Diffusive fractionation of Li isotopes in wet, highly silicic melts
Affiliations | Corresponding Author | Cite as | Funding informationHolycross, M.E., Watson, E.B., Richter, F.M., Villeneuve, J. (2018) Diffusive fractionation of Li isotopes in wet, highly silicic melts. Geochem. Persp. Let. 6, 39–42.
National Science Foundation.
- Share this article





Article views:4,612Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
for Li isotope diffusion in a dry basalt-rhyolite couple at 1350 °C. The similarity of the two values indicates little or no dependence of βLi in silicate melts on either temperature or melt composition. The new data confirm a very high potential for diffusive fractionation of 6Li from 7Li and can be confidently used to model deviations in δ7Li to determine the time-temperature histories of natural rhyolite samples.Figures and Tables
 Table 1 Experiment conditions for diffusion couple runs and measured 7Li diffusion coefficients. | 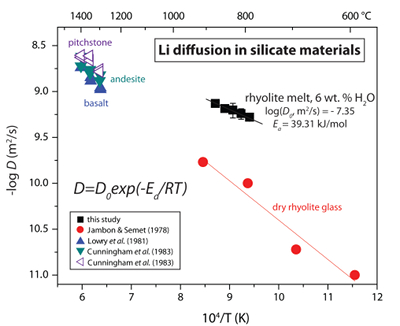 Figure 1 Arrhenius plot showing temperature dependence of Li diffusion in silicate materials. Lithium diffusion in wet rhyolite melt is significantly faster than in dry obsidian glass due to the decrease in viscosity from glass to liquid and from the addition of 6 wt. % dissolved H2O to the melt. |  Figure 2 δ7Li profiles produced from SIMS analyses. 6Li diffuses into the “low” Li glass (x > 0) faster than 7Li, fractionating the isotopes in the melt. If the Li isotopes were diffusing at the same speed in rhyolitic melt (β = 0), a smoothly varying isotope gradient would be present near x = 0. When β ≠ 0, calculated profiles show a shallow or reversed slope near the diffusion couple interface at x = 0, as is seen in the data in both panels. The kinetic fractionation of Li isotopes in hydrous rhyolitic melt is best fit by an average β = 0.228. |  Figure 3 The reported β factors for isotopes of an element i vary with its Si-normalised diffusivity, Di/DSi. Data point in red is for rhyolitic melt containing 6 wt. % H2O at 810 ºC. Silicon is an extremely slow diffuser in rhyolite while Li is extremely fast. This suggests diffusion of Li may be decoupled from the melt network and exhibit a greater mass discrimination. Figure after Watkins et al. (2017). |
| Table 1 | Figure 1 | Figure 2 | Figure 3 |
top
Introduction and Experimental Approach
The detection of large lithium isotope variations in variety of terrestrial and planetary materials has driven significant advancements in Li isotope geochemistry over the past two decades (see recent reviews by Tomascak et al., 2016
Tomascak, P.B., Magna, T., Dohmen, R. (2016) Advances in Lithium Isotopes Geochemistry. Springer-Verlag, Berlin.
and Parkinson et al., 2007Penniston-Dorland, S., Liu, X.M., Rudnick, R.L. (2017) Lithium Isotope Geochemistry. Reviews in Mineralogy and Geochemistry 82, 165-217.
for details). The ~17 % mass difference between the two isotopes of lithium (6Li and 7Li) can lead to considerable equilibrium and kinetic fractionation in Earth systems. Experimental demonstrations have shown that Li diffusion in melts and minerals is subject to a large mass effect (Richter et al., 2003Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
, 2014Richter, F., Watson, E.B., Chaussidon, M., Mendybaev, R., Ruscitto, D. (2014) Lithium isotope fractionation by diffusion in minerals. Part 1: Pyroxenes. Geochimica et Cosmochimica Acta 126, 352-370.
, 2017Richter, F. Chaussidon, M., Watson, E.B., Mendybaev, R., Homolova, V. (2017) Lithium isotope fractionation by diffusion in minerals. Part 2: Olivine. Geochimica et Cosmochimica Acta 219, 124-142.
; Dohmen et al., 2010Dohmen, R., Kasemann, S.A., Coogan, L.A., Chakraborty, S. (2010) Diffusion of Li in olivine. Part I: Experimental observations and a multi species diffusion model. Geochimica et Cosmochimica Acta 74, 274-292.
). In natural samples, diffusive fractionation of Li isotopes has been recorded at the µm to m scale (e.g., Barrat et al., 2005Barrat, J.A., Chaussidon, M., Bohn, M., Gillet, P., Gopel, C., Lesourd, M. (2005) Lithium behavior during cooling of a dry basalt: An ion-microprobe study of the lunar meteorite Northwest Africa 479 (NWA 479). Geochimica et Cosmochimica Acta 69, 5597-5609.
; Lundstrom et al., 2005Lundstrom, C.C., Chaussidon, M., Hsui, A.T., Kelemen, P., Zimmerma, M. (2005) Observations of Li isotopic variations in the Trinity Ophiolite: Evidence for isotopic fractionation by diffusion during mantle melting. Geochimica et Cosmochimica Acta 69, 735-751.
; Teng et al., 2006Teng, F.Z., McDonough, W.F., Rudnick, R.L., Walker, R.J. (2006) Diffusion-driven extreme lithium isotopic fractionation in country rocks of the Tin Mountain pegmatite. Earth and Planetary Science Letters 243, 701-710.
; Jeffcoate et al., 2007Jeffcoate, A.B., Elliott, T., Kasemann, S.A., Ionov, D., Cooper, K., Brooker, R. (2007) Li isotope fractionation in peridotites and mafic melts. Geochimica et Cosmochimica Acta 71, 202-218.
; Gao et al., 2011Gao, Y.J., Snow, J.E., Casey, J.F., Yu, J.B. (2011) Cooling-induced fractionation of mantle Li isotopes from the ultra-slow spreading Gakkel Ridge. Earth and Planetary Science Letters 301, 231-240.
). The relative diffusivities of the two isotopes of Li are expressed by the empirical constant β in the equationEq. 1
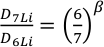
where D is the diffusivity of the individual isotopes (Richter et al., 1999
Richter, F.M., Liang, Y., Davis, A.M. (1999) Isotope fractionation by diffusion in molten oxides. Geochimica et Cosmochimica Acta 63, 2853-2861.
); the larger the value of β, the more sensitive the diffusivity is to isotope mass.Lithium’s small ionic radius and +1 valence make it an exceptionally fast diffuser in silicate melts (Jambon and Semet, 1978
Jambon, A., Semet, M.P. (1978) Lithium diffusion in silicate glasses of albite, orthoclase and obsidian composition: an ion-microprobe determination. Earth and Planetary Science Letters 37, 445-450.
