Direct precipitation of siderite in ferruginous environments
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:665Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
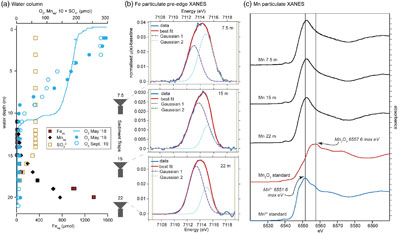 Figure 1 Geochemical and mineralogical characteristics of water column solutes and solid phases. (a) Dissolved solutes in the CL water column. The oxycline (decrease in dissolved oxygen) occurs between 7 and 12 m, and the chemocline (increase in dissolved Fe) occurs between 17 and 19 m. Sediment traps were deployed at 7.5, 15 and 22 m. (b) Sediment trap Fe XANES analyses. Pre-edge centroid energies are ∼7114 eV, consistent with a mixture of Fe(II) and Fe(III). (c) Sediment trap Mn K-edge XANES analyses. Peaks have lower energy (∼6552 eV) compared to Mn-oxides (∼6558 eV), consistent with Mn(II) minerals. | 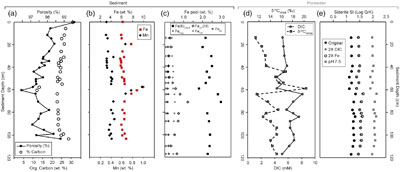 Figure 2 Bulk sediment and porewater geochemistry for the CL sediment core. (a) Porosity and organic carbon content of the sediments. The high organic carbon content of the sediments contributes to their high porosity. (b) Bulk sediment Fe and Mn content showing peaks at ∼60 cm depth, which correspond to the presence of siderite (Fig. 3). (c) Iron speciation analysis demonstrating that much of the sediment Fe is contained in the Fe(II)unsulf pool. (d) Porewater dissolved inorganic carbon (DIC) concentration and isotope composition, showing a positive δ13C signature consistent with the mass balance of depleted carbon lost to methane. (e) Siderite saturation index (SI), which assumes the Fe concentration (1.689 mmol/L) and pH (6.8) of deepest ferruginous water and measured porewater DIC values. Sensitivity scenarios were based on doubling DIC and Fe concentrations, and increasing pH to 7.5. | 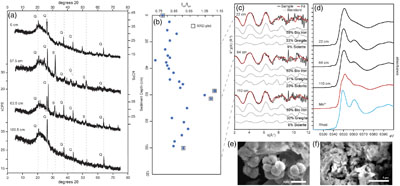 Figure 3 Sediment Fe phases. (a) Example XRD scans showing the emergence of siderite in mid-core depths (57.5 and 63.5 cm scans; Q = quartz peaks, S = siderite peaks). (b) Relative XRD intensity of the siderite peak normalised to the quartz peak (Isid/Iqtz), showing an increase in siderite abundance mid-core. (c) Sediment Fe EXAFS spectra fit with linear contribution, showing values for Bio Fe, greigite, and siderite, with the greatest contribution (20 %) of siderite to fits in the 64 cm samples (Table S-5a). (d) Sediment Mn XANES dominated by reduced Mn(II), and the emergence of a distinctive double-peak of rhodochrosite in the 64 and 110 cm samples. (e, f) Example SEM images of twinned sphere and dumbbell crystal morphologies from the siderite-enriched interval, consistent with experimental precipitates of the mineral. | 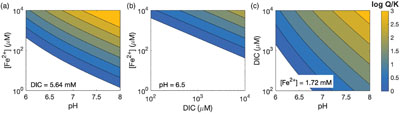 Figure 4 Relative influence of changes in porewater Fe concentration, pH and DIC on the saturation index of siderite. Inputs are based on water chemistry at 20 m, which is assumed to represent a minimum threshold for porewater concentrations. In general, iron concentrations above ∼100 μM seem to be required for siderite precipitation, unless the pH is high. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Siderite (FeCO3) occurs in sediments throughout Earth’s history (Ohmoto et al., 2004
Ohmoto, H., Watanabe, Y., Kumazawa, K. (2004) Evidence from massive siderite beds for a CO2-rich atmosphere before ∼ 1.8 billion years ago. Nature 429, 395–399. https://doi.org/10.1038/nature02573
) and is a common component in Precambrian iron formations (IFs; Konhauser et al., 2017Konhauser, K.O., Planavsky, N.J., Hardisty, D.S., Robbins, L.J., Warchola, T.J., Haugaard, R., Lalonde, S.V., Partin, C.A., Oonk, P.B.H., Tsikos, H., Lyons, T.W., Bekker, A., Johnson, C.M. (2017) Iron Formations: A global record of Neoarchean to Paleoproterozoic environmental history. Earth-Science Reviews 172, 140–177. https://doi.org/10.1016/j.earscirev.2017.06.012
). Despite the diversity of environments in which siderite occurs, two mechanisms are generally considered to drive its formation: 1) direct precipitation from an anoxic and iron-enriched (ferruginous) fluid; and 2) as a diagenetic product derived from reduction of primary iron (oxyhydr)oxides coupled to microbial organic carbon remineralisation (Heimann et al., 2010Heimann, A., Johnson, C.M., Beard, B.L., Valley, J.W., Roden, E.E., Spicuzza, M.J., Beukes, N.J. (2010) Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ∼2.5 Ga marine environments. Earth and Planetary Science Letters 294, 8–18. https://doi.org/10.1016/j.epsl.2010.02.015
). While dissimilatory reduction of reactive iron (oxyhydr)oxides, such as ferrihydrite, may explain the negative C-isotopic composition and textures of many lacustrine siderite occurrences (Vuillemin et al., 2019Vuillemin, A., Wirth, R., Kemnitz, H., Schleicher, A.M., Friese, A., Bauer, K.W., Simister, R., Nomosatryo, A., Ordonez, L., Ariztegui, D., Henny, C., Crowe, S.A., Benning, L.G., Kallmeyer, J., Russell, J.M., Bijaksana, S., Vogel, H., Towuti Drilling Project Science Team (2019) Formation of diagenetic siderite in modern ferruginous sediments. Geology 47, 540–544. https://doi.org/10.1130/G46100.1
), the textures and C-isotope signatures of well-preserved Precambrian IFs are also consistent with precipitation from ferruginous seawater (Beukes et al., 1990Beukes, N.J., Klein, C., Kaufman, A.J., Hayes, J.M. (1990) Carbonate petrography, kerogen distribution, and carbon and oxygen isotope variations in an Early Proterozoic transition from limestone to iron-formation deposition, Transvaal Supergroup, South Africa. Economic Geology 85, 663–690. https://doi.org/10.2113/gsecongeo.85.4.663
; Siahi et al., 2020Siahi, M., Tsikos, H., Rafuza, S., Oonk, P.B.H., Mhlanga, X.R., van Niekerk, D., Mason, P.R.D., Harris, C. (2020) Insights into the processes and controls on the absolute abundance and distribution of manganese in Precambrian iron formations. Precambrian Research 350, 105878. https://doi.org/10.1016/j.precamres.2020.105878
; Riding et al., 2022Riding, R., Liang, L., Fralick, P. (2022) Oxygen-induced chemocline precipitation between Archean Fe-rich and Fe-poor carbonate seas. Precambrian Research 383, 106902. https://doi.org/10.1016/j.precamres.2022.106902
), perhaps under a strong hydrothermal influence (Jiang and Tosca, 2019Jiang, C.Z., Tosca, N.J. (2019) Fe (II)-carbonate precipitation kinetics and the chemistry of anoxic ferruginous seawater. Earth and Planetary Science Letters 506, 231–242. https://doi.org/10.1016/j.epsl.2018.11.010
).To constrain the conditions that govern natural siderite precipitation, we examined iron (Fe) and manganese (Mn) phases in water column particulates and recent sediments from ferruginous Canyon Lake (CL) in Michigan, USA. Modern ferruginous lakes serve as analogue systems, informing our understanding of biogeochemical dynamics in anoxic Precambrian oceans (Swanner et al., 2020
Swanner, E.D., Lambrecht, N., Wittkop, C., Harding, C., Katsev, S., Torgeson, J., Poulton, S.W. (2020) The biogeochemistry of ferruginous lakes and past ferruginous oceans. Earth-Science Reviews 211, 1–43. https://doi.org/10.1016/j.earscirev.2020.103430
). Canyon Lake is an ideal site to investigate these processes, as its ferruginous bottom waters are poised near siderite supersaturation, and its water column has been chemically stable for at least the last 80 years (Lambrecht et al., 2018Lambrecht, N., Wittkop, C., Katsev, S., Fakhraee, M., Swanner, E.D. (2018) Geochemical Characterization of Two Ferruginous Meromictic Lakes in the Upper Midwest, USA. Journal of Geophysical Research: Biogeosciences 123, 3403–3422. http://dx.doi.org/10.1029/2018JG004587
). Found in a boreal shield setting, groundwater supplies the small CL basin with dissolved iron, and its water column chemistry and methane cycling embed processes similar to those thought to have dominated Precambrian oceans (Lambrecht et al., 2020Lambrecht, N., Katsev, S., Wittkop, C., Hall, S.J., Sheilk, C.S., Picarad, A., Fakhraee, M., Swanner, E.D. (2020) Biogeochemical and physical controls on methane fluxes from two ferruginous meromictic lakes. Geobiology 18, 54–69. https://doi.org/10.1111/gbi.12365
).top
Methods
Multiparameter sondes were used to determine CL water column properties in 2018 and 2019, and water samples for cation and anion analysis were collected in May 2018, following standard procedures. A sediment freeze core was collected in February 2018, and sediment traps were deployed in 2019. Samples for iron speciation, XANES, and EXAFS were collected, processed under an N2 atmosphere and stored anoxically until analysis. See Supplementary Information for Site Description, Methods, and full Results.
