Activity coefficients of siderophile elements in Fe-Si liquids at high pressure
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:341Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
 ). Comparison of 1 GPa and 10 GPa data shows no difference except for Nb. Epsilon parameters derived from low pressure experiments can thus be used to calculate activity coefficients for application to higher pressure processes (at least to 10 GPa).
). Comparison of 1 GPa and 10 GPa data shows no difference except for Nb. Epsilon parameters derived from low pressure experiments can thus be used to calculate activity coefficients for application to higher pressure processes (at least to 10 GPa).Figures and Tables
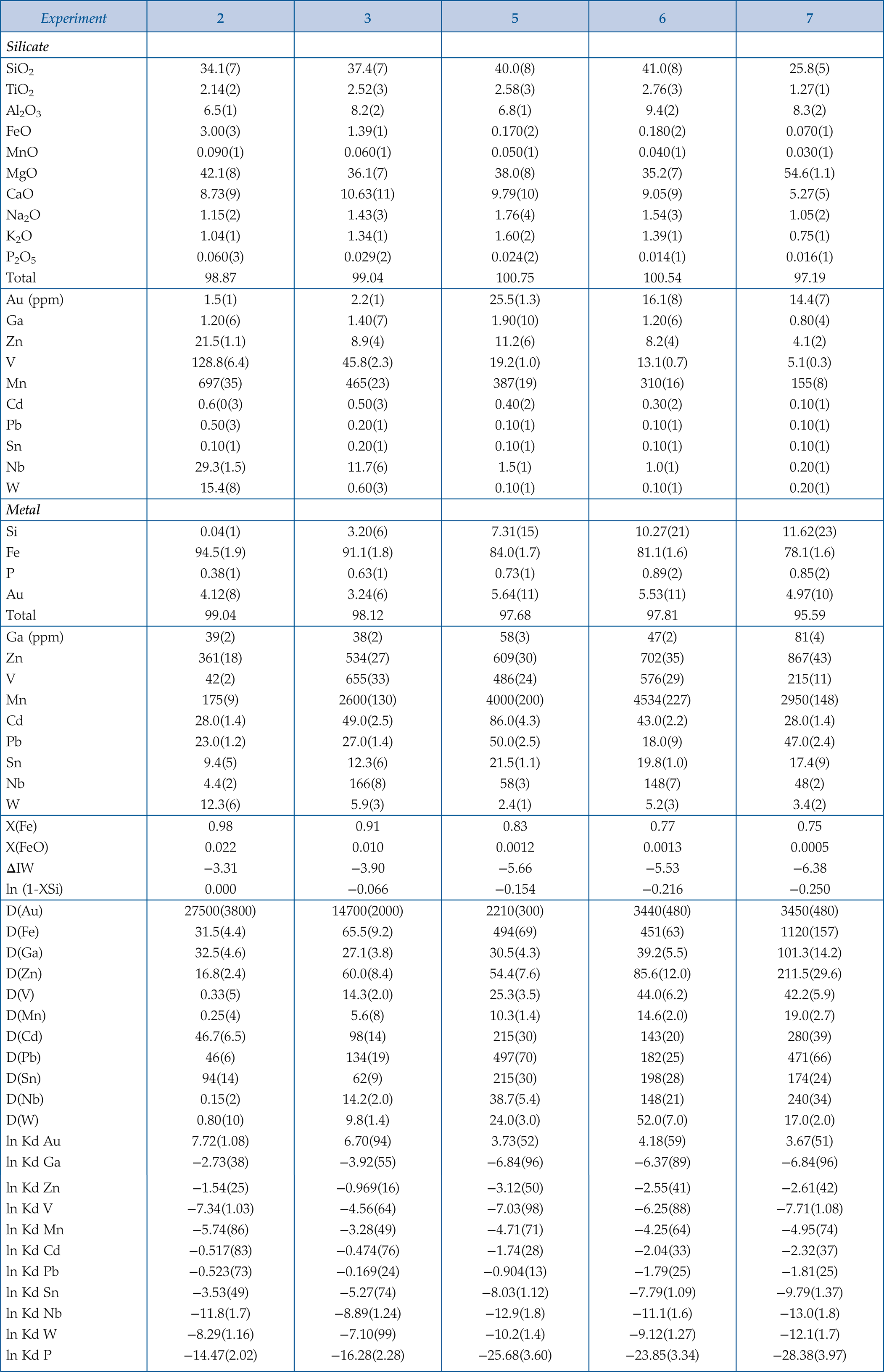 Table 1 Silicate and metal compositions and partition and exchange coefficient summary (values in parentheses represent 2σ error). | 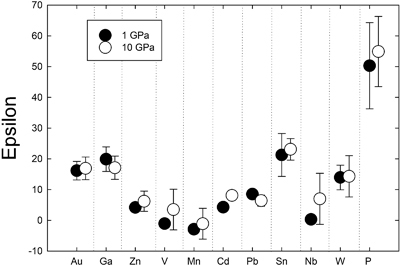 Figure 1 Comparison of 1 GPa and 10 GPa epsilon interaction parameters, with the 1 GPa values re-calculated from 1873 to 2373 K as discussed in the text. P, Au, Ga, Sn, Cd, Pb, Nb, W, Zn and V all have positive , indicating that Si will cause a decrease in D metal/silicate with Si present in the metallic liquid. Mn has a negative , indicating that Si will cause a very slight increase in D(Mn) metal/silicate with Si present in the metallic liquid. Nb exhibits the largest difference in measured in | 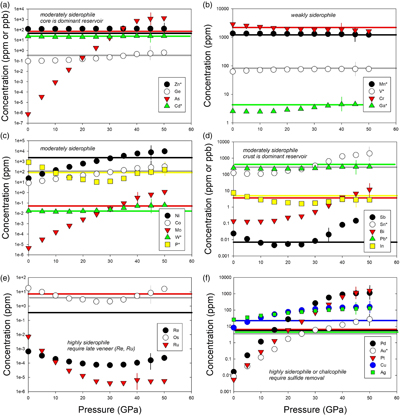 Figure 2 Evolution of siderophile element content of a terrestrial magma ocean as accretion proceeds and the PT conditions of metal-silicate equilibrium increase. Primitive upper mantle (PUM) siderophile element concentrations (from Palme and O’Neill, 2014) are horizontal lines with colour matching the symbols of the calculated values in each panel. The concentrations of 19 elements can be explained by metal-silicate equilibrium in a near 30–40 GPa magma ocean (Ni, Co, Mo, W, P, Mn, V, Cr, Ga, Zn, In, Ge, Sb, As, Sn, Bi, Cd, and Pb). Calculated concentrations of some elements such as Ag, Cu, Au, Pd, Pt become higher than PUM values, indicating the need for a removal mechanism such as a sulfide matte or late metallic segregation (see Righter et al. 2018, for detailed discussion). These 5 elements, together with Re, Os, and Ru, ultimately have their mantle concentrations set by addition of chondritic material after core formation and sulfide segregation. | 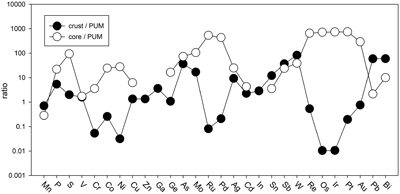 Figure 3 Concentrations of siderophile elements in the crust (Rudnick and Gao, 2014) and core (McDonough, 2003) of the Earth, normalised to values in the primitive upper mantle (PUM) (Palme and O’Neill, 2014). This diagram demonstrates that most siderophile elements are concentrated into the core, but there is an important and significant subset that is more highly concentrated into the crust, including Mn, Bi, Pb, In, Sn, Sb, and W. |
| Table 1 | Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Light elements can alloy with Fe in the cores of terrestrial planetary bodies. Due to density considerations, Earth’s core likely contains ∼10 % of a light element, which could be a combination of S, C, Si, and O with Si probably being the most abundant (Hirose et al., 2013)
Hirose, K., Labrosse, S., Hernlund, J. (2013) Composition and state of the core. Annual Review of Earth and Planetary Sciences 41, 657–691.
. The dissolution of these elements, and in particular Si, is dependent upon oxygen fugacity (Righter et al., 2020Righter, K., Herd, C.D., Boujibar, A. (2020) Redox processes in early Earth accretion and in terrestrial bodies. Elements: An International Magazine of Mineralogy, Geochemistry, and Petrology 16, 161–166.
