Calcium isotope fractionation during melt immiscibility and carbonatite petrogenesis
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




Article views:617Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Figures and Tables
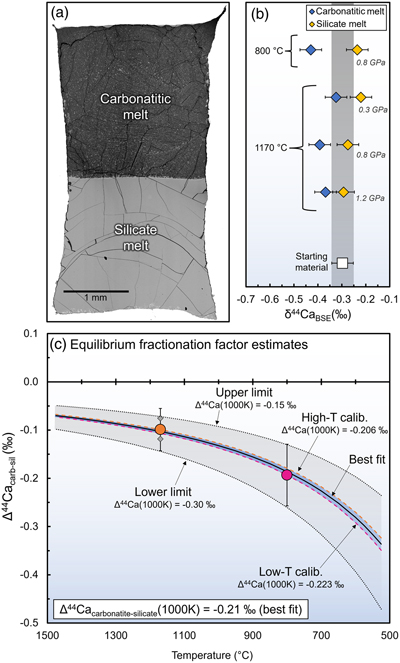 Figure 1 Experimental results of carbonatite-silicate melt immiscibility. (a) Back scattered electron image of an exemplary experimental run product (GS8-28) in a welded Au80Pd20 capsule where the lower density carbonatite melt (dark grey, now quench minerals) migrates to the gravitational top, while the silicate melt (now silicate glass), accumulates at the bottom. (b) Stable Ca isotope results. (c) Estimates of Ca isotope fractionation factor between conjugate carbonatite and silicate melts. Error bars represent 2σ internal uncertainty based on multiple measurements of USGS standards W-2a and COQ-1, where tSE (the t standard error, where ‘t’ is the critical value for a 95% confidence interval in a t-distribution) and 2 s.e. uncertainties both yield similar estimates of ±0.04 ‰ for δ44Ca and ±0.06 ‰ for Δ44Ca (see Supplementary Information). These uncertainty estimates are also similar to our calculated measurement repeatability (±0.04 ‰, based on the 22 samples measured in duplicate in this work). Long term external reproducibility, which is most important for comparisons of our natural δ44Ca data (Fig. 2b) with previous/future work is estimated as ± 0.12 ‰ (2 s.d.) based on 21 measurements of W-2a performed over three years. | 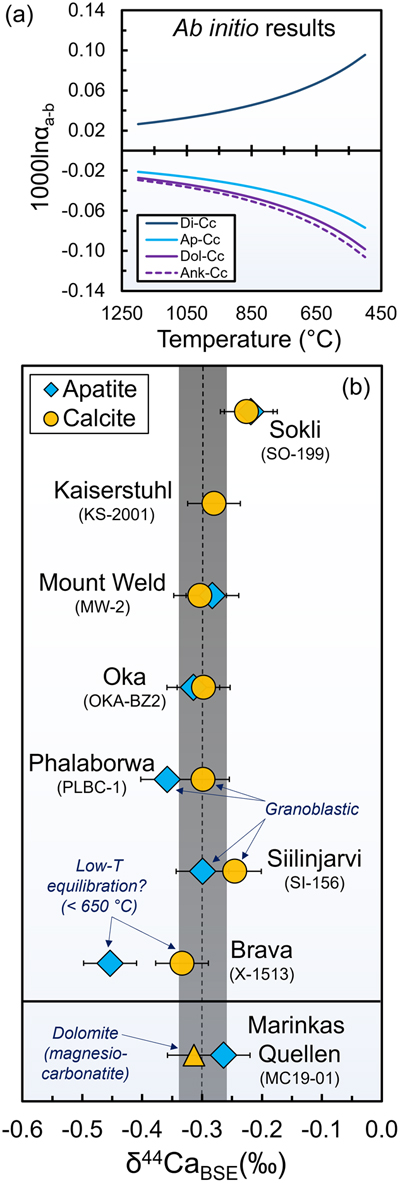 Figure 2 Ab initio estimates for equilibrium between various minerals and summary of our δ44Ca measurements in calcite and apatite from natural carbonatites. (a) Predicted inter-mineral fractionation factors (1000lnαmineral-calcite) vs. temperature. (b) δ44Ca data for pristine apatite and calcite separated from our carbonatite samples. In panel (b) 2σ uncertainties for δ44Ca are the same as described in the caption of Figure 1. | 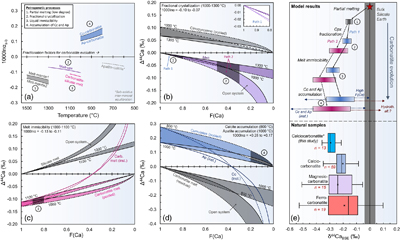 Figure 3 Calcium isotope evolution during the various stages of carbonatite petrogenesis compared to data for carbonatites from this and previous studies. (a) Fractionation factors for the four stages: partial melting (Step 1), melt differentiation by crystal fractionation (Step 2), carbonatite-silicate melt immiscibility (Step 3), and accumulation of calcite and apatite (Step 4). Partial melting results (Step 1) come from Antonelli et al. (2023a) and encompass the largest and smallest predicted fractionations during melting of carbon-bearing garnet lherzolite at 1400−1300 °C. (b) Clinopyroxene fractionation from CO2-rich alkaline mafic melt (Step 2), showing the two possible paths (nephelinite “path 1” and syenite “path 2”) leading to two-liquid immiscibility. (c) Carbonatite-silicate melt immiscibility (Step 3). (d) Calcite and apatite accumulation from a carbonatitic melt (Step 4). Shaded regions in (b-d) represent predicted F(Ca) values. In (e) we show the model results from Step 1 to 4 and compare them with natural carbonatite data (Supplementary Information). | 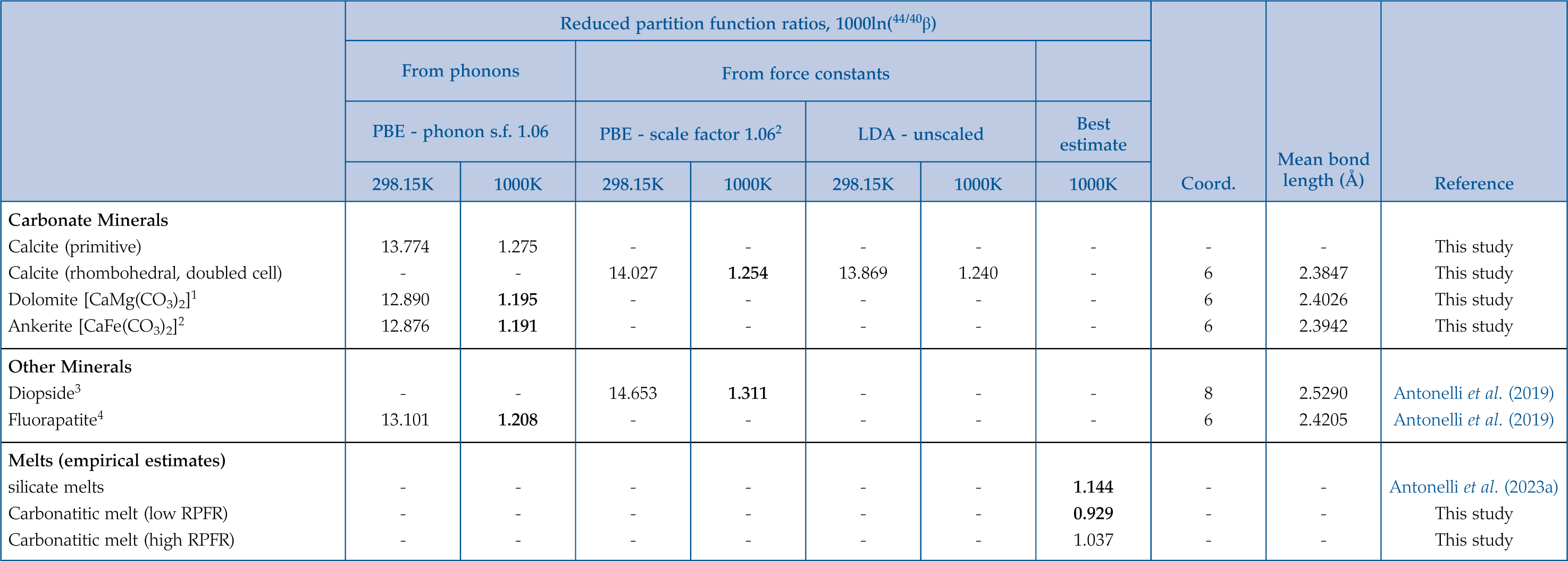 Table 1 Ab initio results for Ca isotope 1000lnβ factors (44Ca/40Ca) in calcite, dolomite, ankerite, diopside, and fluorapatite, along with empirical estimates for melts used in our carbonatite petrogenesis models (preferred values in bold). |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
top
Introduction
Carbonatites are rare igneous rocks that contain large amounts (>50 %) of carbonate minerals and represent the most CO2-rich magmas in the geologic record (Yaxley et al., 2022
Yaxley, G.M., Anenburg, M., Tappe, S., Decree, S., Guzmics, T. (2022) Carbonatites: Classification, Sources, Evolution, and Emplacement. Annual Review of Earth and Planetary Sciences 50, 261–293. https://doi.org/10.1146/annurev-earth-032320-104243
). Although they provide a majority of the world’s Rare Earth Elements (REE) and are intimately linked to the deep Earth carbon cycle, their principal formation mechanism(s) are still debated. Possible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023Berkesi, M., Myovela, J.L., Yaxley, G.M., Guzmics, T. (2023) Carbonatite formation in continental settings via high pressure – high temperature liquid immiscibility. Geochimica et Cosmochimica Acta 349, 41–54. https://doi.org/10.1016/j.gca.2023.03.027
; Berndt and Klemme, 2022Berndt, J., Klemme, S. (2022) Origin of carbonatites—liquid immiscibility caught in the act. Nature Communications 13, 7–14. https://doi.org/10.1038/s41467-022-30500-7
; Brooker and Kjarsgaard, 2011Brooker, R.A., Kjarsgaard, B.A. (2011) Silicate-carbonate liquid immiscibility and phase relations in the system SiO2-Na2O-Al2O3-CaO-Co2 at 0·1-2·5 GPa with applications to carbonatite genesis. Journal of Petrology 52, 1281–1305. https://doi.org/10.1093/petrology/egq081
; Guzmics et al., 2015Guzmics, T., Zajacz, Z., Mitchell, R.H., Szab´o, C., Wälle, M. (2015) The role of liquid–liquid immiscibility and crystal fractionation in the genesis of carbonatite magmas: insights from Kerimasi melt inclusions. Contributions to Mineralogy and Petrology 169, 17. https://doi.org/10.1007/s00410-014-1093-4
; Weidendorfer et al., 2017Weidendorfer, D., Schmidt, M.W., Mattsson, H.B. (2017) A common origin of carbonatite magmas. Geology 45, 507–510. https://doi.org/10.1130/G38801.1
)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998Harmer, R.E., Gittins, J. (1998) The case for primary, mantle-derived carbonatite magma. Journal of Petrology 39, 1895–1903. https://doi.org/10.1093/petroj/39.11-12.1895
; Yaxley and Brey, 2004Yaxley, G.M., Brey, G.P. (2004) Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contributions to Mineralogy and Petrology 146, 606–619. https://doi.org/10.1007/s00410-003-0517-3
). There are also significant debates regarding the role of carbonatite magmas within the carbon cycle, with some recent stable isotope studies suggesting that carbonatites may represent the return of subducted marine carbonates back to the surface (Hulett et al., 2016Hulett, S.R.W., Simonetti, A., Rasbury, E.T., Hemming, N.G. (2016) Recycling of subducted crustal components into carbonatite melts revealed by boron isotopes. Nature Geoscience 9, 904–908. https://doi.org/10.1038/ngeo2831
; Amsellem et al., 2020Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6. https://doi.org/10.1126/sciadv.aba3269
; Banerjee et al., 2021Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
). In contrast, δ13C in most carbonatites overlap with those of peridotitic diamonds (Stachel et al., 2022Stachel, T., Aulbach, S., Harris, J.W. (2022) Mineral inclusions in lithospheric diamonds. Reviews in Mineralogy and Geochemistry 88, 307–391. https://doi.org/10.2138/rmg.2022.88.06
). The carbon in these magmas is thus mantle-derived or, if recycled, could result from a ∼4:1 mix between carbonates and organic carbon sources, rendering it indistinguishable from primordial carbon.Given the uncertainties surrounding the interpretation of δ13C data, stable Ca isotopes have recently gained popularity as a potential tool for tracing subducted marine carbonates in mantle-derived magmas. Their use as a recycled carbonate tracer depends on δ44CaBSE in marine carbonates (Phanerozoic average of −0.35 ‰) being typically lower than found in mantle rocks [≈0 ‰ (see Antonelli and Simon, 2020