top
Results and Discussion
Iron and manganese phases in ferruginous waters. Centroid energies for Fe XANES pre-edge peaks from sediment trap material from 7.5, 15 and 20 m were ∼7114 eV (Figs. 1, S-3), consistent with a mixture of Fe(II) and Fe(III). Best fits from linear combination fitting of Fe extended X-ray absorption fine structure (EXAFS) spectra for the sediment trap samples included, in order of contribution to fits, a biogenic Fe-oxyhydroxide (“Bio Fe”; Toner et al., 2009
Toner, B.M., Santelli, C.M., Marcus, M.A., Wirth, R., Chan, C.S., McCollom, T., Bach, W., Edwards, K.J. (2009) Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochimica et Cosmochimica Acta 73, 388–403. https://doi.org/10.1016/j.gca.2008.09.035
), siderite, magnetite (Hansel et al., 2005Hansel, C.M., Benner, S.G., Fendorf, S. (2005) Competing Fe(II)-Induced Mineralization Pathways of Ferrihydrite. Environmental Science & Technology 39, 7147–7153. https://doi.org/10.1021/es050666z
), green rust, and greigite. However, the quality of these fits was low, likely due to the limited amount of sample producing spectra with low signal to noise, or the presence of poorly crystalline phases. The main Mn K-edge peak energy in sediment trap samples (∼6552 eV) was consistent with Mn(II) rather than Mn(III) or Mn(IV) (∼6558 eV), indicating that Mn-oxides are not present in the water column (Fig. 1).
Figure 1 Geochemical and mineralogical characteristics of water column solutes and solid phases. (a) Dissolved solutes in the CL water column. The oxycline (decrease in dissolved oxygen) occurs between 7 and 12 m, and the chemocline (increase in dissolved Fe) occurs between 17 and 19 m. Sediment traps were deployed at 7.5, 15 and 22 m. (b) Sediment trap Fe XANES analyses. Pre-edge centroid energies are ∼7114 eV, consistent with a mixture of Fe(II) and Fe(III). (c) Sediment trap Mn K-edge XANES analyses. Peaks have lower energy (∼6552 eV) compared to Mn-oxides (∼6558 eV), consistent with Mn(II) minerals.
Sediment geochemistry and mineralogy. The sediments contain a large pool of highly reactive Fe (FeHR; average 3.59 ± 0.52 wt. %), with the FeHR pool dominated by unsulfidised Fe(II) phases (Fe(II)unsulf), with a generally low concentration of poorly crystalline ferric (oxyhydr)oxide phases, such as ferrihydrite (Feox1). The remaining Fe phases, comprising crystalline Fe (oxyhydr)oxides (Feox2), magnetite (Femag) and pyrite (Fepy), were less significant throughout the core (Fig. 2; Table S-1).

Figure 2 Bulk sediment and porewater geochemistry for the CL sediment core. (a) Porosity and organic carbon content of the sediments. The high organic carbon content of the sediments contributes to their high porosity. (b) Bulk sediment Fe and Mn content showing peaks at ∼60 cm depth, which correspond to the presence of siderite (Fig. 3). (c) Iron speciation analysis demonstrating that much of the sediment Fe is contained in the Fe(II)unsulf pool. (d) Porewater dissolved inorganic carbon (DIC) concentration and isotope composition, showing a positive δ13C signature consistent with the mass balance of depleted carbon lost to methane. (e) Siderite saturation index (SI), which assumes the Fe concentration (1.689 mmol/L) and pH (6.8) of deepest ferruginous water and measured porewater DIC values. Sensitivity scenarios were based on doubling DIC and Fe concentrations, and increasing pH to 7.5.
Calculations using porewater dissolved inorganic carbon (DIC) and the solute chemistry of the deepest CL waters show siderite to be supersaturated throughout the porewater (Fig. 2). A sensitivity analysis adding additional dissolved Fe or DIC raised the saturation index only slightly; the most significant increase in siderite saturation scenarios came from increasing pH from 6.8 (the assumed porewater value) to 7.5 (Fig. 2), consistent with observations from ferruginous porewaters, where alkalinity increases due to organic carbon remineralisation (Vuillemin et al., 2023
Vuillemin, A., Mayr, C., Schuessler, J.A., Friese, A., Bauer, K.W., Lücke, A., Heuer, V.B., Glombitza, C., Henny, C., von Blanckenburg, F., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J. (2023) A one-million-year isotope record from siderites formed in modern ferruginous sediments. GSA Bulletin 135, 504–522. https://doi.org/10.1130/B36211.1
).Siderite was detected by XRD, with primary and secondary peaks observed at 31.62° and 52° 2θ (Fig. 3). A semi-quantitative ratio of siderite to quartz (Isid/Iqtz) suggests siderite is concentrated mid-core (∼60 cm), despite being supersaturated throughout the porewaters. SEM images from this interval detected <5 μm globular clumps with both dumbbell and spherical egg-shaped siderite morphologies that are consistent with growth in an organic matrix (Dupraz et al., 2009
Dupraz, C., Reid, R.P., Braissant, O., Decho, A.W., Norman, R.S., Visscher, P.T. (2009) Processes of carbonate precipitation in modern microbial mats. Earth-Science Reviews 96, 141–162. https://doi.org/10.1016/j.earscirev.2008.10.005
); a few crystals also exhibited rhomb-like shapes. All observed crystal forms were consistent with siderite crystals grown in lab experiments (e.g., Jiang and Tosca, 2019Jiang, C.Z., Tosca, N.J. (2019) Fe (II)-carbonate precipitation kinetics and the chemistry of anoxic ferruginous seawater. Earth and Planetary Science Letters 506, 231–242. https://doi.org/10.1016/j.epsl.2018.11.010
; Lin et al., 2019Lin, C.Y., Turchyn, A.V., Krylov, A., Antler, G. (2019) The microbially driven formation of siderite in salt marsh sediments. Geobiology 18, 207–224. https://doi.org/10.1111/gbi.12371
) or observed in lacustrine settings (Wittkop et al., 2014Wittkop, C., Teranes, J., Lubenow, B., Dean, W.E. (2014) Carbon- and oxygen-stable isotope signatures of methanogenesis, temperature, and water column stratification in Holocene siderite varves. Chemical Geology 389, 153–166. https://doi.org/10.1016/j.chemgeo.2014.09.016
). Pre-edge peak fitting of sediment Fe XANES indicated predominantly Fe(II) (see Supplementary Information). Iron EXAFS of sediments were best fit by combinations of Bio Fe and siderite, with siderite having the greatest fit contribution (20.8 %) at 64 cm. Manganese XANES spectra were consistent with rhodochrosite (MnCO3, which forms solid solutions with siderite) in samples from 64 and 110 cm.
Figure 3 Sediment Fe phases. (a) Example XRD scans showing the emergence of siderite in mid-core depths (57.5 and 63.5 cm scans; Q = quartz peaks, S = siderite peaks). (b) Relative XRD intensity of the siderite peak normalised to the quartz peak (Isid/Iqtz), showing an increase in siderite abundance mid-core. (c) Sediment Fe EXAFS spectra fit with linear contribution, showing values for Bio Fe, greigite, and siderite, with the greatest contribution (20 %) of siderite to fits in the 64 cm samples (Table S-5a). (d) Sediment Mn XANES dominated by reduced Mn(II), and the emergence of a distinctive double-peak of rhodochrosite in the 64 and 110 cm samples. (e, f) Example SEM images of twinned sphere and dumbbell crystal morphologies from the siderite-enriched interval, consistent with experimental precipitates of the mineral.
