Past endolithic life in metamorphic ocean crust
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:446Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
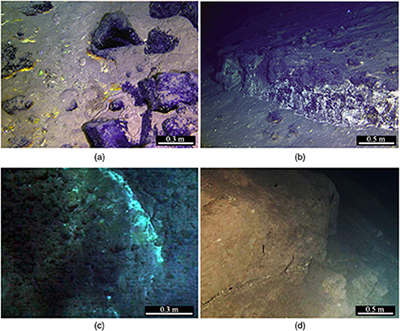 Figure 1 Representative metamorphic ocean crust on the north (a-b) and south (c-d) slopes of the SMT. (a) Yellow-green zeolite rocks coated by black manganese crusts (Dive JL118; 6695 mbsl). (b) White-yellow zeolite rock terrace (Dive JL119; 6001 mbsl). (c) Bluish-green celadonite rock outcrop (Dive JL122; 6300 mbsl). (d) Yellowish zeolite mound (Dive JL122; 6296 mbsl). Zeolite rocks in yellow indicate the presence of ferric iron. | 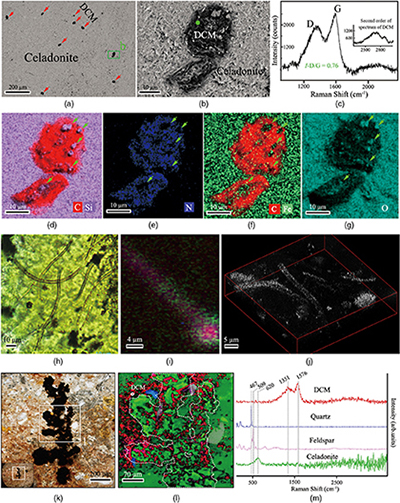 Figure 2 Representative carbonaceous spheroids (a-g; sample JL122-G01), filaments (h-j; sample JL122-G01), and Frutexites-like structures (k-m; sample JL122-G02) preserved in metamorphic rocks. (a) SEM-BSE image showing the distribution of spheroids composed of disordered carbonaceous matter (DCM, red arrows) in celadonite. (b) Enlarged view of DCM shown with a green rectangle in (a). (c) Raman spectrum collected at location marked by a green spot in (b), showing the characteristic first order (D band at ∼1365 cm−1 and G band at ∼1590 cm−1) and second order (between ∼2500 and 3000 cm−1) bands of DCM. (d–g) SEM-EDS images corresponding to (b), showing that DCM comprising of carbon (C) and nitrogen (N) is embedded by celadonite with celadonite grains on the inside (green arrows). (h) Transmitted white light photomicrograph of filaments. (i) Micro-Raman image of a filament. Reds and greens represent DCM and celadonite, respectively. (j) Three dimensional CLSM image of filaments using 488 nm laser excitation. (k) Transmitted white light photomicrographs of Frutexites-like structures. (l) Micro-Raman image of the white box shown in (k). White dotted lines indicate the contour of Frutexites-like structure. Reds, greens, blues and pinks represent DCM, celadonite, quartz and feldspar, respectively. (m) Average Raman spectra of DCM and minerals from (l). | 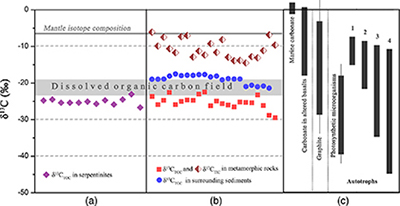 Figure 3 (a) Total organic carbon isotopes in serpentinites from Iberian Margin (Schwarzenbach et al., 2013). (b) Carbon isotopes in zeolite-rich metamorphic rocks and surrounding sediments from the SMT. (c) Carbon isotope variations found in nature (Schidlowski, 2001). The right hand column shows carbon isotope fractionation variations in four different carbon fixation pathways by autotrophic microorganisms (1: 3-hydroxypropionate cycle; 2: reductive tricarboxylic acid (rTCA) cycle; 3: Calvin-Benson-Bassham cycle; 4: reductive acetyl-CoA pathway). Data for the dissolved organic carbon field are from Bauer (2002). | 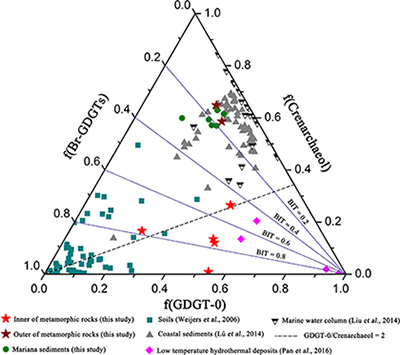 Figure 4 Ternary diagram showing the relationships of the fractional abundances of acyclic glycerol dibiphytanyl glycerol tetraether lipids (GDGT-0), crenarchaeol and sum of all branched GDGTs (Br-GDGTs) in different environments. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Letter
The deep subsurface biosphere on Earth exists in a variety of hydrated subsurface regions with sufficient energy gradients (Orsi, 2018
Orsi, W.D. (2018) Ecology and evolution of seafloor and subseafloor microbial communities. Nature Reviews Microbiology, doi:10.1038/s41579-018-0046-8.
). Furnes et al. (2008)Furnes, H., Mcloughlin, N., Muehlenbachs, K., Banerjee, N., Staudigel, H., Dilek, Y, De Wit, M., Van Kranendonk, M., Schiffman, P. (2008) Oceanic pillow lavas and hyaloclastites as habitats formicrobial life through time – a review. In: Dilek, Y., Furnes, H., Muehlenbachs, K. (Eds.) Links Between Geological Processes, Microbial Activities and Evolution of Life. Springer Verlag, Berlin, 1–68.
reviewed the microbial alteration textures in the meta-volcanic glasses and crusts of pillow lavas from submarine environments, showing that textural signatures and chemical tracers of microbial life can be well preserved in metamorphic rocks. Based on a heat conduction model, Plümper et al. (2017)Plümper, O., King, H.E., Geisler, T., Liu, Y., Pabst, S., Savov, I.P., Rost, D., Zack, T. (2017) Subduction zone forearc serpentinites as incubators for deep microbial life. Proceedings of the National Academy of Sciences of the United States of America 114, 4324–4329.
estimated that microbial life could exist as deep as ∼10 km below the seafloor. However, little is known about whether microbial life thrives in metamorphic rocks that form under low temperature, high pressure conditions at a convergent margin during the descent of a subducting plate. Among these metamorphic rocks, zeolite facies rocks (Fig. S-1) are first formed from metamorphism of basaltic rocks in subduction zones at depths of 1–14 km beneath the seafloor and temperatures of approximately 40–300 °C (Marshak, 2009Marshak, S. (2009) Essentials of Geology.
Takai, K., Nakamura, K., Toki, T., Tsunogai, U., Miyazaki, M., Miyazaki, J., Hirayama, H., Nakagawa, S., Nunoura, T., Horikoshi, K. (2008) Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proceedings of the National Academy of Sciences of the United States of America 105, 10949–10954.
