and Geophysics 33, 1-10.
), which may represent oxic depositional conditions favouring lower δ98/95Mo values. In the Mackenzie Basin, black shales deposited under euxinic conditions may have higher δ98/95Mo (Johnston et al., 2012Johnston, D.T., Macdonald, F.A., Gill, B.C., Hoffman, P.F., Schrag, D.P. (2012) Uncovering the Neoproterozoic carbon cycle. Nature 483, 320.
). When we compare δ98/95Modiss values of rivers at our study sites alongside published measurements, we find that river water δ98/95Modiss values are ~0.2 ‰ to >1 ‰ higher than their complementary solids (Fig. 1). General shifts in δ98/95Modiss values between locations can be explained by shifting rock compositions, but the systematically higher δ98/95Modiss values in streams and rivers requires further explanation.Previous work has suggested that incongruent weathering of phases, such as sulfide and sulfate minerals, may play a role in setting the δ98/95Modiss values of rivers (Neubert et al., 2011
Neubert, N., Heri, A.R., Voegelin, A.R., Nägler, T.F., Schlunegger, F., Villa, I.M. (2011) The molybdenum isotopic composition in river water: constraints from small catchments. Earth and Planetary Science Letters 304, 180-190.
; Voegelin et al., 2012Voegelin, A.R., Nägler, T.F., Pettke, T., Neubert, N., Steinmann, M., Pourret, O., Villa, I.M. (2012) The impact of igneous bedrock weathering on the Mo isotopic composition of stream waters: Natural samples and laboratory experiments. Geochimica et Cosmochimica Acta 86, 150-165.
) and groundwaters (Neely et al., 2018Neely, R.A., Gislason, S.R., Ólafsson, M., McCoy-West, A.J., Pearce, C.R., Burton, K.W. (2018) Molybdenum isotope behaviour in groundwaters and terrestrial hydrothermal systems, Iceland. Earth and Planetary Science Letters 486, 108-118.
). To explore this, we examine δ98/95Mo values alongside concentration ratios of [Mo] to rhenium, [Re], in rivers, soils and sediments from the Southern Alps (Fig. 2). Rhenium is a mobile and soluble element that is also sourced from organic and sulfide phases, yet in contrast to Mo, Re is not thought to be incorporated into secondary weathering products (Miller et al., 2011Miller, C.A., Peucker-Ehrenbrink, B., Walker, B.D., Marcantonio, F. (2011) Re-assessing the surface cycling of molybdenum and rhenium. Geochimica et Cosmochimica Acta 75, 7146-7179.
). If preferential weathering of sulfide phases is responsible for the fractionation patterns, we would expect waters to have sulfide-like compositions (high δ98/95Mo, high [Mo]/[Re]) (Miller et al., 2011Miller, C.A., Peucker-Ehrenbrink, B., Walker, B.D., Marcantonio, F. (2011) Re-assessing the surface cycling of molybdenum and rhenium. Geochimica et Cosmochimica Acta 75, 7146-7179.
; Neely et al., 2018Neely, R.A., Gislason, S.R., Ólafsson, M., McCoy-West, A.J., Pearce, C.R., Burton, K.W. (2018) Molybdenum isotope behaviour in groundwaters and terrestrial hydrothermal systems, Iceland. Earth and Planetary Science Letters 486, 108-118.
), while the residue in soils would have lower δ98/95Mo and [Mo]/[Re] values than parent materials. Our data lie perpendicular to this (Fig. 2), with soils having Mo enrichment relative to Re when compared to river bed materials. A negative pattern between δ98/95Mo and [Mo]/[Re] across our sample set is consistent with a process that preferentially removes light Mo isotopes from waters, and leaves a complementary pool of light Mo isotopes in soils.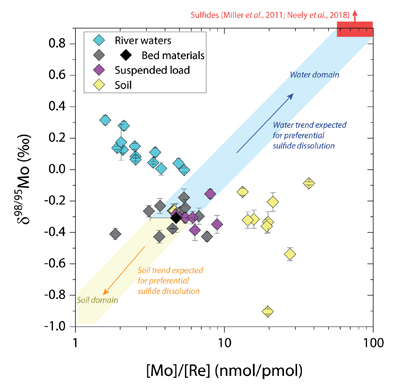
Figure 2 The Mo isotope ratios of materials from the western Southern Alps, New Zealand, versus the Mo to Re concentration ratios for river waters (light blue), river bed materials (grey), suspended load (purple) and soils (yellow). Black diamond is the mean of the bed material samples. Shaded domains show the expected fields of soil and water compositions if preferential dissolution of sulfides was occuring, but data lie perpendicular to this trend implying an alternative mechanism is responsible for fractionation patterns observed.
top
Chemical Weathering Imprint on River Water δ98/95Mo
Field observations and experiments suggest Mo can be removed from solution during Fe-Mn (oxyhydr)oxide formation (Barling and Anbar, 2004
Barling, J., Anbar, A.D. (2004) Molybdenum isotope fractionation during adsorption by manganese oxides. Earth and Planetary Science Letters 217, 315-329.
; Goldberg et al., 2009Goldberg, T., Archer, C., Vance, D., Poulton, S.W. (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr) oxides. Geochimica et Cosmochimica Acta 73, 6502-6516.
; Pearce et al., 2010Pearce, C.R., Burton, K.W., von Strandmann, P.A.P., James, R.H., Gíslason, S.R. (2010) Molybdenum isotope behaviour accompanying weathering and riverine transport in a basaltic terrain. Earth and Planetary Science Letters 295, 104-114.
) and can be adsorbed onto organic matter (Siebert et al., 2015Siebert, C., Pett-Ridge, J.C., Opfergelt, S., Guicharnaud, R.A., Halliday, A.N., Burton, K.W. (2015) Molybdenum isotope fractionation in soils: Influence of redox conditions, organic matter, and atmospheric inputs. Geochimica et Cosmochimica Acta 162, 1-24.