Primary iron phases in Canyon Lake. Despite the highly reducing nature of the CL water column and sediments, some Feox1 persists in the sediment core. XANES spectra are consistent with the presence of mixed Fe(II)-Fe(III) phases (see Supplementary Information), perhaps green rust, which has been found in other ferruginous settings (Zegeye et al., 2012
Zegeye, A., Bonneville, S., Benning, L.G., Sturm, A., Fowle, D.A., Jones, C., Canfield, D.E., Ruby, C., MacLean, L.C., Nomosatryo, S., Crowe, S.A., Poulton, S.W. (2012) Green rust formation controls nutrient availability in a ferruginous water column. Geology 40, 599–602. https://doi.org/10.1130/g32959.1
). While authigenic magnetite has also been shown to be an early precipitate in ferruginous settings (Bauer et al., 2020Bauer, K.W., Byrne, J.M., Kenward, P., Simister, R.L., Michiels, C.C., Friese, A., Vuillemin, A., Henny, C., Nomosatryo, S., Kallmeyer, J., Kappler, A., Smit, M.A., Francois, R., Crowe, S.A. (2020) Magnetite biomineralization in ferruginous waters and early Earth evolution. Earth and Planetary Science Letters 549, 116495. https://doi.org/10.1016/j.epsl.2020.116495
), the concentrations in our extractions were very low (Fig. 2), and magnetite was not conclusively detected by EXAFS or XRD. Our EXAFS results suggest greigite is present in the sediments, but Fe extractions imply that this mixed-valence sulfide is a minor component of the FeHR pool (i.e. low concentrations of Fe sulfides), suggesting that the EXAFS analysis is highly sensitive to the presence of sulfides, or is perhaps interfered with by an unknown phase. Sulfide abundance is limited by the small sulfate reservoir in the water column available for reduction (Lambrecht et al., 2018Lambrecht, N., Wittkop, C., Katsev, S., Fakhraee, M., Swanner, E.D. (2018) Geochemical Characterization of Two Ferruginous Meromictic Lakes in the Upper Midwest, USA. Journal of Geophysical Research: Biogeosciences 123, 3403–3422. http://dx.doi.org/10.1029/2018JG004587
), though a contribution from an organic sulfur reservoir cannot be ruled out (e.g., Phillips et al., 2023Phillips, A.A., Ulloa, I., Hyde, E., Agnich, J., Sharpnack, L., O’Malley, K.G., Webb, S.M., Schreiner, K.M., Sheik, C.S., Katsev, S., Raven, M.R. (2023) Organic sulfur from source to sink in low-sulfate Lake Superior. Limnology and Oceanography 68, 2716–2732. https://doi.org/10.1002/lno.12454
).The persistence of reduced Mn phases throughout the CL water column and sediments (Figs. 1 and 2) underscores the limited ability of this system to oxidise the large reservoir of Fe and Mn from the ferruginous portion of the water column. The dominance of reduced Mn phases in sediment trap materials perhaps points to an external source of reduced solutes—in this case, groundwater input to the lake (Lambrecht et al., 2018
Lambrecht, N., Wittkop, C., Katsev, S., Fakhraee, M., Swanner, E.D. (2018) Geochemical Characterization of Two Ferruginous Meromictic Lakes in the Upper Midwest, USA. Journal of Geophysical Research: Biogeosciences 123, 3403–3422. http://dx.doi.org/10.1029/2018JG004587
). Thus, Fe(III) delivery to CL sediments is limited, likely in the form of a poorly ordered Fe-(oxyhydr)oxide (Bio Fe), potentially supplemented by small quantities of greigite and/or green rust; a small component of Fe(III) may be added through detrital phases.Controls on siderite occurrence. Although equilibrium modelling indicates siderite supersaturation throughout CL porewaters, carbonate minerals were only confirmed in one relatively restricted horizon, suggesting an inhibiting factor in the remaining intervals. The relatively slow kinetics of siderite precipitation, especially in the cold (∼5 °C) bottom waters of CL, is a likely constraint; hence, a metastable precursor to siderite, such as green rust, may precipitate first. Carbonate green rust is closely associated with diagenetic siderite precipitation (Vuillemin et al., 2019
Vuillemin, A., Wirth, R., Kemnitz, H., Schleicher, A.M., Friese, A., Bauer, K.W., Simister, R., Nomosatryo, A., Ordonez, L., Ariztegui, D., Henny, C., Crowe, S.A., Benning, L.G., Kallmeyer, J., Russell, J.M., Bijaksana, S., Vogel, H., Towuti Drilling Project Science Team (2019) Formation of diagenetic siderite in modern ferruginous sediments. Geology 47, 540–544. https://doi.org/10.1130/G46100.1
), and has been previously identified in the sediment traps from ferruginous Lake Matano (Zegeye et al., 2012Zegeye, A., Bonneville, S., Benning, L.G., Sturm, A., Fowle, D.A., Jones, C., Canfield, D.E., Ruby, C., MacLean, L.C., Nomosatryo, S., Crowe, S.A., Poulton, S.W. (2012) Green rust formation controls nutrient availability in a ferruginous water column. Geology 40, 599–602. https://doi.org/10.1130/g32959.1
). Green rust ages to siderite in laboratory experiments (Halevy et al., 2017Halevy, I., Alesker, M., Schuster, E.M., Popovitz-Biro, R., Feldman, Y. (2017) A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nature Geoscience 10, 577–581. https://doi.org/10.1038/NGEO2978
), but the mechanisms which govern this transformation are not well understood (e.g., Wiesli et al., 2004Wiesli, R.A., Beard, B.L., Johnson, C.M. (2004) Experimental determination of Fe isotope fractionation between aqueous Fe(II), siderite and “green rust” in abiotic systems. Chemical Geology 211, 343–362. https://doi.org/10.1016/j.chemgeo.2004.07.002
), though pH is an important control on its behavior (e.g., Guilbaud et al., 2013Guilbaud, R., White, M.L., Poulton, S.W. (2013) Surface charge and growth of sulphate and carbonate green rust in aqueous media. Geochimica et Cosmochimica Acta 108, 141–153. https://doi.org/10.1016/j.gca.2013.01.017
). Although green rust potentially plays a role in the CL system, we were not able to conclusively identify it in this study.The organic carbon-rich CL sediments also create an environment that inhibits carbonate precipitation. Over longer timescales, porewater alkalinity and pH are known to increase due to continued OM fermentation (Vuillemin et al., 2023
Vuillemin, A., Mayr, C., Schuessler, J.A., Friese, A., Bauer, K.W., Lücke, A., Heuer, V.B., Glombitza, C., Henny, C., von Blanckenburg, F., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J. (2023) A one-million-year isotope record from siderites formed in modern ferruginous sediments. GSA Bulletin 135, 504–522. https://doi.org/10.1130/B36211.1
), but organic substrates may both inhibit and promote carbonate precipitation, dependent on the composition of functional groups, and the pH of the environment (Dupraz et al., 2009Dupraz, C., Reid, R.P., Braissant, O., Decho, A.W., Norman, R.S., Visscher, P.T. (2009) Processes of carbonate precipitation in modern microbial mats. Earth-Science Reviews 96, 141–162. https://doi.org/10.1016/j.earscirev.2008.10.005
). In CL sediments, intense fermentation (Lambrecht et al., 2020Lambrecht, N., Katsev, S., Wittkop, C., Hall, S.J., Sheilk, C.S., Picarad, A., Fakhraee, M., Swanner, E.D. (2020) Biogeochemical and physical controls on methane fluxes from two ferruginous meromictic lakes. Geobiology 18, 54–69. https://doi.org/10.1111/gbi.12365
) likely contributes to the inhibition of siderite precipitation by adding CO2 to the pore fluids, buffering against significant increases in pH.The appearance of crystalline siderite (XRD-detectable) ∼60 cm below the ferruginous sediment–water interface suggests a precipitation barrier was overcome in this horizon, likely by an environmental change. While porewater diffusion smoothes the concentration profiles of solutes, diagenetic siderite precipitation may occur episodically due to environmental changes, such as lake level fluctuations (e.g., Vuillemin et al., 2023
Vuillemin, A., Mayr, C., Schuessler, J.A., Friese, A., Bauer, K.W., Lücke, A., Heuer, V.B., Glombitza, C., Henny, C., von Blanckenburg, F., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J. (2023) A one-million-year isotope record from siderites formed in modern ferruginous sediments. GSA Bulletin 135, 504–522. https://doi.org/10.1130/B36211.1
). However, CL has been stably stratified at least since the late 1930s (Lambrecht et al., 2018Lambrecht, N., Wittkop, C., Katsev, S., Fakhraee, M., Swanner, E.D. (2018) Geochemical Characterization of Two Ferruginous Meromictic Lakes in the Upper Midwest, USA. Journal of Geophysical Research: Biogeosciences 123, 3403–3422. http://dx.doi.org/10.1029/2018JG004587
), implying that climate-driven changes in lake mixing (e.g., drought) were an unlikely influence in the interval where siderite occurs (see Supplementary Information). Wildfires are a more likely influence, as both historical accounts and tree ring records indicate that significant fires occurred in the forests surrounding CL as recently as the early 1900s (Muzika et al., 2015Muzika, R.M., Guyette, R.P., Stambaugh, M.C., Marschall, J.M. (2015) Fire, Drought, and Humans in a Heterogeneous Lake Superior Landscape. Journal of Sustainable Forestry 34, 49–70. https://doi.org/1080/10549811.2014.973991
). Calcium carbonate is a known product of wood combustion, and wildfire mineral ash is known to increase environmental pH in a variety of contexts (e.g., Brito et al., 2021Brito, D.Q., Santos, L.H.G., Passos, C.J.S., Oliveira-Filho, E.C. (2021) Short-Term Effects of Wildfire Ash on Water Quality Parameters: A Laboratory Approach. Bulletin of Environmental Contamination and Toxicology 107, 500–505. https://doi.org/10.1007/s00128-021-03220-9
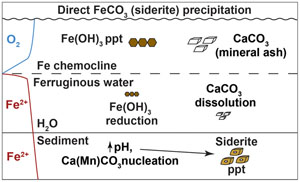
).Mineral ash deposition would stimulate an increase in porewater pH and deliver CaCO3 to sediments. These changes would catalyse siderite precipitation by enhancing sorbtion of Fe and Mn ions to ash particles (Brito et al., 2021
Brito, D.Q., Santos, L.H.G., Passos, C.J.S., Oliveira-Filho, E.C. (2021) Short-Term Effects of Wildfire Ash on Water Quality Parameters: A Laboratory Approach. Bulletin of Environmental Contamination and Toxicology 107, 500–505. https://doi.org/10.1007/s00128-021-03220-9
), increasing the activity of CO32− (Fig. 4), and providing nucleation sites for crystal growth, overcoming kinetic barriers to siderite precipitation (Jiang and Tosca, 2019Jiang, C.Z., Tosca, N.J. (2019) Fe (II)-carbonate precipitation kinetics and the chemistry of anoxic ferruginous seawater. Earth and Planetary Science Letters 506, 231–242. https://doi.org/10.1016/j.epsl.2018.11.010
; Lin et al., 2019Lin, C.Y., Turchyn, A.V., Krylov, A., Antler, G. (2019) The microbially driven formation of siderite in salt marsh sediments. Geobiology 18, 207–224. https://doi.org/10.1111/gbi.12371
). The increase in abundance of bulk Fe and Mn in the siderite layer (Fig. 2) is consistent with a sorbtive process, and an ash layer may have resulted in reduced sediment porosity in the same interval, impeding porewater flow, leading to a localised increase in solute concentrations, further enhancing mineral precipitation potential. These observations suggest that the siderite layer in Canyon Lake sediments derives from a combination of depositional (ashfall, sorbtion) and diagenetic (post-depositional crystal growth) processes.
