A genetic metasomatic link between eclogitic and peridotitic diamond inclusions
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:494Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
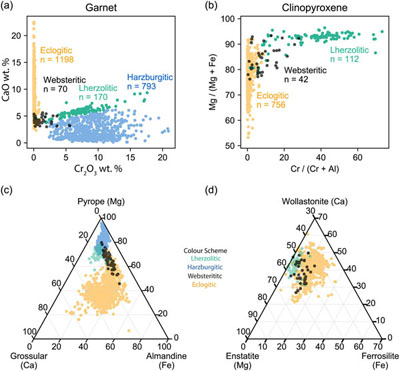 Figure 1 Inclusion parageneses (a,b) and end member compositions for garnets (c) and clinopyroxenes (d) from mantle diamond inclusions. Diamond inclusion data are from numerous sources provided in the Supplementary Information. Inclusion parageneses were assigned based on the original publisher’s characterisation (see Supplementary Information). Ternaries plotted using the Python package python-ternary (Harper et al., 2019). | 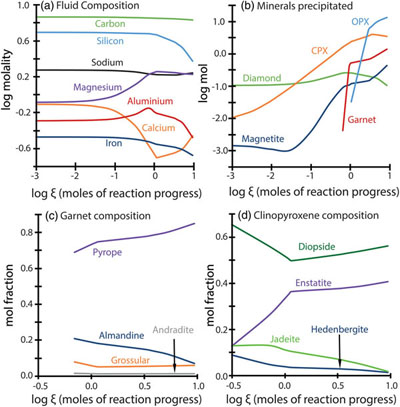 Figure 2 Model results for the reaction pathway during metasomatism of lherzolite by an eclogitic fluid (Run 27; Tables 1, S-1–S-4). Each unit of the reaction progress variable (ξ) corresponds to destruction of 1.0 mole of each of the reactant minerals per 1.0 kg of H2O in the initial fluid. (a) Changes in the total dissolved concentration of the major elements in the fluid; (b) Moles of new minerals precipitated from the fluid during the continuous reaction pathway; (c) and (d) The compositions of garnets and clinopyroxenes, respectively, as a function of reaction progress. Full results for all models are provided in the output files (available upon request). Olivine precipitates in the final stage of the model (Table 1) and is therefore absent in Figure 2b. Note change in scale for the x axis for a–b vs. c–d. | 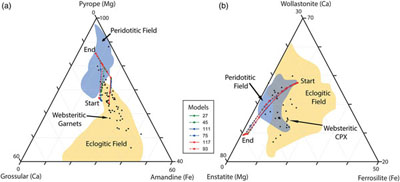 Figure 3 Selected model results for predicted (a) garnet and (b) clinopyroxene compositions during progressive metasomatism. Each model run refers to a single peridotite metasomatised by a single eclogitic fluid. Key parameters for the models shown are detailed in Tables 1, S-1–S-5, and the input and output files are available upon request. | 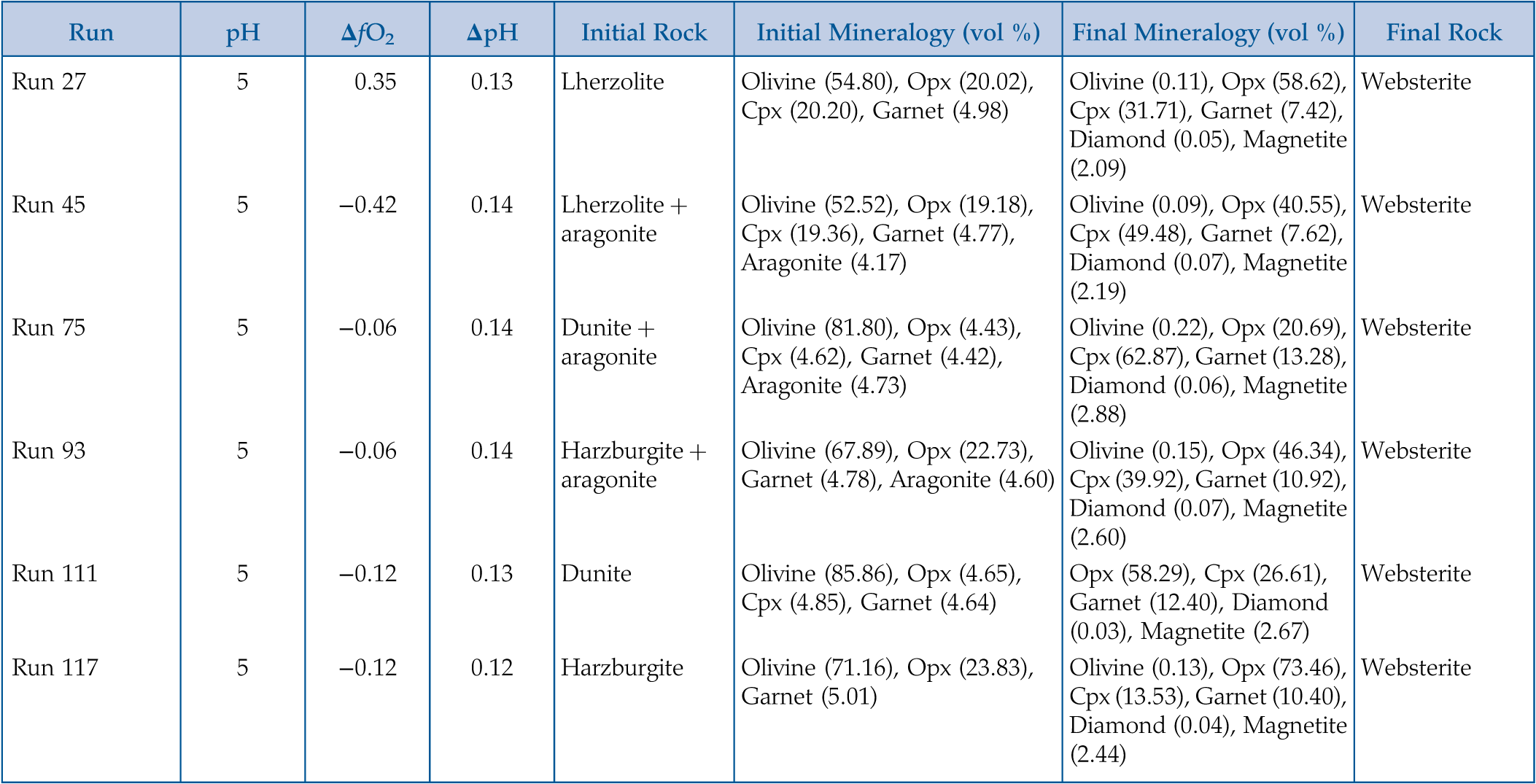 Table 1 Key parameters for representative metasomatic mass transfer modes presented in Figures 2, 3. The pressure and temperature for all runs was fixed at 5 GPa and 1000 °C with the starting fluid fO2 and fluid pH fixed at ΔFMQ −3 and pH 5.0, respectively. ΔfO2 and ΔpH express changes in the final fluid relative to the initial. Note that these changes are very small even though the mineralogical changes in the rock are very large. A larger suite of results is provided in Table S-5. |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
top
Introduction
Mineral inclusions in diamonds provide the geological context for diamond formation. These invaluable samples reveal that diamond formation spans more than 75 % of Earth’s history (Gurney et al., 2010
Gurney, J.J., Helmstaedt, H.H., Richardson., S.H., Shirey, S.B. (2010) Diamonds through time. Economic Geology 105, 689–712.