; King et al., 2018King, E.K., Perakis, S.S., Pett-Ridge, J.C. (2018) Molybdenum isotope fractionation during adsorption to organic matter. Geochimica et Cosmochimica Acta 222, 584-598.
). To explore the potential imprint of this process in both the western Southern Alps and our wider sample set, we use [Mo]:[Re] ratios to quantify Mo removal from solution (Supplementary Information). Following an approach taken for several other isotope systems (Millot et al., 2010Millot, R., Vigier, N., Gaillardet, J. (2010) Behaviour of lithium and its isotopes during weathering in the Mackenzie Basin, Canada. Geochimica et Cosmochimica Acta 74, 3897-3912.
; Dellinger et al., 2015Dellinger, M., Gaillardet, J., Bouchez, J., Calmels, D., Louvat, P., Dosseto, A., Gorge, C., Alanoca, L., Maurice, L. (2015) Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes. Geochimica et Cosmochimica Acta 164, 71-93.
), the fraction of Mo left in solution after secondary mineral formation (fModiss) is:Eq. 1
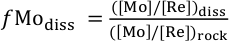
where ([Mo]/[Re])diss is the ratio of Mo to Re in the dissolved products of weathering (river water), and ([Mo]/[Re])rock is the ratio of the elements in the unweathered parent. A value of fModiss = 1 suggests Mo is released congruently to the dissolved phase alongside Re. A value of fModiss < 1 suggests less Mo loss relative to Re from the dissolved phase (i.e. Mo retention in secondary minerals).
To account for lithological controls on δ98/95Modiss between basins (Fig. 1), we calculate the difference between river water and source rock: Δ98/95Modiss-BM = δ98/95Modiss - δ98/95MoBM (Table S-3). Despite the diversity of our studied catchments in terms of geology, climate and scale, the Δ98/95Modiss-BM values are correlated with fModiss (Fig. 3): as the fraction of Mo left in solution decreases, Δ98/95Modiss-BM values increase. Notwithstanding the uncertainties on fModiss (Supplementary Information), the data suggest a common process across all of our study sites that modifies δ98/95Modiss values from those of the parent materials and decreases Mo/Re ratios as δ98/95Modiss values increase (Fig. 2). Adsorption of Mo to Fe and Mn (oxyhydr)oxides and/or organic matter removes Mo from solution (Goldberg et al., 1996
Goldberg, S., Forster, H.S., Godfrey, C.L. (1996) Molybdenum Adsorption on Oxides, Clay Minerals, and Soils. Soil Science Society of America Journal 60, 425-432.
) and preferentially scavenges light isotopes (Barling and Anbar, 2004Barling, J., Anbar, A.D. (2004) Molybdenum isotope fractionation during adsorption by manganese oxides. Earth and Planetary Science Letters 217, 315-329.
; Goldberg et al., 2009Goldberg, T., Archer, C., Vance, D., Poulton, S.W. (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr) oxides. Geochimica et Cosmochimica Acta 73, 6502-6516.
; King et al., 2018King, E.K., Perakis, S.S., Pett-Ridge, J.C. (2018) Molybdenum isotope fractionation during adsorption to organic matter. Geochimica et Cosmochimica Acta 222, 584-598.
). We find that experimentally derived fractionation factors for Mo uptake by Fe and Mn (oxyhydr)oxides are consistent with our new data (Fig. 3), supporting inferences from a granitic weathering profile (Wang et al., 2018Wang, Z., Ma, J., Li, J., Wei, G., Zeng, T., Li, L., Zhang, L., Deng, W., Xie, L., Liu, Z. (2018) Fe (hydro) oxide controls Mo isotope fractionation during the weathering of granite. Geochimica et Cosmochimica Acta 226, 1-17.
).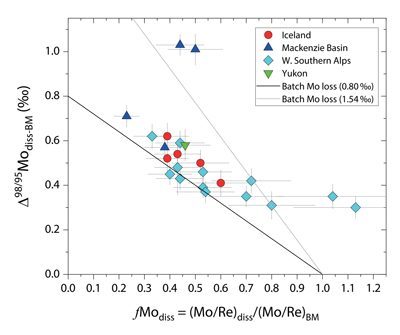
Figure 3 The fraction of Mo remaining in river water, fModiss, estimated using the ratio of Mo to rhenium (Re) in the dissolved load relative to parent materials, versus the difference in δ98/95Mo between river water and river bed materials. Lines are a batch fractionation model using fractionation factors between a solution and secondary mineral phases, based on fractionation factors of -0.8 ‰ (black) to -1.4 ‰ (grey) (Goldberg et al., 2009
Goldberg, T., Archer, C., Vance, D., Poulton, S.W. (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr) oxides. Geochimica et Cosmochimica Acta 73, 6502-6516.
; Barling and Anbar, 2004Barling, J., Anbar, A.D. (2004) Molybdenum isotope fractionation during adsorption by manganese oxides. Earth and Planetary Science Letters 217, 315-329.
). Error bars indicated for fModiss are the propagated 2 s.e. errors on (Mo/Re)BM, which is the main source of uncertainty. Error bars for Δ98/95Modiss-BM incorporate the 2 s.d analytical error on δ98/95Modiss and the 2 s.e. of the mean δ98/95MoBM.Biological processes could influence δ98/95Modiss values if plants fractionate Mo during uptake (Malinovsky and Kashulin, 2018
Malinovsky, D., Kashulin, N.A. (2018) Molybdenum isotope fractionation in plants measured by MC-ICPMS. Analytical Methods 10, 131-137.
) and previous observations on organic rich soils document a net enrichment in heavier isotopes compared to the original bedrock (Siebert et al., 2015Siebert, C., Pett-Ridge, J.C., Opfergelt, S., Guicharnaud, R.A., Halliday, A.N., Burton, K.W. (2015) Molybdenum isotope fractionation in soils: Influence of redox conditions, organic matter, and atmospheric inputs. Geochimica et Cosmochimica Acta 162, 1-24.