Antonelli, M.A., Simon, J.I. (2020) Calcium isotopes in high-temperature terrestrial processes. Chemical Geology 548, 119651. https://doi.org/10.1016/j.chemgeo.2020.119651
)]. Previous studies exploring δ44Ca in carbonatites argue that subducted marine carbonates occur in the sources of essentially all (Amsellem et al., 2020Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6. https://doi.org/10.1126/sciadv.aba3269
), some (Banerjee et al., 2021Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
), or no (Sun et al., 2021Sun, J., Zhu, X.K., Belshaw, N.S., Chen, W., Doroshkevich, A.G., Luo, W.J., Song, W.L., Chen, B.B., Cheng, Z.G., Li, Z.H. and Wang, Y. (2021) Ca isotope systematics of carbonatites: Insights into carbonatite source and evolution. Geochemical Perspectives Letters 17, 11–15. https://doi.org/10.7185/geochemlet.2107
) carbonatites. Amsellem et al. (2020)Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6. https://doi.org/10.1126/sciadv.aba3269
found ubiquitously low δ44CaBSE in carbonatites (average of −0.7 ‰), while the two more recent studies found similar average values of approximately −0.2 ‰ (using two different analytical approaches), and argue that the data of Amsellem et al. (2020)Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6. https://doi.org/10.1126/sciadv.aba3269
were affected by analytical issues (Banerjee et al., 2021Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
; Sun et al., 2021Sun, J., Zhu, X.K., Belshaw, N.S., Chen, W., Doroshkevich, A.G., Luo, W.J., Song, W.L., Chen, B.B., Cheng, Z.G., Li, Z.H. and Wang, Y. (2021) Ca isotope systematics of carbonatites: Insights into carbonatite source and evolution. Geochemical Perspectives Letters 17, 11–15. https://doi.org/10.7185/geochemlet.2107
). Nevertheless, the latter two studies disagree on the potential effects of magmatic processes (Supplementary Information) and find significantly different total ranges for carbonatite δ44Ca, where lower values [found in Banerjee et al. (2021)Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
] are argued to result from subducted marine carbonates.Unlike radiogenic isotope tracers, stable Ca isotope ratios may be significantly affected by magmatic processes that are often poorly constrained and lead to large uncertainties regarding their application as source tracers. To constrain the δ44Ca of carbonatites and their parental melts, we explore δ44Ca variations from experimental, natural, and theoretical perspectives. We first demonstrate that conjugate carbonatite-silicate melts in high pressure, high temperature experiments have significant δ44Ca differences at equilibrium. We then combine these results with novel ab initio and empirical constraints to develop quantitative models for Ca isotope fractionation during carbonatite petrogenesis.
top
Calcium Isotope Fractionation During Carbonatite-Silicate Melt Immiscibility
Although mineral-mineral and mineral-melt equilibrium Ca isotope fractionations are relatively well constrained from both ab initio and empirical investigations (Antonelli and Simon, 2020
Antonelli, M.A., Simon, J.I. (2020) Calcium isotopes in high-temperature terrestrial processes. Chemical Geology 548, 119651. https://doi.org/10.1016/j.chemgeo.2020.119651
), Ca isotope fractionation between immiscible melts has not been explored. Constraining this process is of the utmost importance for carbonatite petrogenesis, as direct melts of carbonate-bearing peridotites in the upper mantle are dolomitic and cannot evolve to the calciocarbonatite melt compositions (Lee and Wyllie, 1998Lee, W.-J., Wyllie, P.J. (1998) Petrogenesis of Carbonatite Magmas from Mantle to Crust, Constrained by the System CaO-(MgO + FeO*)-(Na2O + K2O)-(SiO2 + Al2O3 + TiO2)-CO2. Journal of Petrology 39, 495–517. https://doi.org/10.1093/petroj/39.3.495
) most frequently observed and readily unmixed from silicate melts (Lee and Wyllie, 1998Lee, W.-J., Wyllie, P.J. (1998) Petrogenesis of Carbonatite Magmas from Mantle to Crust, Constrained by the System CaO-(MgO + FeO*)-(Na2O + K2O)-(SiO2 + Al2O3 + TiO2)-CO2. Journal of Petrology 39, 495–517. https://doi.org/10.1093/petroj/39.3.495
; Brooker and Kjarsgaard, 2011Brooker, R.A., Kjarsgaard, B.A. (2011) Silicate-carbonate liquid immiscibility and phase relations in the system SiO2-Na2O-Al2O3-CaO-Co2 at 0·1-2·5 GPa with applications to carbonatite genesis. Journal of Petrology 52, 1281–1305. https://doi.org/10.1093/petrology/egq081
; Martin et al., 2013Martin, L.H.J., Schmidt, M.W., Mattsson, H.B., Guenther, D. (2013) Element Partitioning between Immiscible Carbonatite and Silicate Melts for Dry and H2O-bearing Systems at 1-3GPa. Journal of Petrology 54, 2301–2338. https://doi.org/10.1093/petrology/egt048
; Guzmics et al., 2015Guzmics, T., Zajacz, Z., Mitchell, R.H., Szab´o, C., Wälle, M. (2015) The role of liquid–liquid immiscibility and crystal fractionation in the genesis of carbonatite magmas: insights from Kerimasi melt inclusions. Contributions to Mineralogy and Petrology 169, 17. https://doi.org/10.1007/s00410-014-1093-4
). To better understand Ca isotope fractionation between immiscible melts, we equilibrated conjugate carbonatite and nephelinitic silicate melts in a centrifuging piston cylinder apparatus at ETH Zurich (Schmidt et al., 2006Schmidt, M.W., Connolly, J.A.D., Günther, D., Bogaerts, M. (2006) Element partitioning: The role of melt structure and composition. Science 312, 1646–1650. https://doi.org/10.1126/science.1126690
). This apparatus allows for near-perfect physical separation of two immiscible melts (Figs. 1a, S-1). We conducted experiments at two different temperatures (800 °C and 1170 °C) and also explored the effects of variable pressure (0.3, 0.8, and ∼1.2 GPa) in the higher temperature experiments (Supplementary Information). After equilibration and centrifugation, experimental run products were quenched and sectioned lengthwise to allow for both destructive (Ca isotope analyses by TIMS) and non-destructive analyses (e.g., EPMA; Table S-1) of the two melts.
Figure 1 Experimental results of carbonatite-silicate melt immiscibility. (a) Back scattered electron image of an exemplary experimental run product (GS8-28) in a welded Au80Pd20 capsule where the lower density carbonatite melt (dark grey, now quench minerals) migrates to the gravitational top, while the silicate melt (now silicate glass), accumulates at the bottom. (b) Stable Ca isotope results. (c) Estimates of Ca isotope fractionation factor between conjugate carbonatite and silicate melts. Error bars represent 2σ internal uncertainty based on multiple measurements of USGS standards W-2a and COQ-1, where tSE (the t standard error, where ‘t’ is the critical value for a 95% confidence interval in a t-distribution) and 2 s.e. uncertainties both yield similar estimates of ±0.04 ‰ for δ44Ca and ±0.06 ‰ for Δ44Ca (see Supplementary Information). These uncertainty estimates are also similar to our calculated measurement repeatability (±0.04 ‰, based on the 22 samples measured in duplicate in this work). Long term external reproducibility, which is most important for comparisons of our natural δ44Ca data (Fig. 2b) with previous/future work is estimated as ± 0.12 ‰ (2 s.d.) based on 21 measurements of W-2a performed over three years.