) with most diamonds forming in the keel of the sub-continental lithospheric mantle (120–180 km; Stachel and Harris, 2008Stachel, T., Harris, J.W. (2008) The origin of cratonic diamonds – constraints from mineral inclusions. Ore Geology Review 34, 5–32.
). The diversity of the petrological characteristics of diamond-hosted mineral inclusions either reflect [1] pre-metasomatic mineralogical heterogeneity in the upper mantle (Cartigny et al., 2001Cartigny, P., Harris, J.W., Javoy, M. (2001) Diamond genesis, mantle fractionations and mantle nitrogen content: a study of δ13C–N concentrations in diamonds. Earth and Planetary Science Letters 185, 85–98.
; Nestola et al., 2017Nestola, F., Jung, H., Taylor, L.A. (2017) Mineral inclusions in diamonds may be synchronous but not syngenetic. Nature Communications 14168, doi: 10.1038/ncomms14168.
), [2] fluid (Mikhail et al., 2019aMikhail, S., McCubbin, F.M., Jenner, F.E., Shirey, S.B., Rumble, D., Bowden, R. (2019a) Diamondites: evidence for a distinct tectono-thermal diamond-forming event beneath the Kaapvaal craton. Contributions to Mineralogy and Petrology 174, 71.
,bMikhail, S., Crosby, J.C., Stuart, F.M., DiNicola, L., Abernethy, F.A.J. (2019b) A secretive mechanical exchange between mantle and crustal volatiles revealed by helium isotopes in 13C-depleted diamonds. Geochemical Perspectives Letters 11, 39–43.
) and/or melt metasomatism (Aulbach et al., 2002Aulbach, S., Stachel, T., Viljoen, K.S., Brey, G.P., Harris, J.W. (2002) Eclogitic and websteritic diamond sources beneath the Limpopo Belt – is slab-melting the link? Contributions to Mineralogy and Petrology 143, 56–70.
; Kiseeva et al., 2016Kiseeva, E.S., Wood, B.J., Ghosh, S., Stachel, T. (2016) The pyroxenite-diamond connection. Geochemical Perspectives Letters 2, 1–9.
) coeval with diamond formation, or [3] a combination of both options. The ambiguity arises because the relationship between diamond and inclusion can be protogenetic (Nestola et al., 2017Nestola, F., Jung, H., Taylor, L.A. (2017) Mineral inclusions in diamonds may be synchronous but not syngenetic. Nature Communications 14168, doi: 10.1038/ncomms14168.
) or syngenetic (Harris, 1968Harris, J.W. (1968) The recognition of diamond inclusions. Part 1: syngenetic mineral inclusions. Industrial Diamond Review 28, 402–410.
; Mikhail et al., 2019aMikhail, S., McCubbin, F.M., Jenner, F.E., Shirey, S.B., Rumble, D., Bowden, R. (2019a) Diamondites: evidence for a distinct tectono-thermal diamond-forming event beneath the Kaapvaal craton. Contributions to Mineralogy and Petrology 174, 71.
). We proceed here without assuming either a single protogenetic or syngenetic origin for all diamond inclusions.Diamond-hosted mineral inclusions are diverse, including, but not limited to, sulfides, silicates, oxides, carbonates, and metallic phases (Stachel and Harris, 2008
Stachel, T., Harris, J.W. (2008) The origin of cratonic diamonds – constraints from mineral inclusions. Ore Geology Review 34, 5–32.
) and several of these mineral families can be sub-divided into three groups, termed inclusion paragenesis. These are [1] peridotitic (i.e. Cr-rich pyrope, diopside, enstatite, olivine), [2] eclogitic (i.e. Cr-poor pyrope-almandine, Na-rich omphacite), and [3] websteritic (intermediate compositions; Meyer and Boyd, 1972Meyer, H.O.A., Boyd, F.R. (1972) Composition and origin of crystalline inclusions in natural diamonds. Geochimica et Cosmochimica Acta 36, 1255–1273.
; Sobolev et al., 1973Sobolev, N.V., Lavrent’ev, Y.G., Pokhilenko, N.P., Usova, L.V. (1973) Chrome-rich garnets from the kimberlites of yakutia and their parageneses. Contributions to Mineralogy and Petrology 40, 39–52.
; Gurney et al., 1984Gurney, J.J., Harris, J.W., Rickard, R.S. (1984) Silicate and oxide inclusions in diamonds from the Orapa mine, Botswana. In: Kornprobst, J. (Ed.) Kimberlites II: The Mantle and Crust-Mantle Relationships. Developments in Petrology, Elsevier, Amsterdam, 3–9.
) (Fig. 1a–d). The assignment of inclusion paragenesis is an empirical and subjective classification scheme which does not inform on process, sensu stricto. Herein, we focus on garnet and clinopyroxene data because these two inclusion types are present in significant abundances in all three paragenetic groups. The boundaries between peridotitic and eclogitic suites are only as clear as the contrast between the symbol shape and colour selected for the plot (Fig. 1a–d). For example, there is less of a distinction between peridotitic and eclogitic clinopyroxenes than is apparent for peridotitic and eclogitic garnets (Fig. 1a–d). In Figure 1c,d it can be seen that garnets and clinopyroxenes show an apparent continuum between the peridotitic and eclogitic suites charted by websteritic garnets (Fig. 1). Herein, we examine two key assumptions: [1] the different silicate inclusion parageneses are genetically distinct, and [2] silicate inclusion and host diamond paragenesis are syngenetic.
Figure 1 Inclusion parageneses (a,b) and end member compositions for garnets (c) and clinopyroxenes (d) from mantle diamond inclusions. Diamond inclusion data are from numerous sources provided in the Supplementary Information. Inclusion parageneses were assigned based on the original publisher’s characterisation (see Supplementary Information). Ternaries plotted using the Python package python-ternary (Harper et al., 2019
Harper, M., Weinstein, B., tgwoodcock, Simon, C., chebee7i, Morgan, W., Knight, V., Swanson-Hysell, N., Evans, M., jl-bernal, ZGainsforth, The Gitter Badger, SaxonAnglo, Greco, M., Zuidhof, G. (2019) marcharper/python-ternary: Version 1.0.6. Zenodo, doi: 10.5281/zenodo.2628066.
).top
Methods and Results
Modelling approach. Our conceptual geologic model involves a fluid that is initially in equilibrium with a mafic eclogite migrating and encountering peridotite with which it is not in equilibrium. As a consequence, irreversible chemical mass transfer occurs. This model is analogous to a fluid migrating from a subducting slab and metasomatising the sub-continental lithospheric mantle. Alternatively, the source of the fluid could be upwelling transition zone (i.e. a plume), exsolving water when hydrous wadsleyite converts to olivine + H2O and the fluid phase would rise buoyantly. We built on the long-standing tradition in theoretical aqueous geochemistry of modelling irreversible chemical mass transfer in crustal hydrothermal systems (Helgeson, 1970
Helgeson, H.C. (1970) A chemical and thermodynamic model of ore deposition in hydrothermal systems. In: Morgan, B.A. (Ed.) Fiftieth Anniversary Symposia: Mineralogy and Petrology of the Upper Mantle; Sulfides; Mineralogy and Geochemistry of Non-Marine Evaporites. Special Paper No. 3, Mineralogical Society of America, Washington, D.C., 155–186.
, 1979Helgeson, H.C. (1979) Mass transfer among minerals and hydrothermal solutions. In: Barnes, H.L. (Ed.) Geochemistry of Hydrothermal Ore Deposits. Wiley, New York, 568–610.
; Sverjensky, 1984Sverjensky, D.A. (1984) Oil-field brines as ore-forming solutions. Economic Geology 79, 23–37.
