Ca isotope systematics of carbonatites: Insights into carbonatite source and evolution
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:806Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
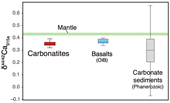
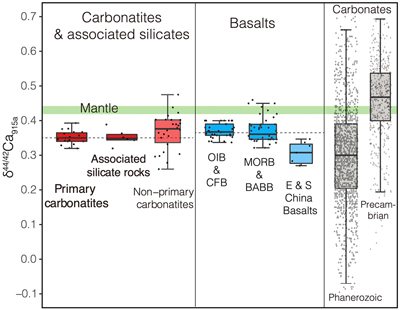 Figure 1 Comparison of δ44/42Ca values between carbonatites, basalts, mantle, and sedimentary carbonates. The dashed lines represent average δ44/42Ca values of carbonatites and basalts, respectively. Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019). | 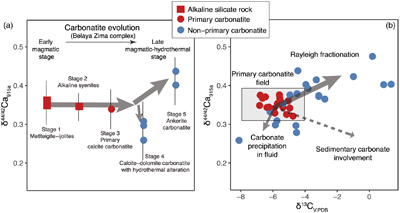 Figure 2 Plots showing δ44/42Ca variation during carbonatite evolution. (a) δ44/42Ca for different types of carbonatites and associated silicate rocks from Belaya Zima complex, Russia. (b) δ44/42Ca vs. δ13C plot for carbonatites. See data sources in Table S-1. | 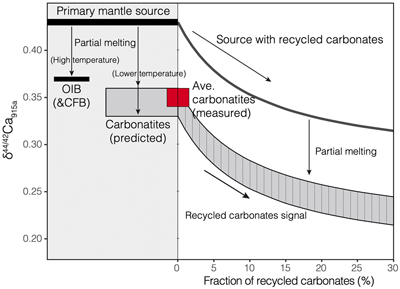 Figure 3 Expected δ44/42Ca values of carbonatites originating from a primary mantle source and mantle sources involved with different fractions of (Phanerozoic) recycled carbonates. The measured δ44/42Ca data for carbonatites is consistent with an origin from the primary mantle source mixed with little (<2 %) recycled carbonates. See detailed description in the main text. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Carbonatite is an exotic igneous rock formed predominantly of carbonates and an important host or source of critical metals, including REE and Nb (Woolley and Kempe, 1989
Woolley, A.R., Kempe, D.R.C. (1989) Carbonatites: nomenclature, average chemical compositions, and element distribution. In: Bell, K. (Ed.) Carbonatites: genesis and evolution. Unwin Hyman, London, 1–14.
; Sun et al., 2013Sun, J., Zhu, X., Chen, Y., Fang, N. (2013) Iron isotopic constraints on the genesis of Bayan Obo ore deposit, Inner Mongolia, China. Precambrian Research 235, 88–106.
; Verplanck et al., 2016Verplanck, P.L., Mariano, A.N., Mariano Jr, A. (2016) Rare Earth Element Ore Geology of Carbonatites. Reviews in Economic Geology, 5–32.
). It is closely related to the deep carbon cycle which can provide insights into mantle-to-crust carbon transfer. Consensus has been made that carbonatite melts are derived from the carbonate-bearing mantle (Dasgupta et al., 2007Dasgupta, R., Hirschmann, M.M., Smith, N.D. (2007) Partial Melting Experiments of Peridotite + CO2 at 3 GPa and Genesis of Alkalic Ocean Island Basalts. Journal of Petrology 48, 2093–2124.
; Bell and Simonetti, 2010Bell, K., Simonetti, A. (2010) Source of parental melts to carbonatites–critical isotopic constraints. Mineralogy and Petrology 98, 77–89.
). However, whether the carbon generating the carbonatite was originally sourced from the primitive mantle or a recycled component remains debated (Barker, 1996Barker, D.S. (1996) Consequences of recycled carbon in carbonatites. Canadian Mineralogist 34, 373–388.
; Hoernle et al., 2002Hoernle, K., Tilton, G., Le Bas, M.J., Duggen, S., Garbe-Schönberg, D. (2002) Geochemistry of oceanic carbonatites compared with continental carbonatites: mantle recycling of oceanic crustal carbonate. Contributions to Mineralogy and Petrology 142, 520–542.
; Bell and Simonetti, 2010Bell, K., Simonetti, A. (2010) Source of parental melts to carbonatites–critical isotopic constraints. Mineralogy and Petrology 98, 77–89.
). C and O isotopes are the most direct tracers for recycled carbonates as carbon and oxygen are the major elements in carbonates. However, their primary isotope signatures tend to be fractionated or affected by late stage fluids and magma degassing (Deines, 1989Deines, P. (1989) Stable isotope variations in carbonatites. In: Bell, K. (Ed.)Carbonatites: Genesis and Evolution. Unwin Hyman, London, 301–359.
).Ca is the most common and abundant metal in carbonatites (Woolley and Kempe, 1989
Woolley, A.R., Kempe, D.R.C. (1989) Carbonatites: nomenclature, average chemical compositions, and element distribution. In: Bell, K. (Ed.) Carbonatites: genesis and evolution. Unwin Hyman, London, 1–14.
). Ca isotopes have emerged as a novel tool for tracing recycled carbonates in the mantle (Huang et al., 2011Huang, S., Farkaš, J., Jacobsen, S.B. (2011) Stable calcium isotopic compositions of Hawaiian shield lavas: Evidence for recycling of ancient marine carbonates into the mantle. Geochimica et Cosmochimica Acta 75, 4987–4997.
; Liu et al., 2017Liu, F., Li, X., Wang, G., Liu, Y., Zhu, H., Kang, J., Huang, F., Sun, W., Xia, X., Zhang, Z. (2017) Marine Carbonate Component in the Mantle Beneath the Southeastern Tibetan Plateau: Evidence From Magnesium and Calcium Isotopes: Carbonates tracked by Mg and Ca isotopes. Journal of Geophysical Research: Solid Earth 122, 9729–9744.
). This is because, 1) Ca isotope compositions of surface carbonate and the mantle peridotite are distinct (Fantle and Tipper, 2014Fantle, M.S., Tipper, E.T. (2014) Calcium isotopes in the global biogeochemical Ca cycle: Implications for development of a Ca isotope proxy. Earth-Science Reviews 129, 148–177.
; Kang et al., 2017Kang, J.-T., Ionov, D.A., Liu, F., Zhang, C.-L., Golovin, A.V., Qin, L.-P., Zhang, Z.-F., Huang, F. (2017) Calcium isotopic fractionation in mantle peridotites by melting and metasomatism and Ca isotope composition of the Bulk Silicate Earth. Earth and Planetary Science Letters 474, 128–137.
), 2) Ca abundance of the former is nearly one order of magnitude higher than that of the later, and 3) Ca isotope fractionation is negligible during (basaltic) magmatic differentiation (Zhang et al., 2018Zhang, H., Wang, Y., He, Y., Teng, F.-Z., Jacobsen, S.B., Helz, R.T., Marsh, B.D., Huang, S. (2018) No measurable calcium isotopic fractionation during crystallization of Kilauea Iki lava lake. Geochemistry, Geophysics, Geosystems 19, 3128–3139.
