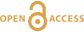In situ redox control and Raman spectroscopic characterisation of solutions below 300 °C
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:377Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
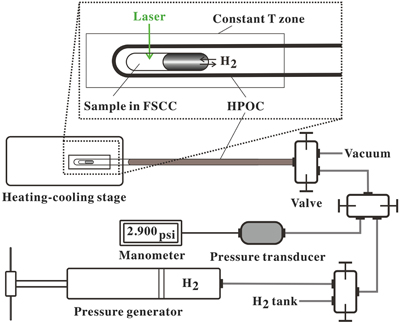 Figure 1 A schematic diagram showing the experimental setup. The sample solution was sealed in an FSCC, which was heated to a fixed T on a heating-cooling stage (Linkam CAP500) and exposed to a fixed external PH2 either during heating or after reaching the target T (see SI for details). |  Figure 2 (a) A schematic diagram (modified from Fig. 1) showing the experimental setup for testing the osmotic equilibrium between PH2 in an FSCC (inner tube 1) and externally imposed PH2. (b) Representative spectra collected from inner tube 1 under an externally imposed PH2 of 1.2 bar (0 to 330 min) and 0.5 bar (480 to 900 min) with the four vibrational bands of H2 marked. (c) Peak height of the Q1(1) vibrational band of H2 (PHH2) measured in a vacuumed FSCC (inner tube 1) at various times after being externally imposed with a PH2 of 1.2 bar (black dots) and then 0.5 bar (red triangles). The values of PHH2 measured from inner tube 2 are shown by squares at 1.2 and 0.5 bar. | 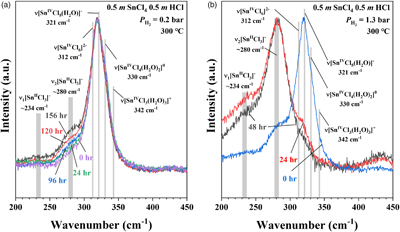 Figure 3 In situ Raman spectra of 0.5 m SnCl4 + 0.5 m HCl solution (300 °C) in an FSCC under vapour saturated pressure after being exposed to (a) a PH2 of 0.2 bar and then (b) 1.3 bar for various durations (shown in hours). The spectrum taken at 0 hr in (b) is the same spectrum taken at 156 hr in (a). According to Schmidt (2018), the major SnIV species was [SnIVCl5(H2O)]-, which was reduced slowly to the SnII species, [SnIICl3]-, under a PH2 of 0.2 bar, as shown in (a), and then quickly under a PH2 of 1.3 bar, as shown in (b). Each spectrum was collected for 200 s with 3 accumulations. The vertical gray lines were taken from Schmidt (2018), showing Raman peak positions of various Sn-Cl species at ambient P-T conditions. | 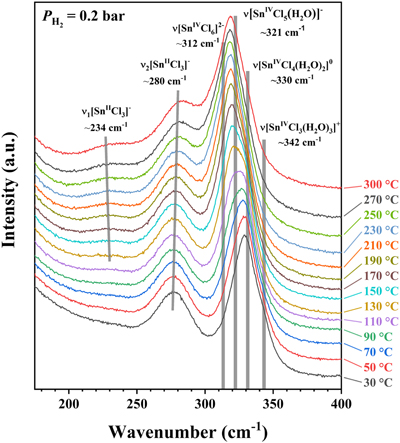 Figure 4 In situ Raman spectra collected at various Ts (30 to 300 °C) from an aqueous solution under vapour saturated pressures and an externally imposed PH2 of 0.2 bar. The sample initially contained 0.5 m SnCl4 and 0.5 m HCl in an FSCC and was quenched from 300 °C after being kept at that T for 311 hr. Each spectrum was collected after the sample was kept at the specified T for at least 10 minutes, which were long enough for reaching equilibrium. The vertical gray lines were taken from Schmidt (2018), showing Raman peak positions of various Sn-bearing species at ambient P-T conditions. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Many geological processes are affected by the redox state of the system, particularly those involving multivalence elements. To simulate these processes in laboratories, several well established redox control techniques have been applied in hydrothermal experiments, including the double capsule (or oxygen buffer) (Eugster, 1957
Eugster, H.P. (1957) Heterogeneous reactions involving oxidation and reduction at high pressures and temperatures. Journal of Chemical Physics 26, 1760–1761.
) and Shaw membrane techniques (Shaw, 1963Shaw, H.R. (1963) Hydrogen-water vapor mixtures: Control of hydrothermal atmospheres by hydrogen osmosis. Science 139, 1220–1222.
). However, even after considerable refinements in the past six decades (Chou, 1987Chou, I.M. (1987) Calibration of the graphite-methane buffer using the fH2 sensors at 2-kbar pressure. American Mineralogist 72, 76–81.
; Taylor et al., 1992Taylor, J., Wall, V., Pownceby, M. (1992) The calibration and application of accurate redox sensors. American Mineralogist 77, 284–295.
; Berndt et al., 2002Berndt, J., Liebske, C., Holtz, F., Freise, M., Nowak, M., Ziegenbein, D., Hurkuck, W., Koepke, J. (2002) A combined rapid-quench and H2-membrane setup for internally heated pressure vessels: Description and application for water solubility in basaltic melts. American Mineralogist 87, 1717–1726.