, 1987Sverjensky, D.A. (1987) The role of migrating oil-field brines in the formation of sediment-hosted Cu-rich deposits. Economic Geology 82, 1130–1141.
). Here we used the aqueous speciation and solubility code EQ3 and the chemical mass transfer code EQ6 (Wolery, 1992Wolery, T.J. (1992) EQ3/6: A software package for geochemical modeling of aqueous systems: package overview and installation guide (version 7.0). Lawrence Livermore National Laboratory, University of California, Livermore, CA.
) modified for upper mantle temperatures and pressures using thermodynamic data from the extended Deep Earth Water model calibrated with experimental solubilities (Huang and Sverjensky, 2019Huang, F., Sverjensky, D.A. (2019) Extended Deep Earth Water Model for predicting major element mantle metasomatism. Geochimica et Cosmochimica Acta 254, 192–230.
) previously applied to the formation of Panda diamonds (Huang and Sverjensky, 2020Huang, F., Sverjensky, D.A. (2020) Mixing of carbonatitic into saline fluid during Panda diamond formation. Geochimica et Cosmochimica Acta, 284, 1–20.
). More detail is given in the Supplementary Information. We ran a series of models to simulate the reactions between eclogitic fluids and a variety of lherzolite, harzburgite, and dunite compositions (Tables 1, S-5) using empirical data for peridotites from the compilation of Pearson and Wittig (2014)Pearson, D.G., Wittig, N. (2014) The formation and evolution of cratonic mantle lithosphere—evidence from mantle xenoliths. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry. Second edition, Elsevier, Oxford, 255–292.
.Table 1 Key parameters for representative metasomatic mass transfer modes presented in Figures 2, 3. The pressure and temperature for all runs was fixed at 5 GPa and 1000 °C with the starting fluid fO2 and fluid pH fixed at ΔFMQ −3 and pH 5.0, respectively. ΔfO2 and ΔpH express changes in the final fluid relative to the initial. Note that these changes are very small even though the mineralogical changes in the rock are very large. A larger suite of results is provided in Table S-5.
| Run | pH | ΔfO2 | ΔpH | Initial Rock | Initial Mineralogy (vol %) | Final Mineralogy (vol %) | Final Rock |
| Run 27 | 5 | 0.35 | 0.13 | Lherzolite | Olivine (54.80), Opx (20.02), Cpx (20.20), Garnet (4.98) | Olivine (0.11), Opx (58.62), Cpx (31.71), Garnet (7.42), Diamond (0.05), Magnetite (2.09) | Websterite |
| Run 45 | 5 | −0.42 | 0.14 | Lherzolite + aragonite | Olivine (52.52), Opx (19.18), Cpx (19.36), Garnet (4.77), Aragonite (4.17) | Olivine (0.09), Opx (40.55), Cpx (49.48), Garnet (7.62), Diamond (0.07), Magnetite (2.19) | Websterite |
| Run 75 | 5 | −0.06 | 0.14 | Dunite + aragonite | Olivine (81.80), Opx (4.43), Cpx (4.62), Garnet (4.42), Aragonite (4.73) | Olivine (0.22), Opx (20.69), Cpx (62.87), Garnet (13.28), Diamond (0.06), Magnetite (2.88) | Websterite |
| Run 93 | 5 | −0.06 | 0.14 | Harzburgite + aragonite | Olivine (67.89), Opx (22.73), Garnet (4.78), Aragonite (4.60) | Olivine (0.15), Opx (46.34), Cpx (39.92), Garnet (10.92), Diamond (0.07), Magnetite (2.60) | Websterite |
| Run 111 | 5 | −0.12 | 0.13 | Dunite | Olivine (85.86), Opx (4.65), Cpx (4.85), Garnet (4.64) | Opx (58.29), Cpx (26.61), Garnet (12.40), Diamond (0.03), Magnetite (2.67) | Websterite |
| Run 117 | 5 | −0.12 | 0.12 | Harzburgite | Olivine (71.16), Opx (23.83), Garnet (5.01) | Olivine (0.13), Opx (73.46), Cpx (13.53), Garnet (10.40), Diamond (0.04), Magnetite (2.44) | Websterite |
Model parameterisation. The input variables are the initial fluid geochemistry, and the abundances and solid solution compositions of minerals in the initial rock, with a fixed pressure of 5 GPa and fixed temperature of 1000 °C. The initial fluids were based on a calibration of the fluid chemistry in equilibrium with a mafic eclogitic measured by Kessel et al. (2015)
Kessel, R., Pettke, T., Fumagalli, P. (2015) Melting of metasomatized peridotite at 4–6 GPa and up to 1200 °C: an experimental approach. Contributions to Mineralogy and Petrology 169, 37.
as documented in Huang and Sverjensky (2019)Huang, F., Sverjensky, D.A. (2019) Extended Deep Earth Water Model for predicting major element mantle metasomatism. Geochimica et Cosmochimica Acta 254, 192–230.
(Table S-1). We assumed ideal site mixing of pyrope, almandine, and grossular in garnet composition and a non-ideal mixing of diopside, hedenbergite, and clinoenstatite in clinopyroxene previously calibrated using known mineral and fluid chemistries to simulate diamond formation beneath the Panda mine in the Slave craton (NW Canada) at 950 °C and 4.5 GPa (Huang and Sverjensky, 2020Huang, F., Sverjensky, D.A. (2020) Mixing of carbonatitic into saline fluid during Panda diamond formation. Geochimica et Cosmochimica Acta, 284, 1–20.
). The pressure-temperature conditions adopted here (5 GPa and 1000 °C) are relevant to lithospheric diamond formation (Stachel and Harris, 2008Stachel, T., Harris, J.W. (2008) The origin of cratonic diamonds – constraints from mineral inclusions. Ore Geology Review 34, 5–32.
). The temperature of 1000 °C is at the lower end of natural systems, where the average inclusion entrapment temperature is 1155 ± 105 °C (n = 444; Stachel and Luth, 2015Stachel, T., Luth, R.W. (2015) Diamond formation – Where, when and how? Lithos 223, 200–220.
). The initial oxygen fugacity (fO2) of the model fluids was varied from −1 to −6 log units relative to the Fayalite-Magnetite-Quartz reaction (abbreviated to ΔFMQ), a greater range than is calculated from lithospheric diamond inclusions (Stachel and Luth, 2015Stachel, T., Luth, R.W. (2015) Diamond formation – Where, when and how? Lithos 223, 200–220.
). Representative initial fluids are given in Tables S-1 and S-2. The model outputs the evolving chemistry of the fluid and the metasomatic minerals finishing when the fluid finally equilibrates with the initial rock. The final fluid compositions and modal abundances of the minerals produced are shown by representative results in Figures 2, 3 and Tables 1, S-3–S-5 (input and output files are available at https://doi.org/10.17630/32ebd3c0-bba6-4aa1-9b6d-c53a0a3b61e0).
Figure 2 Model results for the reaction pathway during metasomatism of lherzolite by an eclogitic fluid (Run 27; Tables 1, S-1–S-4). Each unit of the reaction progress variable (ξ) corresponds to destruction of 1.0 mole of each of the reactant minerals per 1.0 kg of H2O in the initial fluid. (a) Changes in the total dissolved concentration of the major elements in the fluid; (b) Moles of new minerals precipitated from the fluid during the continuous reaction pathway; (c) and (d) The compositions of garnets and clinopyroxenes, respectively, as a function of reaction progress. Full results for all models are provided in the output files (available upon request). Olivine precipitates in the final stage of the model (Table 1) and is therefore absent in Figure 2b. Note change in scale for the x axis for a–b vs. c–d.

