Termination of Cryogenian ironstone deposition by deep ocean euxinia
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:629Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
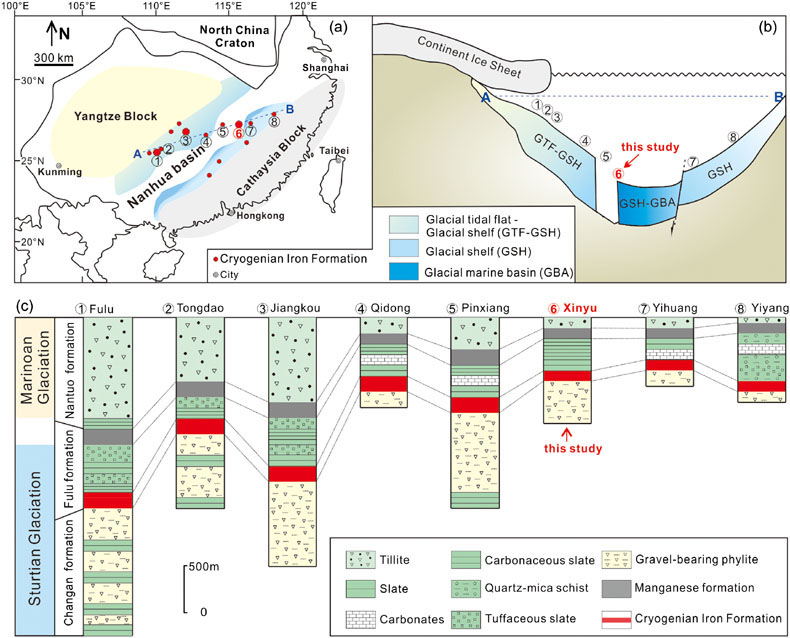
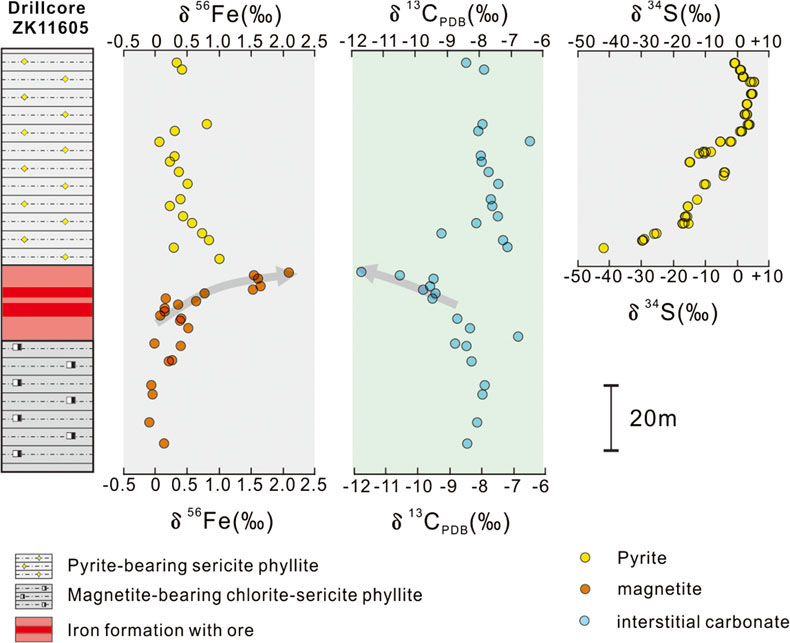
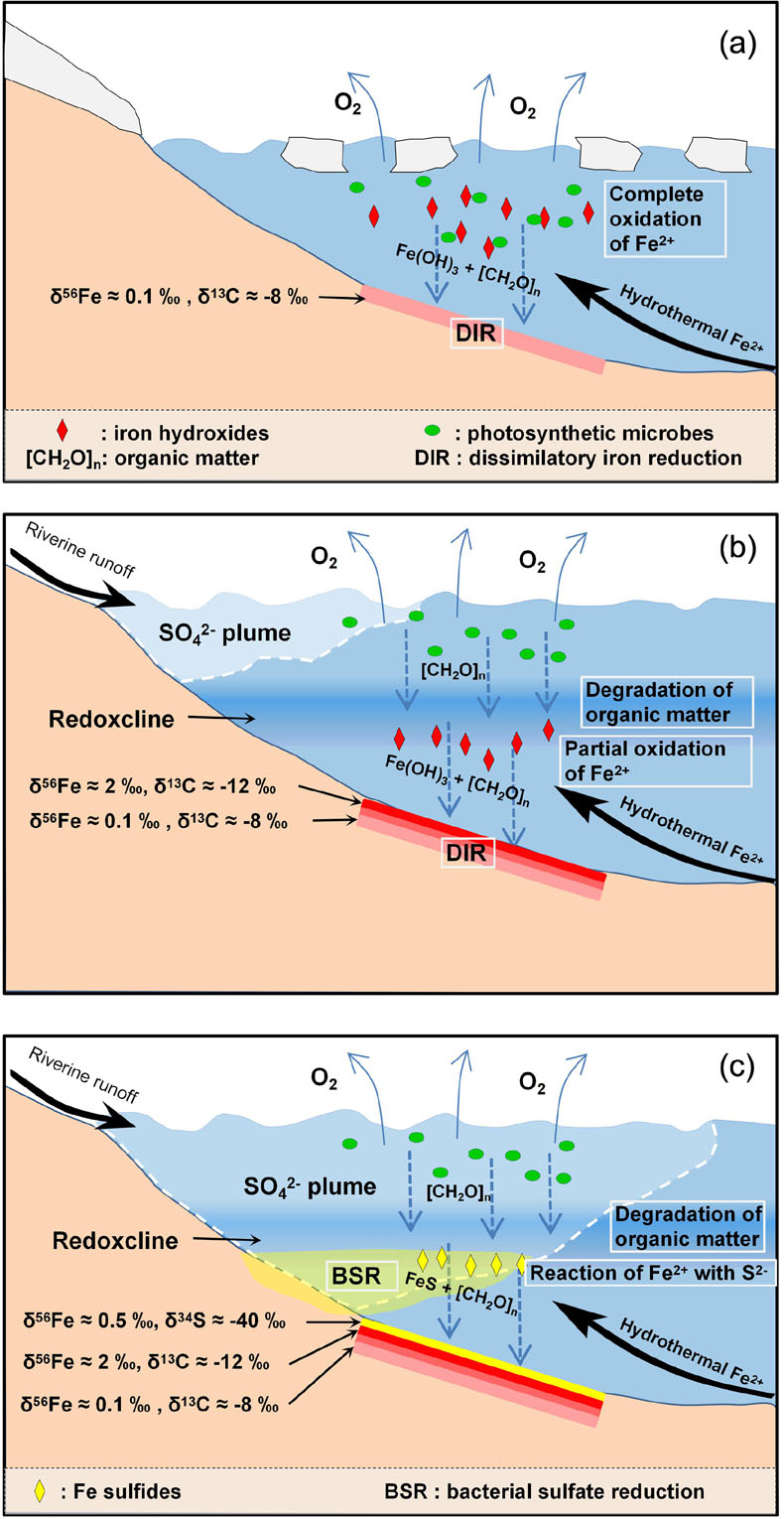
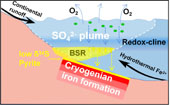
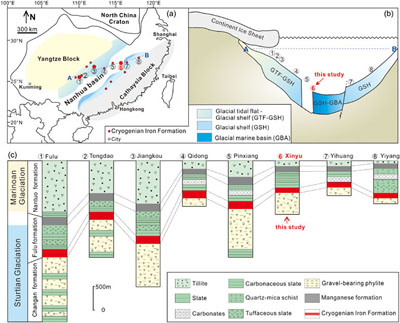 Figure 1 (a) Distribution of Cryogenian Iron Formations in South China and their tectonic setting during the Neoproterozoic. (b) Tectonic background and palaeo-depth of the Cryogenian iron formations, after Wang and Li (2003). (c) Stratigraphic correlations of different CIFs from South China, after Tang et al. (1987). The thickness of iron formations, manganese formations, and carbonate in between are magnified for illustration, and do not represent true thickness. | 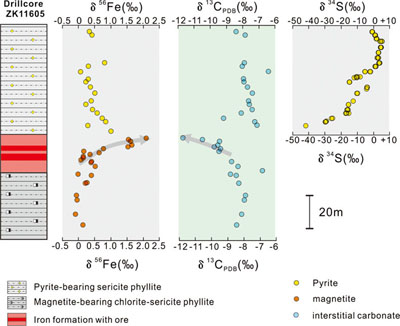 Figure 2 Stratigraphic column and Fe-C-S isotope data along a drill core of the Xinyu CIF. | 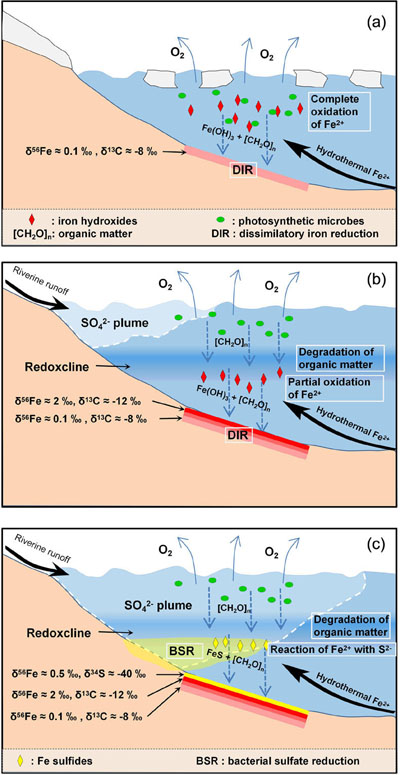 Figure 3 A conceptual model for initiation, development, and termination of Cryogenian iron formations. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
