Correcting for vital effects in coral carbonate using triple oxygen isotopes
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:653Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
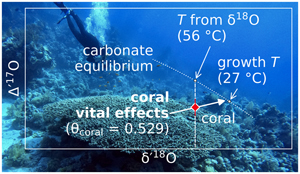
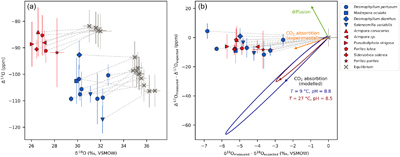 Figure 1 Triple oxygen isotope data for cold- and warm-water corals presented (a) in Δ’17O vs. δ’18O space and (b) as an offset plot. The expected equilibrium positions for each sample (grey crosses) are based on respective seawater δ18O and T estimates. The dashed grey lines represent the effective vital effect slopes, moving away the coral carbonate from equilibrium. The CO2 absorption loops are made using the isoDIC model, simulating conditions resembling the internal calcification environment of cold- and warm-water corals (see Figure S-4 for details). The experimental CO2 absorption vector considers a slope of 0.532, as discussed in the text. The P. porites sample is from Passey et al. (2014). | 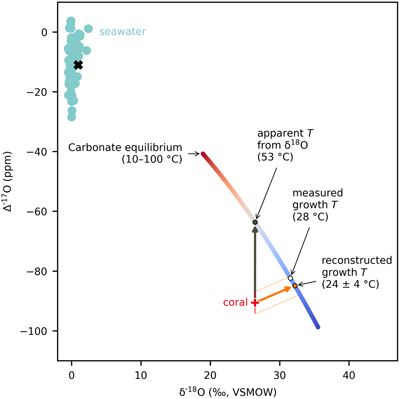 Figure 2 The concept of correcting vital effects using triple oxygen isotopes demonstrated on a warm-water coral (Siderastrea siderea; SK-SA5, red cross). The δ18O of the coral yields an apparent temperature estimate, which is too warm (grey arrow and circle). A back-extrapolation to the equilibrium curve yields a corrected temperature estimate (orange arrow and circle) that is indistinguishable from the actual coral growth temperature (white circle). Error propagation considers the measurement error but not the error of the seawater isotope composition (see text). | 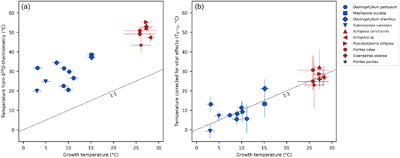 Figure 3 Apparent growth temperatures reconstructed from isotope thermometry compared to actual growth temperatures. (a) Temperature estimates from classical δ18O thermometry are too warm compared to the actual growth temperatures. (b) Temperatures corrected for vital effects using Δ’17O match the actual growth temperatures within error. The P. porites sample is from Passey et al. (2014). |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Classic oxygen isotope thermometry typically assumes that respective carbonates form in thermodynamic equilibrium with the ambient seawater, and thus, the carbonates’ isotope composition only depends on temperature (T) and the composition of the ambient water. In biogenic carbonates, however, vital effects exert additional control on the magnitude of the total fractionation. Vital effects are a combination of kinetic and metabolic effects that influence the isotope composition of many biogenic minerals, including coral carbonate. While coral carbonate serves as a valuable high resolution climate archive, interpreting their isotopic code remains challenging due to the complexity of their biomineralisation mechanism.
It has long been noticed that most modern coral carbonate comprises much lower δ18O and δ13C than expected from equilibrium (Weber and Woodhead, 1972
Weber, J.N., Woodhead, P.M.J. (1972) Temperature dependence of oxygen-18 concentration in reef coral carbonates. Journal of Geophysical Research 77, 463–473. https://doi.org/10.1029/JC077i003p00463
; McConnaughey, 1989aMcConnaughey, T. (1989a) 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns. Geochimica et Cosmochimica Acta 53, 151–162. https://doi.org/10.1016/0016-7037(89)90282-2
; Smith et al., 2000Smith, J.E., Schwarcz, H.P., Risk, M.J., McConnaughey, T.A., Keller, N. (2000) Paleotemperatures from deep-sea corals: Overcoming “vital effects.” Palaios 15, 25–32. https://doi.org/10.1669/0883-1351(2000)015<0025:pfdsco>2.0.co;2
; Adkins et al., 2003Adkins, J.F., Boyle, E.A., Curry, W.B., Lutringer, A. (2003) Stable isotopes in deep-sea corals and a new mechanism for “vital effects.” Geochimica et Cosmochimica Acta 67, 1129–1143. https://doi.org/10.1016/s0016-7037(02)01203-6
). Temperature estimates from equilibrium δ18O–T calibration curves indicate higher T (by >10 °C) compared to actual growth T. Although species-specific δ18O–T calibrations can partly account for vital effects (Weber and Woodhead, 1972Weber, J.N., Woodhead, P.M.J. (1972) Temperature dependence of oxygen-18 concentration in reef coral carbonates. Journal of Geophysical Research 77, 463–473. https://doi.org/10.1029/JC077i003p00463
; Smith et al., 2000Smith, J.E., Schwarcz, H.P., Risk, M.J., McConnaughey, T.A., Keller, N. (2000) Paleotemperatures from deep-sea corals: Overcoming “vital effects.” Palaios 15, 25–32. https://doi.org/10.1669/0883-1351(2000)015<0025:pfdsco>2.0.co;2
), the reconstructed temperatures can remain inaccurate (Marali et al., 2013Marali, S., Wisshak, M., López Correa, M., Freiwald, A. (2013) Skeletal microstructure and stable isotope signature of three bathyal solitary cold-water corals from the Azores. Palaeogeography, Palaeoclimatology, Palaeoecology 373, 25–38. https://doi.org/10.1016/j.palaeo.2012.06.017
). Likewise, coral growth T determined using clumped isotope thermometry (Δ47) is also biased by >10 °C, but towards lower T (Thiagarajan et al., 2011Thiagarajan, N., Adkins, J., Eiler, J. (2011) Carbonate clumped isotope thermometry of deep-sea corals and implications for vital effects. Geochimica et Cosmochimica Acta 75, 4416–4425. https://doi.org/10.1016/j.gca.2011.05.004
; Saenger et al., 2012Saenger, C., Affek, H.P., Felis, T., Thiagarajan, N., Lough, J.M., Holcomb, M. (2012) Carbonate clumped isotope variability in shallow water corals: Temperature dependence and growth-related vital effects. Geochimica et Cosmochimica Acta 99, 224–242. https://doi.org/10.1016/j.gca.2012.09.035
). Accurate coral thermometry will only become feasible if the isotopic bias is accounted for (e.g., Davies et al., 2022Davies, A.J., Guo, W., Bernecker, M., Tagliavento, M., Raddatz, J., Gischler, E., Flögel, S., Fiebig, J. (2022) Dual clumped isotope thermometry of coral carbonate. Geochimica et Cosmochimica Acta 338, 66–78. https://doi.org/10.1016/j.gca.2022.10.015
).Dual clumped isotope thermometry (Δ47 and Δ48) is a new approach currently explored to extract quantitative temperature information from carbonate samples affected by kinetic effects (Bajnai et al., 2020
Bajnai, D., Guo, W., Spötl, C., Coplen, T.B., Methner, K., Löffler, N., Krsnik, E., Gischler, E., Hansen, M., Henkel, D., Price, G.D., Raddatz, J., Scholz, D., Fiebig, J. (2020) Dual clumped isotope thermometry resolves kinetic biases in carbonate formation temperatures. Nature Communications 11, 4005. https://doi.org/10.1038/s41467-020-17501-0
). For coral carbonates, Davies et al. (2022)Davies, A.J., Guo, W., Bernecker, M., Tagliavento, M., Raddatz, J., Gischler, E., Flögel, S., Fiebig, J. (2022) Dual clumped isotope thermometry of coral carbonate. Geochimica et Cosmochimica Acta 338, 66–78. https://doi.org/10.1016/j.gca.2022.10.015
show systematic deviations from clumped isotope equilibrium along slopes characteristic of the underlying kinetic mechanisms. Apparent temperatures are corrected by back-extrapolating to the equilibrium line, providing accurate growth temperature reconstructions with a precision better than ±3 °C (1 s.e.). Here, we propose a similar concept for attaining accurate carbonate growth temperatures but using triple oxygen isotopes.Triple oxygen isotope analyses involve the simultaneous analysis of δ17O and δ18O. Most Earth surface processes result in mass-dependent fractionation, which leads to a nearly perfect correlation between δ17O and δ18O values with a slope of approximately 0.5. The mass-dependent fractionation between two phases is described by Equation 1, where θ represents the triple oxygen isotope exponent characteristic of the process and temperature, and α is the equilibrium isotope fractionation factor:
Eq. 1
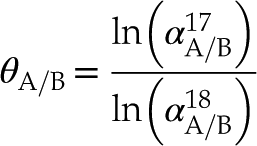
Because the θ values of mass-dependent processes vary within a small range, the Δ’17O notation (in ppm) is used to visualise differences between them:
Eq. 2
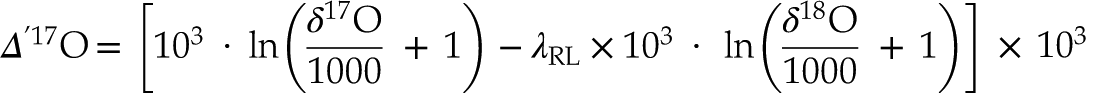
Here, we chose 0.528 for λRL (Luz and Barkan, 2010
Luz, B., Barkan, E. (2010) Variations of 17O/16O and 18O/16O in meteoric waters. Geochimica et Cosmochimica Acta 74, 6276–6286. https://doi.org/10.1016/j.gca.2010.08.016