Figure 4 Relative influence of changes in porewater Fe concentration, pH and DIC on the saturation index of siderite. Inputs are based on water chemistry at 20 m, which is assumed to represent a minimum threshold for porewater concentrations. In general, iron concentrations above ∼100 μM seem to be required for siderite precipitation, unless the pH is high.
We cannot eliminate the possibility that another process, such as an overturn of lake stratification, drove enhanced Fe(III) delivery to CL sediments in the past, but it seems unlikely. Lake overturn would presumably be accompanied by oxidation of the CL’s large isotopically light methane reservoir, which is inconsistent with our carbon isotope data. The association between mineral ashfall and sidertite precipitation we suggest is testable with high-resolution sediment chronology, advanced microscopy (e.g., TEM), and/or charcoal analysis.
The pronounced influence of pH on siderite precipitation is linked to the dependence of siderite saturation on the concentration of the CO32− ion (Fig. 4). Carbonate equilibrium dictates that [CO32−] scales linearly with total DIC but nonlinearly with [H+], due to the presence of a quadratic term (Supplementary Information). Hence, changing the pH by 1 unit has a stronger effect than a more substantial change in DIC. In CL porewaters where organic carbon remineralisation buffers H+ fluctuations by increasing alkalinity (and consumption of H+ by acetogens and methanogens), an external agent (i.e. mineral ash) is required to drive more substantial pH changes.
The detection of rhodochrosite by XANES in the same horizon where XRD indicates siderite illustrates the heterogeneous nature of carbonate precipitation in CL. Lacustrine siderites are commonly Mn-substituted (Swanner et al., 2020
Swanner, E.D., Lambrecht, N., Wittkop, C., Harding, C., Katsev, S., Torgeson, J., Poulton, S.W. (2020) The biogeochemistry of ferruginous lakes and past ferruginous oceans. Earth-Science Reviews 211, 1–43. https://doi.org/10.1016/j.earscirev.2020.103430
), possibly linked to the more readily reducible nature of Mn-oxides relative to Fe-(oxyhydr)oxides (Vuillemin et al., 2019Vuillemin, A., Wirth, R., Kemnitz, H., Schleicher, A.M., Friese, A., Bauer, K.W., Simister, R., Nomosatryo, A., Ordonez, L., Ariztegui, D., Henny, C., Crowe, S.A., Benning, L.G., Kallmeyer, J., Russell, J.M., Bijaksana, S., Vogel, H., Towuti Drilling Project Science Team (2019) Formation of diagenetic siderite in modern ferruginous sediments. Geology 47, 540–544. https://doi.org/10.1130/G46100.1
). However, the lack of an Mn-oxide flux in CL suggests that a fundamental control on precipitation governs this occurrence of Mn-siderite, with competition from nucleation inhibitors (e.g., Mg(II); Vuillemin et al., 2019Vuillemin, A., Wirth, R., Kemnitz, H., Schleicher, A.M., Friese, A., Bauer, K.W., Simister, R., Nomosatryo, A., Ordonez, L., Ariztegui, D., Henny, C., Crowe, S.A., Benning, L.G., Kallmeyer, J., Russell, J.M., Bijaksana, S., Vogel, H., Towuti Drilling Project Science Team (2019) Formation of diagenetic siderite in modern ferruginous sediments. Geology 47, 540–544. https://doi.org/10.1130/G46100.1
) or the differential solubilities of Ca-Mn-Fe carbonates (e.g., Wittkop et al., 2020Wittkop, C., Swanner, E., Grengs, A., Lambrecht, N., Fakrhaee, M., Myrbo, A., Bray, A., Poulton, S., Katsev, S. (2020) Evaluating a primary carbonate pathway for manganese enrichments in reducing environments. Earth and Planetary Science Letters 538, 116201. https://doi.org/10.1016/j.epsl.2020.116201
) offering potential explanations. Thus, the occurrence of Mn-rich siderite in the geologic record (e.g., Siahi et al, 2020Siahi, M., Tsikos, H., Rafuza, S., Oonk, P.B.H., Mhlanga, X.R., van Niekerk, D., Mason, P.R.D., Harris, C. (2020) Insights into the processes and controls on the absolute abundance and distribution of manganese in Precambrian iron formations. Precambrian Research 350, 105878. https://doi.org/10.1016/j.precamres.2020.105878
; Swanner et al., 2020Swanner, E.D., Lambrecht, N., Wittkop, C., Harding, C., Katsev, S., Torgeson, J., Poulton, S.W. (2020) The biogeochemistry of ferruginous lakes and past ferruginous oceans. Earth-Science Reviews 211, 1–43. https://doi.org/10.1016/j.earscirev.2020.103430
) may be interpreted to reflect passive incorporation of dissolved Mn into the crystal structure, rather than reduction of Mn-oxides.Biogeochemical Implications. Although siderite occurs in sediments throughout the geologic record, direct precipitation from Fe-enriched anoxic fluids is thought to be rare due to the slow kinetics of siderite precipitation (Jiang and Tosca, 2019
Jiang, C.Z., Tosca, N.J. (2019) Fe (II)-carbonate precipitation kinetics and the chemistry of anoxic ferruginous seawater. Earth and Planetary Science Letters 506, 231–242. https://doi.org/10.1016/j.epsl.2018.11.010
). Modern process studies in oxygenated surface environments tend to encounter siderite only in early diagenetic settings (e.g., Lin et al., 2019Lin, C.Y., Turchyn, A.V., Krylov, A., Antler, G. (2019) The microbially driven formation of siderite in salt marsh sediments. Geobiology 18, 207–224. https://doi.org/10.1111/gbi.12371
). Hence it is often assumed that siderite precipitates following the deposition of an Fe-(oxyhydr)oxide precursor such as ferrihydrite, which is subsequently reduced in the sediments through microbial respiration, providing both dissolved Fe and the alkalinity needed to precipitate carbonate phases (Heimann et al., 2010Heimann, A., Johnson, C.M., Beard, B.L., Valley, J.W., Roden, E.E., Spicuzza, M.J., Beukes, N.J. (2010) Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ∼2.5 Ga marine environments. Earth and Planetary Science Letters 294, 8–18. https://doi.org/10.1016/j.epsl.2010.02.015
). However, our observations are not consistent with this traditional model, as the sediment Fe reservoir in CL is dominated by Fe(II) phases occurring below a persistently ferruginous water column.While Fe-(oxyhydr)oxides are often invoked as precursors for Precambrian IF deposition (e.g., Konhauser et al., 2017
Konhauser, K.O., Planavsky, N.J., Hardisty, D.S., Robbins, L.J., Warchola, T.J., Haugaard, R., Lalonde, S.V., Partin, C.A., Oonk, P.B.H., Tsikos, H., Lyons, T.W., Bekker, A., Johnson, C.M. (2017) Iron Formations: A global record of Neoarchean to Paleoproterozoic environmental history. Earth-Science Reviews 172, 140–177. https://doi.org/10.1016/j.earscirev.2017.06.012
), recent work has focused on the role of Fe-silicates in IF genesis (e.g., Hinz et al., 2021Hinz, I., Nims, C., Theuer, S., Templeton, A.S., Johnson, J.E. (2021) Ferric iron triggers greenalite formation in simulated Archean seawater. Geology 49, 905–910. https://doi.org/10.1130/G48495.1
). In CL, direct precipitation of siderite below a ferruginous water column represents a significant Fe burial pathway that does not require precursor Fe-(oxyhydr)oxides or silicates. Hence, our work demonstrates that the presence of siderite in sediments should be carefully considered when interpreting past redox conditions, as direct precipitation pathways imply much lower oxygen levels at the sediment-water interface than diagenetic pathways involving Fe-(oxyhydr)oxide reduction.Multiple studies highlight the importance of pH in governing both Fe-carbonate and Fe-silicate systems (Halevy et al., 2017
Halevy, I., Alesker, M., Schuster, E.M., Popovitz-Biro, R., Feldman, Y. (2017) A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nature Geoscience 10, 577–581. https://doi.org/10.1038/NGEO2978
; Jiang and Tosca, 2019Jiang, C.Z., Tosca, N.J. (2019) Fe (II)-carbonate precipitation kinetics and the chemistry of anoxic ferruginous seawater. Earth and Planetary Science Letters 506, 231–242. https://doi.org/10.1016/j.epsl.2018.11.010
; Hinz et al., 2021Hinz, I., Nims, C., Theuer, S., Templeton, A.S., Johnson, J.E. (2021) Ferric iron triggers greenalite formation in simulated Archean seawater. Geology 49, 905–910. https://doi.org/10.1130/G48495.1
), as well as the evolution of stable phases from green rust precursors (Zegeye et al., 2012Zegeye, A., Bonneville, S., Benning, L.G., Sturm, A., Fowle, D.A., Jones, C., Canfield, D.E., Ruby, C., MacLean, L.C., Nomosatryo, S., Crowe, S.A., Poulton, S.W. (2012) Green rust formation controls nutrient availability in a ferruginous water column. Geology 40, 599–602. https://doi.org/10.1130/g32959.1
