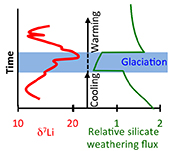
 | Global climate stabilisation by chemical weathering during the Hirnantian glaciation Abstract: Chemical weathering of silicate rocks is a primary drawdown mechanism of atmospheric carbon dioxide. The processes that affect weathering are therefore central in controlling global climate. A temperature-controlled “weathering thermostat” has long been proposed in stabilising long-term climate, but without definitive evidence from the geologic record. Here we use lithium isotopes (δ7Li) to assess the impact of silicate weathering across a significant climate-cooling period, the end-Ordovician Hirnantian glaciation (~445 Ma). We find a positive δ7Li excursion, suggestive of a silicate weathering decline. Using a coupled lithium-carbon model, we show that initiation of the glaciation was likely caused by declining CO2 degassing, which triggered abrupt global cooling, and much lower weathering rates. This lower CO2 drawdown during the glaciation allowed climatic recovery and deglaciation. Combined, the data and model provide support from the geological record for the operation of the weathering thermostat. |
 | Not so non-marine? Revisiting the Stoer Group and the Mesoproterozoic biosphere Abstract: The Poll a’Mhuilt Member of the Stoer Group (Torridonian Supergroup) in Scotland has been heralded as a rare window into the ecology of Mesoproterozoic terrestrial environments. Its unusually high molybdenum concentrations and large sulphur isotope fractionations have been used as evidence to suggest that lakes 1.2 billion years ago were better oxygenated and enriched in key nutrients relative to contemporaneous oceans, making them ideal habitats for the evolution of eukaryotes. Here we show with new Sr and Mo isotope data, supported by sedimentological evidence, that the depositional setting of this unit was likely connected to the ocean and that the elevated Mo and S contents can be explained by evapo-concentration of seawater. Thus, it remains unresolved if Mesoproterozoic lakes were important habitats for early eukaryotic life. |
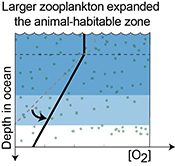 | Reorganisation of Earth’s biogeochemical cycles briefly oxygenated the oceans 520 Myr ago Abstract: The Phanerozoic radiation of bilaterian animals has been linked to oxygenation of Earth's oceans, due to the oxygen demand of the evolving animal ecosystems. However, how early animals may have regulated Earth’s surface oxygen budget via self-stabilising feedbacks is poorly understood. Here, we report parallel positive uranium, carbon, and sulphur isotope excursions from carbonate successions in Siberia that document a brief global oxygenation episode 521–520 Myr ago, at the onset of diversification of larger arthropods known from the fossil record. Our data and model indicate that an abrupt increase in the sinking rate of marine organic matter expanded the oxygenated zone in the oceans and that reducing conditions returned 1.3 ± 0.8 Myr after the onset of this transient oxygenation episode, necessitating a strong negative feedback to the increasing levels of oxygen. We speculate that larger zooplankton could have sourced both oxygen and food to the seafloor, fueling bioturbation over wider areas and, thereby, stabilising O2-rich habitats in the oceans. Thus, this reorganisation exemplifies how animal ecosystems might have influenced oxygen availability in Earth's surface environment soon after their establishment. |
 | The heterogeneous nature of Fe delivery from melting icebergs Abstract: The micronutrient iron (Fe) can be transported from marine terminating glaciers to the ocean by icebergs. There are however few observations of iceberg Fe content, and the flux of Fe from icebergs to the offshore surface ocean is poorly constrained. Here we report the dissolved Fe (DFe), total dissolvable Fe (TdFe) and ascorbic acid extractable Fe (FeAsc) sediment content of icebergs from Kongsfjorden, Svalbard. The concentrations of DFe (range 0.63 nM – 536 nM, mean 37 nM, median 6.5 nM) and TdFe (range 46 nM – 57 µM, mean 3.6 µM, median 144 nM) both demonstrated highly heterogeneous distributions and there was no significant correlation between these two fractions. FeAsc (range 0.0042 to 0.12 wt. %) was low compared to both previous measurements in Kongsfjorden and to current estimates of the global mean. FeAsc content per volume ice did however, as expected, show a significant relationship with sediment loading (which ranged from < 0.1 – 234 g L-1 of meltwater). In the Arctic, icebergs lose their sediment load faster than ice volume due to the rapid loss of basal ice after calving. We therefore suggest that the loss of basal ice is a potent mechanism for the reduction of mean TdFe and FeAsc per volume of iceberg. Delivery of TdFe and FeAsc to the ocean is thereby biased towards coastal waters where, in Kongsfjorden, DFe (18 ± 17 nM) and TdFe (mean 8.1 µM, median 3.7 µM) concentrations were already elevated. |