; Lowry et al., 1981Lowry, R.K., Reed, S.J.B., Nolan, J., Henderson, P., Long, J.V.P. (1981) Lithium tracer-diffusion in an alkali-basaltic melt—an ion-microprobe determination. Earth and Planetary Science Letters 53, 36-40.
; Cunningham et al., 1983Cunningham, G.J., Henderson, P., Lowry, R.K., Nolan, J., Reed, S.J.B., Long, J.V.P. (1983) Lithium diffusion in silicate melts. Earth and Planetary Science Letters 65, 203-205.
; Richter et al., 2003Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
). In recent years, lithium diffusion gradients in silicate rocks have been increasingly used as geospeedometers of short-lived heating events (e.g., Coogan et al., 2005Coogan, L.A., Kasemann, S.A., Chakraborty, S. (2005) Rates of hydrothermal cooling of new oceanic crust derived from lithium-geospeedometry. Earth and Planetary Science Letters 240, 415-424.
; Parkinson et al., 2007Parkinson, I.J., Hammond, S.J., James, R.H., Rogers, N.W. (2007) High-temperature lithium isotope fractionation: Insights from lithium isotope diffusion in magmatic systems. Earth and Planetary Science Letters 257, 609-621.
; Charlier et al., 2012Charlier, B.L.A., Morgan, D.J., Wilson, C.J.N., Wooden, J.L., Allan, A.S.R., Baker, J.A. (2012) Lithium concentration gradients in feldspar and quartz record the final minutes of magma ascent in an explosive supereruption. Earth and Planetary Science Letters 319, 218-227.
; Richter et al., 2016Richter, F., Chaussidon, M., Mendybaev, R., Kite, E. (2016) Reassessing the cooling rate and geologic setting of Martian meteorites MIL 03346 and NWA 817. Geochimica et Cosmochimica Acta 182, 1-23.
). Quantitative application of Li-geospeedometry requires that the gradients used for this purpose are demonstrably due to diffusion along with knowledge of Li kinetic isotope behaviour (i.e. β factors). Richter et al. (2003)Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
reported β = 0.215 for the diffusive fractionation of Li isotopes in a dry basalt-rhyolite diffusion couple in a single experiment at 1350 ºC. It was unclear if this β value applies to Li isotope fractionation in hydrous, silicic magmatic systems in which the behaviour of Li has drawn particular attention. In view of the widespread interest in the Li geochemistry of such systems, there is a compelling need for constraints on β in pertinent melt compositions and temperatures. Additionally, the work of Watkins et al. (2009Watkins, J.M., DePaolo, D.J., Huber, C., Ryerson, F.J. (2009) Liquid composition-dependence of calcium isotope fractionation during diffusion in molten silicates. Geochimica et Cosmochimica Acta 73, 7341-7359.
, 2011Watkins, J.M., DePaolo, D.J., Ryerson, F.J., Peterson, B.T. (2011) Influence of liquid structure on diffusive isotope separation in molten silicates and aqueous solutions. Geochimica et Cosmochimica Acta 75, 3103-3118.
, 2017Watkins, J.M., DePaolo, D.J., Watson, E.B. (2017) Kinetic fractionation of non-traditional stable isotopes by diffusion and crystal growth reactions. Reviews in Mineralogy and Geochemistry 82, 85-125.
) showed significant compositional dependence of β for isotope diffusion in simple silicate liquids, providing further motivation for determining the kinetic isotope fractionation of Li in a common but not previously studied molten silicate system.To address the question of the general applicability of the earlier result of Richter et al. (2003)
Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
, we performed piston-cylinder experiments to measure the relative diffusivities of Li isotopes in a melt composition and temperature range directly relevant to highly silicic volcanism (790-875 ºC). Lithium diffusion couples were fabricated in Ag containers from two polished and juxtaposed cylinders of pre-synthesised hydrous rhyolite glass with different amounts of Li (Table S-1). The couples were placed in ¾” NaCl-Pyrex®-MgO assemblies, cold pressurised to ~12 kb and allowed to settle for 1 hr in the piston-cylinder before heating to the desired run temperature. This necessitated only minimal adjustments to the sample pressure once the run had reached the final temperature. A two-part ramping routine was used to heat the experiments quickly without significantly overshooting the desired temperature (see Supplementary Information). Experiment conditions are recorded in Table 1.Table 1 Experiment conditions for diffusion couple runs and measured 7Li diffusion coefficients.
| experiment | T (°C) | t (s) | D7Li (m2/s) | 2σ SE |
| LiDiff2 | 875 | 492 | 7.40E-10 | ~7.4 E-11 |
| LiDiff3 | 850 | 261 | 6.50E-10 | ~6.5 E-11 |
| LiDiff4* | 810 | 266 | 5.74E-10 | 7.53E-11 |
| LiDiff5 | 790 | 205 | 5.25E-10 | 5.02E-11 |
| LiDiff6* | 830 | 133 | 6.28E-10 | 1.14E-10 |
*SIMS analysis
Download in Exceltop
Lithium Diffusion Coefficients in Wet Rhyolitic Melt
Experimental glasses were analysed for 7Li using the laser ablation ICP-MS at Rensselaer Polytechnic Institute. 7Li diffusivities were derived by fitting the concentration profiles obtained from LA-ICP-MS measurements (Fig. S-2) with the time-dependent solution for diffusion in an infinite diffusion couple
Eq. 2
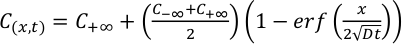
Resulting 7Li diffusivities are listed in Table 1. All diffusion couples were ramped to the final temperature as quickly as possible to limit Li diffusion in the melt during run-up. However, it is inevitable that some Li diffusion occurred during the heating interval. The effects of diffusion during ramp up are accounted for in our data reduction scheme (see Supplementary Information). In two instances where the rapid diffusivity of Li resulted in an increase in Li concentrations at the end of the diffusion couple (experiments LiDiff2 and LiDiff3), an explicit finite-difference method was used to determine Li diffusion coefficients in a confined rhyolite melt system with zero-flux boundaries.
Calculated diffusion coefficients are fit to the Arrhenius equation to demonstrate the temperature dependence of Li transport in silicate melt (Fig. 1). A linear regression fit to DLi vs. T-1 yields the Arrhenius parameters log(D0, m2/s) = -7.35 ± 0.14 and Ea = 39.31 ± 2.91 kJ/mol for Li diffusion in hydrous rhyolitic liquid. Figure 1 shows the Arrhenius relationship for Li diffusion in hydrous rhyolite melt compared to Li diffusivities in rhyolite glass and various silicate melt compositions. Lithium diffusion is very fast even in dry rhyolite glass and the significant decrease in viscosity that occurs across the glass transition and from the addition of 6 wt. % H2O to the melt network increases Li diffusivities by ~10x over the investigated T-1 range (cf. Jambon and Semet, 1978
Jambon, A., Semet, M.P. (1978) Lithium diffusion in silicate glasses of albite, orthoclase and obsidian composition: an ion-microprobe determination. Earth and Planetary Science Letters 37, 445-450.