; Matthews et al., 2003Matthews, W., Linnen, R.L., Guo, Q. (2003) A filler-rod technique for controlling redox conditions in cold-seal pressure vessels. American Mineralogist 88, 701–707.
; Alex and Zajacz, 2020Alex, A., Zajacz, Z. (2020) A new method to quantitatively control oxygen fugacity in externally heated pressure vessel experiments. European Journal of Mineralogy 32, 219–234.
), these techniques cannot be applied at low temperatures (T < 400 °C) because the precious metal hydrogen membranes that are commonly used in these techniques, such as Pt or Ag-Pd alloys, are not effective at these Ts (Chou, 1986Chou, I.M. (1986) Permeability of precious metals to hydrogen at 2-Kb total pressure and elevated-temperatures. American Journal of Science 286, 638–658.
).To overcome this difficulty, other experimental techniques that do not require precious metal hydrogen membranes were developed (see Supplementary Information, SI). However, these methods may suffer from leakage of H2 from the autoclave, contamination by the buffer materials and possibly slow reaction kinetics of the redox buffer at low Ts. On the other hand, due to its high permeability to H2, fused silica has been adopted as a hydrogen membrane for T < 400 °C hydrothermal experiments (Chou et al., 2008
Chou, I.M., Song, Y.C., Burruss, R.C. (2008) A new method for synthesizing fluid inclusions in fused silica capillaries containing organic and inorganic material. Geochimica et Cosmochimica Acta 72, 5217–5231.
; Shang et al., 2009Shang, L.B., Chou, I.M., Lu, W.J., Burruss, R.C., Zhang, Y.X. (2009) Determination of diffusion coefficients of hydrogen in fused silica between 296 and 523 K by Raman spectroscopy and application of fused silica capillaries in studying redox reactions. Geochimica et Cosmochimica Acta 73, 5435–5443.
; Fang and Chou, 2021Fang, J., Chou, I.M. (2021) Redox control and measurement in low-temperature (< 450 °C) hydrothermal experiments. American Mineralogist 106, 1333–1340.
). A vacuumed FSCC has been employed as a hydrogen fugacity (ƒH2) sensor, which was sealed together with a redox buffer (either Ni-NiO or Co-CoO) in an Au capsule and pressurised externally in a cold seal pressure vessel (CSPV) at 100 MPa and equilibrated between 250 and 400 °C (Fang and Chou, 2021Fang, J., Chou, I.M. (2021) Redox control and measurement in low-temperature (< 450 °C) hydrothermal experiments. American Mineralogist 106, 1333–1340.
). The H2 pressures (PH2) equilibrated at elevated P-T conditions, calculated based on the ideal gas law from the PH2 values measured from quenched FSCCs at room T, were in good agreement with those predicted from available thermodynamic data. However, in situ Raman spectroscopic measurements are not possible because neither Au nor CSPV are transparent. Nevertheless, this technique opens perspectives for simultaneous in situ measurements that will be tackled in the present study.Before the use of optical cells (e.g., hydrothermal diamond anvil cells [HDACs], HPOCs, FSCCs), the quench method was used in most hydrothermal experiments, and the interpretation of the observed quenched products was always a great challenge, especially when non-quenchable species were involved. For example, trisulfur ion (S3-) plays important role during the hydrothermal transport and mineralisation of gold, but it is stable only at elevated P-Ts (Pokrovski and Dubrovinsky, 2011
Pokrovski, G.S., Dubrovinsky, L.S. (2011) The S3– ion is stable in geological fluids at elevated temperatures and pressures. Science 331, 1052–1054.
; Pokrovski and Dubessy, 2015Pokrovski, G.S., Dubessy, J. (2015) Stability and abundance of the trisulfur radical ion S3− in hydrothermal fluids. Earth and Planetary Science Letters 411, 298–309.
; Pokrovski et al., 2015Pokrovski, G.S., Kokh, M.A., Guillaume, D., Borisova, A.Y., Gisquet, P., Hazemann, J.-L., Lahera, E., Del Net, W., Proux, O., Testemale, D. (2015) Sulfur radical species form gold deposits on Earth. Proceedings of the National Academy of Sciences 112, 13484–13489.
).Therefore, this study proposes a new design combining HPOC and FSCC optical cells with a quantitative Raman spectroscopic analysis technique to perform in situ redox control and spectroscopic analyses for hydrothermal experiments below 300 °C. The sample is loaded in an FSCC, which is exposed to and equilibrated with H2 at two different fixed Ps in an HPOC. A case study on Sn-Cl complexes using this design is also reported.
top
Experimental Procedure
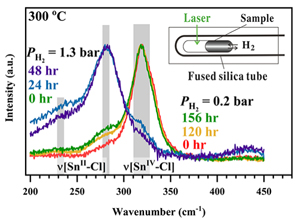
A schematic diagram of the experimental setup is shown in Figure 1. Detailed descriptions of optical cell setup, sample preparation, experimental procedures, collection and processing of Raman spectra are given in SI.