), which also controls the solubility of trace elements in metallic and silicate melts. Because Si is a major alloying agent in metallic cores, its solution in Fe metallic liquids can have a significant influence on the activity coefficients of siderophile elements, and thus the partitioning behaviour of those elements between the core and mantle (Tuff et al., 2011Tuff, J., Wood, B.J., Wade, J. (2011) The effect of Si on metal-silicate partitioning of siderophile elements and implications for the conditions of core formation Geochimica et Cosmochimica Acta 75, 673–690.
; Righter et al., 2018Righter, K., Pando, K., Humayun, M., Waeselmann, N., Yang, S., Boujibar, A., Danielson, L.R. (2018) Effect of silicon on activity coefficients of siderophile elements (Au, Pd, Pt, P, Ga, Cu, Zn, and Pb) in liquid Fe: Roles of core formation, late sulfide matte, and late veneer in shaping terrestrial mantle geochemistry. Geochimica et Cosmochimica Acta 232, 101–123.
).The Earth’s core is estimated to have formed at pressures between 40–60 GPa (Wade and Wood, 2005
Wade, J., Wood, B.J. (2005) Core formation and the oxidation state of the Earth. Earth and Planetary Science Letters 236, 78–95.
; Righter, 2011Righter, K. (2011) Prediction of metal-silicate partition coefficients for siderophile elements: An update and assessment of PT conditions for metal-silicate equilibrium during accretion of the Earth. Earth and Planetary Science Letters 304 158–167.
; Siebert et al., 2011Siebert, J., Corgne, A., Ryerson, F.J. (2011) Systematics of metal-silicate partitioning for many siderophile elements applied to Earth’s core formation. Geochimica et Cosmochimica Acta 75, 1451–1489.
). Pressure is known to influence volumetric properties of metallic and silicate liquids (e.g., Armstrong et al., 2019Armstrong, K., Frost, D.J., McCammon, C.A., Rubie, D.C., Ballaran, T.B. (2019) Deep magma ocean formation set the oxidation state of Earth’s mantle. Science 365, 903–906.
), and also affect oxygen fugacity (e.g., Righter, 2016Righter, K. (2016) Metal-Silicate Partitioning of Siderophile Elements and Core-Mantle Segregation. Deep Earth: Physics and Chemistry of the Lower Mantle and Core, Geophysical Monograph 217, 161–174.
), but less is known about the effect of pressure on activity coefficients (e.g., Steenstra et al., 2020Steenstra, E.S., Seegers, A.X., Putter, R., Berndt, J., Klemme, S., Matveev, S., Bullock, E.S., van Westrenen, W. (2020) Metal-silicate partitioning systematics of siderophile elements at reducing conditions: A new experimental database. Icarus 335, 113391.
). If, for example, the activity coefficient of a moderately siderophile element (like W) in metallic Fe doubled, the corresponding concentration of that element in the silicate melt would nearly double as well. Thus even a modest change in activity coefficient will have a significant effect on mantle concentrations of siderophile element modelling outcomes. Understanding the effect of pressure on activity coefficients in the Fe-Si system, and in metallic liquids in general, is thus important for modelling core formation in the Earth and other terrestrial-like planets.In this work, we carried out a series of experiments (See Supplementary Information Part 1, Fig. S-1, and Table 1) at 10 GPa to investigate the effect variable Si content has on the activity coefficients of Au, P, V, Mn, Ga, Zn, Cd, Sn, W, Pb, and Nb in Fe-Si alloys at 10 GPa and 2373 K. We derive interaction parameters for Fe-Si liquids for comparison to behaviour already investigated at 1 GPa, using the same starting materials (Righter et al., 2018
Righter, K., Pando, K., Humayun, M., Waeselmann, N., Yang, S., Boujibar, A., Danielson, L.R. (2018) Effect of silicon on activity coefficients of siderophile elements (Au, Pd, Pt, P, Ga, Cu, Zn, and Pb) in liquid Fe: Roles of core formation, late sulfide matte, and late veneer in shaping terrestrial mantle geochemistry. Geochimica et Cosmochimica Acta 232, 101–123.
).Table 1 Silicate and metal compositions and partition and exchange coefficient summary (values in parentheses represent 2σ error).
| Experiment | 2 | 3 | 5 | 6 | 7 |
| Silicate | |||||
| SiO2 | 34.1(7) | 37.4(7) | 40.0(8) | 41.0(8) | 25.8(5) |
| TiO2 | 2.14(2) | 2.52(3) | 2.58(3) | 2.76(3) | 1.27(1) |
| Al2O3 | 6.5(1) | 8.2(2) | 6.8(1) | 9.4(2) | 8.3(2) |
| FeO | 3.00(3) | 1.39(1) | 0.170(2) | 0.180(2) | 0.070(1) |
| MnO | 0.090(1) | 0.060(1) | 0.050(1) | 0.040(1) | 0.030(1) |
| MgO | 42.1(8) | 36.1(7) | 38.0(8) | 35.2(7) | 54.6(1.1) |
| CaO | 8.73(9) | 10.63(11) | 9.79(10) | 9.05(9) | 5.27(5) |
| Na2O | 1.15(2) | 1.43(3) | 1.76(4) | 1.54(3) | 1.05(2) |
| K2O | 1.04(1) | 1.34(1) | 1.60(2) | 1.39(1) | 0.75(1) |
| P2O5 | 0.060(3) | 0.029(2) | 0.024(2) | 0.014(1) | 0.016(1) |
| Total | 98.87 | 99.04 | 100.75 | 100.54 | 97.19 |
| Au (ppm) | 1.5(1) | 2.2(1) | 25.5(1.3) | 16.1(8) | 14.4(7) |
| Ga | 1.20(6) | 1.40(7) | 1.90(10) | 1.20(6) | 0.80(4) |
| Zn | 21.5(1.1) | 8.9(4) | 11.2(6) | 8.2(4) | 4.1(2) |
| V | 128.8(6.4) | 45.8(2.3) | 19.2(1.0) | 13.1(0.7) | 5.1(0.3) |
| Mn | 697(35) | 465(23) | 387(19) | 310(16) | 155(8) |
| Cd | 0.6(0(3) | 0.50(3) | 0.40(2) | 0.30(2) | 0.10(1) |
| Pb | 0.50(3) | 0.20(1) | 0.10(1) | 0.10(1) | 0.10(1) |
| Sn | 0.10(1) | 0.20(1) | 0.10(1) | 0.10(1) | 0.10(1) |
| Nb | 29.3(1.5) | 11.7(6) | 1.5(1) | 1.0(1) | 0.20(1) |
| W | 15.4(8) | 0.60(3) | 0.10(1) | 0.10(1) | 0.20(1) |
| Metal | |||||
| Si | 0.04(1) | 3.20(6) | 7.31(15) | 10.27(21) | 11.62(23) |
| Fe | 94.5(1.9) | 91.1(1.8) | 84.0(1.7) | 81.1(1.6) | 78.1(1.6) |
| P | 0.38(1) | 0.63(1) | 0.73(1) | 0.89(2) | 0.85(2) |
| Au | 4.12(8) | 3.24(6) | 5.64(11) | 5.53(11) | 4.97(10) |
| Total | 99.04 | 98.12 | 97.68 | 97.81 | 95.59 |
| Ga (ppm) | 39(2) | 38(2) | 58(3) | 47(2) | 81(4) |
| Zn | 361(18) | 534(27) | 609(30) | 702(35) | 867(43) |
| V | 42(2) | 655(33) | 486(24) | 576(29) | 215(11) |
| Mn | 175(9) | 2600(130) | 4000(200) | 4534(227) | 2950(148) |
| Cd | 28.0(1.4) | 49.0(2.5) | 86.0(4.3) | 43.0(2.2) | 28.0(1.4) |
| Pb | 23.0(1.2) | 27.0(1.4) | 50.0(2.5) | 18.0(9) | 47.0(2.4) |
| Sn | 9.4(5) | 12.3(6) | 21.5(1.1) | 19.8(1.0) | 17.4(9) |
| Nb | 4.4(2) | 166(8) | 58(3) | 148(7) | 48(2) |
| W | 12.3(6) | 5.9(3) | 2.4(1) | 5.2(3) | 3.4(2) |
| X(Fe) | 0.98 | 0.91 | 0.83 | 0.77 | 0.75 |
| X(FeO) | 0.022 | 0.010 | 0.0012 | 0.0013 | 0.0005 |
| ΔIW | −3.31 | −3.90 | −5.66 | −5.53 | −6.38 |
| ln (1-XSi) | 0.000 | −0.066 | −0.154 | −0.216 | −0.250 |
| D(Au) | 27500(3800) | 14700(2000) | 2210(300) | 3440(480) | 3450(480) |
| D(Fe) | 31.5(4.4) | 65.5(9.2) | 494(69) | 451(63) | 1120(157) |
| D(Ga) | 32.5(4.6) | 27.1(3.8) | 30.5(4.3) | 39.2(5.5) | 101.3(14.2) |
| D(Zn) | 16.8(2.4) | 60.0(8.4) | 54.4(7.6) | 85.6(12.0) | 211.5(29.6) |
| D(V) | 0.33(5) | 14.3(2.0) | 25.3(3.5) | 44.0(6.2) | 42.2(5.9) |
| D(Mn) | 0.25(4) | 5.6(8) | 10.3(1.4) | 14.6(2.0) | 19.0(2.7) |