We find that carbonatitic melts always have lower δ44Ca than coexisting silicate melts, with the 800 °C (0.8 GPa) experiment yielding Δ44Cacarb-sil of −0.19 ± 0.06 ‰ and the three 1170 °C experiments giving statistically indistinguishable results, regardless of pressure (Δ44Cacarb-sil between −0.08 ‰ and −0.12 ‰; Fig. 1b, Table S-2). Several independent lines of evidence suggest that our experiments achieved Ca isotope equilibrium. Along with the high Ca diffusivities in silicate and carbonatitic melts relative to the length scales and durations of our experiments, the equilibrium fractionation factor [1000lnα; Fig. 1c] calculated from the lower T experiment (−0.22 at 1000K, using a 106/T2 law), is strikingly similar to that derived from the average of the higher T experiments (−0.21 at 1000K; Fig. S2). The three different 1170 °C experiments also represent significant variations in the Ca fraction [F(Ca)] in carbonatitic vs. silicate melts, yet they yield statistically indistinguishable Δ44Cacarb-sil, suggesting closed system equilibrium fractionation (Fig. S-3). Our finding that carbonatitic melts have lower δ44Ca than coexisting silicate melts is consistent with predictions for a weaker bonding environment for Ca in ionic carbonatitic melts vs. predominantly covalent silicate melts (see Genge et al., 1995
Genge, M.J., Price, G.D., Jones, A.P. (1995) Molecular dynamics simulations of CaCO3 melts to mantle pressures and temperatures: implications for carbonatite magmas. Earth and Planetary Science Letters 131, 225–238. https://doi.org/10.1016/0012-821X(95)00020-D
).top
Natural and Theoretical Constraints on δ44Ca in Carbonatite Minerals
Most carbonatites represent cumulate rocks (Yaxley et al., 2022
Yaxley, G.M., Anenburg, M., Tappe, S., Decree, S., Guzmics, T. (2022) Carbonatites: Classification, Sources, Evolution, and Emplacement. Annual Review of Earth and Planetary Sciences 50, 261–293. https://doi.org/10.1146/annurev-earth-032320-104243
). In calciocarbonatites, the Ca budget is dominated by calcite (plus minor apatite ± diopside), whereas magnesiocarbonatites and ferrocarbonatites are dominated by dolomite and ankerite, respectively. We therefore present novel ab initio predictions (PBE functionals) for equilibrium Ca isotope fractionation between these minerals using the Quantum Espresso software package, following established methods (Antonelli et al., 2019Antonelli, M.A., Schiller, M., Schauble, E.A., Mittal, T., DePaolo, D.J., Chacko, T., Grew, E.S., Tripoli, B. (2019) Kinetic and equilibrium Ca isotope effects in high-T rocks and minerals. Earth and Planetary Science Letters 517, 71–82. https://doi.org/10.1016/j.epsl.2019.04.013
, 2023bAntonelli, M.A., Yakymchuk, C., Schauble, E.A., Foden, J., Janoušek, V., Moyen, J.-F., Hoffmann, J., Moynier, F., Bachmann, O. (2023b) Granite petrogenesis and the δ44Ca of continental crust. Earth and Planetary Science Letters 608, 118080. https://doi.org/10.1016/j.epsl.2023.118080
) (Supplementary Information; Fig. S-4). At isotopic equilibrium, we find that calcite is slightly lighter than diopside (by −0.06 ‰ at 1000K) and slightly heavier than fluorapatite (by +0.05‰ at 1000K), in agreement with previous predictions based on LDA functionals (Xiao et al., 2022Xiao, Z.-C., Zhou, C., Kang, J.-T., Wu, Z.-Q., Huang, F. (2022) The factors controlling equilibrium inter-mineral Ca isotope fractionation: Insights from first-principles calculations. Geochimica et Cosmochimica Acta 333, 373–389. https://doi.org/10.1016/j.gca.2022.07.021
). Dolomite and ankerite both have lower 1000lnβ values, similar to those of fluorapatite (Fig. 2a, Table 1).
Figure 2 Ab initio estimates for equilibrium between various minerals and summary of our δ44Ca measurements in calcite and apatite from natural carbonatites. (a) Predicted inter-mineral fractionation factors (1000lnαmineral-calcite) vs. temperature. (b) δ44Ca data for pristine apatite and calcite separated from our carbonatite samples. In panel (b) 2σ uncertainties for δ44Ca are the same as described in the caption of Figure 1.
Table 1 Ab initio results for Ca isotope 1000lnβ factors (44Ca/40Ca) in calcite, dolomite, ankerite, diopside, and fluorapatite, along with empirical estimates for melts used in our carbonatite petrogenesis models (preferred values in bold).
1PBE/GBRV1.4 40-200Ry phonon-based two phonon wave vectors
2PBE/GBRV1.4(Ca,C,O)-PSLibrary(Fe) 90-1080Ry Hubbard correction U = 3.5 eV phonon-based two phonon wave vectors
3Full C2/c cell with doubled c
4Ca1, *7-10th Ca-O neighbours at 2.84Å; Ca2, 5O+1F, and *7th Ca-O bond at 2.80Å
To better constrain δ44Ca in natural carbonatite minerals, we extracted pristine calcite (or dolomite) and apatite crystals from seven calcio- and one magnesiocarbonatite that have equilibrium calcite-apatite REE distributions reflecting primary magmatic signatures (in all but one sample), characterised in previous work (Sartori et al., 2023
Sartori, G., Galli, A., Weidendorfer, D., Schmidt, M.W. (2023) A tool to distinguish magmatic from secondarily recrystallized carbonatites—Calcite/apatite rare earth element partitioning. Geology 51, 54–58. https://doi.org/10.1130/G50416.1
) (Tables S-3, S-4). We chose these minerals to test whether equilibrium was attained between the most abundant Ca minerals in carbonatites, or whether kinetic effects could be leading to some of the observed δ44Ca variability. Along with δ44Ca analyses by TIMS, we also measured radiogenic 40Ca abundances in several samples (and carbonatite standard COQ-1; Table S-5), all of which yield ɛCa within error of the BSE value (Antonelli et al., 2021Antonelli, M.A., DePaolo, D.J., Christensen, J.N., Wotzlaw, J.-F., Pester, N.J., Bachmann, O. (2021) Radiogenic 40Ca in seawater: implications for modern and ancient Ca cycles. ACS Earth and Space Chemistry 5, 2481–2492. https://doi.org/10.1021/acsearthspacechem.1c00179
).We find a relatively restricted δ44CaBSE range in our calcite separates [−0.33 to −0.23 ‰, average of −0.29 ± 0.03 ‰ (tSE, n = 8)] and slightly larger range (but similar average) in our apatite separates [−0.45 to −0.22 ‰, average of −0.31 ± 0.07 ‰ (tSE, n = 7); Fig. 2b, Table S-2]. In most calciocarbonatite samples, apatite is marginally lighter than calcite, consistent with isotopic equilibrium at temperatures above ∼700 °C. One calciocarbonatite sample (X-1513), however, has Δ44Caapatite-calcite of −0.12 ± 0.06 ‰, potentially suggesting lower temperature inter-mineral equilibration (e.g., <650 °C for a calcite-apatite difference >0.06 ‰). Dolomite and apatite from our magnesiocarbonatite sample (MC-1901) are also consistent with inter-mineral isotopic equilibrium and have δ44Ca indistinguishable from those of our calciocarbonatites. Overall, our mineral δ44Ca analyses yield results that are slightly lower (Fig. 3e; Supplementary Information, Supplementary Data-1) but generally similar to whole rock calciocarbonatite analyses from recent studies (Banerjee et al., 2021
Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
; Sun et al., 2021Sun, J., Zhu, X.K., Belshaw, N.S., Chen, W., Doroshkevich, A.G., Luo, W.J., Song, W.L., Chen, B.B., Cheng, Z.G., Li, Z.H. and Wang, Y. (2021) Ca isotope systematics of carbonatites: Insights into carbonatite source and evolution. Geochemical Perspectives Letters 17, 11–15. https://doi.org/10.7185/geochemlet.2107
). Previous work exploring carbonatite calcite separates, however, found a larger range in δ44CaBSE with a slightly higher average [−0.10 ± 0.08 ‰ (tSE, n = 17)] than found in calciocarbonatite whole rocks (Banerjee et al., 2021Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
), but the primary magmatic nature of the calcite in this data set was not investigated. Despite their subtle differences, all these values are significantly different from the earlier report of ubiquitously low δ44CaBSE [average of −0.69 ± 0.04 ‰ (tSE, n = 50)] in carbonatite whole rocks (Amsellem et al., 2020Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6. https://doi.org/10.1126/sciadv.aba3269
). Additional discussion of our data in the context of previous isotopic studies can be found in the Supplementary Information.