; Chen et al., 2019Chen, C., Dai, W., Wang, Z., Liu, Y., Li, M., Becker, H., Foley, S.F. (2019) Calcium isotope fractionation during magmatic processes in the upper mantle. Geochimica et Cosmochimica Acta 249, 121–137.
).Previous studies indicate the potential of Ca isotopes in mantle-derived rocks for tracing recycled carbonates but the Ca isotope composition of carbonatites has remained poorly constrained, and the reported δ44/42Ca values are inconsistent among different groups (Amini et al., 2009
Amini, M., Eisenhauer, A., Böhm, F., Holmden, C., Kreissig, K., Hauff, F., Jochum, K.P. (2009) Calcium Isotopes (δ44/40Ca) in MPI-DING Reference Glasses, USGS Rock Powders and Various Rocks: Evidence for Ca Isotope Fractionation in Terrestrial Silicates. Geostandards and Geoanalytical Research 33, 231–247.
; Maloney, 2018Maloney, M. (2018) Calcium Isotopes in Natural and Experimental Carbonated Silicate Melts. PhD Thesis, Western University, Electronic Thesis and Dissertation Repository, 5256.
; Banerjee and Chakrabarti, 2019Banerjee, A., Chakrabarti, R. (2019) A geochemical and Nd, Sr and stable Ca isotopic study of carbonatites and associated silicate rocks from the ∼65 Ma old Ambadongar carbonatite complex and the Phenai Mata igneous complex, Gujarat, India: Implications for crustal contamination, carbonate recycling, hydrothermal alteration and source-mantle mineralogy. Lithos 326–327, 572–585.
; Amsellem et al., 2020Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6, eaba3269.
). Here, based on a refined analytical protocol, the Ca isotope composition of worldwide carbonatites and associated silicate rocks is investigated, Ca isotope behaviour during carbonatite evolution is studied, and the role of recycled carbonates in producing carbonatite is assessed.top
Basalt-like Ca Isotope Compositions for Carbonatites
Previously reported Ca isotope data of carbonatites have significant δ44/42Ca variations and the values are inconsistent among different groups (Fig. S-1). The scatter may partly be related to inter-laboratory bias. Carbonatites are unusual rocks (extremely enriched in critical metals such as rare earth elements), and no analytical method developed specifically for this kind of sample has been reported so far. To ensure the accuracy of Ca isotope measurements using the SSB-MC-ICPMS method, much effort has been spent on methodology in this study. These include: (1) detailed investigation on effects of matrix elements, particularly REE, on Ca isotope analysis, which were largely ignored previously but turn out to be serious, (2) a new protocol of column chemistry developed based on cation ion exchange resin (with higher stability than the DGA extraction resin that is often used), where all possible matrix elements are examined to be sure of being eliminated effectively (to a level of Element/Ca <0.0001). Any other possible analytical pitfalls, including a “column effect”, Sr effect or “column fractionation”, were avoided (see details of analytical method in SI), (3) inter-laboratory comparison was made by measuring eight standard reference materials and four carbonatite samples both performed in CAGS lab using this rigorous method and performed in CUGB lab using a DS-TIMS method reported by He et al. (2017)
He, Y., Wang, Y., Zhu, C., Huang, S., Li, S. (2017) Mass-Independent and Mass-Dependent Ca Isotopic Compositions of Thirteen Geological Reference Materials Measured by Thermal Ionisation Mass Spectrometry. Geostandards and Geoanalytical Research 41, 283–302.
, where all measured δ44/42Ca values are consistent within analytical precision (Figs. S-1, S-2, Tables S-1, S-2).Using the refined Ca isotope analytical method, we analysed 47 samples of carbonatites and associated silicate rocks from 15 occurrences from Canada, America, East Africa, Russia, Mongolia, Brazil and China (see details of sample information and their geological background in SI), along with analyses of their major elements and C-O isotope compositions (see analytical methods in SI). The results of δ44/42Ca fall within the range previously reported (Fig. S-1). To avoid any possible inter-laboratory bias and make a better estimate of Ca isotope composition of carbonatites, only those previously reported data sets with their accuracies demonstrated by carbonatite standards/samples and with the analytical precision similar or better than ours are used. The available data (Table S-1) give a range of 0.26 ‰ to 0.47 ‰ for δ44/42Ca in carbonatites, with most clustering around 0.35 ‰ (Figs. 1, S-3, Table S-1). Notably, primary carbonatites and associated silicate rocks are homogeneous in Ca isotope composition with an average of 0.35 ± 0.01 ‰ (2 s.e., n = 30) (Fig. 1), close to those of worldwide basalts (average δ44/42Ca = 0.37 ± 0.01 ‰, 2 s.e., n = 84); while non-primary carbonatites have detectable δ44/42Ca variations (Figs. 1, S-3). This estimate is significantly different to that made by Amsellem et al. (2020)
Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6, eaba3269.
, and accordingly leads to a different understanding on the nature of the carbonatite source as discussed below.
Figure 1 Comparison of δ44/42Ca values between carbonatites, basalts, mantle, and sedimentary carbonates. The dashed lines represent average δ44/42Ca values of carbonatites and basalts, respectively. Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009
Amini, M., Eisenhauer, A., Böhm, F., Holmden, C., Kreissig, K., Hauff, F., Jochum, K.P. (2009) Calcium Isotopes (δ44/40Ca) in MPI-DING Reference Glasses, USGS Rock Powders and Various Rocks: Evidence for Ca Isotope Fractionation in Terrestrial Silicates. Geostandards and Geoanalytical Research 33, 231–247.
; Simon and DePaolo, 2010Simon, J.I., DePaolo, D.J. (2010) Stable calcium isotopic composition of meteorites and rocky planets. Earth and Planetary Science Letters 289, 457–466.
; Fantle and Tipper, 2014Fantle, M.S., Tipper, E.T. (2014) Calcium isotopes in the global biogeochemical Ca cycle: Implications for development of a Ca isotope proxy. Earth-Science Reviews 129, 148–177.
; Jacobson et al., 2015Jacobson, A.D., Grace Andrews, M., Lehn, G.O., Holmden, C. (2015) Silicate versus carbonate weathering in Iceland: New insights from Ca isotopes. Earth and Planetary Science Letters 416, 132–142.
; Blättler and Higgins, 2017Blättler, C.L., Higgins, J.A. (2017) Testing Urey’s carbonate–silicate cycle using the calcium isotopic composition of sedimentary carbonates. Earth and Planetary Science Letters 479, 241–251.
; Kang et al., 2017Kang, J.-T., Ionov, D.A., Liu, F., Zhang, C.-L., Golovin, A.V., Qin, L.-P., Zhang, Z.-F., Huang, F. (2017) Calcium isotopic fractionation in mantle peridotites by melting and metasomatism and Ca isotope composition of the Bulk Silicate Earth. Earth and Planetary Science Letters 474, 128–137.