| D(Cd) | 46.7(6.5) | 98(14) | 215(30) | 143(20) | 280(39) |
| D(Pb) | 46(6) | 134(19) | 497(70) | 182(25) | 471(66) |
| D(Sn) | 94(14) | 62(9) | 215(30) | 198(28) | 174(24) |
| D(Nb) | 0.15(2) | 14.2(2.0) | 38.7(5.4) | 148(21) | 240(34) |
| D(W) | 0.80(10) | 9.8(1.4) | 24.0(3.0) | 52.0(7.0) | 17.0(2.0) |
| ln Kd Au | 7.72(1.08) | 6.70(94) | 3.73(52) | 4.18(59) | 3.67(51) |
| ln Kd Ga | −2.73(38) | −3.92(55) | −6.84(96) | −6.37(89) | −6.84(96) |
| ln Kd Zn | −1.54(25) | −0.969(16) | −3.12(50) | −2.55(41) | −2.61(42) |
| ln Kd V | −7.34(1.03) | −4.56(64) | −7.03(98) | −6.25(88) | −7.71(1.08) |
| ln Kd Mn | −5.74(86) | −3.28(49) | −4.71(71) | −4.25(64) | −4.95(74) |
| ln Kd Cd | −0.517(83) | −0.474(76) | −1.74(28) | −2.04(33) | −2.32(37) |
| ln Kd Pb | −0.523(73) | −0.169(24) | −0.904(13) | −1.79(25) | −1.81(25) |
| ln Kd Sn | −3.53(49) | −5.27(74) | −8.03(1.12) | −7.79(1.09) | −9.79(1.37) |
| ln Kd Nb | −11.8(1.7) | −8.89(1.24) | −12.9(1.8) | −11.1(1.6) | −13.0(1.8) |
| ln Kd W | −8.29(1.16) | −7.10(99) | −10.2(1.4) | −9.12(1.27) | −12.1(1.7) |
| ln Kd P | −14.47(2.02) | −16.28(2.28) | −25.68(3.60) | −23.85(3.34) | −28.38(3.97) |
top
Results
Determination of epsilon interaction parameters. The epsilon interaction parameter (
 ) is a measure of the interaction between a trace element M and the solute Si in a Fe metallic liquid. The
) is a measure of the interaction between a trace element M and the solute Si in a Fe metallic liquid. The  approach for calculating activity coefficients allows effects of solutes like Si, S, and C to be quantified for a multicomponent metallic liquid (Supplementary Information Part 3). Positive
approach for calculating activity coefficients allows effects of solutes like Si, S, and C to be quantified for a multicomponent metallic liquid (Supplementary Information Part 3). Positive  values indicate dissolved Si causes a decrease in partition coefficients, whereas negative values indicate an increase. Mn is the only element to exhibit a negative
values indicate dissolved Si causes a decrease in partition coefficients, whereas negative values indicate an increase. Mn is the only element to exhibit a negative  of −1.1 ± 5.0 (standard error), and even this value is only marginally negative when error is considered. V and Zn have small, positive
of −1.1 ± 5.0 (standard error), and even this value is only marginally negative when error is considered. V and Zn have small, positive  of 3.5 ± 6.6 and 6.2 ± 3.3, respectively. Cd, Pb, Nb, and W all have positive but slightly lower
of 3.5 ± 6.6 and 6.2 ± 3.3, respectively. Cd, Pb, Nb, and W all have positive but slightly lower  of 8.1 ± 1.3, 6.4 ± 1.8, 7.0 ± 8.3 and 14.3 ± 6.7, respectively (Figs. 1, S-2). Au, Ga, and Sn have moderate, positive
of 8.1 ± 1.3, 6.4 ± 1.8, 7.0 ± 8.3 and 14.3 ± 6.7, respectively (Figs. 1, S-2). Au, Ga, and Sn have moderate, positive  of 16.9 ± 3.7, 17.1 ± 3.8, and 23.1 ± 3.5, respectively, whereas P yielded the highest value of
of 16.9 ± 3.7, 17.1 ± 3.8, and 23.1 ± 3.5, respectively, whereas P yielded the highest value of  at 55.0 ± 11.4 (Figs. 1, S-2).
at 55.0 ± 11.4 (Figs. 1, S-2).
Figure 1 Comparison of 1 GPa and 10 GPa epsilon interaction parameters, with the 1 GPa values re-calculated from 1873 to 2373 K as discussed in the text. P, Au, Ga, Sn, Cd, Pb, Nb, W, Zn and V all have positive
 , indicating that Si will cause a decrease in D metal/silicate with Si present in the metallic liquid. Mn has a negative
, indicating that Si will cause a decrease in D metal/silicate with Si present in the metallic liquid. Mn has a negative  , indicating that Si will cause a very slight increase in D(Mn) metal/silicate with Si present in the metallic liquid. Nb exhibits the largest difference in measured in
, indicating that Si will cause a very slight increase in D(Mn) metal/silicate with Si present in the metallic liquid. Nb exhibits the largest difference in measured in  at low pressure and 10 GPa, suggesting there might be a measurable pressure effect at even higher pressures.
at low pressure and 10 GPa, suggesting there might be a measurable pressure effect at even higher pressures.top
Discussion
Comparison to low pressure epsilon parameters. Comparison of our newly determined 10 GPa
 to values at 1 GPa (re-calculated to 2373 K using the following equation:
to values at 1 GPa (re-calculated to 2373 K using the following equation: 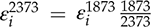 ) from previous studies) shows very similar values at both pressures (Fig. 1). Zn, V, Cd, and Nb are all shifted to higher values at 10 GPa, but the differences are within error. Similarly, Pb is slightly lower than the 1 GPa value, but still within error. Nb has the largest difference in measured epsilon values at low pressure and 10 GPa (albeit still within error), suggesting there might be a measurable pressure effect at even higher pressures. However, the low pressure value in Figure 1 was taken from the Steelmaking Data Sourcebook (1988)
) from previous studies) shows very similar values at both pressures (Fig. 1). Zn, V, Cd, and Nb are all shifted to higher values at 10 GPa, but the differences are within error. Similarly, Pb is slightly lower than the 1 GPa value, but still within error. Nb has the largest difference in measured epsilon values at low pressure and 10 GPa (albeit still within error), suggesting there might be a measurable pressure effect at even higher pressures. However, the low pressure value in Figure 1 was taken from the Steelmaking Data Sourcebook (1988)Steelmaking Data Sourcebook (1988) The Japan Society for the Promotion of Science: The 19th Committee on Steelmaking. Gordon and Breach Science Publishers, Montreux.
and is a value of −0.66, compared to our 10 GPa value of 7.0 ± 8.3. These experimental differences in the two studies are likely important and thus make difficult a comparison between low and high pressure values to assess a pressure effect. Despite these small differences for a few elements, our 10 GPa data are largely indistinguishable from the 1 GPa data and indicate that activity coefficients are not strongly dependent upon pressure.Steenstra et al. (2020)
Steenstra, E.S., Seegers, A.X., Putter, R., Berndt, J., Klemme, S., Matveev, S., Bullock, E.S., van Westrenen, W. (2020) Metal-silicate partitioning systematics of siderophile elements at reducing conditions: A new experimental database. Icarus 335, 113391.
examine the effect of pressure on combining their data at 1 to 4 GPa with 11 GPa data from Vogel et al. (2018)
combining their data at 1 to 4 GPa with 11 GPa data from Vogel et al. (2018)Vogel, A.K., Jennings, E.S., Laurenz, V., Rubie, D.C., Frost, D.J. (2018) The dependence of metal-silicate partitioning of moderately volatile elements on oxygen fugacity and Si contents of Fe metal: Implications for their valence states in silicate liquids. Geochimica et Cosmochimica Acta 237, 275–293.
