Copper isotope fractionation during asteroid core solidification
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:560Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
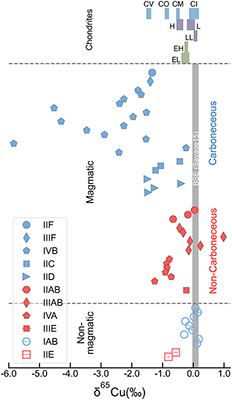 Figure 1 Copper isotope data for chondrites and iron meteorites. Data for different types of chondrites from Savage et al. (2015) and iron meteorites from Luck et al. (2005), Bishop et al. (2012), and Chen et al. (2016) are plotted. The bulk silicate Earth (BSE) composition is from Savage et al. (2015). | 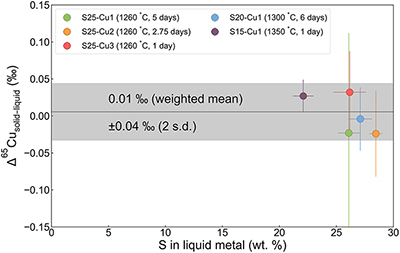 Figure 2 Equilibrium Cu isotope fractionation between solid and liquid metal during core crystallisation. A plot of all experimental data is available in Figure S-5. Error bars represent 2 standard deviations of the mean. | 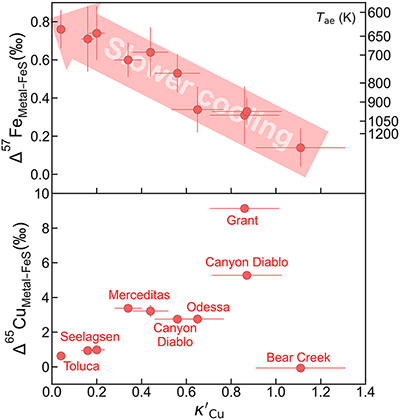 Figure 3 Iron and Cu isotope fractionation between metal and troilite in iron meteorites. Figure reproduced based on Williams and Archer (2011). The apparent partition coefficient of Cu is defined as κʹCu = CFeS(Cu)/CMeteorite(Cu). Calculated apparent equilibrium temperature (Tae) for Fe isotope fractionation is plotted on the right side of y-axis (see Supplementary Information). | 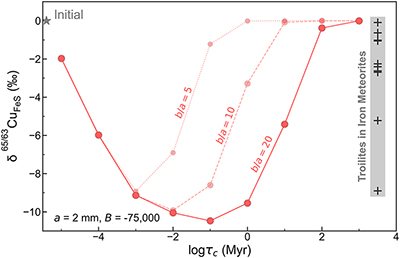 Figure 4 Modelled Cu isotope fractionation in troilite as a function of cooling time scale. The model was conducted assuming a troilite radius (a) of 2 mm, and a temperature dependence of the partition coefficient (B) of −75,000. Measured Cu isotope composition of troilites in iron meteorites is plotted on the right-hand side of the figure for comparison. Details of the model are described in the main text and in the Supplementary Information. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
The terrestrial planets in our Solar System are vastly different from each other in internal structure and composition due to their distinct accretion and formation histories. Earth, when compared with the bulk Solar System composition as represented by the CI chondrites, exhibits a prominent depletion in moderately volatile elements, such as alkali elements, Cu, and Zn (McDonough and Sun, 1995
McDonough, W.F., Sun, S.-s. (1995) The composition of the Earth. Chemical Geology 120, 223–253. https://doi.org/10.1016/0009-2541(94)00140-4
). With a half condensation temperature of ∼1030 K (Lodders, 2003Lodders, K. (2003) Solar System Abundances and Condensation Temperatures of the Elements. The Astrophysical Journal 591, 1220. https://doi.org/10.1086/375492
; Wood et al., 2019Wood, B.J., Smythe, D.J., Harrison, T. (2019) The condensation temperatures of the elements: A reappraisal. American Mineralogist 104, 844–856. https://doi.org/10.2138/am-2019-6852CCBY
), Cu is one of the moderately volatile elements that could be susceptible to evaporative loss during the early stages of Earth accretion. On the other hand, Cu is also a moderately siderophile element (e.g., Mahan et al., 2018Mahan, B., Siebert, J., Blanchard, I., Badro, J., Kubik, E., Sossi, P., Moynier, F. (2018) Investigating Earth’s Formation History Through Copper and Sulfur Metal-Silicate Partitioning During Core-Mantle Differentiation. Journal of Geophysical Research: Solid Earth 123, 8349–8363. https://doi.org/10.1029/2018JB015991
), indicating that its depletion in the Bulk Silicate Earth (BSE) could be due to core formation.One potential way to understand the relative importance of the above two effects on Cu abundance in the BSE is to examine Cu isotope fractionation. Measurements of mid-ocean ridge basalts, ocean island basalts, komatiites, and peridotites defined a robust and precise BSE δ65Cu (defined as the per mil difference in 65Cu/63Cu with respect to NIST SRM 976) value of 0.07 ± 0.10 ‰ (2 s.d.), significantly heavier than the estimated initial bulk Earth composition of −0.24 ± 0.09 ‰ (2 s.d.) based on chondrites (Savage et al., 2015
Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
).Despite the potential application of Cu isotopes to tracing planetary processes experienced by Earth, experimental studies on equilibrium Cu isotope fractionation are limited to only a few studies on silicate–metal and silicate–sulphide fractionation. These studies found limited fractionation between silicate melt and metallic liquid, but relatively large fractionations between silicate melt and sulphide liquid (up to 0.33 ‰, Xia et al., 2019
Xia, Y., Kiseeva, E., Wade, J., Huang, F. (2019) The effect of core segregation on the Cu and Zn isotope composition of the silicate Moon. Geochemical Perspectives Letters 12, 12–17. https://doi.org/10.7185/geochemlet.1928
; ∼1 ‰, Savage et al., 2015Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
), leading the authors to conclude that late segregation of a sulphide-rich liquid to the core has contributed to the heavy Cu isotope composition of the BSE (Savage et al., 2015Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
), and the bulk silicate Moon (BSM) (Xia et al., 2019Xia, Y., Kiseeva, E., Wade, J., Huang, F. (2019) The effect of core segregation on the Cu and Zn isotope composition of the silicate Moon. Geochemical Perspectives Letters 12, 12–17. https://doi.org/10.7185/geochemlet.1928
).Core crystallisation is another process commonly experienced by differentiated asteroids and terrestrial bodies. Recent experiments by Ni et al. (2020)
Ni, P., Chabot, N.L., Ryan, C.J., Shahar, A. (2020) Heavy iron isotope composition of iron meteorites explained by core crystallization. Nature Geoscience 13, 611–615. https://doi.org/10.1038/s41561-020-0617-y
demonstrated that core crystallisation could introduce Fe isotope fractionation due to solid metal–liquid metal differentiation. This fractionation trend was observed in magmatic iron meteorites originated from the metallic cores of differentiated extinct asteroids (Ni et al., 2020Ni, P., Chabot, N.L., Ryan, C.J., Shahar, A. (2020) Heavy iron isotope composition of iron meteorites explained by core crystallization. Nature Geoscience 13, 611–615. https://doi.org/10.1038/s41561-020-0617-y
). Compiled copper isotope data of iron meteorites show large variations in their δ65Cu from −6 ‰ to +1 ‰ (Figure 1), raising the need to quantify the effect of core crystallisation on causing the variation. In this study, we conducted solid–liquid metal equilibrium experiments to constrain the degree of Cu isotope fractionation during core crystallisation in iron meteorite parent bodies. The experimental data are combined with a kinetic model to explain Cu isotope variations in iron meteorites. Our results are also used to indirectly assess existing Cu isotope exchange experiments that suggested a late-stage sulphide segregation in causing the bulk silicate Earth and Moon to be heavy in Cu isotopic composition.