Figure 3 Selected model results for predicted (a) garnet and (b) clinopyroxene compositions during progressive metasomatism. Each model run refers to a single peridotite metasomatised by a single eclogitic fluid. Key parameters for the models shown are detailed in Tables 1, S-1–S-5, and the input and output files are available upon request.
top
The Nature of Diamond Inclusion Formation
Connecting eclogitic to peridotitic inclusions along a single reaction pathway. Fluid-rock interaction results in the compositional evolution of the fluid phase coupled to the continual precipitation of mineral phases with progressively more Mg-rich garnet and clinopyroxene compositions (Fig. 2a–d). In fact, the major element geochemistry of garnets precipitated in our models traverses from eclogitic to peridotitic along the websteritic ‘bridge’ during a single reaction pathway (Fig. 3a). Importantly, it can be seen in Figure 3a that reaction pathways in lherzolite, harzburgite, and dunite follow the same compositional trend. Metasomatism of a single peridotite by a single eclogitic fluid will result in the progressive precipitation of garnets starting with eclogitic, then websteritic, and finally pyrope-rich peridotitic compositions (Fig. 3a). A similar observation is true for clinopyroxenes, where our models all travel from deep inside the eclogitic field and cross over the (poorly defined) websteritic data cluster and into the (also poorly defined) peridotitic cluster along a single reaction pathway (Fig. 3b). Our results for garnet and clinopyroxene compositional trends may not apply to all diamond-hosted mineral inclusions, but they demonstrate that the paragenesis for the inclusion and host diamond can be syngenetic, and that the different inclusion parageneses can be genetically related and preserve a reaction pathway. Therefore, and on occasion, inclusion paragenesis does not serve as a bona fide proxy for provenance. For example, a peridotitic inclusion can precipitate from an eclogitic fluid, while an eclogitic inclusion will precipitate from an eclogitic fluid within a peridotite. Without diamond to preserve the reaction products as inclusions there would be no evidence for this pathway (i.e. the websteritic bridge) because the high temperatures of the mantle are in excess of the blocking temperature for these phases (Pearson and Wittig, 2014
Pearson, D.G., Wittig, N. (2014) The formation and evolution of cratonic mantle lithosphere—evidence from mantle xenoliths. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry. Second edition, Elsevier, Oxford, 255–292.
) which would result in post-formation homogenisation via diffusion. However, the highly refractory, low diffusivity, and inert nature of diamond, results in archives of this reaction pathway occasionally, and globally, in the geochemistry of diamond inclusions (Fig. 3a,b).The formation of diamond along single reaction pathways (shown in Fig. 2b) involving subducted fluids reacting with ambient mantle peridotite, as presented here, is consistent with the several observations for the geochemistry of diamonds and their inclusions. Our models provide a single mechanistic explanation for the situation where single natural diamonds host disequilibrium inclusion assemblages, such as diamonds hosting inclusions of mixed parageneses (Gurney and Boyd, 1982
Gurney, J.J., Boyd, F.R. (1982) Mineral intergrowths with polycrystalline diamonds from Orapa Mine, Botswana. Carnegie Institution of Washington Yearbook 81, 267–273.
; Wang, 1998Wang, W. (1998) Formation of diamond with mineral inclusions of “mixed” eclogite and peridotite paragenesis. Earth and Planetary Science Letters 160, 831–834.
; Dobosi and Kurat, 2010Dobosi, G., Kurat, G. (2010) On the origin of silicate-bearing diamondites. Mineralogy and Petrology 99, 29–42.
; Mikhail et al., 2019aMikhail, S., McCubbin, F.M., Jenner, F.E., Shirey, S.B., Rumble, D., Bowden, R. (2019a) Diamondites: evidence for a distinct tectono-thermal diamond-forming event beneath the Kaapvaal craton. Contributions to Mineralogy and Petrology 174, 71.
). Furthermore, our models satisfy some stable isotope data, such as strong isotopic evidence for the role of both mantle and crustal sources within single samples and single sample populations. For example, mantle-like high 3He/4He is observed for diamonds with crustal-like 15N-enrichment and 13C-depletion (Gautheron et al., 2005Gautheron, C., Cartigny, P., Moreira, M., Harris, J.W., Allègre, C.J. (2005) Evidence for a mantle component shown by rare gases, C and N isotopes in polycrystalline diamonds from Orapa (Botswana). Earth and Planetary Science Letters 240, 559–572.
; Mikhail et al., 2019bMikhail, S., Crosby, J.C., Stuart, F.M., DiNicola, L., Abernethy, F.A.J. (2019b) A secretive mechanical exchange between mantle and crustal volatiles revealed by helium isotopes in 13C-depleted diamonds. Geochemical Perspectives Letters 11, 39–43.
; Timmerman et al., 2019Timmerman, S., Honda, M., Burnham, A.D., Amelin, Y., Woodland, S., Pearson, D.G., Jaques, A.L., Le Losq, C., Bennett, V.C., Bulanova, G.P., Smith, C.B., Harris, J.W., Tohver, E. (2019) Primordial and recycled helium isotope signatures in the mantle transition zone. Science 365, 692–694.
) and the occasional observation for coupled and progressive 13C-depletion and 18O-enrichment trends between diamond and inclusion (Schulze et al., 2013Schulze, D.J., Harte, B., Page, F.Z., Valley, J.W., Channer, D.M.D., Jaques, A.L. (2013) Anticorrelation between low δ13C of eclogitic diamonds and high δ18O of their coesite and garnet inclusions requires a subduction origin. Geology 41, 455–458.
).top
Other Implications
Isothermal and redox neutral diamond formation. Diamond formation by the reduction or oxidation of carbon is difficult to express because lithospheric peridotites can only buffer fO2 for systems where the fluid component is present in trace quantities (<50 ppm; Luth and Stachel, 2014
Luth, R.W., Stachel, T. (2014) The buffering capacity of lithospheric mantle: implications for diamond formation. Contributions to Mineralogy and Petrology 168, 1083.
). This led Stachel and Luth (2015)Stachel, T., Luth, R.W. (2015) Diamond formation – Where, when and how? Lithos 223, 200–220.
to propose a redox neutral mechanism where diamond forms during the cooling of a COH fluid as it crosses thermal gradient. Our data also show that diamond formation is feasible without the need for any shift in redox or pH of the fluid (logfO2 in our models shifts by ≤0.4 log units; Table 1). Contrary to the model in Stachel and Luth (2015)Stachel, T., Luth, R.W. (2015) Diamond formation – Where, when and how? Lithos 223, 200–220.
, our dataset also demonstrates that a thermal gradient is not required. Our models predict isothermal and isobaric diamond formation during irreversible chemical mass transfer. The fundamental driving force for the metasomatism during the chemical mass transfer is provided by the initial chemical disequilibrium when a fluid leaves a mafic eclogitic rock and encounters a peridotite with which it must react.A metasomatic origin for mantle pyroxenites These data inadvertently predict that fluid metasomatism is a potential candidate for the sub-solidus conversion of refractory peridotite into fertile websterite (Fig. 2 and Table 1). The final amount of olivine is less than 1 % in volume and produced only in the very last step of the models (Tables 1, S-5). Our models show the reaction pathway for mafic eclogitic fluids with garnet-bearing and garnet-free dunites, harzburgites, or lherzolites always results in the precipitation of a two-pyroxene dominated mineral assemblage (i.e. websterite; Tables 1, S-5). Interestingly, our metasomatic model producing pyroxenite and diamond is closely analogous to a pyroxenite-diamond connection previously suggested (Kiseeva et al., 2016
Kiseeva, E.S., Wood, B.J., Ghosh, S., Stachel, T. (2016) The pyroxenite-diamond connection. Geochemical Perspectives Letters 2, 1–9.