The Cryogenian Period (ca. 720–635 Ma) marks a turning point in the history of Earth’s surficial environment, when seawater changed from a generally anoxic, ferruginous and sulfate-poor state (Canfield et al., 2008
Canfield, D.E., Poulton, S.W., Knoll, A.H., Narbonne, G.M., Ross, G., Goldberg, T., Strauss, H. (2008) Ferruginous Conditions Dominated Later Neoproterozoic Deep-Water Chemistry. Science 321, 949.
; Guilbaud et al., 2015Guilbaud, R., Poulton, S.W., Butterfield, N.J., Zhu, M., Shields-Zhou, G.A. (2015) A global transition to ferruginous conditions in the early Neoproterozoic oceans. Nature Geoscience 8, 466–470.
) to a more oxygenated, Fe-poor and sulfate-rich state (Sahoo et al., 2012Sahoo, S.K., Planavsky, N.J., Kendall, B., Wang, X., Shi, X., Scott, C, Anbar, A.D., Lyons, T.W., Jiang, G. (2012) Ocean oxygenation in the wake of the Marinoan glaciation. Nature 489, 546–549.
; Shields et al., 2019Shields, G.A., Mills, B.J.W., Zhu, M., Raub, T.D., Daines, S.J., Lenton, T.M. (2019) Unique Neoproterozoic carbon isotope excursions sustained by coupled evaporite dissolution and pyrite burial. Nature Geoscience 12, 823–827.
) that progressively came to dominate through the ensuing Phanerozoic Eon (Och and Shields-Zhou, 2012Och, L.M., Shields-Zhou, G.A. (2012) The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Science Reviews 110, 26–57.