), making it the maximum metamorphic grade that can potentially host life in subduction zones. Thus, zeolite facies rocks in subduction zones may provide promising habitats for subsurface piezophilic microorganisms.To test this hypothesis, we recovered metamorphic rocks that have been tectonically extruded through deep faults at the seafloor from the subduction zone of the southern Mariana trench (SMT) at a water depth of 5500 to 6800 m (Fig. S-2, Table S-1), using the manned submersible Jiaolong during two R/V XYH09 cruises (June–July 2016 and May–June 2017). Extensive outcrops of metamorphic rocks were observed on the south and north slopes of the SMT (Fig. 1). X-ray diffraction (XRD) analyses show that these metamorphic rocks mainly consist of phillipsite, heulandite, gmelinite, analcite, natrolite, chabasite, chlorite, and celadonite (Table S-1), a mineral assemblage indicative of low grade zeolite facies metamorphism. The oxygen isotopic composition of carbonates extracted from these rocks (δ18OSMOW = 13.9 to 25.3 ‰) reveals that they have been altered at temperatures between 20 °C and 89 °C assuming equilibrium with 18O-depleted fluids (−4 ‰; Table S-2).

Figure 1 Representative metamorphic ocean crust on the north (a-b) and south (c-d) slopes of the SMT. (a) Yellow-green zeolite rocks coated by black manganese crusts (Dive JL118; 6695 mbsl). (b) White-yellow zeolite rock terrace (Dive JL119; 6001 mbsl). (c) Bluish-green celadonite rock outcrop (Dive JL122; 6300 mbsl). (d) Yellowish zeolite mound (Dive JL122; 6296 mbsl). Zeolite rocks in yellow indicate the presence of ferric iron.
Transmitted white light and scanning electron microscopy (SEM) observations revealed a high abundance of spheroidal objects with diameters of ca. 2–150 μm in zeolite and celadonite (Figs. 2a,b, S-3, S-4). They exhibit loose, net-like, or condensed-gel structures (Fig. S-4d). Nano-scale secondary ion mass spectrometry (NanoSIMS) and energy dispersive X-ray spectroscopy (EDS) element maps show that the spheroids are chiefly composed of carbon (C), nitrogen (N) and phosphorus (P), whereas the matrix is zeolite or celadonite (Figs. 2d–g, S-3c,f, S-4e, S-5). Raman spectra of the carbonaceous spheroids are characterised by two broad and overlapped first order bands, assigned as D and G, and a very wide second order band of disordered carbonaceous matter (DCM; Fig. 2c). The morphologies and chemical compositions of the SMT carbonaceous spheroids resemble those of a fossilised microbial community found in serpentinised mantle rocks (Klein et al., 2015
Klein, F., Humphris, S.E., Guo, W., Schubotz, F., Schwarzenbach, E.M., Orsi, W.D. (2015) Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. Proceedings of the National Academy of Sciences of the United States of America 112, 12036–12041.
; Plümper et al., 2017Plümper, O., King, H.E., Geisler, T., Liu, Y., Pabst, S., Savov, I.P., Rost, D., Zack, T. (2017) Subduction zone forearc serpentinites as incubators for deep microbial life. Proceedings of the National Academy of Sciences of the United States of America 114, 4324–4329.
).Microscopic filaments, which are composed of DCM (Figs. 2i,j, S-6b; Video S-1), are also present in the SMT metamorphic rocks (Figs. 2h, S-6a,c,d). These filaments, ∼2.0 μm wide and ∼30–200 μm long, are typically tubular, non-septate and unbranched. The tubular nature of the carbonaceous filaments, the existence of a “hollow” lumina shown in Video S-1, and the range of widths exhibited by the filaments, are consistent with a biological origin (Buick, 1990
Buick, R. (1990) Microfossil Recognition in Archean Rocks: An Appraisal of Spheroids and Filaments from a 3500 M.Y. Old Chert-Barite Unit at North Pole, Western Australia. Palaios 5, 441–459.
). Morphologically, they resemble microbial filaments found in zeolite-carbonate interfaces in sub-seafloor basalts (Ivarsson et al., 2008Ivarsson, M., Lindblom, S., Broman, C., Holm, N.G. (2008) Fossilized microorganisms associated with zeolite–carbonate interfaces in sub-seafloor hydrothermal environments. Geobiology 6, 155–170.
).Millimetre scale Frutexites-like structures composed of zeolite and celadonite also occur in the matrix (Figs. 2k,l, S-7; see Supplementary Information). The close spatial associations and bulbous arborescent morphology of the SMT Frutexites-like structures, as well as the occurrence of carbonaceous matter concentrated in zeolite and celadonite minerals, indicate that the organic matter may have been formed by microbial activity during subduction metamorphism. Similar Frutexites-like structures were previously found in subsurface rock samples and attributed to chemoautotrophic communities (Heim et al., 2017
Heim, C., Queâric, N-V., Ionescu, D., Schaèfer, N., Reitner, J. (2017) Frutexites-like structures formed by iron oxidizing biofilms in the continental subsurface (Äspö Hard Rock Laboratory, Sweden). PLoS ONE 12, e0177542.
).
Figure 2 Representative carbonaceous spheroids (a-g; sample JL122-G01), filaments (h-j; sample JL122-G01), and Frutexites-like structures (k-m; sample JL122-G02) preserved in metamorphic rocks. (a) SEM-BSE image showing the distribution of spheroids composed of disordered carbonaceous matter (DCM, red arrows) in celadonite. (b) Enlarged view of DCM shown with a green rectangle in (a). (c) Raman spectrum collected at location marked by a green spot in (b), showing the characteristic first order (D band at ∼1365 cm−1 and G band at ∼1590 cm−1) and second order (between ∼2500 and 3000 cm−1) bands of DCM. (d–g) SEM-EDS images corresponding to (b), showing that DCM comprising of carbon (C) and nitrogen (N) is embedded by celadonite with celadonite grains on the inside (green arrows). (h) Transmitted white light photomicrograph of filaments. (i) Micro-Raman image of a filament. Reds and greens represent DCM and celadonite, respectively. (j) Three dimensional CLSM image of filaments using 488 nm laser excitation. (k) Transmitted white light photomicrographs of Frutexites-like structures. (l) Micro-Raman image of the white box shown in (k). White dotted lines indicate the contour of Frutexites-like structure. Reds, greens, blues and pinks represent DCM, celadonite, quartz and feldspar, respectively. (m) Average Raman spectra of DCM and minerals from (l).
Geochemical analyses show that contents of total organic carbon (TOC) in metamorphic rocks range from 0.008 wt. % to 0.379 wt. %, while contents of total inorganic carbon (TIC) range from 0.004 wt. % to 0.141 wt. %. δ13CTOC values vary between −22.4 ‰ and −29.5 ‰ (average = −25.2 ‰), whereas TIC is more enriched in 13C (δ13CTIC = −14.5 ‰ to −6.1 ‰; Table S-2). Carbonates derived from circulating unmodified seawater and the mantle typically have δ13C values of approximately 0 and −7 ‰, respectively (Fig. 3; Deines, 2002
Deines, P. (2002) The carbon isotope geochemistry of mantle xenoliths. Earth-Science Reviews 58, 247–278.
). The low δ13CTIC values and their large shift in the SMT metamorphic rocks indicate that organic matter was oxidised into 12C-enriched CO2, which shifted the isotopic values of the dissolved inorganic carbon pool from which carbonate precipitated. Similar δ13CTIC values were also recorded in altered basalts on the Costa Rica Rift and attributed to microbial metabolic processes (Torsvik et al., 1998Torsvik, T., Furnes, H., Muehlenbachs, K., Thorseth, I.H., Tumyr, O. (1998) Evidence for microbial activity at the glass-alteration interface in oceanic basalts. Earth and Planetary Science Letters 162, 165–176.