Figure 3 Calcium isotope evolution during the various stages of carbonatite petrogenesis compared to data for carbonatites from this and previous studies. (a) Fractionation factors for the four stages: partial melting (Step 1), melt differentiation by crystal fractionation (Step 2), carbonatite-silicate melt immiscibility (Step 3), and accumulation of calcite and apatite (Step 4). Partial melting results (Step 1) come from Antonelli et al. (2023a)
Antonelli, M.A., Giuliani, A., Wang, Z., Wang, M., Zhou, L., Feng, L., Li, M., Zhang, Z., Liu, F. and Drysdale, R.N. (2023a) Subducted carbonates not required: Deep mantle melting explains stable Ca isotopes in kimberlite magmas. Geochimica et Cosmochimica Acta 348, 410–427. https://doi.org/10.1016/j.gca.2023.03.025
and encompass the largest and smallest predicted fractionations during melting of carbon-bearing garnet lherzolite at 1400−1300 °C. (b) Clinopyroxene fractionation from CO2-rich alkaline mafic melt (Step 2), showing the two possible paths (nephelinite “path 1” and syenite “path 2”) leading to two-liquid immiscibility. (c) Carbonatite-silicate melt immiscibility (Step 3). (d) Calcite and apatite accumulation from a carbonatitic melt (Step 4). Shaded regions in (b-d) represent predicted F(Ca) values. In (e) we show the model results from Step 1 to 4 and compare them with natural carbonatite data (Supplementary Information).top
Four Stage Carbonatite Petrogenesis Model
To evaluate whether incorporation of recycled marine carbonates is required by the δ44Ca of carbonatites, we must first evaluate Ca isotope fractionations related to the two foremost models for carbonatite petrogenesis: (i) differentiation of an alkaline CO2-rich silicate melt, resulting in carbonatite-silicate melt immiscibility, and (ii) direct formation of carbonatitic melt through partial melting of carbonate-bearing mantle. Given that the latter model (explored in the Supplementary Information; Fig. S-5) is unable to produce calcic carbonatitic melts (Lee and Wyllie, 1998
Lee, W.-J., Wyllie, P.J. (1998) Petrogenesis of Carbonatite Magmas from Mantle to Crust, Constrained by the System CaO-(MgO + FeO*)-(Na2O + K2O)-(SiO2 + Al2O3 + TiO2)-CO2. Journal of Petrology 39, 495–517. https://doi.org/10.1093/petroj/39.3.495
), we focus our main discussion on the first model (Fig. 3). This model consists of four major steps: (i) low degree partial melting of carbon-bearing mantle to form a primitive CO2-rich alkaline mafic melt, (ii) fractional crystallisation (mainly of olivine and clinopyroxene) to enrich the differentiated melt in alkalis and reach the carbonatite-silicate melt miscibility gap, (iii) carbonatite-silicate melt immiscibility in the mid-to-lower crust, and (iv) fractionation of calcite (and apatite) from carbonatitic melts to form intrusive (cumulate) calciocarbonatites in the upper crust.Partial melting of carbon-bearing garnet lherzolite to form CO2-rich ultramafic alkaline melts (Step 1) was explored in a recent study (Antonelli et al., 2023a
Antonelli, M.A., Giuliani, A., Wang, Z., Wang, M., Zhou, L., Feng, L., Li, M., Zhang, Z., Liu, F. and Drysdale, R.N. (2023a) Subducted carbonates not required: Deep mantle melting explains stable Ca isotopes in kimberlite magmas. Geochimica et Cosmochimica Acta 348, 410–427. https://doi.org/10.1016/j.gca.2023.03.025
). Using the fractionation factors and assumptions from that study, we predict δ44Ca shifts of −0.13 ‰ to −0.18 ‰ (at 1400−1300 °C) for the partial melts. These values agree well with the average δ44CaBSE composition of kimberlites [−0.16 ± 0.03 ‰ (Antonelli et al., 2023aAntonelli, M.A., Giuliani, A., Wang, Z., Wang, M., Zhou, L., Feng, L., Li, M., Zhang, Z., Liu, F. and Drysdale, R.N. (2023a) Subducted carbonates not required: Deep mantle melting explains stable Ca isotopes in kimberlite magmas. Geochimica et Cosmochimica Acta 348, 410–427. https://doi.org/10.1016/j.gca.2023.03.025
)], which are likely to be similar to the melts generated in the first stages of carbonatite formation. Fractionation of minerals from this melt (Step 2) to reach carbonatite-silicate two-melt immiscibility can occur via two different pathways, entailing different amounts of fractional crystallisation, most importantly of clinopyroxene (Supplementary Information). We predict δ44Ca shifts of −0.01 ‰ to −0.02 ‰ for CO2-rich nephelinitic melts (‘path 1’) and of −0.03 ‰ to −0.12 ‰ for CO2-rich foid-syenite melts (‘path 2’), at temperatures of 1300−1000 °C (Fig. 3b). Carbonatite-silicate melt immiscibility (Step 3) is then assumed to occur at 1100−1000 °C and leads to δ44Ca shifts of −0.09 to −0.12 ‰ for carbonatitic melts (Fig. 3c) and relatively little change in the δ44Ca of the conjugate silicate melts (<+0.03 ‰, because more than ∼80 % of the total Ca remains in the silicate melts). The final stage, accumulation of calcite and apatite from carbonatitic melt at 1000−800 °C to form an intrusive calciocarbonatite body (Step 4), leads to slight positive shifts in the accumulated minerals (Fig. 3d) relative to the carbonatite melt (ranging from 0.00 to +0.11 ‰, depending on the Ca fraction remaining in the melt). This final positive shift in the carbonatite cumulates is of a similar magnitude to the negative shift induced by melt immiscibility, and may explain similar δ44Ca in some carbonatites and conjugate silicates (Sun et al., 2021Sun, J., Zhu, X.K., Belshaw, N.S., Chen, W., Doroshkevich, A.G., Luo, W.J., Song, W.L., Chen, B.B., Cheng, Z.G., Li, Z.H. and Wang, Y. (2021) Ca isotope systematics of carbonatites: Insights into carbonatite source and evolution. Geochemical Perspectives Letters 17, 11–15. https://doi.org/10.7185/geochemlet.2107
).Summing the maximum and minimum estimates from the four sequential stages in our model (described in the Supplementary Information; Fig. 3e, Supplementary Data-2) leads to calciocarbonatite cumulates with estimated δ44CaBSE ranging between −0.32 ‰ and −0.12 ‰, for those derived from nephelinites (‘path 1’), and from −0.43 ‰ to −0.14 ‰, for those derived from foid-syenites (‘path 2’). This four step model can also produce magnesiocarbonatites (once enough calcite precipitates to saturate dolomite), and we predict that these magnesiocarbonatites cumulates would have lower δ44Ca (by at least −0.1 ‰) than genetically-associated calciocarbonatite cumulates (Supplementary Information), due to the lower 1000lnβ of dolomite (relative to calcite) and to open system processes (i.e. melt segregation after calcite fractionation). After the final accumulation of calcite (or dolomite) and apatite, sub-solidus equilibration may further shift apatite δ44Ca to lower values (e.g., X-1513), but this is unlikely to change the δ44Ca of calcite (given that apatite is a relatively minor phase) and cannot change the δ44Ca of whole rocks.
top
Discussion
As shown in Figure 3e, our four stage carbonatite petrogenesis model accounts for the δ44Ca variability observed in our samples and in recent previous studies (Banerjee et al., 2021
Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
; Sun et al., 2021Sun, J., Zhu, X.K., Belshaw, N.S., Chen, W., Doroshkevich, A.G., Luo, W.J., Song, W.L., Chen, B.B., Cheng, Z.G., Li, Z.H. and Wang, Y. (2021) Ca isotope systematics of carbonatites: Insights into carbonatite source and evolution. Geochemical Perspectives Letters 17, 11–15. https://doi.org/10.7185/geochemlet.2107
) without requiring any variability in mantle source compositions (i.e. δ44CaBSE ≈ 0 ‰). Ca isotope ratios lower than predicted in our models (e.g., 3 samples with δ44CaBSE down to −0.53 ± 0.06 ‰) have only been found in magnesio- and ferrocarbonatites (Banerjee et al., 2021Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
). Given that we predict lower δ44Ca (by <−0.1‰; Supplementary Information) in magnesiocarbonatite cumulates (relative to calciocarbonatite cumulates) these values are also well explained by our four stage model.Our two stage model for direct partial melting of carbonated garnet lherzolite (which cannot produce calciocarbonatitic melts; Supplementary Information) followed by dolomite accumulation, yields δ44CaBSE ranging from −0.41 ‰ to −0.05 ‰ (assuming 1−5 wt. % carbonates in the mantle, with BSE-like δ44Ca) at temperatures of 1050−950 °C for partial melting (Foley and Pintér, 2018
Foley, S.F., Pintér, Z. (2018) Primary melt compositions in the earth’s mantle. In: Kono, Y. and Sanloup, C. (Eds.) Magmas Under Pressure: Advances in High-Pressure Experiments on Structure and Properties of Melts, 3–42. Elsevier. https://doi.org/10.1016/B978-0-12-811301-1.00001-0
) and of 1000−800 °C for dolomite accumulation (Fig. S-5). This predicted range also agrees well with δ44Ca in magnesiocarbonatites (Banerjee et al., 2021Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
). Using δ44CaBSE significantly lower than BSE for the carbonates [e.g., −0.35 ‰, Phanerozoic average marine carbonates, see Antonelli and Simon (2020)Antonelli, M.A., Simon, J.I. (2020) Calcium isotopes in high-temperature terrestrial processes. Chemical Geology 548, 119651. https://doi.org/10.1016/j.chemgeo.2020.119651
and references therein], however, yields carbonatite compositions that are mostly lower than observed in nature (Fig. S-5). Thus, if recycled marine carbonates are involved in the sources of some magnesiocarbonatites, they most likely have δ44CaBSE ≈ 0 ‰ [the average value of Precambrian carbonates (Blättler and Higgins, 2017Blättler, C.L., Higgins, J.A. (2017) Testing Urey’s carbonate–silicate cycle using the calcium isotopic composition of sedimentary carbonates. Earth and Planetary Science Letters 479, 241–251. https://doi.org/10.1016/j.epsl.2017.09.033