). However, the organic rich soil layers from the western Southern Alps have similar δ98/95Mo values to river bed materials (Figs. 2 and S-5). Surface soil litters with organic carbon contents >4 wt. % have a mean δ98/95Mo = -0.23 ± 0.11 ‰ (n = 5), which is the same within uncertainty as the river bed material at this location (δ98/95Mo = -0.26 ± 0.04 ‰; Table S-4).In contrast, the weathered colluvium sediments with low organic matter contents (<1.5 %) have a mean δ98/95Mo = -0.52 ± 0.28 ‰ (n = 4), with δ98/95Mo values reaching -0.90 ± 0.07 ‰ (Figs. 2 and S-5). Weathered materials in the surface environment thus offer a complementary reservoir of Mo to river water (Figs. 1 and 2). These data are comparable to those of Siebert et al. (2015)
Siebert, C., Pett-Ridge, J.C., Opfergelt, S., Guicharnaud, R.A., Halliday, A.N., Burton, K.W. (2015) Molybdenum isotope fractionation in soils: Influence of redox conditions, organic matter, and atmospheric inputs. Geochimica et Cosmochimica Acta 162, 1-24.
, who found lower δ98/95Mo in the deeper portions of soil horizons from Hawaii, Iceland and Puerto Rico. A light Mo reservoir in mineral soils is consistent with δ98/95Mo measurements on soil and root samples from the Massif Central (Voegelin et al., 2012Voegelin, A.R., Nägler, T.F., Pettke, T., Neubert, N., Steinmann, M., Pourret, O., Villa, I.M. (2012) The impact of igneous bedrock weathering on the Mo isotopic composition of stream waters: Natural samples and laboratory experiments. Geochimica et Cosmochimica Acta 86, 150-165.
, Fig. 1) and soil samples paired to local bedrock samples in Hawaii (King et al., 2016King, E.K., Thompson, A., Chadwick, O.A., Pett-Ridge, J.C. (2016) Molybdenum sources and isotopic composition during early stages of pedogenesis along a basaltic climate transect. Chemical Geology 445, 54-67.
).In the Mackenzie Basin, we find the highest average Δ98/95Modiss-BM value (0.78 ± 0.23 ‰) (Fig. 3). This would suggest a weathering regime that promotes Mo removal from solution, potentially by Fe-Mn (oxyhydr)oxide formation. In contrast, the Southern Alps have a lower mean value of Δ98/95Modiss-BM (0.42 ± 0.09 ‰). The higher erosion rates in this setting drive high oxidative weathering fluxes (Horan et al., 2017
Horan, K., Hilton, R.G., Selby, D., Ottley, C.J., Gröcke, D.R., Hicks, M., Burton, K.W. (2017) Mountain glaciation drives rapid oxidation of rock-bound organic carbon. Science Advances 3, e1701107.
) but a lower extent of primary and secondary weathering compared to the Mackenzie Basin (Supplementary Information). We acknowledge that the dataset of Mo isotope ratios is limited in size compared to other isotope systems (Dellinger et al., 2015Dellinger, M., Gaillardet, J., Bouchez, J., Calmels, D., Louvat, P., Dosseto, A., Gorge, C., Alanoca, L., Maurice, L. (2015) Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes. Geochimica et Cosmochimica Acta 164, 71-93.
) and for the published datasets (Fig. 1) fModiss cannot be estimated without complementary Re analyses. In addition, understanding temporal and spatial changes in water flow paths and Mo flux at the catchment scale requires flux weighted δ98/95Mo values (King and Pett-Ridge, 2018King, E.K., Pett-Ridge, J.C. (2018) Reassessing the dissolved molybdenum isotopic composition of ocean inputs: The effect of chemical weathering and groundwater. Geology 46, 955-958.
). Nevertheless, the contrast between our study locations suggests that primary weathering coupled to the formation of specific mineral phases (which are likely to be linked to bioclimatic regimes, erosion rates, lithology) could play a role in setting differences in Δ98/95Modiss-BM.top
Wider Implications
Our approach attempts to tease apart the source (lithology) versus process (secondary mineral formation) controls on δ98/95Modiss in rivers. Although lithological differences account for ~1 ‰ variability (Fig. 1), we find that the partitioning of Mo between the dissolved load and solid weathering products (fModiss) can produce an additional ~1 ‰ offset (Fig. 3). These findings indicate that changes in primary and secondary weathering patterns could give rise to changes in δ98/95Modiss values. Over geological time, this could influence the Mo isotope ratios of lakes, coastal regions and the δ98/95Mo values of seawater. Shifts of as little as ~0.3 ‰ in continental runoff impact how δ98/95Mo values of sedimentary rocks are used to reconstruct palaeoredox conditions (Dickson, 2017
Dickson, A.J. (2017) A molybdenum-isotope perspective on Phanerozoic deoxygenation events. Nature Geoscience 10, 721-726.
). Global changes in chemical weathering on land are reflected in seawater lithium isotope records over the Cenozoic (e.g., Misra and Froelich, 2012Misra, S., Froelich, P.N. (2012) Lithium Isotope History of Cenozoic Seawater: Changes in Silicate Weathering and Reverse Weathering. Science 335, 818-823.
; Dellinger et al., 2015Dellinger, M., Gaillardet, J., Bouchez, J., Calmels, D., Louvat, P., Dosseto, A., Gorge, C., Alanoca, L., Maurice, L. (2015) Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes. Geochimica et Cosmochimica Acta 164, 71-93.
). Our data raise the intriguing possibility that secular trends in δ98/95Modiss could also result from changes in the extent of primary and secondary weathering (Fig. 3), and call for future work to better constrain δ98/95Mo fractionation in large rivers catchments to understand spatio-temporal variability.top
Acknowledgements
KH was funded by a Natural Environment Research Council (NERC, UK) PhD award. RGH was supported by a European Research Council Starting Grant (ERC-StG, 678779, ROC-CO2). Fieldwork in New Zealand was funded by a Durham University Grant (Building Research Links in New Zealand) to RGH. Fieldwork support for Canada came from the British Society for Geomorphology (RGH) and the Carnegie Trusts (ETT). Research in the Mackenzie Basin was carried out under Scientific Research License No. 14802 issued by the Aurora Research Institute and in the Yukon River under License No. 13-47S&E. We are thankful to Mathieu Dellinger for discussions and Christopher Pearce, Rebecca Neely, Geoff Nowell, Chris Ottley and Amanda Hayton for additional laboratory support.