). Iron formations were deposited globally during the Cryogenian Period, following an interval of over 1 billion years, during which iron formations were rare (Cox et al., 2013Cox, G.M., Halverson, G.P., Minarik, W.G., Le Heron, D.P., Macdonald, F.A., Bellefroid, E.J., Strauss, J.V. (2013) Neoproterozoic iron formation: An evaluation of its temporal, environmental and tectonic significance. Chemical Geology 362, 232–249.
; Hoffman et al., 2017Hoffman, P.F., Abbot, D.S., Ashkenazy, Y., Benn, D.I., Brocks, J.J., Cohen, P.A., Cox, G.M., Creveling, J.R., Donnadieu, Y., Erwin, D.H., et al. (2017) Snowball Earth climate dynamics and Cryogenian geology-geobiology. Science Advances 3, e1600983.
), but not entirely absent (Canfield et al., 2018Canfield, D.E., Zhang, S., Wang, H., Wang, X., Zhao, W., Su, J., Bjerrum, C.J., Haxen, E.R., and Hammarlund, E.U., Hammarlund, E.U. (2018) A Mesoproterozoic iron formation. Proceedings of the National Academy of Sciences 115, E3895.
). Iron formation deposition requires a reduced ocean to allow effective transport of iron in its soluble form, Fe2+, as well as a mechanism for oxidation of the Fe2+ and accumulation of large masses of Fe oxides/hydroxides. Reappearance of widespread iron deposits during the Cryogenian Period has been taken, therefore, as supporting evidence for the “Snowball Earth” hypothesis, as global ice sheet cover could have led to widespread ocean anoxia (Hoffman and Schrag, 2002Hoffman, P.F., Schrag, D.P. (2002) The snowball Earth hypothesis: testing the limits of global change. Terra Nova 14, 129–155.
; Kirschvink, 1992Kirschvink, J.L. (1992) Late Proterozoic low-latitude global glaciation: The snowball Earth. In: Schopf, J.W., Klein, C. (Eds.) Cambridge University Press, Cambridge.
). The appearance of Cryogenian iron formations (CIFs) provides robust evidence for the return of Fe(II)-rich, anoxic oceans, and by the same token, termination of CIF deposition was likely linked to some fundamental change in ocean chemistry.Was disappearance of the CIFs caused by a decrease in Fe2+ concentration due to ocean oxygenation as seen in modern oceans? Or was it caused by the depletion of free oxygen and development of euxinic (H2S-rich) environments as has been proposed for the Mesoproterozoic (Canfield, 1998
Canfield, D.E. (1998) A new model for Proterozoic ocean chemistry. Nature 396, 450–453.
) and Ediacaran oceans (Li et al., 2010Li, C., Love, G.D., Lyons, T.W., Fike, D.A., Sessions, A.L., Chu, X. (2010) A Stratified Redox Model for the Ediacaran Ocean. Science 328, 80.
)? The details of CIF termination bear on our understanding of the chemistry of Cryogenian oceans, and are critical for a better understanding of the biological radiations and diversification that followed. However, the termination of CIF deposition, its cause and related environmental implications, have been little discussed to date (Cox et al., 2013Cox, G.M., Halverson, G.P., Minarik, W.G., Le Heron, D.P., Macdonald, F.A., Bellefroid, E.J., Strauss, J.V. (2013) Neoproterozoic iron formation: An evaluation of its temporal, environmental and tectonic significance. Chemical Geology 362, 232–249.
). Iron, carbon and sulfur are major players in natural redox processes and show significant isotopic differences between their reduced and oxidised forms. In this study, we present an integrated study of C-S-Fe isotope data from a new drill core record for a large Sturtian-aged CIF from South China that was deposited in a deep marine setting. The completeness of the drill core record is unprecedented for Cryogenian iron formations and the isotopic signatures hold clues about the onset, development, and particularly, termination of CIF deposition.top
Clues from a Deep Water Cryogenian Iron Formation
The CIF from Xinyu, Jiangxi Province, China presents a remarkable opportunity to study ocean chemistry during the Cryogenian glaciation (Tang et al., 1987
Tang, J., Fu, H., Yu, Z. (1987) Stratigraphy, type and formation conditions of the late precambrian banded iron ores in south China. Chinese Journal of Geochemistry 6, 331–341.
; Zhu et al., 2019Zhu, X., Sun, J., Li, Z. (2019) Iron isotopic variations of the Cryogenian banded iron formations: A new model. Precambrian Research 331, 105359.
). The southern margin of the Yangtze Craton of South China hosts a number of stratigraphically correlative CIFs that are associated with successions assigned to the ∼55 Myr, early Cryogenian ‘Sturtian’ ice age (Figs. 1, S-1). The Xinyu CIF, which is the largest among the South China deposits, was deposited offshore within the Nanhua rift basin that separated the Yangtze Craton and the Cathaysia Block during the Neoproterozoic Era. It was deposited in deep water (Wang and Li, 2003Wang, J., Li, Z. (2003) History of Neoproterozoic rift basins in South China: implications for Rodinia break-up. Precambrian Research 122, 141–158.
), and contains magnetite as the major Fe oxide mineral. The Xinyu CIF is uniform in its stratigraphic sequence and can be traced continuously for over 60 km (Fig. S-2), despite local deformation and greenschist facies metamorphism. The Xinyu CIF in the ore district is underlain by a magnetite-bearing, chlorite-sericite phyllite and overlain by a pyrite-bearing sericite phyllite (Figs. 2, S-3). The pyrite-bearing phyllite horizon is used as a faithful marker for the termination of Xinyu CIF by the local iron ore exploration geologists. Magnetite and pyrite in the sericite phyllite are generally disseminated and euhedral to subhedral in shape (Figs. S-4 to S-6). These rocks also contain platy hematite and irregular interstitial carbonates (mostly ankerite) as minor constituents (Figs. S-7, S-8). The lack of overgrowth texture in magnetite and occurrence of minute Fe oxide inclusions in ankerite suggest that the two minerals were products of early diagenesis, rather than late metamorphic events (Figs. S-9, S-10).