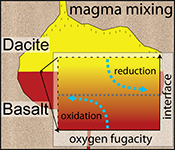 | A magma mixing redox trap that moderates mass transfer of sulphur and metals Abstract: Mixing and juxtaposition of chemically distinct magmatic systems are key processes for the evolution of Earth’s crust. Yet, the physicochemical nature at mixing interfaces remains poorly described, as crystallisation, melting, heat transfer, and diffusion are interconnected and lead to complex mass transfer processes driving unique patterns of element fractionation. Here, we use diffusion couple experiments between felsic and mafic magmas (melt + crystals ± volatiles) to document the formation of large gradients in oxygen fugacity at the magma-magma mixing interface. Reducing and oxidising boundary layers at the interface develop rapidly and remain in dynamic disequilibrium for days to possibly weeks. We suggest that the observed transient redox gradient is caused by cation transfer across the interface where the required counter flux of electron holes is insufficient to compensate an evolving electron hole gradient. Such boundary layer redox effects may control fractionation of polyvalent and chalcophile elements and moderate, for example, Cu/Au ratios in arc-related porphyry ore deposits. |
 | Temporal variation in relative zircon abundance throughout Earth history Abstract: Zircon is the preeminent chronometer of deep time on Earth, informing models of crustal growth and providing our only direct window into the Hadean Eon. However, the quantity of zircon crystallised per unit mass of magma is highly variable, complicating interpretation of the terrestrial zircon record. Here we combine zircon saturation simulations with a dataset of ∼52,000 igneous whole rock geochemical analyses to quantify secular variation in relative zircon abundance throughout Earth history. We find dramatically increasing zircon abundance per mass of magma through geologic time, suggesting that the zircon record underestimates past crustal volume even if preservation bias is eliminated. Similarly, zircons were even less likely to crystallise from low-silica magmas in early Earth history than they are today, together suggesting that the observed Hadean zircon record may require a larger volume of generally felsic Hadean crust than previously expected. |
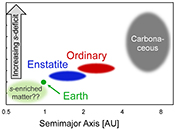 | The cosmic molybdenum-neodymium isotope correlation and the building material of the Earth Abstract: The isotopic similarity of enstatite chondrites and Earth has often been cited as evidence that the Earth is made of enstatite chondrite-like material. Here we show, however, that enstatite chondrites exhibit nucleosynthetic molybdenum (Mo) isotope anomalies and, therefore, cannot represent the sole building blocks of the Earth. Enstatite and ordinary chondrites together with the Earth’s mantle plot on a cosmic Mo-Nd isotope correlation line that reflects varying proportions of s-process matter in these samples. This correlation indicates that the nucleosynthetic makeup of Earth’s building material did not change over time and that Earth, on average, accreted from bodies that originated closer to the Sun and were enriched in s-process matter compared to known chondrites. As such, any contribution of chondrites to Earth’s accreting material must be compensated by the addition of s-process enriched bodies. This material is not present in our meteorite collections, but may have been sampled by Venus or Mercury. The s-process enriched nature of the Earth can fully account for its higher 142Nd compared to chondrites, which therefore does not require an early differentiation of Earth’s mantle. |
 | Determining subduction-zone fluid composition using a tourmaline mineral probe Abstract: Subduction zones are the sites where crustal materials are recycled into the mantle. In response to increasing pressures and temperatures during this process, hydrated minerals break down and release solute-bearing fluids and, above ~750 ˚C, form hydrous melts. Magmas generated by interaction of these melts and fluids with the mantle have a characteristic arc elemental signature. Here, a zoned refractory tourmaline grain, formed during Alpine subduction and uplift, was used to reconstruct the compositions of the fluids involved in element transfer. The reconstructed compositions confirm that slab-released fluids carry the arc-signature, and suggest that mineral–fluid element partitioning controls their compositions. However, these fluids are calculated to be dilute. To reconcile this with higher element-to-water ratios required for arc magmas, a two-stage arc-magma genesis model is favoured where fluids imprint their compositional signature progressively on a slab mélange that is subsequently transferred to, and interacts with the mantle to generate arc magmas. |