). As water is added to silicate liquid, OH- molecules break bridging oxygen bonds, decreasing melt viscosity and increasing cation diffusivities (e.g., Watson, 1979Watson, E.B. (1979) Diffusion of cesium ions in H2O-saturated granitic melt. Science 205, 1259-1260.
, 1981Watson, E.B. (1981) Diffusion in magmas at depth in the Earth: The effects of pressure and dissolved H2O. Earth and Planetary Science Letters 52, 291-301.
; Zhang et al., 2003Zhang, Y., Xu, Z., Liu, Y. (2003) Viscosity of hydrous rhyolitic melts inferred from kinetic experiments, and a new viscosity model. American Mineralogist 88, 1741-1752.
and many others). The rapid diffusion of Li in hydrous, highly silicic melts implies that any gradients in total Li in rhyolite magma systems will be quickly homogenised while other trace element concentration gradients may persist (Holycross and Watson, 2016aHolycross, M.E., Watson, E.B. (2016a) Diffusive fractionation of 25 trace elements in basaltic and rhyolitic melts (abstract). 2016 AGU Fall Meeting, San Francisco, CA, http://adsabs.harvard.edu/abs/2016AGUFMMR51A2689H.
).
Figure 1 Arrhenius plot showing temperature dependence of Li diffusion in silicate materials. Lithium diffusion in wet rhyolite melt is significantly faster than in dry obsidian glass due to the decrease in viscosity from glass to liquid and from the addition of 6 wt. % dissolved H2O to the melt.
top
Lithium Isotope Fractionation in Wet Rhyolitic Melt
To assess possible effects of melt composition on βLi, we determined the relative diffusivities of the two Li isotopes in our hydrous rhyolite melts for comparison with the results of Richter et al. (2003)
Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
. Lithium isotopic analyses were carried out on a Cameca IMS 1270-E7 ion probe at the Centre de Recherches Pétrographiques et Géochimiques (CRPG) in Nancy, France. Information about the data collection routine is listed in the Supplementary Information. Lithium isotope fractionation profiles for two experiments are presented in Figure 2. Ion probe analyses revealed the two glasses used to form the diffusion couples had different 7Li/6Li compositions. Diffusion between the two glasses with different 7Li/6Li produced a somewhat unconventional δ7Li profile characterised by a pronounced shoulder in the diffusion couple.The β value for Li isotope diffusion in rhyolite was determined by generating individual model concentration profiles of 7Li and 6Li and then comparing the model 7Li/6Li values to the SIMS data. Model Li isotope profiles for experiments LiDiff4 and LiDiff6 were calculated using the solutions given by Equations 1 and 2. 7Li diffusion coefficients obtained from least-squares fits to measured LA-ICP-MS profiles were kept constant while the diffusivity of 6Li was varied so that the output of the 7Li/6Li model profile best matched the 7Li/6Li SIMS profile, as evaluated by obtaining the smallest χ2 parameter for each ratio profile (Holycross and Watson, 2016b
Holycross, M.E., Watson, E.B. (2016b) Diffusive fractionation of trace elements in basaltic melt. Contributions to Mineralogy and Petrology 171, 1-15.
).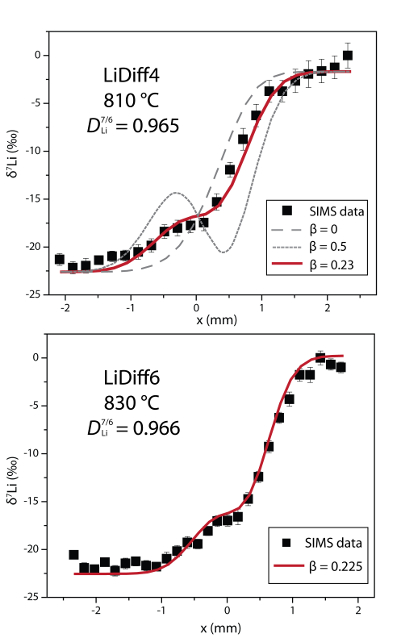
Figure 2 δ7Li profiles produced from SIMS analyses. 6Li diffuses into the “low” Li glass (x > 0) faster than 7Li, fractionating the isotopes in the melt. If the Li isotopes were diffusing at the same speed in rhyolitic melt (β = 0), a smoothly varying isotope gradient would be present near x = 0. When β ≠ 0, calculated profiles show a shallow or reversed slope near the diffusion couple interface at x = 0, as is seen in the data in both panels. The kinetic fractionation of Li isotopes in hydrous rhyolitic melt is best fit by an average β = 0.228.
In Figure 2, the Li fractionation data from experiment LiDiff4 is compared to profiles calculated with various β factors showing that β = 0.23 is the best fit. When β ≠ 0, the different diffusivities of 7Li and 6Li create a shoulder or reversal in the δ7Li profile near the couple interface. In the present experiments a δ7Li sigmoidal profile is superimposed on the gradient due to the two end members having different δ7Li. The overall good fit of the calculated Li fractionation profiles to the measured data confirms that 7Li and 6Li diffuse at different rates. Kinetic fractionation profiles from LiDiff4 and LiDiff6 are best fit when 6Li diffuses ~3.5 % faster than 7Li in the melt (i.e. DLi7/6 = 0.965), corresponding to an average β of 0.228.
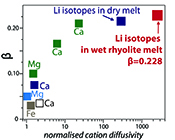
The global dataset of experimentally-determined β values (β > 0) for fractionation in silicate liquids spans βCa = 0.035 to our new value of βLi = 0.228 (compiled by Watkins et al., 2017
Watkins, J.M., DePaolo, D.J., Watson, E.B. (2017) Kinetic fractionation of non-traditional stable isotopes by diffusion and crystal growth reactions. Reviews in Mineralogy and Geochemistry 82, 85-125.
). The βLi determined here for diffusion in hydrous obsidian melt at 790-875 °C is close to the value of Richter et al. (2003)Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
, who found β = 0.215 for Li isotopes in dry silicate melt in a single experiment at 1350 ºC. It is unclear if our new value and that of Richter et al. (2003)Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
are statistically different by a small amount given the difficulty of assigning realistic uncertainties for such a small number of experiments. Regardless, the key point is that the βLi values are remarkably similar despite very different system compositions and temperatures.The similarity of βLi for both systems may be a consequence of Li decoupling from the silicate melt network during diffusion. Watkins et al. (2009
Watkins, J.M., DePaolo, D.J., Huber, C., Ryerson, F.J. (2009) Liquid composition-dependence of calcium isotope fractionation during diffusion in molten silicates. Geochimica et Cosmochimica Acta 73, 7341-7359.