Figure 1 (a) Distribution of Cryogenian Iron Formations in South China and their tectonic setting during the Neoproterozoic. (b) Tectonic background and palaeo-depth of the Cryogenian iron formations, after Wang and Li (2003)
Wang, J., Li, Z. (2003) History of Neoproterozoic rift basins in South China: implications for Rodinia break-up. Precambrian Research 122, 141–158.
. (c) Stratigraphic correlations of different CIFs from South China, after Tang et al. (1987)Tang, J., Fu, H., Yu, Z. (1987) Stratigraphy, type and formation conditions of the late precambrian banded iron ores in south China. Chinese Journal of Geochemistry 6, 331–341.
. The thickness of iron formations, manganese formations, and carbonate in between are magnified for illustration, and do not represent true thickness.We characterised the Fe isotope data of magnetite and pyrite, S isotope data of pyrite, and C isotope data of interstitial carbonates from a representative drill core that intersects the strata bearing the Xinyu CIF (Fig. 2). Details of analytical methods are provided in the Supplementary Information. The δ56Fe values cluster around a crustal baseline value of +0.1 ‰ for disseminated magnetite grains in the chlorite-sericite phyllite (Fig. 2) underlying the Xinyu CIF, but values increase monotonically up-section within the Xinyu CIF, reaching a maximum value of +2 ‰ at the top of the CIF. This trend is mirrored by the C isotope values of interstitial carbonates (δ13Ccarb), which decrease from −7 ‰ in the lower magnetite-bearing, chlorite-sericite phyllite to −12 ‰ at the top of the Xinyu CIF. These δ13Ccarb values abruptly increase to around −8 ‰ across the boundary between CIF and the overlying sericite phyllite. This is echoed by a fundamental change in the mineralogy of the disseminated Fe-bearing minerals in the sericite phyllite, whereby magnetite changes to pyrite. The δ56Fe values of the pyrite grains scatter about +0.5 ‰, whereas δ34S values increase from a very low value of −40 ‰ to a high point of +7 ‰, before returning to around 0 ‰ up-section (Fig. 2).

Figure 2 Stratigraphic column and Fe-C-S isotope data along a drill core of the Xinyu CIF.
top
Discussion
The upward trend towards higher δ56Fe values within the Xinyu CIF is similar to previously reported Fe isotope patterns from Xinyu and other ‘Sturtian’ CIFs worldwide (Zhu et al., 2019
Zhu, X., Sun, J., Li, Z. (2019) Iron isotopic variations of the Cryogenian banded iron formations: A new model. Precambrian Research 331, 105359.
). The source of iron in the iron formations is believed to have been hydrothermal fluids that have δ56Fe values close to 0 ‰ or slightly lower (Johnson et al., 2008Johnson, C.M., Beard, B.L., Roden, E.E. (2008) The Iron Isotope Fingerprints of Redox and Biogeochemical Cycling in Modern and Ancient Earth. Annual Review of Earth and Planetary Sciences 36, 457–493.
). The low δ56Fe values in the lower Xinyu CIF likely record periods of efficient oxidation and quantitative removal of hydrothermal Fe(II) that occurred during the early stages of CIF deposition (Fig. 3a). Given that the primary precipitates of Fe(II) oxidation (i.e. Fe(III) oxides/hydroxides) enrich heavy Fe isotopes over aqueous Fe(II) by ∼3–4 ‰ at equilibrium (Johnson et al., 2008Johnson, C.M., Beard, B.L., Roden, E.E. (2008) The Iron Isotope Fingerprints of Redox and Biogeochemical Cycling in Modern and Ancient Earth. Annual Review of Earth and Planetary Sciences 36, 457–493.
), the positive δ56Fe values in iron formations are most parsimoniously explained by partial oxidation of hydrothermal Fe(II) (Johnson et al., 2008Johnson, C.M., Beard, B.L., Roden, E.E. (2008) The Iron Isotope Fingerprints of Redox and Biogeochemical Cycling in Modern and Ancient Earth. Annual Review of Earth and Planetary Sciences 36, 457–493.
; Li et al., 2013Li, W., Czaja, A.D., Van Kranendonk, M.J., Beard, B.L., Roden, E.E., Johnson, C.M. (2013) An anoxic, Fe(II)-rich, U-poor ocean 3.46 billion years ago. Geochimica et Cosmochimica Acta 120, 65–79.
). Increasing δ56Fe up-section in the CIFs, therefore, reflects a decreasing availability of free O2 to oxidise Fe(II)aq in the Cryogenian oceans (Fig. 3b). Decreased O2 production seems unlikely in deglacial oceans, as removal of ice sheets would have allowed photosynthetic organisms to thrive, and moreover, newly resumed global hydrological cycles would have delivered abundant nutrient elements (e.g., P) into the oceans to boost biological productivity (Shields et al., 1997Shields, G., Stille, P., Brasier, M.D., Atudorei, N. (1997) Stratified oceans and oxygenation of the late Precambrian environment: a post glacial geochemical record from the Neoproterozoic of W. Mongolia. Terra Nova 9, 218–222.
; Och and Shields-Zhou, 2012Och, L.M., Shields-Zhou, G.A. (2012) The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Science Reviews 110, 26–57.
). Therefore, development of a redoxcline and decrease in oxidation efficiency (i.e. increase in partial oxidation) of deep water Fe(II) seems a more plausible explanation for the Fe isotope trend recorded in the Xinyu CIF (Fig. 3b).
Figure 3 A conceptual model for initiation, development, and termination of Cryogenian iron formations.