Figure 1 A schematic diagram showing the experimental setup. The sample solution was sealed in an FSCC, which was heated to a fixed T on a heating-cooling stage (Linkam CAP500) and exposed to a fixed external PH2 either during heating or after reaching the target T (see SI for details).
top
Osmotic Equilibrium and In Situ Redox Control in an FSCC
To achieve in situ redox control in an FSCC at elevated P-T conditions, we determined the experimental duration required to reach osmotic equilibrium between the sample in an FSCC and the externally imposed H2 pressure (PH2) in an HPOC at a fixed T. The experimental setup shown in Figure 1 was modified by replacing the solution-containing FSCC with a vacuumed FSCC (inner tube 1 in Fig. 2a) and inserting a short fused silica capillary tube with the same ID and OD as the FSCC, but with two ends open (inner tube 2 in Fig. 2a). In situ Raman spectra were collected from inner tube 1 at 300 °C, first at a PH2 of 1.2 bar (0 to 470 min) and then reduced to 0.5 bar (480 to 1050 min); the representative spectra collected at 0 to 900 min are shown in Figure 2b. The peak heights of Q1(1) vibrations of H2 (PHH2) as a function of time are shown in Figure 2c. Results show that approximately 300 minutes were required for the vacuumed FSCC to reach the externally imposed PH2 of 1.2 bar, and approximately 400 minutes to achieve osmotic equilibrium when the external PH2 was reduced to 0.5 bar.

Figure 2 (a) A schematic diagram (modified from Fig. 1) showing the experimental setup for testing the osmotic equilibrium between PH2 in an FSCC (inner tube 1) and externally imposed PH2. (b) Representative spectra collected from inner tube 1 under an externally imposed PH2 of 1.2 bar (0 to 330 min) and 0.5 bar (480 to 900 min) with the four vibrational bands of H2 marked. (c) Peak height of the Q1(1) vibrational band of H2 (PHH2) measured in a vacuumed FSCC (inner tube 1) at various times after being externally imposed with a PH2 of 1.2 bar (black dots) and then 0.5 bar (red triangles). The values of PHH2 measured from inner tube 2 are shown by squares at 1.2 and 0.5 bar.
top
Application to the Investigation of Hydrothermal Sn Species
Schmidt (2018
Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511.
, and references therein) showed that Sn-Cl complexes in hydrothermal solutions have characteristic Raman spectra for species with Sn in either the +2 (SnII) or +4 (SnIV) valence state. To demonstrate the current in situ redox control technique, a 0.5 m (mole/kg H2O) SnCl4 + 0.5 m HCl solution was sealed in an FSCC together with a vapour phase and exposed to a PH2 of 0.2 bar in an HPOC after being heated to 300 °C (Fig. 1). Raman spectra of the solution were collected in situ under vapour saturation conditions as time elapsed from 0 to 156 hr (Fig. 3a).
Figure 3 In situ Raman spectra of 0.5 m SnCl4 + 0.5 m HCl solution (300 °C) in an FSCC under vapour saturated pressure after being exposed to (a) a PH2 of 0.2 bar and then (b) 1.3 bar for various durations (shown in hours). The spectrum taken at 0 hr in (b) is the same spectrum taken at 156 hr in (a). According to Schmidt (2018)
Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511.
, the major SnIV species was [SnIVCl5(H2O)]-, which was reduced slowly to the SnII species, [SnIICl3]-, under a PH2 of 0.2 bar, as shown in (a), and then quickly under a PH2 of 1.3 bar, as shown in (b). Each spectrum was collected for 200 s with 3 accumulations. The vertical gray lines were taken from Schmidt (2018)Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511.
, showing Raman peak positions of various Sn-Cl species at ambient P-T conditions.The results show that all of the Sn was in the SnIV state at the beginning, and the amount of SnII species increased slowly with time up to 156 hr; at least 276 hr is needed to reach the reaction equilibrium (Figs. S-3, S-7). However, when the external PH2 in the HPOC was increased to 1.3 bar, almost all of the SnIV were converted to SnII species within 48 hours (Fig. 3b); the tail around 321 cm-1 in the 48 hr spectrum may indicate a minor amount of remaining SnIV. Figure 4 shows in situ Raman spectra collected at various Ts (30 to 300 °C) from an aqueous solution under vapour saturated pressures and an external PH2 of 0.2 bar. For details see SI.

Figure 4 In situ Raman spectra collected at various Ts (30 to 300 °C) from an aqueous solution under vapour saturated pressures and an externally imposed PH2 of 0.2 bar. The sample initially contained 0.5 m SnCl4 and 0.5 m HCl in an FSCC and was quenched from 300 °C after being kept at that T for 311 hr. Each spectrum was collected after the sample was kept at the specified T for at least 10 minutes, which were long enough for reaching equilibrium. The vertical gray lines were taken from Schmidt (2018)
Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511.
, showing Raman peak positions of various Sn-bearing species at ambient P-T conditions.Deconvolution of Raman bands of SnII & IV-Cl complexes collected at 30 °C before and after heating and reduction. Due to uncertain T effects on Raman shift for different SnII & IV-Cl complexes, two spectra collected from the second FSCC sample solution at a constant temperature of 30 °C were selected for processing, in order to evaluate the contributions of different Sn-Cl complex species to the Raman bands before and after heating and reduction. Results were shown in Figure S-4; for details see SI.