). Could subtle variations in seawater silica concentration and pH have generated the silicate-carbonate banding observed in many Precambrian IFs (e.g., James et al., 1968James, H.J., Dutton, C.E., Pettijohn, F.J., Wier, K.L. (1968) Geology and Ore Deposits of the Iron River-Crystal Falls District, Iron Country, Michigan. USGS Professional Paper 570, United States Geological Survey, Washington, D.C. https://doi.org/10.3133/pp570
; Beukes et al., 1990Beukes, N.J., Klein, C., Kaufman, A.J., Hayes, J.M. (1990) Carbonate petrography, kerogen distribution, and carbon and oxygen isotope variations in an Early Proterozoic transition from limestone to iron-formation deposition, Transvaal Supergroup, South Africa. Economic Geology 85, 663–690. https://doi.org/10.2113/gsecongeo.85.4.663
)? A growing body of evidence supports this possibility, but additional experiments are needed to evaluate the role of pH changes and the presence of nucleation substrates in the direct precipitation of siderite (and associated Mn-carbonate).In ancient seas, the role of wildfire ash in CL may have been played by processes that introduce calcium carbonate to more acidic ferruginous waters, including transgression over previously deposited carbonates, turbidites, or crystals settling from whiting events (e.g., Morse et al., 2003
Morse, J.W., Gledhill, D.K., Millero, F.J. (2003) CaCO3 precipitation kinetics in waters from the great Bahama bank: Implications for the relationship between bank hydrochemistry and whitings. Geochimica et Cosmochimica Acta 67, 2819–2826. https://doi.org/10.1016/S0016-7037(03)00103-0
). The novel link between wildfire ash and enhanced siderite precipitation identified here may also imply a new pathway for enhancing carbon sequestration in methane-rich, ferruginous environments that appear to be widespread in boreal shield settings and postglacial lakes (Schiff et al., 2017Schiff, S.L., Tsuji, J.M., Wu, L., Venkiteswaran, J.J., Molot, L.A., Elgood, R.J., Paterson, M.J., Neufeld, J.D. (2017) Millions of boreal shield lakes can be used to probe Archean ocean biogeochemistry. Scientific Reports 7, 46708. https://doi.org/10.1038/srep46708
).top
Acknowledgements
We thank Ben Harrison, Lance Hybben, Michael Mondry, and the Huron Mountain Club for field assistance. Jessica Heck, Kristina Brady Shannon, Ryan O’Grady and Amy Myrbo of the Continental Scientific Drilling Facility assisted with handling of the freeze core. Rose-Marie Muzika, Kerry Woods and Daniel Cziczo provided helpful discussion of fire history. This research utilized the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Tianpin Wu and George Sterbinsky at 9BM assisted with data collection and analysis at the APS. Funding was provided by NSF-EAR 1660691, 1660761 and 1660873. Open access was supported by a Minnesota State University – Mankato Faculty Scholarship Grant.
Editor: Liane G. Benning
top
References
Bauer, K.W., Byrne, J.M., Kenward, P., Simister, R.L., Michiels, C.C., Friese, A., Vuillemin, A., Henny, C., Nomosatryo, S., Kallmeyer, J., Kappler, A., Smit, M.A., Francois, R., Crowe, S.A. (2020) Magnetite biomineralization in ferruginous waters and early Earth evolution. Earth and Planetary Science Letters 549, 116495. https://doi.org/10.1016/j.epsl.2020.116495
 Show in context
Show in context While authigenic magnetite has also been shown to be an early precipitate in ferruginous settings (Bauer et al., 2020), the concentrations in our extractions were very low (Fig. 2), and magnetite was not conclusively detected by EXAFS or XRD. Our EXAFS results suggest greigite is present in the sediments, but Fe extractions imply that this mixed-valence sulfide is a minor component of the FeHR pool (i.e. low concentrations of Fe sulfides), suggesting that the EXAFS analysis is highly sensitive to the presence of sulfides, or is perhaps interfered with by an unknown phase.
View in article
Beukes, N.J., Klein, C., Kaufman, A.J., Hayes, J.M. (1990) Carbonate petrography, kerogen distribution, and carbon and oxygen isotope variations in an Early Proterozoic transition from limestone to iron-formation deposition, Transvaal Supergroup, South Africa. Economic Geology 85, 663–690. https://doi.org/10.2113/gsecongeo.85.4.663
 Show in context
Show in context While dissimilatory reduction of reactive iron (oxyhydr)oxides, such as ferrihydrite, may explain the negative C-isotopic composition and textures of many lacustrine siderite occurrences (Vuillemin et al., 2019), the textures and C-isotope signatures of well-preserved Precambrian IFs are also consistent with precipitation from ferruginous seawater (Beukes et al., 1990; Siahi et al., 2020; Riding et al., 2022), perhaps under a strong hydrothermal influence (Jiang and Tosca, 2019).
View in article
Could subtle variations in seawater silica concentration and pH have generated the silicate-carbonate banding observed in many Precambrian IFs (e.g., James et al., 1968; Beukes et al., 1990)?
View in article
Brito, D.Q., Santos, L.H.G., Passos, C.J.S., Oliveira-Filho, E.C. (2021) Short-Term Effects of Wildfire Ash on Water Quality Parameters: A Laboratory Approach. Bulletin of Environmental Contamination and Toxicology 107, 500–505. https://doi.org/10.1007/s00128-021-03220-9
 Show in context
Show in context Calcium carbonate is a known product of wood combustion, and wildfire mineral ash is known to increase environmental pH in a variety of contexts (e.g., Brito et al., 2021).
View in article
These changes would catalyse siderite precipitation by enhancing sorbtion of Fe and Mn ions to ash particles (Brito et al., 2021), increasing the activity of CO32− (Fig. 4), and providing nucleation sites for crystal growth, overcoming kinetic barriers to siderite precipitation (Jiang and Tosca, 2019; Lin et al., 2019).
View in article
Dupraz, C., Reid, R.P., Braissant, O., Decho, A.W., Norman, R.S., Visscher, P.T. (2009) Processes of carbonate precipitation in modern microbial mats. Earth-Science Reviews 96, 141–162. https://doi.org/10.1016/j.earscirev.2008.10.005
 Show in context
Show in context SEM images from this interval detected <5 μm globular clumps with both dumbbell and spherical egg-shaped siderite morphologies that are consistent with growth in an organic matrix (Dupraz et al., 2009); a few crystals also exhibited rhomb-like shapes.
View in article
Over longer timescales, porewater alkalinity and pH are known to increase due to continued OM fermentation (Vuillemin et al., 2023), but organic substrates may both inhibit and promote carbonate precipitation, dependent on the composition of functional groups, and the pH of the environment (Dupraz et al., 2009).
View in article
Guilbaud, R., White, M.L., Poulton, S.W. (2013) Surface charge and growth of sulphate and carbonate green rust in aqueous media. Geochimica et Cosmochimica Acta 108, 141–153. https://doi.org/10.1016/j.gca.2013.01.017
 Show in context
Show in context Green rust ages to siderite in laboratory experiments (Halevy et al., 2017), but the mechanisms which govern this transformation are not well understood (e.g., Wiesli et al., 2004), though pH is an important control on its behavior (e.g., Guilbaud et al., 2013).
View in article
Halevy, I., Alesker, M., Schuster, E.M., Popovitz-Biro, R., Feldman, Y. (2017) A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nature Geoscience 10, 577–581. https://doi.org/10.1038/NGEO2978
 Show in context
Show in context Green rust ages to siderite in laboratory experiments (Halevy et al., 2017), but the mechanisms which govern this transformation are not well understood (e.g., Wiesli et al., 2004), though pH is an important control on its behavior (e.g., Guilbaud et al., 2013).
View in article
Multiple studies highlight the importance of pH in governing both Fe-carbonate and Fe-silicate systems (Halevy et al., 2017; Jiang and Tosca, 2019; Hinz et al., 2021), as well as the evolution of stable phases from green rust precursors (Zegeye et al., 2012).
View in article
Hansel, C.M., Benner, S.G., Fendorf, S. (2005) Competing Fe(II)-Induced Mineralization Pathways of Ferrihydrite. Environmental Science & Technology 39, 7147–7153. https://doi.org/10.1021/es050666z
 Show in context
Show in context Best fits from linear combination fitting of Fe extended X-ray absorption fine structure (EXAFS) spectra for the sediment trap samples included, in order of contribution to fits, a biogenic Fe-oxyhydroxide (“Bio Fe”; Toner et al., 2009), siderite, magnetite (Hansel et al., 2005), green rust, and greigite.