, 2011Watkins, J.M., DePaolo, D.J., Ryerson, F.J., Peterson, B.T. (2011) Influence of liquid structure on diffusive isotope separation in molten silicates and aqueous solutions. Geochimica et Cosmochimica Acta 75, 3103-3118.
, 2017Watkins, J.M., DePaolo, D.J., Watson, E.B. (2017) Kinetic fractionation of non-traditional stable isotopes by diffusion and crystal growth reactions. Reviews in Mineralogy and Geochemistry 82, 85-125.
) proposed that the β factor for isotopic fractionation of an element i in simple silicate melt may be a function of its Si-normalised diffusivity, Di/DSi (Fig. 3). Faster diffusing elements may be moving as single atoms in the melt network and thus show a greater isotope mass discrimination because their diffusion is not correlated with the mobilities of other network elements. At very large values of Di/DSi, changes in βi in different melt compositions are minimal. This is observed comparing our new data to that of Richter et al. (2003)Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
. At 810 ºC in a rhyolite melt with 6 wt. % H2O, DLi/DSi≈ 2560 (Si value from Baker and Bossányi, 1994Baker, D.R., Bossányi, H. (1994) The combined effect of F and H2O on interdiffusion between peralkaline dacitic and rhyolitic melts. Contributions to Mineralogy and Petrology 117, 203-214.
), roughly an order of magnitude greater than DLi/DSi in the experiment of Richter et al. (2003)Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
and yet they have very similar values of βLi. The high diffusivity of Li in wet rhyolite and the insensitivity of βLi to liquid composition indicates Li isotope diffusion is independent of melt structure. This suggests that Li may jump primarily between coordinated sites in the melt network, perhaps not unlike the fast diffusion of Li via an interstitial mechanism in crystalline mineral phases (e.g., Mullen, 1961Mullen, J.G. (1961) Isotope effect in intermetallic diffusion. Physical Review 121, 1649.
; Dohmen et al., 2010Dohmen, R., Kasemann, S.A., Coogan, L.A., Chakraborty, S. (2010) Diffusion of Li in olivine. Part I: Experimental observations and a multi species diffusion model. Geochimica et Cosmochimica Acta 74, 274-292.
; Richter et al. 2014Richter, F., Watson, E.B., Chaussidon, M., Mendybaev, R., Ruscitto, D. (2014) Lithium isotope fractionation by diffusion in minerals. Part 1: Pyroxenes. Geochimica et Cosmochimica Acta 126, 352-370.
, 2017Richter, F. Chaussidon, M., Watson, E.B., Mendybaev, R., Homolova, V. (2017) Lithium isotope fractionation by diffusion in minerals. Part 2: Olivine. Geochimica et Cosmochimica Acta 219, 124-142.
).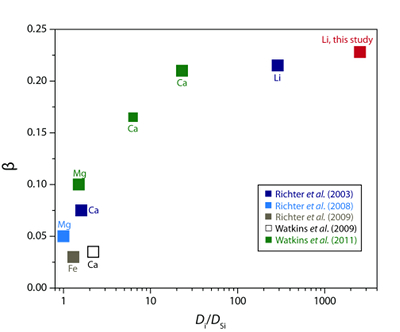
Figure 3 The reported β factors for isotopes of an element i vary with its Si-normalised diffusivity, Di/DSi. Data point in red is for rhyolitic melt containing 6 wt. % H2O at 810 ºC. Silicon is an extremely slow diffuser in rhyolite while Li is extremely fast. This suggests diffusion of Li may be decoupled from the melt network and exhibit a greater mass discrimination. Figure after Watkins et al. (2017)
Watkins, J.M., DePaolo, D.J., Watson, E.B. (2017) Kinetic fractionation of non-traditional stable isotopes by diffusion and crystal growth reactions. Reviews in Mineralogy and Geochemistry 82, 85-125.
.The vital aspect of our measurements is that they can be confidently applied to Li isotope diffusion profiles in rhyolitic rock specimens to determine the timescales of rapid heating events in highly silicic volcanic systems. Diffusive fractionation of Li isotopes in our experiments demonstrate the potential for creating considerable variations in the δ7Li of natural magmas during kinetically controlled growth of bubbles or crystalline mineral phases (Koga et al., 2011
Koga, K.T., Rose, E.F., Laporte, D., Cluzel, N., Shimizu, N. (2011) Lithium-boron isotope fractionation during degassing of rhyolitic magma. Mineralogical Magazine 75, 1211.
; Watson, 2017Watson, E.B. (2017) Diffusive fractionation of volatiles and their isotopes during bubble growth in magmas. Contributions to Mineralogy and Petrology 172, 61.
). This raises the possibility that the isotopic composition of Li in rhyolitic rock systems may record significant kinetic fractionation, which can be modelled using βLi derived from the experiments reported here.top
Acknowledgements
This study was supported in part by NSF grant no. EAR-0948204 to EBW. We thank Jared Singer for assistance with the LA-ICPMS. Comments by Ming Tang and an anonymous reviewer improved an earlier version of this manuscript.
Editor: Helen Williams
top
References
Baker, D.R., Bossányi, H. (1994) The combined effect of F and H2O on interdiffusion between peralkaline dacitic and rhyolitic melts. Contributions to Mineralogy and Petrology 117, 203-214.
 Show in context
Show in context At 810 ºC in a rhyolite melt with 6 wt. % H2O, DLi/DSi ≈ 2560 (Si value from Baker and Bossányi, 1994), roughly an order of magnitude greater than DLi/DSi in the experiment of Richter et al. (2003) and yet they have very similar values of βLi.
View in article
Barrat, J.A., Chaussidon, M., Bohn, M., Gillet, P., Gopel, C., Lesourd, M. (2005) Lithium behavior during cooling of a dry basalt: An ion-microprobe study of the lunar meteorite Northwest Africa 479 (NWA 479). Geochimica et Cosmochimica Acta 69, 5597-5609.
 Show in context
Show in contextIn natural samples, diffusive fractionation of Li isotopes has been recorded at the µm to m scale (e.g., Barrat et al., 2005; Lundstrom et al., 2005; Teng et al., 2006; Jeffcoate et al., 2007; Gao et al., 2011).
View in article
Charlier, B.L.A., Morgan, D.J., Wilson, C.J.N., Wooden, J.L., Allan, A.S.R., Baker, J.A. (2012) Lithium concentration gradients in feldspar and quartz record the final minutes of magma ascent in an explosive supereruption. Earth and Planetary Science Letters 319, 218-227.
 Show in context
Show in contextIn recent years, lithium diffusion gradients in silicate rocks have been increasingly used as geospeedometers of short-lived heating events (e.g., Coogan et al., 2005; Parkinson et al., 2007; Charlier et al., 2012; Richter et al., 2016).