)] and are thus isotopically indistinguishable from the mantle.Ca isotope ratios higher than predicted in our models, on the other hand, could result from several processes, including late stage alteration (Mitchell and Gittins, 2022
Mitchell, R.H., Gittins, J. (2022) Carbonatites and carbothermalites: A revised classification. Lithos 430–431, 106861. https://doi.org/10.1016/j.lithos.2022.106861
), and it is well established that ferrocarbonatite formation involves secondary alteration. Given that δ44Ca in ferrocarbonatites range to slightly higher values than other types (Fig. 3e), this suggests that carbo-hydrothermal alteration is a mechanism that may potentially push δ44Ca to more positive values (Supplementary Information).top
Conclusions
We experimentally determined that carbonatite-silicate melt immiscibility leads to significantly lower δ44Ca in carbonatitic melts [1000lnα of −0.21 ± 0.06 (tSE, n = 4) at 1000K] and does not produce particularly high δ44Ca in conjugate silicates. Our finding of lower δ44Ca in carbonatitic vs. silicate melts at equilibrium is consistent with empirical predictions for Mg and Fe isotopes (Johnson et al., 2010
Johnson, C.M., Bell, K., Beard, B.L., Shultis, A.I. (2010) Iron isotope compositions of carbonatites record melt generation, crystallization, and late-stage volatile-transport processes. Mineralogy and Petrology 98, 91–110. https://doi.org/10.1007/s00710-009-0055-4
; Li et al., 2016Li, W.Y., Teng, F.Z., Halama, R., Keller, J., Klaudius, J. (2016) Magnesium isotope fractionation during carbonatite magmatism at Oldoinyo Lengai, Tanzania. Earth and Planetary Science Letters 444, 26–33. https://doi.org/10.1016/j.epsl.2016.03.034
) and indicates a weaker Ca bonding environment in carbonatitic melts compared to silicate melts. In turn, this suggests that lighter isotopes of other similarly bound and coordinated cations (e.g., Mg, Fe, Sr, Ba, Zn) should also be preferentially partitioned into carbonatitic melts during liquid immiscibility.Although direct melting of carbonate-bearing mantle with δ44CaBSE ≈ 0 can produce δ44Ca values that agree with natural magnesiocarbonatite data, without requiring melt immiscibility, this process cannot produce calciocarbonatites (Lee and Wyllie, 1998
Lee, W.-J., Wyllie, P.J. (1998) Petrogenesis of Carbonatite Magmas from Mantle to Crust, Constrained by the System CaO-(MgO + FeO*)-(Na2O + K2O)-(SiO2 + Al2O3 + TiO2)-CO2. Journal of Petrology 39, 495–517. https://doi.org/10.1093/petroj/39.3.495
). Building on our best current understanding of carbonatite petrogenesis (Weidendorfer and Asimow, 2022Weidendorfer, D., Asimow, P.D. (2022) Experimental constraints on truly conjugate alkaline silicate – carbonatite melt pairs. Earth and Planetary Science Letters 584, 117500. https://doi.org/10.1016/j.epsl.2022.117500
; Yaxley et al., 2022Yaxley, G.M., Anenburg, M., Tappe, S., Decree, S., Guzmics, T. (2022) Carbonatites: Classification, Sources, Evolution, and Emplacement. Annual Review of Earth and Planetary Sciences 50, 261–293. https://doi.org/10.1146/annurev-earth-032320-104243
; Berkesi et al., 2023Berkesi, M., Myovela, J.L., Yaxley, G.M., Guzmics, T. (2023) Carbonatite formation in continental settings via high pressure – high temperature liquid immiscibility. Geochimica et Cosmochimica Acta 349, 41–54. https://doi.org/10.1016/j.gca.2023.03.027
; Sartori and Schmidt, 2023Sartori, G., Schmidt, M.W. (2023) Phosphorous-solubility in carbonatite melts: Apatite crystallization modeled via its solubility product. Geochimica et Cosmochimica Acta 352, 122–132. https://doi.org/10.1016/j.gca.2023.04.034
), we combined our experimental constraints on melt immiscibility with ab initio and empirical predictions to develop a four stage Ca isotope fractionation model that successfully explains natural δ44Ca variations in essentially all primary carbonatites, including those from this study. Although subducted marine carbonates have been invoked to explain δ44Ca in carbonatites (Amsellem et al., 2020Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6. https://doi.org/10.1126/sciadv.aba3269
; Banerjee et al., 2021Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
), we have shown that magmatic processes can generate the entire range of observed δ44Ca, without requiring any mantle-source variations. Carbonatitic melts, therefore, do not necessarily require the isotopic signature of subducted marine carbonates in their mantle sources at any point in Earth history.top
Acknowledgements
We thank Senan Oesch for help with mineral separation and bulk rock analyses, two anonymous reviewers for their useful comments, and Helen Williams for efficient editorial handling. This work was supported by ETH post-doctoral fellowship (19-2 FEL-33) to MAA, Swiss National Science Foundation (SNSF) Ambizione Fellowship (PZ00P2_180126/1) to AG, and SNSF grant 2000020-178948/1 to MWS. The work of EAS was funded through NSF grants EAR1524811 and EAR1530306.
Editor: Helen Williams
top
References
Amsellem, E., Moynier, F., Bertrand, H., Bouyon, A., Mata, J., Tappe, S., Day, J.M.D. (2020) Calcium isotopic evidence for the mantle sources of carbonatites. Science Advances 6. https://doi.org/10.1126/sciadv.aba3269
 Show in context
Show in contextThere are also significant debates regarding the role of carbonatite magmas within the carbon cycle, with some recent stable isotope studies suggesting that carbonatites may represent the return of subducted marine carbonates back to the surface (Hulett et al., 2016; Amsellem et al., 2020; Banerjee et al., 2021).
View in article
Previous studies exploring δ44Ca in carbonatites argue that subducted marine carbonates occur in the sources of essentially all (Amsellem et al., 2020), some (Banerjee et al., 2021), or no (Sun et al., 2021) carbonatites.
View in article
Amsellem et al. (2020) found ubiquitously low δ44CaBSE in carbonatites (average of −0.7 ‰), while the two more recent studies found similar average values of approximately −0.2 ‰ (using two different analytical approaches), and argue that the data of Amsellem et al. (2020) were affected by analytical issues (Banerjee et al., 2021; Sun et al., 2021).
View in article
Despite their subtle differences, all these values are significantly different from the earlier report of ubiquitously low δ44CaBSE [average of −0.69 ± 0.04 ‰ (tSE, n = 50)] in carbonatite whole rocks (Amsellem et al., 2020).
View in article
Although subducted marine carbonates have been invoked to explain δ44Ca in carbonatites (Amsellem et al., 2020; Banerjee et al., 2021), we have shown that magmatic processes can generate the entire range of observed δ44Ca, without requiring any mantle-source variations. Carbonatitic melts, therefore, do not necessarily require the isotopic signature of subducted marine carbonates in their mantle sources at any point in Earth history.
View in article
Antonelli, M.A., Giuliani, A., Wang, Z., Wang, M., Zhou, L., Feng, L., Li, M., Zhang, Z., Liu, F. and Drysdale, R.N. (2023a) Subducted carbonates not required: Deep mantle melting explains stable Ca isotopes in kimberlite magmas. Geochimica et Cosmochimica Acta 348, 410–427. https://doi.org/10.1016/j.gca.2023.03.025
 Show in context
Show in context(a) Fractionation factors for the four stages: partial melting (Step 1), melt differentiation by crystal fractionation (Step 2), carbonatite-silicate melt immiscibility (Step 3), and accumulation of calcite and apatite (Step 4). Partial melting results (Step 1) come from Antonelli et al. (2023a) and encompass the largest and smallest predicted fractionations during melting of carbon-bearing garnet lherzolite at 1400−1300 °C.
View in article
Partial melting of carbon-bearing garnet lherzolite to form CO2-rich ultramafic alkaline melts (Step 1) was explored in a recent study (Antonelli et al., 2023a).
View in article
These values agree well with the average δ44CaBSE composition of kimberlites [−0.16 ± 0.03 ‰ (Antonelli et al., 2023a)], which are likely to be similar to the melts generated in the first stages of carbonatite formation.
View in article
Antonelli, M.A., Yakymchuk, C., Schauble, E.A., Foden, J., Janoušek, V., Moyen, J.-F., Hoffmann, J., Moynier, F., Bachmann, O. (2023b) Granite petrogenesis and the δ44Ca of continental crust. Earth and Planetary Science Letters 608, 118080. https://doi.org/10.1016/j.epsl.2023.118080
 Show in context
Show in contextWe therefore present novel ab initio predictions (PBE functionals) for equilibrium Ca isotope fractionation between these minerals using the Quantum Espresso software package, following established methods (Antonelli et al., 2019, 2023b) (Supplementary Information; Fig. S-4).
View in article
Antonelli, M.A., DePaolo, D.J., Christensen, J.N., Wotzlaw, J.-F., Pester, N.J., Bachmann, O. (2021) Radiogenic 40Ca in seawater: implications for modern and ancient Ca cycles. ACS Earth and Space Chemistry 5, 2481–2492. https://doi.org/10.1021/acsearthspacechem.1c00179
 Show in context
Show in contextAlong with δ44Ca analyses by TIMS, we also measured radiogenic 40Ca abundances in several samples (and carbonatite standard COQ-1; Table S-5), all of which yield ɛCa within error of the BSE value (Antonelli et al., 2021).
View in article
Antonelli, M.A., Simon, J.I. (2020) Calcium isotopes in high-temperature terrestrial processes. Chemical Geology 548, 119651. https://doi.org/10.1016/j.chemgeo.2020.119651
 Show in context
Show in contextTheir use as a recycled carbonate tracer depends on δ44CaBSE in marine carbonates (Phanerozoic average of −0.35 ‰) being typically lower than found in mantle rocks [≈0 ‰ (see Antonelli and Simon, 2020)]
View in article
Although mineral-mineral and mineral-melt equilibrium Ca isotope fractionations are relatively well constrained from both ab initio and empirical investigations (Antonelli and Simon, 2020), Ca isotope fractionation between immiscible melts has not been explored.