Editor: Sophie Opfergelt
top
Author Contributions
KH, RGH and KB conceived the research and designed the study. KH, RGH, ETT, SH and KB collected the samples. KH undertook the geochemical analyses under the supervision of AMW. KH interpreted the data with RGH and in discussion with AMW, KB, DS and ETT. KH and RGH wrote the manuscript with input from co-authors.
top
References
Archer, C., Vance, D. (2008) The isotopic signature of the global riverine molybdenum flux and anoxia in the ancient oceans. Nature Geoscience 1, 597-600.
 Show in context
Show in contextConsequently, the isotope ratios of dissolved Mo in rivers (δ98/95Modiss) control the δ98/95Mo values of seawater and estimations of the extent of past seawater euxinia from geochemical records (Archer and Vance, 2008).
View in article
This is primarily because we lack information on the δ98/95Mo values of rocks and soils in many river catchments (Archer and Vance, 2008).
View in article
Arnold, G.L., Anbar, A.D., Barling, J., Lyons, T.W. (2004) Molybdenum isotope evidence for widespread anoxia in mid-proterozoic oceans. Science 304, 87-90.
 Show in context
Show in contextThe cycling of molybdenum (Mo) in Earth’s surface environments holds key information on the weathering and redox reactions that control atmospheric gas concentrations (Arnold et al., 2004; Dickson, 2017).
View in article
Barling, J., Anbar, A.D. (2004) Molybdenum isotope fractionation during adsorption by manganese oxides. Earth and Planetary Science Letters 217, 315-329.
 Show in context
Show in context Field observations and experiments suggest Mo can be removed from solution during Fe-Mn (oxyhydr)oxide formation (Barling and Anbar, 2004; Goldberg et al., 2009; Pearce et al., 2010) and can be adsorbed onto organic matter (Siebert et al., 2015; King et al., 2018).
View in article
Adsorption of Mo to Fe and Mn (oxyhydr)oxides and/or organic matter removes Mo from solution (Goldberg et al., 1996) and preferentially scavenges light isotopes (Barling and Anbar, 2004; Goldberg et al., 2009; King et al., 2018).
View in article
Figure 3 [...] Lines are a batch fractionation model using fractionation factors between a solution and secondary mineral phases, based on fractionation factors of -0.8 ‰ (black) to -1.4 ‰ (grey) (Goldberg et al., 2009; Barling and Anbar, 2004).
View in article
Dellinger, M., Gaillardet, J., Bouchez, J., Calmels, D., Louvat, P., Dosseto, A., Gorge, C., Alanoca, L., Maurice, L. (2015) Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes. Geochimica et Cosmochimica Acta 164, 71-93.
 Show in context
Show in contextFollowing an approach taken for several other isotope systems (Millot et al., 2010; Dellinger et al., 2015), the fraction of Mo left in solution after secondary mineral formation (fModiss) is: 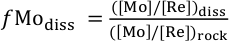
where ([Mo]/[Re])diss is the ratio of Mo to Re in the dissolved products of weathering (river water), and ([Mo]/[Re])rock is the ratio of the elements in the unweathered parent.
View in article
We acknowledge that the dataset of Mo isotope ratios is limited in size compared to other isotope systems (Dellinger et al., 2015) and for the published datasets (Fig. 1) fModiss cannot be estimated without complementary Re analyses.
View in article
Global changes in chemical weathering on land are reflected in seawater lithium isotope records over the Cenozoic (e.g., Misra and Froelich, 2012; Dellinger et al., 2015).
View in article
Dickson, A.J. (2017) A molybdenum-isotope perspective on Phanerozoic deoxygenation events. Nature Geoscience 10, 721-726.
 Show in context
Show in context The cycling of molybdenum (Mo) in Earth’s surface environments holds key information on the weathering and redox reactions that control atmospheric gas concentrations (Arnold et al., 2004; Dickson, 2017).
View in article
For instance, changes in the extent of primary and secondary weathering could lead to changes in the δ98/95Modiss of rivers over geological timescales, which may leave an imprint on seawater chemistry (Dickson, 2017).
View in article
Shifts of as little as ~0.3 ‰ in continental runoff impact how δ98/95Mo values of sedimentary rocks are used to reconstruct palaeoredox conditions (Dickson, 2017).
View in article
Goldberg, S., Forster, H.S., Godfrey, C.L. (1996) Molybdenum Adsorption on Oxides, Clay Minerals, and Soils. Soil Science Society of America Journal 60, 425-432.
 Show in context
Show in context Adsorption of Mo to Fe and Mn (oxyhydr)oxides and/or organic matter removes Mo from solution (Goldberg et al., 1996) and preferentially scavenges light isotopes (Barling and Anbar, 2004; Goldberg et al., 2009; King et al., 2018).
View in article
Goldberg, T., Archer, C., Vance, D., Poulton, S.W. (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr) oxides. Geochimica et Cosmochimica Acta 73, 6502-6516.
 Show in context
Show in context Field observations and experiments suggest Mo can be removed from solution during Fe-Mn (oxyhydr)oxide formation (Barling and Anbar, 2004; Goldberg et al., 2009; Pearce et al., 2010) and can be adsorbed onto organic matter (Siebert et al., 2015; King et al., 2018).
View in article
Adsorption of Mo to Fe and Mn (oxyhydr)oxides and/or organic matter removes Mo from solution (Goldberg et al., 1996) and preferentially scavenges light isotopes (Barling and Anbar, 2004; Goldberg et al., 2009; King et al., 2018).