, for 12 elements, some of which we have examined here. Our results for Cd, Pb, Mn, V, and Sn are in overall agreement – none of these elements shows discernable change between 1 and 10 GPa. Steenstra et al. (2020)Steenstra, E.S., Seegers, A.X., Putter, R., Berndt, J., Klemme, S., Matveev, S., Bullock, E.S., van Westrenen, W. (2020) Metal-silicate partitioning systematics of siderophile elements at reducing conditions: A new experimental database. Icarus 335, 113391.
also present some evidence for change at high pressure for for Ni, In, As and Sb, but change in those series occurs between 1 and 2 GPa and change above that is negligible (i.e., 2 GPa = 11 GPa values for As, Sb, Sn). Pb, Cu, Cr, Mn, V, and Cd
for Ni, In, As and Sb, but change in those series occurs between 1 and 2 GPa and change above that is negligible (i.e., 2 GPa = 11 GPa values for As, Sb, Sn). Pb, Cu, Cr, Mn, V, and Cd  all show no trend at higher pressures, similar to most elements we have examined here.
all show no trend at higher pressures, similar to most elements we have examined here.Implications. The accretion of terrestrial planets is a process that begins in the earliest Solar System as differentiated bodies form within a few million years after the start of the Solar System or T0 (Kleine et al., 2009
Kleine, T., Touboul, M., Bourdon, B., Nimmo, F., Mezger, K., Palme, H. (2009) Hf-W chronology of the accretion and early evolution of asteroids and terrestrial planets. Geochimica et Cosmochimica Acta 73, 5150–5188, doi: 10.1016/j.gca.2008.11.047.
; Levison et al., 2015Levison, H.F., Kretke, K.A., Walsh, K.J., Bottke, W.F. (2015) Growing the terrestrial planets from the gradual accumulation of sub-meter-sized objects. Proceedings of the National Academy of Sciences 112, 14,180–14,185, doi: 10.1073/pnas.1513364112
). The process continues with oligarchic growth of planetesimals (Kokubo and Ida, 1998Kokubo, E., Ida, S. (1998) Oligarchic growth of protoplanets. Icarus 131, 171–178.
) and then transitions to growth by merging and impact of planetesimals into planets (e.g., Chambers, 2013Chambers, J.E. (2013) Late-stage planetary accretion including hit-and-run collisions and fragmentation. Icarus 224, 43–56.
). As this process unfolds, the energetics of accretion increase steadily providing sufficient energy to melt – either partially or completely – planetary mantles and metal-silicate mixtures (e.g., de Vries et al., 2016de Vries, J., Nimmo, F., Melosh, H.J., Jacobson, S.A., Morbidelli, A., Rubie, D.C. (2016) Impact-induced melting during accretion of the Earth. Progress in Earth and Planetary Science 3, 7.
). The equilibration of metal and silicate melts is rapid and complete during this process (Kendall and Melosh, 2016Kendall, J.D., Melosh, H.J. (2016) Differentiated planetesimal impacts into a terrestrial magma ocean: Fate of the iron core. Earth and Planetary Science Letters 448, 24–33.
; Lherm and Deguen, 2018Lherm, V., Deguen, R. (2018) Small-Scale Metal/Silicate Equilibration During Core Formation: The Influence of Stretching Enhanced Diffusion on Mixing. Journal of Geophysical Research: Solid Earth 123, 10,496–10,516.
) and thus the siderophile element content of the molten portion of the planet will change as the depth and associated pressure of metal-silicate equilibrium increases (e.g., Rubie et al., 2003Rubie, D.C., Melosh, H.J., Reid, J.E., Liebske, C., Righter, K. (2003) Mechanisms of metal–silicate equilibration in the terrestrial magma ocean. Earth and Planetary Science Letters 205, 239–255.
). Modelling of siderophile element contents of molten upper mantle melts in equilibrium with Fe-Si-S-C metallic liquids during accretion now includes 26 elements (Fig. 2), including moderately siderophile and refractory (Ni, Co, Mo, and W), moderately siderophile and volatile (P, Ga, Cu, Ge, Sb, As, In, Bi, Sn, Ag, Cd, and Pb), weakly siderophile (Mn, Cr, V, Zn), and highly siderophile (Au, Pd, Pt, Re, Os, Ru) elements. The number of elements that can be modelled for Fe-Si-S-C liquids using this approach has nearly tripled since 2016.
Figure 2 Evolution of siderophile element content of a terrestrial magma ocean as accretion proceeds and the PT conditions of metal-silicate equilibrium increase. Primitive upper mantle (PUM) siderophile element concentrations (from Palme and O’Neill, 2014
Palme, H., O’Neill, H.S.C. (2014) Cosmochemical estimates of mantle composition. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 3. Second edition, Elsevier, Oxford, 1–39.
) are horizontal lines with colour matching the symbols of the calculated values in each panel. The concentrations of 19 elements can be explained by metal-silicate equilibrium in a near 30–40 GPa magma ocean (Ni, Co, Mo, W, P, Mn, V, Cr, Ga, Zn, In, Ge, Sb, As, Sn, Bi, Cd, and Pb). Calculated concentrations of some elements such as Ag, Cu, Au, Pd, Pt become higher than PUM values, indicating the need for a removal mechanism such as a sulfide matte or late metallic segregation (see Righter et al. 2018Righter, K., Pando, K., Humayun, M., Waeselmann, N., Yang, S., Boujibar, A., Danielson, L.R. (2018) Effect of silicon on activity coefficients of siderophile elements (Au, Pd, Pt, P, Ga, Cu, Zn, and Pb) in liquid Fe: Roles of core formation, late sulfide matte, and late veneer in shaping terrestrial mantle geochemistry. Geochimica et Cosmochimica Acta 232, 101–123.
, for detailed discussion). These 5 elements, together with Re, Os, and Ru, ultimately have their mantle concentrations set by addition of chondritic material after core formation and sulfide segregation.A complete understanding of this large group of elements is desirable for a number of reasons. First, some of these elements are critical to biochemical processes and the origin of life, being part of energetically favourable enzymes or participants in chemical processes (e.g., W, Mo, P, Ni, Co; Falkowski et al., 2008
Falkowski, P.G., Fenchel, T., Delong, E.F. (2008) The microbial engines that drive Earth’s biogeochemical cycles. Science 320, 1034–1039.
; Kim et al., 2013Kim J., Senn S., Harel A., Jelen B., Falkowski P. (2013) Discovering the electronic circuit diagram of life: structural relationships among transition metal binding sites in oxidoreductases. Philosophical Transactions of the Royal Society B 368, doi: 10.1098/rstb.2012.0257.
; Benner et al., 2019Benner, S.A., Bell, E.A., Biondi, E., Brasser, R., Carell, T., Kim, H.-J., Mojzsis, S.J., Omran, A., Pasek, M.A., Trail, D. (2019) When Did Life Likely Emerge on Earth in an RNA-First Process? ChemSystemsChem, doi: 10.1002/syst.201900035.
), taking part in biochemical processes at black smokers, or involved in respiration (V, Cu, and Zn). For example, enhanced concentration of P in terrestrial or extrasolar planetary mantles is expected in equilibrium with Fe-Si liquids (Righter et al., 2018Righter, K., Pando, K., Humayun, M., Waeselmann, N., Yang, S., Boujibar, A., Danielson, L.R. (2018) Effect of silicon on activity coefficients of siderophile elements (Au, Pd, Pt, P, Ga, Cu, Zn, and Pb) in liquid Fe: Roles of core formation, late sulfide matte, and late veneer in shaping terrestrial mantle geochemistry. Geochimica et Cosmochimica Acta 232, 101–123.
). Higher concentrations in the mantle would aid the eventual transfer of P to the crust (Supplementary Information Part 4), thus influencing the stability of P-bearing biochemicals such as ADP, RNA, and DNA in habitable planets. Second, the highly siderophile elements (e.g., Au, Ru, Ir, Os), chalcophile elements (Cu, Ag), and volatile trace metals are frequently employed in constraining post core formation and late accretion processes (e.g., Walker, 2009Walker, R.J. (2009) Highly siderophile elements in the Earth, Moon and Mars: update and implications for planetary accretion and differentiation. Chemie der Erde-Geochemistry 69, 101–125.