Chemical vs. osmotic equilibrium. Our experimental results at 300 °C indicate that reaching chemical equilibrium in the Sn-H-Cl system requires a much longer time than that for the osmotic equilibrium for H2 across the fused silica membrane (Figs. 2, 3). However, this conclusion is system specific and may not be valid for (a) other chemical systems, (b) the use of FSCC with different dimensions (i.e. OD, ID and length), or (c) other P-T experimental conditions. Therefore, more testing is required when applying this method.
The presence of unknown SnIV species between 30 and 75 °C. Figure S-5 shows that, at a constant T between 30 and 75 °C, the intensities of Raman spectroscopic band of SnIV-Cl species collected from an aqueous solution in an FSCC-containing 0.8 m SnCl4 + 0.5 m HCl + 0.8 m NaCl during heating (blue) are lower than those collected during cooling (red). This may result from the presence of unknown SnIV species. For details, see SI.
Reduction kinetics of SnIV-Cl to SnII-Cl complexes at 300 °C. The reduction reaction of SnIV-Cl to SnII-Cl complexes at 300 °C under externally imposed PH2 of 0.2 bar can be described by Eq. S-1, and the details on the derivation of reaction rate constant are given in SI.
top
Advantages of In Situ Observation and Raman Spectroscopic Characterisation
In situ observations show that the initial sample solution (0.5 m SnCl4 + 0.5 m HCl) was a homogeneous solution at 25 °C, presumably consisting mainly of Sn-Cl complexes and other unknown species, and they were saturated with respect to cassiterite at 125 °C (Fig. S-1a). However, the cassiterite precipitate could not be detected after being heated at 300 °C under an external PH2 of 0.2 bar for 72 hours (Fig. S-1h), indicating that the formation and stabilisation of SnII & IV-Cl complexes (and also the increase in acidity) increase the solubility of the cassiterite. These SnII & IV-Cl complexes are still present after quenching (Fig. S-4b), and no cassiterite precipitation occurred during or after quenching. Even though these SnII & IV-Cl complexes are quenchable, there is no guarantee that the proportions of these complexes remain the same. In contrast to the use of the conventional quench method for the study of cassiterite solubility, the coexistence of SnII and SnIV complexes and their quantities equilibrated at a specified P-T-pH-PH2 experimental condition can be identified using our in situ method, when quantitative Raman spectroscopic analysis technique is eventually developed, such that the chemical equation for the dissolution of cassiterite in hydrothermal solutions can be correctly expressed.
top
Conclusions
Here we present an experimental technique for in situ redox control and Raman spectroscopic characterisation of complexes (i.e. SnII & IV-Cl interactions) in vapour saturated aqueous solutions. We demonstrated that (a) the time required to reach osmotic equilibrium for H2 across the fused silica membrane is shorter than that for chemical equilibrium between SnII and SnIV, (b) a 0.5 m SnCl4 + 0.5 m HCl solution was saturated with respect to cassiterite (SnO2) between 125 and 300 °C, before the species in the solution were converted to stable SnII & IV-Cl complexes under an externally imposed PH2 of 0.2 bar, and (c) the reduction rate from SnIV-Cl to SnII-Cl complexes at 300 °C under a PH2 of 0.2 bar (equivalent to NNO buffer) was slow, but the rate increased substantially when PH2 increased to 1.3 bar (equivalent to FMQ buffer). These findings are critical for the construction of a sound Sn mineralising mechanism. Furthermore, by using in situ Raman spectroscopic characterisation of the sample in an FSCC under a fixed P-T and redox state, we avoided the possible quench effects on the distribution of dissolved species and greatly increased our capabilities of performing rigorous hydrothermal experiments involving redox reactions at low temperatures, possibly even down to 200 °C (Fang and Chou, 2021
Fang, J., Chou, I.M. (2021) Redox control and measurement in low-temperature (< 450 °C) hydrothermal experiments. American Mineralogist 106, 1333–1340.
).top
Acknowledgements
We thank Dr. Y. Wan, Ms. Y. Chen and Ms. H.Y. Zhang in the Institute of Deep-sea Science and Engineering (IDSSE), the Chinese Academy of Sciences (CAS) for their assistance in our experiments. Constructive reviews were provided by Dr. Xiaolin Wang of Nanjing University, and Dr. Y. Wan and Dr. Nanfei Cheng of IDSSE, CAS. We thank Prof. Gleb Pokrovski and an anonymous reviewer for their critical and constructive reviews. This study was funded by the Key Frontier Science Program (QYZDY-SSW-DQC008) of CAS, and the National Natural Science Foundation of China (No. 41973055; No. 4213000358).
Editor: Satish Myneni
top
References
Alex, A., Zajacz, Z. (2020) A new method to quantitatively control oxygen fugacity in externally heated pressure vessel experiments. European Journal of Mineralogy 32, 219–234. https://doi.org/10.5194/ejm-32-219-2020
 Show in context
Show in context However, even after considerable refinements in the past six decades (Chou, 1987; Taylor et al., 1992; Berndt et al., 2002; Matthews et al., 2003; Alex and Zajacz, 2020), these techniques cannot be applied at low temperatures (T < 400 °C) because the precious metal hydrogen membranes that are commonly used in these techniques, such as Pt or Ag-Pd alloys, are not effective at these Ts (Chou, 1986).