View in article
Figure 3 [...] Lines are a batch fractionation model using fractionation factors between a solution and secondary mineral phases, based on fractionation factors of -0.8 ‰ (black) to -1.4 ‰ (grey) (Goldberg et al., 2009; Barling and Anbar, 2004).
View in article
Hilton, R.G., Galy, A., Hovius, N., Horng, M.J. and Chen, H. (2010) The isotopic composition of particulate organic carbon in mountain rivers of Taiwan. Geochimica et Cosmochimica Acta 74, 3164-3181.
 Show in context
Show in context To constrain the composition of the rocks undergoing weathering, the most unweathered parts of river sediment loads can be used; these are typically found in the sand and silts of river bed materials in erosive settings (e.g., Hilton et al., 2010).
View in article
Horan, K., Hilton, R.G., Selby, D., Ottley, C.J., Gröcke, D.R., Hicks, M., Burton, K.W. (2017) Mountain glaciation drives rapid oxidation of rock-bound organic carbon. Science Advances 3, e1701107.
 Show in context
Show in context The higher erosion rates in this setting drive high oxidative weathering fluxes (Horan et al., 2017) but a lower extent of primary and secondary weathering compared to the Mackenzie Basin (Supplementary Information).
View in article
Johnston, D.T., Macdonald, F.A., Gill, B.C., Hoffman, P.F., Schrag, D.P. (2012) Uncovering the Neoproterozoic carbon cycle. Nature 483, 320.
 Show in context
Show in contextIn the Mackenzie Basin, black shales deposited under euxinic conditions may have higher δ98/95Mo (Johnston et al., 2012).
View in article
Kendall, B., Dahl, T.W. Anbar, A.D. (2017) The stable isotope geochemistry of molybdenum. Reviews in Mineralogy and Geochemistry 82, 683-732.
 Show in context
Show in context The starting isotope ratios of Mo-bearing phases can vary, with contrasts between igneous and sedimentary rocks, where the δ98/95Mo values of the latter depend on the redox state of the depositional environment, and can vary by ~2 ‰ at the outcrop scale (Pearce et al., 2010; Yang et al., 2015; Kendall et al., 2017; Neely et al., 2018; Li et al., 2019).
View in article
Kerl, C.F., Lohmayer, R., Bura-Nakić, E., Vance, D., Planer-Friedrich, B. (2017) Experimental confirmation of isotope fractionation in thiomolybdates using ion chromatographic separation and detection by multicollector ICPMS. Analytical Chemistry 89, 3123-3129.
 Show in context
Show in contextThis is because Mo isotopes (reported here as δ98/95Mo = [((98Mo/95Mo)sample/ (98Mo/95Mo)NIST-SRM-3134) - 1 ] × 1000 [‰]) can be fractionated during Mo removal from seawater, depending on the redox conditions of the sediment pore waters and overlying water column, and dissolved Mo speciation (Kerl et al., 2017).
View in article
King, E.K., Pett-Ridge, J.C. (2018) Reassessing the dissolved molybdenum isotopic composition of ocean inputs: The effect of chemical weathering and groundwater. Geology 46, 955-958.
 Show in context
Show in context Untangling the dual controls of source and process on river δ98/95Modiss values is challenging (King and Pett-Ridge, 2018).
View in article
In addition, understanding temporal and spatial changes in water flow paths and Mo flux at the catchment scale requires flux weighted δ98/95Mo values (King and Pett-Ridge, 2018).
View in article
King, E.K., Thompson, A., Chadwick, O.A., Pett-Ridge, J.C. (2016) Molybdenum sources and isotopic composition during early stages of pedogenesis along a basaltic climate transect. Chemical Geology 445, 54-67.
 Show in context
Show in contextA light Mo reservoir in mineral soils is consistent with δ98/95Mo measurements on soil and root samples from the Massif Central (Voegelin et al., 2012, Fig. 1) and soil samples paired to local bedrock samples in Hawaii (King et al., 2016).
View in article
King, E.K., Perakis, S.S., Pett-Ridge, J.C. (2018) Molybdenum isotope fractionation during adsorption to organic matter. Geochimica et Cosmochimica Acta 222, 584-598.
 Show in context
Show in context Field observations and experiments suggest Mo can be removed from solution during Fe-Mn (oxyhydr)oxide formation (Barling and Anbar, 2004; Goldberg et al., 2009; Pearce et al., 2010) and can be adsorbed onto organic matter (Siebert et al., 2015; King et al., 2018).
View in article
Adsorption of Mo to Fe and Mn (oxyhydr)oxides and/or organic matter removes Mo from solution (Goldberg et al., 1996) and preferentially scavenges light isotopes (Barling and Anbar, 2004; Goldberg et al., 2009; King et al., 2018).
View in article
Malinovsky, D., Kashulin, N.A. (2018) Molybdenum isotope fractionation in plants measured by MC-ICPMS. Analytical Methods 10, 131-137.
 Show in context
Show in contextBiological processes could influence δ98/95Modiss values if plants fractionate Mo during uptake (Malinovsky and Kashulin, 2018) and previous observations on organic rich soils document a net enrichment in heavier isotopes compared to the original bedrock (Siebert et al., 2015).
View in article
Miller, C.A., Peucker-Ehrenbrink, B., Walker, B.D., Marcantonio, F. (2011) Re-assessing the surface cycling of molybdenum and rhenium. Geochimica et Cosmochimica Acta 75, 7146-7179.
 Show in context
Show in contextWe use the trace element rhenium (Re), which is hosted in similar phases as Mo but is not susceptible to uptake during Fe-Mn-(oxyhydr)oxide formation (Miller et al., 2011), to help track the imprint of Mo isotope fractionation during chemical weathering.
View in article
Chemical weathering can oxidise Mo in rocks to the soluble MoO42- anion, which can be leached from soils and delivered as dissolved Mo to rivers (Miller et al., 2011).