) as well as how Earth (and other bodies) acquired volatiles in general (e.g., Halliday, 2013Halliday, A.N. (2013) The origins of volatiles in the terrestrial planets. Geochimica et Cosmochimica Acta 105, 146–171.
; Righter et al., 2019Righter, K., Pando, K., Ross, D.K., Righter, M., Lapen, T.J. (2019) Effect of silicon on activity coefficients of Bi, Cd, Sn, and Ag in liquid Fe-Si, and implications for differentiation and core formation. Meteoritics & Planetary Science 54, 1379–1394.
). And third, the distribution of most siderophile elements between core, mantle and crust is dominated by the core, but the distribution of several exceptional elements is actually dominated by the crust (e.g., Bi, Sn, As, Sb, Mo, W, Pb, In; Fig. 3). All of these elements exhibit positive values, indicating their mantle concentrations would be elevated by equilibration with Fe-Si core-forming alloy. The higher mantle concentrations would also enhance the ultimate transfer of these elements to the crust and produce similar or higher concentrations in the crust compared to the core (Fig. 3). Thus, a complete understanding of how all these siderophile elements became established in the primitive mantle and ultimately in the crust is essential to understanding the basic geochemistry of the early Earth.
values, indicating their mantle concentrations would be elevated by equilibration with Fe-Si core-forming alloy. The higher mantle concentrations would also enhance the ultimate transfer of these elements to the crust and produce similar or higher concentrations in the crust compared to the core (Fig. 3). Thus, a complete understanding of how all these siderophile elements became established in the primitive mantle and ultimately in the crust is essential to understanding the basic geochemistry of the early Earth.
Figure 3 Concentrations of siderophile elements in the crust (Rudnick and Gao, 2014
Rudnick, R.L., Gao, S. (2014) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 4. Second edition, Elsevier, Oxford, 1–51.
) and core (McDonough, 2003McDonough, W.F. (2003) Compositional model for the Earth’s core. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 2. Pergamon, Oxford, 547–568.
) of the Earth, normalised to values in the primitive upper mantle (PUM) (Palme and O’Neill, 2014Palme, H., O’Neill, H.S.C. (2014) Cosmochemical estimates of mantle composition. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 3. Second edition, Elsevier, Oxford, 1–39.
). This diagram demonstrates that most siderophile elements are concentrated into the core, but there is an important and significant subset that is more highly concentrated into the crust, including Mn, Bi, Pb, In, Sn, Sb, and W.The current work includes moderately siderophile and refractory (W), moderately siderophile and volatile (P, Cd, Sn, Pb), weakly siderophile (Mn, V, Ga, Zn, Nb), and highly siderophile (Au) elements. Most elements in these 4 groups exhibit no pressure effect on the activity coefficients in Fe-Si liquids, perhaps a surprising result because some have argued that structural changes in metallic liquids in this pressure range may lead to changes in partitioning behaviour in the Fe-C and Fe-Si liquids (5.2 GPa in Fe-C; Sanloup et al., 2011
Sanloup, C., Van Westrenen, W., Dasgupta, R., Maynard-Casely, H., Perrillat, J.P. (2011) Compressibility change in iron-rich melt and implications for core formation models. Earth and Planetary Science Letters 306, 118–122.
, and 1-4 GPa in Fe-Si; Shibazaki et al. 2015Shibazaki, Y., Kono, Y., Fei, Y. (2015) Microscopic structural change in a liquid Fe-C alloy of ∼5 GPa. Geophysical Research Letters 42, 5236–5242.
). Although one might expect changes in epsilon parameters (and thus activity coefficients) due to such structural changes, our results suggest that there is no effect up to 10 GPa. It is possible that Fe-Si liquids do not undergo such structural changes below 10 GPa (e.g., Sanloup et al., 2004Sanloup, C., Fiquet, G., Gregoryanz, E., Morard, G., Mezouar, M. (2004) Effect of Si on liquid Fe compressibility: Implications for sound velocity in core materials. Geophysical Research Letters 31, L07604.
), and thus we do not see a change in activity coefficients. Some changes are proposed at higher pressures (e.g., Morard et al., 2007Morard, G., Sanloup, C., Fiquet, G., Mezouar, M., Rey, N., Poloni, R., Beck, P. (2007) Structure of eutectic Fe–FeS melts to pressures up to 17 GPa: implications for planetary cores. Earth and Planetary Science Letters 263, 128–139.
; Fe-S), and so detection of changes in activity coefficients at higher pressures should be pursued as well. In the meantime, because our results show no evidence for such structural changes in Fe-Si liquids below 10 GPa, the epsilon parameters can be employed in modelling equilibria at pressures up to 10 GPa.Further studies of other siderophile elements at 10 GPa should be undertaken for comparison to the current results and to measure potential pressure effects on activity coefficients. Most elements should also be studied to pressures higher than 10 GPa and up to 70 GPa – pressures more applicable to those during the accretion of larger terrestrial planets. In particular, Cu, Mo, Bi, As, Sb, as well as the highly siderophile elements (HSE) Pt and Pd all exhibit pressure dependence, and thus pressure effects on activity coefficients must be thoroughly understood and studied in more detail. Additionally, a better understanding of the effect of pressure on Nb would be important to evaluating the Nb content of the mantle of differentiated bodies and the overall distribution cores and mantles in differentiated bodies (e.g., Nebel et al., 2010
Nebel, O., van Westrenen, W., Vroon, P.Z., Wille, M., Raith, M.M. (2010) Deep mantle storage of the Earth’s missing niobium in late-stage residual melts from a magma ocean. Geochimica et Cosmochimica Acta 74, 4392–4404.
; Münker et al., 2017Münker, C., Fonseca, R.O., Schulz, T. (2017) Silicate Earth’s missing niobium may have been sequestered into asteroidal cores. Nature Geoscience 10, 822–826.
).top
Acknowledgements
This work was supported by NASA Planetary Science Division (MH), and an RTOP to KR from the NASA Cosmochemistry program.
Editor: Anat Shahar
top
References
Armstrong, K., Frost, D.J., McCammon, C.A., Rubie, D.C., Ballaran, T.B. (2019) Deep magma ocean formation set the oxidation state of Earth’s mantle. Science 365, 903–906.
 Show in context
Show in context Pressure is known to influence volumetric properties of metallic and silicate liquids (e.g., Armstrong et al., 2019), and also affect oxygen fugacity (e.g., Righter, 2016), but less is known about the effect of pressure on activity coefficients (e.g., Steenstra et al., 2020).
View in article
Benner, S.A., Bell, E.A., Biondi, E., Brasser, R., Carell, T., Kim, H.-J., Mojzsis, S.J., Omran, A., Pasek, M.A., Trail, D. (2019) When Did Life Likely Emerge on Earth in an RNA-First Process? ChemSystemsChem, doi: 10.1002/syst.201900035.
 Show in context
Show in context First, some of these elements are critical to biochemical processes and the origin of life, being part of energetically favourable enzymes or participants in chemical processes (e.g., W, Mo, P, Ni, Co; Falkowski et al., 2008; Kim et al., 2013; Benner et al., 2019), taking part in biochemical processes at black smokers, or involved in respiration (V, Cu, and Zn).
View in article
Chambers, J.E. (2013) Late-stage planetary accretion including hit-and-run collisions and fragmentation. Icarus 224, 43–56.
 Show in context
Show in context The process continues with oligarchic growth of planetesimals (Kokubo and Ida, 1998) and then transitions to growth by merging and impact of planetesimals into planets (e.g., Chambers, 2013).
View in article
de Vries, J., Nimmo, F., Melosh, H.J., Jacobson, S.A., Morbidelli, A., Rubie, D.C. (2016) Impact-induced melting during accretion of the Earth. Progress in Earth and Planetary Science 3, 7.
 Show in context
Show in context As this process unfolds, the energetics of accretion increase steadily providing sufficient energy to melt – either partially or completely – planetary mantles and metal-silicate mixtures (e.g., de Vries et al., 2016).
View in article
Falkowski, P.G., Fenchel, T., Delong, E.F. (2008) The microbial engines that drive Earth’s biogeochemical cycles. Science 320, 1034–1039.
 Show in context
Show in context First, some of these elements are critical to biochemical processes and the origin of life, being part of energetically favourable enzymes or participants in chemical processes (e.g., W, Mo, P, Ni, Co; Falkowski et al., 2008; Kim et al., 2013; Benner et al., 2019), taking part in biochemical processes at black smokers, or involved in respiration (V, Cu, and Zn).