Concomitant with the up-section increase in magnetite δ56Fe, there is a decrease in δ13Ccarb from mantle-like values of around −8 ‰ in the sericite phyllite to values as low as −12 ‰ at the top of the Xinyu CIF. Low δ13Ccarb values in carbonates frequently reflect contributions from organic C that has significantly lower δ13C values than dissolved inorganic carbon. Dissimilatory iron reduction (DIR) by microbes in soft sediments has been identified as an effective mechanism that transforms Fe(III) hydroxides into magnetite and remineralises organic carbon to form low δ13C diagenetic carbonates (Heimann et al., 2010
Heimann, A., Johnson, C.M., Beard, B.L., Valley, J.W., Roden, E.E., Spicuzza, M.J., Beukes, N.J. (2010) Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ∼ 2.5 Ga marine environments. Earth and Planetary Science Letters 294, 8–18.
; Craddock and Dauphas, 2011Craddock, P.R., Dauphas, N. (2011) Iron and carbon isotope evidence for microbial iron respiration throughout the Archean. Earth and Planetary Science Letters 303, 121–132.
). The decreasing δ13Ccarb values within the Xinyu CIF could therefore reflect increasing delivery to the seafloor of organic matter and efficient remineralisation by DIR microbes. The activity of O2-respiring heterotrophic microbes in the water column in the presence of abundant organic matter supply would consume dissolved O2, producing mid-column anoxia, which lowered the intensity of aqueous Fe(II) oxidation, producing high δ56Fe Fe(III) precipitates (Fig. 3b). Therefore, the C isotope record corroborates the Fe isotope evidence for a strengthening of redox stratification during precipitation of the upper part of the Xinyu CIF.The termination of CIF deposition at Xinyu was immediately followed by deposition of rocks containing pyrite with very low (−40 ‰) δ34S values (Fig. 2). Deposition of pyrite requires S2−, which could be provided by microbial reduction of
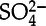 in the presence of organic matter. It is widely accepted that sulfate was depleted in oceans during the Snowball Earth Events (Kump and Seyfried, 2005
in the presence of organic matter. It is widely accepted that sulfate was depleted in oceans during the Snowball Earth Events (Kump and Seyfried, 2005Kump, L.R., Seyfried, W.E. (2005) Hydrothermal Fe fluxes during the Precambrian: Effect of low oceanic sulfate concentrations and low hydrostatic pressure on the composition of black smokers. Earth and Planetary Science Letters 235, 654–662.
). Deglaciation, however, could have provided a source of sulfate via renewed riverine input from continents. The dissolved sulfate was delivered to oceans by rivers, but due to density differences, the sulfate likely spread initially within plumes of freshwater at the surface mixing zone, separated from the organic-rich water body and seafloor below the redoxcline (Fig. 3b). As soon as sulfate could reach beneath the redoxcline, microbes would have been capable of metabolising organic matter via the bacterial sulfate reduction pathway, releasing H2S that reacted with hydrothermal Fe2+ to form low solubility Fe sulfide precipitates (Fig. 3c). Predominance of bacterial sulfate reduction therefore terminated the deposition of Fe(III) hydroxides in CIF.The pyrite grains that precipitated immediately after the CIF have very low (−40 ‰) δ34S values. Experiments have shown that the magnitude of S isotope fractionation associated with bacterial sulfate reduction is controlled by the amount of sulfate, whereby the maximum Δ34Ssulfate-sulfide fractionation factor of ∼45 ‰ occurs in the presence of >1 mM sulfate in water (Habicht and Canfield, 1997
Habicht, K.S., Canfield, D.E. (1997) Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochimica et Cosmochimica Acta 61, 5351–5361.
). Therefore, the very low δ34S signatures of pyrite require that bacterial sulfate reduction started with abundant (>1 mM) dissolved sulfate. The onset of pyrite precipitation after CIF termination implies a rapid delivery of abundant sulfate to deeper oceans. The increase in pyrite δ34S values implies that the consumption of dissolved sulfate by bacterial sulfate reduction outpaced replenishment of sulfate from rivers, leading to a decrease in the global ocean sulfate reservoir, and progressively higher δ34S in residual sulfate.The Fe-C-S isotope record presents a compelling case for termination of CIF deposition by sulfur cycling and extensive development of euxinia in the water column of deglacial Cryogenian oceans. It has been proposed that the termination of major banded iron formations around 1.8 Ga was caused by the development of euxinic oceans (Poulton et al., 2004
Poulton, S.W., Fralick, P.W., Canfield, D.E. (2004) The transition to a sulphidic ocean ∼1.84 billion years ago. Nature 431, 173–177.
). Deposition of pyrite-bearing sediments following ironstone deposition has not been reported from CIF sequences in other parts of the world, so it is possible that the Xinyu CIF is a special case that is different from other CIFs in terms of the pathway of IF termination. This can be explained by the differences in the depth of IF deposition. Xinyu CIF was deposited in a deep marine setting based on sedimentary facies analysis (Tang et al., 1987Tang, J., Fu, H., Yu, Z. (1987) Stratigraphy, type and formation conditions of the late precambrian banded iron ores in south China. Chinese Journal of Geochemistry 6, 331–341.
) and palaeogeographic reconstruction (Wang and Li, 2003Wang, J., Li, Z. (2003) History of Neoproterozoic rift basins in South China: implications for Rodinia break-up. Precambrian Research 122, 141–158.
), therefore the spread of ocean euxinia is recorded by the deep Xinyu CIF, but not in other shallow CIFs as Fe(II) delivery was cut in deeper seawater. A recent study reported widespread euxinia at the end of Marinoan glaciation, whereby the abundance of pyrite nodules was significantly greater in deep settings than in shallow settings (Lang et al., 2018Lang, X., Shen, B., Peng, Y., Xiao, S., Zhou, C., Bao, H., Kaufman, A.J., Huang, K., Crockford, P. W., Liu, Y., et al., (2018) Transient marine euxinia at the end of the terminal Cryogenian glaciation. Nature Communications 9, 3019.