View in article
Rhenium is a mobile and soluble element that is also sourced from organic and sulfide phases, yet in contrast to Mo, Re is not thought to be incorporated into secondary weathering products (Miller et al., 2011).
View in article
If preferential weathering of sulfide phases is responsible for the fractionation patterns, we would expect waters to have sulfide-like compositions (high δ98/95Mo, high [Mo]/[Re]) (Miller et al., 2011; Neely et al., 2018), while the residue in soils would have lower δ98/95Mo and [Mo]/[Re] values than parent materials.
View in article
Millot, R., Vigier, N., Gaillardet, J. (2010) Behaviour of lithium and its isotopes during weathering in the Mackenzie Basin, Canada. Geochimica et Cosmochimica Acta 74, 3897-3912.
 Show in context
Show in context Following an approach taken for several other isotope systems (Millot et al., 2010; Dellinger et al., 2015), the fraction of Mo left in solution after secondary mineral formation (fModiss) is: 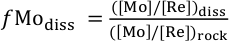
where ([Mo]/[Re])diss is the ratio of Mo to Re in the dissolved products of weathering (river water), and ([Mo]/[Re])rock is the ratio of the elements in the unweathered parent.
View in article
Misra, S., Froelich, P.N. (2012) Lithium Isotope History of Cenozoic Seawater: Changes in Silicate Weathering and Reverse Weathering. Science 335, 818-823.
 Show in context
Show in context Global changes in chemical weathering on land are reflected in seawater lithium isotope records over the Cenozoic (e.g., Misra and Froelich, 2012; Dellinger et al., 2015).
View in article
Li, Y., McCoy-West, A.J., Zhang, S., Selby, D., Burton, K.W., Horan, K. (2019) Controlling mechanisms for molybdenum isotope fractionation in porphyry deposits: the Qulong example. Economic Geology 114, 981-992.
 Show in context
Show in context The starting isotope ratios of Mo-bearing phases can vary, with contrasts between igneous and sedimentary rocks, where the δ98/95Mo values of the latter depend on the redox state of the depositional environment, and can vary by ~2 ‰ at the outcrop scale (Pearce et al., 2010; Yang et al., 2015; Kendall et al., 2017; Neely et al., 2018; Li et al., 2019).
View in article
Neely, R.A., Gislason, S.R., Ólafsson, M., McCoy-West, A.J., Pearce, C.R., Burton, K.W. (2018) Molybdenum isotope behaviour in groundwaters and terrestrial hydrothermal systems, Iceland. Earth and Planetary Science Letters 486, 108-118.
 Show in context
Show in context However, other studies have emphasised the role of lithology and weathering of labile phases, such as sulfide minerals, in setting the δ98/95Modiss of rivers (Voegelin et al., 2012; Neely et al., 2018).
View in article
The starting isotope ratios of Mo-bearing phases can vary, with contrasts between igneous and sedimentary rocks, where the δ98/95Mo values of the latter depend on the redox state of the depositional environment, and can vary by ~2 ‰ at the outcrop scale (Pearce et al., 2010; Yang et al., 2015; Kendall et al., 2017; Neely et al., 2018; Li et al., 2019).
View in article
Previous work has suggested that incongruent weathering of phases, such as sulfide and sulfate minerals, may play a role in setting the δ98/95Modiss values of rivers (Neubert et al., 2011; Voegelin et al., 2012) and groundwaters (Neely et al., 2018).
View in article
If preferential weathering of sulfide phases is responsible for the fractionation patterns, we would expect waters to have sulfide-like compositions (high δ98/95Mo, high [Mo]/[Re]) (Miller et al., 2011; Neely et al., 2018), while the residue in soils would have lower δ98/95Mo and [Mo]/[Re] values than parent materials.
View in article
Neubert, N., Heri, A.R., Voegelin, A.R., Nägler, T.F., Schlunegger, F., Villa, I.M. (2011) The molybdenum isotopic composition in river water: constraints from small catchments. Earth and Planetary Science Letters 304, 180-190.
 Show in context
Show in contextPrevious work has suggested that incongruent weathering of phases, such as sulfide and sulfate minerals, may play a role in setting the δ98/95Modiss values of rivers (Neubert et al., 2011; Voegelin et al., 2012) and groundwaters (Neely et al., 2018).
View in article
Pearce, C.R., Cohen, A.S., Coe, A.L., Burton, K.W. (2008) Molybdenum isotope evidence for global ocean anoxia coupled with perturbations to the carbon cycle during the Early Jurassic. Geology 36, 231-234.
 Show in context
Show in contextReconstructing the δ98/95Mo values of seawater is a recognised method for assessing the extent of past euxinic conditions and is linked to ocean oxygenation (Pearce et al., 2008).
View in article
Pearce, C.R., Burton, K.W., von Strandmann, P.A.P., James, R.H., Gíslason, S.R. (2010) Molybdenum isotope behaviour accompanying weathering and riverine transport in a basaltic terrain. Earth and Planetary Science Letters 295, 104-114.
 Show in context
Show in context Some of this variability has been linked to the Mo isotope fractionation occurring during chemical weathering and the formation of secondary minerals, such as iron (Fe) and manganese (Mn) (oxyhydr)oxides (Pearce et al., 2010; Wang et al., 2015, 2018).
View in article
The starting isotope ratios of Mo-bearing phases can vary, with contrasts between igneous and sedimentary rocks, where the δ98/95Mo values of the latter depend on the redox state of the depositional environment, and can vary by ~2 ‰ at the outcrop scale (Pearce et al., 2010; Yang et al., 2015; Kendall et al., 2017; Neely et al., 2018; Li et al., 2019).
View in article
Field observations and experiments suggest Mo can be removed from solution during Fe-Mn (oxyhydr)oxide formation (Barling and Anbar, 2004; Goldberg et al., 2009; Pearce et al., 2010) and can be adsorbed onto organic matter (Siebert et al., 2015; King et al., 2018).
View in article
Roser, B.P., Cooper, A.F. (1990) Geochemistry and terrane affiliation of Haast Schist from the western Southern Alps, New Zealand. New Zealand Journal of Geology and Geophysics 33, 1-10.