Figure 1 Copper isotope data for chondrites and iron meteorites. Data for different types of chondrites from Savage et al. (2015)
Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
and iron meteorites from Luck et al. (2005)Luck, J.-M., Othman, D.B., Albarède, F. (2005) Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochimica et Cosmochimica Acta 69, 5351–5363. https://doi.org/10.1016/j.gca.2005.06.018
, Bishop et al. (2012)Bishop, M.C., Moynier, F., Weinstein, C., Fraboulet, J.-G., Wang, K., Foriel, J. (2012) The Cu isotopic composition of iron meteorites. Meteoritics and Planetary Science 47, 268–276. https://doi.org/10.1111/j.1945-5100.2011.01326.x
, and Chen et al. (2016)Chen, H., Moynier, F., Humayun, M., Bishop, M.C., Williams, J.T. (2016) Cosmogenic effects on Cu isotopes in IVB iron meteorites. Geochimica et Cosmochimica Acta 182, 145–154. https://doi.org/10.1016/j.gca.2016.03.006
are plotted. The bulk silicate Earth (BSE) composition is from Savage et al. (2015)Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
.top
Methods
Solid–liquid metal equilibrium experiments were conducted at the Johns Hopkins University Applied Physics Laboratory to simulate the core crystallisation process following the approach in Chabot et al. (2017)
Chabot, N.L., Wollack, E.A., McDonough, W.F., Ash, R.D., Saslow, S.A. (2017) Experimental determination of partitioning in the Fe-Ni system for applications to modeling meteoritic metals. Meteoritics and Planetary Science 52, 1133–1145. https://doi.org/10.1111/maps.12864
utilising vacuum-sealed silica tubes and vertical furnaces. Major element composition of the phases was analysed with a JEOL 8530F electron microprobe at the Carnegie Institution for Science. The quenched solid and liquid metal phases were sampled for Cu isotope measurements using a micromill equipped with tungsten carbide drill bits. Column purification of Cu was performed using in-house custom-made quartz glass columns following the procedure previously reported by Ni et al. (2021)Ni, P., Macris, C.A., Darling, E.A., Shahar, A. (2021) Evaporation-induced copper isotope fractionation: Insights from laser levitation experiments. Geochimica et Cosmochimica Acta 298, 131–148. https://doi.org/10.1016/j.gca.2021.02.007
. Isotopic compositions of the purified Cu solution of the solid and liquid metal phases were measured using a Nu Plasma II Multi-Collector ICP-MS at the Carnegie Institution for Science in wet plasma mode. Analyses were conducted using ERM-AE633 as the bracketing standard, and all isotope results are reported relative to NIST SRM 976 after correcting a −0.01 ‰ difference between ERM-AE633 and NIST SRM 976 (Moeller et al., 2012Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177–199. https://doi.org/10.1111/j.1751-908X.2011.00153.x
):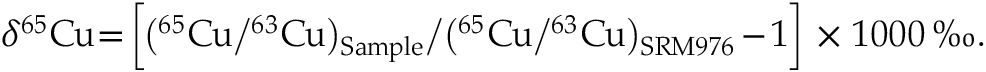
Modelling of the partitioning, diffusion, and isotopic fractionation of Cu between metal and troilite grains in iron meteorites was performed assuming one-dimensional diffusion in spherical coordinates and an asymptotic cooling history. Details about the experimental and analytical methods and the kinetic model are available in the Supplementary Information.