View in article
Berndt, J., Liebske, C., Holtz, F., Freise, M., Nowak, M., Ziegenbein, D., Hurkuck, W., Koepke, J. (2002) A combined rapid-quench and H2-membrane setup for internally heated pressure vessels: Description and application for water solubility in basaltic melts. American Mineralogist 87, 1717–1726. https://doi.org/10.2138/am-2002-11-1222
 Show in context
Show in context However, even after considerable refinements in the past six decades (Chou, 1987; Taylor et al., 1992; Berndt et al., 2002; Matthews et al., 2003; Alex and Zajacz, 2020), these techniques cannot be applied at low temperatures (T < 400 °C) because the precious metal hydrogen membranes that are commonly used in these techniques, such as Pt or Ag-Pd alloys, are not effective at these Ts (Chou, 1986).
View in article
Chou, I.M. (1986) Permeability of precious metals to hydrogen at 2-Kb total pressure and elevated-temperatures. American Journal of Science 286, 638–658. https://doi.org/10.2475/ajs.286.8.638
 Show in context
Show in context However, even after considerable refinements in the past six decades (Chou, 1987; Taylor et al., 1992; Berndt et al., 2002; Matthews et al., 2003; Alex and Zajacz, 2020), these techniques cannot be applied at low temperatures (T < 400 °C) because the precious metal hydrogen membranes that are commonly used in these techniques, such as Pt or Ag-Pd alloys, are not effective at these Ts (Chou, 1986).
View in article
Chou, I.M. (1987) Calibration of the graphite-methane buffer using the fH2 sensors at 2-kbar pressure. American Mineralogist 72, 76–81.
 Show in context
Show in context However, even after considerable refinements in the past six decades (Chou, 1987; Taylor et al., 1992; Berndt et al., 2002; Matthews et al., 2003; Alex and Zajacz, 2020), these techniques cannot be applied at low temperatures (T < 400 °C) because the precious metal hydrogen membranes that are commonly used in these techniques, such as Pt or Ag-Pd alloys, are not effective at these Ts (Chou, 1986).
View in article
Chou, I.M., Song, Y.C., Burruss, R.C. (2008) A new method for synthesizing fluid inclusions in fused silica capillaries containing organic and inorganic material. Geochimica et Cosmochimica Acta 72, 5217–5231. https://doi.org/10.1016/j.gca.2008.07.030
 Show in context
Show in context On the other hand, due to its high permeability to H2, fused silica has been adopted as a hydrogen membrane for T < 400 °C hydrothermal experiments (Chou et al., 2008; Shang et al., 2009; Fang and Chou, 2021).
View in article
Eugster, H.P. (1957) Heterogeneous reactions involving oxidation and reduction at high pressures and temperatures. Journal of Chemical Physics 26, 1760–1761. https://doi.org/10.1063/1.1743626
 Show in context
Show in context To simulate these processes in laboratories, several well established redox control techniques have been applied in hydrothermal experiments, including the double capsule (or oxygen buffer) (Eugster, 1957) and Shaw membrane techniques (Shaw, 1963).
View in article
Fang, J., Chou, I.M. (2021) Redox control and measurement in low-temperature (< 450 °C) hydrothermal experiments. American Mineralogist 106, 1333–1340. https://doi.org/10.2138/am-2021-7687
 Show in context
Show in context A vacuumed FSCC has been employed as a hydrogen fugacity (ƒH2) sensor, which was sealed together with a redox buffer (either Ni-NiO or Co-CoO) in an Au capsule and pressurised externally in a cold seal pressure vessel (CSPV) at 100 MPa and equilibrated between 250 and 400 °C (Fang and Chou, 2021).
View in article
Furthermore, by using in situ Raman spectroscopic characterisation of the sample in an FSCC under a fixed P-T and redox state, we avoided the possible quench effects on the distribution of dissolved species and greatly increased our capabilities of performing rigorous hydrothermal experiments involving redox reactions at low temperatures, possibly even down to 200 °C (Fang and Chou, 2021).
View in article
On the other hand, due to its high permeability to H2, fused silica has been adopted as a hydrogen membrane for T < 400 °C hydrothermal experiments (Chou et al., 2008; Shang et al., 2009; Fang and Chou, 2021).
View in article
Matthews, W., Linnen, R.L., Guo, Q. (2003) A filler-rod technique for controlling redox conditions in cold-seal pressure vessels. American Mineralogist 88, 701–707. https://doi.org/10.2138/am-2003-0424
 Show in context
Show in context However, even after considerable refinements in the past six decades (Chou, 1987; Taylor et al., 1992; Berndt et al., 2002; Matthews et al., 2003; Alex and Zajacz, 2020), these techniques cannot be applied at low temperatures (T < 400 °C) because the precious metal hydrogen membranes that are commonly used in these techniques, such as Pt or Ag-Pd alloys, are not effective at these Ts (Chou, 1986).
View in article
Pokrovski, G.S., Dubrovinsky, L.S. (2011) The S3– ion is stable in geological fluids at elevated temperatures and pressures. Science 331, 1052–1054. https://doi.org/10.1126/science.1199911
 Show in context
Show in context For example, trisulfur ion (S3-) plays important role during the hydrothermal transport and mineralisation of gold, but it is stable only at elevated P-Ts (Pokrovski and Dubrovinsky, 2011; Pokrovski and Dubessy, 2015; Pokrovski et al., 2015).