View in article
Coogan, L.A., Kasemann, S.A., Chakraborty, S. (2005) Rates of hydrothermal cooling of new oceanic crust derived from lithium-geospeedometry. Earth and Planetary Science Letters 240, 415-424.
 Show in context
Show in contextIn recent years, lithium diffusion gradients in silicate rocks have been increasingly used as geospeedometers of short-lived heating events (e.g., Coogan et al., 2005; Parkinson et al., 2007; Charlier et al., 2012; Richter et al., 2016).
View in article
Cunningham, G.J., Henderson, P., Lowry, R.K., Nolan, J., Reed, S.J.B., Long, J.V.P. (1983) Lithium diffusion in silicate melts. Earth and Planetary Science Letters 65, 203-205.
 Show in context
Show in context Lithium’s small ionic radius and +1 valence make it an exceptionally fast diffuser in silicate melts (Jambon and Semet, 1978; Lowry et al., 1981; Cunningham et al., 1983; Richter et al., 2003).
View in article
Figure 1
View in article
Dohmen, R., Kasemann, S.A., Coogan, L.A., Chakraborty, S. (2010) Diffusion of Li in olivine. Part I: Experimental observations and a multi species diffusion model. Geochimica et Cosmochimica Acta 74, 274-292.
 Show in context
Show in contextExperimental demonstrations have shown that Li diffusion in melts and minerals is subject to a large mass effect (Richter et al., 2003, 2014, 2017; Dohmen et al., 2010).
View in article
This suggests that Li may jump primarily between coordinated sites in the melt network, perhaps not unlike the fast diffusion of Li via an interstitial mechanism in crystalline mineral phases (e.g., Mullen, 1961; Dohmen et al., 2010; Richter et al. 2014, 2017).
View in article
Gao, Y.J., Snow, J.E., Casey, J.F., Yu, J.B. (2011) Cooling-induced fractionation of mantle Li isotopes from the ultra-slow spreading Gakkel Ridge. Earth and Planetary Science Letters 301, 231-240.
 Show in context
Show in context In natural samples, diffusive fractionation of Li isotopes has been recorded at the µm to m scale (e.g., Barrat et al., 2005; Lundstrom et al., 2005; Teng et al., 2006; Jeffcoate et al., 2007; Gao et al., 2011).
View in article
Holycross, M.E., Watson, E.B. (2016a) Diffusive fractionation of 25 trace elements in basaltic and rhyolitic melts (abstract). 2016 AGU Fall Meeting, San Francisco, CA, http://adsabs.harvard.edu/abs/2016AGUFMMR51A2689H.
 Show in context
Show in contextThe rapid diffusion of Li in hydrous, highly silicic melts implies that any gradients in total Li in rhyolite magma systems will be quickly homogenised while other trace element concentration gradients may persist (Holycross and Watson, 2016a).
View in article
Holycross, M.E., Watson, E.B. (2016b) Diffusive fractionation of trace elements in basaltic melt. Contributions to Mineralogy and Petrology 171, 1-15.
 Show in context
Show in context 7Li diffusion coefficients obtained from least-squares fits to measured LA-ICP-MS profiles were kept constant while the diffusivity of 6Li was varied so that the output of the 7Li/6Li model profile best matched the 7Li/6Li SIMS profile, as evaluated by obtaining the smallest χ2 parameter for each ratio profile (Holycross and Watson, 2016b).
View in article
Jambon, A., Semet, M.P. (1978) Lithium diffusion in silicate glasses of albite, orthoclase and obsidian composition: an ion-microprobe determination. Earth and Planetary Science Letters 37, 445-450.
 Show in context
Show in context Lithium’s small ionic radius and +1 valence make it an exceptionally fast diffuser in silicate melts (Jambon and Semet, 1978; Lowry et al., 1981; Cunningham et al., 1983; Richter et al., 2003).
View in article
Lithium diffusion is very fast even in dry rhyolite glass and the significant decrease in viscosity that occurs across the glass transition and from the addition of 6 wt. % H2O to the melt network increases Li diffusivities by ~10x over the investigated T-1 range (cf. Jambon and Semet, 1978).
View in article
Figure 1
View in article
Jeffcoate, A.B., Elliott, T., Kasemann, S.A., Ionov, D., Cooper, K., Brooker, R. (2007) Li isotope fractionation in peridotites and mafic melts. Geochimica et Cosmochimica Acta 71, 202-218.
 Show in context
Show in contextIn natural samples, diffusive fractionation of Li isotopes has been recorded at the µm to m scale (e.g., Barrat et al., 2005; Lundstrom et al., 2005; Teng et al., 2006; Jeffcoate et al., 2007; Gao et al., 2011).
View in article
Koga, K.T., Rose, E.F., Laporte, D., Cluzel, N., Shimizu, N. (2011) Lithium-boron isotope fractionation during degassing of rhyolitic magma. Mineralogical Magazine 75, 1211.
 Show in context
Show in context Diffusive fractionation of Li isotopes in our experiments demonstrate the potential for creating considerable variations in the δ7Li of natural magmas during kinetically controlled growth of bubbles or crystalline mineral phases (Koga et al., 2011; Watson, 2017).
View in article
Lowry, R.K., Reed, S.J.B., Nolan, J., Henderson, P., Long, J.V.P. (1981) Lithium tracer-diffusion in an alkali-basaltic melt—an ion-microprobe determination. Earth and Planetary Science Letters 53, 36-40.
 Show in context
Show in contextLithium’s small ionic radius and +1 valence make it an exceptionally fast diffuser in silicate melts (Jambon and Semet, 1978; Lowry et al., 1981; Cunningham et al., 1983; Richter et al., 2003).
View in article
Figure 1
View in article
Lundstrom, C.C., Chaussidon, M., Hsui, A.T., Kelemen, P., Zimmerma, M. (2005) Observations of Li isotopic variations in the Trinity Ophiolite: Evidence for isotopic fractionation by diffusion during mantle melting. Geochimica et Cosmochimica Acta 69, 735-751.
 Show in context
Show in context In natural samples, diffusive fractionation of Li isotopes has been recorded at the µm to m scale (e.g., Barrat et al., 2005; Lundstrom et al., 2005; Teng et al., 2006; Jeffcoate et al., 2007; Gao et al., 2011).
View in article
Mullen, J.G. (1961) Isotope effect in intermetallic diffusion. Physical Review 121, 1649.
 Show in context
Show in context This suggests that Li may jump primarily between coordinated sites in the melt network, perhaps not unlike the fast diffusion of Li via an interstitial mechanism in crystalline mineral phases (e.g., Mullen, 1961; Dohmen et al., 2010; Richter et al. 2014, 2017).