View in article
Using δ44CaBSE significantly lower than BSE for the carbonates [e.g., −0.35 ‰, Phanerozoic average marine carbonates, see Antonelli and Simon (2020) and references therein], however, yields carbonatite compositions that are mostly lower than observed in nature (Fig. S-5).
View in article
Antonelli, M.A., Schiller, M., Schauble, E.A., Mittal, T., DePaolo, D.J., Chacko, T., Grew, E.S., Tripoli, B. (2019) Kinetic and equilibrium Ca isotope effects in high-T rocks and minerals. Earth and Planetary Science Letters 517, 71–82. https://doi.org/10.1016/j.epsl.2019.04.013
 Show in context
Show in contextWe therefore present novel ab initio predictions (PBE functionals) for equilibrium Ca isotope fractionation between these minerals using the Quantum Espresso software package, following established methods (Antonelli et al., 2019, 2023b) (Supplementary Information; Fig. S-4).
View in article
Banerjee, A., Chakrabarti, R., Simonetti, A. (2021) Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: implications for crustal recycling through time. Geochimica et Cosmochimica Acta 307, 168–191. https://doi.org/10.1016/j.gca.2021.05.046
 Show in context
Show in contextThere are also significant debates regarding the role of carbonatite magmas within the carbon cycle, with some recent stable isotope studies suggesting that carbonatites may represent the return of subducted marine carbonates back to the surface (Hulett et al., 2016; Amsellem et al., 2020; Banerjee et al., 2021).
View in article
Previous studies exploring δ44Ca in carbonatites argue that subducted marine carbonates occur in the sources of essentially all (Amsellem et al., 2020), some (Banerjee et al., 2021), or no (Sun et al., 2021) carbonatites.
View in article
Amsellem et al. (2020) found ubiquitously low δ44CaBSE in carbonatites (average of −0.7 ‰), while the two more recent studies found similar average values of approximately −0.2 ‰ (using two different analytical approaches), and argue that the data of Amsellem et al. (2020) were affected by analytical issues (Banerjee et al., 2021; Sun et al., 2021).
View in article
Nevertheless, the latter two studies disagree on the potential effects of magmatic processes (Supplementary Information) and find significantly different total ranges for carbonatite δ44Ca, where lower values [found in Banerjee et al. (2021)] are argued to result from subducted marine carbonates.
View in article
Overall, our mineral δ44Ca analyses yield results that are slightly lower (Fig. 3e; Supplementary Information, Supplementary Data-1) but generally similar to whole rock calciocarbonatite analyses from recent studies (Banerjee et al., 2021; Sun et al., 2021).
View in article
Previous work exploring carbonatite calcite separates, however, found a larger range in δ44CaBSE with a slightly higher average [−0.10 ± 0.08 ‰ (tSE, n = 17)] than found in calciocarbonatite whole rocks (Banerjee et al., 2021), but the primary magmatic nature of the calcite in this data set was not investigated.
View in article
As shown in Figure 3e, our four stage carbonatite petrogenesis model accounts for the δ44Ca variability observed in our samples and in recent previous studies (Banerjee et al., 2021; Sun et al., 2021) without requiring any variability in mantle source compositions (i.e. δ44CaBSE ≈ 0 ‰).
View in article
Ca isotope ratios lower than predicted in our models (e.g., 3 samples with δ44CaBSE down to −0.53 ± 0.06 ‰) have only been found in magnesio- and ferrocarbonatites (Banerjee et al., 2021).
View in article
This predicted range also agrees well with δ44Ca in magnesiocarbonatites (Banerjee et al., 2021).
View in article
Although subducted marine carbonates have been invoked to explain δ44Ca in carbonatites (Amsellem et al., 2020; Banerjee et al., 2021), we have shown that magmatic processes can generate the entire range of observed δ44Ca, without requiring any mantle-source variations. Carbonatitic melts, therefore, do not necessarily require the isotopic signature of subducted marine carbonates in their mantle sources at any point in Earth history.
View in article
Berkesi, M., Myovela, J.L., Yaxley, G.M., Guzmics, T. (2023) Carbonatite formation in continental settings via high pressure – high temperature liquid immiscibility. Geochimica et Cosmochimica Acta 349, 41–54. https://doi.org/10.1016/j.gca.2023.03.027
 Show in context
Show in contextPossible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023; Berndt and Klemme, 2022; Brooker and Kjarsgaard, 2011; Guzmics et al., 2015; Weidendorfer et al., 2017)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998; Yaxley and Brey, 2004).
View in article
Building on our best current understanding of carbonatite petrogenesis (Weidendorfer and Asimow, 2022; Yaxley et al., 2022; Berkesi et al., 2023; Sartori and Schmidt, 2023), we combined our experimental constraints on melt immiscibility with ab initio and empirical predictions to develop a four stage Ca isotope fractionation model that successfully explains natural δ44Ca variations in essentially all primary carbonatites, including those from this study.
View in article
Berndt, J., Klemme, S. (2022) Origin of carbonatites—liquid immiscibility caught in the act. Nature Communications 13, 7–14. https://doi.org/10.1038/s41467-022-30500-7
 Show in context
Show in contextPossible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023; Berndt and Klemme, 2022; Brooker and Kjarsgaard, 2011; Guzmics et al., 2015; Weidendorfer et al., 2017)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998; Yaxley and Brey, 2004).
View in article
Blättler, C.L., Higgins, J.A. (2017) Testing Urey’s carbonate–silicate cycle using the calcium isotopic composition of sedimentary carbonates. Earth and Planetary Science Letters 479, 241–251. https://doi.org/10.1016/j.epsl.2017.09.033
 Show in context
Show in contextThus, if recycled marine carbonates are involved in the sources of some magnesiocarbonatites, they most likely have δ44CaBSE ≈ 0 ‰ [the average value of Precambrian carbonates (Blättler and Higgins, 2017)] and are thus isotopically indistinguishable from the mantle.
View in article
Brooker, R.A., Kjarsgaard, B.A. (2011) Silicate-carbonate liquid immiscibility and phase relations in the system SiO2-Na2O-Al2O3-CaO-Co2 at 0·1-2·5 GPa with applications to carbonatite genesis. Journal of Petrology 52, 1281–1305. https://doi.org/10.1093/petrology/egq081
 Show in context
Show in contextPossible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023; Berndt and Klemme, 2022; Brooker and Kjarsgaard, 2011; Guzmics et al., 2015; Weidendorfer et al., 2017)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998; Yaxley and Brey, 2004).
View in article
Constraining this process is of the utmost importance for carbonatite petrogenesis, as direct melts of carbonate-bearing peridotites in the upper mantle are dolomitic and cannot evolve to the calciocarbonatite melt compositions (Lee and Wyllie, 1998) most frequently observed and readily unmixed from silicate melts (Lee and Wyllie, 1998; Brooker and Kjarsgaard, 2011; Martin et al., 2013; Guzmics et al., 2015).
View in article
Foley, S.F., Pintér, Z. (2018) Primary melt compositions in the earth’s mantle. In: Kono, Y. and Sanloup, C. (Eds.) Magmas Under Pressure: Advances in High-Pressure Experiments on Structure and Properties of Melts, 3–42. Elsevier. https://doi.org/10.1016/B978-0-12-811301-1.00001-0
 Show in context
Show in contextOur two stage model for direct partial melting of carbonated garnet lherzolite (which cannot produce calciocarbonatitic melts; Supplementary Information) followed by dolomite accumulation, yields δ44CaBSE ranging from −0.41 ‰ to −0.05 ‰ (assuming 1−5 wt. % carbonates in the mantle, with BSE-like δ44Ca) at temperatures of 1050−950 °C for partial melting (Foley and Pintér, 2018) and of 1000−800 °C for dolomite accumulation (Fig. S-5).
View in article
Genge, M.J., Price, G.D., Jones, A.P. (1995) Molecular dynamics simulations of CaCO3 melts to mantle pressures and temperatures: implications for carbonatite magmas. Earth and Planetary Science Letters 131, 225–238. https://doi.org/10.1016/0012-821X(95)00020-D
 Show in context
Show in contextOur finding that carbonatitic melts have lower δ44Ca than coexisting silicate melts is consistent with predictions for a weaker bonding environment for Ca in ionic carbonatitic melts vs. predominantly covalent silicate melts (see Genge et al., 1995).
View in article
Guzmics, T., Zajacz, Z., Mitchell, R.H., Szab´o, C., Wälle, M. (2015) The role of liquid–liquid immiscibility and crystal fractionation in the genesis of carbonatite magmas: insights from Kerimasi melt inclusions. Contributions to Mineralogy and Petrology 169, 17. https://doi.org/10.1007/s00410-014-1093-4
 Show in context
Show in contextPossible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023; Berndt and Klemme, 2022; Brooker and Kjarsgaard, 2011; Guzmics et al., 2015; Weidendorfer et al., 2017)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998; Yaxley and Brey, 2004).
View in article
Constraining this process is of the utmost importance for carbonatite petrogenesis, as direct melts of carbonate-bearing peridotites in the upper mantle are dolomitic and cannot evolve to the calciocarbonatite melt compositions (Lee and Wyllie, 1998) most frequently observed and readily unmixed from silicate melts (Lee and Wyllie, 1998; Brooker and Kjarsgaard, 2011; Martin et al., 2013; Guzmics et al., 2015).
View in article
Harmer, R.E., Gittins, J. (1998) The case for primary, mantle-derived carbonatite magma. Journal of Petrology 39, 1895–1903. https://doi.org/10.1093/petroj/39.11-12.1895
 Show in context
Show in contextPossible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023; Berndt and Klemme, 2022; Brooker and Kjarsgaard, 2011; Guzmics et al., 2015; Weidendorfer et al., 2017)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998; Yaxley and Brey, 2004).