) albeit with a sub-solidus fluid rather than a melt. Furthermore, the major element geochemistry of mid-ocean ridge and ocean island basalts has been used to posit a pyroxenite-bearing mantle source (very high pyroxene to olivine ratio; Hirschmann and Stolper, 1996Hirschmann, M.M., Stolper, E.M. (1996) A possible role for garnet pyroxenite in the origin of the ‘garnet signature’ in MORB. Contributions to Mineralogy and Petrology 124, 185–208.
; Sobolev et al., 2007Sobolev, A.V., Hofmann, A.W., Kuzmin, D.V., Yaxley, G.M., Arndt, N.T., Chung, S.L., Danyushevsky, L.V., Elliott, T., Frey, F.A., Garcia, M.O., Gurenko, A.A., Kamenetsky, V.S., Kerr, A.C., Krivolutskaya, N.A., Matvienkov, V.V., Nikogosian, I.K., Rocholl, A., Sigurdsson, I.A., Sushchevskaya, N.M., Teklay, M. (2007) The amount of recycled crust in sources of mantle-derived melts. Science 316, 412–417.
). Thus pyroxenites produced by fluid metasomatism (this study) may not be common or vast in scale, but our model demonstrates a mechanism for the generation of a a pyroxenitic source via the metasomatic conversion of peridotite into websterite, without the need to champion a mechanism involving high temperature melting of refractory eclogite.top
Acknowledgements
We are extremely grateful to Thomas Stachel (Univ. of Alberta) for generously sharing the extensive database for mineral inclusions in diamonds without which this study would have taken several years to complete. Funding for SM, ERM, and MR is provided by NERC standard grant (NE/PO12167/1) and UK Space Agency Aurora grant (ST/T001763/1). Funding for DAS is provided by the US DOE (DE-FG-02-96ER-14616) and the US NSF (EAR 1624325 and ACI 1550346). We are grateful to the constructive peer review process handled by Dr Helen Williams and provided by Dr Sonja Aulbach, Dr Janne Koorneef, and two anonymous reviewers, which improved the clarity of this contribution.
Editor: Helen Williams
top
References
Aulbach, S., Stachel, T., Viljoen, K.S., Brey, G.P., Harris, J.W. (2002) Eclogitic and websteritic diamond sources beneath the Limpopo Belt – is slab-melting the link? Contributions to Mineralogy and Petrology 143, 56–70.
 Show in context
Show in context The diversity of the petrological characteristics of diamond-hosted mineral inclusions either reflect [1] pre-metasomatic mineralogical heterogeneity in the upper mantle (Cartigny et al., 2001; Nestola et al., 2017), [2] fluid (Mikhail et al., 2019a,b) and/or melt metasomatism (Aulbach et al., 2002; Kiseeva et al., 2016) coeval with diamond formation, or [3] a combination of both options.
View in article
Cartigny, P., Harris, J.W., Javoy, M. (2001) Diamond genesis, mantle fractionations and mantle nitrogen content: a study of δ13C–N concentrations in diamonds. Earth and Planetary Science Letters 185, 85–98.
 Show in context
Show in context The diversity of the petrological characteristics of diamond-hosted mineral inclusions either reflect [1] pre-metasomatic mineralogical heterogeneity in the upper mantle (Cartigny et al., 2001; Nestola et al., 2017), [2] fluid (Mikhail et al., 2019a,b) and/or melt metasomatism (Aulbach et al., 2002; Kiseeva et al., 2016) coeval with diamond formation, or [3] a combination of both options.
View in article
Dobosi, G., Kurat, G. (2010) On the origin of silicate-bearing diamondites. Mineralogy and Petrology 99, 29–42.
 Show in context
Show in context Our models provide a single mechanistic explanation for the situation where single natural diamonds host disequilibrium inclusion assemblages, such as diamonds hosting inclusions of mixed parageneses (Gurney and Boyd, 1982; Wang, 1998; Dobosi and Kurat, 2010; Mikhail et al., 2019a).
View in article
Gautheron, C., Cartigny, P., Moreira, M., Harris, J.W., Allègre, C.J. (2005) Evidence for a mantle component shown by rare gases, C and N isotopes in polycrystalline diamonds from Orapa (Botswana). Earth and Planetary Science Letters 240, 559–572.
 Show in context
Show in context For example, mantle-like high 3He/4He is observed for diamonds with crustal-like 15N-enrichment and 13C-depletion (Gautheron et al., 2005; Mikhail et al., 2019b; Timmerman et al., 2019) and the occasional observation for coupled and progressive 13C-depletion and 18O-enrichment trends between diamond and inclusion (Schulze et al., 2013).
View in article
Gurney, J.J., Boyd, F.R. (1982) Mineral intergrowths with polycrystalline diamonds from Orapa Mine, Botswana. Carnegie Institution of Washington Yearbook 81, 267–273.
 Show in context
Show in context Our models provide a single mechanistic explanation for the situation where single natural diamonds host disequilibrium inclusion assemblages, such as diamonds hosting inclusions of mixed parageneses (Gurney and Boyd, 1982; Wang, 1998; Dobosi and Kurat, 2010; Mikhail et al., 2019a).
View in article
Gurney, J.J., Harris, J.W., Rickard, R.S. (1984) Silicate and oxide inclusions in diamonds from the Orapa mine, Botswana. In: Kornprobst, J. (Ed.) Kimberlites II: The Mantle and Crust-Mantle Relationships. Developments in Petrology, Elsevier, Amsterdam, 3–9.
 Show in context
Show in context These are [1] peridotitic (i.e. Cr-rich pyrope, diopside, enstatite, olivine), [2] eclogitic (i.e. Cr-poor pyrope-almandine, Na-rich omphacite), and [3] websteritic (intermediate compositions; Meyer and Boyd, 1972; Sobolev et al., 1973; Gurney et al., 1984) (Fig. 1a–d).
View in article
Gurney, J.J., Helmstaedt, H.H., Richardson., S.H., Shirey, S.B. (2010) Diamonds through time. Economic Geology 105, 689–712.
 Show in context
Show in context These invaluable samples reveal that diamond formation spans more than 75 % of Earth’s history (Gurney et al., 2010) with most diamonds forming in the keel of the sub-continental lithospheric mantle (120–180 km; Stachel and Harris, 2008).
View in article
Furthermore, the major element geochemistry of mid-ocean ridge and ocean island basalts has been used to posit a pyroxenite-bearing mantle source (very high pyroxene to olivine ratio; Hirschmann and Stolper, 1996; Sobolev et al., 2007).
View in article
Harper, M., Weinstein, B., tgwoodcock, Simon, C., chebee7i, Morgan, W., Knight, V., Swanson-Hysell, N., Evans, M., jl-bernal, ZGainsforth, The Gitter Badger, SaxonAnglo, Greco, M., Zuidhof, G. (2019) marcharper/python-ternary: Version 1.0.6. Zenodo, doi: 10.5281/zenodo.2628066.
 Show in context
Show in context Ternaries plotted using the Python package python-ternary (Harper et al., 2019).
View in article
Harris, J.W. (1968) The recognition of diamond inclusions. Part 1: syngenetic mineral inclusions. Industrial Diamond Review 28, 402–410.
 Show in context
Show in context The ambiguity arises because the relationship between diamond and inclusion can be protogenetic (Nestola et al., 2017) or syngenetic (Harris, 1968; Mikhail et al., 2019a).