top
Results and Discussion
A total of ten solid–liquid metal equilibrium experiments were conducted at 1260–1425 °C to study Cu isotope fractionation during iron meteorite core crystallisation. Sulphur content of the liquid metal was intrinsically controlled by the temperature following the Fe-Ni-S phase diagram (Hsieh and Chang, 1987
Hsieh, K.-C., Chang, Y.A. (1987) Thermochemical Description of the Ternary Iron-Nickel-Sulfur System. Canadian Metallurgical Quarterly 26, 311–327. https://doi.org/10.1179/cmq.1987.26.4.311
), with higher sulphur in the liquid at lower temperatures. Microscopic images of one experiment (S20-Cu1) are shown in Figure S-1 as an example. The quenched sample charges show distinct regions of solid metal (Fe-Ni alloy) and liquid metal (Fe-Ni-S), the latter with characteristic dendritic textures formed during quench. The quenched liquid metal contains 0.8 to 3.3 wt. % Cu while the solid metal contains 0.3 to 0.6 wt. % Cu (Table S-1). Calculated Cu partition coefficients between the solid and liquid metal vary from 0.11 to 0.62 as a function of sulphur content, generally consistent with literature data (Figure S-2; Chabot and Jones, 2003Chabot, N.L., Jones, J.H. (2003) The parameterization of solid metal-liquid metal partitioning of siderophile elements. Meteoritics and Planetary Science 38, 1425–1436. https://doi.org/10.1111/j.1945-5100.2003.tb00248.x
; Chabot et al., 2009Chabot, N.L., Saslow, S.A., McDonough, W.F., Jones, J.H. (2009) An investigation of the behavior of Cu and Cr during iron meteorite crystallization. Meteoritics and Planetary Science 44, 505–519. https://doi.org/10.1111/j.1945-5100.2009.tb00747.x
).Although the Cu partitioning data indicate chemical equilibrium between the solid and liquid metal, it was not the case for Cu isotope exchange. Copper isotopic difference between the solid and liquid metal phases in these ten experiments vary significantly from 0.35 ± 0.04 ‰ to 1.75 ± 0.08 ‰. Further investigation of the experimental data showed that isotopic equilibrium was only reached for high-sulphur experiments at temperatures lower than 1350 °C (S15-Cu1, S20-Cu1, S25-Cu1, S25-Cu2, S25-Cu3). For low-sulphur experiments at higher temperatures (S5-Cu1, S5-Cu3, S10-Cu1, S15-Cu2, S15-Cu6), Cu isotopic composition was significantly affected by evaporation of Cu to the interior of the vacuum-sealed quartz glass tube. The initial bulk δ65Cu was 0.45 ± 0.02 ‰ as measured on the Cu powder used for doping the experiments and the starting mixture for S15-Cu1 (Table S-2). The low-sulphur, high-temperature experiments reached isotopic compositions as heavy as 1.39 ± 0.19 ‰ for the solid metal and 1.75 ± 0.08 ‰ for the liquid metal (Figure S-3). Evaporative loss of the Cu occurs primarily in the liquid metal with higher mobility for the elements, driving it towards heavier δ65Cu compositions. Copper diffusion in the solid metal limits its isotopic exchange with the liquid metal, causing its Cu isotope composition to exhibit a lighter value. This is evidenced by duplicated sampling and analyses of the same experiments, which yielded identical δ65Cu composition for the liquid metal but different compositions for the solid metal in both cases (S5-Cu1, S10-Cu1; Table S-2). The evaporative loss of Cu is further proved by measuring the acid leachate of the tube inner wall of the most affected experiment (S15-Cu2). The Cu leachate yielded an extremely light Cu isotopic composition of −1.51 ± 0.06 ‰, consistent with light Cu being preferentially lost during evaporation and later condensed on the low-temperature end of the quartz glass tube.
Due to the complications caused by Cu evaporation, equilibrium Cu isotope fractionation data are limited to the low-temperature experiments with 20 wt. % or more sulphur in the liquid metal (Figure S-4). Isotopic equilibrium between the solid and liquid metal was assessed by: 1) a set of time-series experiments conducted at the lowest temperature (1260 °C) for durations of 1, 2.75, and 5 days; 2) departure of the bulk Cu isotopic composition from the estimated initial composition (δ65Cu = 0.45‰). The time-series experiments yielded fractionation factors within error from each other, suggesting that isotopic equilibrium was reached within one day at 1260 °C (Figure S-3). For the low-temperature experiments with 20 wt. % or more sulphur, the bulk δ65Cu varies between 0.36 ± 0.04 ‰ and 0.69 ± 0.03 ‰, within ∼0.2 ‰ from the estimated initial of 0.45 ± 0.02 ‰ (Table S-2). We suspect the offset to the light composition was the consequence of isotopic heterogeneity within the Cu powder used for doping the experiments because Cu evaporation would only drive the bulk composition toward heavy values. The shift toward the heavy composition can be attributed to either isotopic heterogeneity or a small degree of Cu evaporation that was compensated by sufficient diffusive re-equilibration.
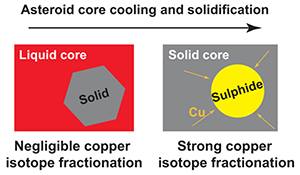
With the above considerations, five of the ten experiments with sulphur contents over 20 wt. % can be concluded to have reached isotopic equilibrium. All five experiments gave Cu isotope fractionation factors of ∼0 ‰, indicating limited fractionation between solid and liquid metal (Figure 2; Table S-2). With a weighted average of 0.01 ± 0.04 ‰ for Δ65Cusolid–liquid metal (Δ65Cusolid–liquid metal = δ65Cusolid–metal − δ65Culiquid–metal), crystallisation of the parent core of magmatic iron meteorites does not cause significant isotope fractionation in Cu. This is different from the case of Fe (Ni et al., 2020
Ni, P., Chabot, N.L., Ryan, C.J., Shahar, A. (2020) Heavy iron isotope composition of iron meteorites explained by core crystallization. Nature Geoscience 13, 611–615. https://doi.org/10.1038/s41561-020-0617-y
), where fractionation of over 0.1 ‰ occurs throughout the core crystallisation process. Such a low Δ65Cusolid–liquid metal conclusively excludes core crystallisation as a potential source of large Cu isotope variations in magmatic irons (Figure 1).
Figure 2 Equilibrium Cu isotope fractionation between solid and liquid metal during core crystallisation. A plot of all experimental data is available in Figure S-5. Error bars represent 2 standard deviations of the mean.
Another process common to the parent bodies of all magmatic iron meteorites that could affect their Cu isotope composition is the diffusive exchange between Fe metal and troilite. This process occurs at much lower temperatures than core crystallisation and would be separate from it. As a chalcophile element, Cu tends to partition into the troilite phase, especially as the temperature decreases. Direct measurements of troilite grains in iron meteorites found them to be as light as −9 ‰ in −9 ‰ (Williams and Archer, 2011
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal–sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166–3178. https://doi.org/10.1016/j.gca.2011.03.010
). Such large fractionation can potentially explain the large Cu isotope variations seen in iron meteorites. According to the authors, this significant fractionation is likely the result of a kinetic process. As shown in Figure 3, Williams and Archer (2011)Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal–sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166–3178. https://doi.org/10.1016/j.gca.2011.03.010
measured both Fe and Cu isotopes of troilite in iron meteorites. The fractionation of iron isotopes between the Fe-Ni alloy matrix and troilite was found to correlate with apparent Cu partition coefficient (calculated as Cu concentration of troilite divided by bulk meteorite Cu concentration) and kamacite bandwidth, which is related to cooling rate.