 Show in context
Show in context The differences are consistent with the relatively organic carbon and sulfide poor greywacke of the Southern Alps (Roser and Cooper, 1990), which may represent oxic depositional conditions favouring lower δ98/95Mo values.
View in article
Siebert, C., Pett-Ridge, J.C., Opfergelt, S., Guicharnaud, R.A., Halliday, A.N., Burton, K.W. (2015) Molybdenum isotope fractionation in soils: Influence of redox conditions, organic matter, and atmospheric inputs. Geochimica et Cosmochimica Acta 162, 1-24.
 Show in context
Show in context Field observations and experiments suggest Mo can be removed from solution during Fe-Mn (oxyhydr)oxide formation (Barling and Anbar, 2004; Goldberg et al., 2009; Pearce et al., 2010) and can be adsorbed onto organic matter (Siebert et al., 2015; King et al., 2018).
View in article
Biological processes could influence δ98/95Modiss values if plants fractionate Mo during uptake (Malinovsky and Kashulin, 2018) and previous observations on organic rich soils document a net enrichment in heavier isotopes compared to the original bedrock (Siebert et al., 2015).
View in article
These data are comparable to those of Siebert et al. (2015), who found lower δ98/95Mo in the deeper portions of soil horizons from Hawaii, Iceland and Puerto Rico.
View in article
Voegelin, A.R., Nägler, T.F., Pettke, T., Neubert, N., Steinmann, M., Pourret, O., Villa, I.M. (2012) The impact of igneous bedrock weathering on the Mo isotopic composition of stream waters: Natural samples and laboratory experiments. Geochimica et Cosmochimica Acta 86, 150-165.
 Show in context
Show in context However, other studies have emphasised the role of lithology and weathering of labile phases, such as sulfide minerals, in setting the δ98/95Modiss of rivers (Voegelin et al., 2012; Neely et al., 2018).
View in article
Previous work has suggested that incongruent weathering of phases, such as sulfide and sulfate minerals, may play a role in setting the δ98/95Modiss values of rivers (Neubert et al., 2011; Voegelin et al., 2012) and groundwaters (Neely et al., 2018).
View in article
A light Mo reservoir in mineral soils is consistent with δ98/95Mo measurements on soil and root samples from the Massif Central (Voegelin et al., 2012, Fig. 1) and soil samples paired to local bedrock samples in Hawaii (King et al., 2016).
View in article
Wang, Z., Ma, J., Li, J., Wei, G., Chen, X., Deng, W., Xie, L., Lu, W., Zou, L. (2015) Chemical weathering controls on variations in the molybdenum isotopic composition of river water: Evidence from large rivers in China. Chemical Geology 410, 201-212.
 Show in context
Show in contextSome of this variability has been linked to the Mo isotope fractionation occurring during chemical weathering and the formation of secondary minerals, such as iron (Fe) and manganese (Mn) (oxyhydr)oxides (Pearce et al., 2010; Wang et al., 2015, 2018).
View in article
Wang, Z., Ma, J., Li, J., Wei, G., Zeng, T., Li, L., Zhang, L., Deng, W., Xie, L., Liu, Z. (2018) Fe (hydro) oxide controls Mo isotope fractionation during the weathering of granite. Geochimica et Cosmochimica Acta 226, 1-17.
 Show in context
Show in context Some of this variability has been linked to the Mo isotope fractionation occurring during chemical weathering and the formation of secondary minerals, such as iron (Fe) and manganese (Mn) (oxyhydr)oxides (Pearce et al., 2010; Wang et al., 2015, 2018).
View in article
We find that experimentally derived fractionation factors for Mo uptake by Fe and Mn (oxyhydr)oxides are consistent with our new data (Fig. 3), supporting inferences from a granitic weathering profile (Wang et al., 2018).
View in article
Yang, J., Siebert, C., Barling, J., Savage, P., Liang, Y.H., Halliday, A.N. (2015) Absence of molybdenum isotope fractionation during magmatic differentiation at Hekla volcano, Iceland. Geochimica et Cosmochimica Acta 162, 126-136.
 Show in context
Show in contextThe starting isotope ratios of Mo-bearing phases can vary, with contrasts between igneous and sedimentary rocks, where the δ98/95Mo values of the latter depend on the redox state of the depositional environment, and can vary by ~2 ‰ at the outcrop scale (Pearce et al., 2010; Yang et al., 2015; Kendall et al., 2017; Neely et al., 2018; Li et al., 2019).
View in article
These contrast with published rocks from Iceland (-0.15 ± 0.01 ‰; Yang et al., 2015) and our river bed materials from the Mackenzie Basin (0.38 ± 0.14 ‰, n = 4) (Table S-1).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1. Sampling Locations
- 2. Sampling Methods
- 3. Analytical Methods
- 4. Supplementary Discussion of Soil and Suspended Sediment δ98/95Mo Values
- 5. Quantifying Mo Removal from Solution and the Mo Fractionation Model
- Tables S-1 to S-5
- Figures S-1 to S-6
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
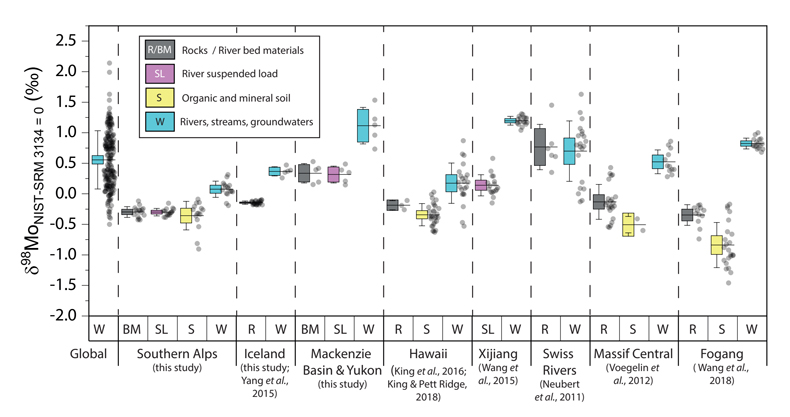
Figure 1 Molybdenum isotope ratios (δ98/95Mo, NIST-SRM3134 = 0 ‰) for this study (Southern Alps, Iceland, Mackenzie Basin and Yukon), alongside published measurements with: R = rocks, BM = river bed materials (grey); SL = suspended load (pink); S = soils (yellow); W = water (blue). Measurements are shown as grey dots, bars show the ±2 s.e. and whiskers ±2 s.d.