View in article
Helgeson, H.C. (1970) A chemical and thermodynamic model of ore deposition in hydrothermal systems. In: Morgan, B.A. (Ed.) Fiftieth Anniversary Symposia: Mineralogy and Petrology of the Upper Mantle; Sulfides; Mineralogy and Geochemistry of Non-Marine Evaporites. Special Paper No. 3, Mineralogical Society of America, Washington, D.C., 155–186.
 Show in context
Show in context We built on the long-standing tradition in theoretical aqueous geochemistry of modelling irreversible chemical mass transfer in crustal hydrothermal systems (Helgeson, 1970, 1979; Sverjensky, 1984, 1987).
View in article
Helgeson, H.C. (1979) Mass transfer among minerals and hydrothermal solutions. In: Barnes, H.L. (Ed.) Geochemistry of Hydrothermal Ore Deposits. Wiley, New York, 568–610.
 Show in context
Show in context We built on the long-standing tradition in theoretical aqueous geochemistry of modelling irreversible chemical mass transfer in crustal hydrothermal systems (Helgeson, 1970, 1979; Sverjensky, 1984, 1987).
View in article
Hirschmann, M.M., Stolper, E.M. (1996) A possible role for garnet pyroxenite in the origin of the ‘garnet signature’ in MORB. Contributions to Mineralogy and Petrology 124, 185–208.
 Show in context
Show in context Furthermore, the major element geochemistry of mid-ocean ridge and ocean island basalts has been used to posit a pyroxenite-bearing mantle source (very high pyroxene to olivine ratio; Hirschmann and Stolper, 1996; Sobolev et al., 2007).
View in article
Huang, F., Sverjensky, D.A. (2019) Extended Deep Earth Water Model for predicting major element mantle metasomatism. Geochimica et Cosmochimica Acta 254, 192–230.
 Show in context
Show in context Here we used the aqueous speciation and solubility code EQ3 and the chemical mass transfer code EQ6 (Wolery, 1992) modified for upper mantle temperatures and pressures using thermodynamic data from the extended Deep Earth Water model calibrated with experimental solubilities (Huang and Sverjensky, 2019) previously applied to the formation of Panda diamonds (Huang and Sverjensky, 2020).
View in article
The initial fluids were based on a calibration of the fluid chemistry in equilibrium with a mafic eclogitic measured by Kessel et al. (2015) as documented in Huang and Sverjensky (2019) (Table S-1).
View in article
Huang, F., Sverjensky, D.A. (2020) Mixing of carbonatitic into saline fluid during Panda diamond formation. Geochimica et Cosmochimica Acta, 284, 1–20.
 Show in context
Show in context We assumed ideal site mixing of pyrope, almandine, and grossular in garnet composition and a non-ideal mixing of diopside, hedenbergite, and clinoenstatite in clinopyroxene previously calibrated using known mineral and fluid chemistries to simulate diamond formation beneath the Panda mine in the Slave craton (NW Canada) at 950 °C and 4.5 GPa (Huang and Sverjensky, 2020).
View in article
Here we used the aqueous speciation and solubility code EQ3 and the chemical mass transfer code EQ6 (Wolery, 1992) modified for upper mantle temperatures and pressures using thermodynamic data from the extended Deep Earth Water model calibrated with experimental solubilities (Huang and Sverjensky, 2019) previously applied to the formation of Panda diamonds (Huang and Sverjensky, 2020).
View in article
Kessel, R., Pettke, T., Fumagalli, P. (2015) Melting of metasomatized peridotite at 4–6 GPa and up to 1200 °C: an experimental approach. Contributions to Mineralogy and Petrology 169, 37.
 Show in context
Show in context The initial fluids were based on a calibration of the fluid chemistry in equilibrium with a mafic eclogitic measured by Kessel et al. (2015) as documented in Huang and Sverjensky (2019) (Table S-1).
View in article
Kiseeva, E.S., Wood, B.J., Ghosh, S., Stachel, T. (2016) The pyroxenite-diamond connection. Geochemical Perspectives Letters 2, 1–9.
 Show in context
Show in context Interestingly, our metasomatic model producing pyroxenite and diamond is closely analogous to a pyroxenite-diamond connection previously suggested (Kiseeva et al., 2016) albeit with a sub-solidus fluid rather than a melt.
View in article
The diversity of the petrological characteristics of diamond-hosted mineral inclusions either reflect [1] pre-metasomatic mineralogical heterogeneity in the upper mantle (Cartigny et al., 2001; Nestola et al., 2017), [2] fluid (Mikhail et al., 2019a,b) and/or melt metasomatism (Aulbach et al., 2002; Kiseeva et al., 2016) coeval with diamond formation, or [3] a combination of both options.
View in article
Luth, R.W., Stachel, T. (2014) The buffering capacity of lithospheric mantle: implications for diamond formation. Contributions to Mineralogy and Petrology 168, 1083.
 Show in context
Show in context Diamond formation by the reduction or oxidation of carbon is difficult to express because lithospheric peridotites can only buffer fO2 for systems where the fluid component is present in trace quantities (<50 ppm; Luth and Stachel, 2014).
View in article
Meyer, H.O.A., Boyd, F.R. (1972) Composition and origin of crystalline inclusions in natural diamonds. Geochimica et Cosmochimica Acta 36, 1255–1273.
 Show in context
Show in context These are [1] peridotitic (i.e. Cr-rich pyrope, diopside, enstatite, olivine), [2] eclogitic (i.e. Cr-poor pyrope-almandine, Na-rich omphacite), and [3] websteritic (intermediate compositions; Meyer and Boyd, 1972; Sobolev et al., 1973; Gurney et al., 1984) (Fig. 1a–d).
View in article
Mikhail, S., Crosby, J.C., Stuart, F.M., DiNicola, L., Abernethy, F.A.J. (2019b) A secretive mechanical exchange between mantle and crustal volatiles revealed by helium isotopes in 13C-depleted diamonds. Geochemical Perspectives Letters 11, 39–43.
 Show in context
Show in context For example, mantle-like high 3He/4He is observed for diamonds with crustal-like 15N-enrichment and 13C-depletion (Gautheron et al., 2005; Mikhail et al., 2019b; Timmerman et al., 2019) and the occasional observation for coupled and progressive 13C-depletion and 18O-enrichment trends between diamond and inclusion (Schulze et al., 2013).
View in article
The diversity of the petrological characteristics of diamond-hosted mineral inclusions either reflect [1] pre-metasomatic mineralogical heterogeneity in the upper mantle (Cartigny et al., 2001; Nestola et al., 2017), [2] fluid (Mikhail et al., 2019a,b) and/or melt metasomatism (Aulbach et al., 2002; Kiseeva et al., 2016) coeval with diamond formation, or [3] a combination of both options.
View in article
Mikhail, S., McCubbin, F.M., Jenner, F.E., Shirey, S.B., Rumble, D., Bowden, R. (2019a) Diamondites: evidence for a distinct tectono-thermal diamond-forming event beneath the Kaapvaal craton. Contributions to Mineralogy and Petrology 174, 71.
 Show in context
Show in context The diversity of the petrological characteristics of diamond-hosted mineral inclusions either reflect [1] pre-metasomatic mineralogical heterogeneity in the upper mantle (Cartigny et al., 2001; Nestola et al., 2017), [2] fluid (Mikhail et al., 2019a,b) and/or melt metasomatism (Aulbach et al., 2002; Kiseeva et al., 2016) coeval with diamond formation, or [3] a combination of both options.
View in article
The ambiguity arises because the relationship between diamond and inclusion can be protogenetic (Nestola et al., 2017) or syngenetic (Harris, 1968; Mikhail et al., 2019a).