View in article
Hulett, S.R.W., Simonetti, A., Rasbury, E.T., Hemming, N.G. (2016) Recycling of subducted crustal components into carbonatite melts revealed by boron isotopes. Nature Geoscience 9, 904–908. https://doi.org/10.1038/ngeo2831
 Show in context
Show in contextThere are also significant debates regarding the role of carbonatite magmas within the carbon cycle, with some recent stable isotope studies suggesting that carbonatites may represent the return of subducted marine carbonates back to the surface (Hulett et al., 2016; Amsellem et al., 2020; Banerjee et al., 2021).
View in article
Johnson, C.M., Bell, K., Beard, B.L., Shultis, A.I. (2010) Iron isotope compositions of carbonatites record melt generation, crystallization, and late-stage volatile-transport processes. Mineralogy and Petrology 98, 91–110. https://doi.org/10.1007/s00710-009-0055-4
 Show in context
Show in contextOur finding of lower δ44Ca in carbonatitic vs. silicate melts at equilibrium is consistent with empirical predictions for Mg and Fe isotopes (Johnson et al., 2010; Li et al., 2016) and indicates a weaker Ca bonding environment in carbonatitic melts compared to silicate melts.
View in article
Lee, W.-J., Wyllie, P.J. (1998) Petrogenesis of Carbonatite Magmas from Mantle to Crust, Constrained by the System CaO-(MgO + FeO*)-(Na2O + K2O)-(SiO2 + Al2O3 + TiO2)-CO2. Journal of Petrology 39, 495–517. https://doi.org/10.1093/petroj/39.3.495
 Show in context
Show in contextConstraining this process is of the utmost importance for carbonatite petrogenesis, as direct melts of carbonate-bearing peridotites in the upper mantle are dolomitic and cannot evolve to the calciocarbonatite melt compositions (Lee and Wyllie, 1998) most frequently observed and readily unmixed from silicate melts (Lee and Wyllie, 1998; Brooker and Kjarsgaard, 2011; Martin et al., 2013; Guzmics et al., 2015).
View in article
Given that the latter model (explored in the Supplementary Information; Fig. S-5) is unable to produce calcic carbonatitic melts (Lee and Wyllie, 1998), we focus our main discussion on the first model (Fig. 3).
View in article
Although direct melting of carbonate-bearing mantle with δ44CaBSE ≈ 0 can produce δ44Ca values that agree with natural magnesiocarbonatite data, without requiring melt immiscibility, this process cannot produce calciocarbonatites (Lee and Wyllie, 1998).
View in article
Li, W.Y., Teng, F.Z., Halama, R., Keller, J., Klaudius, J. (2016) Magnesium isotope fractionation during carbonatite magmatism at Oldoinyo Lengai, Tanzania. Earth and Planetary Science Letters 444, 26–33. https://doi.org/10.1016/j.epsl.2016.03.034
 Show in context
Show in contextOur finding of lower δ44Ca in carbonatitic vs. silicate melts at equilibrium is consistent with empirical predictions for Mg and Fe isotopes (Johnson et al., 2010; Li et al., 2016) and indicates a weaker Ca bonding environment in carbonatitic melts compared to silicate melts.
View in article
Martin, L.H.J., Schmidt, M.W., Mattsson, H.B., Guenther, D. (2013) Element Partitioning between Immiscible Carbonatite and Silicate Melts for Dry and H2O-bearing Systems at 1-3GPa. Journal of Petrology 54, 2301–2338. https://doi.org/10.1093/petrology/egt048
 Show in context
Show in contextConstraining this process is of the utmost importance for carbonatite petrogenesis, as direct melts of carbonate-bearing peridotites in the upper mantle are dolomitic and cannot evolve to the calciocarbonatite melt compositions (Lee and Wyllie, 1998) most frequently observed and readily unmixed from silicate melts (Lee and Wyllie, 1998; Brooker and Kjarsgaard, 2011; Martin et al., 2013; Guzmics et al., 2015).
View in article
Mitchell, R.H., Gittins, J. (2022) Carbonatites and carbothermalites: A revised classification. Lithos 430–431, 106861. https://doi.org/10.1016/j.lithos.2022.106861
 Show in context
Show in contextCa isotope ratios higher than predicted in our models, on the other hand, could result from several processes, including late stage alteration (Mitchell and Gittins, 2022), and it is well established that ferrocarbonatite formation involves secondary alteration.
View in article
Sartori, G., Galli, A., Weidendorfer, D., Schmidt, M.W. (2023) A tool to distinguish magmatic from secondarily recrystallized carbonatites—Calcite/apatite rare earth element partitioning. Geology 51, 54–58. https://doi.org/10.1130/G50416.1
 Show in context
Show in contextTo better constrain δ44Ca in natural carbonatite minerals, we extracted pristine calcite (or dolomite) and apatite crystals from seven calcio- and one magnesiocarbonatite that have equilibrium calcite-apatite REE distributions reflecting primary magmatic signatures (in all but one sample), characterised in previous work (Sartori et al., 2023) (Tables S-3, S-4).
View in article
Sartori, G., Schmidt, M.W. (2023) Phosphorous-solubility in carbonatite melts: Apatite crystallization modeled via its solubility product. Geochimica et Cosmochimica Acta 352, 122–132. https://doi.org/10.1016/j.gca.2023.04.034
 Show in context
Show in contextBuilding on our best current understanding of carbonatite petrogenesis (Weidendorfer and Asimow, 2022; Yaxley et al., 2022; Berkesi et al., 2023; Sartori and Schmidt, 2023), we combined our experimental constraints on melt immiscibility with ab initio and empirical predictions to develop a four stage Ca isotope fractionation model that successfully explains natural δ44Ca variations in essentially all primary carbonatites, including those from this study.
View in article
Schmidt, M.W., Connolly, J.A.D., Günther, D., Bogaerts, M. (2006) Element partitioning: The role of melt structure and composition. Science 312, 1646–1650. https://doi.org/10.1126/science.1126690
 Show in context
Show in contextTo better understand Ca isotope fractionation between immiscible melts, we equilibrated conjugate carbonatite and nephelinitic silicate melts in a centrifuging piston cylinder apparatus at ETH Zurich (Schmidt et al., 2006).
View in article
Stachel, T., Aulbach, S., Harris, J.W. (2022) Mineral inclusions in lithospheric diamonds. Reviews in Mineralogy and Geochemistry 88, 307–391. https://doi.org/10.2138/rmg.2022.88.06
 Show in context
Show in contextIn contrast, δ13C in most carbonatites overlap with those of peridotitic diamonds (Stachel et al., 2022).
View in article
Sun, J., Zhu, X.K., Belshaw, N.S., Chen, W., Doroshkevich, A.G., Luo, W.J., Song, W.L., Chen, B.B., Cheng, Z.G., Li, Z.H. and Wang, Y. (2021) Ca isotope systematics of carbonatites: Insights into carbonatite source and evolution. Geochemical Perspectives Letters 17, 11–15. https://doi.org/10.7185/geochemlet.2107
 Show in context
Show in contextPrevious studies exploring δ44Ca in carbonatites argue that subducted marine carbonates occur in the sources of essentially all (Amsellem et al., 2020), some (Banerjee et al., 2021), or no (Sun et al., 2021) carbonatites.
View in article
Amsellem et al. (2020) found ubiquitously low δ44CaBSE in carbonatites (average of −0.7 ‰), while the two more recent studies found similar average values of approximately −0.2 ‰ (using two different analytical approaches), and argue that the data of Amsellem et al. (2020) were affected by analytical issues (Banerjee et al., 2021; Sun et al., 2021).
View in article
Overall, our mineral δ44Ca analyses yield results that are slightly lower (Fig. 3e; Supplementary Information, Supplementary Data-1) but generally similar to whole rock calciocarbonatite analyses from recent studies (Banerjee et al., 2021; Sun et al., 2021).
View in article
This final positive shift in the carbonatite cumulates is of a similar magnitude to the negative shift induced by melt immiscibility, and may explain similar δ44Ca in some carbonatites and conjugate silicates (Sun et al., 2021).
View in article
As shown in Figure 3e, our four stage carbonatite petrogenesis model accounts for the δ44Ca variability observed in our samples and in recent previous studies (Banerjee et al., 2021; Sun et al., 2021) without requiring any variability in mantle source compositions (i.e. δ44CaBSE ≈ 0 ‰).
View in article
Weidendorfer, D., Asimow, P.D. (2022) Experimental constraints on truly conjugate alkaline silicate – carbonatite melt pairs. Earth and Planetary Science Letters 584, 117500. https://doi.org/10.1016/j.epsl.2022.117500
 Show in context
Show in contextBuilding on our best current understanding of carbonatite petrogenesis (Weidendorfer and Asimow, 2022; Yaxley et al., 2022; Berkesi et al., 2023; Sartori and Schmidt, 2023), we combined our experimental constraints on melt immiscibility with ab initio and empirical predictions to develop a four stage Ca isotope fractionation model that successfully explains natural δ44Ca variations in essentially all primary carbonatites, including those from this study.
View in article
Weidendorfer, D., Schmidt, M.W., Mattsson, H.B. (2017) A common origin of carbonatite magmas. Geology 45, 507–510. https://doi.org/10.1130/G38801.1
 Show in context
Show in contextPossible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023; Berndt and Klemme, 2022; Brooker and Kjarsgaard, 2011; Guzmics et al., 2015; Weidendorfer et al., 2017)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998; Yaxley and Brey, 2004).