View in article
Pokrovski, G.S., Dubessy, J. (2015) Stability and abundance of the trisulfur radical ion S3− in hydrothermal fluids. Earth and Planetary Science Letters 411, 298–309. https://doi.org/10.1016/j.epsl.2014.11.035
 Show in context
Show in context For example, trisulfur ion (S3-) plays important role during the hydrothermal transport and mineralisation of gold, but it is stable only at elevated P-Ts (Pokrovski and Dubrovinsky, 2011; Pokrovski and Dubessy, 2015; Pokrovski et al., 2015).
View in article
Pokrovski, G.S., Kokh, M.A., Guillaume, D., Borisova, A.Y., Gisquet, P., Hazemann, J.-L., Lahera, E., Del Net, W., Proux, O., Testemale, D. (2015) Sulfur radical species form gold deposits on Earth. Proceedings of the National Academy of Sciences 112, 13484–13489. https://doi.org/10.1073/pnas.1506378112
 Show in context
Show in context For example, trisulfur ion (S3-) plays important role during the hydrothermal transport and mineralisation of gold, but it is stable only at elevated P-Ts (Pokrovski and Dubrovinsky, 2011; Pokrovski and Dubessy, 2015; Pokrovski et al., 2015).
View in article
Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511. https://doi.org/10.1016/j.gca.2017.10.011
 Show in context
Show in context Schmidt (2018, and references therein) showed that Sn-Cl complexes in hydrothermal solutions have characteristic Raman spectra for species with Sn in either the +2 (SnII) or +4 (SnIV) valence state.
View in article
According to Schmidt (2018), the major SnIV species was [SnIVCl5(H2O)]-, which was reduced slowly to the SnII species, [SnIICl3]-, under a PH2 of 0.2 bar, as shown in (a), and then quickly under a PH2 of 1.3 bar, as shown in (b)
View in article
The vertical gray lines were taken from Schmidt (2018), showing Raman peak positions of various Sn-Cl species at ambient P-T conditions.
View in article
The vertical gray lines were taken from Schmidt (2018), showing Raman peak positions of various Sn-bearing species at ambient P-T conditions.
View in article
Shang, L.B., Chou, I.M., Lu, W.J., Burruss, R.C., Zhang, Y.X. (2009) Determination of diffusion coefficients of hydrogen in fused silica between 296 and 523 K by Raman spectroscopy and application of fused silica capillaries in studying redox reactions. Geochimica et Cosmochimica Acta 73, 5435–5443. https://doi.org/10.1016/j.gca.2009.06.001
 Show in context
Show in context On the other hand, due to its high permeability to H2, fused silica has been adopted as a hydrogen membrane for T < 400 °C hydrothermal experiments (Chou et al., 2008; Shang et al., 2009; Fang and Chou, 2021).
View in article
Shaw, H.R. (1963) Hydrogen-water vapor mixtures: Control of hydrothermal atmospheres by hydrogen osmosis. Science 139, 1220–1222. https://doi.org/10.1126/science.139.3560.1220
 Show in context
Show in context To simulate these processes in laboratories, several well established redox control techniques have been applied in hydrothermal experiments, including the double capsule (or oxygen buffer) (Eugster, 1957) and Shaw membrane techniques (Shaw, 1963).
View in article
Taylor, J., Wall, V., Pownceby, M. (1992) The calibration and application of accurate redox sensors. American Mineralogist 77, 284–295.
 Show in context
Show in context However, even after considerable refinements in the past six decades (Chou, 1987; Taylor et al., 1992; Berndt et al., 2002; Matthews et al., 2003; Alex and Zajacz, 2020), these techniques cannot be applied at low temperatures (T < 400 °C) because the precious metal hydrogen membranes that are commonly used in these techniques, such as Pt or Ag-Pd alloys, are not effective at these Ts (Chou, 1986).