View in article
Parkinson, I.J., Hammond, S.J., James, R.H., Rogers, N.W. (2007) High-temperature lithium isotope fractionation: Insights from lithium isotope diffusion in magmatic systems. Earth and Planetary Science Letters 257, 609-621.
 Show in context
Show in context In recent years, lithium diffusion gradients in silicate rocks have been increasingly used as geospeedometers of short-lived heating events (e.g., Coogan et al., 2005; Parkinson et al., 2007; Charlier et al., 2012; Richter et al., 2016).
View in article
Penniston-Dorland, S., Liu, X.M., Rudnick, R.L. (2017) Lithium Isotope Geochemistry. Reviews in Mineralogy and Geochemistry 82, 165-217.
 Show in context
Show in context The detection of large lithium isotope variations in variety of terrestrial and planetary materials has driven significant advancements in Li isotope geochemistry over the past two decades (see recent reviews by Tomascak et al., 2016 and Penniston-Dorland et al., 2017 for details).
View in article
Richter, F.M., Liang, Y., Davis, A.M. (1999) Isotope fractionation by diffusion in molten oxides. Geochimica et Cosmochimica Acta 63, 2853-2861.
 Show in context
Show in context The relative diffusivities of the two isotopes of Li are expressed by the empirical constant β in the equation 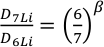 where D is the diffusivity of the individual isotopes (Richter et al., 1999); the larger the value of β, the more sensitive the diffusivity is to isotope mass.
where D is the diffusivity of the individual isotopes (Richter et al., 1999); the larger the value of β, the more sensitive the diffusivity is to isotope mass.
View in article
Richter, F.M., Davis, A.M., DePaolo, D.J., Watson, E.B. (2003) Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochimica et Cosmochimica Acta 67, 3905-3923.
 Show in context
Show in context This value is very close to β = 0.215 determined by Richter et al. (2003) for Li isotope diffusion in a dry basalt-rhyolite couple at 1350 °C.
View in article
Experimental demonstrations have shown that Li diffusion in melts and minerals is subject to a large mass effect (Richter et al., 2003, 2014, 2017; Dohmen et al., 2010).
View in article
Lithium’s small ionic radius and +1 valence make it an exceptionally fast diffuser in silicate melts (Jambon and Semet, 1978; Lowry et al., 1981; Cunningham et al., 1983; Richter et al., 2003).
View in article
Richter et al. (2003) reported β = 0.215 for the diffusive fractionation of Li isotopes in a dry basalt-rhyolite diffusion couple in a single experiment at 1350 ºC.
View in article
To address the question of the general applicability of the earlier result of Richter et al. (2003), we performed piston-cylinder experiments to measure the relative diffusivities of Li isotopes in a melt composition and temperature range directly relevant to highly silicic volcanism (790-875 ºC).
View in article
To assess possible effects of melt composition on βLi, we determined the relative diffusivities of the two Li isotopes in our hydrous rhyolite melts for comparison with the results of Richter et al. (2003).
View in article
The βLi determined here for diffusion in hydrous obsidian melt at 790-875 °C is close to the value of Richter et al. (2003), who found β = 0.215 for Li isotopes in dry silicate melt in a single experiment at 1350 ºC.
View in article
It is unclear if our new value and that of Richter et al. (2003) are statistically different by a small amount given the difficulty of assigning realistic uncertainties for such a small number of experiments.
View in article
This is observed comparing our new data to that of Richter et al. (2003).
View in article
At 810 ºC in a rhyolite melt with 6 wt. % H2O, DLi/DSi ≈ 2560 (Si value from Baker and Bossányi, 1994), roughly an order of magnitude greater than DLi/DSi in the experiment of Richter et al. (2003) and yet they have very similar values of βLi.
View in article
Figure 3
View in article
Richter, F.M., Watson, E.B., Mendybaev, R.A., Teng, F.Z., Janney, P.E. (2008) Magnesium isotope fractionation in silicate melts by chemical and thermal diffusion. Geochimica et Cosmochimica Acta 72, 206-220.
 Show in context
Show in context Figure 3
View in article
Richter, F.M., Watson, E.B., Mendybaev, R., Dauphas, N., Georg, B., Watkins, J., Valley, J. (2009) Isotopic fractionation of the major elements of molten basalt by chemical and thermal diffusion. Geochimica et Cosmochimica Acta 73, 4250-4263.
 Show in context
Show in contextFigure 3
View in article
Richter, F., Watson, E.B., Chaussidon, M., Mendybaev, R., Ruscitto, D. (2014) Lithium isotope fractionation by diffusion in minerals. Part 1: Pyroxenes. Geochimica et Cosmochimica Acta 126, 352-370.
 Show in context
Show in context Experimental demonstrations have shown that Li diffusion in melts and minerals is subject to a large mass effect (Richter et al., 2003, 2014, 2017; Dohmen et al., 2010).
View in article
This suggests that Li may jump primarily between coordinated sites in the melt network, perhaps not unlike the fast diffusion of Li via an interstitial mechanism in crystalline mineral phases (e.g., Mullen, 1961; Dohmen et al., 2010; Richter et al. 2014, 2017).
View in article
Richter, F., Chaussidon, M., Mendybaev, R., Kite, E. (2016) Reassessing the cooling rate and geologic setting of Martian meteorites MIL 03346 and NWA 817. Geochimica et Cosmochimica Acta 182, 1-23.
 Show in context
Show in contextIn recent years, lithium diffusion gradients in silicate rocks have been increasingly used as geospeedometers of short-lived heating events (e.g., Coogan et al., 2005; Parkinson et al., 2007; Charlier et al., 2012; Richter et al., 2016).
View in article
Richter, F. Chaussidon, M., Watson, E.B., Mendybaev, R., Homolova, V. (2017) Lithium isotope fractionation by diffusion in minerals. Part 2: Olivine. Geochimica et Cosmochimica Acta 219, 124-142.
 Show in context
Show in context Experimental demonstrations have shown that Li diffusion in melts and minerals is subject to a large mass effect (Richter et al., 2003, 2014, 2017; Dohmen et al., 2010).
View in article
This suggests that Li may jump primarily between coordinated sites in the melt network, perhaps not unlike the fast diffusion of Li via an interstitial mechanism in crystalline mineral phases (e.g., Mullen, 1961; Dohmen et al., 2010; Richter et al. 2014, 2017).
View in article
Teng, F.Z., McDonough, W.F., Rudnick, R.L., Walker, R.J. (2006) Diffusion-driven extreme lithium isotopic fractionation in country rocks of the Tin Mountain pegmatite. Earth and Planetary Science Letters 243, 701-710.
 Show in context
Show in contextIn natural samples, diffusive fractionation of Li isotopes has been recorded at the µm to m scale (e.g., Barrat et al., 2005; Lundstrom et al., 2005; Teng et al., 2006; Jeffcoate et al., 2007; Gao et al., 2011).