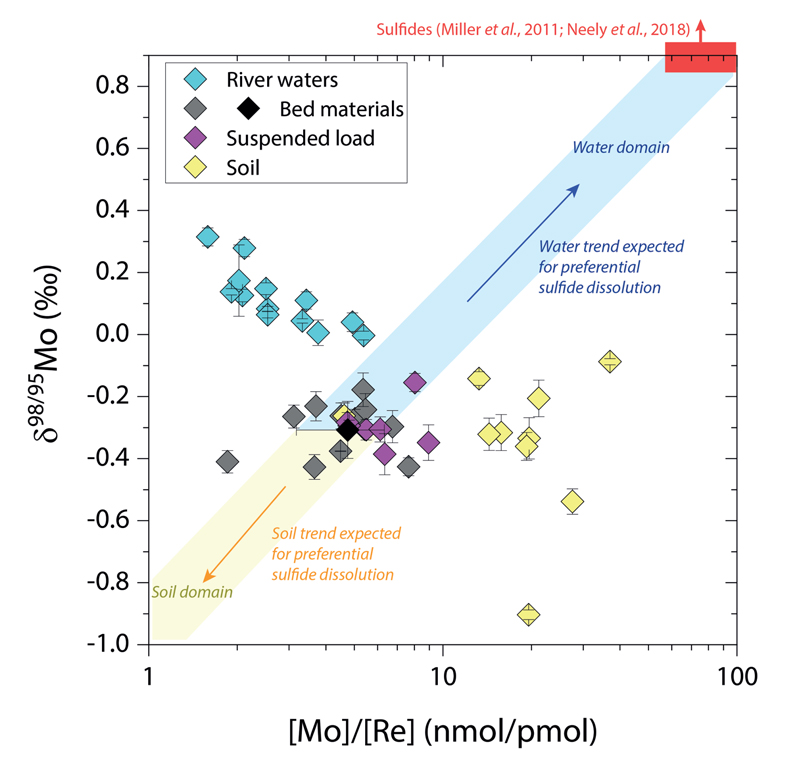
Figure 2 The Mo isotope ratios of materials from the western Southern Alps, New Zealand, versus the Mo to Re concentration ratios for river waters (light blue), river bed materials (grey), suspended load (purple) and soils (yellow). Black diamond is the mean of the bed material samples. Shaded domains show the expected fields of soil and water compositions if preferential dissolution of sulfides was occuring, but data lie perpendicular to this trend implying an alternative mechanism is responsible for fractionation patterns observed.
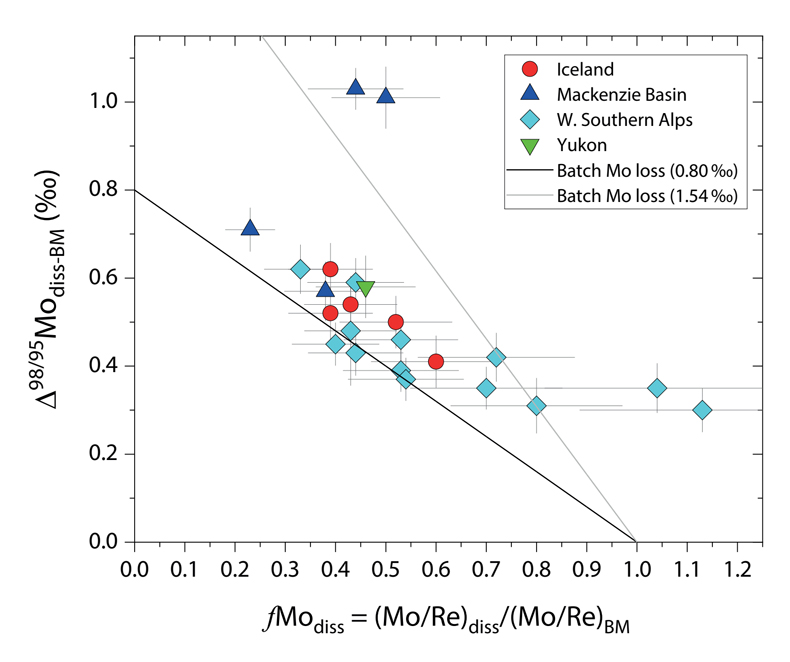
Figure 3 The fraction of Mo remaining in river water, fModiss, estimated using the ratio of Mo to rhenium (Re) in the dissolved load relative to parent materials, versus the difference in δ98/95Mo between river water and river bed materials. Lines are a batch fractionation model using fractionation factors between a solution and secondary mineral phases, based on fractionation factors of -0.8 ‰ (black) to -1.4 ‰ (grey) (Goldberg et al., 2009
Goldberg, T., Archer, C., Vance, D., Poulton, S.W. (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr) oxides. Geochimica et Cosmochimica Acta 73, 6502-6516.
; Barling and Anbar, 2004Barling, J., Anbar, A.D. (2004) Molybdenum isotope fractionation during adsorption by manganese oxides. Earth and Planetary Science Letters 217, 315-329.
). Error bars indicated for fModiss are the propagated 2 s.e. errors on (Mo/Re)BM, which is the main source of uncertainty. Error bars for Δ98/95Modiss-BM incorporate the 2 s.d analytical error on δ98/95Modiss and the 2 s.e. of the mean δ98/95MoBM.