). The logarithmic transformation of the δ-values in Equation 2 is commonly abbreviated as δ’. All oxygen isotope values in this study are reported on the VSMOW (Vienna Standard Mean Ocean Water) scale.Carbonates forming in thermodynamic equilibrium must fall on the equilibrium curve in Δ’17O vs. δ’18O space. Kinetic processes simultaneously drive δ18O and Δ’17O away from this curve. The slope (or θ) of any given kinetic process is a physical constant. Yet, apparent slopes may result from a combination of processes (Guo and Zhou, 2019
Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
; Wostbrock et al., 2020aWostbrock, J.A.G., Brand, U., Coplen, T.B., Swart, P.K., Carlson, S.J., Brearley, A.J., Sharp, Z.D. (2020a) Calibration of carbonate-water triple oxygen isotope fractionation: Seeing through diagenesis in ancient carbonates. Geochimica et Cosmochimica Acta 288, 369–388. https://doi.org/10.1016/j.gca.2020.07.045
; Herwartz, 2021Herwartz, D. (2021) Triple oxygen isotope variations in Earth’s crust. Reviews in Mineralogy and Geochemistry 86, 291–322. https://doi.org/10.2138/rmg.2021.86.09
; Bajnai et al., 2024Bajnai, D., Cao, X., Klipsch, S., Pack, A., Herwartz, D. (2024) Triple oxygen isotope systematics of CO2 hydroxylation. Chemical Geology 654, 122059. https://doi.org/10.1016/j.chemgeo.2024.122059
). Constraining an empirical slope for the vital effects in corals and using it for back-extrapolation to the equilibrium curve makes accurate paleotemperature reconstructions feasible. In addition, the kinetic slope in triple oxygen isotope space can help in the mechanistic understanding of the underlying vital effects.top
Coral Triple Oxygen Isotope Analyses
We analysed the triple oxygen isotope composition of 17 modern scleractinian (aragonitic) corals, representing four cold-water and five warm-water species. We retrieved seawater temperature and δ18Osw for each sample from a global gridded database (Breitkreuz et al., 2018
Breitkreuz, C., Paul, A., Kurahashi‐Nakamura, T., Losch, M., Schulz, M. (2018) A dynamical reconstruction of the global monthly mean oxygen isotopic composition of seawater. Journal of Geophysical Research: Oceans 123, 7206–7219. https://doi.org/10.1029/2018JC014300
), using the interpolation method outlined in Daëron and Gray (2023)Daëron, M., Gray, W.R. (2023) Revisiting oxygen‐18 and clumped isotopes in planktic and benthic foraminifera. Paleoceanography and Paleoclimatology 38, e2023PA004660. https://doi.org/10.1029/2023PA004660
. For some samples, in situ measured T and δ18Osw values were also available, which we used to assess the accuracy of the database estimates. The database and in situ measured values showed close agreement, typically within ±1 °C and ±0.2 ‰ (Fig. S–1). We regarded these average differences as indicative of the precision of the database and additionally factored in the interpolation error (on average ±1 °C and ±0.2 ‰) to obtain the final uncertainty of the database’s seawater T and δ18O estimates. We used the database values and their uncertainties to calculate the expected equilibrium δ18O and Δ’17O values. Seawater Δ’17O values were not directly measured but universally assumed to be Δ’17Osw = −11 ± 6 ppm, which covers most modern seawater data (Luz and Barkan, 2010Luz, B., Barkan, E. (2010) Variations of 17O/16O and 18O/16O in meteoric waters. Geochimica et Cosmochimica Acta 74, 6276–6286. https://doi.org/10.1016/j.gca.2010.08.016
; Lin et al., 2021Lin, Y., Wu, N., Ta, K., Landais, A., Peng, X. (2021) Triple oxygen isotopic compositions of ocean water from the Mariana Trench. ACS Earth and Space Chemistry 5, 3087–3096. https://doi.org/10.1021/acsearthspacechem.1c00187
). Information on the samples is summarised in Tables S-1 and S-3.
Figure 1 Triple oxygen isotope data for cold- and warm-water corals presented (a) in Δ’17O vs. δ’18O space and (b) as an offset plot. The expected equilibrium positions for each sample (grey crosses) are based on respective seawater δ18O and T estimates. The dashed grey lines represent the effective vital effect slopes, moving away the coral carbonate from equilibrium. The CO2 absorption loops are made using the isoDIC model, simulating conditions resembling the internal calcification environment of cold- and warm-water corals (see Figure S-4 for details). The experimental CO2 absorption vector considers a slope of 0.532, as discussed in the text. The P. porites sample is from Passey et al. (2014)
Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
.The triple oxygen isotope analyses were conducted at the University of Göttingen using a tuneable infrared laser direct absorption spectrometer (TILDAS), detailed in Bajnai et al. (2023)
Bajnai, D., Pack, A., Arduin Rode, F., Seefeld, M., Surma, J., Di Rocco, T. (2023) A dual inlet system for laser spectroscopy of triple oxygen isotopes in carbonate-derived and air CO2. Geochemistry, Geophysics, Geosystems 24, e2023GC010976. https://doi.org/10.1029/2023GC010976
. The CO2 analyte gas was derived from acid digestion at 25 °C. Carbonate-derived CO2 data were normalised relative to two in-house standards: heavy CO2 and light CO2 measured alongside the samples, as detailed in Bajnai et al. (2024)Bajnai, D., Cao, X., Klipsch, S., Pack, A., Herwartz, D. (2024) Triple oxygen isotope systematics of CO2 hydroxylation. Chemical Geology 654, 122059. https://doi.org/10.1016/j.chemgeo.2024.122059
(Figs. S–2, S–3). Briefly, the triple oxygen isotope composition of these two reference gases was determined relative to NBS-18 and IAEA-603 measurements and the values reported by Wostbrock et al. (2020b)Wostbrock, J.A.G., Cano, E.J., Sharp, Z.D. (2020b) An internally consistent triple oxygen isotope calibration of standards for silicates, carbonates and air relative to VSMOW2 and SLAP2. Chemical Geology 533, 119432. https://doi.org/10.1016/j.chemgeo.2019.119432
. A 25 °C acid fractionation factor was applied to the CO2 data to calculate the isotope composition of the coral aragonite (i.e. 18α25°C-acid-CO2/aragonite = 1.01063 (Kim et al., 2007Kim, S.-T., Mucci, A., Taylor, B.E. (2007) Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75 °C: Revisited. Chemical Geology 246, 135–146. https://doi.org/10.1016/j.chemgeo.2007.08.005
) and θ25°C-acid-CO2/carbonate = 0.523 (Wostbrock et al., 2020bWostbrock, J.A.G., Cano, E.J., Sharp, Z.D. (2020b) An internally consistent triple oxygen isotope calibration of standards for silicates, carbonates and air relative to VSMOW2 and SLAP2. Chemical Geology 533, 119432. https://doi.org/10.1016/j.chemgeo.2019.119432
)).In our final dataset, we included the warm-water coral data point reported by Passey et al. (2014)
Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
(sample JBC03, Porites porites). To normalise this data point to our carbonate reference frame, we followed the steps outlined in Huth et al. (2022)Huth, T.E., Passey, B.H., Cole, J.E., Lachniet, M.S., McGee, D., Denniston, R.F., Truebe, S., Levin, N.E. (2022) A framework for triple oxygen isotopes in speleothem paleoclimatology. Geochimica et Cosmochimica Acta 319, 191–219. https://doi.org/10.1016/j.gca.2021.11.002
. Specifically, we applied empirical fractionation factors to the reported O2/CaCO3 values to account for fractionations induced by acid digestion and fluorination (18αempirical = 1.00812 and θempirical = 0.5234). Additionally, to account for the difference in the acid fractionation factors between aragonite and calcite, we shifted the corrected δ18O value by 0.41 ‰ (Kim et al., 2007Kim, S.-T., Mucci, A., Taylor, B.E. (2007) Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75 °C: Revisited. Chemical Geology 246, 135–146. https://doi.org/10.1016/j.chemgeo.2007.08.005
). The respective seawater temperature and δ18Osw values were determined in the same manner as for our samples.top
Results
The results of the triple oxygen isotope analyses are presented in Tables S-2 and S-3 and shown in Figure 1a. The long-term repeatability of the Δ’17O measurements of the heavy CO2 and light CO2 reference gases was 8–12 ppm. For the coral samples, the standard deviation of 2 to 7 replicate analyses was generally ±5 ppm for Δ’17O, while for δ18O it was ±0.1 ‰.