View in article
Halliday, A.N. (2013) The origins of volatiles in the terrestrial planets. Geochimica et Cosmochimica Acta 105, 146–171.
 Show in context
Show in context Second, the highly siderophile elements (e.g., Au, Ru, Ir, Os), chalcophile elements (Cu, Ag), and volatile trace metals are frequently employed in constraining post core formation and late accretion processes (e.g., Walker, 2009) as well as how Earth (and other bodies) acquired volatiles in general (e.g., Halliday, 2013; Righter et al., 2019).
View in article
Hirose, K., Labrosse, S., Hernlund, J. (2013) Composition and state of the core. Annual Review of Earth and Planetary Sciences 41, 657–691.
 Show in context
Show in context Due to density considerations, Earth’s core likely contains ∼10 % of a light element, which could be a combination of S, C, Si, and O with Si probably being the most abundant (Hirose et al., 2013).
View in article
Kendall, J.D., Melosh, H.J. (2016) Differentiated planetesimal impacts into a terrestrial magma ocean: Fate of the iron core. Earth and Planetary Science Letters 448, 24–33.
 Show in context
Show in context The equilibration of metal and silicate melts is rapid and complete during this process (Kendall and Melosh, 2016; Lherm and Deguen, 2018) and thus the siderophile element content of the molten portion of the planet will change as the depth and associated pressure of metal-silicate equilibrium increases (e.g., Rubie et al., 2003).
View in article
Kim J., Senn S., Harel A., Jelen B., Falkowski P. (2013) Discovering the electronic circuit diagram of life: structural relationships among transition metal binding sites in oxidoreductases. Philosophical Transactions of the Royal Society B 368, doi: 10.1098/rstb.2012.0257.
 Show in context
Show in context First, some of these elements are critical to biochemical processes and the origin of life, being part of energetically favourable enzymes or participants in chemical processes (e.g., W, Mo, P, Ni, Co; Falkowski et al., 2008; Kim et al., 2013; Benner et al., 2019), taking part in biochemical processes at black smokers, or involved in respiration (V, Cu, and Zn).
View in article
Kleine, T., Touboul, M., Bourdon, B., Nimmo, F., Mezger, K., Palme, H. (2009) Hf-W chronology of the accretion and early evolution of asteroids and terrestrial planets. Geochimica et Cosmochimica Acta 73, 5150–5188, doi: 10.1016/j.gca.2008.11.047.
 Show in context
Show in context The accretion of terrestrial planets is a process that begins in the earliest Solar System as differentiated bodies form within a few million years after the start of the Solar System or T0 (Kleine et al., 2009; Levison et al., 2015).
View in article
Kokubo, E., Ida, S. (1998) Oligarchic growth of protoplanets. Icarus 131, 171–178.
 Show in context
Show in context The process continues with oligarchic growth of planetesimals (Kokubo and Ida, 1998) and then transitions to growth by merging and impact of planetesimals into planets (e.g., Chambers, 2013).
View in article
Levison, H.F., Kretke, K.A., Walsh, K.J., Bottke, W.F. (2015) Growing the terrestrial planets from the gradual accumulation of sub-meter-sized objects. Proceedings of the National Academy of Sciences 112, 14,180–14,185, doi: 10.1073/pnas.1513364112.
 Show in context
Show in context The accretion of terrestrial planets is a process that begins in the earliest Solar System as differentiated bodies form within a few million years after the start of the Solar System or T0 (Kleine et al., 2009; Levison et al., 2015).
View in article
Lherm, V., Deguen, R. (2018) Small-Scale Metal/Silicate Equilibration During Core Formation: The Influence of Stretching Enhanced Diffusion on Mixing. Journal of Geophysical Research: Solid Earth 123, 10,496–10,516.
 Show in context
Show in context The equilibration of metal and silicate melts is rapid and complete during this process (Kendall and Melosh, 2016; Lherm and Deguen, 2018) and thus the siderophile element content of the molten portion of the planet will change as the depth and associated pressure of metal-silicate equilibrium increases (e.g., Rubie et al., 2003).
View in article
McDonough, W.F. (2003) Compositional model for the Earth’s core. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 2. Pergamon, Oxford, 547–568.
 Show in context
Show in context Concentrations of siderophile elements in the crust (Rudnick and Gao, 2014) and core (McDonough, 2003) of the Earth, normalised to values in the primitive upper mantle (PUM) (Palme and O’Neill, 2014).
View in article
Morard, G., Sanloup, C., Fiquet, G., Mezouar, M., Rey, N., Poloni, R., Beck, P. (2007) Structure of eutectic Fe–FeS melts to pressures up to 17 GPa: implications for planetary cores. Earth and Planetary Science Letters 263, 128–139.
 Show in context
Show in context Some changes are proposed at higher pressures (e.g., Morard et al., 2007; Fe-S), and so detection of changes in activity coefficients at higher pressures should be pursued as well.
View in article
Münker, C., Fonseca, R.O., Schulz, T. (2017) Silicate Earth’s missing niobium may have been sequestered into asteroidal cores. Nature Geoscience 10, 822–826.
 Show in context
Show in context Additionally, a better understanding of the effect of pressure on Nb would be important to evaluating the Nb content of the mantle of differentiated bodies and the overall distribution cores and mantles in differentiated bodies (e.g., Nebel et al., 2010; Münker et al., 2017).
View in article
Nebel, O., van Westrenen, W., Vroon, P.Z., Wille, M., Raith, M.M. (2010) Deep mantle storage of the Earth’s missing niobium in late-stage residual melts from a magma ocean. Geochimica et Cosmochimica Acta 74, 4392–4404.
 Show in context
Show in context Additionally, a better understanding of the effect of pressure on Nb would be important to evaluating the Nb content of the mantle of differentiated bodies and the overall distribution cores and mantles in differentiated bodies (e.g., Nebel et al., 2010; Münker et al., 2017).
View in article
Palme, H., O’Neill, H.S.C. (2014) Cosmochemical estimates of mantle composition. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 3. Second edition, Elsevier, Oxford, 1–39.
 Show in context
Show in context Primitive upper mantle (PUM) siderophile element concentrations (from Palme and O’Neill, 2014) are horizontal lines with colour matching the symbols of the calculated values in each panel.
View in article
Concentrations of siderophile elements in the crust (Rudnick and Gao, 2014) and core (McDonough, 2003) of the Earth, normalised to values in the primitive upper mantle (PUM) (Palme and O’Neill, 2014).
View in article
Righter, K. (2011) Prediction of metal-silicate partition coefficients for siderophile elements: An update and assessment of PT conditions for metal-silicate equilibrium during accretion of the Earth. Earth and Planetary Science Letters 304 158–167.
 Show in context
Show in context The Earth’s core is estimated to have formed at pressures between 40–60 GPa (Wade and Wood, 2005; Righter, 2011; Siebert et al., 2011).
View in article
Righter, K. (2016) Metal-Silicate Partitioning of Siderophile Elements and Core-Mantle Segregation. Deep Earth: Physics and Chemistry of the Lower Mantle and Core, Geophysical Monograph 217, 161–174.
 Show in context
Show in context Pressure is known to influence volumetric properties of metallic and silicate liquids (e.g., Armstrong et al., 2019), and also affect oxygen fugacity (e.g., Righter, 2016), but less is known about the effect of pressure on activity coefficients (e.g., Steenstra et al., 2020).
View in article
Righter, K., Pando, K., Humayun, M., Waeselmann, N., Yang, S., Boujibar, A., Danielson, L.R. (2018) Effect of silicon on activity coefficients of siderophile elements (Au, Pd, Pt, P, Ga, Cu, Zn, and Pb) in liquid Fe: Roles of core formation, late sulfide matte, and late veneer in shaping terrestrial mantle geochemistry. Geochimica et Cosmochimica Acta 232, 101–123.
 Show in context
Show in context Because Si is a major alloying agent in metallic cores, its solution in Fe metallic liquids can have a significant influence on the activity coefficients of siderophile elements, and thus the partitioning behaviour of those elements between the core and mantle (Tuff et al., 2011; Righter et al., 2018).
View in article
We derive interaction parameters for Fe-Si liquids for comparison to behaviour already investigated at 1 GPa, using the same starting materials (Righter et al., 2018).
View in article
Calculated concentrations of some elements such as Ag, Cu, Au, Pd, Pt become higher than PUM values, indicating the need for a removal mechanism such as a sulfide matte or late metallic segregation (see Righter et al. 2018, for detailed discussion).