). The δ13CTOC values recorded in the SMT metamorphic rocks are typically lower than δ13CTOC values (−17.5 to −21.3 ‰) of the surrounding sediments (Fig. 3b; Table S-2). This clearly indicates that the source of organic carbon in such rocks is different from those in the surrounding sediments. Abiotic organic synthesis in the oceanic lithosphere, including Fischer-Tropsch-type reactions (150–350 °C), re-speciation and high temperature reactions (>400–500 °C) of C-O-H fluids during magma cooling, and carbonate decomposition to CH4 (<800 °C), may also produce organic matter with a similar range of δ13C values, but they dominantly form methane and short chain hydrocarbons (Andreani and Ménez, 2019Andreani, M., Ménez, B. (2019) New Perspectives on Abiotic Organic Synthesis and Processing during Hydrothermal Alteration of the Oceanic Lithosphere. In: Orcutt, B., Daniel, I., Dasgupta, R. (Eds.) Deep Carbon: Past to Present. Cambridge University Press, Cambridge, 447–479.
) and their required temperatures are inconsistent with the low temperature (20–89 °C) zeolite facies rocks studied here. Although some evidence suggests abiotic carbonaceous matter can be formed during low temperature alteration of ocean crust (<150 °C), it would have a distinct chemical composition containing aromatic carbon and short aliphatic chains (Sforna et al., 2018Sforna, M.C., Brunelli, D., Pisapia, C., Pasini, V., Malferrari, D., Ménez, B. (2018) Abiotic formation of condensed carbonaceous matter in the hydrating oceanic crust. Nature Communications 9, 5049.
). Raman analysis shows that the intensity ratio of the D and G bands for DCM is 0.76 (Fig. 2c), which is distinctly higher than that for graphite from the Loch Maree Group (0.21), but falls within those for organic matter from the Applecross, Aultbea and Cailleach Head Formations (0.47–1.02; Muirhead et al., 2016Muirhead, D.K., Parnell, J., Spinks, S., Bowden, S.A. (2016) Characterization of organic matter in the Torridonian using Raman spectroscopy. In: Brasier, A.T., McIlroy, D., McLoughlin, N. (Eds.) Earth System Evolution and Early Life: A Celebration of the Work of Martin Brasier. Geological Society, London, Special Publications 448, 71–80.
). This clearly indicates that such organic carbon in SMT metamorphic rocks is not graphite. Similar δ13CTOC values were found in serpentinite muds from mud volcanoes on the Mariana forearc (Eickenbusch et al., 2019Eickenbusch, P., Takai, K., Sissman, O., Suzuki, S., Menzies, C., Sakai, S., Sansjofre, P., Tasumi, E., Bernasconi, S.M., Glombitza, C., Jørgensen, B.B., Morono, Y., Lever, M.A. (2019) Origin of Short-Chain Organic Acids in Serpentinite Mud Volcanoes of the Mariana Convergent Margin. Frontiers in Microbiology 10, 1729.
), and serpentinites from the Iberian Margin (Schwarzenbach et al., 2013Schwarzenbach, E.M., Früh-Green, G.L., Bernasconi, S.M., Alt, J.C., Plas, A. (2013) Serpentinization and carbon sequestration: A study of two ancient peridotite-hosted hydrothermal systems. Chemical Geology 351, 115–133.
). Alongside an isotopic offset of TOC relative to marine dissolved organic carbon, Raman and lipid biomarker analyses provide strong evidence that such organic carbon is primarily derived from chemolithoautotrophic microorganisms instead of abiotic production (Schwarzenbach et al., 2013Schwarzenbach, E.M., Früh-Green, G.L., Bernasconi, S.M., Alt, J.C., Plas, A. (2013) Serpentinization and carbon sequestration: A study of two ancient peridotite-hosted hydrothermal systems. Chemical Geology 351, 115–133.
; Klein et al., 2015Klein, F., Humphris, S.E., Guo, W., Schubotz, F., Schwarzenbach, E.M., Orsi, W.D. (2015) Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. Proceedings of the National Academy of Sciences of the United States of America 112, 12036–12041.
). Thus, carbon isotope data suggest that this organic carbon in SMT metamorphic rocks is of biological origin.
Figure 3 (a) Total organic carbon isotopes in serpentinites from Iberian Margin (Schwarzenbach et al., 2013
Schwarzenbach, E.M., Früh-Green, G.L., Bernasconi, S.M., Alt, J.C., Plas, A. (2013) Serpentinization and carbon sequestration: A study of two ancient peridotite-hosted hydrothermal systems. Chemical Geology 351, 115–133.
). (b) Carbon isotopes in zeolite-rich metamorphic rocks and surrounding sediments from the SMT. (c) Carbon isotope variations found in nature (Schidlowski, 2001Schidlowski, M. (2001) Carbon isotopes as biogeochemical recorders of life over 3.8 Ga of Earth history: evolution of a concept. Precambrian Research 106, 17–134.
). The right hand column shows carbon isotope fractionation variations in four different carbon fixation pathways by autotrophic microorganisms (1: 3-hydroxypropionate cycle; 2: reductive tricarboxylic acid (rTCA) cycle; 3: Calvin-Benson-Bassham cycle; 4: reductive acetyl-CoA pathway). Data for the dissolved organic carbon field are from Bauer (2002)Bauer, J.E. (2002) In: Hansell, D.A., Carlson, C.A. (Eds.) Biogeochemistry of Marine Dissolved Organic Matter: Carbon isotopic composition of DOM. Academic Press, Cambridge, 405–455.
.Glycerol dibiphytanyl glycerol tetraether lipids (GDGTs) analyses reveal that the GDGTs concentrations in the outer portions of metamorphic rocks (OPMR; GDGT-0: 5.445–8.784 ng/g; crenarchaeol: 14.527–17.427 ng/g; branched GDGT: 2.471–3.538 ng/g) are comparable to those of the surrounding sediments (GDGT-0: 4.939–11.385 ng/g; crenarchaeol: 10.783–26.826 ng/g; branched GDGT: 2.196–7.665 ng/g), but notably higher than those in the inner portions of metamorphic rocks (IPMR; GDGT-0: 0.086–0.800 ng/g; crenarchaeol: 0.012–0.192 ng/g; branched GDGT: 0.044–0.597 ng/g; Table S-3). Crenarchaeol is considered to be a specific biomarker for Thaumarchaeota that grows chemoautotrophically by utilising ammonium or other electron donors (Pester et al., 2011
Pester, M., Schleper, C., Wagner, M. (2011) The Thaumarchaeota: an emerging view of their phylogeny and Ecophysiology. Current Opinion in Microbiology 14, 300–306.
), whereas GDGT-0 is most likely to be synthesised by both Thaumarchaeota and methanogenic archaea in deep subseafloor environments that grow chemoautotrophically by utilising H2 (Weijers et al., 2009Weijers, J.W.H., Blaga, C.I., Werne, J.P., Sinninghe Damsté, J.S. (2009) Microbial membrane lipids in lake sediments as a paleothermometer. PAGES news 17, 102–104.
). Crenarchaeol and branched GDGTs can be produced in sub-seafloor environments (Lincoln et al., 2013Lincoln, S.A., Bradley, A.S., Newman, S.A., Summons, R.E. (2013) Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in chimneys of the Lost City Hydrothermal Field. Organic Geochemistry 60, 45–53.