top
Discussion
Seeing through vital effects. We chose the theoretical aragonite calibration of Guo and Zhou (2019)
Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
as representative of thermodynamic equilibrium. In triple oxygen isotope space, all cold- and warm-water corals fall below their respective equilibrium lines (Figs. 1, 2). These offsets indicate that these corals did not form carbonate in equilibrium with seawater. Thus, respective δ18O-temperature estimates are inaccurate (Fig. 3a) and attaining true T requires a correction for vital effects.
Figure 2 The concept of correcting vital effects using triple oxygen isotopes demonstrated on a warm-water coral (Siderastrea siderea; SK-SA5, red cross). The δ18O of the coral yields an apparent temperature estimate, which is too warm (grey arrow and circle). A back-extrapolation to the equilibrium curve yields a corrected temperature estimate (orange arrow and circle) that is indistinguishable from the actual coral growth temperature (white circle). Error propagation considers the measurement error but not the error of the seawater isotope composition (see text).

Figure 3 Apparent growth temperatures reconstructed from isotope thermometry compared to actual growth temperatures. (a) Temperature estimates from classical δ18O thermometry are too warm compared to the actual growth temperatures. (b) Temperatures corrected for vital effects using Δ’17O match the actual growth temperatures within error. The P. porites sample is from Passey et al. (2014)
Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
.For each coral, we calculated the effective vital effect slope (θcoral) in triple oxygen isotope space, by connecting the measured and the expected equilibrium values (Fig. 1). The θcoral values ranged between 0.527 and 0.531, with a mean of 0.529 and a standard deviation of ±0.001 ppm. To evaluate the accuracy of each θcoral estimate, we used Monte Carlo error propagation, considering measurement errors and growth parameter uncertainties. The error of the individual θcoral values was approximately ±0.002.
There was no resolvable difference in the vital effect slopes between species or among cold- and warm-water corals (Fig. S-5). In contrast, in dual clumped isotope space, cold- and warm-water corals exhibit distinct kinetic trajectories, explained by differences in the calcification parameters, such as the carbonic anhydrase activity, growth temperature, and the pH of the calcifying fluid (Davies et al., 2022
Davies, A.J., Guo, W., Bernecker, M., Tagliavento, M., Raddatz, J., Gischler, E., Flögel, S., Fiebig, J. (2022) Dual clumped isotope thermometry of coral carbonate. Geochimica et Cosmochimica Acta 338, 66–78. https://doi.org/10.1016/j.gca.2022.10.015
). Variations in these calcification parameters have a smaller impact on the kinetic slope in triple oxygen isotope space because the offset in δ18O is an order of magnitude larger than in Δ’17O (Figs. 1b, S-4). The vital effect slopes are, thus, primarily driven by the kinetic fractionation exerting the largest effect while species-specific variations in calcification parameters appear to be less important.To calculate growth temperatures corrected for vital effects, we back-extrapolated individual data points to their respective equilibrium line using unique vital effect slopes (TΔ’17O; Figs. 2, 3b, S-6). Rather than employing the previously established mean θcoral, we calculated a distinct slope for each coral, derived from the data of all other corals except the one under analysis, thereby avoiding circularity in our calculations (Table S-3). The unique slopes were indistinguishable from the mean θcoral of 0.529 within ±0.001. The reconstructed TΔ’17O match the respective database T generally within ±3 °C (Fig. 3b). Considering the uncertainty of the isotope analyses and the seawater parameters, the accuracy of TΔ’17O is much larger, ca. ±10 °C. Sensitivity analyses show that every 1 ppm decrease in Δ’17O errors corresponds to an approximately 1 °C reduction in the TΔ’17O errors. Therefore, the ±6 ppm uncertainty in the seawater Δ’17O alone already accounts for an uncertainty over ±5 °C. Currently, only limited data exist for the triple oxygen isotope composition of seawater (Luz and Barkan, 2010
Luz, B., Barkan, E. (2010) Variations of 17O/16O and 18O/16O in meteoric waters. Geochimica et Cosmochimica Acta 74, 6276–6286. https://doi.org/10.1016/j.gca.2010.08.016
; Lin et al., 2021Lin, Y., Wu, N., Ta, K., Landais, A., Peng, X. (2021) Triple oxygen isotopic compositions of ocean water from the Mariana Trench. ACS Earth and Space Chemistry 5, 3087–3096. https://doi.org/10.1021/acsearthspacechem.1c00187
). The inherent uncertainty of global variations in seawater Δ’17O is reduced when focusing on individual locations and effectively cancels out when reconstructing relative temperature shifts. Disregarding the uncertainty in seawater Δ’17O improves the accuracy of TΔ’17O to ±5 °C.Similar to Wostbrock et al. (2020a)
Wostbrock, J.A.G., Brand, U., Coplen, T.B., Swart, P.K., Carlson, S.J., Brearley, A.J., Sharp, Z.D. (2020a) Calibration of carbonate-water triple oxygen isotope fractionation: Seeing through diagenesis in ancient carbonates. Geochimica et Cosmochimica Acta 288, 369–388. https://doi.org/10.1016/j.gca.2020.07.045
, who explain how to ‘see through’ diagenesis using triple oxygen isotopes, the correction scheme outlined above allows us to see through vital effects. Importantly, a consistent but not necessarily “correct” reference frame is required for reconstructing growth temperatures. That is, the choice of equilibrium calibration does not significantly affect the TΔ’17O estimates because the empirical slope obtained for a particular equilibrium curve is later used to back-extrapolate to the same curve, resulting in identical TΔ’17O estimates. The lack of variability in the θcoral between species means that triple oxygen isotopes can be well suited for correcting vital effects in extinct organisms. This is not limited by the uncertainties in past seawater Δ’17O values because it was modelled to change only within ±10 ppm during the Phanerozoic (Guo et al., 2022Guo, M., Wostbrock, J.A.G., Planavsky, N.J., Korenaga, J. (2022) Reconstructing seawater δ18O and Δ′17O values with solid Earth system evolution. Earth and Planetary Science Letters 592, 117637. https://doi.org/10.1016/j.epsl.2022.117637
).Looking at vital effects. Coral calcification models aim to reproduce observed δ18O–δ13C covariations in carbonates and mainly consider: 1) CO2 absorption leading to disequilibrium between H2O and DIC in the calcifying fluid; 2) isotope effects at the mineral water interface; and 3) CO2 (aq) diffusion across the cell membranes. Besides the diffusive flux of CO2 (aq) through the lipid membrane, carbon is also sourced directly from seawater. The calcifying fluid is not perfectly isolated from seawater but isolated enough to significantly up-regulate pH via the enzyme Ca-ATPase that pumps Ca2+ into and H+ out of the calcifying fluid (Rollion-Bard et al., 2003
Rollion-Bard, C., Chaussidon, M., France-Lanord, C. (2003) pH control on oxygen isotopic composition of symbiotic corals. Earth and Planetary Science Letters 215, 275–288. https://doi.org/10.1016/S0012-821X(03)00391-1