View in article
For example, enhanced concentration of P in terrestrial or extrasolar planetary mantles is expected in equilibrium with Fe-Si liquids (Righter et al., 2018).
View in article
Righter, K., Pando, K., Ross, D.K., Righter, M., Lapen, T.J. (2019) Effect of silicon on activity coefficients of Bi, Cd, Sn, and Ag in liquid Fe-Si, and implications for differentiation and core formation. Meteoritics & Planetary Science 54, 1379–1394.
 Show in context
Show in context Second, the highly siderophile elements (e.g., Au, Ru, Ir, Os), chalcophile elements (Cu, Ag), and volatile trace metals are frequently employed in constraining post core formation and late accretion processes (e.g., Walker, 2009) as well as how Earth (and other bodies) acquired volatiles in general (e.g., Halliday, 2013; Righter et al., 2019).
View in article
Righter, K., Herd, C.D., Boujibar, A. (2020) Redox processes in early Earth accretion and in terrestrial bodies. Elements: An International Magazine of Mineralogy, Geochemistry, and Petrology 16, 161–166.
 Show in context
Show in context The dissolution of these elements, and in particular Si, is dependent upon oxygen fugacity (Righter et al., 2020), which also controls the solubility of trace elements in metallic and silicate melts.
View in article
Rubie, D.C., Melosh, H.J., Reid, J.E., Liebske, C., Righter, K. (2003) Mechanisms of metal–silicate equilibration in the terrestrial magma ocean. Earth and Planetary Science Letters 205, 239–255.
 Show in context
Show in context The equilibration of metal and silicate melts is rapid and complete during this process (Kendall and Melosh, 2016; Lherm and Deguen, 2018) and thus the siderophile element content of the molten portion of the planet will change as the depth and associated pressure of metal-silicate equilibrium increases (e.g., Rubie et al., 2003).
View in article
Rudnick, R.L., Gao, S. (2014) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 4. Second edition, Elsevier, Oxford, 1–51.
 Show in context
Show in context Concentrations of siderophile elements in the crust (Rudnick and Gao, 2014) and core (McDonough, 2003) of the Earth, normalised to values in the primitive upper mantle (PUM) (Palme and O’Neill, 2014).
View in article
Sanloup, C., Fiquet, G., Gregoryanz, E., Morard, G., Mezouar, M. (2004) Effect of Si on liquid Fe compressibility: Implications for sound velocity in core materials. Geophysical Research Letters 31, L07604.
 Show in context
Show in context It is possible that Fe-Si liquids do not undergo such structural changes below 10 GPa (e.g., Sanloup et al., 2004), and thus we do not see a change in activity coefficients.
View in article
Sanloup, C., Van Westrenen, W., Dasgupta, R., Maynard-Casely, H., Perrillat, J.P. (2011) Compressibility change in iron-rich melt and implications for core formation models. Earth and Planetary Science Letters 306, 118–122.
 Show in context
Show in context Most elements in these 4 groups exhibit no pressure effect on the activity coefficients in Fe-Si liquids, perhaps a surprising result because some have argued that structural changes in metallic liquids in this pressure range may lead to changes in partitioning behaviour in the Fe-C and Fe-Si liquids (5.2 GPa in Fe-C; Sanloup et al., 2011, and 1-4 GPa in Fe-Si; Shibazaki et al. 2015).
View in article
Shibazaki, Y., Kono, Y., Fei, Y. (2015) Microscopic structural change in a liquid Fe-C alloy of ∼5 GPa. Geophysical Research Letters 42, 5236–5242.
 Show in context
Show in context Most elements in these 4 groups exhibit no pressure effect on the activity coefficients in Fe-Si liquids, perhaps a surprising result because some have argued that structural changes in metallic liquids in this pressure range may lead to changes in partitioning behaviour in the Fe-C and Fe-Si liquids (5.2 GPa in Fe-C; Sanloup et al., 2011, and 1-4 GPa in Fe-Si; Shibazaki et al. 2015).
View in article
Siebert, J., Corgne, A., Ryerson, F.J. (2011) Systematics of metal-silicate partitioning for many siderophile elements applied to Earth’s core formation. Geochimica et Cosmochimica Acta 75, 1451–1489.
 Show in context
Show in context The Earth’s core is estimated to have formed at pressures between 40–60 GPa (Wade and Wood, 2005; Righter, 2011; Siebert et al., 2011).
View in article
Steenstra, E.S., Seegers, A.X., Putter, R., Berndt, J., Klemme, S., Matveev, S., Bullock, E.S., van Westrenen, W. (2020) Metal-silicate partitioning systematics of siderophile elements at reducing conditions: A new experimental database. Icarus 335, 113391.
 Show in context
Show in context Pressure is known to influence volumetric properties of metallic and silicate liquids (e.g., Armstrong et al., 2019), and also affect oxygen fugacity (e.g., Righter, 2016), but less is known about the effect of pressure on activity coefficients (e.g., Steenstra et al., 2020).
View in article
Steenstra et al. (2020) examine the effect of pressure on  combining their data at 1 to 4 GPa with 11 GPa data from Vogel et al. (2018), for 12 elements, some of which we have examined here. Our results for Cd, Pb, Mn, V, and Sn are in overall agreement – none of these elements shows discernable change between 1 and 10 GPa.
combining their data at 1 to 4 GPa with 11 GPa data from Vogel et al. (2018), for 12 elements, some of which we have examined here. Our results for Cd, Pb, Mn, V, and Sn are in overall agreement – none of these elements shows discernable change between 1 and 10 GPa.
View in article
Steenstra et al. (2020) also present some evidence for change at high pressure for  for Ni, In, As and Sb, but change in those series occurs between 1 and 2 GPa and change above that is negligible (i.e., 2 GPa = 11 GPa values for As, Sb, Sn). Pb, Cu, Cr, Mn, V, and Cd
for Ni, In, As and Sb, but change in those series occurs between 1 and 2 GPa and change above that is negligible (i.e., 2 GPa = 11 GPa values for As, Sb, Sn). Pb, Cu, Cr, Mn, V, and Cd  all show no trend at higher pressures, similar to most elements we have examined here.
all show no trend at higher pressures, similar to most elements we have examined here.
View in article
Steelmaking Data Sourcebook (1988) The Japan Society for the Promotion of Science: The 19th Committee on Steelmaking. Gordon and Breach Science Publishers, Montreux.
 Show in context
Show in context However, the low pressure value in Figure 1 was taken from the Steelmaking Data Sourcebook (1988) and is a value of −0.66, compared to our 10 GPa value of 7.0 ± 8.3.
View in article
Tuff, J., Wood, B.J., Wade, J. (2011) The effect of Si on metal-silicate partitioning of siderophile elements and implications for the conditions of core formation Geochimica et Cosmochimica Acta 75, 673–690.
 Show in context
Show in context Because Si is a major alloying agent in metallic cores, its solution in Fe metallic liquids can have a significant influence on the activity coefficients of siderophile elements, and thus the partitioning behaviour of those elements between the core and mantle (Tuff et al., 2011; Righter et al., 2018).
View in article
Vogel, A.K., Jennings, E.S., Laurenz, V., Rubie, D.C., Frost, D.J. (2018) The dependence of metal-silicate partitioning of moderately volatile elements on oxygen fugacity and Si contents of Fe metal: Implications for their valence states in silicate liquids. Geochimica et Cosmochimica Acta 237, 275–293.
 Show in context
Show in context Steenstra et al. (2020) examine the effect of pressure on  combining their data at 1 to 4 GPa with 11 GPa data from Vogel et al. (2018), for 12 elements, some of which we have examined here. Our results for Cd, Pb, Mn, V, and Sn are in overall agreement – none of these elements shows discernable change between 1 and 10 GPa.
combining their data at 1 to 4 GPa with 11 GPa data from Vogel et al. (2018), for 12 elements, some of which we have examined here. Our results for Cd, Pb, Mn, V, and Sn are in overall agreement – none of these elements shows discernable change between 1 and 10 GPa.
View in article
Wade, J., Wood, B.J. (2005) Core formation and the oxidation state of the Earth. Earth and Planetary Science Letters 236, 78–95.
 Show in context
Show in context The Earth’s core is estimated to have formed at pressures between 40–60 GPa (Wade and Wood, 2005; Righter, 2011; Siebert et al., 2011).
View in article
Walker, R.J. (2009) Highly siderophile elements in the Earth, Moon and Mars: update and implications for planetary accretion and differentiation. Chemie der Erde-Geochemistry 69, 101–125.