; Liu et al., 2017Liu, F., Li, X., Wang, G., Liu, Y., Zhu, H., Kang, J., Huang, F., Sun, W., Xia, X., Zhang, Z. (2017) Marine Carbonate Component in the Mantle Beneath the Southeastern Tibetan Plateau: Evidence From Magnesium and Calcium Isotopes: Carbonates tracked by Mg and Ca isotopes. Journal of Geophysical Research: Solid Earth 122, 9729–9744.
; Zhang et al., 2018Zhang, H., Wang, Y., He, Y., Teng, F.-Z., Jacobsen, S.B., Helz, R.T., Marsh, B.D., Huang, S. (2018) No measurable calcium isotopic fractionation during crystallization of Kilauea Iki lava lake. Geochemistry, Geophysics, Geosystems 19, 3128–3139.
; Zhu et al., 2018Zhu, H., Liu, F., Li, X., Wang, G., Zhang, Z., Sun, W. (2018) Calcium Isotopic Compositions of Normal Mid-Ocean Ridge Basalts From the Southern Juan de Fuca Ridge. Journal of Geophysical Research: Solid Earth 123, 1303–1313.
, 2020Zhu, H., Du, L., Li, X., Zhang, Z., Sun, W. (2020) Calcium isotopic fractionation during plate subduction: Constraints from back-arc basin basalts. Geochimica et Cosmochimica Acta 270, 379–393.
; Chen et al., 2019Chen, C., Dai, W., Wang, Z., Liu, Y., Li, M., Becker, H., Foley, S.F. (2019) Calcium isotope fractionation during magmatic processes in the upper mantle. Geochimica et Cosmochimica Acta 249, 121–137.
).top
Ca Isotope Fractionation during Carbonatite Evolution and Implications for Carbonatite Petrogenesis
The processes relevant to the formation of carbonatite may involve partial melting of the mantle, magmatic differentiation, magmatic-hydrothermal processes and secondary hydrothermal alteration. Understanding Ca isotope behaviour during these processes is fundamental for tracing the carbonatite source using Ca isotopes.
The extent of Ca isotope fractionation during carbonatite evolution is investigated through a suite of carbonatite and associated silicate rocks from the Belaya Zima complex, a typical “nephelinite-clan carbonatite”, the most common carbonatite group worldwide (see details in SI). Associated rocks include early magmatic alkaline silicate rocks and primary calcite carbonatites (both containing melt inclusions) through to more evolved late magmatic-hydrothermal calcite-dolomite carbonatites and ferro-carbonatites (see descriptions in SI). The rocks of early magmatic stages show homogeneous δ44/42Ca values (around 0.35 ‰), while the later stages exhibit either lower or higher δ44/42Ca values (0.26 ‰ to 0.44 ‰) (Fig. 2a), suggesting that Ca isotopes fractionate insignificantly during magmatic processes but moderately during late stage magmatic-hydrothermal processes.

Figure 2 Plots showing δ44/42Ca variation during carbonatite evolution. (a) δ44/42Ca for different types of carbonatites and associated silicate rocks from Belaya Zima complex, Russia. (b) δ44/42Ca vs. δ13C plot for carbonatites. See data sources in Table S-1.
The extent of Ca isotope fractionation during secondary meteoric alteration is examined from Songwe carbonatite samples that have suffered variable degrees of low temperature meteoric alteration (see descriptions in SI). Although δ18O values vary significantly, the δ44/42Ca variation is small and falls at the edge of the range of primary carbonatite (Table S-1), implying that Ca isotope fractionation during this process is subtle.
Ca and C are normally associated in carbonatite evolution. Using coupled Ca-C stable isotope data, Ca isotope behaviour during carbonatite evolution is further assessed. In the Ca-C isotope plot, it is interesting to note that there exists a correlation between δ44/42Ca and δ13C for worldwide carbonatites (Fig. 2b). A “primary carbonatite field” can be defined based on the Ca-C isotope data of primary carbonatites. Most non-primary carbonatites tend to have higher δ44/42Ca and δ13C values, and a few tend to have lower δ44/42Ca and δ13C values. A δ13C higher than the “mantle values” for carbonatites may result from, (1) Rayleigh fractionation processes in fluid-rich carbonatite magma, and (2) incorporation of sedimentary carbonates before or after carbonatite emplacement. The latter case, however, would result in coupled higher δ13C-lower δ44/42Ca data, which are not observed (Fig. 2b). Hence, the correlation between δ44/42Ca and δ13C implies that Ca isotope variation in carbonatites is dominated by Rayleigh fractionation processes in fluid-rich carbonatite magma—a process also resulting in correlated C-O isotope variation in carbonatites (Deines, 1989
Deines, P. (1989) Stable isotope variations in carbonatites. In: Bell, K. (Ed.)Carbonatites: Genesis and Evolution. Unwin Hyman, London, 301–359.
).The little δ44/42Ca variation in Belaya Zima and other worldwide primary carbonatites and associated silicate rocks suggest that Ca fractionation is negligible during carbonatite magmatic differentiation, as that has been observed for basaltic magmatic differentiation (Zhang et al., 2018
Zhang, H., Wang, Y., He, Y., Teng, F.-Z., Jacobsen, S.B., Helz, R.T., Marsh, B.D., Huang, S. (2018) No measurable calcium isotopic fractionation during crystallization of Kilauea Iki lava lake. Geochemistry, Geophysics, Geosystems 19, 3128–3139.
; Chen et al., 2019Chen, C., Dai, W., Wang, Z., Liu, Y., Li, M., Becker, H., Foley, S.F. (2019) Calcium isotope fractionation during magmatic processes in the upper mantle. Geochimica et Cosmochimica Acta 249, 121–137.
). This is consistent with the theoretical prediction that the equilibrium Ca isotope fractionation between Ca-dominated minerals in high temperature conditions is minimal, e.g., Δ44/42Cacalcite-cpx < 0.02 ‰ at a temperature over 800 °C (Wang et al., 2017Wang, W., Qin, T., Zhou, C., Huang, S., Wu, Z., Huang, F. (2017) Concentration effect on equilibrium fractionation of Mg-Ca isotopes in carbonate minerals: Insights from first-principles calculations. Geochimica et Cosmochimica Acta 208, 185–197.
; Chen et al., 2019Chen, C., Dai, W., Wang, Z., Liu, Y., Li, M., Becker, H., Foley, S.F. (2019) Calcium isotope fractionation during magmatic processes in the upper mantle. Geochimica et Cosmochimica Acta 249, 121–137.
). Hence, the Ca isotope signature of primary carbonatites and associated silicate rocks reflects the composition of their parental carbonatite melts, which is δ44/42Ca = 0.35 ± 0.01 ‰ (2 s.e., n = 30).Detectable Ca isotope fractionation during late stages of carbonatite evolution is likely related to the involvement of fluids. Precipitation of carbonate from the CO2-fluid-bearing carbonatite melt/liquid likely leads to Ca and C isotope fractionation (possibly kinetic), driving the evolved carbonatite melt/liquid isotopically heavier by Rayleigh fractionation. Ca isotopes are thus a useful tool for studying the petrogenesis of carbonatite and may further provide insights in relevant REE mineralisation with which it is normally associated. For example, Ca isotopes may help to discriminate between magmatic and magmatic-hydrothermal carbonatite, which is a critical for understanding REE mineralisation in carbonatites. It may also help to constrain the origin of late stage ankerite carbonatite (ferrocarbonatite), i.e. whether a primary product crystallised from the melt/liquid or a metasomatic product replacing earlier calcite-carbonatite (see details in SI).
top
No Requirement for Recycled Carbonate in a Carbonatite Source
The source of carbonatite has commonly believed to be carbonate-bearing, but whether the carbon is primitive or recycled surface carbonate remains controversial.