View in article
Tomascak, P.B., Magna, T., Dohmen, R. (2016) Advances in Lithium Isotopes Geochemistry. Springer-Verlag, Berlin.
 Show in context
Show in context The detection of large lithium isotope variations in variety of terrestrial and planetary materials has driven significant advancements in Li isotope geochemistry over the past two decades (see recent reviews by Tomascak et al., 2016 and Penniston-Dorland et al., 2017 for details).
View in article
Watkins, J.M., DePaolo, D.J., Huber, C., Ryerson, F.J. (2009) Liquid composition-dependence of calcium isotope fractionation during diffusion in molten silicates. Geochimica et Cosmochimica Acta 73, 7341-7359.
 Show in context
Show in context Additionally, the work of Watkins et al. (2009, 2011, 2017) showed significant compositional dependence of β for isotope diffusion in simple silicate liquids, providing further motivation for determining the kinetic isotope fractionation of Li in a common but not previously studied molten silicate system.
View in article
Watkins et al. (2009, 2011, 2017) proposed that the β factor for isotopic fractionation of an element i in simple silicate melt may be a function of its Si-normalised diffusivity, Di/DSi (Fig. 3).
View in article
Figure 3
View in article
Watkins, J.M., DePaolo, D.J., Ryerson, F.J., Peterson, B.T. (2011) Influence of liquid structure on diffusive isotope separation in molten silicates and aqueous solutions. Geochimica et Cosmochimica Acta 75, 3103-3118.
 Show in context
Show in contextAdditionally, the work of Watkins et al. (2009, 2011, 2017) showed significant compositional dependence of β for isotope diffusion in simple silicate liquids, providing further motivation for determining the kinetic isotope fractionation of Li in a common but not previously studied molten silicate system.
View in article
Watkins et al. (2009, 2011, 2017) proposed that the β factor for isotopic fractionation of an element i in simple silicate melt may be a function of its Si-normalised diffusivity, Di/DSi (Fig. 3).
View in article
Figure 3
View in article
Watkins, J.M., DePaolo, D.J., Watson, E.B. (2017) Kinetic fractionation of non-traditional stable isotopes by diffusion and crystal growth reactions. Reviews in Mineralogy and Geochemistry 82, 85-125.
 Show in context
Show in context Additionally, the work of Watkins et al. (2009, 2011, 2017) showed significant compositional dependence of β for isotope diffusion in simple silicate liquids, providing further motivation for determining the kinetic isotope fractionation of Li in a common but not previously studied molten silicate system.
View in article
The global dataset of experimentally-determined β values (β > 0) for fractionation in silicate liquids spans βCa = 0.035 to our new value of βLi = 0.228 (compiled by Watkins et al., 2017).
View in article
Watkins et al. (2009, 2011, 2017) proposed that the β factor for isotopic fractionation of an element i in simple silicate melt may be a function of its Si-normalised diffusivity, Di/DSi (Fig. 3).
View in article
Figure 3 [...] Figure after Watkins et al. (2017).
View in article
Watson, E.B. (1979) Diffusion of cesium ions in H2O-saturated granitic melt. Science 205, 1259-1260.
 Show in context
Show in context As water is added to silicate liquid, OH- molecules break bridging oxygen bonds, decreasing melt viscosity and increasing cation diffusivities (e.g., Watson, 1979, 1981; Zhang et al., 2003 and many others).
View in article
Watson, E.B. (1981) Diffusion in magmas at depth in the Earth: The effects of pressure and dissolved H2O. Earth and Planetary Science Letters 52, 291-301.
 Show in context
Show in context As water is added to silicate liquid, OH- molecules break bridging oxygen bonds, decreasing melt viscosity and increasing cation diffusivities (e.g., Watson, 1979, 1981; Zhang et al., 2003 and many others).
View in article
Watson, E.B. (2017) Diffusive fractionation of volatiles and their isotopes during bubble growth in magmas. Contributions to Mineralogy and Petrology 172, 61.
 Show in context
Show in context Diffusive fractionation of Li isotopes in our experiments demonstrate the potential for creating considerable variations in the δ7Li of natural magmas during kinetically controlled growth of bubbles or crystalline mineral phases (Koga et al., 2011; Watson, 2017).
View in article
Zhang, Y., Xu, Z., Liu, Y. (2003) Viscosity of hydrous rhyolitic melts inferred from kinetic experiments, and a new viscosity model. American Mineralogist 88, 1741-1752.
 Show in context
Show in context As water is added to silicate liquid, OH- molecules break bridging oxygen bonds, decreasing melt viscosity and increasing cation diffusivities (e.g., Watson, 1979, 1981; Zhang et al., 2003 and many others).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Experimental Methods
- Analytical Methods
- Table S-1
- Figures S-1 and S-2
- Supplementary Information References
Figures and Tables
Table 1 Experiment conditions for diffusion couple runs and measured 7Li diffusion coefficients.
| experiment | T (°C) | t (s) | D7Li (m2/s) | 2σ SE |
| LiDiff2 | 875 | 492 | 7.40E-10 | ~7.4 E-11 |
| LiDiff3 | 850 | 261 | 6.50E-10 | ~6.5 E-11 |
| LiDiff4* | 810 | 266 | 5.74E-10 | 7.53E-11 |
| LiDiff5 | 790 | 205 | 5.25E-10 | 5.02E-11 |
| LiDiff6* | 830 | 133 | 6.28E-10 | 1.14E-10 |
*SIMS analysis
Back to article | Download in Excel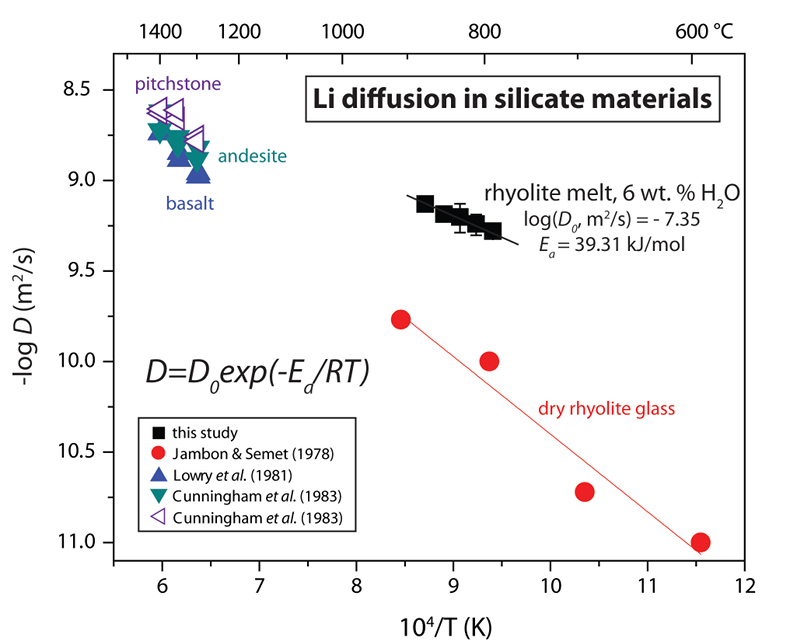
Figure 1 Arrhenius plot showing temperature dependence of Li diffusion in silicate materials. Lithium diffusion in wet rhyolite melt is significantly faster than in dry obsidian glass due to the decrease in viscosity from glass to liquid and from the addition of 6 wt. % dissolved H2O to the melt.