; Pan et al., 2016Pan, A.Y., Yang, Q.H., Zhou, H.Y., Ji, F.W., Wang, H., Pancost, R.D. (2016) A diagnostic GDGT signature for the impact of hydrothermal activity on surface deposits at the Southwest Indian Ridge. Organic Geochemistry 99, 90–101.
), even though they are commonly regarded as typical water column and soil biomarkers, respectively. In the OPMR, the GDGT-0 vs. crenarchaeol ratios (GCR; 0.4 to 0.5) are similar to those of the surrounding sediments (0.3–0.5), indicating that Thaumarchaeota is a preponderant group of Archaea (Fig. 4; Schouten et al., 2002Schouten, S., Hopmans, E.C., Schefuss, E., Sinninghe Damste, J.S. (2002) Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth and Planetary Science Letters 204, 265–274.
). In the IPMR, the GCR ranges from 1.4 to 59.5 indicating a substantial methanogenic source for GDGT-0 (Weijers et al., 2009Weijers, J.W.H., Blaga, C.I., Werne, J.P., Sinninghe Damsté, J.S. (2009) Microbial membrane lipids in lake sediments as a paleothermometer. PAGES news 17, 102–104.
), as detected in Lost City chimneys and Iberia Margin brucite-calcite veins (Lincoln et al., 2013Lincoln, S.A., Bradley, A.S., Newman, S.A., Summons, R.E. (2013) Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in chimneys of the Lost City Hydrothermal Field. Organic Geochemistry 60, 45–53.
; Klein et al., 2015Klein, F., Humphris, S.E., Guo, W., Schubotz, F., Schwarzenbach, E.M., Orsi, W.D. (2015) Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. Proceedings of the National Academy of Sciences of the United States of America 112, 12036–12041.
). In addition, the branched vs. isoprenoid tetraether (BIT) values for the IPMR range from 0.42 to 0.97, with an average value of 0.72 (n = 5; Fig. 4). These values are distinctly higher than those observed for the OPMR (average = 0.15, n = 2) and surrounding sediments (average = 0.19, n = 6), but almost identical to those observed for low temperature hydrothermal deposits (average = 0.63, n = 4; Pan et al., 2016Pan, A.Y., Yang, Q.H., Zhou, H.Y., Ji, F.W., Wang, H., Pancost, R.D. (2016) A diagnostic GDGT signature for the impact of hydrothermal activity on surface deposits at the Southwest Indian Ridge. Organic Geochemistry 99, 90–101.
). Collectively, these tetraether lipid signatures further corroborate the in situ contribution of organic carbon by chemolithoautotrophic life in the IPMR.
Figure 4 Ternary diagram showing the relationships of the fractional abundances of acyclic glycerol dibiphytanyl glycerol tetraether lipids (GDGT-0), crenarchaeol and sum of all branched GDGTs (Br-GDGTs) in different environments.
H2 generated by anaerobic basalt-fluid reactions during low grade metamorphism can function as an electron donor to provide energy for known chemolithoautotrophic life in metamorphic ocean crusts (Du et al., 2019
Du, M., Peng, X., Seyfried Jr, W.E., Ta, K., Guo, Z., Chen, S., Chou, I.M., Li, J., Xu, H. (2019) Fluid discharge linked to bending of the incoming plate at the Mariana subduction zone. Geochemical Perspectives Letters 11, 1–5.
). Ferrous iron in basaltic minerals, such as pyroxene and olivine, can be oxidised to ferric iron through the dissociation of water during metamorphism and result in the generation of H2 (Bach, 2016Bach, W. (2016) Some compositional and kinetic controls on the bioenergetics landscapes in oceanic basement. Frontiers in Microbiology 7, 1–8.
). Previous laboratory experiments demonstrated that H2 could be generated through anaerobic basaltic mineral-water reaction at low to moderate temperatures (30 °C to 200 °C; Stevens and McKinley, 2000Stevens, T.O., McKinley, J.P. (2000) Abiotic controls on H2 production from basalt-water reactions and implications for aquifer biogeochemistry. Environmental Science and Technology 34, 826–831.
). High GCR ranging from 1.4 to 59.5 (Fig. 4), together with trace iron in zeolite facies rocks, strongly support this hypothesis. Besides H2, ammonium could provide another potential electron donor for chemolithoautotrophic life in metamorphic rocks, as indicated by high contents of crenarchaeol and NH4+ (average = 8.8 ppm; Table S-4) in some of metamorphic rocks. Ammonia can be derived from the abiotic mineral catalysed reduction of N2, NO2− and NO3− (Brandes et al., 1998Brandes, J.A., Boctor, N.Z., Cody, G.D., Cooper, B.A., Hazen, R.M., Yoder Jr, H.S. (1998) Abiotic nitrogen reduction on the early Earth. Nature 395, 365–367.
) or microbial nitrate reduction coupled with H2 oxidation (Cowen et al., 2003Cowen, J.P, Giovannoni, S.J., Kenig, F., Johnson, H.P., Butterfield, D., Rappe, M.S., Hutnak, M., Lam, P. (2003) Fluids from aging ocean crust that support microbial life. Science 299, 120–123.
) in the upper ocean crust. These autotrophic metabolisms in the metamorphic crust may evolve during rock aging and decrease with decreasing intensity of fluid-rock reactions (Türke et al., 2018Türke, A., Menez, B., Bach, W. (2018) Comparing biosignatures in aged basalt glass from North Pond, Mid-Atlantic Ridge and the Louisville Seamount Trail, off New Zealand. PLoS ONE 13, e0190053.
).The low grade metamorphic ocean crust described here represents previously underexplored habitats for chemolithoautotrophic life on Earth. From the P-T phase diagram (Fig. S-1), this habitable niche, with temperatures below the upper limit for life (122 °C; Takai et al., 2008
Takai, K., Nakamura, K., Toki, T., Tsunogai, U., Miyazaki, M., Miyazaki, J., Hirayama, H., Nakagawa, S., Nunoura, T., Horikoshi, K. (2008) Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proceedings of the National Academy of Sciences of the United States of America 105, 10949–10954.
), is expected to extend up to approximately 14 km into the ocean lithosphere of the subduction zone, far deeper than either the known basalt-hosted deep biosphere that is dominant in the upper 300–500 m of the ocean crust (Furnes et al., 2008Furnes, H., Mcloughlin, N., Muehlenbachs, K., Banerjee, N., Staudigel, H., Dilek, Y, De Wit, M., Van Kranendonk, M., Schiffman, P. (2008) Oceanic pillow lavas and hyaloclastites as habitats formicrobial life through time – a review. In: Dilek, Y., Furnes, H., Muehlenbachs, K. (Eds.) Links Between Geological Processes, Microbial Activities and Evolution of Life. Springer Verlag, Berlin, 1–68.
), or the ultramafic rock-hosted deep biosphere that is predicted as deep as ∼10 km below the seafloor (Plümper et al., 2017Plümper, O., King, H.E., Geisler, T., Liu, Y., Pabst, S., Savov, I.P., Rost, D., Zack, T. (2017) Subduction zone forearc serpentinites as incubators for deep microbial life. Proceedings of the National Academy of Sciences of the United States of America 114, 4324–4329.