). Another prominent enzyme, carbonic anhydrase, accelerates CO2 (aq)–HCO3− interconversion. Respective enzyme activities moderate both the carbonate precipitation rates and the isotope exchange rates. Biomineralisation models focusing on isotope effects reproduce the observed δ18O–δ13C covariations (McConnaughey, 1989bMcConnaughey, T. (1989b) 13C and 18O isotopic disequilibrium in biological carbonates: II. In vitro simulation of kinetic isotope effects. Geochimica et Cosmochimica Acta 53, 163–171. https://doi.org/10.1016/0016-7037(89)90283-4
; Adkins et al., 2003Adkins, J.F., Boyle, E.A., Curry, W.B., Lutringer, A. (2003) Stable isotopes in deep-sea corals and a new mechanism for “vital effects.” Geochimica et Cosmochimica Acta 67, 1129–1143. https://doi.org/10.1016/s0016-7037(02)01203-6
; Chen et al., 2018Chen, S., Gagnon, A.C., Adkins, J.F. (2018) Carbonic anhydrase, coral calcification and a new model of stable isotope vital effects. Geochimica et Cosmochimica Acta 236, 179–197. https://doi.org/10.1016/j.gca.2018.02.032
). Davies et al. (2022)Davies, A.J., Guo, W., Bernecker, M., Tagliavento, M., Raddatz, J., Gischler, E., Flögel, S., Fiebig, J. (2022) Dual clumped isotope thermometry of coral carbonate. Geochimica et Cosmochimica Acta 338, 66–78. https://doi.org/10.1016/j.gca.2022.10.015
compare dual clumped isotope data of corals with a respective theoretical CO2 absorption model and show that certain mechanisms (e.g., DIC speciation effects — the classic pH effect) are inconsistent with observed dual clumped isotope fractionation trends. Although contributions from kinetic isotope fractionation at the carbonate–water interface cannot be excluded (Watson, 2004Watson, E.B. (2004) A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochimica et Cosmochimica Acta 68, 1473–1488. https://doi.org/10.1016/j.gca.2003.10.003
; Watkins et al., 2014Watkins, J.M., Hunt, J.D., Ryerson, F.J., DePaolo, D.J. (2014) The influence of temperature, pH, and growth rate on the δ18O composition of inorganically precipitated calcite. Earth and Planetary Science Letters 404, 332–343. https://doi.org/10.1016/j.epsl.2014.07.036
), the almost perfect fit of the theoretical model with analytical results led these authors to suggest that CO2 absorption is the main mechanism inducing kinetic effects. Guo and Zhou (2019)Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
also attribute the offset from the expected equilibrium in the warm water coral data of Passey et al. (2014)Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
to CO2 absorption kinetics. The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989aMcConnaughey, T. (1989a) 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns. Geochimica et Cosmochimica Acta 53, 151–162. https://doi.org/10.1016/0016-7037(89)90282-2
, 1989bMcConnaughey, T. (1989b) 13C and 18O isotopic disequilibrium in biological carbonates: II. In vitro simulation of kinetic isotope effects. Geochimica et Cosmochimica Acta 53, 163–171. https://doi.org/10.1016/0016-7037(89)90283-4
; Adkins et al., 2003Adkins, J.F., Boyle, E.A., Curry, W.B., Lutringer, A. (2003) Stable isotopes in deep-sea corals and a new mechanism for “vital effects.” Geochimica et Cosmochimica Acta 67, 1129–1143. https://doi.org/10.1016/s0016-7037(02)01203-6
; Thiagarajan et al., 2011Thiagarajan, N., Adkins, J., Eiler, J. (2011) Carbonate clumped isotope thermometry of deep-sea corals and implications for vital effects. Geochimica et Cosmochimica Acta 75, 4416–4425. https://doi.org/10.1016/j.gca.2011.05.004
; Saenger et al., 2012Saenger, C., Affek, H.P., Felis, T., Thiagarajan, N., Lough, J.M., Holcomb, M. (2012) Carbonate clumped isotope variability in shallow water corals: Temperature dependence and growth-related vital effects. Geochimica et Cosmochimica Acta 99, 224–242. https://doi.org/10.1016/j.gca.2012.09.035
; Chen et al., 2018Chen, S., Gagnon, A.C., Adkins, J.F. (2018) Carbonic anhydrase, coral calcification and a new model of stable isotope vital effects. Geochimica et Cosmochimica Acta 236, 179–197. https://doi.org/10.1016/j.gca.2018.02.032
; Guo and Zhou, 2019Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
; Davies et al., 2023Davies, A.J., Brand, U., Tagliavento, M., Bitner, M.A., Bajnai, D., Staudigel, P., Bernecker, M., Fiebig, J. (2023) Isotopic disequilibrium in brachiopods disentangled with dual clumped isotope thermometry. Geochimica et Cosmochimica Acta 359, 135–147. https://doi.org/10.1016/j.gca.2023.08.005
), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.The choice of equilibrium calibration affects the θcoral values and, consequently, how these slopes are interpreted with respect to vital effects. The mean θcoral value obtained using the Guo and Zhou (2019)
Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
aragonite equilibrium calibration is 0.529 ± 0.001 (within a range of 0.527 to 0.531), reflecting the net effect of several superimposed processes. Using other calibrations affects the mean θcoral estimates but does not affect the main conclusions drawn here (see Supplementary Information). Although a consistently used reference frame has no impact on the reconstructed TΔ’17O, only the correct reference frame will provide accurate empirical triple oxygen isotope slope values required to interpret the biomineralisation mechanisms correctly.Guo and Zhou (2019)
Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
presented theoretical slopes for CO2 absorption (θabsorption = 0.538 to 0.541 at pH between 8.4 and 9, respectively), which differ significantly from our observed θcoral = 0.529 ± 0.001. Assuming that normalisation issues are insignificant, this discrepancy implies that vital effects in corals are not dominated by CO2 absorption. Shallower slopes hint towards considerable proportions of CO2 (aq) diffusion, which follows much shallower slopes (i.e. θdiffusion = 0.506; Herwartz, 2021Herwartz, D. (2021) Triple oxygen isotope variations in Earth’s crust. Reviews in Mineralogy and Geochemistry 86, 291–322. https://doi.org/10.2138/rmg.2021.86.09
). Diffusion has been suggested to be an important contributor to the vital effects in brachiopods (Davies et al., 2023Davies, A.J., Brand, U., Tagliavento, M., Bitner, M.A., Bajnai, D., Staudigel, P., Bernecker, M., Fiebig, J. (2023) Isotopic disequilibrium in brachiopods disentangled with dual clumped isotope thermometry. Geochimica et Cosmochimica Acta 359, 135–147. https://doi.org/10.1016/j.gca.2023.08.005
). The theoretical θabsorption range of 0.538 to 0.541 could imply that 34–39 % of the total vital effect is induced by diffusion. An alternative explanation for the observed discrepancy between the observed vital effect slopes and CO2 absorption in triple oxygen isotope space is that the theoretical CO2 absorption slopes are inaccurate.The slope for the hydroxylation reaction (CO2 + OH− −> HCO3−) hinges on the triple oxygen isotope compositions of the reacting OH− and CO2 (aq). The model of Guo and Zhou (2019)
Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
considers parameters derived from experimental fractionation factors, which are inconsistent with the most recent theoretical models. Experimental work by Bajnai et al. (2024Bajnai, D., Cao, X., Klipsch, S., Pack, A., Herwartz, D. (2024) Triple oxygen isotope systematics of CO2 hydroxylation. Chemical Geology 654, 122059. https://doi.org/10.1016/j.chemgeo.2024.122059