View in article
top
Supplementary Information
The Supplementary Information includes:
- S-1. Previous Redox Buffer Techniques Without Precious Metal Hydrogen Membranes
- S-2. Optical Cell Setup and Sample Preparation
- S-3. Collection and Processing of Raman Spectra
- S-4. Temperature Effect on the Sn-Cl Complexes in a Vapour-saturated Sn-H-Cl Aqueous Solution
- S-5. Deconvolution of Raman Bands of SnII & IV-Cl Complexes Collected at 30 °C Before and After Heating and Reduction
- S-6. The Presence of Unknown SnIV Species between 30 and 75 °C
- S-7. Reduction Kinetics of SnIV-Cl to SnII-Cl Complexes at 300 °C
- Figures S-1 to S-7
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
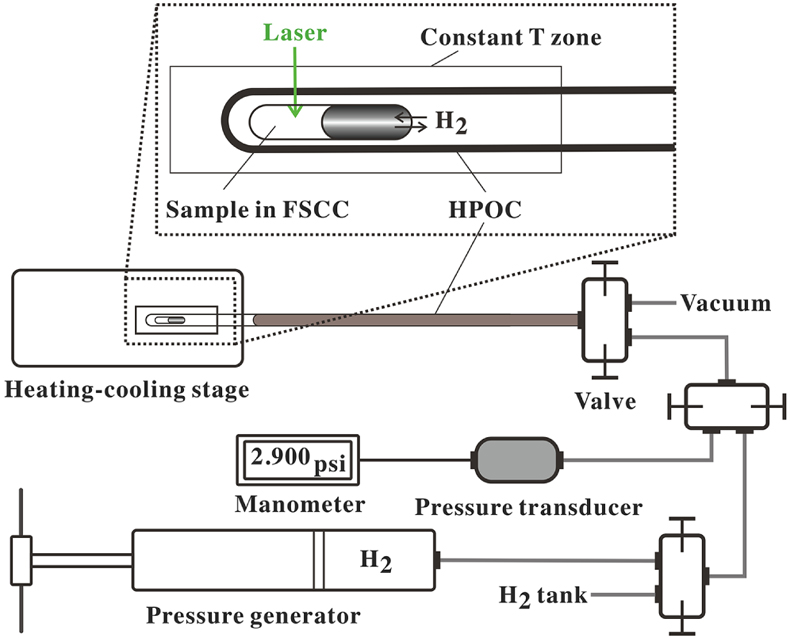
Figure 1 A schematic diagram showing the experimental setup. The sample solution was sealed in an FSCC, which was heated to a fixed T on a heating-cooling stage (Linkam CAP500) and exposed to a fixed external PH2 either during heating or after reaching the target T (see SI for details).
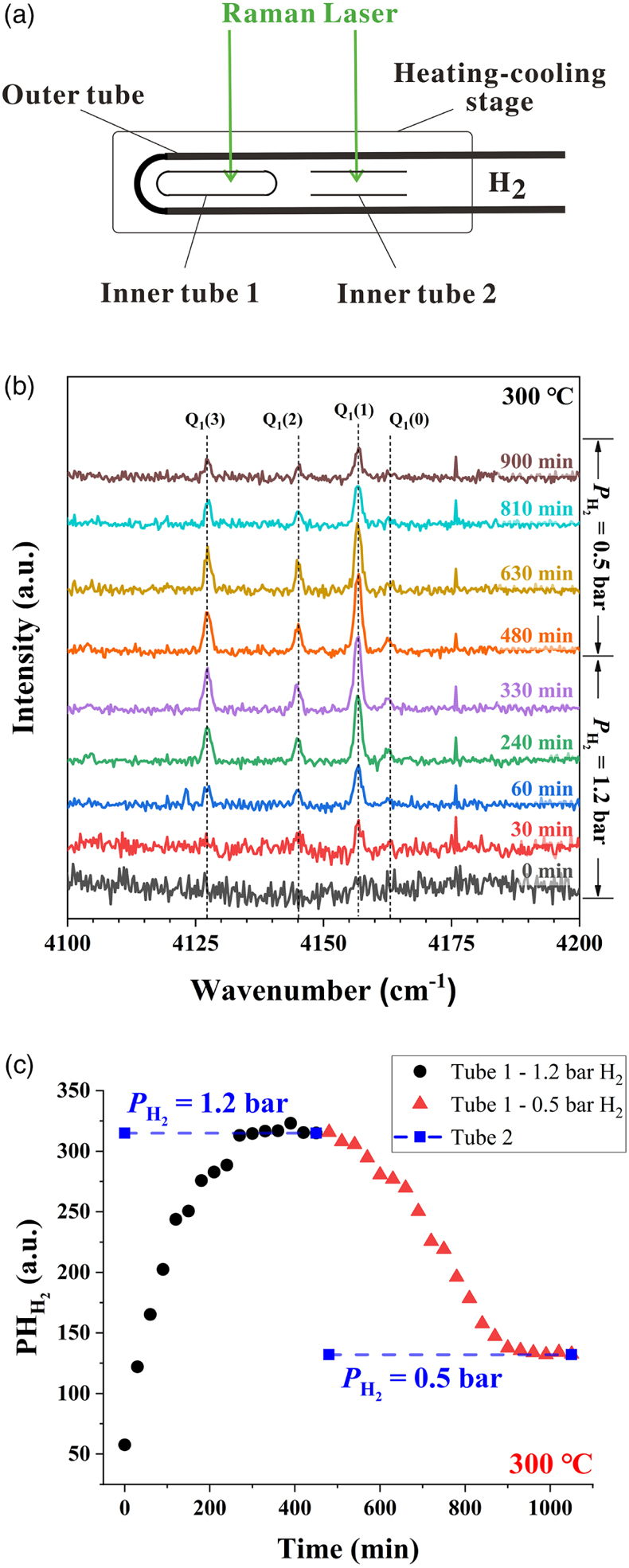
Figure 2 (a) A schematic diagram (modified from Fig. 1) showing the experimental setup for testing the osmotic equilibrium between PH2 in an FSCC (inner tube 1) and externally imposed PH2. (b) Representative spectra collected from inner tube 1 under an externally imposed PH2 of 1.2 bar (0 to 330 min) and 0.5 bar (480 to 900 min) with the four vibrational bands of H2 marked. (c) Peak height of the Q1(1) vibrational band of H2 (PHH2) measured in a vacuumed FSCC (inner tube 1) at various times after being externally imposed with a PH2 of 1.2 bar (black dots) and then 0.5 bar (red triangles). The values of PHH2 measured from inner tube 2 are shown by squares at 1.2 and 0.5 bar.