). However, it still remains unclear how long this endolithic life was sustained and to what extent it impacts crustal biogeochemical cycles. Future works in other deep sea localities are needed to further comprehend this deep subsurface ecosystem and its potential role in the deep carbon cycle in the region scale geological context.top
Acknowledgements
We are very grateful to the pilots and crew of the RVXYH09-Jiaolong for their professional service during the two cruises in June to July 2016 and in May to June 2017. Funding for this study was provided by the National key research and development plan of China (2018YFC0309902, 2018YFC0309802 and 2016YFC0302301) and the Doctoral Fund of Hebei Normal University (L2020B24). We acknowledge the Editor Prof. Liane G. Benning, as well as four anonymous reviewers, for their constructive comments that greatly improved the manuscript.
Editor: Liane G. Benning
top
Author Contributions
XP designed research. XP, ZG, MD, KT and HX participated in the cruise. XP, ZG, MD, KT, ADC, DP and HX performed research and analysed data. All authors discussed the results and wrote the paper. XP, ZG, and MD contributed equally to this work.
top
References
Andreani, M., Ménez, B. (2019) New Perspectives on Abiotic Organic Synthesis and Processing during Hydrothermal Alteration of the Oceanic Lithosphere. In: Orcutt, B., Daniel, I., Dasgupta, R. (Eds.) Deep Carbon: Past to Present. Cambridge University Press, Cambridge, 447–479.
 Show in context
Show in contextAbiotic organic synthesis in the oceanic lithosphere, including Fischer-Tropsch-type reactions (150–350 °C), re-speciation and high temperature reactions (>400–500 °C) of C-O-H fluids during magma cooling, and carbonate decomposition to CH4 (<800 °C), may also produce organic matter with a similar range of δ13C values, but they dominantly form methane and short chain hydrocarbons (Andreani and Ménez, 2019) and their required temperatures are inconsistent with the low temperature (20–89 °C) zeolite facies rocks studied here.
View in article
Bach, W. (2016) Some compositional and kinetic controls on the bioenergetics landscapes in oceanic basement. Frontiers in Microbiology 7, 1–8.
 Show in context
Show in contextFerrous iron in basaltic minerals, such as pyroxene and olivine, can be oxidised to ferric iron through the dissociation of water during metamorphism and result in the generation of H2 (Bach, 2016).
View in article
Bauer, J.E. (2002) In: Hansell, D.A., Carlson, C.A. (Eds.) Biogeochemistry of Marine Dissolved Organic Matter: Carbon isotopic composition of DOM. Academic Press, Cambridge, 405–455.
 Show in context
Show in contextThe right hand column shows carbon isotope fractionation variations in four different carbon fixation pathways by autotrophic microorganisms (1: 3-hydroxypropionate cycle; 2: reductive tricarboxylic acid (rTCA) cycle; 3: Calvin-Benson-Bassham cycle; 4: reductive acetyl-CoA pathway). Data for the dissolved organic carbon field are from Bauer (2002).
View in article
Brandes, J.A., Boctor, N.Z., Cody, G.D., Cooper, B.A., Hazen, R.M., Yoder Jr, H.S. (1998) Abiotic nitrogen reduction on the early Earth. Nature 395, 365–367.
 Show in context
Show in contextAmmonia can be derived from the abiotic mineral catalysed reduction of N2, NO2− and NO3− (Brandes et al., 1998) or microbial nitrate reduction coupled with H2 oxidation (Cowen et al., 2003) in the upper ocean crust.
View in article
Buick, R. (1990) Microfossil Recognition in Archean Rocks: An Appraisal of Spheroids and Filaments from a 3500 M.Y. Old Chert-Barite Unit at North Pole, Western Australia. Palaios 5, 441–459.
 Show in context
Show in contextThe tubular nature of the carbonaceous filaments, the existence of a “hollow” lumina shown in Video S-1, and the range of widths exhibited by the filaments, are consistent with a biological origin (Buick, 1990).
View in article
Cowen, J.P, Giovannoni, S.J., Kenig, F., Johnson, H.P., Butterfield, D., Rappe, M.S., Hutnak, M., Lam, P. (2003) Fluids from aging ocean crust that support microbial life. Science 299, 120–123.
 Show in context
Show in contextAmmonia can be derived from the abiotic mineral catalysed reduction of N2, NO2− and NO3− (Brandes et al., 1998) or microbial nitrate reduction coupled with H2 oxidation (Cowen et al., 2003) in the upper ocean crust.
View in article
Deines, P. (2002) The carbon isotope geochemistry of mantle xenoliths. Earth-Science Reviews 58, 247–278.
 Show in context
Show in contextCarbonates derived from circulating unmodified seawater and the mantle typically have δ13C values of approximately 0 and −7 ‰, respectively (Fig. 3; Deines, 2002).
View in article
Du, M., Peng, X., Seyfried Jr, W.E., Ta, K., Guo, Z., Chen, S., Chou, I.M., Li, J., Xu, H. (2019) Fluid discharge linked to bending of the incoming plate at the Mariana subduction zone. Geochemical Perspectives Letters 11, 1–5.
 Show in context
Show in contextH2 generated by anaerobic basalt-fluid reactions during low grade metamorphism can function as an electron donor to provide energy for known chemolithoautotrophic life in metamorphic ocean crusts (Du et al., 2019).
View in article
Eickenbusch, P., Takai, K., Sissman, O., Suzuki, S., Menzies, C., Sakai, S., Sansjofre, P., Tasumi, E., Bernasconi, S.M., Glombitza, C., Jørgensen, B.B., Morono, Y., Lever, M.A. (2019) Origin of Short-Chain Organic Acids in Serpentinite Mud Volcanoes of the Mariana Convergent Margin. Frontiers in Microbiology 10, 1729.
 Show in context
Show in contextSimilar δ13CTOC values were found in serpentinite muds from mud volcanoes on the Mariana forearc (Eickenbusch et al., 2019), and serpentinites from the Iberian Margin (Schwarzenbach et al., 2013).
View in article
Furnes, H., Mcloughlin, N., Muehlenbachs, K., Banerjee, N., Staudigel, H., Dilek, Y, De Wit, M., Van Kranendonk, M., Schiffman, P. (2008) Oceanic pillow lavas and hyaloclastites as habitats formicrobial life through time – a review. In: Dilek, Y., Furnes, H., Muehlenbachs, K. (Eds.) Links Between Geological Processes, Microbial Activities and Evolution of Life. Springer Verlag, Berlin, 1–68.
 Show in context
Show in contextThe deep subsurface biosphere on Earth exists in a variety of hydrated subsurface regions with sufficient energy gradients (Orsi, 2018). Furnes et al. (2008) reviewed the microbial alteration textures in the meta-volcanic glasses and crusts of pillow lavas from submarine environments, showing that textural signatures and chemical tracers of microbial life can be well preserved in metamorphic rocks.
View in article
The low grade metamorphic ocean crust described here represents previously underexplored habitats for chemolithoautotrophic life on Earth. From the P-T phase diagram (Fig. S-1), this habitable niche, with temperatures below the upper limit for life (122 °C; Takai et al., 2008), is expected to extend up to approximately 14 km into the ocean lithosphere of the subduction zone, far deeper than either the known basalt-hosted deep biosphere that is dominant in the upper 300–500 m of the ocean crust (Furnes et al., 2008), or the ultramafic rock-hosted deep biosphere that is predicted as deep as ∼10 km below the seafloor (Plümper et al., 2017).
View in article
Heim, C., Queâric, N-V., Ionescu, D., Schaèfer, N., Reitner, J. (2017) Frutexites-like structures formed by iron oxidizing biofilms in the continental subsurface (Äspö Hard Rock Laboratory, Sweden). PLoS ONE 12, e0177542.