) recently constrained the Δ’17O values of the two reacting species in CO2 absorption and yielded consistent results with theory. Accordingly, these authors’ revised estimates for hydroxylation and hydration in seawater are θhydroxylation ≈ 0.532 and θhydration ≈ 0.531 (Fig. S-7). The corrected estimate for the CO2 absorption slope at pH 8 is 0.532, which is more consistent with the observed empirical slopes but still allows for some superimposed diffusion of up to 19 % of the total vital effect.From a triple oxygen isotope perspective, it is not possible to distinguish between the diffusion of CO2 (aq) through the membrane and diffusion happening at the mineral–water interface. It is worth pointing out that surface effects are not necessarily related to diffusion (Watson, 2004
Watson, E.B. (2004) A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochimica et Cosmochimica Acta 68, 1473–1488. https://doi.org/10.1016/j.gca.2003.10.003
; Watkins et al., 2014Watkins, J.M., Hunt, J.D., Ryerson, F.J., DePaolo, D.J. (2014) The influence of temperature, pH, and growth rate on the δ18O composition of inorganically precipitated calcite. Earth and Planetary Science Letters 404, 332–343. https://doi.org/10.1016/j.epsl.2014.07.036
). The θ values for other processes, such as entrapment or the inheritance of isotope signals from amorphous calcium carbonate precursors, are presently unknown and may significantly contribute to coral vital effects without necessarily affecting the observed slope.Outlook. Robust estimates for the slopes in triple oxygen isotope space for all potentially relevant kinetic processes will allow the evaluation of coral calcification models and corroborate the theoretical triple oxygen isotope framework. Theoretical approaches can calculate full thermodynamic equilibrium (e.g., Guo and Zhou, 2019
Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
), but their confirmation using natural samples remains challenging. This is at least partly related to the diverse suite of methods applied for carbonate Δ’17O analyses (Passey et al., 2014Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
; Bergel et al., 2020Bergel, S.J., Barkan, E., Stein, M., Affek, H.P. (2020) Carbonate 17Oexcess as a paleo-hydrology proxy: Triple oxygen isotope fractionation between H2O and biogenic aragonite, derived from freshwater mollusks. Geochimica et Cosmochimica Acta 275, 36–47. https://doi.org/10.1016/j.gca.2020.02.005
; Wostbrock et al., 2020aWostbrock, J.A.G., Brand, U., Coplen, T.B., Swart, P.K., Carlson, S.J., Brearley, A.J., Sharp, Z.D. (2020a) Calibration of carbonate-water triple oxygen isotope fractionation: Seeing through diagenesis in ancient carbonates. Geochimica et Cosmochimica Acta 288, 369–388. https://doi.org/10.1016/j.gca.2020.07.045
, 2020bWostbrock, J.A.G., Cano, E.J., Sharp, Z.D. (2020b) An internally consistent triple oxygen isotope calibration of standards for silicates, carbonates and air relative to VSMOW2 and SLAP2. Chemical Geology 533, 119432. https://doi.org/10.1016/j.chemgeo.2019.119432
; Huth et al., 2022Huth, T.E., Passey, B.H., Cole, J.E., Lachniet, M.S., McGee, D., Denniston, R.F., Truebe, S., Levin, N.E. (2022) A framework for triple oxygen isotopes in speleothem paleoclimatology. Geochimica et Cosmochimica Acta 319, 191–219. https://doi.org/10.1016/j.gca.2021.11.002
). One way to test equilibrium calibrations is to check if experimental data referenced to them reproduce theoretical θKIE calculations. Also, compared to equilibrium models, kinetic isotope models work with far more parameters. In turn, kinetic models provide information on parameters such as precipitation rates and pH, while equilibrium models “only” provide temperature information. The concept of seeing through vital effects aims to reconstruct equilibrium conditions and, consequently, palaeotemperatures. However, triple oxygen isotopes also allow us to look at vital effects and help constrain calcification parameters beyond temperature. By combining triple oxygen isotopes with other isotope systems, such as carbon isotopes and dual clumped isotopes, along with elemental concentrations, it becomes possible to constrain the numerous variables in kinetic models.top
Acknowledgements
The authors are grateful to A. Freiwald, D. Tracey, S. Mills, and G. Bernasconi-Green for providing cold-water coral samples to this study, and J. Fiebig for comments on an earlier version of this manuscript. The S. variabilis sample was collected by NIWA (New Zealand) as part of the ‘Impact of resource use on vulnerable deep-sea communities’ project, funded by the MBIA with support from the Ministry for Primary Industries and NIWA’s ‘Enabling the Management of Marine Mining’ project. S. Klipsch and D. Bajnai contributed equally to this manuscript. This research was funded by the DFG via grants HE 6357/2-2 and HE 6357/4-1.
Editor: Gavin Foster
top
References
Adkins, J.F., Boyle, E.A., Curry, W.B., Lutringer, A. (2003) Stable isotopes in deep-sea corals and a new mechanism for “vital effects.” Geochimica et Cosmochimica Acta 67, 1129–1143. https://doi.org/10.1016/s0016-7037(02)01203-6
 Show in context
Show in context It has long been noticed that most modern coral carbonate comprises much lower δ18O and δ13C than expected from equilibrium (Weber and Woodhead, 1972; McConnaughey, 1989a; Smith et al., 2000; Adkins et al., 2003).
View in article
Biomineralisation models focusing on isotope effects reproduce the observed δ18O–δ13C covariations (McConnaughey, 1989b; Adkins et al., 2003; Chen et al., 2018).
View in article
The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
Bajnai, D., Cao, X., Klipsch, S., Pack, A., Herwartz, D. (2024) Triple oxygen isotope systematics of CO2 hydroxylation. Chemical Geology 654, 122059. https://doi.org/10.1016/j.chemgeo.2024.122059
 Show in context
Show in context Yet, apparent slopes may result from a combination of processes (Guo and Zhou, 2019; Wostbrock et al., 2020a; Herwartz, 2021; Bajnai et al., 2024).
View in article
Carbonate-derived CO2 data were normalised relative to two in-house standards: heavy CO2 and light CO2 measured alongside the samples, as detailed in Bajnai et al. (2024) (Figs. S–2, S–3).
View in article
Experimental work by Bajnai et al. (2024) recently constrained the Δ’17O values of the two reacting species in CO2 absorption and yielded consistent results with theory.
View in article
Bajnai, D., Guo, W., Spötl, C., Coplen, T.B., Methner, K., Löffler, N., Krsnik, E., Gischler, E., Hansen, M., Henkel, D., Price, G.D., Raddatz, J., Scholz, D., Fiebig, J. (2020) Dual clumped isotope thermometry resolves kinetic biases in carbonate formation temperatures. Nature Communications 11, 4005. https://doi.org/10.1038/s41467-020-17501-0
 Show in context
Show in context Dual clumped isotope thermometry (Δ47 and Δ48) is a new approach currently explored to extract quantitative temperature information from carbonate samples affected by kinetic effects (Bajnai et al., 2020).
View in article
Bajnai, D., Pack, A., Arduin Rode, F., Seefeld, M., Surma, J., Di Rocco, T. (2023) A dual inlet system for laser spectroscopy of triple oxygen isotopes in carbonate-derived and air CO2. Geochemistry, Geophysics, Geosystems 24, e2023GC010976. https://doi.org/10.1029/2023GC010976
 Show in context
Show in context The triple oxygen isotope analyses were conducted at the University of Göttingen using a tuneable infrared laser direct absorption spectrometer (TILDAS), detailed in Bajnai et al. (2023).