Assuming a carbonatite melt is derived from the “normal” mantle, its expected Ca isotope composition can be modelled and calculated using available equilibrium Ca isotope fractionation factors of minerals in the literature (Table S-5). The partial melting conditions are assumed to OIB-like in pressure but with a lower temperature, as the presence of CO2 (which is required to generate carbonatite melts) may depress the solidus of peridotite by ∼100–400 °C (Dasgupta et al., 2007
Dasgupta, R., Hirschmann, M.M., Smith, N.D. (2007) Partial Melting Experiments of Peridotite + CO2 at 3 GPa and Genesis of Alkalic Ocean Island Basalts. Journal of Petrology 48, 2093–2124.
; Foley and Pintér, 2018Foley, S.F., Pintér, Z. (2018) Primary Melt Compositions in the Earth’s Mantle. In: Kono, Y., Sanloup, C. (Eds.) Magmas Under Pressure: advances in high-pressure experiments on structure and properties of melts. Elsevier, Netherlands, 3–42.
). The results show that Ca isotope fractionation during partial melting of carbonatite melt is expected to larger than that of OIB melt by ∼0.01–0.04 ‰ in δ44/42Ca due to the temperature effect (Table S-5). Given average δ44/42Ca values of 0.37 ‰ and 0.43 ‰ for OIB and BSE (Fig. 1 and references therein), Ca isotope fractionation during partial melting of mantle for OIB melts and carbonatite melts is Δ44/42Caperidotite-OIB melt = 0.06 ‰ and Δ44/42Caperidotite-carbonatite melt = ∼0.07–0.10 ‰, respectively. Accordingly, the carbonatite melt derived from the normal mantle source is expected to have δ44/42Ca values of ∼0.33–0.36 ‰. The δ44/42Ca of carbonatite melts inferred from the measured values of carbonatites (an average of 0.35 ± 0.01 ‰, 2 s.e.; Fig. 1) are in accordance with these predicted values, supporting a normal mantle source for their origin.If carbonatites are sourced from the mantle involving recycled surface carbonates, they should have “anomalous” δ44/42Ca signatures, due to the difference of δ44/42Ca between mantle and surface carbonates (Fig. 1). This is observed in Cenozoic basalts from East and South China (Tengchong basalts and Zhangjiakou basalt, GSR-3) (He et al., 2017
He, Y., Wang, Y., Zhu, C., Huang, S., Li, S. (2017) Mass-Independent and Mass-Dependent Ca Isotopic Compositions of Thirteen Geological Reference Materials Measured by Thermal Ionisation Mass Spectrometry. Geostandards and Geoanalytical Research 41, 283–302.
; Liu et al., 2017Liu, F., Li, X., Wang, G., Liu, Y., Zhu, H., Kang, J., Huang, F., Sun, W., Xia, X., Zhang, Z. (2017) Marine Carbonate Component in the Mantle Beneath the Southeastern Tibetan Plateau: Evidence From Magnesium and Calcium Isotopes: Carbonates tracked by Mg and Ca isotopes. Journal of Geophysical Research: Solid Earth 122, 9729–9744.
), whose sources are commonly believed to involve recycled carbonates (Li et al., 2017Li, S.-G., Yang, W., Ke, S., Meng, X., Tian, H., Xu, L., He, Y., Huang, J., Wang, X.-C., Xia, Q. (2017) Deep carbon cycles constrained by a large-scale mantle Mg isotope anomaly in eastern China. National Science Review 4, 111–120.
), with δ44/42Ca (0.31 ‰ on average) lower than those of “normal” global basalts (Fig. 1). The effect of recycled carbonates on the change in the Ca isotope composition of carbonatites is assessed by a simple model using a Phanerozoic carbonate component (CaO = 40 %, δ44/42Ca = 0.29 ‰; Fantle and Tipper, 2014Fantle, M.S., Tipper, E.T. (2014) Calcium isotopes in the global biogeochemical Ca cycle: Implications for development of a Ca isotope proxy. Earth-Science Reviews 129, 148–177.
) as one end member and a BSE component as another (CaO = 3.65 %, δ44/42Ca = 0.43 ‰; Kang et al., 2017Kang, J.-T., Ionov, D.A., Liu, F., Zhang, C.-L., Golovin, A.V., Qin, L.-P., Zhang, Z.-F., Huang, F. (2017) Calcium isotopic fractionation in mantle peridotites by melting and metasomatism and Ca isotope composition of the Bulk Silicate Earth. Earth and Planetary Science Letters 474, 128–137.
; Simon and DePaolo, 2010Simon, J.I., DePaolo, D.J. (2010) Stable calcium isotopic composition of meteorites and rocky planets. Earth and Planetary Science Letters 289, 457–466.
). The results show that involvement of the Phanerozoic carbonates would exert a significant effect on the Ca isotope compositions of carbonatite melts (Fig. 3), and that for the studied carbonatites with δ44/42Ca values within analytical uncertainty, the contribution of surface carbonates to their sources, if it occurred, would be 2 % at most (Fig. 3). Incorporation of recycled carbonate in the mantle source may also result in a “mixing trend” coupled with δ44/42Ca and other proxies (e.g., 87Sr/86Sr or δ13C) for mantle-derived rocks. A δ44/42Ca variation correlated to 87Sr/86Sr and Sr/Nb signatures has been observed in Hawaiian lavas (Huang et al., 2011Huang, S., Farkaš, J., Jacobsen, S.B. (2011) Stable calcium isotopic compositions of Hawaiian shield lavas: Evidence for recycling of ancient marine carbonates into the mantle. Geochimica et Cosmochimica Acta 75, 4987–4997.
). Although “anomalous” δ44/42Ca values, e.g., as low as 0.26 ‰, and correlated δ44/42Ca-δ13C variations are observed for carbonatites (including non-primary types), the coupled Ca-C isotope pattern is inconsistent with involvement of sedimentary carbonates (Fig. 2b).
Figure 3 Expected δ44/42Ca values of carbonatites originating from a primary mantle source and mantle sources involved with different fractions of (Phanerozoic) recycled carbonates. The measured δ44/42Ca data for carbonatites is consistent with an origin from the primary mantle source mixed with little (<2 %) recycled carbonates. See detailed description in the main text.