 Show in context
Show in contextSimilar Frutexites-like structures were previously found in subsurface rock samples and attributed to chemoautotrophic communities (Heim et al., 2017).
View in article
Ivarsson, M., Lindblom, S., Broman, C., Holm, N.G. (2008) Fossilized microorganisms associated with zeolite–carbonate interfaces in sub-seafloor hydrothermal environments. Geobiology 6, 155–170.
 Show in context
Show in contextMorphologically, they resemble microbial filaments found in zeolite-carbonate interfaces in sub-seafloor basalts (Ivarsson et al., 2008).
View in article
Klein, F., Humphris, S.E., Guo, W., Schubotz, F., Schwarzenbach, E.M., Orsi, W.D. (2015) Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. Proceedings of the National Academy of Sciences of the United States of America 112, 12036–12041.
 Show in context
Show in contextThe morphologies and chemical compositions of the SMT carbonaceous spheroids resemble those of a fossilised microbial community found in serpentinised mantle rocks (Klein et al., 2015; Plümper et al., 2017).
View in article
Alongside an isotopic offset of TOC relative to marine dissolved organic carbon, Raman and lipid biomarker analyses provide strong evidence that such organic carbon is primarily derived from chemolithoautotrophic microorganisms instead of abiotic production (Schwarzenbach et al., 2013; Klein et al., 2015).
View in article
In the IPMR, the GCR ranges from 1.4 to 59.5 indicating a substantial methanogenic source for GDGT-0 (Weijers et al., 2009), as detected in Lost City chimneys and Iberia Margin brucite-calcite veins (Lincoln et al., 2013; Klein et al., 2015).
View in article
Lincoln, S.A., Bradley, A.S., Newman, S.A., Summons, R.E. (2013) Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in chimneys of the Lost City Hydrothermal Field. Organic Geochemistry 60, 45–53.
 Show in context
Show in contextCrenarchaeol is considered to be a specific biomarker for Thaumarchaeota that grows chemoautotrophically by utilising ammonium or other electron donors (Pester et al., 2011), whereas GDGT-0 is most likely to be synthesised by both Thaumarchaeota and methanogenic archaea in deep subseafloor environments that grow chemoautotrophically by utilising H2 (Weijers et al., 2009). Crenarchaeol and branched GDGTs can be produced in sub-seafloor environments (Lincoln et al., 2013; Pan et al., 2016), even though they are commonly regarded as typical water column and soil biomarkers, respectively.
View in article
In the IPMR, the GCR ranges from 1.4 to 59.5 indicating a substantial methanogenic source for GDGT-0 (Weijers et al., 2009), as detected in Lost City chimneys and Iberia Margin brucite-calcite veins (Lincoln et al., 2013; Klein et al., 2015).
View in article
Liu, X., Zhu, C., Wakeham, S.G., Hinrichs, K.-U. (2014) In situ production of branched glycerol dialkyl glycerol tetraethers in anoxic marine water columns. Marine Chemistry 166, 1–8.
 Show in context
Show in contextFigure 4
View in article
Lü, X., Yang, H., Song, J., Versteegh, G.J.M., Li, X., Yuan, H., Li, N., Yang, C., Yang, Y., Ding, W., Xie, S. (2014) Sources and distribution of isoprenoid glycerol dialkyl glycerol tetraethers (GDGTs) in sediments from the east coastal sea of China: Application of GDGT-based paleothermometry to a shallow marginal sea. Organic Geochemistry 75, 24–35.
 Show in context
Show in contextFigure 4
View in article
Marshak, S. (2009) Essentials of Geology.
 Show in context
Show in contextAmong these metamorphic rocks, zeolite facies rocks (Fig. S-1) are first formed from metamorphism of basaltic rocks in subduction zones at depths of 1–14 km beneath the seafloor and temperatures of approximately 40–300 °C (Marshak, 2009).
View in article
Muirhead, D.K., Parnell, J., Spinks, S., Bowden, S.A. (2016) Characterization of organic matter in the Torridonian using Raman spectroscopy. In: Brasier, A.T., McIlroy, D., McLoughlin, N. (Eds.) Earth System Evolution and Early Life: A Celebration of the Work of Martin Brasier. Geological Society, London, Special Publications 448, 71–80.
 Show in context
Show in contextRaman analysis shows that the intensity ratio of the D and G bands for DCM is 0.76 (Fig. 2c), which is distinctly higher than that for graphite from the Loch Maree Group (0.21), but falls within those for organic matter from the Applecross, Aultbea and Cailleach Head Formations (0.47–1.02; Muirhead et al., 2016).
View in article
Orsi, W.D. (2018) Ecology and evolution of seafloor and subseafloor microbial communities. Nature Reviews Microbiology, doi:10.1038/s41579-018-0046-8.
 Show in context
Show in contextThe deep subsurface biosphere on Earth exists in a variety of hydrated subsurface regions with sufficient energy gradients (Orsi, 2018).
View in article
Pan, A.Y., Yang, Q.H., Zhou, H.Y., Ji, F.W., Wang, H., Pancost, R.D. (2016) A diagnostic GDGT signature for the impact of hydrothermal activity on surface deposits at the Southwest Indian Ridge. Organic Geochemistry 99, 90–101.
 Show in context
Show in contextCrenarchaeol and branched GDGTs can be produced in sub-seafloor environments (Lincoln et al., 2013; Pan et al., 2016), even though they are commonly regarded as typical water column and soil biomarkers, respectively.
View in article
These values are distinctly higher than those observed for the OPMR (average = 0.15, n = 2) and surrounding sediments (average = 0.19, n = 6), but almost identical to those observed for low temperature hydrothermal deposits (average = 0.63, n = 4; Pan et al., 2016).
View in article
Pester, M., Schleper, C., Wagner, M. (2011) The Thaumarchaeota: an emerging view of their phylogeny and Ecophysiology. Current Opinion in Microbiology 14, 300–306.
 Show in context
Show in contextCrenarchaeol is considered to be a specific biomarker for Thaumarchaeota that grows chemoautotrophically by utilising ammonium or other electron donors (Pester et al., 2011), whereas GDGT-0 is most likely to be synthesised by both Thaumarchaeota and methanogenic archaea in deep subseafloor environments that grow chemoautotrophically by utilising H2 (Weijers et al., 2009).
View in article
Plümper, O., King, H.E., Geisler, T., Liu, Y., Pabst, S., Savov, I.P., Rost, D., Zack, T. (2017) Subduction zone forearc serpentinites as incubators for deep microbial life. Proceedings of the National Academy of Sciences of the United States of America 114, 4324–4329.
 Show in context
Show in contextThe deep subsurface biosphere on Earth exists in a variety of hydrated subsurface regions with sufficient energy gradients (Orsi, 2018). Furnes et al. (2008) reviewed the microbial alteration textures in the meta-volcanic glasses and crusts of pillow lavas from submarine environments, showing that textural signatures and chemical tracers of microbial life can be well preserved in metamorphic rocks. Based on a heat conduction model, Plümper et al. (2017) estimated that microbial life could exist as deep as ∼10 km below the seafloor.
View in article
The morphologies and chemical compositions of the SMT carbonaceous spheroids resemble those of a fossilised microbial community found in serpentinised mantle rocks (Klein et al., 2015; Plümper et al., 2017).