). We therefore argue that the spread of euxinia into deep oceans and titration of hydrothermal Fe2+ by sulfides is a viable mechanism to explain the disappearance of IFs after the Cryogenian Period. If so, the mineralogical and isotopic records of the Xinyu CIF pinpoint the onset of euxinia, which was to be become widespread along productive margins until the Cambrian (Li et al., 2010Li, C., Love, G.D., Lyons, T.W., Fike, D.A., Sessions, A.L., Chu, X. (2010) A Stratified Redox Model for the Ediacaran Ocean. Science 328, 80.
). Isotopic records from the Xinyu CIF also testify to the diversity and prosperity of the ocean microbial community. A microbial ecosystem of diverse metabolisms, including oxygenic photosynthesis, O2-respiring heterotrophism, DIR, and BSR thrived through cycling of redox elements (O-C-S-Fe) in Cryogenian oceans upon deglaciation.top
Acknowledgements
This manuscript benefited from constructive comments from two anonymous reviewers, as well as editorial comments from Dr. Sophie Opfergelt. The authors thank Tianyu Chen and Xuelei Chu for discussions. This study is supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB26020101) to WL, National Key R&D Program of China (Grant No. 2018YFC0603703) to CZW, and Natural Science Foundation of China (No. 41622301 to WL and No. 41872077 to CZW).
Editor: Eric H. Oelkers
top
References
Canfield, D.E. (1998) A new model for Proterozoic ocean chemistry. Nature 396, 450–453.
 Show in context
Show in contextWas disappearance of the CIFs caused by a decrease in Fe2+ concentration due to ocean oxygenation as seen in modern oceans? Or was it caused by the depletion of free oxygen and development of euxinic (H2S-rich) environments as has been proposed for the Mesoproterozoic (Canfield, 1998) and Ediacaran oceans (Li et al., 2010)?
View in article
Canfield, D.E., Poulton, S.W., Knoll, A.H., Narbonne, G.M., Ross, G., Goldberg, T., Strauss, H. (2008) Ferruginous Conditions Dominated Later Neoproterozoic Deep-Water Chemistry. Science 321, 949.
 Show in context
Show in contextThe Cryogenian Period (ca. 720–635 Ma) marks a turning point in the history of Earth’s surficial environment, when seawater changed from a generally anoxic, ferruginous and sulfate-poor state (Canfield et al., 2008; Guilbaud et al., 2015) to a more oxygenated, Fe-poor and sulfate-rich state (Sahoo et al., 2012; Shields et al., 2019) that progressively came to dominate through the ensuing Phanerozoic Eon (Och and Shields-Zhou, 2012).
View in article
Canfield, D.E., Zhang, S., Wang, H., Wang, X., Zhao, W., Su, J., Bjerrum, C.J., Haxen, E.R., and Hammarlund, E.U., Hammarlund, E.U. (2018) A Mesoproterozoic iron formation. Proceedings of the National Academy of Sciences 115, E3895.
 Show in context
Show in contextIron formations were deposited globally during the Cryogenian Period, following an interval of over 1 billion years, during which iron formations were rare (Cox et al., 2013; Hoffman et al., 2017), but not entirely absent (Canfield et al., 2018).
View in article
Cox, G.M., Halverson, G.P., Minarik, W.G., Le Heron, D.P., Macdonald, F.A., Bellefroid, E.J., Strauss, J.V. (2013) Neoproterozoic iron formation: An evaluation of its temporal, environmental and tectonic significance. Chemical Geology 362, 232–249.
 Show in context
Show in contextHowever, the termination of CIF deposition, its cause and related environmental implications, have been little discussed to date (Cox et al., 2013).
View in article
Iron formations were deposited globally during the Cryogenian Period, following an interval of over 1 billion years, during which iron formations were rare (Cox et al., 2013; Hoffman et al., 2017), but not entirely absent (Canfield et al., 2018).
View in article
Craddock, P.R., Dauphas, N. (2011) Iron and carbon isotope evidence for microbial iron respiration throughout the Archean. Earth and Planetary Science Letters 303, 121–132.
 Show in context
Show in contextDissimilatory iron reduction (DIR) by microbes in soft sediments has been identified as an effective mechanism that transforms Fe(III) hydroxides into magnetite and remineralises organic carbon to form low δ13C diagenetic carbonates (Heimann et al., 2010; Craddock and Dauphas, 2011).
View in article
Guilbaud, R., Poulton, S.W., Butterfield, N.J., Zhu, M., Shields-Zhou, G.A. (2015) A global transition to ferruginous conditions in the early Neoproterozoic oceans. Nature Geoscience 8, 466–470.
 Show in context
Show in contextThe Cryogenian Period (ca. 720–635 Ma) marks a turning point in the history of Earth’s surficial environment, when seawater changed from a generally anoxic, ferruginous and sulfate-poor state (Canfield et al., 2008; Guilbaud et al., 2015) to a more oxygenated, Fe-poor and sulfate-rich state (Sahoo et al., 2012; Shields et al., 2019) that progressively came to dominate through the ensuing Phanerozoic Eon (Och and Shields-Zhou, 2012).
View in article
Habicht, K.S., Canfield, D.E. (1997) Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochimica et Cosmochimica Acta 61, 5351–5361.
 Show in context
Show in contextExperiments have shown that the magnitude of S isotope fractionation associated with bacterial sulfate reduction is controlled by the amount of sulfate, whereby the maximum Δ34Ssulfate-sulfide fractionation factor of ∼45 ‰ occurs in the presence of >1 mM sulfate in water (Habicht and Canfield, 1997).