View in article
Heimann, A., Johnson, C.M., Beard, B.L., Valley, J.W., Roden, E.E., Spicuzza, M.J., Beukes, N.J. (2010) Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ∼2.5 Ga marine environments. Earth and Planetary Science Letters 294, 8–18. https://doi.org/10.1016/j.epsl.2010.02.015
 Show in context
Show in context Despite the diversity of environments in which siderite occurs, two mechanisms are generally considered to drive its formation: 1) direct precipitation from an anoxic and iron-enriched (ferruginous) fluid; and 2) as a diagenetic product derived from reduction of primary iron (oxyhydr)oxides coupled to microbial organic carbon remineralisation (Heimann et al., 2010).
View in article
Hence it is often assumed that siderite precipitates following the deposition of an Fe-(oxyhydr)oxide precursor such as ferrihydrite, which is subsequently reduced in the sediments through microbial respiration, providing both dissolved Fe and the alkalinity needed to precipitate carbonate phases (Heimann et al., 2010).
View in article
Hinz, I., Nims, C., Theuer, S., Templeton, A.S., Johnson, J.E. (2021) Ferric iron triggers greenalite formation in simulated Archean seawater. Geology 49, 905–910. https://doi.org/10.1130/G48495.1
 Show in context
Show in context While Fe-(oxyhydr)oxides are often invoked as precursors for Precambrian IF deposition (e.g., Konhauser et al., 2017), recent work has focused on the role of Fe-silicates in IF genesis (e.g., Hinz et al., 2021).
View in article
Multiple studies highlight the importance of pH in governing both Fe-carbonate and Fe-silicate systems (Halevy et al., 2017; Jiang and Tosca, 2019; Hinz et al., 2021), as well as the evolution of stable phases from green rust precursors (Zegeye et al., 2012).
View in article
James, H.J., Dutton, C.E., Pettijohn, F.J., Wier, K.L. (1968) Geology and Ore Deposits of the Iron River-Crystal Falls District, Iron Country, Michigan. USGS Professional Paper 570, United States Geological Survey, Washington, D.C. https://doi.org/10.3133/pp570
 Show in context
Show in context Could subtle variations in seawater silica concentration and pH have generated the silicate-carbonate banding observed in many Precambrian IFs (e.g., James et al., 1968; Beukes et al., 1990)?
View in article
Jiang, C.Z., Tosca, N.J. (2019) Fe (II)-carbonate precipitation kinetics and the chemistry of anoxic ferruginous seawater. Earth and Planetary Science Letters 506, 231–242. https://doi.org/10.1016/j.epsl.2018.11.010
 Show in context
Show in context While dissimilatory reduction of reactive iron (oxyhydr)oxides, such as ferrihydrite, may explain the negative C-isotopic composition and textures of many lacustrine siderite occurrences (Vuillemin et al., 2019), the textures and C-isotope signatures of well-preserved Precambrian IFs are also consistent with precipitation from ferruginous seawater (Beukes et al., 1990; Siahi et al., 2020; Riding et al., 2022), perhaps under a strong hydrothermal influence (Jiang and Tosca, 2019).
View in article
All observed crystal forms were consistent with siderite crystals grown in lab experiments (e.g., Jiang and Tosca, 2019; Lin et al., 2019) or observed in lacustrine settings (Wittkop et al., 2014).
View in article
These changes would catalyse siderite precipitation by enhancing sorbtion of Fe and Mn ions to ash particles (Brito et al., 2021), increasing the activity of CO32− (Fig. 4), and providing nucleation sites for crystal growth, overcoming kinetic barriers to siderite precipitation (Jiang and Tosca, 2019; Lin et al., 2019).
View in article
Although siderite occurs in sediments throughout the geologic record, direct precipitation from Fe-enriched anoxic fluids is thought to be rare due to the slow kinetics of siderite precipitation (Jiang and Tosca, 2019).
View in article
Multiple studies highlight the importance of pH in governing both Fe-carbonate and Fe-silicate systems (Halevy et al., 2017; Jiang and Tosca, 2019; Hinz et al., 2021), as well as the evolution of stable phases from green rust precursors (Zegeye et al., 2012).
View in article
Konhauser, K.O., Planavsky, N.J., Hardisty, D.S., Robbins, L.J., Warchola, T.J., Haugaard, R., Lalonde, S.V., Partin, C.A., Oonk, P.B.H., Tsikos, H., Lyons, T.W., Bekker, A., Johnson, C.M. (2017) Iron Formations: A global record of Neoarchean to Paleoproterozoic environmental history. Earth-Science Reviews 172, 140–177. https://doi.org/10.1016/j.earscirev.2017.06.012
 Show in context
Show in context Siderite (FeCO3) occurs in sediments throughout Earth’s history (Ohmoto et al., 2004) and is a common component in Precambrian iron formations (IFs; Konhauser et al., 2017).
View in article
While Fe-(oxyhydr)oxides are often invoked as precursors for Precambrian IF deposition (e.g., Konhauser et al., 2017), recent work has focused on the role of Fe-silicates in IF genesis (e.g., Hinz et al., 2021).
View in article
Lambrecht, N., Wittkop, C., Katsev, S., Fakhraee, M., Swanner, E.D. (2018) Geochemical Characterization of Two Ferruginous Meromictic Lakes in the Upper Midwest, USA. Journal of Geophysical Research: Biogeosciences 123, 3403–3422. https://dx.doi.org/10.1029/2018JG004587
 Show in context
Show in context Canyon Lake is an ideal site to investigate these processes, as its ferruginous bottom waters are poised near siderite supersaturation, and its water column has been chemically stable for at least the last 80 years (Lambrecht et al., 2018).
View in article
Sulfide abundance is limited by the small sulfate reservoir in the water column available for reduction (Lambrecht et al., 2018), though a contribution from an organic sulfur reservoir cannot be ruled out (e.g., Phillips et al., 2023).
View in article
The dominance of reduced Mn phases in sediment trap materials perhaps points to an external source of reduced solutes—in this case, groundwater input to the lake (Lambrecht et al., 2018).
View in article
However, CL has been stably stratified at least since the late 1930s (Lambrecht et al., 2018), implying that climate-driven changes in lake mixing (e.g., drought) were an unlikely influence in the interval where siderite occurs (see Supplementary Information).
View in article
Lambrecht, N., Katsev, S., Wittkop, C., Hall, S.J., Sheilk, C.S., Picarad, A., Fakhraee, M., Swanner, E.D. (2020) Biogeochemical and physical controls on methane fluxes from two ferruginous meromictic lakes. Geobiology 18, 54–69. https://doi.org/10.1111/gbi.12365
 Show in context
Show in context Found in a boreal shield setting, groundwater supplies the small CL basin with dissolved iron, and its water column chemistry and methane cycling embed processes similar to those thought to have dominated Precambrian oceans (Lambrecht et al., 2020).
View in article
In CL sediments, intense fermentation (Lambrecht et al., 2020) likely contributes to the inhibition of siderite precipitation by adding CO2 to the pore fluids, buffering against significant increases in pH.
View in article
Lin, C.Y., Turchyn, A.V., Krylov, A., Antler, G. (2019) The microbially driven formation of siderite in salt marsh sediments. Geobiology 18, 207–224. https://doi.org/10.1111/gbi.12371
 Show in context
Show in context All observed crystal forms were consistent with siderite crystals grown in lab experiments (e.g., Jiang and Tosca, 2019; Lin et al., 2019) or observed in lacustrine settings (Wittkop et al., 2014).
View in article
These changes would catalyse siderite precipitation by enhancing sorbtion of Fe and Mn ions to ash particles (Brito et al., 2021), increasing the activity of CO32− (Fig. 4), and providing nucleation sites for crystal growth, overcoming kinetic barriers to siderite precipitation (Jiang and Tosca, 2019; Lin et al., 2019).
View in article
Modern process studies in oxygenated surface environments tend to encounter siderite only in early diagenetic settings (e.g., Lin et al., 2019).
View in article
Morse, J.W., Gledhill, D.K., Millero, F.J. (2003) CaCO3 precipitation kinetics in waters from the great Bahama bank: Implications for the relationship between bank hydrochemistry and whitings. Geochimica et Cosmochimica Acta 67, 2819–2826. https://doi.org/10.1016/S0016-7037(03)00103-0
 Show in context
Show in context In ancient seas, the role of wildfire ash in CL may have been played by processes that introduce calcium carbonate to more acidic ferruginous waters, including transgression over previously deposited carbonates, turbidites, or crystals settling from whiting events (e.g., Morse et al., 2003).
View in article
Muzika, R.M., Guyette, R.P., Stambaugh, M.C., Marschall, J.M. (2015) Fire, Drought, and Humans in a Heterogeneous Lake Superior Landscape. Journal of Sustainable Forestry 34, 49–70. https://doi.org/1080/10549811.2014.973991
 Show in context
Show in context Wildfires are a more likely influence, as both historical accounts and tree ring records indicate that significant fires occurred in the forests surrounding CL as recently as the early 1900s (Muzika et al., 2015).
View in article
Ohmoto, H., Watanabe, Y., Kumazawa, K. (2004) Evidence from massive siderite beds for a CO2-rich atmosphere before ∼ 1.8 billion years ago. Nature 429, 395–399. https://doi.org/10.1038/nature02573
 Show in context
Show in context Siderite (FeCO3) occurs in sediments throughout Earth’s history (Ohmoto et al., 2004) and is a common component in Precambrian iron formations (IFs; Konhauser et al., 2017).