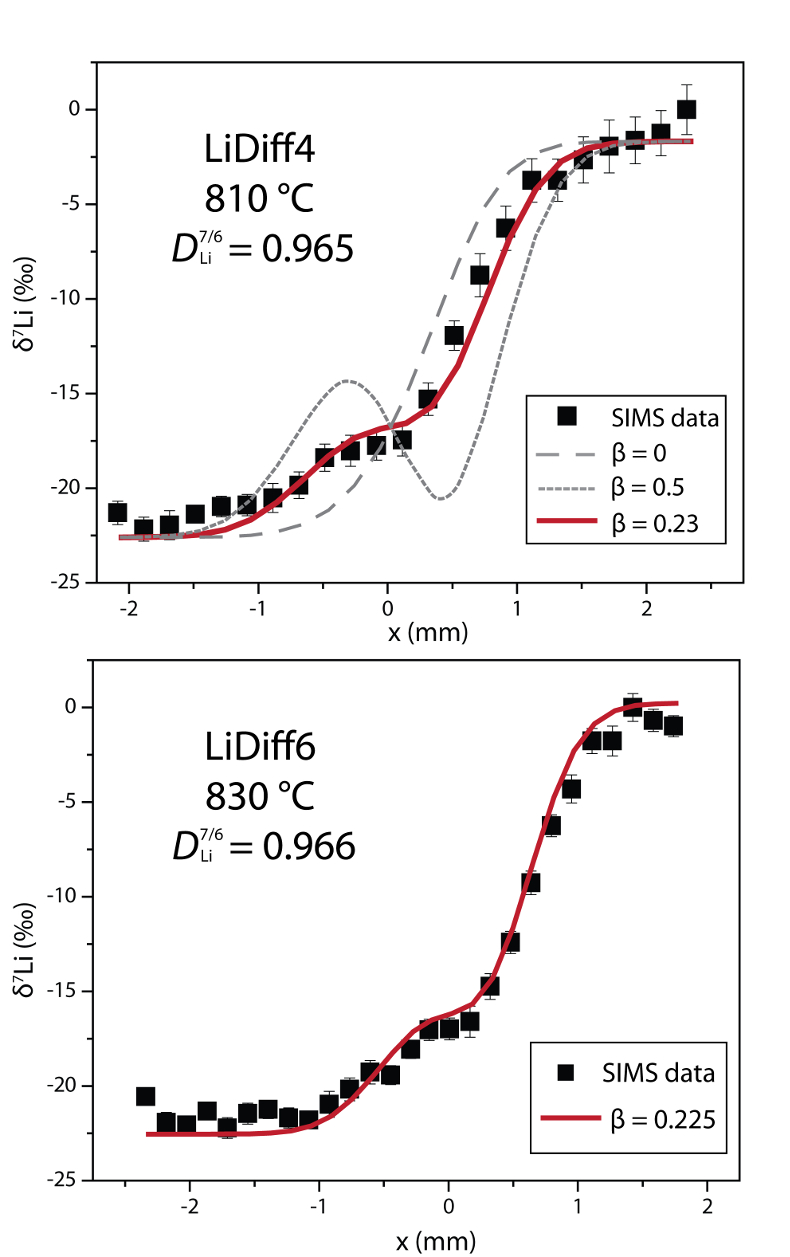
Figure 2 δ7Li profiles produced from SIMS analyses. 6Li diffuses into the “low” Li glass (x > 0) faster than 7Li, fractionating the isotopes in the melt. If the Li isotopes were diffusing at the same speed in rhyolitic melt (β = 0), a smoothly varying isotope gradient would be present near x = 0. When β ≠ 0, calculated profiles show a shallow or reversed slope near the diffusion couple interface at x = 0, as is seen in the data in both panels. The kinetic fractionation of Li isotopes in hydrous rhyolitic melt is best fit by an average β = 0.228.
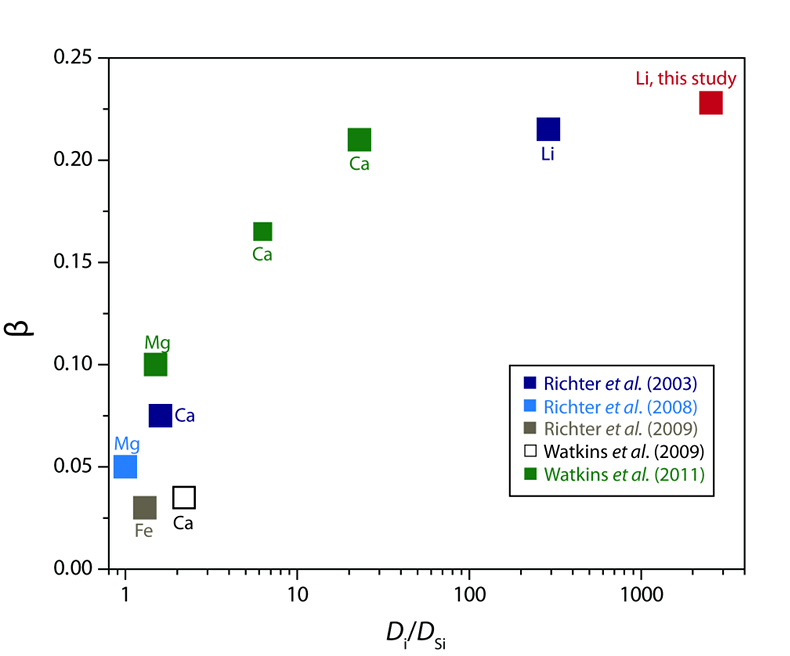
Figure 3 The reported β factors for isotopes of an element i vary with its Si-normalised diffusivity, Di/DSi. Data point in red is for rhyolitic melt containing 6 wt. % H2O at 810 ºC. Silicon is an extremely slow diffuser in rhyolite while Li is extremely fast. This suggests diffusion of Li may be decoupled from the melt network and exhibit a greater mass discrimination. Figure after Watkins et al. (2017)
Watkins, J.M., DePaolo, D.J., Watson, E.B. (2017) Kinetic fractionation of non-traditional stable isotopes by diffusion and crystal growth reactions. Reviews in Mineralogy and Geochemistry 82, 85-125.
.