 Show in context
Show in context Second, the highly siderophile elements (e.g., Au, Ru, Ir, Os), chalcophile elements (Cu, Ag), and volatile trace metals are frequently employed in constraining post core formation and late accretion processes (e.g., Walker, 2009) as well as how Earth (and other bodies) acquired volatiles in general (e.g., Halliday, 2013; Righter et al., 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1) Experimental and Analytical Techniques
- 2) Phase Equilibria and Equilibrium
- 3) Determination of Epsilon Interaction Parameters
- 4) Stability of Phosphides in Early Earth Mantle
- Figures S-1 to S-3
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
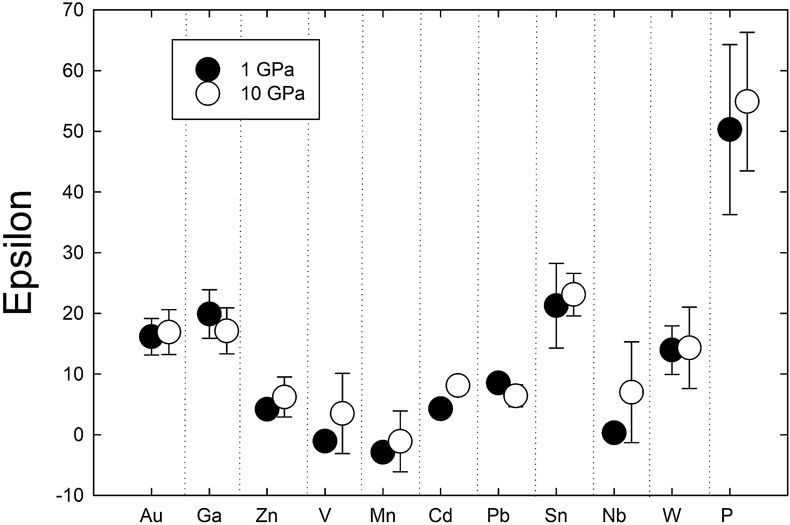
Figure 1 Comparison of 1 GPa and 10 GPa epsilon interaction parameters, with the 1 GPa values re-calculated from 1873 to 2373 K as discussed in the text. P, Au, Ga, Sn, Cd, Pb, Nb, W, Zn and V all have positive
 , indicating that Si will cause a decrease in D metal/silicate with Si present in the metallic liquid. Mn has a negative
, indicating that Si will cause a decrease in D metal/silicate with Si present in the metallic liquid. Mn has a negative  , indicating that Si will cause a very slight increase in D(Mn) metal/silicate with Si present in the metallic liquid. Nb exhibits the largest difference in measured in
, indicating that Si will cause a very slight increase in D(Mn) metal/silicate with Si present in the metallic liquid. Nb exhibits the largest difference in measured in  at low pressure and 10 GPa, suggesting there might be a measurable pressure effect at even higher pressures.
at low pressure and 10 GPa, suggesting there might be a measurable pressure effect at even higher pressures.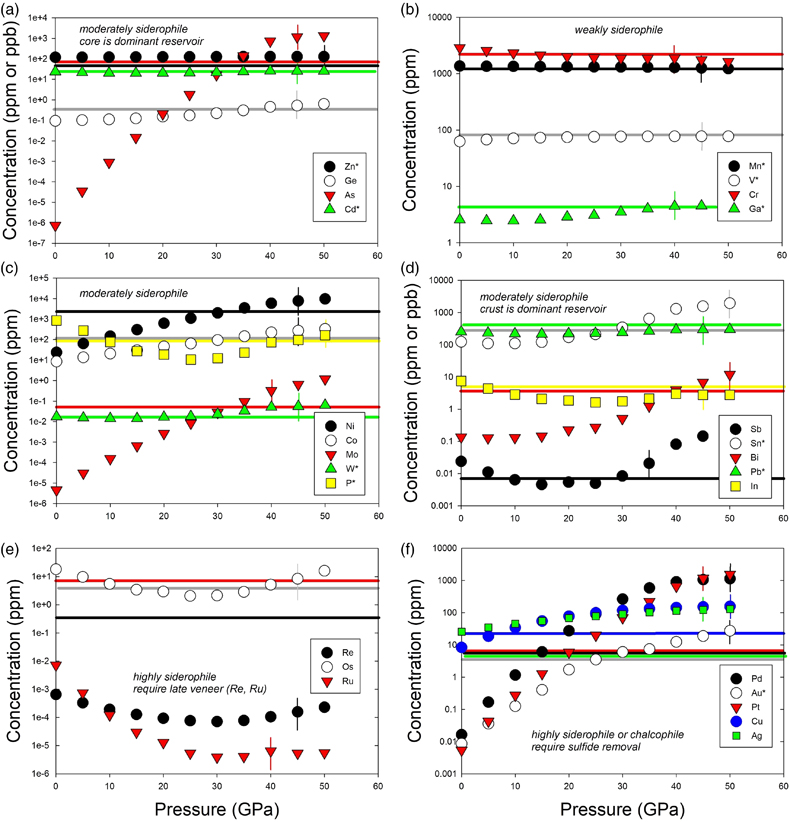
Figure 2 Evolution of siderophile element content of a terrestrial magma ocean as accretion proceeds and the PT conditions of metal-silicate equilibrium increase. Primitive upper mantle (PUM) siderophile element concentrations (from Palme and O’Neill, 2014
Palme, H., O’Neill, H.S.C. (2014) Cosmochemical estimates of mantle composition. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 3. Second edition, Elsevier, Oxford, 1–39.
) are horizontal lines with colour matching the symbols of the calculated values in each panel. The concentrations of 19 elements can be explained by metal-silicate equilibrium in a near 30–40 GPa magma ocean (Ni, Co, Mo, W, P, Mn, V, Cr, Ga, Zn, In, Ge, Sb, As, Sn, Bi, Cd, and Pb). Calculated concentrations of some elements such as Ag, Cu, Au, Pd, Pt become higher than PUM values, indicating the need for a removal mechanism such as a sulfide matte or late metallic segregation (see Righter et al. 2018Righter, K., Pando, K., Humayun, M., Waeselmann, N., Yang, S., Boujibar, A., Danielson, L.R. (2018) Effect of silicon on activity coefficients of siderophile elements (Au, Pd, Pt, P, Ga, Cu, Zn, and Pb) in liquid Fe: Roles of core formation, late sulfide matte, and late veneer in shaping terrestrial mantle geochemistry. Geochimica et Cosmochimica Acta 232, 101–123.
, for detailed discussion). These 5 elements, together with Re, Os, and Ru, ultimately have their mantle concentrations set by addition of chondritic material after core formation and sulfide segregation.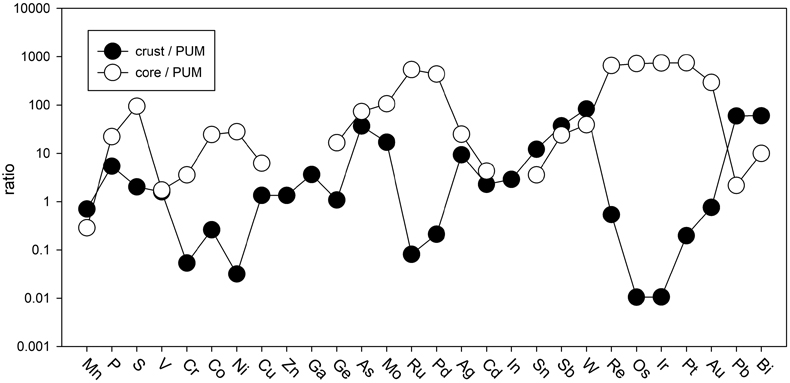
Figure 3 Concentrations of siderophile elements in the crust (Rudnick and Gao, 2014
Rudnick, R.L., Gao, S. (2014) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 4. Second edition, Elsevier, Oxford, 1–51.
) and core (McDonough, 2003McDonough, W.F. (2003) Compositional model for the Earth’s core. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 2. Pergamon, Oxford, 547–568.
) of the Earth, normalised to values in the primitive upper mantle (PUM) (Palme and O’Neill, 2014Palme, H., O’Neill, H.S.C. (2014) Cosmochemical estimates of mantle composition. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 3. Second edition, Elsevier, Oxford, 1–39.
). This diagram demonstrates that most siderophile elements are concentrated into the core, but there is an important and significant subset that is more highly concentrated into the crust, including Mn, Bi, Pb, In, Sn, Sb, and W.