View in article
Our models provide a single mechanistic explanation for the situation where single natural diamonds host disequilibrium inclusion assemblages, such as diamonds hosting inclusions of mixed parageneses (Gurney and Boyd, 1982; Wang, 1998; Dobosi and Kurat, 2010; Mikhail et al., 2019a).
View in article
Nestola, F., Jung, H., Taylor, L.A. (2017) Mineral inclusions in diamonds may be synchronous but not syngenetic. Nature Communications 14168, doi: 10.1038/ncomms14168.
 Show in context
Show in context The ambiguity arises because the relationship between diamond and inclusion can be protogenetic (Nestola et al., 2017) or syngenetic (Harris, 1968; Mikhail et al., 2019a).
View in article
The diversity of the petrological characteristics of diamond-hosted mineral inclusions either reflect [1] pre-metasomatic mineralogical heterogeneity in the upper mantle (Cartigny et al., 2001; Nestola et al., 2017), [2] fluid (Mikhail et al., 2019a,b) and/or melt metasomatism (Aulbach et al., 2002; Kiseeva et al., 2016) coeval with diamond formation, or [3] a combination of both options.
View in article
Pearson, D.G., Wittig, N. (2014) The formation and evolution of cratonic mantle lithosphere—evidence from mantle xenoliths. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry. Second edition, Elsevier, Oxford, 255–292.
 Show in context
Show in context We ran a series of models to simulate the reactions between eclogitic fluids and a variety of lherzolite, harzburgite, and dunite compositions (Tables 1, S-5) using empirical data for peridotites from the compilation of Pearson and Wittig (2014).
View in article
Without diamond to preserve the reaction products as inclusions there would be no evidence for this pathway (i.e. the websteritic bridge) because the high temperatures of the mantle are in excess of the blocking temperature for these phases (Pearson and Wittig, 2014) which would result in post-formation homogenisation via diffusion.
View in article
Schulze, D.J., Harte, B., Page, F.Z., Valley, J.W., Channer, D.M.D., Jaques, A.L. (2013) Anticorrelation between low δ13C of eclogitic diamonds and high δ18O of their coesite and garnet inclusions requires a subduction origin. Geology 41, 455–458.
 Show in context
Show in context For example, mantle-like high 3He/4He is observed for diamonds with crustal-like 15N-enrichment and 13C-depletion (Gautheron et al., 2005; Mikhail et al., 2019b; Timmerman et al., 2019) and the occasional observation for coupled and progressive 13C-depletion and 18O-enrichment trends between diamond and inclusion (Schulze et al., 2013).
View in article
Sobolev, N.V., Lavrent’ev, Y.G., Pokhilenko, N.P., Usova, L.V. (1973) Chrome-rich garnets from the kimberlites of yakutia and their parageneses. Contributions to Mineralogy and Petrology 40, 39–52.
 Show in context
Show in context These are [1] peridotitic (i.e. Cr-rich pyrope, diopside, enstatite, olivine), [2] eclogitic (i.e. Cr-poor pyrope-almandine, Na-rich omphacite), and [3] websteritic (intermediate compositions; Meyer and Boyd, 1972; Sobolev et al., 1973; Gurney et al., 1984) (Fig. 1a–d).
View in article
Sobolev, A.V., Hofmann, A.W., Kuzmin, D.V., Yaxley, G.M., Arndt, N.T., Chung, S.L., Danyushevsky, L.V., Elliott, T., Frey, F.A., Garcia, M.O., Gurenko, A.A., Kamenetsky, V.S., Kerr, A.C., Krivolutskaya, N.A., Matvienkov, V.V., Nikogosian, I.K., Rocholl, A., Sigurdsson, I.A., Sushchevskaya, N.M., Teklay, M. (2007) The amount of recycled crust in sources of mantle-derived melts. Science 316, 412–417.
 Show in context
Show in context Furthermore, the major element geochemistry of mid-ocean ridge and ocean island basalts has been used to posit a pyroxenite-bearing mantle source (very high pyroxene to olivine ratio; Hirschmann and Stolper, 1996; Sobolev et al., 2007).
View in article
Stachel, T., Harris, J.W. (2008) The origin of cratonic diamonds – constraints from mineral inclusions. Ore Geology Review 34, 5–32.
 Show in context
Show in context Diamond-hosted mineral inclusions are diverse, including, but not limited to, sulfides, silicates, oxides, carbonates, and metallic phases (Stachel and Harris, 2008) and several of these mineral families can be sub-divided into three groups, termed inclusion paragenesis.
View in article
The pressure-temperature conditions adopted here (5 GPa and 1000 °C) are relevant to lithospheric diamond formation (Stachel and Harris, 2008).
View in article
These invaluable samples reveal that diamond formation spans more than 75 % of Earth’s history (Gurney et al., 2010) with most diamonds forming in the keel of the sub-continental lithospheric mantle (120–180 km; Stachel and Harris, 2008).
View in article
Stachel, T., Luth, R.W. (2015) Diamond formation – Where, when and how? Lithos 223, 200–220.
 Show in context
Show in context The temperature of 1000 °C is at the lower end of natural systems, where the average inclusion entrapment temperature is 1155 ± 105 °C (n = 444; Stachel and Luth, 2015).
View in article
The initial oxygen fugacity (fO2) of the model fluids was varied from −1 to −6 log units relative to the Fayalite-Magnetite-Quartz reaction (abbreviated to ΔFMQ), a greater range than is calculated from lithospheric diamond inclusions (Stachel and Luth, 2015).
View in article
This led Stachel and Luth (2015) to propose a redox neutral mechanism where diamond forms during the cooling of a COH fluid as it crosses thermal gradient.
View in article
Contrary to the model in Stachel and Luth (2015), our dataset also demonstrates that a thermal gradient is not required.
View in article
Sverjensky, D.A. (1984) Oil-field brines as ore-forming solutions. Economic Geology 79, 23–37.
 Show in context
Show in context We built on the long-standing tradition in theoretical aqueous geochemistry of modelling irreversible chemical mass transfer in crustal hydrothermal systems (Helgeson, 1970, 1979; Sverjensky, 1984, 1987).
View in article
Sverjensky, D.A. (1987) The role of migrating oil-field brines in the formation of sediment-hosted Cu-rich deposits. Economic Geology 82, 1130–1141.
 Show in context
Show in context We built on the long-standing tradition in theoretical aqueous geochemistry of modelling irreversible chemical mass transfer in crustal hydrothermal systems (Helgeson, 1970, 1979; Sverjensky, 1984, 1987).
View in article
Timmerman, S., Honda, M., Burnham, A.D., Amelin, Y., Woodland, S., Pearson, D.G., Jaques, A.L., Le Losq, C., Bennett, V.C., Bulanova, G.P., Smith, C.B., Harris, J.W., Tohver, E. (2019) Primordial and recycled helium isotope signatures in the mantle transition zone. Science 365, 692–694.
 Show in context
Show in context For example, mantle-like high 3He/4He is observed for diamonds with crustal-like 15N-enrichment and 13C-depletion (Gautheron et al., 2005; Mikhail et al., 2019b; Timmerman et al., 2019) and the occasional observation for coupled and progressive 13C-depletion and 18O-enrichment trends between diamond and inclusion (Schulze et al., 2013).
View in article
Wang, W. (1998) Formation of diamond with mineral inclusions of “mixed” eclogite and peridotite paragenesis. Earth and Planetary Science Letters 160, 831–834.
 Show in context
Show in context Our models provide a single mechanistic explanation for the situation where single natural diamonds host disequilibrium inclusion assemblages, such as diamonds hosting inclusions of mixed parageneses (Gurney and Boyd, 1982; Wang, 1998; Dobosi and Kurat, 2010; Mikhail et al., 2019a).