View in article
Xiao, Z.-C., Zhou, C., Kang, J.-T., Wu, Z.-Q., Huang, F. (2022) The factors controlling equilibrium inter-mineral Ca isotope fractionation: Insights from first-principles calculations. Geochimica et Cosmochimica Acta 333, 373–389. https://doi.org/10.1016/j.gca.2022.07.021
 Show in context
Show in contextAt isotopic equilibrium, we find that calcite is slightly lighter than diopside (by −0.06 ‰ at 1000K) and slightly heavier than fluorapatite (by +0.05‰ at 1000K), in agreement with previous predictions based on LDA functionals (Xiao et al., 2022). Dolomite and ankerite both have lower 1000lnβ values, similar to those of fluorapatite (Fig. 2a, Table 1).
View in article
Yaxley, G.M., Anenburg, M., Tappe, S., Decree, S., Guzmics, T. (2022) Carbonatites: Classification, Sources, Evolution, and Emplacement. Annual Review of Earth and Planetary Sciences 50, 261–293. https://doi.org/10.1146/annurev-earth-032320-104243
 Show in context
Show in contextCarbonatites are rare igneous rocks that contain large amounts (>50 %) of carbonate minerals and represent the most CO2-rich magmas in the geologic record (Yaxley et al., 2022).
View in article
Most carbonatites represent cumulate rocks (Yaxley et al., 2022).
View in article
Building on our best current understanding of carbonatite petrogenesis (Weidendorfer and Asimow, 2022; Yaxley et al., 2022; Berkesi et al., 2023; Sartori and Schmidt, 2023), we combined our experimental constraints on melt immiscibility with ab initio and empirical predictions to develop a four stage Ca isotope fractionation model that successfully explains natural δ44Ca variations in essentially all primary carbonatites, including those from this study.
View in article
Yaxley, G.M., Brey, G.P. (2004) Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contributions to Mineralogy and Petrology 146, 606–619. https://doi.org/10.1007/s00410-003-0517-3
 Show in context
Show in contextPossible models of carbonatite formation include (i) exsolution from moderately-to-strongly evolved CO2-rich alkaline silicate melts [i.e. carbonatite-silicate melt immiscibility (Berkesi et al., 2023; Berndt and Klemme, 2022; Brooker and Kjarsgaard, 2011; Guzmics et al., 2015; Weidendorfer et al., 2017)], or (ii) direct partial melting of carbonate-bearing mantle rocks (Harmer and Gittins, 1998; Yaxley and Brey, 2004).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Centrifuging piston-cylinder experiments
- Ca isotope analyses by thermal-ionization mass spectrometry (TIMS)
- 2a. Radiogenic Ca isotope analyses and implications
- 2b. Stable Ca isotope analyses
- Density functional theory (ab-initio) estimates
- Natural carbonatite samples
- Two-stage (direct-melting) model for magnesiocarbonatites
- Four-stage melt immiscibility model for calciocarbonatites and magnesiocarbonatites
- Comparisons with previous δ44Ca data and other isotopic studies
- Figures S-1 to S-5
- Tables S-1 to S-5
- Datasets S-1 to S-3
- Supplementary References
Download the Supplementary Information (PDF)
Download Table S-3 and S-4 (xlsx)
Download Data S-1(xlsx)
Download Data S-2 (xlsx)
Download Data S-3 (xlsx)
Figures

Figure 1 Experimental results of carbonatite-silicate melt immiscibility. (a) Back scattered electron image of an exemplary experimental run product (GS8-28) in a welded Au80Pd20 capsule where the lower density carbonatite melt (dark grey, now quench minerals) migrates to the gravitational top, while the silicate melt (now silicate glass), accumulates at the bottom. (b) Stable Ca isotope results. (c) Estimates of Ca isotope fractionation factor between conjugate carbonatite and silicate melts. Error bars represent 2σ internal uncertainty based on multiple measurements of USGS standards W-2a and COQ-1, where tSE (the t standard error, where ‘t’ is the critical value for a 95% confidence interval in a t-distribution) and 2 s.e. uncertainties both yield similar estimates of ±0.04 ‰ for δ44Ca and ±0.06 ‰ for Δ44Ca (see Supplementary Information). These uncertainty estimates are also similar to our calculated measurement repeatability (±0.04 ‰, based on the 22 samples measured in duplicate in this work). Long term external reproducibility, which is most important for comparisons of our natural δ44Ca data (Fig. 2b) with previous/future work is estimated as ± 0.12 ‰ (2 s.d.) based on 21 measurements of W-2a performed over three years.
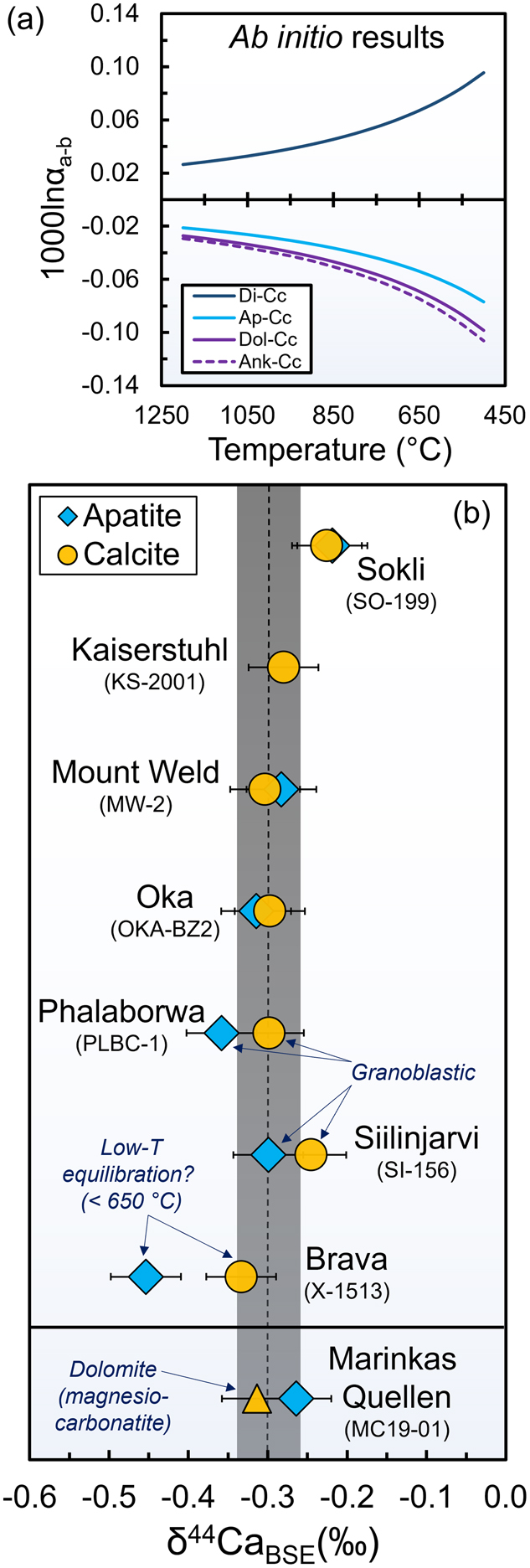
Figure 2 Ab initio estimates for equilibrium between various minerals and summary of our δ44Ca measurements in calcite and apatite from natural carbonatites. (a) Predicted inter-mineral fractionation factors (1000lnαmineral-calcite) vs. temperature. (b) δ44Ca data for pristine apatite and calcite separated from our carbonatite samples. In panel (b) 2σ uncertainties for δ44Ca are the same as described in the caption of Figure 1.
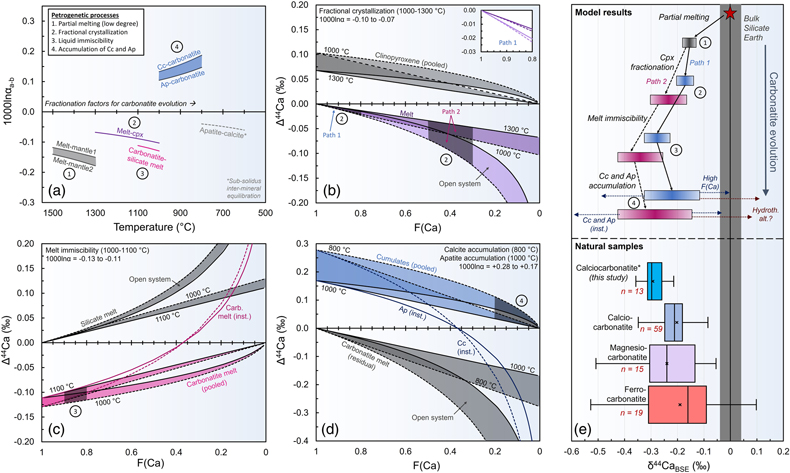
Figure 3 Calcium isotope evolution during the various stages of carbonatite petrogenesis compared to data for carbonatites from this and previous studies. (a) Fractionation factors for the four stages: partial melting (Step 1), melt differentiation by crystal fractionation (Step 2), carbonatite-silicate melt immiscibility (Step 3), and accumulation of calcite and apatite (Step 4). Partial melting results (Step 1) come from Antonelli et al. (2023a)
Antonelli, M.A., Giuliani, A., Wang, Z., Wang, M., Zhou, L., Feng, L., Li, M., Zhang, Z., Liu, F. and Drysdale, R.N. (2023a) Subducted carbonates not required: Deep mantle melting explains stable Ca isotopes in kimberlite magmas. Geochimica et Cosmochimica Acta 348, 410–427. https://doi.org/10.1016/j.gca.2023.03.025
and encompass the largest and smallest predicted fractionations during melting of carbon-bearing garnet lherzolite at 1400−1300 °C. (b) Clinopyroxene fractionation from CO2-rich alkaline mafic melt (Step 2), showing the two possible paths (nephelinite “path 1” and syenite “path 2”) leading to two-liquid immiscibility. (c) Carbonatite-silicate melt immiscibility (Step 3). (d) Calcite and apatite accumulation from a carbonatitic melt (Step 4). Shaded regions in (b-d) represent predicted F(Ca) values. In (e) we show the model results from Step 1 to 4 and compare them with natural carbonatite data (Supplementary Information).