Therefore, Ca isotope data imply that carbonatites originate from a normal mantle source, with no requirement for any contribution of recycled carbonates into their source.
top
Acknowledgements
We thank Yu-Te Alan Hsieh, Robert Owen, Jane Barling, Phil Holdship, Yongsheng He, Suohan Tang, Shuo Chen, Jianxiong Ma, Chunhong Wang, Pengfei Li, Lixin Zhang and Zilong Zhou for their help with isotope and element analyses and their helpful discussions. Yike Li is thanked for providing a sample from Cataolo. Shuijiong Wang is thanked for insightful discussions. Shichun Huang and another anonymous reviewer are gratefully appreciated for their constructive comments. Cin-Ty Lee is thanked for editorial handling. This study is financially supported by the National Natural Science Foundation of China (41773018), the National Key R&D Programmes of China (2019YFA0708604, 2019YFA0708404), the National Natural Science Foundation of China (42072113), the Key Laboratory of Deep-Earth Dynamics of Ministry of Natural Resources (J1901-13, J1901-20-1) and the Geological Survey Program of the China Geological Survey (DD20211358).
Editor: Cin-Ty Lee
top
Author contributions
X-KZ designed the research project. NSB and GMH provided expertise and facility in the establishment of the Ca isotope analytical method. JS, W-JL, B-BC and Z-HL performed the MC-ICPMS-method Ca isotope measurements and YW performed the TIMS Ca isotope measurements. JK, WC, AGD, W-LS, Z-GC and W-JL provided samples. JS and X-KZ interpreted the data and wrote the paper, with additional input from all the co-authors.
top
References
Amini, M., Eisenhauer, A., Böhm, F., Holmden, C., Kreissig, K., Hauff, F., Jochum, K.P. (2009) Calcium Isotopes (δ44/40Ca) in MPI-DING Reference Glasses, USGS Rock Powders and Various Rocks: Evidence for Ca Isotope Fractionation in Terrestrial Silicates. Geostandards and Geoanalytical Research 33, 231–247.
 Show in context
Show in context Previous studies indicate the potential of Ca isotopes in mantle-derived rocks for tracing recycled carbonates but the Ca isotope composition of carbonatites has remained poorly constrained, and the reported δ44/42Ca values are inconsistent among different groups (Amini et al., 2009; Maloney, 2018; Banerjee and Chakrabarti, 2019; Amsellem et al., 2020).
View in article
Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6, eaba3269.
 Show in context
Show in context Previous studies indicate the potential of Ca isotopes in mantle-derived rocks for tracing recycled carbonates but the Ca isotope composition of carbonatites has remained poorly constrained, and the reported δ44/42Ca values are inconsistent among different groups (Amini et al., 2009; Maloney, 2018; Banerjee and Chakrabarti, 2019; Amsellem et al., 2020).
View in article
This estimate is significantly different to that made by Amsellem et al. (2020), and accordingly leads to a different understanding on the nature of the carbonatite source as discussed below.
View in article
Banerjee, A., Chakrabarti, R. (2019) A geochemical and Nd, Sr and stable Ca isotopic study of carbonatites and associated silicate rocks from the ∼65 Ma old Ambadongar carbonatite complex and the Phenai Mata igneous complex, Gujarat, India: Implications for crustal contamination, carbonate recycling, hydrothermal alteration and source-mantle mineralogy. Lithos 326–327, 572–585.
 Show in context
Show in context Previous studies indicate the potential of Ca isotopes in mantle-derived rocks for tracing recycled carbonates but the Ca isotope composition of carbonatites has remained poorly constrained, and the reported δ44/42Ca values are inconsistent among different groups (Amini et al., 2009; Maloney, 2018; Banerjee and Chakrabarti, 2019; Amsellem et al., 2020).
View in article
Barker, D.S. (1996) Consequences of recycled carbon in carbonatites. Canadian Mineralogist 34, 373–388.
 Show in context
Show in context However, whether the carbon generating the carbonatite was originally sourced from the primitive mantle or a recycled component remains debated (Barker, 1996; Hoernle et al., 2002; Bell and Simonetti, 2010).
View in article
Bell, K., Simonetti, A. (2010) Source of parental melts to carbonatites–critical isotopic constraints. Mineralogy and Petrology 98, 77–89.
 Show in context
Show in context Consensus has been made that carbonatite melts are derived from the carbonate-bearing mantle (Dasgupta et al., 2007; Bell and Simonetti, 2010).
View in article
However, whether the carbon generating the carbonatite was originally sourced from the primitive mantle or a recycled component remains debated (Barker, 1996; Hoernle et al., 2002; Bell and Simonetti, 2010).
View in article
Blättler, C.L., Higgins, J.A. (2017) Testing Urey’s carbonate–silicate cycle using the calcium isotopic composition of sedimentary carbonates. Earth and Planetary Science Letters 479, 241–251.
 Show in context
Show in context Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
Chen, C., Dai, W., Wang, Z., Liu, Y., Li, M., Becker, H., Foley, S.F. (2019) Calcium isotope fractionation during magmatic processes in the upper mantle. Geochimica et Cosmochimica Acta 249, 121–137.
 Show in context
Show in context This is because, 1) Ca isotope compositions of surface carbonate and the mantle peridotite are distinct (Fantle and Tipper, 2014; Kang et al., 2017), 2) Ca abundance of the former is nearly one order of magnitude higher than that of the later, and 3) Ca isotope fractionation is negligible during (basaltic) magmatic differentiation (Zhang et al., 2018; Chen et al., 2019).
View in article
Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
The little δ44/42Ca variation in Belaya Zima and other worldwide primary carbonatites and associated silicate rocks suggest that Ca fractionation is negligible during carbonatite magmatic differentiation, as that has been observed for basaltic magmatic differentiation (Zhang et al., 2018; Chen et al., 2019).
View in article
This is consistent with the theoretical prediction that the equilibrium Ca isotope fractionation between Ca-dominated minerals in high temperature conditions is minimal, e.g., Δ44/42Cacalcite-cpx < 0.02 ‰ at a temperature over 800 °C (Wang et al., 2017; Chen et al., 2019).
View in article
Dasgupta, R., Hirschmann, M.M., Smith, N.D. (2007) Partial Melting Experiments of Peridotite + CO2 at 3 GPa and Genesis of Alkalic Ocean Island Basalts. Journal of Petrology 48, 2093–2124.
 Show in context
Show in context Consensus has been made that carbonatite melts are derived from the carbonate-bearing mantle (Dasgupta et al., 2007; Bell and Simonetti, 2010).
View in article
The partial melting conditions are assumed to OIB-like in pressure but with a lower temperature, as the presence of CO2 (which is required to generate carbonatite melts) may depress the solidus of peridotite by ∼100–400 °C (Dasgupta et al., 2007; Foley and Pintér, 2018).
View in article
Deines, P. (1989) Stable isotope variations in carbonatites. In: Bell, K. (Ed.)Carbonatites: Genesis and Evolution. Unwin Hyman, London, 301–359.
 Show in context
Show in context However, their primary isotope signatures tend to be fractionated or affected by late stage fluids and magma degassing (Deines, 1989).
View in article
Hence, the correlation between δ44/42Ca and δ13C implies that Ca isotope variation in carbonatites is dominated by Rayleigh fractionation processes in fluid-rich carbonatite magma—a process also resulting in correlated C-O isotope variation in carbonatites (Deines, 1989).
View in article
Fantle, M.S., Tipper, E.T. (2014) Calcium isotopes in the global biogeochemical Ca cycle: Implications for development of a Ca isotope proxy. Earth-Science Reviews 129, 148–177.