Figure 3 Iron and Cu isotope fractionation between metal and troilite in iron meteorites. Figure reproduced based on Williams and Archer (2011)
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal–sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166–3178. https://doi.org/10.1016/j.gca.2011.03.010
. The apparent partition coefficient of Cu is defined as κʹCu = CFeS(Cu)/CMeteorite(Cu). Calculated apparent equilibrium temperature (Tae) for Fe isotope fractionation is plotted on the right side of y-axis (see Supplementary Information).Iron meteorites that cooled down faster had less time to develop the Widmanstätten pattern and therefore tend to show narrower kamacite bands in general, although Ni and P concentrations also play a role. Chemically, a faster cooling rate would also limit the length scale of diffusion, “freezing” equilibrium Fe isotope fractionation and Cu partitioning that happened at higher temperatures. Iron meteorites with slower cooling rates, on the other hand, would have time to grow wider kamacite bands and allow for chemical re-equilibrium between the metal and troilite untill much lower temperatures. Our calculated apparent equilibrium temperatures based on Fe isotope fractionation factors from Dauphas et al. (2012)
Dauphas, N., Roskosz, M., Alp, E.E., Golden, D.C., Sio, C.K., Tissot, F.L.H., Hu, M.Y., Zhao, J., Gao, L., Morris, R.V. (2012) A general moment NRIXS approach to the determination of equilibrium Fe isotopic fractionation factors: Application to goethite and jarosite. Geochimica et Cosmochimica Acta 94, 254–275. https://doi.org/10.1016/j.gca.2012.06.013
(see Supplementary Information) vary between 630 and 1200 K, which are generally consistent with the upper limit of troilite stability (Hsieh and Chang, 1987Hsieh, K.-C., Chang, Y.A. (1987) Thermochemical Description of the Ternary Iron-Nickel-Sulfur System. Canadian Metallurgical Quarterly 26, 311–327. https://doi.org/10.1179/cmq.1987.26.4.311
) and the lower end of Widmanstätten pattern nucleation (Yang and Goldstein, 2005Yang, J., Goldstein, J.I. (2005) The formation of the Widmanstätten structure in meteorites. Meteoritics and Planetary Science 40, 239–253. https://doi.org/10.1111/j.1945-5100.2005.tb00378.x
).Copper isotope compositions measured in the same samples (Williams and Archer, 2011
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal–sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166–3178. https://doi.org/10.1016/j.gca.2011.03.010
) show much larger fractionations than possible equilibrium effects at magmatic temperatures (Figure 3). Unlike Fe, which is a primary component of both the metal and troilite phases in iron meteorites, Cu is a trace element that experiences significant redistribution between these phases as it becomes more chalcophile upon cooling (Figure 3). Such redistribution requires mass transfer by diffusion in solid metal, potentially leading to large kinetic isotope fractionation. It has been proposed by Williams and Archer (2011)Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal–sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166–3178. https://doi.org/10.1016/j.gca.2011.03.010
that the range of Cu isotope composition for troilites was the product of partial isotope equilibrium from an initial kinetically-caused, light signature.We developed a quantitative model to simulate the diffusive exchange of Cu between the metal and troilite phases, aimed at assessing the extent of light Cu enrichment in the troilite from the cooling and redistribution of Cu within the iron meteorite parent body. The isotopic composition of the troilite is modelled as a function of cooling time scale (τc) and shown in Figure 4. The simulations were conducted assuming a troilite radius (a) of 2 mm, a metal matrix radius (b) of 40 mm, and a B value of −75,000 to characterise the temperature dependence of the partition coefficient (κ). At 1100–1200 K when troilites first form, Cu is approximately evenly distributed between metal and troilite, as evidenced by the highest apparent partition coefficient (κ) of ∼1 in the meteorite Bear Creek (Figure 3) and solid metal–liquid metal–troilite equilibrium experiments (Shread et al., 2024
Shread, E.E., Chabot, N.L., Hamill, C.D., Ash, R.D., Corrigan, C.M. (2024) The crystallization of iron meteorites and the effect of troilite on trace element chemistry. 55th Lunar and Planetary Science Conference, abstract #1024. https://www.hou.usra.edu/meetings/lpsc2024/pdf/1024.pdf
).
Figure 4 Modelled Cu isotope fractionation in troilite as a function of cooling time scale. The model was conducted assuming a troilite radius (a) of 2 mm, and a temperature dependence of the partition coefficient (B) of −75,000. Measured Cu isotope composition of troilites in iron meteorites is plotted on the right-hand side of the figure for comparison. Details of the model are described in the main text and in the Supplementary Information.
If an iron meteorite cooled down at extremely high cooling rates (e.g., τc = 10 years), the troilite exhibits only minor Cu isotopic fractionation due to the limited Cu exchange between the metal and troilite, “freezing” its elemental partition and Cu isotope fractionation at high-temperature equilibrium. This could be the case for the Bear Creek meteorite, which recorded a high Cu partition coefficient and low degrees of Fe and Cu isotope fractionation (Figure 3). With slightly longer cooling time scales, ranging from 0.1 to 100 ka, Cu will have time to diffuse from metal to sulphide. The diffusion front entering the troilite phase would be enriched with light Cu isotopes, quickly overwriting the initial Cu isotope signal that was dominated by equilibrium fractionation. This would decrease the δ65Cu composition of the troilite to be as light as −10 ‰. At even longer cooling time scales, from 1 to 1000 Ma, the heavier Cu isotope in the metal matrix has more time to diffuse into the troilite, thus bringing its isotopic composition back to ∼0 ‰, representing equilibrium fractionation (Figure S-5).
Simulations assuming smaller b/a ratios of 10 and 5 produced similar outcomes, albeit with the peak fractionation occurring at different cooling time scales. Our model offers a semi-quantitative explanation for the Cu isotope compositions observed in troilites from iron meteorites, demonstrating a non-monatomic dependence on cooling rate, as shown in Figure 4. The modelling results align with the observed trend turnover and the overall magnitude of fractionation depicted in Figure 3b.