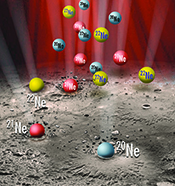 | Solar wind implantation supplied light volatiles during the first stage of Earth accretion Abstract: The isotopic and elemental compositions of noble gases constitute a powerful tool to study volatile origin and evolution, due to their inertness, and can thus provide crucial information about the early stage of planetary formation. Two models are proposed to explain the light noble gas origin on Earth: the solar wind implantation model and the solar nebula gas dissolution model. However, noble gas measurements often show addition of air to the mantle-derived gas, which complicates the determination of mantle isotopic ratios. We analysed the noble gas isotopic compositions of single vesicles in samples from the Galápagos hotspot with laser ablation, in order to understand and remove this atmospheric component, as well as discriminate between the two scenarios. Based on the new high precision results and a new statistical approach, we show that the solar wind implantation model is more likely to explain the terrestrial He, Ne and Ar composition. This scenario could bring important constraints on the solar system environment during the early stage of planetary formation. |
 | Distribution of Zn isotopes during Alzheimer’s disease Abstract: Alzheimer’s disease is associated with abnormal homeostasis of Zn, because of deposits of Zn-rich amyloid-β fibrils. This connection between Zn and brain aging provides a possibility to use changes in Zn homeostasis to study the evolution of the disease. Here, we studied the evolution of the Zn isotopic composition of brain, serum and red blood cells from APPswe/PSEN1dE9 transgenic mice, which develop Alzheimer’s-like disease (including Aβ deposition starting after 6 months), in comparison to wild type controls. We found that wild type brains become progressively enriched in the lighter isotopes of Zn between 6 and 12 months. We interpret this enrichment in terms of changes in Zn speciation in the cytoplasm, where isotopically heavy unbound Zn2+ is bound to glutamate released by presynaptic vesicles of neurons. Brains from APPswe/PSEN1dE9 mice were enriched in the heavier isotopes of Zn when compared to wild type suggesting an increase in Zn content of the brain associated with Aβ plaques where Zn binds preferentially to isotopically heavy amino acids such as histidine and glutamate. |
| Reply to Comment on “A cometary origin for martian atmospheric methane” by Crismani et al., 2017 Reply to Comment on “A cometary origin for martian atmospheric methane” by Crismani et al., 2017 First, I would like to extend thanks to Crismani et al.. for their commentary, which highlights an important uncertainty acknowledged in Fries et al.. (2016), namely whether the total mass of infall material required to produce the observed methane is on par with that available from meteor showers. I would like to disagree on two points presented in the letter, and then discuss the implications of their findings. | |
 | Comment on “A cometary origin for atmospheric martian methane” by Fries by Fries et al., 2016 Comment on “A cometary origin for atmospheric martian methane” by Fries et al., 2016 Reports of transient plumes of martian atmospheric methane (Mummaet al., 2009; Webster et al., 2015) have led to suggestions of biologic or abiotic surface sources. Schuerger et al. (2012) examined the production of methane near the surface from interplanetary dust particles. They found this mechanism was capable of yielding the background value of methane, but could not reproduce plume densities by bolide, airburst or other meteor impact process. Fries et al. (2016) draw on the work of Schuerger et al. (2012) and propose that the methane plumes are sourced instead from intense meteor showers with conversion at high altitudes. |
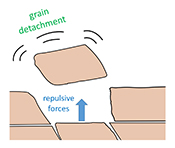 | Repulsion between calcite crystals and grain detachment during water–rock interaction Abstract: Weathering in carbonate rocks is often thought to be governed by chemical dissolution. However, recent studies have shown that mechanical detachment of tiny grains contributes significantly to the overall surface retreat. Whether this detachment is caused by shear forces acting at the surface, or repulsive forces acting between grains, was not known. In this study, we used atomic force microscopy to examine the mechanism of grain detachment and we demonstrate that it occurs even in the absence of shearing fluid flow. This suggests that the removal of grains from rock surfaces can be caused by repulsive forces between calcite grains. Although these repulsive forces are expected to be sensitive to the ionic strength of the solution, we did not find enough evidence to demonstrate a correlation between salinity and the frequency of grain detachment. Importantly, our findings suggest that grain detachment occurs during water-rock interaction under low flow conditions over a range of salinities, with potential impacts on geological carbon sequestration and enhanced oil recovery in carbonate formations. |
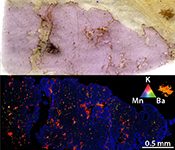 | Evidence of sub-arc mantle oxidation by sulphur and carbon Abstract: The oxygen fugacity (ƒO2) of the Earth’s mantle at subduction zones exerts a primary control on the genesis of mineral deposits in the overlying magmatic arcs and on speciation of volcanic gases emitted into the atmosphere. However, the processes governing mantle ƒO2 such as the introduction of oxidised material by subduction are still unresolved. Here, we present evidence for the reduction of oxidised fluid-borne sulphur and carbon during alteration of depleted mantle by slab fluids at ultra-high pressure in the Bardane peridotite (Western Gneiss Region, Norway). Elevated ferric iron in metasomatic garnet, determined using synchrotron X-ray absorption near edge structure (XANES) spectroscopy, indicates that this process drove oxidation of the silicate assemblage. Our finding indicates that subduction oxidises the Earth’s mantle by cycling of sulphur and carbon. |
 | Water in alkali feldspar: The effect of rhyolite generation on the lunar hydrogen budget Abstract: Recent detection of indigenous hydrogen in a diversity of lunar materials, including volcanic glass (Saal et al., 2008), melt inclusions (Hauri et al., 2011), apatite (Boyce et al., 2010; McCubbin et al., 2010), and plagioclase (Hui et al., 2013) suggests water played a role in the chemical differentiation of the Moon. Water contents measured in plagioclase feldspar, a dominant mineral in the ancient crustal lunar highlands have been used to predict that 320 ppm water initially existed in the lunar magma ocean (Hui et al., 2013) whereas measurements in apatite, found as a minor mineral in lunar rocks, representing younger potassium-enriched melt predict a bulk Moon with <100 ppm water. Here we show that water in alkali feldspar, a common mineral in potassium-enriched rocks, can have ~20 ppm water, which implies magmatic water contents of ~1 wt. % in chemically evolved rhyolitic magmas. The source for these wet, potassium-rich magmas probably contained ~1000 ppm H2O. Thus, lunar granites with ages from 4.3–3.9 Ga (Meyer et al., 1996) likely crystallised from relatively wet melts that degassed upon crystallisation. Geochemical surveys by the Lunar Prospector (Jolliff et al., 2011) and Diviner Lunar Radiometer Experiment (Glotch et al., 2010; Jolliff et al., 2011) indicating the global significance of evolved igneous rocks suggest that the formation of these granites removed water from some mantle source regions, helping to explain the existence of mare basalts with <10 ppm water, but must have left regions of the interior relatively wet as seen by the water content in volcanic glass and melt inclusions. Although these early-formed evolved melts were water-rich, their petrogenesis supports the conclusion that the Moon’s mantle had <100 ppm water for most of its history. |
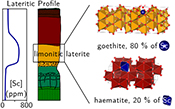 | Scandium speciation in a world-class lateritic deposit Abstract: Scandium (Sc) has unique properties, highly valued for many applications. Future supply is expected to rely on unusually high-grade (up to 1000 ppm) lateritic Sc ores discovered in Eastern Australia. To understand the origin of such exceptional concentrations, we investigated Sc speciation in one of these deposits. The major factors are unusually high concentrations in the parent rock together with lateritic weathering over long time scales in a stable tectonic context. At microscopic and atomic scales, by combining X-ray absorption near-edge structure spectroscopy, X-ray diffraction and microscopic and chemical analyses, we show that Sc-rich volumes are associated with iron oxides. In particular, Sc adsorbed on goethite accounts for ca. 80 % of the Sc budget in our samples. The remaining Sc is incorporated in the crystal structure of haematite, substituting for Fe3+. Scandium grades reflect the high capacity of goethite to adsorb this element. In contrast, the influence of haematite is limited by the low levels of Sc that its structure can incorporate. These crystal-chemical controls play a major role in lateritic Sc deposits developed over ultramafic–mafic rocks. |
<< Previous issueNext issue >>