 Show in context
Show in context This is because, 1) Ca isotope compositions of surface carbonate and the mantle peridotite are distinct (Fantle and Tipper, 2014; Kang et al., 2017), 2) Ca abundance of the former is nearly one order of magnitude higher than that of the later, and 3) Ca isotope fractionation is negligible during (basaltic) magmatic differentiation (Zhang et al., 2018; Chen et al., 2019).
View in article
Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
The effect of recycled carbonates on the change in the Ca isotope composition of carbonatites is assessed by a simple model using a Phanerozoic carbonate component (CaO = 40 %, δ44/42Ca = 0.29 ‰; Fantle and Tipper, 2014) as one end member and a BSE component as another (CaO = 3.65 %, δ44/42Ca = 0.43 ‰; Kang et al., 2017; Simon and DePaolo, 2010).
View in article
Foley, S.F., Pintér, Z. (2018) Primary Melt Compositions in the Earth’s Mantle. In: Kono, Y., Sanloup, C. (Eds.) Magmas Under Pressure: advances in high-pressure experiments on structure and properties of melts. Elsevier, Netherlands, 3–42.
 Show in context
Show in context The partial melting conditions are assumed to OIB-like in pressure but with a lower temperature, as the presence of CO2 (which is required to generate carbonatite melts) may depress the solidus of peridotite by ∼100–400 °C (Dasgupta et al., 2007; Foley and Pintér, 2018).
View in article
He, Y., Wang, Y., Zhu, C., Huang, S., Li, S. (2017) Mass-Independent and Mass-Dependent Ca Isotopic Compositions of Thirteen Geological Reference Materials Measured by Thermal Ionisation Mass Spectrometry. Geostandards and Geoanalytical Research 41, 283–302.
 Show in context
Show in context Any other possible analytical pitfalls, including a “column effect”, Sr effect or “column fractionation”, were avoided (see details of analytical method in SI), (3) inter-laboratory comparison was made by measuring eight standard reference materials and four carbonatite samples both performed in CAGS lab using this rigorous method and performed in CUGB lab using a DS-TIMS method reported by He et al. (2017), where all measured δ44/42Ca values are consistent within analytical precision (Figs. S-1, S-2, Tables S-1, S-2).
View in article
This is observed in Cenozoic basalts from East and South China (Tengchong basalts and Zhangjiakou basalt, GSR-3) (He et al., 2017; Liu et al., 2017), whose sources are commonly believed to involve recycled carbonates (Li et al., 2017), with δ44/42Ca (0.31 ‰ on average) lower than those of “normal” global basalts (Fig. 1).
View in article
Hoernle, K., Tilton, G., Le Bas, M.J., Duggen, S., Garbe-Schönberg, D. (2002) Geochemistry of oceanic carbonatites compared with continental carbonatites: mantle recycling of oceanic crustal carbonate. Contributions to Mineralogy and Petrology 142, 520–542.
 Show in context
Show in context However, whether the carbon generating the carbonatite was originally sourced from the primitive mantle or a recycled component remains debated (Barker, 1996; Hoernle et al., 2002; Bell and Simonetti, 2010).
View in article
Huang, F., Zhou, C., Wang, W., Kang, J., Wu, Z. (2019) First-principles calculations of equilibrium Ca isotope fractionation: Implications for oldhamite formation and evolution of lunar magma ocean. Earth and Planetary Science Letters 510, 153–160.
Huang, S., Farkaš, J., Jacobsen, S.B. (2011) Stable calcium isotopic compositions of Hawaiian shield lavas: Evidence for recycling of ancient marine carbonates into the mantle. Geochimica et Cosmochimica Acta 75, 4987–4997.
 Show in context
Show in context A δ44/42Ca variation correlated to 87Sr/86Sr and Sr/Nb signatures has been observed in Hawaiian lavas (Huang et al., 2011).
View in article
Ca isotopes have emerged as a novel tool for tracing recycled carbonates in the mantle (Huang et al., 2011; Liu et al., 2017).
View in article
Jacobson, A.D., Grace Andrews, M., Lehn, G.O., Holmden, C. (2015) Silicate versus carbonate weathering in Iceland: New insights from Ca isotopes. Earth and Planetary Science Letters 416, 132–142.
 Show in context
Show in context Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
Kang, J.-T., Ionov, D.A., Liu, F., Zhang, C.-L., Golovin, A.V., Qin, L.-P., Zhang, Z.-F., Huang, F. (2017) Calcium isotopic fractionation in mantle peridotites by melting and metasomatism and Ca isotope composition of the Bulk Silicate Earth. Earth and Planetary Science Letters 474, 128–137.
 Show in context
Show in context This is because, 1) Ca isotope compositions of surface carbonate and the mantle peridotite are distinct (Fantle and Tipper, 2014; Kang et al., 2017), 2) Ca abundance of the former is nearly one order of magnitude higher than that of the later, and 3) Ca isotope fractionation is negligible during (basaltic) magmatic differentiation (Zhang et al., 2018; Chen et al., 2019).
View in article
Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
The effect of recycled carbonates on the change in the Ca isotope composition of carbonatites is assessed by a simple model using a Phanerozoic carbonate component (CaO = 40 %, δ44/42Ca = 0.29 ‰; Fantle and Tipper, 2014) as one end member and a BSE component as another (CaO = 3.65 %, δ44/42Ca = 0.43 ‰; Kang et al., 2017; Simon and DePaolo, 2010).
View in article
Li, S.-G., Yang, W., Ke, S., Meng, X., Tian, H., Xu, L., He, Y., Huang, J., Wang, X.-C., Xia, Q. (2017) Deep carbon cycles constrained by a large-scale mantle Mg isotope anomaly in eastern China. National Science Review 4, 111–120.
 Show in context
Show in context This is observed in Cenozoic basalts from East and South China (Tengchong basalts and Zhangjiakou basalt, GSR-3) (He et al., 2017; Liu et al., 2017), whose sources are commonly believed to involve recycled carbonates (Li et al., 2017), with δ44/42Ca (0.31 ‰ on average) lower than those of “normal” global basalts (Fig. 1).
View in article
Liu, F., Li, X., Wang, G., Liu, Y., Zhu, H., Kang, J., Huang, F., Sun, W., Xia, X., Zhang, Z. (2017) Marine Carbonate Component in the Mantle Beneath the Southeastern Tibetan Plateau: Evidence From Magnesium and Calcium Isotopes: Carbonates tracked by Mg and Ca isotopes. Journal of Geophysical Research: Solid Earth 122, 9729–9744.
 Show in context
Show in context Ca isotopes have emerged as a novel tool for tracing recycled carbonates in the mantle (Huang et al., 2011; Liu et al., 2017).
View in article
Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
This is observed in Cenozoic basalts from East and South China (Tengchong basalts and Zhangjiakou basalt, GSR-3) (He et al., 2017; Liu et al., 2017), whose sources are commonly believed to involve recycled carbonates (Li et al., 2017), with δ44/42Ca (0.31 ‰ on average) lower than those of “normal” global basalts (Fig. 1).