View in article
Phillips, A.A., Ulloa, I., Hyde, E., Agnich, J., Sharpnack, L., O’Malley, K.G., Webb, S.M., Schreiner, K.M., Sheik, C.S., Katsev, S., Raven, M.R. (2023) Organic sulfur from source to sink in low-sulfate Lake Superior. Limnology and Oceanography 68, 2716–2732. https://doi.org/10.1002/lno.12454
 Show in context
Show in context Sulfide abundance is limited by the small sulfate reservoir in the water column available for reduction (Lambrecht et al., 2018), though a contribution from an organic sulfur reservoir cannot be ruled out (e.g., Phillips et al., 2023).
View in article
Riding, R., Liang, L., Fralick, P. (2022) Oxygen-induced chemocline precipitation between Archean Fe-rich and Fe-poor carbonate seas. Precambrian Research 383, 106902. https://doi.org/10.1016/j.precamres.2022.106902
 Show in context
Show in context While dissimilatory reduction of reactive iron (oxyhydr)oxides, such as ferrihydrite, may explain the negative C-isotopic composition and textures of many lacustrine siderite occurrences (Vuillemin et al., 2019), the textures and C-isotope signatures of well-preserved Precambrian IFs are also consistent with precipitation from ferruginous seawater (Beukes et al., 1990; Siahi et al., 2020; Riding et al., 2022), perhaps under a strong hydrothermal influence (Jiang and Tosca, 2019).
View in article
Schiff, S.L., Tsuji, J.M., Wu, L., Venkiteswaran, J.J., Molot, L.A., Elgood, R.J., Paterson, M.J., Neufeld, J.D. (2017) Millions of boreal shield lakes can be used to probe Archean ocean biogeochemistry. Scientific Reports 7, 46708. https://doi.org/10.1038/srep46708
 Show in context
Show in context The novel link between wildfire ash and enhanced siderite precipitation identified here may also imply a new pathway for enhancing carbon sequestration in methane-rich, ferruginous environments that appear to be widespread in boreal shield settings and postglacial lakes (Schiff et al., 2017).
View in article
Siahi, M., Tsikos, H., Rafuza, S., Oonk, P.B.H., Mhlanga, X.R., van Niekerk, D., Mason, P.R.D., Harris, C. (2020) Insights into the processes and controls on the absolute abundance and distribution of manganese in Precambrian iron formations. Precambrian Research 350, 105878. https://doi.org/10.1016/j.precamres.2020.105878
 Show in context
Show in context While dissimilatory reduction of reactive iron (oxyhydr)oxides, such as ferrihydrite, may explain the negative C-isotopic composition and textures of many lacustrine siderite occurrences (Vuillemin et al., 2019), the textures and C-isotope signatures of well-preserved Precambrian IFs are also consistent with precipitation from ferruginous seawater (Beukes et al., 1990; Siahi et al., 2020; Riding et al., 2022), perhaps under a strong hydrothermal influence (Jiang and Tosca, 2019).
View in article
However, the lack of an Mn-oxide flux in CL suggests that a fundamental control on precipitation governs this occurrence of Mn-siderite, with competition from nucleation inhibitors (e.g., Mg(II); Vuillemin et al., 2019) or the differential solubilities of Ca-Mn-Fe carbonates (e.g., Wittkop et al., 2020) offering potential explanations. Thus, the occurrence of Mn-rich siderite in the geologic record (e.g., Siahi et al, 2020; Swanner et al., 2020) may be interpreted to reflect passive incorporation of dissolved Mn into the crystal structure, rather than reduction of Mn-oxides.
View in article
Swanner, E.D., Lambrecht, N., Wittkop, C., Harding, C., Katsev, S., Torgeson, J., Poulton, S.W. (2020) The biogeochemistry of ferruginous lakes and past ferruginous oceans. Earth-Science Reviews 211, 1–43. https://doi.org/10.1016/j.earscirev.2020.103430
 Show in context
Show in context To constrain the conditions that govern natural siderite precipitation, we examined iron (Fe) and manganese (Mn) phases in water column particulates and recent sediments from ferruginous Canyon Lake (CL) in Michigan, USA. Modern ferruginous lakes serve as analogue systems, informing our understanding of biogeochemical dynamics in anoxic Precambrian oceans (Swanner et al., 2020).
View in article
Lacustrine siderites are commonly Mn-substituted (Swanner et al., 2020), possibly linked to the more readily reducible nature of Mn-oxides relative to Fe-(oxyhydr)oxides (Vuillemin et al., 2019).
View in article
However, the lack of an Mn-oxide flux in CL suggests that a fundamental control on precipitation governs this occurrence of Mn-siderite, with competition from nucleation inhibitors (e.g., Mg(II); Vuillemin et al., 2019) or the differential solubilities of Ca-Mn-Fe carbonates (e.g., Wittkop et al., 2020) offering potential explanations. Thus, the occurrence of Mn-rich siderite in the geologic record (e.g., Siahi et al, 2020; Swanner et al., 2020) may be interpreted to reflect passive incorporation of dissolved Mn into the crystal structure, rather than reduction of Mn-oxides.
View in article
Toner, B.M., Santelli, C.M., Marcus, M.A., Wirth, R., Chan, C.S., McCollom, T., Bach, W., Edwards, K.J. (2009) Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochimica et Cosmochimica Acta 73, 388–403. https://doi.org/10.1016/j.gca.2008.09.035
 Show in context
Show in context Best fits from linear combination fitting of Fe extended X-ray absorption fine structure (EXAFS) spectra for the sediment trap samples included, in order of contribution to fits, a biogenic Fe-oxyhydroxide (“Bio Fe”; Toner et al., 2009), siderite, magnetite (Hansel et al., 2005), green rust, and greigite.
View in article
Vuillemin, A., Wirth, R., Kemnitz, H., Schleicher, A.M., Friese, A., Bauer, K.W., Simister, R., Nomosatryo, A., Ordonez, L., Ariztegui, D., Henny, C., Crowe, S.A., Benning, L.G., Kallmeyer, J., Russell, J.M., Bijaksana, S., Vogel, H., Towuti Drilling Project Science Team (2019) Formation of diagenetic siderite in modern ferruginous sediments. Geology 47, 540–544. https://doi.org/10.1130/G46100.1
 Show in context
Show in context While dissimilatory reduction of reactive iron (oxyhydr)oxides, such as ferrihydrite, may explain the negative C-isotopic composition and textures of many lacustrine siderite occurrences (Vuillemin et al., 2019), the textures and C-isotope signatures of well-preserved Precambrian IFs are also consistent with precipitation from ferruginous seawater (Beukes et al., 1990; Siahi et al., 2020; Riding et al., 2022), perhaps under a strong hydrothermal influence (Jiang and Tosca, 2019).
View in article
Carbonate green rust is closely associated with diagenetic siderite precipitation (Vuillemin et al., 2019), and has been previously identified in the sediment traps from ferruginous Lake Matano (Zegeye et al., 2012).
View in article
Lacustrine siderites are commonly Mn-substituted (Swanner et al., 2020), possibly linked to the more readily reducible nature of Mn-oxides relative to Fe-(oxyhydr)oxides (Vuillemin et al., 2019).
View in article
However, the lack of an Mn-oxide flux in CL suggests that a fundamental control on precipitation governs this occurrence of Mn-siderite, with competition from nucleation inhibitors (e.g., Mg(II); Vuillemin et al., 2019) or the differential solubilities of Ca-Mn-Fe carbonates (e.g., Wittkop et al., 2020) offering potential explanations. Thus, the occurrence of Mn-rich siderite in the geologic record (e.g., Siahi et al, 2020; Swanner et al., 2020) may be interpreted to reflect passive incorporation of dissolved Mn into the crystal structure, rather than reduction of Mn-oxides.
View in article
Vuillemin, A., Mayr, C., Schuessler, J.A., Friese, A., Bauer, K.W., Lücke, A., Heuer, V.B., Glombitza, C., Henny, C., von Blanckenburg, F., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J. (2023) A one-million-year isotope record from siderites formed in modern ferruginous sediments. GSA Bulletin 135, 504–522. https://doi.org/10.1130/B36211.1
 Show in context
Show in context A sensitivity analysis adding additional dissolved Fe or DIC raised the saturation index only slightly; the most significant increase in siderite saturation scenarios came from increasing pH from 6.8 (the assumed porewater value) to 7.5 (Fig. 2), consistent with observations from ferruginous porewaters, where alkalinity increases due to organic carbon remineralisation (Vuillemin et al., 2023).
View in article
While porewater diffusion smoothes the concentration profiles of solutes, diagenetic siderite precipitation may occur episodically due to environmental changes, such as lake level fluctuations (e.g., Vuillemin et al., 2023).
View in article
Over longer timescales, porewater alkalinity and pH are known to increase due to continued OM fermentation (Vuillemin et al., 2023), but organic substrates may both inhibit and promote carbonate precipitation, dependent on the composition of functional groups, and the pH of the environment (Dupraz et al., 2009).
View in article
Wiesli, R.A., Beard, B.L., Johnson, C.M. (2004) Experimental determination of Fe isotope fractionation between aqueous Fe(II), siderite and “green rust” in abiotic systems. Chemical Geology 211, 343–362. https://doi.org/10.1016/j.chemgeo.2004.07.002
 Show in context
Show in context Green rust ages to siderite in laboratory experiments (Halevy et al., 2017), but the mechanisms which govern this transformation are not well understood (e.g., Wiesli et al., 2004), though pH is an important control on its behavior (e.g., Guilbaud et al., 2013).