View in article
Wolery, T.J. (1992) EQ3/6: A software package for geochemical modeling of aqueous systems: package overview and installation guide (version 7.0). Lawrence Livermore National Laboratory, University of California, Livermore, CA.
 Show in context
Show in context Here we used the aqueous speciation and solubility code EQ3 and the chemical mass transfer code EQ6 (Wolery, 1992) modified for upper mantle temperatures and pressures using thermodynamic data from the extended Deep Earth Water model calibrated with experimental solubilities (Huang and Sverjensky, 2019) previously applied to the formation of Panda diamonds (Huang and Sverjensky, 2020).
View in article
top
Supplementary Information
The Supplementary Information includes:
- S1. Diamond Inclusion Database
- S2. Reaction Path Modelling and the Extended Deep Earth Water Model
- Tables S-1 to S-5
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
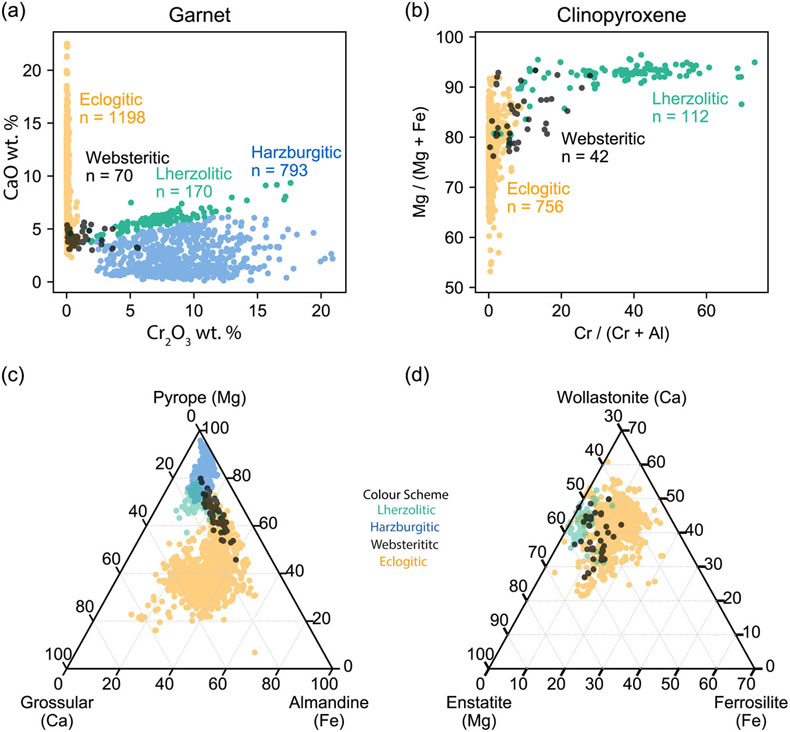
Figure 1 Inclusion parageneses (a,b) and end member compositions for garnets (c) and clinopyroxenes (d) from mantle diamond inclusions. Diamond inclusion data are from numerous sources provided in the Supplementary Information. Inclusion parageneses were assigned based on the original publisher’s characterisation (see Supplementary Information). Ternaries plotted using the Python package python-ternary (Harper et al., 2019
Harper, M., Weinstein, B., tgwoodcock, Simon, C., chebee7i, Morgan, W., Knight, V., Swanson-Hysell, N., Evans, M., jl-bernal, ZGainsforth, The Gitter Badger, SaxonAnglo, Greco, M., Zuidhof, G. (2019) marcharper/python-ternary: Version 1.0.6. Zenodo, doi: 10.5281/zenodo.2628066.
).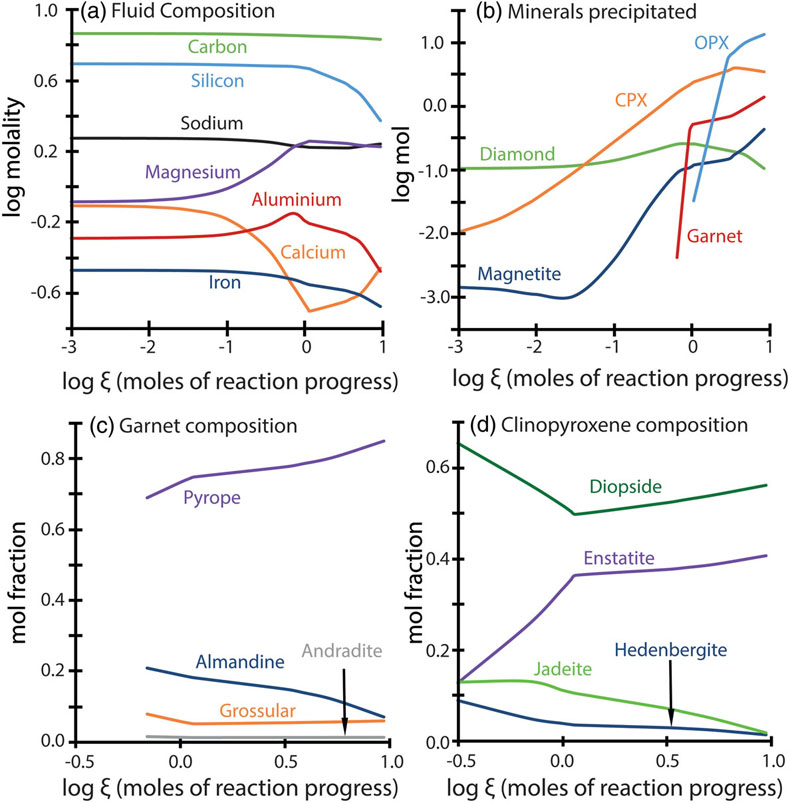
Figure 2 Model results for the reaction pathway during metasomatism of lherzolite by an eclogitic fluid (Run 27; Tables 1, S-1–S-4). Each unit of the reaction progress variable (ξ) corresponds to destruction of 1.0 mole of each of the reactant minerals per 1.0 kg of H2O in the initial fluid. (a) Changes in the total dissolved concentration of the major elements in the fluid; (b) Moles of new minerals precipitated from the fluid during the continuous reaction pathway; (c) and (d) The compositions of garnets and clinopyroxenes, respectively, as a function of reaction progress. Full results for all models are provided in the output files (available upon request). Olivine precipitates in the final stage of the model (Table 1) and is therefore absent in Figure 2b. Note change in scale for the x axis for a–b vs. c–d.
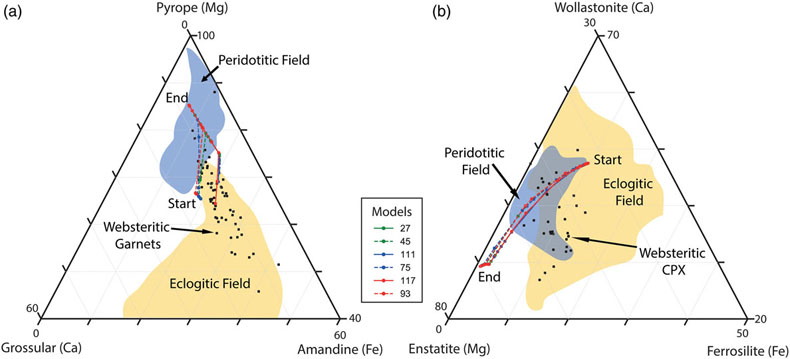
Figure 3 Selected model results for predicted (a) garnet and (b) clinopyroxene compositions during progressive metasomatism. Each model run refers to a single peridotite metasomatised by a single eclogitic fluid. Key parameters for the models shown are detailed in Tables 1, S-1–S-5, and the input and output files are available upon request.