View in article
Maloney, M. (2018) Calcium Isotopes in Natural and Experimental Carbonated Silicate Melts. PhD Thesis, Western University, Electronic Thesis and Dissertation Repository, 5256.
 Show in context
Show in context Previous studies indicate the potential of Ca isotopes in mantle-derived rocks for tracing recycled carbonates but the Ca isotope composition of carbonatites has remained poorly constrained, and the reported δ44/42Ca values are inconsistent among different groups (Amini et al., 2009; Maloney, 2018; Banerjee and Chakrabarti, 2019; Amsellem et al., 2020).
View in article
Simon, J.I., DePaolo, D.J. (2010) Stable calcium isotopic composition of meteorites and rocky planets. Earth and Planetary Science Letters 289, 457–466.
 Show in context
Show in context Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
The effect of recycled carbonates on the change in the Ca isotope composition of carbonatites is assessed by a simple model using a Phanerozoic carbonate component (CaO = 40 %, δ44/42Ca = 0.29 ‰; Fantle and Tipper, 2014) as one end member and a BSE component as another (CaO = 3.65 %, δ44/42Ca = 0.43 ‰; Kang et al., 2017; Simon and DePaolo, 2010).
View in article
Sun, J., Zhu, X., Chen, Y., Fang, N. (2013) Iron isotopic constraints on the genesis of Bayan Obo ore deposit, Inner Mongolia, China. Precambrian Research 235, 88–106.
 Show in context
Show in context Carbonatite is an exotic igneous rock formed predominantly of carbonates and an important host or source of critical metals, including REE and Nb (Woolley and Kempe, 1989; Sun et al., 2013; Verplanck et al., 2016).
View in article
Verplanck, P.L., Mariano, A.N., Mariano Jr, A. (2016) Rare Earth Element Ore Geology of Carbonatites. Reviews in Economic Geology, 5–32.
 Show in context
Show in context Carbonatite is an exotic igneous rock formed predominantly of carbonates and an important host or source of critical metals, including REE and Nb (Woolley and Kempe, 1989; Sun et al., 2013; Verplanck et al., 2016).
View in article
Wang, W., Qin, T., Zhou, C., Huang, S., Wu, Z., Huang, F. (2017) Concentration effect on equilibrium fractionation of Mg-Ca isotopes in carbonate minerals: Insights from first-principles calculations. Geochimica et Cosmochimica Acta 208, 185–197.
 Show in context
Show in context This is consistent with the theoretical prediction that the equilibrium Ca isotope fractionation between Ca-dominated minerals in high temperature conditions is minimal, e.g., Δ44/42Cacalcite-cpx < 0.02 ‰ at a temperature over 800 °C (Wang et al., 2017; Chen et al., 2019).
View in article
Woolley, A.R., Kempe, D.R.C. (1989) Carbonatites: nomenclature, average chemical compositions, and element distribution. In: Bell, K. (Ed.) Carbonatites: genesis and evolution. Unwin Hyman, London, 1–14.
 Show in context
Show in context Carbonatite is an exotic igneous rock formed predominantly of carbonates and an important host or source of critical metals, including REE and Nb (Woolley and Kempe, 1989; Sun et al., 2013; Verplanck et al., 2016).
View in article
Ca is the most common and abundant metal in carbonatites (Woolley and Kempe, 1989).
View in article
Zhang, H., Wang, Y., He, Y., Teng, F.-Z., Jacobsen, S.B., Helz, R.T., Marsh, B.D., Huang, S. (2018) No measurable calcium isotopic fractionation during crystallization of Kilauea Iki lava lake. Geochemistry, Geophysics, Geosystems 19, 3128–3139.
 Show in context
Show in context This is because, 1) Ca isotope compositions of surface carbonate and the mantle peridotite are distinct (Fantle and Tipper, 2014; Kang et al., 2017), 2) Ca abundance of the former is nearly one order of magnitude higher than that of the later, and 3) Ca isotope fractionation is negligible during (basaltic) magmatic differentiation (Zhang et al., 2018; Chen et al., 2019).
View in article
Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
The little δ44/42Ca variation in Belaya Zima and other worldwide primary carbonatites and associated silicate rocks suggest that Ca fractionation is negligible during carbonatite magmatic differentiation, as that has been observed for basaltic magmatic differentiation (Zhang et al., 2018; Chen et al., 2019).
View in article
Zhu, H., Liu, F., Li, X., Wang, G., Zhang, Z., Sun, W. (2018) Calcium Isotopic Compositions of Normal Mid-Ocean Ridge Basalts From the Southern Juan de Fuca Ridge. Journal of Geophysical Research: Solid Earth 123, 1303–1313.
 Show in context
Show in context Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
Zhu, H., Du, L., Li, X., Zhang, Z., Sun, W. (2020) Calcium isotopic fractionation during plate subduction: Constraints from back-arc basin basalts. Geochimica et Cosmochimica Acta 270, 379–393.
 Show in context
Show in context Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009; Simon and DePaolo, 2010; Fantle and Tipper, 2014; Jacobson et al., 2015; Blättler and Higgins, 2017; Kang et al., 2017; Liu et al., 2017; Zhang et al., 2018; Zhu et al., 2018, 2020; Chen et al., 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1. Ca Isotope Analytical Methods
- 2. Analytical Methods for Major Elements and C-O Isotope Compositions
- 3. Geological Contexts of Carbonatites and Related Alkaline Rocks and Sampling
- 4. Ca Isotope Constraints on the Origin of Ankerite Carbonatite
- Tables S-1 to S-5
- Figures S-1 to S-7
- Supplementary Information References
Download Tables S-1 and S-5 (Excel).
Download the Supplementary Information (PDF).
Figures
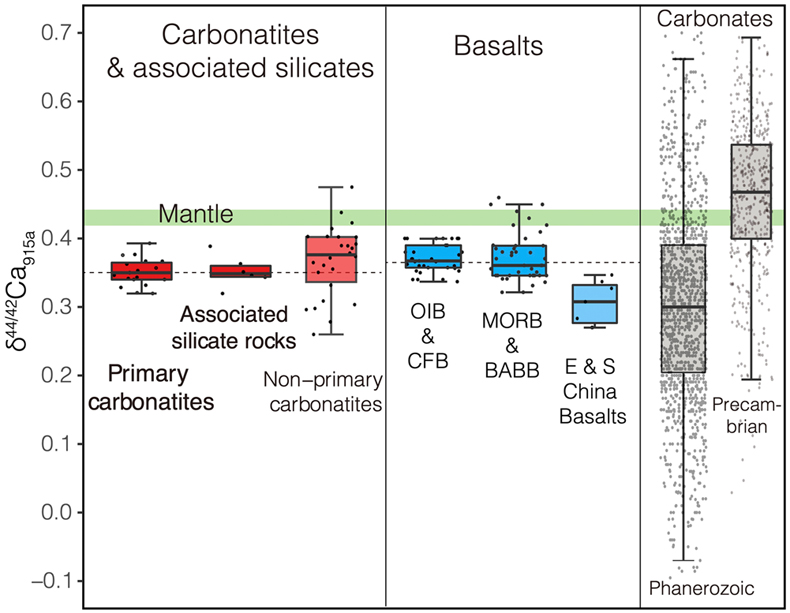
Figure 1 Comparison of δ44/42Ca values between carbonatites, basalts, mantle, and sedimentary carbonates. The dashed lines represent average δ44/42Ca values of carbonatites and basalts, respectively. Data sources are from this study and references (see Tables S-1, S-2; Amini et al., 2009
Amini, M., Eisenhauer, A., Böhm, F., Holmden, C., Kreissig, K., Hauff, F., Jochum, K.P. (2009) Calcium Isotopes (δ44/40Ca) in MPI-DING Reference Glasses, USGS Rock Powders and Various Rocks: Evidence for Ca Isotope Fractionation in Terrestrial Silicates. Geostandards and Geoanalytical Research 33, 231–247.