View in article
Bergel, S.J., Barkan, E., Stein, M., Affek, H.P. (2020) Carbonate 17Oexcess as a paleo-hydrology proxy: Triple oxygen isotope fractionation between H2O and biogenic aragonite, derived from freshwater mollusks. Geochimica et Cosmochimica Acta 275, 36–47. https://doi.org/10.1016/j.gca.2020.02.005
 Show in context
Show in context This is at least partly related to the diverse suite of methods applied for carbonate Δ’17O analyses (Passey et al., 2014; Bergel et al., 2020; Wostbrock et al., 2020a, 2020b; Huth et al., 2022).
View in article
Breitkreuz, C., Paul, A., Kurahashi‐Nakamura, T., Losch, M., Schulz, M. (2018) A dynamical reconstruction of the global monthly mean oxygen isotopic composition of seawater. Journal of Geophysical Research: Oceans 123, 7206–7219. https://doi.org/10.1029/2018JC014300
 Show in context
Show in context We retrieved seawater temperature and δ18Osw for each sample from a global gridded database (Breitkreuz et al., 2018), using the interpolation method outlined in Daëron and Gray (2023).
View in article
Chen, S., Gagnon, A.C., Adkins, J.F. (2018) Carbonic anhydrase, coral calcification and a new model of stable isotope vital effects. Geochimica et Cosmochimica Acta 236, 179–197. https://doi.org/10.1016/j.gca.2018.02.032
 Show in context
Show in context Biomineralisation models focusing on isotope effects reproduce the observed δ18O–δ13C covariations (McConnaughey, 1989b; Adkins et al., 2003; Chen et al., 2018).
View in article
The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
Daëron, M., Gray, W.R. (2023) Revisiting oxygen‐18 and clumped isotopes in planktic and benthic foraminifera. Paleoceanography and Paleoclimatology 38, e2023PA004660. https://doi.org/10.1029/2023PA004660
 Show in context
Show in context We retrieved seawater temperature and δ18Osw for each sample from a global gridded database (Breitkreuz et al., 2018), using the interpolation method outlined in Daëron and Gray (2023).
View in article
Davies, A.J., Brand, U., Tagliavento, M., Bitner, M.A., Bajnai, D., Staudigel, P., Bernecker, M., Fiebig, J. (2023) Isotopic disequilibrium in brachiopods disentangled with dual clumped isotope thermometry. Geochimica et Cosmochimica Acta 359, 135–147. https://doi.org/10.1016/j.gca.2023.08.005
 Show in context
Show in context The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
Diffusion has been suggested to be an important contributor to the vital effects in brachiopods (Davies et al., 2023).
View in article
Davies, A.J., Guo, W., Bernecker, M., Tagliavento, M., Raddatz, J., Gischler, E., Flögel, S., Fiebig, J. (2022) Dual clumped isotope thermometry of coral carbonate. Geochimica et Cosmochimica Acta 338, 66–78. https://doi.org/10.1016/j.gca.2022.10.015
 Show in context
Show in context Accurate coral thermometry will only become feasible if the isotopic bias is accounted for (e.g., Davies et al., 2022).
View in article
For coral carbonates, Davies et al. (2022) show systematic deviations from clumped isotope equilibrium along slopes characteristic of the underlying kinetic mechanisms.
View in article
In contrast, in dual clumped isotope space, cold- and warm-water corals exhibit distinct kinetic trajectories, explained by differences in the calcification parameters, such as the carbonic anhydrase activity, growth temperature, and the pH of the calcifying fluid (Davies et al., 2022).
View in article
Davies et al. (2022) compare dual clumped isotope data of corals with a respective theoretical CO2 absorption model and show that certain mechanisms (e.g., DIC speciation effects — the classic pH effect) are inconsistent with observed dual clumped isotope fractionation trends.
View in article
Guo, M., Wostbrock, J.A.G., Planavsky, N.J., Korenaga, J. (2022) Reconstructing seawater δ18O and Δ′17O values with solid Earth system evolution. Earth and Planetary Science Letters 592, 117637. https://doi.org/10.1016/j.epsl.2022.117637
 Show in context
Show in context This is not limited by the uncertainties in past seawater Δ’17O values because it was modelled to change only within ±10 ppm during the Phanerozoic (Guo et al., 2022).
View in article
Guo, W., Zhou, C. (2019) Triple oxygen isotope fractionation in the DIC-H2O-CO2 system: A numerical framework and its implications. Geochimica et Cosmochimica Acta 246, 541–564. https://doi.org/10.1016/j.gca.2018.11.018
 Show in context
Show in context Yet, apparent slopes may result from a combination of processes (Guo and Zhou, 2019; Wostbrock et al., 2020a; Herwartz, 2021; Bajnai et al., 2024).
View in article
We chose the theoretical aragonite calibration of Guo and Zhou (2019) as representative of thermodynamic equilibrium. In triple oxygen isotope space, all cold- and warm-water corals fall below their respective equilibrium lines (Figs. 1, 2).
View in article
Guo and Zhou (2019) also attribute the offset from the expected equilibrium in the warm water coral data of Passey et al. (2014) to CO2 absorption kinetics.
View in article
The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
The mean θcoral value obtained using the Guo and Zhou (2019) aragonite equilibrium calibration is 0.529 ± 0.001 (within a range of 0.527 to 0.531), reflecting the net effect of several superimposed processes.
View in article
Guo and Zhou (2019) presented theoretical slopes for CO2 absorption (θabsorption = 0.538 to 0.541 at pH between 8.4 and 9, respectively), which differ significantly from our observed θcoral = 0.529 ± 0.001.
View in article
The model of Guo and Zhou (2019) considers parameters derived from experimental fractionation factors, which are inconsistent with the most recent theoretical models.
View in article
Theoretical approaches can calculate full thermodynamic equilibrium (e.g., Guo and Zhou, 2019), but their confirmation using natural samples remains challenging.
View in article
Herwartz, D. (2021) Triple oxygen isotope variations in Earth’s crust. Reviews in Mineralogy and Geochemistry 86, 291–322. https://doi.org/10.2138/rmg.2021.86.09
 Show in context
Show in context Yet, apparent slopes may result from a combination of processes (Guo and Zhou, 2019; Wostbrock et al., 2020a; Herwartz, 2021; Bajnai et al., 2024).
View in article
Shallower slopes hint towards considerable proportions of CO2 (aq) diffusion, which follows much shallower slopes (i.e. θdiffusion = 0.506; Herwartz, 2021).
View in article
Huth, T.E., Passey, B.H., Cole, J.E., Lachniet, M.S., McGee, D., Denniston, R.F., Truebe, S., Levin, N.E. (2022) A framework for triple oxygen isotopes in speleothem paleoclimatology. Geochimica et Cosmochimica Acta 319, 191–219. https://doi.org/10.1016/j.gca.2021.11.002
 Show in context
Show in context To normalise this data point to our carbonate reference frame, we followed the steps outlined in Huth et al. (2022).
View in article
This is at least partly related to the diverse suite of methods applied for carbonate Δ’17O analyses (Passey et al., 2014; Bergel et al., 2020; Wostbrock et al., 2020a, 2020b; Huth et al., 2022).
View in article
Kim, S.-T., Mucci, A., Taylor, B.E. (2007) Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75 °C: Revisited. Chemical Geology 246, 135–146. https://doi.org/10.1016/j.chemgeo.2007.08.005
 Show in context
Show in context A 25 °C acid fractionation factor was applied to the CO2 data to calculate the isotope composition of the coral aragonite (i.e. 18α25°C-acid-CO2/aragonite = 1.01063 (Kim et al., 2007) and θ25°C-acid-CO2/carbonate = 0.523 (Wostbrock et al., 2020b)).
View in article
Additionally, to account for the difference in the acid fractionation factors between aragonite and calcite, we shifted the corrected δ18O value by 0.41 ‰ (Kim et al., 2007).