View in article
Heimann, A., Johnson, C.M., Beard, B.L., Valley, J.W., Roden, E.E., Spicuzza, M.J., Beukes, N.J. (2010) Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ∼ 2.5 Ga marine environments. Earth and Planetary Science Letters 294, 8–18.
 Show in context
Show in contextDissimilatory iron reduction (DIR) by microbes in soft sediments has been identified as an effective mechanism that transforms Fe(III) hydroxides into magnetite and remineralises organic carbon to form low δ13C diagenetic carbonates (Heimann et al., 2010; Craddock and Dauphas, 2011).
View in article
Hoffman, P.F., Schrag, D.P. (2002) The snowball Earth hypothesis: testing the limits of global change. Terra Nova 14, 129–155.
 Show in context
Show in contextReappearance of widespread iron deposits during the Cryogenian Period has been taken, therefore, as supporting evidence for the “Snowball Earth” hypothesis, as global ice sheet cover could have led to widespread ocean anoxia (Hoffman and Schrag, 2002; Kirschvink, 1992).
View in article
Hoffman, P.F., Abbot, D.S., Ashkenazy, Y., Benn, D.I., Brocks, J.J., Cohen, P.A., Cox, G.M., Creveling, J.R., Donnadieu, Y., Erwin, D.H., et al. (2017) Snowball Earth climate dynamics and Cryogenian geology-geobiology. Science Advances 3, e1600983.
 Show in context
Show in contextIron formations were deposited globally during the Cryogenian Period, following an interval of over 1 billion years, during which iron formations were rare (Cox et al., 2013; Hoffman et al., 2017), but not entirely absent (Canfield et al., 2018).
View in article
Johnson, C.M., Beard, B.L., Roden, E.E. (2008) The Iron Isotope Fingerprints of Redox and Biogeochemical Cycling in Modern and Ancient Earth. Annual Review of Earth and Planetary Sciences 36, 457–493.
 Show in context
Show in contextThe source of iron in the iron formations is believed to have been hydrothermal fluids that have δ56Fe values close to 0 ‰ or slightly lower (Johnson et al., 2008).
View in article
Given that the primary precipitates of Fe(II) oxidation (i.e. Fe(III) oxides/hydroxides) enrich heavy Fe isotopes over aqueous Fe(II) by ∼3–4 ‰ at equilibrium (Johnson et al., 2008), the positive δ56Fe values in iron formations are most parsimoniously explained by partial oxidation of hydrothermal Fe(II) (Johnson et al., 2008; Li et al., 2013).
View in article
Kirschvink, J.L. (1992) Late Proterozoic low-latitude global glaciation: The snowball Earth. In: Schopf, J.W., Klein, C. (Eds.) Cambridge University Press, Cambridge.
 Show in context
Show in contextReappearance of widespread iron deposits during the Cryogenian Period has been taken, therefore, as supporting evidence for the “Snowball Earth” hypothesis, as global ice sheet cover could have led to widespread ocean anoxia (Hoffman and Schrag, 2002; Kirschvink, 1992).
View in article
Kump, L.R., Seyfried, W.E. (2005) Hydrothermal Fe fluxes during the Precambrian: Effect of low oceanic sulfate concentrations and low hydrostatic pressure on the composition of black smokers. Earth and Planetary Science Letters 235, 654–662.
 Show in context
Show in contextDeposition of pyrite requires S2−, which could be provided by microbial reduction of 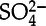 in the presence of organic matter. It is widely accepted that sulfate was depleted in oceans during the Snowball Earth Events (Kump and Seyfried, 2005).
in the presence of organic matter. It is widely accepted that sulfate was depleted in oceans during the Snowball Earth Events (Kump and Seyfried, 2005).
View in article
Lang, X., Shen, B., Peng, Y., Xiao, S., Zhou, C., Bao, H., Kaufman, A.J., Huang, K., Crockford, P. W., Liu, Y., et al., (2018) Transient marine euxinia at the end of the terminal Cryogenian glaciation. Nature Communications 9, 3019.
 Show in context
Show in contextA recent study reported widespread euxinia at the end of Marinoan glaciation, whereby the abundance of pyrite nodules was significantly greater in deep settings than in shallow settings (Lang et al., 2018).
View in article
Li, C., Love, G.D., Lyons, T.W., Fike, D.A., Sessions, A.L., Chu, X. (2010) A Stratified Redox Model for the Ediacaran Ocean. Science 328, 80.
 Show in context
Show in contextIf so, the mineralogical and isotopic records of the Xinyu CIF pinpoint the onset of euxinia, which was to be become widespread along productive margins until the Cambrian (Li et al., 2010).
View in article
Was disappearance of the CIFs caused by a decrease in Fe2+ concentration due to ocean oxygenation as seen in modern oceans? Or was it caused by the depletion of free oxygen and development of euxinic (H2S-rich) environments as has been proposed for the Mesoproterozoic (Canfield, 1998) and Ediacaran oceans (Li et al., 2010)?
View in article
Li, W., Czaja, A.D., Van Kranendonk, M.J., Beard, B.L., Roden, E.E., Johnson, C.M. (2013) An anoxic, Fe(II)-rich, U-poor ocean 3.46 billion years ago. Geochimica et Cosmochimica Acta 120, 65–79.
 Show in context
Show in contextGiven that the primary precipitates of Fe(II) oxidation (i.e. Fe(III) oxides/hydroxides) enrich heavy Fe isotopes over aqueous Fe(II) by ∼3–4 ‰ at equilibrium (Johnson et al., 2008), the positive δ56Fe values in iron formations are most parsimoniously explained by partial oxidation of hydrothermal Fe(II) (Johnson et al., 2008; Li et al., 2013).
View in article
Och, L.M., Shields-Zhou, G.A. (2012) The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Science Reviews 110, 26–57.