Previous studies report a small positive fractionation between metal–silicate and relatively large negative fractionation between sulphide–silicate (Savage et al., 2015
Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
; Xia et al., 2019Xia, Y., Kiseeva, E., Wade, J., Huang, F. (2019) The effect of core segregation on the Cu and Zn isotope composition of the silicate Moon. Geochemical Perspectives Letters 12, 12–17. https://doi.org/10.7185/geochemlet.1928
). Combining those results and assuming equilibrium conditions at ∼1350 °C in all experiments would predict a 0.4 ‰ fractionation in δ65Cu between metal and Ni-free sulphide, and 0.1 ‰ between metal and Ni-rich (∼27 wt. %) sulphide. This contrasts with our measured fractionation of 0.01 ± 0.04 ‰ at 1260 °C. This discrepancy can be attributed to the lack of isotope equilibrium in previous experiments (see Supplementary discussion), a composition dependence that is not yet fully understood, or systematic differences between different experiment setups. A careful re-evaluation of Cu isotope fractionation will be necessary to support the current models of sulphide segregation in explaining the heavy Cu isotope composition of the bulk silicate Earth and the Moon.top
Conclusion
We demonstrate through solid–liquid metal equilibrium experiments that core crystallisation of iron meteorite parent bodies does not result in measurable Cu isotope fractionation. Instead, the observed variations in Cu isotopes among iron meteorites are likely attributable to diffusive Cu enrichment into troilite from the metal matrix as the parent bodies cooled. Our quantitative model illustrates that this process can account for the observed magnitude of Cu isotope fractionation in troilite, as well as the general relationship between the fractionation and the cooling rate of iron meteorites. Interpreting the Cu isotope composition of iron meteorites’ parent cores requires careful evaluation, correction, or possibly complete avoidance of the influence of troilites. Our experiments also illustrate the potential for isotopic disequilibrium, even when elemental partitioning appears to have reached equilibrium. This highlights the necessity of verifying existing experimental data on Cu isotope fractionation between silicate–metal and silicate–sulfide, which is crucial for substantiating the prevailing theory that late-stage sulphide segregation explains the Cu isotope composition of the bulk silicate Earth and the Moon.
top
Acknowledgements
We are grateful to M.F. Horan for assistance with column chemistry work and T.D. Mock for help with the MC-ICPMS measurements. We thank Steve Elardo, Kelsey Prissel, and an anonymous reviewer for their thoughtful comments and Francis McCubbin for editorial handling. This work is partly supported by NSF grant EAR-2025779 to AS and SBS and NASA Emerging Worlds Grant 80NSSC19K1613 to NLC.
Editor: Francis McCubbin
top
References
Bishop, M.C., Moynier, F., Weinstein, C., Fraboulet, J.-G., Wang, K., Foriel, J. (2012) The Cu isotopic composition of iron meteorites. Meteoritics and Planetary Science 47, 268–276. https://doi.org/10.1111/j.1945-5100.2011.01326.x
 Show in context
Show in context Data for different types of chondrites from Savage et al. (2015) and iron meteorites from Luck et al. (2005), Bishop et al. (2012), and Chen et al. (2016) are plotted.
View in article
Chabot, N.L., Jones, J.H. (2003) The parameterization of solid metal-liquid metal partitioning of siderophile elements. Meteoritics and Planetary Science 38, 1425–1436. https://doi.org/10.1111/j.1945-5100.2003.tb00248.x
 Show in context
Show in context Calculated Cu partition coefficients between the solid and liquid metal vary from 0.11 to 0.62 as a function of sulphur content, generally consistent with literature data (Figure S-2; Chabot and Jones, 2003; Chabot et al., 2009).
View in article
Chabot, N.L., Saslow, S.A., McDonough, W.F., Jones, J.H. (2009) An investigation of the behavior of Cu and Cr during iron meteorite crystallization. Meteoritics and Planetary Science 44, 505–519. https://doi.org/10.1111/j.1945-5100.2009.tb00747.x
 Show in context
Show in context Calculated Cu partition coefficients between the solid and liquid metal vary from 0.11 to 0.62 as a function of sulphur content, generally consistent with literature data (Figure S-2; Chabot and Jones, 2003; Chabot et al., 2009).
View in article
Chabot, N.L., Wollack, E.A., McDonough, W.F., Ash, R.D., Saslow, S.A. (2017) Experimental determination of partitioning in the Fe-Ni system for applications to modeling meteoritic metals. Meteoritics and Planetary Science 52, 1133–1145. https://doi.org/10.1111/maps.12864
 Show in context
Show in context Solid–liquid metal equilibrium experiments were conducted at the Johns Hopkins University Applied Physics Laboratory to simulate the core crystallisation process following the approach in Chabot et al. (2017) utilising vacuum-sealed silica tubes and vertical furnaces.
View in article
Chen, H., Moynier, F., Humayun, M., Bishop, M.C., Williams, J.T. (2016) Cosmogenic effects on Cu isotopes in IVB iron meteorites. Geochimica et Cosmochimica Acta 182, 145–154. https://doi.org/10.1016/j.gca.2016.03.006
 Show in context
Show in context Data for different types of chondrites from Savage et al. (2015) and iron meteorites from Luck et al. (2005), Bishop et al. (2012), and Chen et al. (2016) are plotted.
View in article
Dauphas, N., Roskosz, M., Alp, E.E., Golden, D.C., Sio, C.K., Tissot, F.L.H., Hu, M.Y., Zhao, J., Gao, L., Morris, R.V. (2012) A general moment NRIXS approach to the determination of equilibrium Fe isotopic fractionation factors: Application to goethite and jarosite. Geochimica et Cosmochimica Acta 94, 254–275. https://doi.org/10.1016/j.gca.2012.06.013
 Show in context
Show in context Our calculated apparent equilibrium temperatures based on Fe isotope fractionation factors from Dauphas et al. (2012) (see Supplementary Information) vary between 630 and 1200 K, which are generally consistent with the upper limit of troilite stability (Hsieh and Chang, 1987) and the lower end of Widmanstätten pattern nucleation (Yang and Goldstein, 2005).
View in article
Hsieh, K.-C., Chang, Y.A. (1987) Thermochemical Description of the Ternary Iron-Nickel-Sulfur System. Canadian Metallurgical Quarterly 26, 311–327. https://doi.org/10.1179/cmq.1987.26.4.311
 Show in context
Show in context Sulphur content of the liquid metal was intrinsically controlled by the temperature following the Fe-Ni-S phase diagram (Hsieh and Chang, 1987), with higher sulphur in the liquid at lower temperatures. Microscopic images of one experiment (S20-Cu1) are shown in Figure S-1 as an example.
View in article
Our calculated apparent equilibrium temperatures based on Fe isotope fractionation factors from Dauphas et al. (2012) (see Supplementary Information) vary between 630 and 1200 K, which are generally consistent with the upper limit of troilite stability (Hsieh and Chang, 1987) and the lower end of Widmanstätten pattern nucleation (Yang and Goldstein, 2005).
View in article
Lodders, K. (2003) Solar System Abundances and Condensation Temperatures of the Elements. The Astrophysical Journal 591, 1220. https://doi.org/10.1086/375492
 Show in context
Show in context With a half condensation temperature of ∼1030 K (Lodders, 2003; Wood et al., 2019), Cu is one of the moderately volatile elements that could be susceptible to evaporative loss during the early stages of Earth accretion.
View in article
Luck, J.-M., Othman, D.B., Albarède, F. (2005) Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochimica et Cosmochimica Acta 69, 5351–5363. https://doi.org/10.1016/j.gca.2005.06.018
 Show in context
Show in context Data for different types of chondrites from Savage et al. (2015) and iron meteorites from Luck et al. (2005), Bishop et al. (2012), and Chen et al. (2016) are plotted.
View in article
Mahan, B., Siebert, J., Blanchard, I., Badro, J., Kubik, E., Sossi, P., Moynier, F. (2018) Investigating Earth’s Formation History Through Copper and Sulfur Metal-Silicate Partitioning During Core-Mantle Differentiation. Journal of Geophysical Research: Solid Earth 123, 8349–8363. https://doi.org/10.1029/2018JB015991
 Show in context
Show in context On the other hand, Cu is also a moderately siderophile element (e.g., Mahan et al., 2018), indicating that its depletion in the Bulk Silicate Earth (BSE) could be due to core formation.
View in article
McDonough, W.F., Sun, S.-s. (1995) The composition of the Earth. Chemical Geology 120, 223–253. https://doi.org/10.1016/0009-2541(94)00140-4
 Show in context
Show in context Earth, when compared with the bulk Solar System composition as represented by the CI chondrites, exhibits a prominent depletion in moderately volatile elements, such as alkali elements, Cu, and Zn (McDonough and Sun, 1995).
View in article
Moeller, K., Schoenberg, R., Pedersen, R.-B., Weiss, D., Dong, S. (2012) Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations. Geostandards and Geoanalytical Research 36, 177–199. https://doi.org/10.1111/j.1751-908X.2011.00153.x
 Show in context
Show in context Analyses were conducted using ERM-AE633 as the bracketing standard, and all isotope results are reported relative to NIST SRM 976 after correcting a −0.01 ‰ difference between ERM-AE633 and NIST SRM 976 (Moeller et al., 2012):
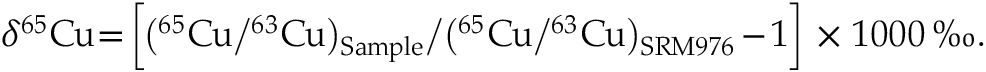 .
.
View in article
Ni, P., Chabot, N.L., Ryan, C.J., Shahar, A. (2020) Heavy iron isotope composition of iron meteorites explained by core crystallization. Nature Geoscience 13, 611–615. https://doi.org/10.1038/s41561-020-0617-y
 Show in context
Show in context Core crystallisation is another process commonly experienced by differentiated asteroids and terrestrial bodies. Recent experiments by Ni et al. (2020) demonstrated that core crystallisation could introduce Fe isotope fractionation due to solid metal–liquid metal differentiation.
View in article
This fractionation trend was observed in magmatic iron meteorites originated from the metallic cores of differentiated extinct asteroids (Ni et al., 2020).
View in article
This is different from the case of Fe (Ni et al., 2020), where fractionation of over 0.1 ‰ occurs throughout the core crystallisation process.
View in article
Ni, P., Macris, C.A., Darling, E.A., Shahar, A. (2021) Evaporation-induced copper isotope fractionation: Insights from laser levitation experiments. Geochimica et Cosmochimica Acta 298, 131–148. https://doi.org/10.1016/j.gca.2021.02.007
 Show in context
Show in context Column purification of Cu was performed using in-house custom-made quartz glass columns following the procedure previously reported by Ni et al. (2021).
View in article
Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
 Show in context
Show in context Measurements of mid-ocean ridge basalts, ocean island basalts, komatiites, and peridotites defined a robust and precise BSE δ65Cu (defined as the per mil difference in 65Cu/63Cu with respect to NIST SRM 976) value of 0.07 ± 0.10 ‰ (2 s.d.), significantly heavier than the estimated initial bulk Earth composition of −0.24 ± 0.09 ‰ (2 s.d.) based on chondrites (Savage et al., 2015).
View in article
These studies found limited fractionation between silicate melt and metallic liquid, but relatively large fractionations between silicate melt and sulphide liquid (up to 0.33 ‰, Xia et al., 2019; ∼1 ‰, Savage et al., 2015), leading the authors to conclude that late segregation of a sulphide-rich liquid to the core has contributed to the heavy Cu isotope composition of the BSE (Savage et al., 2015), and the bulk silicate Moon (BSM) (Xia et al., 2019).
View in article
Data for different types of chondrites from Savage et al. (2015) and iron meteorites from Luck et al. (2005), Bishop et al. (2012), and Chen et al. (2016) are plotted.
View in article
The bulk silicate Earth (BSE) composition is from Savage et al. (2015).
View in article
Previous studies report a small positive fractionation between metal–silicate and relatively large negative fractionation between sulphide–silicate (Savage et al., 2015; Xia et al., 2019).