View in article
Lin, Y., Wu, N., Ta, K., Landais, A., Peng, X. (2021) Triple oxygen isotopic compositions of ocean water from the Mariana Trench. ACS Earth and Space Chemistry 5, 3087–3096. https://doi.org/10.1021/acsearthspacechem.1c00187
 Show in context
Show in context Seawater Δ’17O values were not directly measured but universally assumed to be Δ’17Osw = −11 ± 6 ppm, which covers most modern seawater data (Luz and Barkan, 2010; Lin et al., 2021).
View in article
Currently, only limited data exist for the triple oxygen isotope composition of seawater (Luz and Barkan, 2010; Lin et al., 2021).
View in article
Luz, B., Barkan, E. (2010) Variations of 17O/16O and 18O/16O in meteoric waters. Geochimica et Cosmochimica Acta 74, 6276–6286. https://doi.org/10.1016/j.gca.2010.08.016
 Show in context
Show in context Here, we chose 0.528 for λRL (Luz and Barkan, 2010).
View in article
Seawater Δ’17O values were not directly measured but universally assumed to be Δ’17Osw = −11 ± 6 ppm, which covers most modern seawater data (Luz and Barkan, 2010; Lin et al., 2021).
View in article
Currently, only limited data exist for the triple oxygen isotope composition of seawater (Luz and Barkan, 2010; Lin et al., 2021).
View in article
Marali, S., Wisshak, M., López Correa, M., Freiwald, A. (2013) Skeletal microstructure and stable isotope signature of three bathyal solitary cold-water corals from the Azores. Palaeogeography, Palaeoclimatology, Palaeoecology 373, 25–38. https://doi.org/10.1016/j.palaeo.2012.06.017
 Show in context
Show in context Temperature estimates from equilibrium δ18O–T calibration curves indicate higher T (by >10 °C) compared to actual growth T. Although species-specific δ18O–T calibrations can partly account for vital effects (Weber and Woodhead, 1972; Smith et al., 2000), the reconstructed temperatures can remain inaccurate (Marali et al., 2013).
View in article
McConnaughey, T. (1989a) 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns. Geochimica et Cosmochimica Acta 53, 151–162. https://doi.org/10.1016/0016-7037(89)90282-2
 Show in context
Show in context It has long been noticed that most modern coral carbonate comprises much lower δ18O and δ13C than expected from equilibrium (Weber and Woodhead, 1972; McConnaughey, 1989a; Smith et al., 2000; Adkins et al., 2003).
View in article
The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
McConnaughey, T. (1989b) 13C and 18O isotopic disequilibrium in biological carbonates: II. In vitro simulation of kinetic isotope effects. Geochimica et Cosmochimica Acta 53, 163–171. https://doi.org/10.1016/0016-7037(89)90283-4
 Show in context
Show in context Biomineralisation models focusing on isotope effects reproduce the observed δ18O–δ13C covariations (McConnaughey, 1989b; Adkins et al., 2003; Chen et al., 2018).
View in article
The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
 Show in context
Show in context The P. porites sample is from Passey et al. (2014).
View in article
In our final dataset, we included the warm-water coral data point reported by Passey et al. (2014) (sample JBC03, Porites porites).
View in article
The P. porites sample is from Passey et al. (2014).
View in article
Guo and Zhou (2019) also attribute the offset from the expected equilibrium in the warm water coral data of Passey et al. (2014) to CO2 absorption kinetics.
View in article
This is at least partly related to the diverse suite of methods applied for carbonate Δ’17O analyses (Passey et al., 2014; Bergel et al., 2020; Wostbrock et al., 2020a, 2020b; Huth et al., 2022).
View in article
Rollion-Bard, C., Chaussidon, M., France-Lanord, C. (2003) pH control on oxygen isotopic composition of symbiotic corals. Earth and Planetary Science Letters 215, 275–288. https://doi.org/10.1016/S0012-821X(03)00391-1
 Show in context
Show in context The calcifying fluid is not perfectly isolated from seawater but isolated enough to significantly up-regulate pH via the enzyme Ca-ATPase that pumps Ca2+ into and H+ out of the calcifying fluid (Rollion-Bard et al., 2003).
View in article
Saenger, C., Affek, H.P., Felis, T., Thiagarajan, N., Lough, J.M., Holcomb, M. (2012) Carbonate clumped isotope variability in shallow water corals: Temperature dependence and growth-related vital effects. Geochimica et Cosmochimica Acta 99, 224–242. https://doi.org/10.1016/j.gca.2012.09.035
 Show in context
Show in context Likewise, coral growth T determined using clumped isotope thermometry (Δ47) is also biased by >10 °C, but towards lower T (Thiagarajan et al., 2011; Saenger et al., 2012).
View in article
The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
Smith, J.E., Schwarcz, H.P., Risk, M.J., McConnaughey, T.A., Keller, N. (2000) Paleotemperatures from deep-sea corals: Overcoming “vital effects.” Palaios 15, 25–32. https://doi.org/10.1669/0883-1351(2000)015<0025:pfdsco>2.0.co;2
 Show in context
Show in context It has long been noticed that most modern coral carbonate comprises much lower δ18O and δ13C than expected from equilibrium (Weber and Woodhead, 1972; McConnaughey, 1989a; Smith et al., 2000; Adkins et al., 2003).
View in article
Temperature estimates from equilibrium δ18O–T calibration curves indicate higher T (by >10 °C) compared to actual growth T. Although species-specific δ18O–T calibrations can partly account for vital effects (Weber and Woodhead, 1972; Smith et al., 2000), the reconstructed temperatures can remain inaccurate (Marali et al., 2013).
View in article
Thiagarajan, N., Adkins, J., Eiler, J. (2011) Carbonate clumped isotope thermometry of deep-sea corals and implications for vital effects. Geochimica et Cosmochimica Acta 75, 4416–4425. https://doi.org/10.1016/j.gca.2011.05.004
 Show in context
Show in context Likewise, coral growth T determined using clumped isotope thermometry (Δ47) is also biased by >10 °C, but towards lower T (Thiagarajan et al., 2011; Saenger et al., 2012).
View in article
The CO2 absorption mechanism is a classic candidate for vital effects in biogenic carbonates (McConnaughey, 1989a, 1989b; Adkins et al., 2003; Thiagarajan et al., 2011; Saenger et al., 2012; Chen et al., 2018; Guo and Zhou, 2019; Davies et al., 2023), providing a starting point for comparing theoretical and empirical triple oxygen isotope slope values.
View in article
Watkins, J.M., Hunt, J.D., Ryerson, F.J., DePaolo, D.J. (2014) The influence of temperature, pH, and growth rate on the δ18O composition of inorganically precipitated calcite. Earth and Planetary Science Letters 404, 332–343. https://doi.org/10.1016/j.epsl.2014.07.036
 Show in context
Show in context Although contributions from kinetic isotope fractionation at the carbonate–water interface cannot be excluded (Watson, 2004; Watkins et al., 2014), the almost perfect fit of the theoretical model with analytical results led these authors to suggest that CO2 absorption is the main mechanism inducing kinetic effects.
View in article
It is worth pointing out that surface effects are not necessarily related to diffusion (Watson, 2004; Watkins et al., 2014).
View in article
Watson, E.B. (2004) A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochimica et Cosmochimica Acta 68, 1473–1488. https://doi.org/10.1016/j.gca.2003.10.003
 Show in context
Show in context Although contributions from kinetic isotope fractionation at the carbonate–water interface cannot be excluded (Watson, 2004; Watkins et al., 2014), the almost perfect fit of the theoretical model with analytical results led these authors to suggest that CO2 absorption is the main mechanism inducing kinetic effects.
View in article
It is worth pointing out that surface effects are not necessarily related to diffusion (Watson, 2004; Watkins et al., 2014).
View in article
Weber, J.N., Woodhead, P.M.J. (1972) Temperature dependence of oxygen-18 concentration in reef coral carbonates. Journal of Geophysical Research 77, 463–473. https://doi.org/10.1029/JC077i003p00463
 Show in context
Show in context It has long been noticed that most modern coral carbonate comprises much lower δ18O and δ13C than expected from equilibrium (Weber and Woodhead, 1972; McConnaughey, 1989a; Smith et al., 2000; Adkins et al., 2003).