 Show in context
Show in contextDecreased O2 production seems unlikely in deglacial oceans, as removal of ice sheets would have allowed photosynthetic organisms to thrive, and moreover, newly resumed global hydrological cycles would have delivered abundant nutrient elements (e.g., P) into the oceans to boost biological productivity (Shields et al., 1997; Och and Shields-Zhou, 2012).
View in article
The Cryogenian Period (ca. 720–635 Ma) marks a turning point in the history of Earth’s surficial environment, when seawater changed from a generally anoxic, ferruginous and sulfate-poor state (Canfield et al., 2008; Guilbaud et al., 2015) to a more oxygenated, Fe-poor and sulfate-rich state (Sahoo et al., 2012; Shields et al., 2019) that progressively came to dominate through the ensuing Phanerozoic Eon (Och and Shields-Zhou, 2012).
View in article
Poulton, S.W., Fralick, P.W., Canfield, D.E. (2004) The transition to a sulphidic ocean ∼1.84 billion years ago. Nature 431, 173–177.
 Show in context
Show in contextIt has been proposed that the termination of major banded iron formations around 1.8 Ga was caused by the development of euxinic oceans (Poulton et al., 2004).
View in article
Sahoo, S.K., Planavsky, N.J., Kendall, B., Wang, X., Shi, X., Scott, C, Anbar, A.D., Lyons, T.W., Jiang, G. (2012) Ocean oxygenation in the wake of the Marinoan glaciation. Nature 489, 546–549.
 Show in context
Show in contextThe Cryogenian Period (ca. 720–635 Ma) marks a turning point in the history of Earth’s surficial environment, when seawater changed from a generally anoxic, ferruginous and sulfate-poor state (Canfield et al., 2008; Guilbaud et al., 2015) to a more oxygenated, Fe-poor and sulfate-rich state (Sahoo et al., 2012; Shields et al., 2019) that progressively came to dominate through the ensuing Phanerozoic Eon (Och and Shields-Zhou, 2012).
View in article
Shields, G., Stille, P., Brasier, M.D., Atudorei, N. (1997) Stratified oceans and oxygenation of the late Precambrian environment: a post glacial geochemical record from the Neoproterozoic of W. Mongolia. Terra Nova 9, 218–222.
 Show in context
Show in contextDecreased O2 production seems unlikely in deglacial oceans, as removal of ice sheets would have allowed photosynthetic organisms to thrive, and moreover, newly resumed global hydrological cycles would have delivered abundant nutrient elements (e.g., P) into the oceans to boost biological productivity (Shields et al., 1997; Och and Shields-Zhou, 2012).
View in article
Shields, G.A., Mills, B.J.W., Zhu, M., Raub, T.D., Daines, S.J., Lenton, T.M. (2019) Unique Neoproterozoic carbon isotope excursions sustained by coupled evaporite dissolution and pyrite burial. Nature Geoscience 12, 823–827.
 Show in context
Show in contextThe Cryogenian Period (ca. 720–635 Ma) marks a turning point in the history of Earth’s surficial environment, when seawater changed from a generally anoxic, ferruginous and sulfate-poor state (Canfield et al., 2008; Guilbaud et al., 2015) to a more oxygenated, Fe-poor and sulfate-rich state (Sahoo et al., 2012; Shields et al., 2019) that progressively came to dominate through the ensuing Phanerozoic Eon (Och and Shields-Zhou, 2012).
View in article
Tang, J., Fu, H., Yu, Z. (1987) Stratigraphy, type and formation conditions of the late precambrian banded iron ores in south China. Chinese Journal of Geochemistry 6, 331–341.
 Show in context
Show in context(c) Stratigraphic correlations of different CIFs from South China, after Tang et al. (1987).
View in article
Xinyu CIF was deposited in a deep marine setting based on sedimentary facies analysis (Tang et al., 1987) and palaeogeographic reconstruction (Wang and Li, 2003), therefore the spread of ocean euxinia is recorded by the deep Xinyu CIF, but not in other shallow CIFs as Fe(II) delivery was cut in deeper seawater.
View in article
The CIF from Xinyu, Jiangxi Province, China presents a remarkable opportunity to study ocean chemistry during the Cryogenian glaciation (Tang et al., 1987; Zhu et al., 2019).
View in article
Wang, J., Li, Z. (2003) History of Neoproterozoic rift basins in South China: implications for Rodinia break-up. Precambrian Research 122, 141–158.
 Show in context
Show in contextIt was deposited in deep water (Wang and Li, 2003), and contains magnetite as the major Fe oxide mineral.
View in article
(b) Tectonic background and palaeo-depth of the Cryogenian iron formations, after Wang and Li (2003).
View in article
Xinyu CIF was deposited in a deep marine setting based on sedimentary facies analysis (Tang et al., 1987) and palaeogeographic reconstruction (Wang and Li, 2003), therefore the spread of ocean euxinia is recorded by the deep Xinyu CIF, but not in other shallow CIFs as Fe(II) delivery was cut in deeper seawater.
View in article
Zhu, X., Sun, J., Li, Z. (2019) Iron isotopic variations of the Cryogenian banded iron formations: A new model. Precambrian Research 331, 105359.
 Show in context
Show in contextThe upward trend towards higher δ56Fe values within the Xinyu CIF is similar to previously reported Fe isotope patterns from Xinyu and other ‘Sturtian’ CIFs worldwide (Zhu et al., 2019).
View in article
The CIF from Xinyu, Jiangxi Province, China presents a remarkable opportunity to study ocean chemistry during the Cryogenian glaciation (Tang et al., 1987; Zhu et al., 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1. Geological Background of the Xinyu Iron Formation
- 2. Petrography and Mineral Chemistry of the Xinyu Iron Formation
- 3. Analytical Methods and Data Tables
- Figures S-1 to S-10
- Tables S-1 to S-3
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures