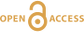Processes controlling carbon cycling in Antarctic glacier surface ecosystems
Affiliations | Corresponding Author | Cite asBagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Dubnick, A., Fitzsimons, S. (2016) Processes controlling carbon cycling in Antarctic glacier surface ecosystems. Geochem. Persp. Let. 2, 44-54.
- Share this article





-
Article views:9,569Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract

Figures and Tables
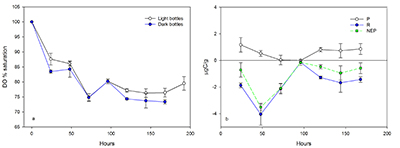 Figure 1 (a) Oxygen concentrations in dark and light bottles during the 200 hr incubation of cryolake sediment from Canada Glacier with (b) corresponding values of P, R and NEP. | 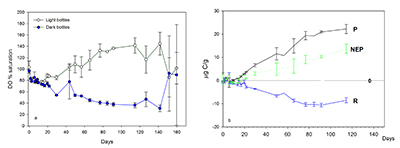 Figure 2 (a) Oxygen concentrations in dark and light bottles during the long term (one year) incubation of cryolake sediment from Joyce Glacier. (b) Corresponding values of P, R and NEP. |  Figure 3 Conceptual model of the exchange of oxygen and DIC between the water column, heterotrophs in the deeper sediment layers and phototrophs concentrated at the sediment surface, during early (a,d) middle (b,e) and later (c,f) stages of the long-term incubation in the dark (a-c) and dark (d-f) bottles. The panels are ordered left to right by time into the experiment, and the x-axis of each panel represents DIC and O2 concentrations relative to starting values (dotted line). |  Table 1 Initial deionised water (DIW) concentration and final concentrations of major ions, DO (% saturation), pH and the Saturation Index (SI) of calcite and aragonite in incubation waters after 360 days. Mean concentration (µeq L-1 minus blanks) of each bottle type is reported. DIC* and SI were calculated from mean concentrations by PHREEQC (phreeqc.dat database), assuming that T = 0 oC, and that Cl- = Na+ in the calculation of DIC. P values for one-tailed paired student’s t-tests show variance of: initial conditions vs. final dark and final light; and final light vs. final dark bottles. |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
Supplementary Figures and Tables
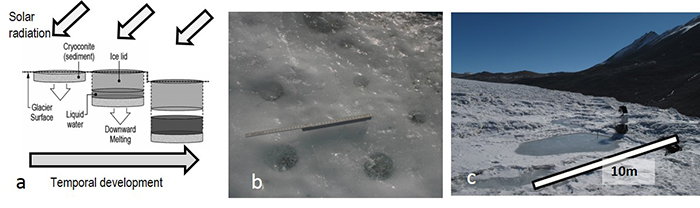 Figure S-1 (a) Absorption of solar radiation by debris results in the formation of cryoconite holes and cryolakes. (b) Examples of the clear refrozen lids of cryoconite holes on Canada Glacier (ruler = 40 cm). (c) An ice-lidded cryolake on Joyce Glacier. |
| Figure S-1 |
top
Introduction
Glacier surface ecosystems are significant sites of biological production in an otherwise nutrient poor landscape (Bagshaw et al., 2013
Bagshaw, E.A., Tranter, M., Fountain, A.G., Welch, K., Basagic, H.J., Lyons, W.B. (2013) Do cryoconite holes have the potential to be significant sources of C, N and P to downstream depauperate ecosystems of Taylor Valley, Antarctica? Arctic Antarctic and Alpine Research 45,4.
). Meltwater flushed from glacier surfaces contains bioavailable carbon, which has the potential to stimulate production in downstream ecosystems (Lawson et al., 2014Lawson, E.C., Wadham, J.L., Tranter, M., Stibal, M., Lis, G.P., Butler, C.E.H., Laybourn-Parry, J., Nienow, P., Chandler, D., Dewsbury, P. (2014) Greenland Ice Sheet exports labile organic carbon to the Arctic oceans. Biogeosciences 11, 14, 4015-4028.
). Glacier surfaces in Antarctica host a variety of ecological niches, including cryoconite holes, cryolakes, and shallow supraglacial streams. The cryoconite holes and cryolakes are formed by rock debris on the surfaces melting into the ice as a consequence of solar heating. The debris is commonly fine-grained and aeolian transported, but can also be coarse material resulting from medial moraines or rock avalanches. Cryoconite holes are small (~1-50 cm diameter, sediment depth 0.5-5 cm), whereas cryolakes are larger (length scales ~2-20 m, sediment depth 0.5-10 cm; Bagshaw et al., 2010Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Basagic, H. (2010) Dynamic behaviour of supraglacial lakes on cold polar glaciers: Canada Glacier, McMurdo Dry Valleys, Antarctica. Journal of Glaciology 56, 196, 366-368.
). Antarctic cryolakes differ from larger and more commonly reported supraglacial lakes on Arctic glaciers by the presence of both a permanent layer of debris at the lake bottom and a persistent ice lid (Bagshaw et al., 2012Bagshaw, E.A., Stibal, M., Anesio, A.M., Bellas, C., Tranter, M., Telling, J., Wadham, J.L. (2012) Glacier Surface Habitats. In: Bell, E.M. (2012) Life at Extremes: Environments, Organisms and Strategies for Survival. CAB International, Dunbeg, Argyll, UK, 537 pp.
), which also differentiates Antarctic cryoconite holes from their Arctic counterparts (Fig. S-1). Antarctic cryoconite holes persist for at least several years (Fountain et al., 2004Fountain, A.G., Tranter, M., Nylen, T.H., Lewis, K.J., Mueller, D.R. (2004) Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. Journal of Glaciology 50, 35-45.
) and host a variety of microorganisms, including cyanobacteria, tardigrades and rotifers (Christner et al., 2003Christner, B.C., Kvitko, B.H., Reeve, J.N. (2003) Molecular identification of Bacteria and Eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7, 177-183.
), likely inoculated by wind-blown fragments of algal mats and desiccated microorganisms (Nkem et al., 2006Nkem, J.N., Wall, D.H., Virginia, R.A., Barrett, J.E., Broos, E.J., Porazinska, D.L., Adams, B.J. (2006) Wind dispersal of soil invertebrates in the McMurdo Dry valleys, Antarctica. Polar Biology 29, 346-352.
). At shallow depths (<1 m) solar heating of the sediment melts the surrounding ice, so water is often present in these habitats, sealed beneath an ice cover of 3-20 cm, even when air temperatures are below 0 °C (Gribbon, 1979Gribbon, P.W. (1979) Cryoconite holes on Sermikaysak, West Greenland. Journal of Glaciology 22, 177-181.
; Fountain et al., 2004Fountain, A.G., Tranter, M., Nylen, T.H., Lewis, K.J., Mueller, D.R. (2004) Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. Journal of Glaciology 50, 35-45.
). Cryolakes are the largest reservoirs of liquid water in the typically frozen supraglacial environment (Fountain et al., 2004Fountain, A.G., Tranter, M., Nylen, T.H., Lewis, K.J., Mueller, D.R. (2004) Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. Journal of Glaciology 50, 35-45.
). The organic carbon and nutrient stored can affect downstream ecosystems when organic compounds and nutrients are flushed from the glacier surface by periodic high melt rates (Bagshaw et al., 2010Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Basagic, H. (2010) Dynamic behaviour of supraglacial lakes on cold polar glaciers: Canada Glacier, McMurdo Dry Valleys, Antarctica. Journal of Glaciology 56, 196, 366-368.
), eventually supporting enhanced biological activity in glacier forefields, lakes and streams (Foreman et al., 2004Foreman, C.M., Wolf, C.F., Priscu, J.C. (2004) Impact of episodic warming events on the physical, chemical and biological relationships of lakes in the McMurdo Dry Valleys, Antarctica. Aquatic Geochemistry 10, 239-268.
; Hood et al., 2015Hood, E., Battin, T.J., Fellman, J., O'Neel, S., Spencer, R.G.M. (2015) Storage and release of organic carbon from glaciers and ice sheets. Nature Geoscience 8, 91-96, doi: 10.1038/ngeo2331.
).The production of organic carbon on glacier surfaces has received considerable attention over the last decade (Anesio and Laybourn-Parry, 2012
Anesio, A.M., Laybourn-Parry, J. (2012) Glaciers and ice sheets as a biome. Trends in Ecology & Evolution 27, 4, 219-225, doi: 10.1016/j.tree.2011.09.012.
). New autochthonous organic carbon accumulates when production (P, Eq. 1) is greater than respiration (R, Eq. 2). Some studies find that P exceeds R in cryoconite holes (Anesio et al., 2009Anesio, A.M., Hodson, A.J., Fritz, A., Psenner, R., Sattler, B. (2009) High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biology 15, 955-960, doi: 10.1111/j.1365-2486.2008.01758.x.
), whilst others find that R exceeds P during the melt season (Stibal and Tranter, 2007Stibal, M., Tranter, M. (2007) Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. Journal of Geophysical Research-Biogeosciences 112, 9.
; Bagshaw et al., 2011Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
).Eq. 1
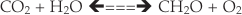
Eq. 2
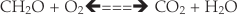
In Antarctica, P is generally much lower in ice-lidded cryoconite holes than in their open Arctic counterparts (Hodson et al., 2010a
Hodson, A., Cameron, K., Boggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., Banwart, S. (2010a) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. Journal of Glaciology 56, 349-362.
). Reported rates of P range from 1.3 (Bagshaw et al., 2011Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
) to 2.4 µg C g-1 d-1 (Hodson et al., 2010aHodson, A., Cameron, K., Boggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., Banwart, S. (2010a) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. Journal of Glaciology 56, 349-362.
), compared with 20.9 µg C g-1 d-1 (Telling et al., 2010Telling, J., Anesio, A.M., Hawkings, J., Tranter, M., Wadham, J.L., Hodson, A.J., Irvine-Fynn, T., Yallop, M.L. (2010) Measuring rates of gross photosynthesis and net community production in cryoconite holes: a comparison of field methods. Annals of Glaciology 51, 153-162.
) to 208 µg C L-1 d-1 (Anesio et al., 2009Anesio, A.M., Hodson, A.J., Fritz, A., Psenner, R., Sattler, B. (2009) High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biology 15, 955-960, doi: 10.1111/j.1365-2486.2008.01758.x.
) in cryoconite on Svalbard glaciers. Telling et al. (2010)Telling, J., Anesio, A.M., Hawkings, J., Tranter, M., Wadham, J.L., Hodson, A.J., Irvine-Fynn, T., Yallop, M.L. (2010) Measuring rates of gross photosynthesis and net community production in cryoconite holes: a comparison of field methods. Annals of Glaciology 51, 153-162.
elaborate on some of the methodological differences between these experiments. A plausible explanation for these apparently contradictory findings is that the microbially-dominated communities within ice-lidded cryoconite holes self-organise to recycle and redistribute resources, to balance P and R over longer timescales and maintain the longevity of the ecosystem. In Arctic systems, individual cryoconite holes do not tend to persist for multiple ablation seasons because sediment is more frequently mobilised by supraglacial meltwaters (Hodson et al., 2008Hodson, A., Anesio, A.M., Tranter, M., Fountain, A., Osborn, M., Priscu, J., Laybourn-Parry, J., Sattler, B. (2008) Glacial ecosystems. Ecological Monographs 78, 41-67.
). Instead, cryoconite granules form, which consist of polymer-bound sediment particles and microorganisms (Langford et al., 2010Langford, H., Hodson, A., Banwart, S., Boggild, C. (2010) The microstructure and biogeochemistry of Arctic cryoconite granules. Annals of Glaciology 51, 87-94.
; Takeuchi et al., 2010Takeuchi, N., Nishiyama, H., Li, Z. (2010) Structure and formation process of cryoconite granules on Ürümqi glacier No. 1, Tien Shan, China. Annals of Glaciology 51, 9-14, doi: 10.3189/172756411795932010.
) and likely maintain ecosystem longevity. Longer term trends in P and R are unlikely to be captured by traditional incubation techniques, which typically have durations of 24 hours. Incubations also disturb and/or mix the surface of the cm-deep sediment layer (Bagshaw et al., 2013Bagshaw, E.A., Tranter, M., Fountain, A.G., Welch, K., Basagic, H.J., Lyons, W.B. (2013) Do cryoconite holes have the potential to be significant sources of C, N and P to downstream depauperate ecosystems of Taylor Valley, Antarctica? Arctic Antarctic and Alpine Research 45,4.
), where autotrophic microorganisms are concentrated, with deeper sediment in which heterotrophic microorganisms flourish. This is similar to observations in cryoconite granules, where photosynthetic organisms are concentrated on the exterior of polymer-bound particles and heterotrophs are found in the interior (Langford et al., 2010Langford, H., Hodson, A., Banwart, S., Boggild, C. (2010) The microstructure and biogeochemistry of Arctic cryoconite granules. Annals of Glaciology 51, 87-94.
; Takeuchi et al., 2010Takeuchi, N., Nishiyama, H., Li, Z. (2010) Structure and formation process of cryoconite granules on Ürümqi glacier No. 1, Tien Shan, China. Annals of Glaciology 51, 9-14, doi: 10.3189/172756411795932010.
). We contend that there is a need to conduct longer term incubations over periods more representative of the whole melt season in order to examine the interplay between autotrophic processes at the sediment surface and heterotrophic processes within the deeper sediment.We report a combination of one week in–field incubations at Canada Glacier (77.6167 °S, 162.9833 °E), and longer term laboratory experiments, equivalent to two melt seasons, on cryolake sediments from Joyce Glacier (78.0243 °S, 163.7788 °E) (Fig. S-1), both in the McMurdo Dry Valleys, Antarctica. These incubations are much longer than those published to date, which are typically 24 hours in duration. Our aim is both to quantify the rates of P and R within cryolake sediment on these timescales, and to assess the interplay of microbial processes on the sediment surface and within the body of the sediment. We use these data to assess whether Antarctic cryolake ecosystems are in a state of net production or consumption of organic carbon.
top
Methods
Standard cryoconite incubation methods using oxygen microoptodes were employed (Hodson et al., 2010a
Hodson, A., Cameron, K., Boggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., Banwart, S. (2010a) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. Journal of Glaciology 56, 349-362.
; Telling et al., 2010Telling, J., Anesio, A.M., Hawkings, J., Tranter, M., Wadham, J.L., Hodson, A.J., Irvine-Fynn, T., Yallop, M.L. (2010) Measuring rates of gross photosynthesis and net community production in cryoconite holes: a comparison of field methods. Annals of Glaciology 51, 153-162.
; Bagshaw et al., 2011Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
), with experiment durations of one week for field incubations and 150 days for laboratory experiments. We equate dissolved oxygen concentration with carbon production (Telling et al., 2010Telling, J., Anesio, A.M., Hawkings, J., Tranter, M., Wadham, J.L., Hodson, A.J., Irvine-Fynn, T., Yallop, M.L. (2010) Measuring rates of gross photosynthesis and net community production in cryoconite holes: a comparison of field methods. Annals of Glaciology 51, 153-162.
). Briefly, the field experiments were run in situ using cryolake sediment from Canada Glacier, and laboratory experiments with incubated cryolake sediment from Joyce Glacier in conditions which mimic the glacier surface in terms of temperature, light and gas exchange. Uniform sediment depth of 1 cm was employed in all incubations. Full details of methods, instruments and analyses are available in the Supplementary Information.top
Results
The mean organic carbon (OC) and inorganic carbon (IC) content of the Joyce Glacier cryolake sediment were 0.12 ± 0.03 % (n = 6) and 0.34 ± 0.03 % (n = 6), respectively. For comparison, Canada Glacier cryoconite OC and IC were 0.18 ± 0.23 % (n = 117) and 0.07 ± 0.11 % (n = 10), respectively. The porosity of the sediment was 34 ± 11 % (n = 117). Heterotrophic processes (R) dominated throughout the 200 hour field experiment (Fig. 1). Oxygen decreased systematically over the first 100 hours in both the dark and light bottles. The absolute values of P, R and NEP are shown in Figure 1b, and are of the order of several µg C per gram of wet sediment throughout the experiment. This is consistent with data reported by Hodson et al. (2010a)
Hodson, A., Cameron, K., Boggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., Banwart, S. (2010a) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. Journal of Glaciology 56, 349-362.
for incubations of cryoconite from Blue Ice in East Antarctica.
Figure 1 (a) Oxygen concentrations in dark and light bottles during the 200 hr incubation of cryolake sediment from Canada Glacier with (b) corresponding values of P, R and NEP.
Dissolved oxygen (DO) variations in the long-term laboratory incubations of cryolake sediment from Joyce Glacier also show net heterotrophy during the first week (200 hr, Fig. 2), and DO concentrations in the light and dark bottles again show strong similarity. The DO concentrations begin to diverge after 380 hr (16 d), with the light bottles eventually becoming supersaturated after 1000 hr (40 d). The DO concentrations in the light bottles increase to 140 % saturation after 250 hr, a value that has been observed in cryoconite holes on Canada Glacier (Tranter et al., 2004
Tranter, M., Fountain, A.G., Fritsen, C.H., Lyons, W.B., Priscu, J.C., Statham, P.J., Welch K.A. (2004) Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrological Processes 18, 379-387.
; Bagshaw et al., 2011Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
). The dark bottles become increasingly undersaturated, falling to values of 23 %.The overall NEP in these long term incubations is 110 µg C. The dissolved inorganic carbon (DIC) content in the deionised water at the start of the experiment was ~32.2 µeq L-1, or 39 µg C, as calculated by PHREEQC assuming that the 100 ml water is at 0 oC and in equilibrium with an atmospheric CO2 concentration of 380 ppm. Since the OC content of the sediment was 0.12 %, the ~25 g of wet sediment contained ~20 mg C (water content of 34 %). These calculations demonstrate that the initial water does not contain sufficient DIC to account for all the new organic matter fixed on the surface, and suggests that carbon must be transferred via respiration in the sediment body to the surface to make up the shortfall in available DIC.

Figure 2 (a) Oxygen concentrations in dark and light bottles during the long term (one year) incubation of cryolake sediment from Joyce Glacier. (b) Corresponding values of P, R and NEP.
Conceptual model. The initial stages of the longer term incubations (Fig. 2) are similar to the shorter term incubations (Fig. 1), in that both sets of incubations show unequivocal net heterotrophy over timescales of ~8 days. This is consistent with other studies which use 24 hr incubation of cryoconite from Antarctica (Hodson et al., 2010a
Hodson, A., Cameron, K., Boggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., Banwart, S. (2010a) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. Journal of Glaciology 56, 349-362.
,bHodson, A., Boggild, C., Hanna, E., Huybrechts, P., Langford, H., Cameron, K., Houldsworth, A. (2010b) The cryoconite ecosystem on the Greenland ice sheet. Annals of Glaciology 51, 123-129.
; Bagshaw et al., 2011Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
; Hodson et al., 2013Hodson, A., Paterson, H., Westwood, K., Cameron, K., Laybourn-Parry, J. (2013) A blue-ice ecosystem on the margins of the East Antarctic ice sheet. Journal of Glaciology 59, 255-268, doi: 10.3189/2013JoG12J052.
). However, the longer term incubations suggest that more complex interactions control the overall C balance, with net heterotrophy shifting to net autotrophy over timescales of >~20 days. Phototrophs in the sediment only fix organic carbon in the illuminated zone, often forming mat-like features (Hodson et al., 2010aHodson, A., Cameron, K., Boggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., Banwart, S. (2010a) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. Journal of Glaciology 56, 349-362.
). The phototrophs were physically disturbed during transfer of the sediment into the incubation bottles at the start of the experiment, so we hypothesise that they can only begin to colonise the surface layer after the experiment begins. Consistent with this were the green-coloured algal mats and granules, visible on the sediment surface by the end of our longer light incubation, which have occasionally been observed in the field. By contrast, the heterotrophic community is less sensitive to physical disturbance and may even be stimulated by the burial of fresh surface organic matter, so it is unsurprising that the incubations show net heterotrophy during the initial stages.A simple conceptual model of O2 and DIC fluxes into and out of the sediment accounts for the observed changes in NEP. Figures 2 and 3(d-f) show that during the first days of the incubation (<50 hr) in the light bottles, rates of heterotrophic activity in the sediment are greater than the rate of photosynthesis in the surface layer. Hence, oxygen concentrations become lower in the sediment, and consequently there is net diffusion of oxygen from the overlying water into the sediment. Simultaneously, the relatively high rate of heterotrophic activity in the sediment results in elevated concentrations of DIC, and so there is diffusion of DIC from the sediment into the overlying water. Gradually, the phototrophs increase in biomass, and start to elevate O2 concentrations in the water, utilising DIC diffusing up through the sediment. Continued heterotrophic activity deeper in the sediment further depletes sediment O2. The rate of heterotrophic activity is likely to diminish over time at depth, as more labile organic matter sources are exhausted and DO concentrations diminish. Near surface layers, in closer diffusive contact with O2 production, maintain higher DO concentrations. In the dark bottles, there are no phototrophic processes, and so concentrations of DO decrease continuously in both the water and sediment (Fig. 3a-c).

Figure 3 Conceptual model of the exchange of oxygen and DIC between the water column, heterotrophs in the deeper sediment layers and phototrophs concentrated at the sediment surface, during early (a,d) middle (b,e) and later (c,f) stages of the long-term incubation in the dark (a-c) and dark (d-f) bottles. The panels are ordered left to right by time into the experiment, and the x-axis of each panel represents DIC and O2 concentrations relative to starting values (dotted line).
Geochemical balancing. Heterotrophic activity in the sediment generates CO2, which is used in photosynthesis, but also dissolves carbonate minerals (Eq. 3). The chemical composition of the final dark and light bottles is consistent with this assertion, since both Ca2+ and HCO3- have increased. The final DIC concentrations are 761 and 241 ueq L-1 respectively, and this presents, superficially, a problem with the calculation of P and R from DIC changes. If all the DIC was derived from heterotrophic reactions in the sediment, and the initial DIC concentration of the water was 32.2 µmol L-1, then R would be equivalent to 913 µg C, and P equivalent to 289 µg C. This implies that the incubation is net heterotrophic, and that 624 µg C net of organic matter has been assimilated within the sediment, which is at odds with the conclusions reached based upon changes in DO alone.
Eq. 3
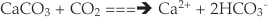
The large discrepancy with the net autotrophy suggested by the DO changes (+110 µg C vs -624 µg C), can be resolved by considering the geochemical balance of the incubation. Heterotrophic activity in the sediment produces CO2 which chemically weathers carbonate and silicate minerals in the sediment. This causes an increase in Ca2+, Mg2+, K+ and HCO3- in the sediment pore waters, which diffuse into the overlying waters. However, the concentration of these species is very different between the final light and dark bottles (Table 1). Photosynthesis in a closed aquatic system increases the pH of the solution and causes increasing saturation with aragonite and calcite (Table 1; Tranter et al., 2004
Tranter, M., Fountain, A.G., Fritsen, C.H., Lyons, W.B., Priscu, J.C., Statham, P.J., Welch K.A. (2004) Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrological Processes 18, 379-387.
). It is notable that Table 1 shows that water is approximately saturated with calcite and aragonite in the light bottles, but is under-saturated in the dark bottles. Hence, as heterotrophic activity within the sediment causes Ca2+ and HCO3- to diffuse through the illuminated sediment surface into the overlying water, photosynthesis, predominantly at the sediment-water interface, saturates the waters and causes carbonate precipitation (Eq. 4). The latter prevents high accumulation of DIC in the light bottles, in contrast to the dark bottles. The actual concentration of Ca2+ and DIC found in solution is thus a kinetic balance between the rate of diffusion of Ca2+ and DIC from the sediment and the rate of photosynthesis. This is a similar scenario to that found at the seafloor (Wilson et al., 1985Wilson, T.R.S., Thomson, J., Colley, S., Hydes, D.J., Higgs, N.C., Sørensen, J. (1985) Early organic diagenesis: The significance of progressive subsurface oxidation fronts in pelagic sediments. Geochimica et Cosmochimica Acta 49, 811-822, doi: 10.1016/0016-7037(85)90174-7.
).Eq. 4
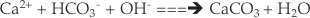
The oxygen depletion in the dark bottle is ~340 µeq L-1. The measured increase in DIC in the dark bottles is ~730 µeq L-1, over double that inferred from the oxygen measurements. This suggests that carbonate minerals are being dissolved in the sediment, rather than silicate minerals, since one mole of rock DIC is liberated for each mole of CO2 generated from oxidation of organic matter. This supports our assertion that carbonate dissolution supplies the additional DIC for the positive NEP that was measured.
Table 1 Initial deionised water (DIW) concentration and final concentrations of major ions, DO (% saturation), pH and the Saturation Index (SI) of calcite and aragonite in incubation waters after 360 days. Mean concentration (µeq L-1 minus blanks) of each bottle type is reported. DIC* and SI were calculated from mean concentrations by PHREEQC (phreeqc.dat database), assuming that T = 0 oC, and that Cl- = Na+ in the calculation of DIC. P values for one-tailed paired student’s t-tests show variance of: initial conditions vs. final dark and final light; and final light vs. final dark bottles.
| Sample | Cl- | NO3- | SO42- | K+ | Mg2+ | Ca2+ | DO | pH | DIC* | SIaragonite | SIcalcite |
| DIW (initial) | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 5.55 | 32.2 | n/a | n/a |
| Light (final) | 4.11 | 0 | 23.3 | 37.1 | 0.25 | 290 | 122 | 9.97 | 241 | -0.08 | 0.09 |
| St dev. | 0.01 | 0.04 | 8.84 | 0.5 | 8.34 | 68.7 | 8.06 | 0.49 | n/a | n/a | n/a |
| T-Test (initial vs. light) | 0 | 0 | 0.08 | 0 | 0.49 | 0.05 | 0.07 | 0.06 | 0.15 | n/a | n/a |
| Dark (final) | 4.84 | 61.9 | 24.9 | 71.1 | 48.6 | 735 | 22.5 | 8.66 | 761 | -0.24 | -0.4 |
| St dev. | 1.02 | 11.75 | 6.18 | 8.32 | 9.54 | 131 | 16.4 | 0.01 | n/a | n/a | n/a |
| T-Test (initial vs. dark) | 0.05 | 0.04 | 0.06 | 0.03 | 0.04 | 0.04 | 0.05 | 0 | 0.02 | n/a | n/a |
| T-Test (light vs. dark) | 0.25 | 0.04 | 0.45 | 0.06 | 0.01 | 0.1 | 0.05 | 0.08 | 0.01 | n/a | n/a |
Significance for carbon budgets. These results demonstrate that glacier surface ecosystems such as cryolakes and cryoconite holes can exist in a state of net production. Processes of carbon cycling have long been documented in cryoconite holes (Tranter et al., 2004
Tranter, M., Fountain, A.G., Fritsen, C.H., Lyons, W.B., Priscu, J.C., Statham, P.J., Welch K.A. (2004) Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrological Processes 18, 379-387.
; Bagshaw et al., 2007Bagshaw, E.A., Tranter, M., Fountain, A.G., Welch, K., Basagic, H.J., Lyons, W.B. (2007) Biogeochemical evolution of cryoconite holes on Canada Glacier, Taylor Valley, Antarctica. Journal of Geophysical Research-Biogeosciences 112, 8, doi: 10.1029/2007jg000442.
; Foreman et al., 2007Foreman, C.M., Sattler, B., Mickuki, J., Porazinska, D., Priscu, J.C., Priscu, L. (2007) Metabolic Activity of Cryoconites in the Taylor Valley, Antarctica. Journal of Geophysical Research: Biogeosciences 112, G04S32 doi: 10.1029/2006JG000358.
; Stibal and Tranter, 2007Stibal, M., Tranter, M. (2007) Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. Journal of Geophysical Research-Biogeosciences 112, 9.
), but the extent of the dependence of photosynthetic organisms concentrated at the surface of the sediment layer on heterotrophic processes deeper in the sediment has not been fully discussed in the literature to date. This inter-dependence may also help explain the link between sediment depth and P:R balance previously observed in short term (6-24 hr) cryoconite incubations (Telling et al., 2012Telling, J., Anesio, A.M., Tranter, M., Stibal, M., Hawkings, J., Irvine-Fynn, T., Hodson, A., Butler, C., Yallop, M.L., Wadham, J. (2012) Controls on the autochthonous production and respiration of organic matter in cryoconite holes on High Arctic glaciers. Journal of Geophysical Research: Biogeosciences 117, G01017, doi: 10.1029/2011JG001828.
). The heterotrophic activity in the deeper sediment serves to relocate organic matter, fixed as CO2, from the deeper layers to the surface of the sediment, and to dissolve carbonate minerals. Should the carbonate minerals be ancient, then the new organic matter fixed from their dissolved DIC will be old in radiocarbon terms. It follows that extracellular exudates and the contents of lysed and partially degraded cells this otherwise new, yet radiocarbon old, organic matter, will produce radiocarbon old dissolved OC (DOC). This combination of processes may help to explain why labile DOC in supraglacial runoff is radiocarbon old (Lawson et al., 2014Lawson, E.C., Wadham, J.L., Tranter, M., Stibal, M., Lis, G.P., Butler, C.E.H., Laybourn-Parry, J., Nienow, P., Chandler, D., Dewsbury, P. (2014) Greenland Ice Sheet exports labile organic carbon to the Arctic oceans. Biogeosciences 11, 14, 4015-4028.
; Hood et al., 2015Hood, E., Battin, T.J., Fellman, J., O'Neel, S., Spencer, R.G.M. (2015) Storage and release of organic carbon from glaciers and ice sheets. Nature Geoscience 8, 91-96, doi: 10.1038/ngeo2331.
). Microbes on glacier surfaces may utilise this ancient DOC in their metabolic processes and augment it with organic matter fixed from the dissolution of ancient carbonates.top
Conclusions
Short-term field and longer-term laboratory incubations demonstrate that autotrophic and heterotrophic processes in glacier surface sediments are closely linked. In the short term, carbon consumption equals or exceeds production if the initial DIC concentrations in the waters are low. This is particularly the case when the sediment depth is sufficient to eliminate light beyond surface layers. If, however, there are sufficient carbonate minerals in the sediment which dissolve to provide additional DIC for fixing, net heterotrophy may eventually give rise to net autotrophy. The rate at which this occurs depends on dissolution of carbonate minerals and diffusion of DIC through the sediment into the surface; hence there is a relationship between primary production in cryoconite and sediment depth, as has previously been reported. We hypothesise that fixation of DIC from carbonate dissolution and the consequence recycling of organic matter has the potential to form radiocarbon old DOC from otherwise young organic matter. These results demonstrate that microbial communities on glacier surfaces do have the potential to accumulate carbon on timescales of months to years.
top
Acknowledgements
This work was supported by EPSRC grant EP/D057620/1, NERC grant NE/I008845/1 and NSF grant ANT-0423595. Fieldwork was conducted with the McMurdo Dry Valleys LTER and Antarctica New Zealand K064 site teams whose support is gratefully acknowledged, with logistics provided by Raytheon Polar Services and PHI Helicopters. Three anonymous reviewers provided helpful suggestions which improved the manuscript.
Editor: Eric H. Oelkers
top
References
Anesio, A.M., Hodson, A.J., Fritz, A., Psenner, R., Sattler, B. (2009) High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biology 15, 955-960, doi: 10.1111/j.1365-2486.2008.01758.x.
 Show in context
Show in context Some studies find that P exceeds R in cryoconite holes (Anesio et al., 2009), whilst others find that R exceeds P during the melt season (Stibal and Tranter, 2007; Bagshaw et al., 2011).
View in article
Reported rates of P range from 1.3 (Bagshaw et al., 2011) to 2.4 µg C g-1 d-1 (Hodson et al., 2010a), compared with 20.9 µg C g-1 d-1 (Telling et al., 2010) to 208 µg C L-1 d-1 (Anesio et al., 2009) in cryoconite on Svalbard glaciers.
View in article
Anesio, A.M., Laybourn-Parry, J. (2012) Glaciers and ice sheets as a biome. Trends in Ecology & Evolution 27, 4, 219-225, doi: 10.1016/j.tree.2011.09.012.
 Show in context
Show in context The production of organic carbon on glacier surfaces has received considerable attention over the last decade (Anesio and Laybourn-Parry, 2012).
View in article
Bagshaw, E.A., Tranter, M., Fountain, A.G., Welch, K., Basagic, H.J., Lyons, W.B. (2007) Biogeochemical evolution of cryoconite holes on Canada Glacier, Taylor Valley, Antarctica. Journal of Geophysical Research-Biogeosciences 112, 8, doi: 10.1029/2007jg000442.
 Show in context
Show in context Processes of carbon cycling have long been documented in cryoconite holes (Tranter et al., 2004; Bagshaw et al., 2007; Foreman et al., 2007; Stibal and Tranter, 2007), but the extent of the dependence of photosynthetic organisms concentrated at the surface of the sediment layer on heterotrophic processes deeper in the sediment has not been fully discussed in the literature to date.
View in article
Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Basagic, H. (2010) Dynamic behaviour of supraglacial lakes on cold polar glaciers: Canada Glacier, McMurdo Dry Valleys, Antarctica. Journal of Glaciology 56, 196, 366-368.
 Show in context
Show in context Cryoconite holes are small (~1-50 cm diameter, sediment depth 0.5-5 cm), whereas cryolakes are larger (length scales ~2-20 m, sediment depth 0.5-10 cm; Bagshaw et al., 2010).
View in article
The organic carbon and nutrient stored can affect downstream ecosystems when organic compounds and nutrients are flushed from the glacier surface by periodic high melt rates (Bagshaw et al., 2010), eventually supporting enhanced biological activity in glacier forefields, lakes and streams (Foreman et al., 2004; Hood et al., 2015).
View in article
Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
 Show in context
Show in context Some studies find that P exceeds R in cryoconite holes (Anesio et al., 2009), whilst others find that R exceeds P during the melt season (Stibal and Tranter, 2007; Bagshaw et al., 2011).
View in article
Reported rates of P range from 1.3 (Bagshaw et al., 2011) to 2.4 µg C g-1 d-1 (Hodson et al., 2010a), compared with 20.9 µg C g-1 d-1 (Telling et al., 2010) to 208 µg C L-1 d-1 (Anesio et al., 2009) in cryoconite on Svalbard glaciers.
View in article
Standard cryoconite incubation methods using oxygen microoptodes were employed (Hodson et al., 2010a; Telling et al., 2010; Bagshaw et al., 2011), with experiment durations of one week for field incubations and 150 days for laboratory experiments.
View in article
The DO concentrations in the light bottles increase to 140 % saturation after 250 hr, a value that has been observed in cryoconite holes on Canada Glacier (Tranter et al., 2004; Bagshaw et al., 2011).
View in article
This is consistent with other studies which use 24 hr incubation of cryoconite from Antarctica (Hodson et al., 2010a,b; Bagshaw et al., 2011; Hodson et al., 2013).
View in article
Bagshaw, E.A., Stibal, M., Anesio, A.M., Bellas, C., Tranter, M., Telling, J., Wadham, J.L. (2012) Glacier Surface Habitats. In: Bell, E.M. (2012) Life at Extremes: Environments, Organisms and Strategies for Survival. CAB International, Dunbeg, Argyll, UK, 537 pp.
 Show in context
Show in context Antarctic cryolakes differ from larger and more commonly reported supraglacial lakes on Arctic glaciers by the presence of both a permanent layer of debris at the lake bottom and a persistent ice lid (Bagshaw et al., 2012), which also differentiates Antarctic cryoconite holes from their Arctic counterparts (Fig. S-1).
View in article
Bagshaw, E.A., Tranter, M., Fountain, A.G., Welch, K., Basagic, H.J., Lyons, W.B. (2013) Do cryoconite holes have the potential to be significant sources of C, N and P to downstream depauperate ecosystems of Taylor Valley, Antarctica? Arctic Antarctic and Alpine Research 45, 4.
 Show in context
Show in context Glacier surface ecosystems are significant sites of biological production in an otherwise nutrient poor landscape (Bagshaw et al., 2013).
View in article
Incubations also disturb and/or mix the surface of the cm-deep sediment layer (Bagshaw et al., 2013), where autotrophic microorganisms are concentrated, with deeper sediment in which heterotrophic microorganisms flourish.
View in article
Christner, B.C., Kvitko, B.H., Reeve, J.N. (2003) Molecular identification of Bacteria and Eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7, 177-183.
 Show in context
Show in context Antarctic cryoconite holes persist for at least several years (Fountain et al., 2004) and host a variety of microorganisms, including cyanobacteria, tardigrades and rotifers (Christner et al., 2003), likely inoculated by wind-blown fragments of algal mats and desiccated microorganisms (Nkem et al., 2006).
View in article
Foreman, C.M., Wolf, C.F., Priscu, J.C. (2004) Impact of episodic warming events on the physical, chemical and biological relationships of lakes in the McMurdo Dry Valleys, Antarctica. Aquatic Geochemistry 10, 239-268.
 Show in context
Show in contextThe organic carbon and nutrient stored can affect downstream ecosystems when organic compounds and nutrients are flushed from the glacier surface by periodic high melt rates (Bagshaw et al., 2010), eventually supporting enhanced biological activity in glacier forefields, lakes and streams (Foreman et al., 2004; Hood et al., 2015).
View in article
Foreman, C.M., Sattler, B., Mickuki, J., Porazinska, D., Priscu, J.C., Priscu, L. (2007) Metabolic Activity of Cryoconites in the Taylor Valley, Antarctica. Journal of Geophysical Research: Biogeosciences 112, G04S32 doi: 10.1029/2006JG000358.
 Show in context
Show in context Processes of carbon cycling have long been documented in cryoconite holes (Tranter et al., 2004; Bagshaw et al., 2007; Foreman et al., 2007; Stibal and Tranter, 2007), but the extent of the dependence of photosynthetic organisms concentrated at the surface of the sediment layer on heterotrophic processes deeper in the sediment has not been fully discussed in the literature to date.
View in article
Fountain, A.G., Tranter, M., Nylen, T.H., Lewis, K.J., Mueller, D.R. (2004) Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. Journal of Glaciology 50, 35-45.
 Show in context
Show in context Antarctic cryoconite holes persist for at least several years (Fountain et al., 2004) and host a variety of microorganisms, including cyanobacteria, tardigrades and rotifers (Christner et al., 2003), likely inoculated by wind-blown fragments of algal mats and desiccated microorganisms (Nkem et al., 2006).
View in article
At shallow depths (<1 m) solar heating of the sediment melts the surrounding ice, so water is often present in these habitats, sealed beneath an ice cover of 3-20 cm, even when air temperatures are below 0 °C (Gribbon, 1979; Fountain et al., 2004).
View in article
Cryolakes are the largest reservoirs of liquid water in the typically frozen supraglacial environment (Fountain et al., 2004).
View in article
Gribbon, P.W. (1979) Cryoconite holes on Sermikaysak, West Greenland. Journal of Glaciology 22, 177-181.
 Show in context
Show in context At shallow depths (<1 m) solar heating of the sediment melts the surrounding ice, so water is often present in these habitats, sealed beneath an ice cover of 3-20 cm, even when air temperatures are below 0 °C (Gribbon, 1979; Fountain et al., 2004).
View in article
Hodson, A., Anesio, A.M., Tranter, M., Fountain, A., Osborn, M., Priscu, J., Laybourn-Parry, J., Sattler, B. (2008) Glacial ecosystems. Ecological Monographs 78, 41-67.
 Show in context
Show in context In Arctic systems, individual cryoconite holes do not tend to persist for multiple ablation seasons because sediment is more frequently mobilised by supraglacial meltwaters (Hodson et al., 2008).
View in article
Hodson, A., Cameron, K., Boggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., Banwart, S. (2010a) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. Journal of Glaciology 56, 349-362.
 Show in context
Show in context
In Antarctica, P is generally much lower in ice-lidded cryoconite holes than in their open Arctic counterparts (Hodson et al., 2010a).
View in article
Reported rates of P range from 1.3 (Bagshaw et al., 2011) to 2.4 µg C g-1 d-1 (Hodson et al., 2010a), compared with 20.9 µg C g-1 d-1 (Telling et al., 2010) to 208 µg C L-1 d-1 (Anesio et al., 2009) in cryoconite on Svalbard glaciers.
View in article
Standard cryoconite incubation methods using oxygen microoptodes were employed (Hodson et al., 2010a; Telling et al., 2010; Bagshaw et al., 2011), with experiment durations of one week for field incubations and 150 days for laboratory experiments.
View in article
This is consistent with data reported by Hodson et al. (2010a) for incubations of cryoconite from Blue Ice in East Antarctica.
View in article
This is consistent with other studies which use 24 hr incubation of cryoconite from Antarctica (Hodson et al., 2010a,b; Bagshaw et al., 2011; Hodson et al., 2013).
View in article
Phototrophs in the sediment only fix organic carbon in the illuminated zone, often forming mat-like features (Hodson et al., 2010a).
View in article
Hodson, A., Boggild, C., Hanna, E., Huybrechts, P., Langford, H., Cameron, K., Houldsworth, A. (2010b) The cryoconite ecosystem on the Greenland ice sheet. Annals of Glaciology 51, 123-129.
 Show in context
Show in context This is consistent with other studies which use 24 hr incubation of cryoconite from Antarctica (Hodson et al., 2010a,b; Bagshaw et al., 2011; Hodson et al., 2013).
View in article
Hodson, A., Paterson, H., Westwood, K., Cameron, K., Laybourn-Parry, J. (2013) A blue-ice ecosystem on the margins of the East Antarctic ice sheet. Journal of Glaciology 59, 255-268, doi: 10.3189/2013JoG12J052.
 Show in context
Show in context This is consistent with other studies which use 24 hr incubation of cryoconite from Antarctica (Hodson et al., 2010a,b; Bagshaw et al., 2011; Hodson et al., 2013).
View in article
Hood, E., Battin, T.J., Fellman, J., O'Neel, S., Spencer, R.G.M. (2015) Storage and release of organic carbon from glaciers and ice sheets. Nature Geoscience 8, 91-96, doi: 10.1038/ngeo2331.
 Show in context
Show in context The organic carbon and nutrient stored can affect downstream ecosystems when organic compounds and nutrients are flushed from the glacier surface by periodic high melt rates (Bagshaw et al., 2010), eventually supporting enhanced biological activity in glacier forefields, lakes and streams (Foreman et al., 2004; Hood et al., 2015).
View in article
This combination of processes may help to explain why labile DOC in supraglacial runoff is radiocarbon old (Lawson et al., 2014; Hood et al., 2015).
View in article
Langford, H., Hodson, A., Banwart, S., Boggild, C. (2010) The microstructure and biogeochemistry of Arctic cryoconite granules. Annals of Glaciology 51, 87-94.
 Show in context
Show in context Instead, cryoconite granules form, which consist of polymer-bound sediment particles and microorganisms (Langford et al., 2010; Takeuchi et al., 2010) and likely maintain ecosystem longevity.
View in article
This is similar to observations in cryoconite granules, where photosynthetic organisms are concentrated on the exterior of polymer-bound particles and heterotrophs are found in the interior (Langford et al., 2010; Takeuchi et al., 2010).
View in article
Lawson, E.C., Wadham, J.L., Tranter, M., Stibal, M., Lis, G.P., Butler, C.E.H., Laybourn-Parry, J., Nienow, P., Chandler, D., Dewsbury, P. (2014) Greenland Ice Sheet exports labile organic carbon to the Arctic oceans. Biogeosciences 11, 14, 4015-4028.
 Show in context
Show in context Meltwater flushed from glacier surfaces contains bioavailable carbon, which has the potential to stimulate production in downstream ecosystems (Lawson et al., 2014).
View in article
This combination of processes may help to explain why labile DOC in supraglacial runoff is radiocarbon old (Lawson et al., 2014; Hood et al., 2015).
View in article
Nkem, J.N., Wall, D.H., Virginia, R.A., Barrett, J.E., Broos, E.J., Porazinska, D.L., Adams, B.J. (2006) Wind dispersal of soil invertebrates in the McMurdo Dry valleys, Antarctica. Polar Biology 29, 346-352.
 Show in context
Show in context Antarctic cryoconite holes persist for at least several years (Fountain et al., 2004) and host a variety of microorganisms, including cyanobacteria, tardigrades and rotifers (Christner et al., 2003), likely inoculated by wind-blown fragments of algal mats and desiccated microorganisms (Nkem et al., 2006).
View in article
Stibal, M., Tranter, M. (2007) Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. Journal of Geophysical Research-Biogeosciences 112, 9.
 Show in context
Show in context Some studies find that P exceeds R in cryoconite holes (Anesio et al., 2009), whilst others find that R exceeds P during the melt season (Stibal and Tranter, 2007; Bagshaw et al., 2011).
View in article
Processes of carbon cycling have long been documented in cryoconite holes (Tranter et al., 2004; Bagshaw et al., 2007; Foreman et al., 2007; Stibal and Tranter, 2007), but the extent of the dependence of photosynthetic organisms concentrated at the surface of the sediment layer on heterotrophic processes deeper in the sediment has not been fully discussed in the literature to date.
View in article
Takeuchi, N., Nishiyama, H., Li, Z. (2010) Structure and formation process of cryoconite granules on Ürümqi glacier No. 1, Tien Shan, China. Annals of Glaciology 51, 9-14, doi: 10.3189/172756411795932010.
 Show in context
Show in context Instead, cryoconite granules form, which consist of polymer-bound sediment particles and microorganisms (Langford et al., 2010; Takeuchi et al., 2010) and likely maintain ecosystem longevity.
View in article
This is similar to observations in cryoconite granules, where photosynthetic organisms are concentrated on the exterior of polymer-bound particles and heterotrophs are found in the interior (Langford et al., 2010; Takeuchi et al., 2010).
View in article
Telling, J., Anesio, A.M., Hawkings, J., Tranter, M., Wadham, J.L., Hodson, A.J., Irvine-Fynn, T., Yallop, M.L. (2010) Measuring rates of gross photosynthesis and net community production in cryoconite holes: a comparison of field methods. Annals of Glaciology 51, 153-162.
 Show in context
Show in context
Reported rates of P range from 1.3 (Bagshaw et al., 2011) to 2.4 µg C g-1 d-1 (Hodson et al., 2010a), compared with 20.9 µg C g-1 d-1 (Telling et al., 2010) to 208 µg C L-1 d-1 (Anesio et al., 2009) in cryoconite on Svalbard glaciers.
View in article
Telling et al. (2010) elaborate on some of the methodological differences between these experiments.
View in article
Standard cryoconite incubation methods using oxygen microoptodes were employed (Hodson et al., 2010a; Telling et al., 2010; Bagshaw et al., 2011), with experiment durations of one week for field incubations and 150 days for laboratory experiments.
View in article
We equate dissolved oxygen concentration with carbon production (Telling et al., 2010).
View in article
Telling, J., Anesio, A.M., Tranter, M., Stibal, M., Hawkings, J., Irvine-Fynn, T., Hodson, A., Butler, C., Yallop, M.L., Wadham, J. (2012) Controls on the autochthonous production and respiration of organic matter in cryoconite holes on High Arctic glaciers. Journal of Geophysical Research: Biogeosciences 117, G01017, doi: 10.1029/2011JG001828.
 Show in context
Show in context This inter-dependence may also help explain the link between sediment depth and P:R balance previously observed in short term (6-24 hr) cryoconite incubations (Telling et al., 2012).
View in article
Tranter, M., Fountain, A.G., Fritsen, C.H., Lyons, W.B., Priscu, J.C., Statham, P.J., Welch K.A. (2004) Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrological Processes 18, 379-387.
 Show in context
Show in context The DO concentrations in the light bottles increase to 140 % saturation after 250 hr, a value that has been observed in cryoconite holes on Canada Glacier (Tranter et al., 2004; Bagshaw et al., 2011).
View in article
Photosynthesis in a closed aquatic system increases the pH of the solution and causes increasing saturation with aragonite and calcite (Table 1; Tranter et al., 2004).
View in article
Processes of carbon cycling have long been documented in cryoconite holes (Tranter et al., 2004; Bagshaw et al., 2007; Foreman et al., 2007; Stibal and Tranter, 2007), but the extent of the dependence of photosynthetic organisms concentrated at the surface of the sediment layer on heterotrophic processes deeper in the sediment has not been fully discussed in the literature to date.
View in article
Wilson, T.R.S., Thomson, J., Colley, S., Hydes, D.J., Higgs, N.C., Sørensen, J. (1985) Early organic diagenesis: The significance of progressive subsurface oxidation fronts in pelagic sediments. Geochimica et Cosmochimica Acta 49, 811-822, doi: 10.1016/0016-7037(85)90174-7.
 Show in context
Show in context This is a similar scenario to that found at the seafloor (Wilson et al., 1985).
View in article
top
Supplementary Information

Figure S-1 (a) Absorption of solar radiation by debris results in the formation of cryoconite holes and cryolakes. (b) Examples of the clear refrozen lids of cryoconite holes on Canada Glacier (ruler = 40 cm). (c) An ice-lidded cryolake on Joyce Glacier.
Methods
Field site. Canada and Joyce Glaciers are small polar glaciers. Both are frozen to their beds and have limited drainage – all hydrological activity is confined to the top 1 m of ice in the supraglacial drainage system, which consists of a network of cracks and crevasses (some subsurface) which link cryoconite holes and cryolakes (Fountain et al., 2004
Fountain, A.G., Tranter, M., Nylen, T.H., Lewis, K.J., Mueller, D.R. (2004) Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. Journal of Glaciology 50, 35-45.
; Bagshaw et al., 2010Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Basagic, H. (2010) Dynamic behaviour of supraglacial lakes on cold polar glaciers: Canada Glacier, McMurdo Dry Valleys, Antarctica. Journal of Glaciology 56, 366-368.
). Meltwater flows for 4-12 weeks per year. Some cryoconite holes remain isolated from the surrounding drainage system for years at a time (Bagshaw et al., 2007Bagshaw, E.A., Tranter, M., Fountain, A.G., Welch, K.A., Basagic, H., Lyons, W.B. (2007) Biogeochemical evolution of cryoconite holes on Canada Glacier, Taylor Valley, Antarctica. Journal of Geophysical Research-Biogeosciences 112, 8, doi: 10.1029/2007jg000442.
). These glaciers are characteristic of the region, and are also the focus of long term studies of stream flow and nutrient flux from the glaciers into the surrounding ecosystems (www.mcmlter.org).Experimental methods. Incubation experiments were conducted using glass bottles with gas-tight stoppers, filled with 1 cm depth of cryolake sediment (selected to mimic that measured in the cryolakes) and brimmed with supraglacial meltwater (field incubations) or deionised water (laboratory). Prior to the experiment, all bottles were rinsed six times with deionised water. Clear glass bottles with small stoppers and a minimal ‘shoulder’ were selected to enhance PAR penetration to the sediment layer. Sediments were collected from the glacier surface using clean plastic gloves and bags, and transferred to the experimental vials within 24 hr (field experiments) or to the freezer (laboratory). Frozen sediment samples were returned to the UK via a temperature controlled shipment. They were melted in the dark at 4 °C prior to the experiment, and then transferred to the glass bottles via ethanol-sterilised spatula.
Three of six 150 ml glass bottles were wrapped in aluminium foil to exclude light, and an additional three bottles were filled with water alone to use as a control. In short-term, one week long field experiments, the bottles were immersed in the melt pond adjacent to Lake Hoare field camp (DLH Pond) to simulate temperature and light conditions in an open cryolake. Aliquots of 50 ml were removed from the bottles for DO analysis each day, and replaced with more supraglacial meltwater, with care taken to avoid disturbing the sediment layer. This mimics, to some extent, the inflow and outflow of meltwater from cryolakes during the height of the ablation season. DO was measured using a YSI 550A electrode, with the solution constantly stirred. The mean coefficient of variance was 0.02. In the laboratory experiments, bottles were immersed in a water bath beneath fan-cooled horticultural lighting (Envirolite) in a cold room at the University of Bristol, where the temperature, monitored throughout the experiment with a Campbell Scientific 107 probe, was maintained between 0 and 1.5 °C. PAR generated by the Envirolite rig was 60 µmol m-2 s-1. This is well below PAR recorded on the ice surface (up to 1200 µmol m-2 s-1), but is within the limits of PAR measured beneath the ice lid of the cryolake, 25 to 475 µmol m-2 s-1 (unpublished data). DO measurements were made using a needle-type optode (PreSens Microx) with a sensing tip of 140 µm, inserted into the bottles daily for the first month of the experiment, and thereafter every 7-14 days up to 145 days. Final measurements of DO, pH and major ions were then taken after 363 days, when the bottle stoppers were removed for the first time. The experiment was consistently illuminated for a whole year, without any addition of nutrients or gas ingress. In a natural system, regular melting of the sediment into the ice liberates new nutrients which are released into solution in the cryolakes (Fountain et al., 2008
Fountain, A.G., Nylen, T., Tranter, M., Bagshaw, E.A. (2008) Temporal variation of cryoconite holes on Canada Glacier, McMurdo Dry Valleys, Antarctica. Journal of Geophysical Research 112, G01S92, doi: 10.1029/2007JG000430.
). In addition, the first spring melt provides a pulse of nutrients which drives biological activity (Telling et al., 2015Telling, J., Anesio, A.M., Tranter, M. , Fountain, A.G., Nylen, T.H., Hawkings, J., Singh, V.B., Kaur, P., Musilova, M., Wadham, J. (2015) Spring thaw ionic pulses boost nutrient availability and microbial growth in entombed Antarctic Dry Valley cryoconite holes. Frontiers in Microbiology 5, 694, doi: 10.3389/fmicb.2014.00694.
). These pulses were absent from our experiment, thus we expect that rates of activity are likely lower than those found in situ.DIC was modelled using PHREEQC water speciation (USGS), assuming Na+ = Cl-. P, R and NEP as µg C g-1 sediment were calculated from the measured oxygen concentrations as in Bagshaw et al. (2011)
Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
. pH was measured using a Beckman Coulter handheld meter, calibrated using low ionic strength buffers (Camlab). Water samples were filtered through 0.45 µm membranes and analysed using a Dionex DX-120 Ion Chromatograph. Precision was <5 % for all ions, except for some Na+ samples which were excluded from later assessment because of contamination. Organic carbon content of the sediment used in the long term incubation experiments was determined using a Eurovector EA3000 Elemental Analyser, calibrated with certified acetanilide (C6H5NH(COCH3)). The detection limit was 0.01 % as organic C. Precision was ~10 % for the range of values encountered (see below). The inorganic carbonate (IC) content was measured on a Strohlein Coulomat 702 Analyser, calibrated with certified barium carbonate. The detection limit was 0.1 % as IC, and precision was ±0.5 %. Output from both instruments was validated with a Eurovector reference soil standard.Supplementary Information References
Bagshaw, E.A., Tranter, M., Fountain, A.G., Welch, K.A., Basagic, H., Lyons, W.B. (2007) Biogeochemical evolution of cryoconite holes on Canada Glacier, Taylor Valley, Antarctica. Journal of Geophysical Research-Biogeosciences 112, 8, doi: 10.1029/2007jg000442.
 Show in context
Show in context Some cryoconite holes remain isolated from the surrounding drainage system for years at a time (Bagshaw et al., 2007).
View in Supplementary Information
Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Basagic, H. (2010) Dynamic behaviour of supraglacial lakes on cold polar glaciers: Canada Glacier, McMurdo Dry Valleys, Antarctica. Journal of Glaciology 56, 366-368.
 Show in context
Show in context Both are frozen to their beds and have limited drainage – all hydrological activity is confined to the top 1 m of ice in the supraglacial drainage system, which consists of a network of cracks and crevasses (some subsurface) which link cryoconite holes and cryolakes (Fountain et al., 2004; Bagshaw et al., 2010).
View in Supplementary Information
Bagshaw, E.A., Tranter, M., Wadham, J.L., Fountain, A.G., Mowlem, M. (2011) High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes 25, 2868-2877, doi: 10.1002/hyp.8049.
 Show in context
Show in contextDIC was modelled using PHREEQC water speciation (USGS), assuming Na+ = Cl-. P, R and NEP as µg C g-1 sediment were calculated from the measured oxygen concentrations as in Bagshaw et al. (2011).
View in Supplementary Information
Fountain, A.G., Tranter, M., Nylen, T.H., Lewis, K.J., Mueller, D.R. (2004) Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. Journal of Glaciology 50, 35-45.
 Show in context
Show in context Both are frozen to their beds and have limited drainage – all hydrological activity is confined to the top 1 m of ice in the supraglacial drainage system, which consists of a network of cracks and crevasses (some subsurface) which link cryoconite holes and cryolakes (Fountain et al., 2004; Bagshaw et al., 2010).
View in Supplementary Information
Fountain, A.G., Nylen, T., Tranter, M., Bagshaw, E.A. (2008) Temporal variation of cryoconite holes on Canada Glacier, McMurdo Dry Valleys, Antarctica. Journal of Geophysical Research 112, G01S92, doi: 10.1029/2007JG000430.
 Show in context
Show in context In a natural system, regular melting of the sediment into the ice liberates new nutrients which are released into solution in the cryolakes (Fountain et al., 2008).
View in Supplementary Information
Telling, J., Anesio, A.M., Tranter, M. , Fountain, A.G., Nylen, T.H., Hawkings, J., Singh, V.B., Kaur, P., Musilova, M., Wadham, J. (2015) Spring thaw ionic pulses boost nutrient availability and microbial growth in entombed Antarctic Dry Valley cryoconite holes. Frontiers in Microbiology 5, 694, doi: 10.3389/fmicb.2014.00694.
 Show in context
Show in context In addition, the first spring melt provides a pulse of nutrients which drives biological activity (Telling et al., 2015).
View in Supplementary Information
Figures and Tables
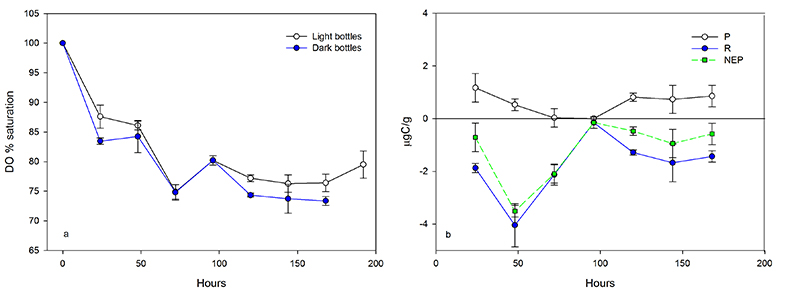
Figure 1 (a) Oxygen concentrations in dark and light bottles during the 200 hr incubation of cryolake sediment from Canada Glacier with (b) corresponding values of P, R and NEP.
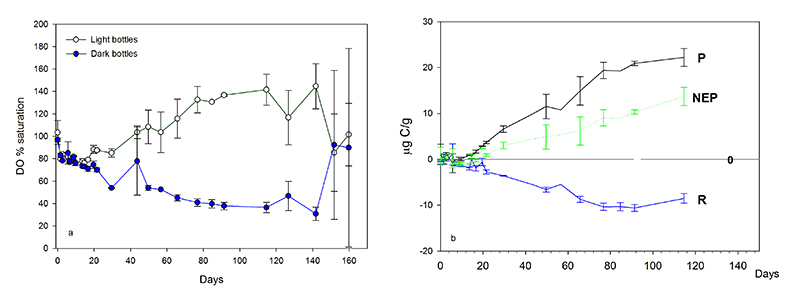
Figure 2 (a) Oxygen concentrations in dark and light bottles during the long term (one year) incubation of cryolake sediment from Joyce Glacier. (b) Corresponding values of P, R and NEP.
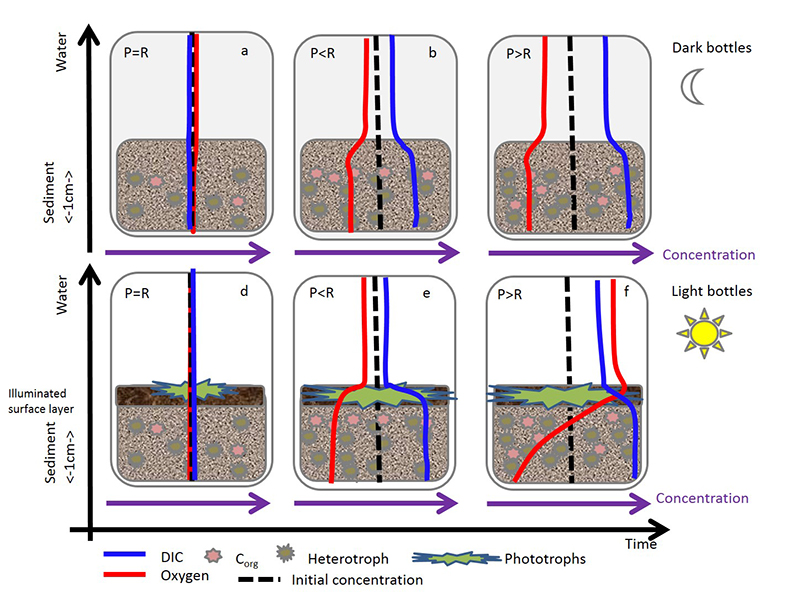
Figure 3 Conceptual model of the exchange of oxygen and DIC between the water column, heterotrophs in the deeper sediment layers and phototrophs concentrated at the sediment surface, during early (a,d) middle (b,e) and later (c,f) stages of the long-term incubation in the dark (a-c) and dark (d-f) bottles. The panels are ordered left to right by time into the experiment, and the x-axis of each panel represents DIC and O2 concentrations relative to starting values (dotted line).
Table 1 Initial deionised water (DIW) concentration and final concentrations of major ions, DO (% saturation), pH and the Saturation Index (SI) of calcite and aragonite in incubation waters after 360 days. Mean concentration (µeq L-1 minus blanks) of each bottle type is reported. DIC* and SI were calculated from mean concentrations by PHREEQC (phreeqc.dat database), assuming that T = 0 oC, and that Cl- = Na+ in the calculation of DIC. P values for one-tailed paired student’s t-tests show variance of: initial conditions vs. final dark and final light; and final light vs. final dark bottles.
| Sample | Cl- | NO3- | SO42- | K+ | Mg2+ | Ca2+ | DO | pH | DIC* | SIaragonite | SIcalcite |
| DIW (initial) | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 5.55 | 32.2 | n/a | n/a |
| Light (final) | 4.11 | 0 | 23.3 | 37.1 | 0.25 | 290 | 122 | 9.97 | 241 | -0.08 | 0.09 |
| St dev. | 0.01 | 0.04 | 8.84 | 0.5 | 8.34 | 68.7 | 8.06 | 0.49 | n/a | n/a | n/a |
| T-Test (initial vs. light) | 0 | 0 | 0.08 | 0 | 0.49 | 0.05 | 0.07 | 0.06 | 0.15 | n/a | n/a |
| Dark (final) | 4.84 | 61.9 | 24.9 | 71.1 | 48.6 | 735 | 22.5 | 8.66 | 761 | -0.24 | -0.4 |
| St dev. | 1.02 | 11.75 | 6.18 | 8.32 | 9.54 | 131 | 16.4 | 0.01 | n/a | n/a | n/a |
| T-Test (initial vs. dark) | 0.05 | 0.04 | 0.06 | 0.03 | 0.04 | 0.04 | 0.05 | 0 | 0.02 | n/a | n/a |
| T-Test (light vs. dark) | 0.25 | 0.04 | 0.45 | 0.06 | 0.01 | 0.1 | 0.05 | 0.08 | 0.01 | n/a | n/a |
Supplementary Figures and Tables
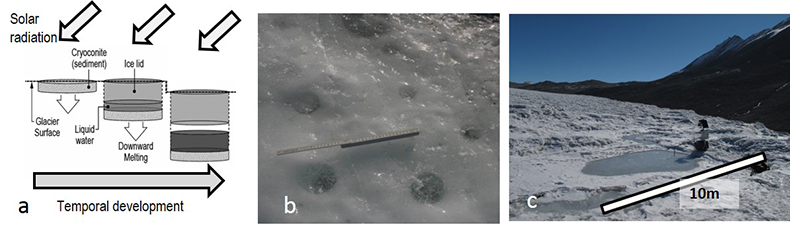
Figure S-1 (a) Absorption of solar radiation by debris results in the formation of cryoconite holes and cryolakes. (b) Examples of the clear refrozen lids of cryoconite holes on Canada Glacier (ruler = 40 cm). (c) An ice-lidded cryolake on Joyce Glacier.