View in article
Temperature estimates from equilibrium δ18O–T calibration curves indicate higher T (by >10 °C) compared to actual growth T. Although species-specific δ18O–T calibrations can partly account for vital effects (Weber and Woodhead, 1972; Smith et al., 2000), the reconstructed temperatures can remain inaccurate (Marali et al., 2013).
View in article
Wostbrock, J.A.G., Brand, U., Coplen, T.B., Swart, P.K., Carlson, S.J., Brearley, A.J., Sharp, Z.D. (2020a) Calibration of carbonate-water triple oxygen isotope fractionation: Seeing through diagenesis in ancient carbonates. Geochimica et Cosmochimica Acta 288, 369–388. https://doi.org/10.1016/j.gca.2020.07.045
 Show in context
Show in context Yet, apparent slopes may result from a combination of processes (Guo and Zhou, 2019; Wostbrock et al., 2020a; Herwartz, 2021; Bajnai et al., 2024).
View in article
Similar to Wostbrock et al. (2020a), who explain how to ‘see through’ diagenesis using triple oxygen isotopes, the correction scheme outlined above allows us to see through vital effects.
View in article
This is at least partly related to the diverse suite of methods applied for carbonate Δ’17O analyses (Passey et al., 2014; Bergel et al., 2020; Wostbrock et al., 2020a, 2020b; Huth et al., 2022).
View in article
Wostbrock, J.A.G., Cano, E.J., Sharp, Z.D. (2020b) An internally consistent triple oxygen isotope calibration of standards for silicates, carbonates and air relative to VSMOW2 and SLAP2. Chemical Geology 533, 119432. https://doi.org/10.1016/j.chemgeo.2019.119432
 Show in context
Show in context Briefly, the triple oxygen isotope composition of these two reference gases was determined relative to NBS-18 and IAEA-603 measurements and the values reported by Wostbrock et al. (2020b).
View in article
A 25 °C acid fractionation factor was applied to the CO2 data to calculate the isotope composition of the coral aragonite (i.e. 18α25°C-acid-CO2/aragonite = 1.01063 (Kim et al., 2007) and θ25°C-acid-CO2/carbonate = 0.523 (Wostbrock et al., 2020b)).
View in article
This is at least partly related to the diverse suite of methods applied for carbonate Δ’17O analyses (Passey et al., 2014; Bergel et al., 2020; Wostbrock et al., 2020a, 2020b; Huth et al., 2022).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Supplementary discussion on systematic errors
- Tables S-1 to S-3
- Figures S-1 to S-7
- Supplementary Information References
Download the Supplementary Information (PDF)
Download Tables S-2 and S-3 (.xlsx)
Figures
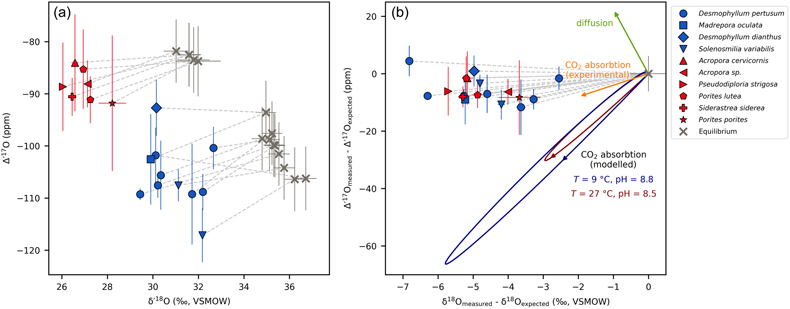
Figure 1 Triple oxygen isotope data for cold- and warm-water corals presented (a) in Δ’17O vs. δ’18O space and (b) as an offset plot. The expected equilibrium positions for each sample (grey crosses) are based on respective seawater δ18O and T estimates. The dashed grey lines represent the effective vital effect slopes, moving away the coral carbonate from equilibrium. The CO2 absorption loops are made using the isoDIC model, simulating conditions resembling the internal calcification environment of cold- and warm-water corals (see Figure S-4 for details). The experimental CO2 absorption vector considers a slope of 0.532, as discussed in the text. The P. porites sample is from Passey et al. (2014)
Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
.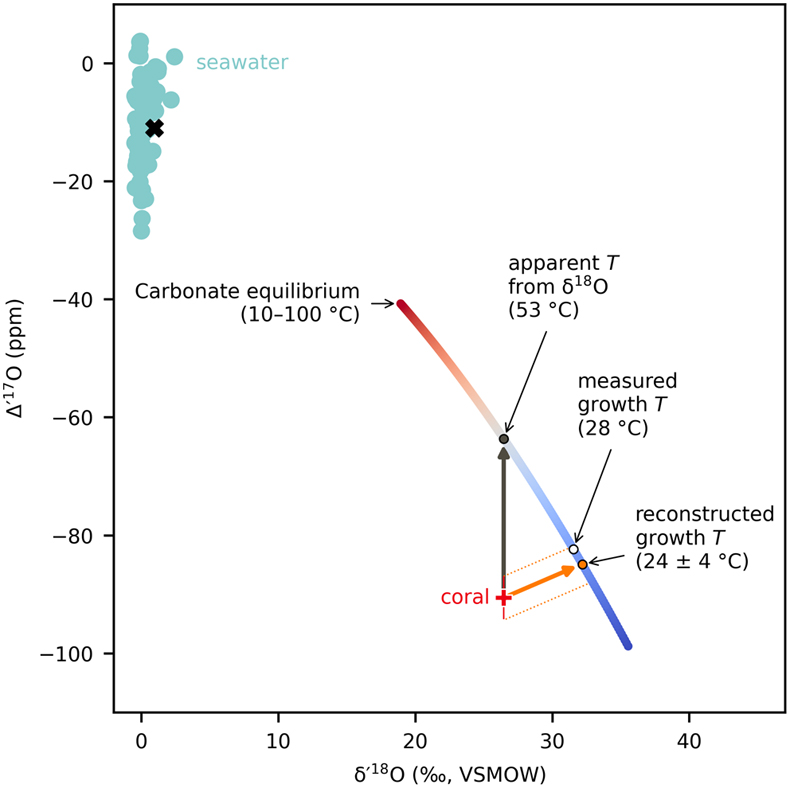
Figure 2 The concept of correcting vital effects using triple oxygen isotopes demonstrated on a warm-water coral (Siderastrea siderea; SK-SA5, red cross). The δ18O of the coral yields an apparent temperature estimate, which is too warm (grey arrow and circle). A back-extrapolation to the equilibrium curve yields a corrected temperature estimate (orange arrow and circle) that is indistinguishable from the actual coral growth temperature (white circle). Error propagation considers the measurement error but not the error of the seawater isotope composition (see text).
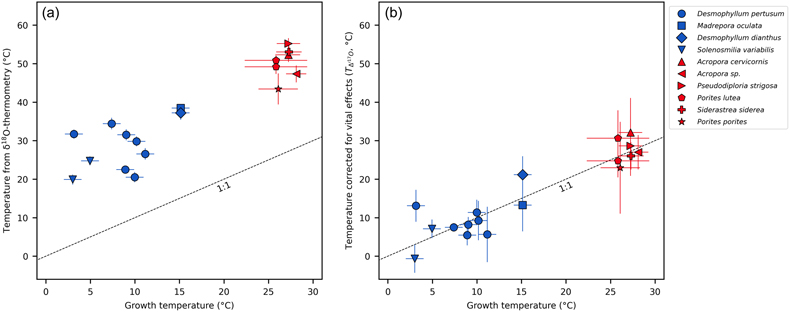
Figure 3 Apparent growth temperatures reconstructed from isotope thermometry compared to actual growth temperatures. (a) Temperature estimates from classical δ18O thermometry are too warm compared to the actual growth temperatures. (b) Temperatures corrected for vital effects using Δ’17O match the actual growth temperatures within error. The P. porites sample is from Passey et al. (2014)
Passey, B.H., Hu, H., Ji, H., Montanari, S., Li, S., Henkes, G.A., Levin, N.E. (2014) Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141, 1–25. https://doi.org/10.1016/j.gca.2014.06.006
.