View in article
Shread, E.E., Chabot, N.L., Hamill, C.D., Ash, R.D., Corrigan, C.M. (2024) The crystallization of iron meteorites and the effect of troilite on trace element chemistry. 55th Lunar and Planetary Science Conference, abstract #1024. https://www.hou.usra.edu/meetings/lpsc2024/pdf/1024.pdf
 Show in context
Show in context At 1100–1200 K when troilites first form, Cu is approximately evenly distributed between metal and troilite, as evidenced by the highest apparent partition coefficient (κ) of ∼1 in the meteorite Bear Creek (Figure 3) and solid metal–liquid metal–troilite equilibrium experiments (Shread et al., 2024).
View in article
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal–sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166–3178. https://doi.org/10.1016/j.gca.2011.03.010
 Show in context
Show in context Direct measurements of troilite grains in iron meteorites found them to be as light as −9 ‰ in δ65Cu (Williams and Archer, 2011).
View in article
As shown in Figure 3, Williams and Archer (2011) measured both Fe and Cu isotopes of troilite in iron meteorites.
View in article
Figure reproduced based on Williams and Archer (2011).
View in article
Copper isotope compositions measured in the same samples (Williams and Archer, 2011) show much larger fractionations than possible equilibrium effects at magmatic temperatures (Figure 3).
View in article
It has been proposed by Williams and Archer (2011) that the range of Cu isotope composition for troilites was the product of partial isotope equilibrium from an initial kinetically-caused, light signature.
View in article
Wood, B.J., Smythe, D.J., Harrison, T. (2019) The condensation temperatures of the elements: A reappraisal. American Mineralogist 104, 844–856. https://doi.org/10.2138/am-2019-6852CCBY
 Show in context
Show in context With a half condensation temperature of ∼1030 K (Lodders, 2003; Wood et al., 2019), Cu is one of the moderately volatile elements that could be susceptible to evaporative loss during the early stages of Earth accretion.
View in article
Xia, Y., Kiseeva, E., Wade, J., Huang, F. (2019) The effect of core segregation on the Cu and Zn isotope composition of the silicate Moon. Geochemical Perspectives Letters 12, 12–17. https://doi.org/10.7185/geochemlet.1928
 Show in context
Show in context These studies found limited fractionation between silicate melt and metallic liquid, but relatively large fractionations between silicate melt and sulphide liquid (up to 0.33 ‰, Xia et al., 2019; ∼1 ‰, Savage et al., 2015), leading the authors to conclude that late segregation of a sulphide-rich liquid to the core has contributed to the heavy Cu isotope composition of the BSE (Savage et al., 2015), and the bulk silicate Moon (BSM) (Xia et al., 2019).
View in article
Previous studies report a small positive fractionation between metal–silicate and relatively large negative fractionation between sulphide–silicate (Savage et al., 2015; Xia et al., 2019).
View in article
Yang, J., Goldstein, J.I. (2005) The formation of the Widmanstätten structure in meteorites. Meteoritics and Planetary Science 40, 239–253. https://doi.org/10.1111/j.1945-5100.2005.tb00378.x
 Show in context
Show in context Our calculated apparent equilibrium temperatures based on Fe isotope fractionation factors from Dauphas et al. (2012) (see Supplementary Information) vary between 630 and 1200 K, which are generally consistent with the upper limit of troilite stability (Hsieh and Chang, 1987) and the lower end of Widmanstätten pattern nucleation (Yang and Goldstein, 2005).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1. Solid-liquid metal equilibrium experiments for Cu
- 2. Electron microprobe analyses
- 3. Copper isotope analyses
- 4. Calculating apparent equilibrium temperature for Fe isotope fractionation between metal and troilite
- 5. Lessons learned for equilibrium Cu isotope fractionation experiments
- 6. Modelling of Cu redistribution between metal and troilite during cooling of the iron meteorite parent body
- Tables S-1 to S-3
- Figures S-1 to S-5
- Supplementary Information References
Download the Supplementary Information (PDF)
Figures
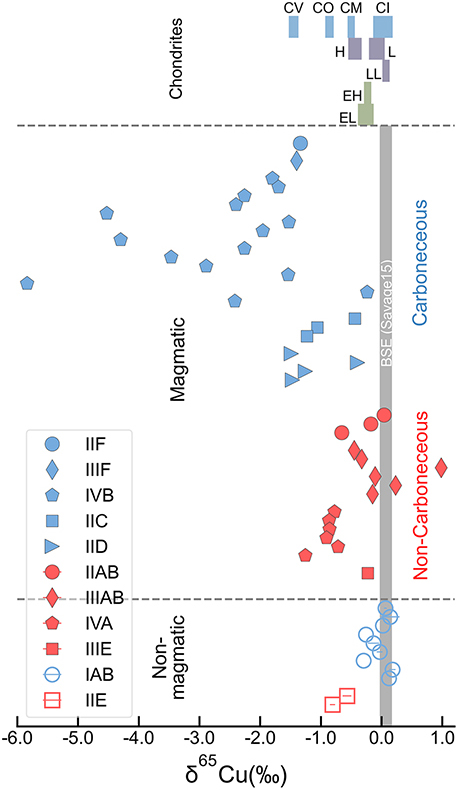
Figure 1 Copper isotope data for chondrites and iron meteorites. Data for different types of chondrites from Savage et al. (2015)
Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
and iron meteorites from Luck et al. (2005)Luck, J.-M., Othman, D.B., Albarède, F. (2005) Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochimica et Cosmochimica Acta 69, 5351–5363. https://doi.org/10.1016/j.gca.2005.06.018
, Bishop et al. (2012)Bishop, M.C., Moynier, F., Weinstein, C., Fraboulet, J.-G., Wang, K., Foriel, J. (2012) The Cu isotopic composition of iron meteorites. Meteoritics and Planetary Science 47, 268–276. https://doi.org/10.1111/j.1945-5100.2011.01326.x
, and Chen et al. (2016)Chen, H., Moynier, F., Humayun, M., Bishop, M.C., Williams, J.T. (2016) Cosmogenic effects on Cu isotopes in IVB iron meteorites. Geochimica et Cosmochimica Acta 182, 145–154. https://doi.org/10.1016/j.gca.2016.03.006
are plotted. The bulk silicate Earth (BSE) composition is from Savage et al. (2015)Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53–64. https://doi.org/10.7185/geochemlet.1506
.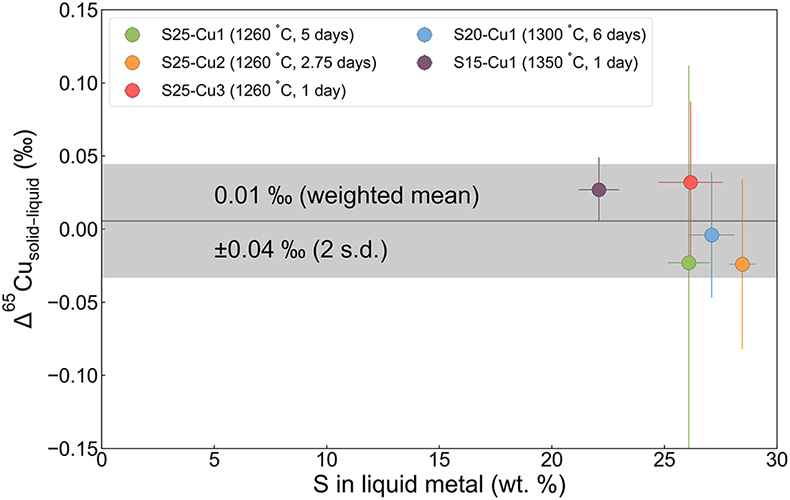
Figure 2 Equilibrium Cu isotope fractionation between solid and liquid metal during core crystallisation. A plot of all experimental data is available in Figure S-5. Error bars represent 2 standard deviations of the mean.