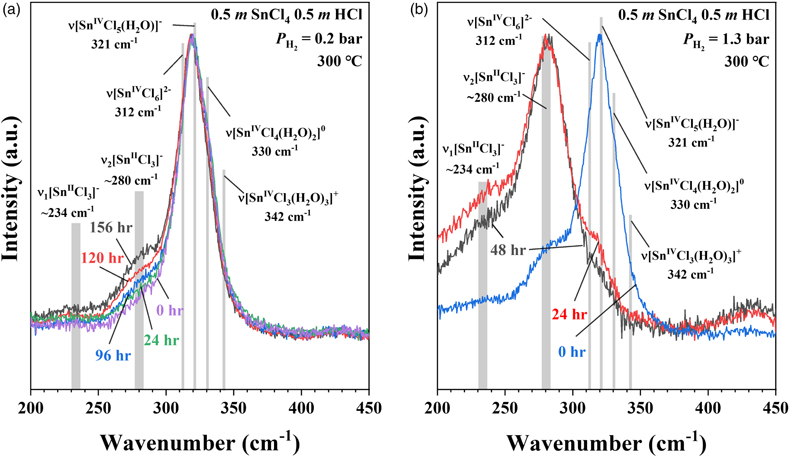
Figure 3 In situ Raman spectra of 0.5 m SnCl4 + 0.5 m HCl solution (300 °C) in an FSCC under vapour saturated pressure after being exposed to (a) a PH2 of 0.2 bar and then (b) 1.3 bar for various durations (shown in hours). The spectrum taken at 0 hr in (b) is the same spectrum taken at 156 hr in (a). According to Schmidt (2018)
Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511.
, the major SnIV species was [SnIVCl5(H2O)]-, which was reduced slowly to the SnII species, [SnIICl3]-, under a PH2 of 0.2 bar, as shown in (a), and then quickly under a PH2 of 1.3 bar, as shown in (b). Each spectrum was collected for 200 s with 3 accumulations. The vertical gray lines were taken from Schmidt (2018)Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511.
, showing Raman peak positions of various Sn-Cl species at ambient P-T conditions.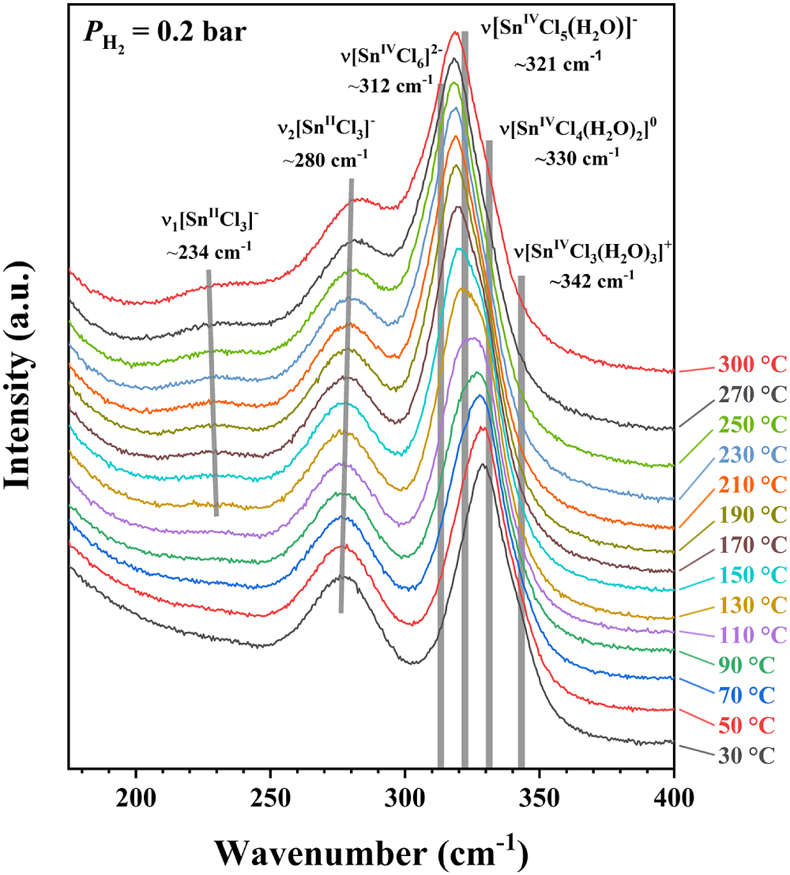
Figure 4 In situ Raman spectra collected at various Ts (30 to 300 °C) from an aqueous solution under vapour saturated pressures and an externally imposed PH2 of 0.2 bar. The sample initially contained 0.5 m SnCl4 and 0.5 m HCl in an FSCC and was quenched from 300 °C after being kept at that T for 311 hr. Each spectrum was collected after the sample was kept at the specified T for at least 10 minutes, which were long enough for reaching equilibrium. The vertical gray lines were taken from Schmidt (2018)
Schmidt, C. (2018) Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an important role of aqueous Sn(IV) species. Geochimica et Cosmochimica Acta 220, 499–511.
, showing Raman peak positions of various Sn-bearing species at ambient P-T conditions.