; Simon and DePaolo, 2010Simon, J.I., DePaolo, D.J. (2010) Stable calcium isotopic composition of meteorites and rocky planets. Earth and Planetary Science Letters 289, 457–466.
; Fantle and Tipper, 2014Fantle, M.S., Tipper, E.T. (2014) Calcium isotopes in the global biogeochemical Ca cycle: Implications for development of a Ca isotope proxy. Earth-Science Reviews 129, 148–177.
; Jacobson et al., 2015Jacobson, A.D., Grace Andrews, M., Lehn, G.O., Holmden, C. (2015) Silicate versus carbonate weathering in Iceland: New insights from Ca isotopes. Earth and Planetary Science Letters 416, 132–142.
; Blättler and Higgins, 2017Blättler, C.L., Higgins, J.A. (2017) Testing Urey’s carbonate–silicate cycle using the calcium isotopic composition of sedimentary carbonates. Earth and Planetary Science Letters 479, 241–251.
; Kang et al., 2017Kang, J.-T., Ionov, D.A., Liu, F., Zhang, C.-L., Golovin, A.V., Qin, L.-P., Zhang, Z.-F., Huang, F. (2017) Calcium isotopic fractionation in mantle peridotites by melting and metasomatism and Ca isotope composition of the Bulk Silicate Earth. Earth and Planetary Science Letters 474, 128–137.
; Liu et al., 2017Liu, F., Li, X., Wang, G., Liu, Y., Zhu, H., Kang, J., Huang, F., Sun, W., Xia, X., Zhang, Z. (2017) Marine Carbonate Component in the Mantle Beneath the Southeastern Tibetan Plateau: Evidence From Magnesium and Calcium Isotopes: Carbonates tracked by Mg and Ca isotopes. Journal of Geophysical Research: Solid Earth 122, 9729–9744.
; Zhang et al., 2018Zhang, H., Wang, Y., He, Y., Teng, F.-Z., Jacobsen, S.B., Helz, R.T., Marsh, B.D., Huang, S. (2018) No measurable calcium isotopic fractionation during crystallization of Kilauea Iki lava lake. Geochemistry, Geophysics, Geosystems 19, 3128–3139.
; Zhu et al., 2018Zhu, H., Liu, F., Li, X., Wang, G., Zhang, Z., Sun, W. (2018) Calcium Isotopic Compositions of Normal Mid-Ocean Ridge Basalts From the Southern Juan de Fuca Ridge. Journal of Geophysical Research: Solid Earth 123, 1303–1313.
, 2020Zhu, H., Du, L., Li, X., Zhang, Z., Sun, W. (2020) Calcium isotopic fractionation during plate subduction: Constraints from back-arc basin basalts. Geochimica et Cosmochimica Acta 270, 379–393.
; Chen et al., 2019Chen, C., Dai, W., Wang, Z., Liu, Y., Li, M., Becker, H., Foley, S.F. (2019) Calcium isotope fractionation during magmatic processes in the upper mantle. Geochimica et Cosmochimica Acta 249, 121–137.
).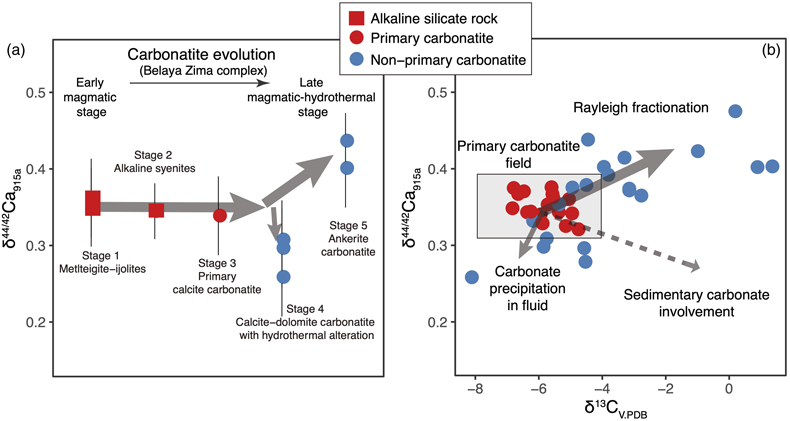
Figure 2 Plots showing δ44/42Ca variation during carbonatite evolution. (a) δ44/42Ca for different types of carbonatites and associated silicate rocks from Belaya Zima complex, Russia. (b) δ44/42Ca vs. δ13C plot for carbonatites. See data sources in Table S-1.
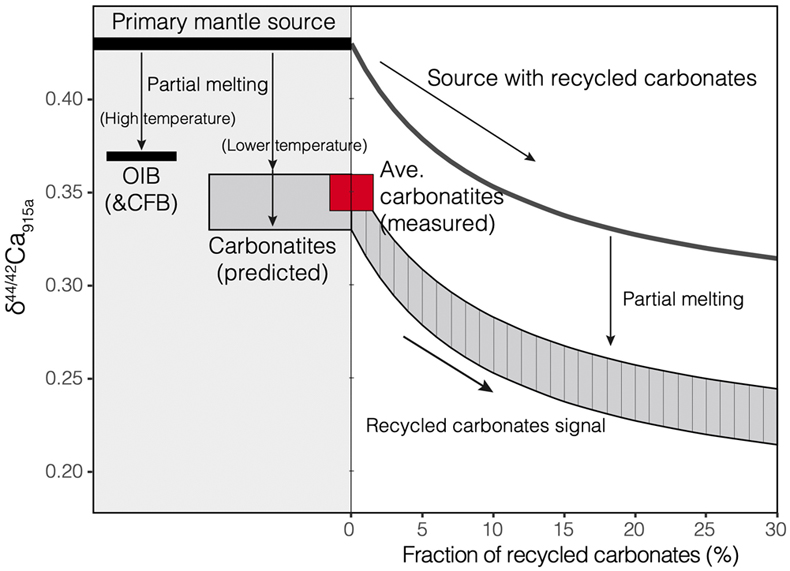
Figure 3 Expected δ44/42Ca values of carbonatites originating from a primary mantle source and mantle sources involved with different fractions of (Phanerozoic) recycled carbonates. The measured δ44/42Ca data for carbonatites is consistent with an origin from the primary mantle source mixed with little (<2 %) recycled carbonates. See detailed description in the main text.