View in article
The low grade metamorphic ocean crust described here represents previously underexplored habitats for chemolithoautotrophic life on Earth. From the P-T phase diagram (Fig. S-1), this habitable niche, with temperatures below the upper limit for life (122 °C; Takai et al., 2008), is expected to extend up to approximately 14 km into the ocean lithosphere of the subduction zone, far deeper than either the known basalt-hosted deep biosphere that is dominant in the upper 300–500 m of the ocean crust (Furnes et al., 2008), or the ultramafic rock-hosted deep biosphere that is predicted as deep as ∼10 km below the seafloor (Plümper et al., 2017).
View in article
Schidlowski, M. (2001) Carbon isotopes as biogeochemical recorders of life over 3.8 Ga of Earth history: evolution of a concept. Precambrian Research 106, 17–134.
 Show in context
Show in context(a) Total organic carbon isotopes in serpentinites from Iberian Margin (Schwarzenbach et al., 2013). (b) Carbon isotopes in zeolite-rich metamorphic rocks and surrounding sediments from the SMT. (c) Carbon isotope variations found in nature (Schidlowski, 2001).
View in article
Schouten, S., Hopmans, E.C., Schefuss, E., Sinninghe Damste, J.S. (2002) Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth and Planetary Science Letters 204, 265–274.
 Show in context
Show in contextCrenarchaeol and branched GDGTs can be produced in sub-seafloor environments (Lincoln et al., 2013; Pan et al., 2016), even though they are commonly regarded as typical water column and soil biomarkers, respectively. In the OPMR, the GDGT-0 vs. crenarchaeol ratios (GCR; 0.4 to 0.5) are similar to those of the surrounding sediments (0.3–0.5), indicating that Thaumarchaeota is a preponderant group of Archaea (Fig. 4; Schouten et al., 2002).
View in article
Schwarzenbach, E.M., Früh-Green, G.L., Bernasconi, S.M., Alt, J.C., Plas, A. (2013) Serpentinization and carbon sequestration: A study of two ancient peridotite-hosted hydrothermal systems. Chemical Geology 351, 115–133.
 Show in context
Show in contextFigure 3 (a) Total organic carbon isotopes in serpentinites from Iberian Margin (Schwarzenbach et al., 2013).
View in article
This clearly indicates that such organic carbon in SMT metamorphic rocks is not graphite. Similar δ13CTOC values were found in serpentinite muds from mud volcanoes on the Mariana forearc (Eickenbusch et al., 2019), and serpentinites from the Iberian Margin (Schwarzenbach et al., 2013).
View in article
Alongside an isotopic offset of TOC relative to marine dissolved organic carbon, Raman and lipid biomarker analyses provide strong evidence that such organic carbon is primarily derived from chemolithoautotrophic microorganisms instead of abiotic production (Schwarzenbach et al., 2013; Klein et al., 2015).
View in article
Sforna, M.C., Brunelli, D., Pisapia, C., Pasini, V., Malferrari, D., Ménez, B. (2018) Abiotic formation of condensed carbonaceous matter in the hydrating oceanic crust. Nature Communications 9, 5049.
 Show in context
Show in contextAlthough some evidence suggests abiotic carbonaceous matter can be formed during low temperature alteration of ocean crust (<150 °C), it would have a distinct chemical composition containing aromatic carbon and short aliphatic chains (Sforna et al., 2018).
View in article
Stevens, T.O., McKinley, J.P. (2000) Abiotic controls on H2 production from basalt-water reactions and implications for aquifer biogeochemistry. Environmental Science and Technology 34, 826–831.
 Show in context
Show in contextPrevious laboratory experiments demonstrated that H2 could be generated through anaerobic basaltic mineral-water reaction at low to moderate temperatures (30 °C to 200 °C; Stevens and McKinley, 2000).
View in article
Takai, K., Nakamura, K., Toki, T., Tsunogai, U., Miyazaki, M., Miyazaki, J., Hirayama, H., Nakagawa, S., Nunoura, T., Horikoshi, K. (2008) Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proceedings of the National Academy of Sciences of the United States of America 105, 10949–10954.
 Show in context
Show in contextThe temperature range of zeolite facies formation is partly within the tolerance of life (Takai et al., 2008), making it the maximum metamorphic grade that can potentially host life in subduction zones.
View in article
The low grade metamorphic ocean crust described here represents previously underexplored habitats for chemolithoautotrophic life on Earth. From the P-T phase diagram (Fig. S-1), this habitable niche, with temperatures below the upper limit for life (122 °C; Takai et al., 2008), is expected to extend up to approximately 14 km into the ocean lithosphere of the subduction zone, far deeper than either the known basalt-hosted deep biosphere that is dominant in the upper 300–500 m of the ocean crust (Furnes et al., 2008), or the ultramafic rock-hosted deep biosphere that is predicted as deep as ∼10 km below the seafloor (Plümper et al., 2017).
View in article
Torsvik, T., Furnes, H., Muehlenbachs, K., Thorseth, I.H., Tumyr, O. (1998) Evidence for microbial activity at the glass-alteration interface in oceanic basalts. Earth and Planetary Science Letters 162, 165–176.
 Show in context
Show in contextSimilar δ13CTIC values were also recorded in altered basalts on the Costa Rica Rift and attributed to microbial metabolic processes (Torsvik et al., 1998).
View in article
Türke, A., Menez, B., Bach, W. (2018) Comparing biosignatures in aged basalt glass from North Pond, Mid-Atlantic Ridge and the Louisville Seamount Trail, off New Zealand. PLoS ONE 13, e0190053.
 Show in context
Show in contextThese autotrophic metabolisms in the metamorphic crust may evolve during rock aging and decrease with decreasing intensity of fluid-rock reactions (Türke et al., 2018).
View in article
Weijers, J.W.H., Schouten, S., Spaargaren, O.C., Sinninghe Damsté, J.S. (2006) Occurrence and distribution of tetraether membrane lipids in soils: implications for the use of the TEX86 proxy and the BIT index. Organic Geochemistry 37, 1680–1693.
 Show in context
Show in contextFigure 4
View in article
Weijers, J.W.H., Blaga, C.I., Werne, J.P., Sinninghe Damsté, J.S. (2009) Microbial membrane lipids in lake sediments as a paleothermometer. PAGES news 17, 102–104.
 Show in context
Show in contextCrenarchaeol is considered to be a specific biomarker for Thaumarchaeota that grows chemoautotrophically by utilising ammonium or other electron donors (Pester et al., 2011), whereas GDGT-0 is most likely to be synthesised by both Thaumarchaeota and methanogenic archaea in deep subseafloor environments that grow chemoautotrophically by utilising H2 (Weijers et al., 2009).