View in article
Wittkop, C., Teranes, J., Lubenow, B., Dean, W.E. (2014) Carbon- and oxygen-stable isotope signatures of methanogenesis, temperature, and water column stratification in Holocene siderite varves. Chemical Geology 389, 153–166. https://doi.org/10.1016/j.chemgeo.2014.09.016
 Show in context
Show in context All observed crystal forms were consistent with siderite crystals grown in lab experiments (e.g., Jiang and Tosca, 2019; Lin et al., 2019) or observed in lacustrine settings (Wittkop et al., 2014).
View in article
Wittkop, C., Swanner, E., Grengs, A., Lambrecht, N., Fakrhaee, M., Myrbo, A., Bray, A., Poulton, S., Katsev, S. (2020) Evaluating a primary carbonate pathway for manganese enrichments in reducing environments. Earth and Planetary Science Letters 538, 116201. https://doi.org/10.1016/j.epsl.2020.116201
 Show in context
Show in context However, the lack of an Mn-oxide flux in CL suggests that a fundamental control on precipitation governs this occurrence of Mn-siderite, with competition from nucleation inhibitors (e.g., Mg(II); Vuillemin et al., 2019) or the differential solubilities of Ca-Mn-Fe carbonates (e.g., Wittkop et al., 2020) offering potential explanations. Thus, the occurrence of Mn-rich siderite in the geologic record (e.g., Siahi et al, 2020; Swanner et al., 2020) may be interpreted to reflect passive incorporation of dissolved Mn into the crystal structure, rather than reduction of Mn-oxides.
View in article
Zegeye, A., Bonneville, S., Benning, L.G., Sturm, A., Fowle, D.A., Jones, C., Canfield, D.E., Ruby, C., MacLean, L.C., Nomosatryo, S., Crowe, S.A., Poulton, S.W. (2012) Green rust formation controls nutrient availability in a ferruginous water column. Geology 40, 599–602. https://doi.org/10.1130/g32959.1
 Show in context
Show in context XANES spectra are consistent with the presence of mixed Fe(II)-Fe(III) phases (see Supplementary Information), perhaps green rust, which has been found in other ferruginous settings (Zegeye et al., 2012).
View in article
Carbonate green rust is closely associated with diagenetic siderite precipitation (Vuillemin et al., 2019), and has been previously identified in the sediment traps from ferruginous Lake Matano (Zegeye et al., 2012).
View in article
Multiple studies highlight the importance of pH in governing both Fe-carbonate and Fe-silicate systems (Halevy et al., 2017; Jiang and Tosca, 2019; Hinz et al., 2021), as well as the evolution of stable phases from green rust precursors (Zegeye et al., 2012).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Study Site
- Methods
- Results
- Discussion
- X-ray Absorption Spectra Supplementary Results and Discussion
- Tables S-1 to S-6
- Figures S-1 to S-7
- Supplementary Information References
Download the Supplementary Information (PDF)
Download Table S-6 (.xlsx)
Figures
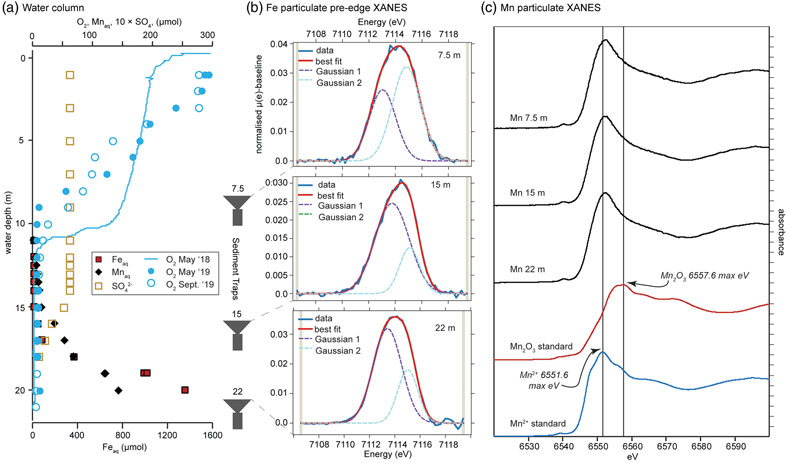
Figure 1 Geochemical and mineralogical characteristics of water column solutes and solid phases. (a) Dissolved solutes in the CL water column. The oxycline (decrease in dissolved oxygen) occurs between 7 and 12 m, and the chemocline (increase in dissolved Fe) occurs between 17 and 19 m. Sediment traps were deployed at 7.5, 15 and 22 m. (b) Sediment trap Fe XANES analyses. Pre-edge centroid energies are ∼7114 eV, consistent with a mixture of Fe(II) and Fe(III). (c) Sediment trap Mn K-edge XANES analyses. Peaks have lower energy (∼6552 eV) compared to Mn-oxides (∼6558 eV), consistent with Mn(II) minerals.
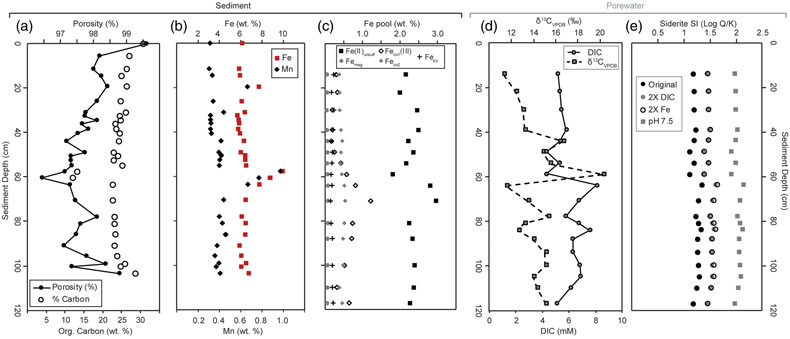
Figure 2 Bulk sediment and porewater geochemistry for the CL sediment core. (a) Porosity and organic carbon content of the sediments. The high organic carbon content of the sediments contributes to their high porosity. (b) Bulk sediment Fe and Mn content showing peaks at ∼60 cm depth, which correspond to the presence of siderite (Fig. 3). (c) Iron speciation analysis demonstrating that much of the sediment Fe is contained in the Fe(II)unsulf pool. (d) Porewater dissolved inorganic carbon (DIC) concentration and isotope composition, showing a positive δ13C signature consistent with the mass balance of depleted carbon lost to methane. (e) Siderite saturation index (SI), which assumes the Fe concentration (1.689 mmol/L) and pH (6.8) of deepest ferruginous water and measured porewater DIC values. Sensitivity scenarios were based on doubling DIC and Fe concentrations, and increasing pH to 7.5.
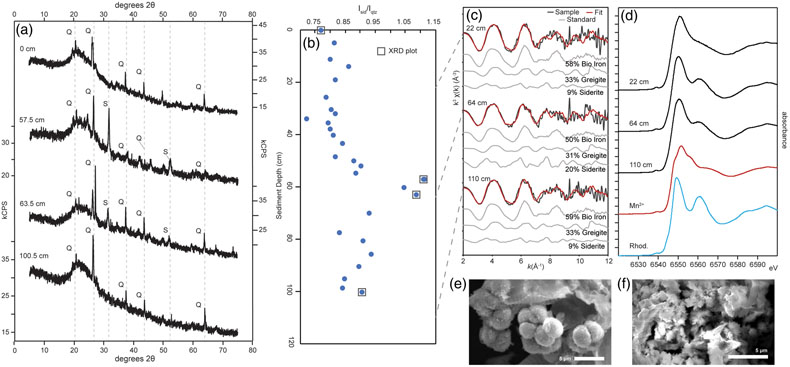
Figure 3 Sediment Fe phases. (a) Example XRD scans showing the emergence of siderite in mid-core depths (57.5 and 63.5 cm scans; Q = quartz peaks, S = siderite peaks). (b) Relative XRD intensity of the siderite peak normalised to the quartz peak (Isid/Iqtz), showing an increase in siderite abundance mid-core. (c) Sediment Fe EXAFS spectra fit with linear contribution, showing values for Bio Fe, greigite, and siderite, with the greatest contribution (20 %) of siderite to fits in the 64 cm samples (Table S-5a). (d) Sediment Mn XANES dominated by reduced Mn(II), and the emergence of a distinctive double-peak of rhodochrosite in the 64 and 110 cm samples. (e, f) Example SEM images of twinned sphere and dumbbell crystal morphologies from the siderite-enriched interval, consistent with experimental precipitates of the mineral.
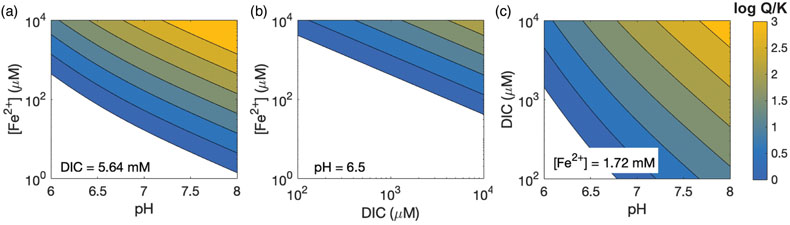
Figure 4 Relative influence of changes in porewater Fe concentration, pH and DIC on the saturation index of siderite. Inputs are based on water chemistry at 20 m, which is assumed to represent a minimum threshold for porewater concentrations. In general, iron concentrations above ∼100 μM seem to be required for siderite precipitation, unless the pH is high.