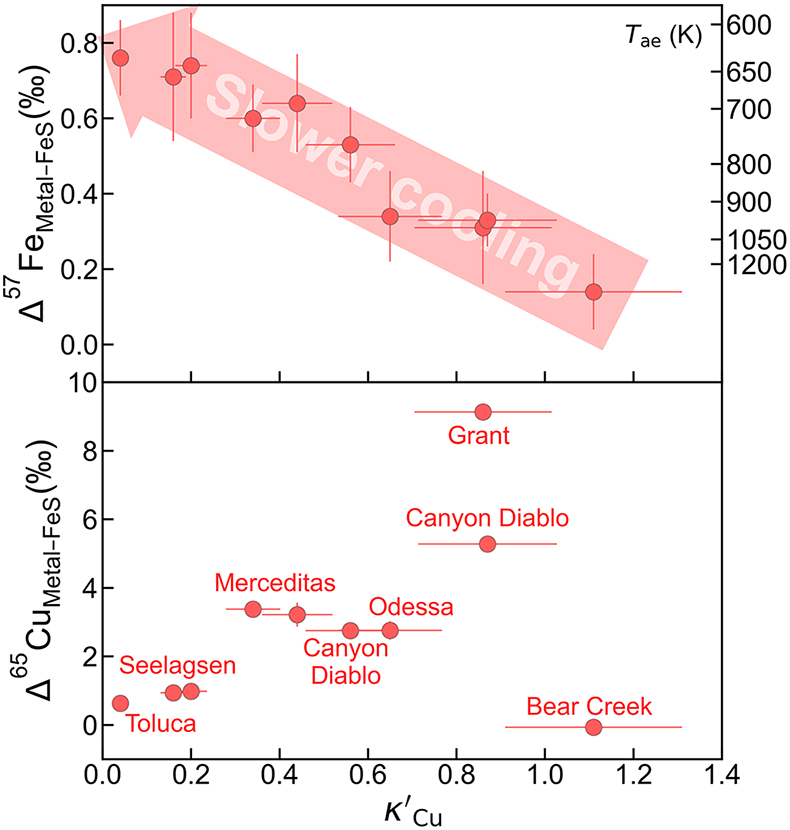
Figure 3 Iron and Cu isotope fractionation between metal and troilite in iron meteorites. Figure reproduced based on Williams and Archer (2011)
Williams, H.M., Archer, C. (2011) Copper stable isotopes as tracers of metal–sulphide segregation and fractional crystallisation processes on iron meteorite parent bodies. Geochimica et Cosmochimica Acta 75, 3166–3178. https://doi.org/10.1016/j.gca.2011.03.010
. The apparent partition coefficient of Cu is defined as κʹCu = CFeS(Cu)/CMeteorite(Cu). Calculated apparent equilibrium temperature (Tae) for Fe isotope fractionation is plotted on the right side of y-axis (see Supplementary Information).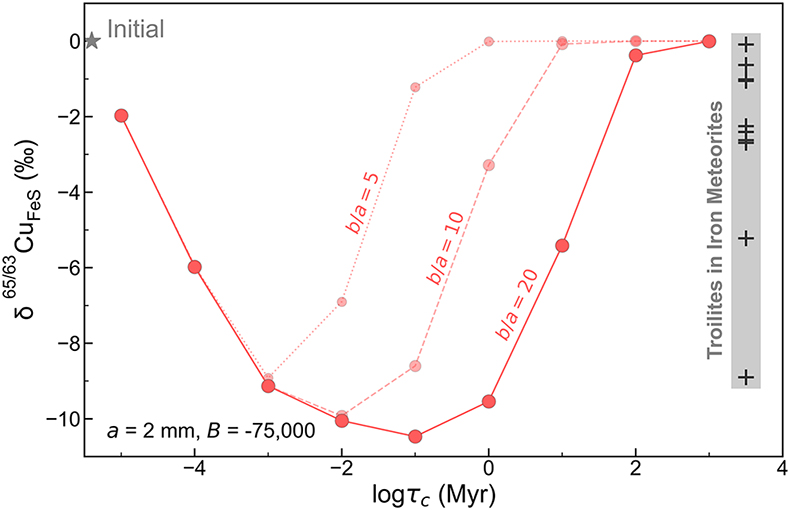
Figure 4 Modelled Cu isotope fractionation in troilite as a function of cooling time scale. The model was conducted assuming a troilite radius (a) of 2 mm, and a temperature dependence of the partition coefficient (B) of −75,000. Measured Cu isotope composition of troilites in iron meteorites is plotted on the right-hand side of the figure for comparison. Details of the model are described in the main text and in the Supplementary Information.