View in article
In the OPMR, the GDGT-0 vs. crenarchaeol ratios (GCR; 0.4 to 0.5) are similar to those of the surrounding sediments (0.3–0.5), indicating that Thaumarchaeota is a preponderant group of Archaea (Fig. 4; Schouten et al., 2002). In the IPMR, the GCR ranges from 1.4 to 59.5 indicating a substantial methanogenic source for GDGT-0 (Weijers et al., 2009), as detected in Lost City chimneys and Iberia Margin brucite-calcite veins (Lincoln et al., 2013; Klein et al., 2015).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Materials and Methods
- Supplementary Text
- Tables S-1 to S-4
- Figures S-1 to S-9
- Video S-1
- Supplementary Information References
Download Video S-1 (.avi)
Download the Supplementary Information (PDF)
Figures
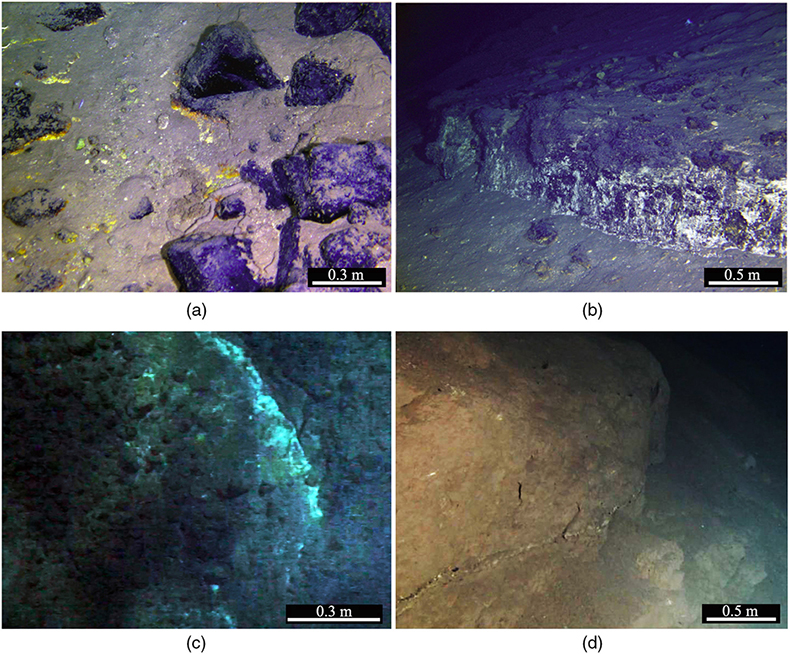
Figure 1 Representative metamorphic ocean crust on the north (a-b) and south (c-d) slopes of the SMT. (a) Yellow-green zeolite rocks coated by black manganese crusts (Dive JL118; 6695 mbsl). (b) White-yellow zeolite rock terrace (Dive JL119; 6001 mbsl). (c) Bluish-green celadonite rock outcrop (Dive JL122; 6300 mbsl). (d) Yellowish zeolite mound (Dive JL122; 6296 mbsl). Zeolite rocks in yellow indicate the presence of ferric iron.
Figure 2 Representative carbonaceous spheroids (a-g; sample JL122-G01), filaments (h-j; sample JL122-G01), and Frutexites-like structures (k-m; sample JL122-G02) preserved in metamorphic rocks. (a) SEM-BSE image showing the distribution of spheroids composed of disordered carbonaceous matter (DCM, red arrows) in celadonite. (b) Enlarged view of DCM shown with a green rectangle in (a). (c) Raman spectrum collected at location marked by a green spot in (b), showing the characteristic first order (D band at ∼1365 cm−1 and G band at ∼1590 cm−1) and second order (between ∼2500 and 3000 cm−1) bands of DCM. (d–g) SEM-EDS images corresponding to (b), showing that DCM comprising of carbon (C) and nitrogen (N) is embedded by celadonite with celadonite grains on the inside (green arrows). (h) Transmitted white light photomicrograph of filaments. (i) Micro-Raman image of a filament. Reds and greens represent DCM and celadonite, respectively. (j) Three dimensional CLSM image of filaments using 488 nm laser excitation. (k) Transmitted white light photomicrographs of Frutexites-like structures. (l) Micro-Raman image of the white box shown in (k). White dotted lines indicate the contour of Frutexites-like structure. Reds, greens, blues and pinks represent DCM, celadonite, quartz and feldspar, respectively. (m) Average Raman spectra of DCM and minerals from (l).
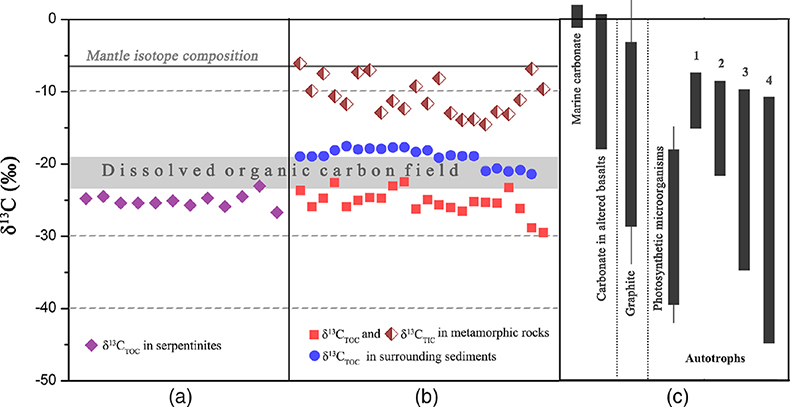
Figure 3 (a) Total organic carbon isotopes in serpentinites from Iberian Margin (Schwarzenbach et al., 2013). (b) Carbon isotopes in zeolite-rich metamorphic rocks and surrounding sediments from the SMT. (c) Carbon isotope variations found in nature (Schidlowski, 2001). The right hand column shows carbon isotope fractionation variations in four different carbon fixation pathways by autotrophic microorganisms (1: 3-hydroxypropionate cycle; 2: reductive tricarboxylic acid (rTCA) cycle; 3: Calvin-Benson-Bassham cycle; 4: reductive acetyl-CoA pathway). Data for the dissolved organic carbon field are from Bauer (2002).
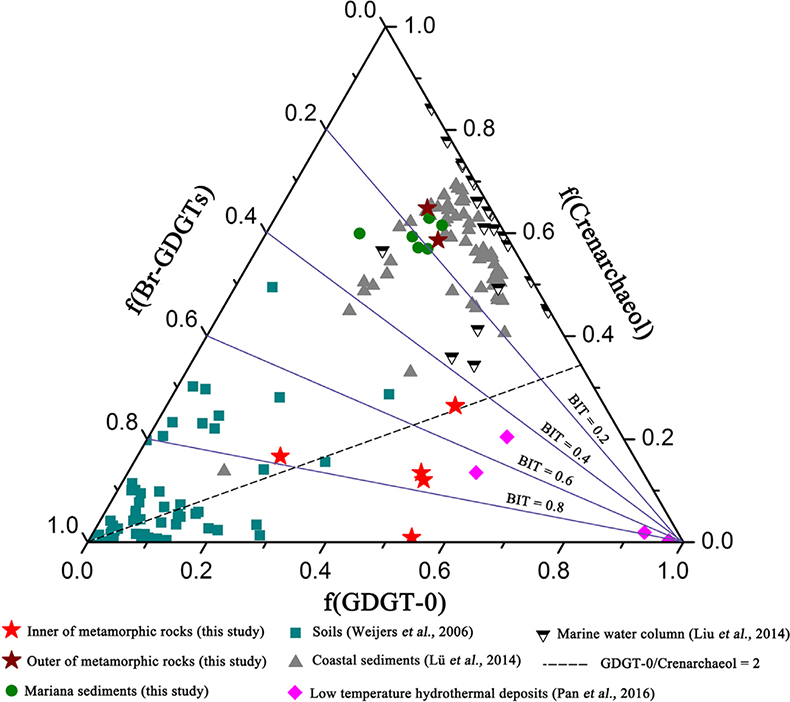
Figure 4 Ternary diagram showing the relationships of the fractional abundances of acyclic glycerol dibiphytanyl glycerol tetraether lipids (GDGT-0), crenarchaeol and sum of all branched GDGTs (Br-GDGTs) in different environments.